Holocene paleohydrology from Lake of the Woods and Shoal ...
The Epifaunal Marine Bivalves and Macrophytes in Merambong Shoal, Pulai River Estuary, Straits of...
Transcript of The Epifaunal Marine Bivalves and Macrophytes in Merambong Shoal, Pulai River Estuary, Straits of...
1 Marine Science Centre, Office of Deputy Vice Chancellor (Research & Innovation),
Universiti Putra
Malaysia, Batu 7, Teluk Kemang, 71050 Port Dickson, Negeri Sembilan, Malaysia.
* Coressponding author e-mail: [email protected]
2 Department of Aquaculture, Faculty of Agriculture, Universiti Putra Malaysia.
3 Faculty of Agriculture and Food Sciences, Universiti Putra Malaysia Kampus Bintulu.
4 School of Environmental & Natural Resource Sciences, Faculty of Science &
Technology, UniversitiKebangsaan Malaysia.
Malayan Nature Journal 2014, 66(1 and 2), 42-51
The Epifaunal Marine Bivalves and Macrophytes in
Merambong Shoal, Pulai River Estuary, Straits of Malacca
WONG N. L. W. S.1*, A. ARSHAD
2, F. M. YUSOFF
2, B. JAPAR
SIDIK3 and A. G. MAZLAN
4
Abstract : The study was carried out to investigate the diversity of
epifaunal bivalves in Merambong seagrass bed, Pulai River Estuary. The
sample collection field trips started from May 2008 to July 2009, 18
transects (from six stations) have been laid perpendicularly along the
elongated shoal during lowest low spring tide. A total of 15 species from
10 bivalve families were recorded throughout the study period. Young
Anadara gubernaculum attached to Enhalus ecoroides showed the
highest abundance throughout the seagrass bed. Veneridae is the most
diverse family with four species recorded during the study followed by 2
species each for Mytilidae and Pinnidae. Negative correlation was
observed between Circe scripta abundance and Ulva spp. coverage (r2= -
0.829, P= 0.042). Station 4 (H’=1.2137; 1-D=0.5807; S=10) and Station
6 (H’=1.5279; 1-D=0.6696; S=13) have higher bivalve diversity and
species richness, and are more heterogeneous compared to other stations.
However, bivalve density was relatively lower in both stations 4 and 6
than the rest of the stations. The result of this study revealed that the
coverage of macrophytes plays an important role in determining the
density and distribution of epifaunal bivalves.
Keywords : Epifaunal bivalve; marine macrophytes; seagrass
INTRODUCTION
Merambong Shoal (1o
19.55’ N, 103o 35.57’ E) seagrass bed is a unique
ecosystem in Pulai River Estuary (Figure 1), southwest of Johor Straits.
It is surrounded by rivers, mangrove, stilts villages, industrial zone and
even small patches of reef around the nearby island.
42
It is a 30 ha sandy-mud subtidal shoals where from nine species
out of a total of 12 seagrass species recorded in Malaysia could be found
here (Japar Sidik et al. 2006). Over the years Merambong Shoal has been
providing food for the local fishing communities in terms of marine
fishes, crabs, and edible molluscs such as bivalves and gastropods. This
valuable ecosystem has major market and non-market economic values
in fisheries, raw materials research, shoreline protection, carbon
sequestration and as an important contributor in biodiversity (MPP-EAS
1999).
However, due to rapid and heavy development activities in the
surrounding area, and with the increasing number of people utilizing the
coast and adjacent waters, this ecosystem is currently under serious
threats (Japar Sidik et al. 2006). Epifaunal invertebrates which include
benthic bivalves and gastropods are organisms which rely on the
aboveground part of seagrass as major habitat throughout their life span
(Nakaoka 2005). Hence they are expected to be more sensitive to
changes in flora abundance and structures compared to the epifaunal
species. However, from previous studies Nakaoka (2005) found that
epifaunal species on different species of seagrasses overlap broadly
within each locality. While a species of epifaunal bivalve might not
completely rely on a single species of macrophyte as their habitat, it is
important to find out the relationship between epifaunal bivalves and the
macrophytes coverage as a whole to determine the influence of
macrophytes coverage on the distribution of epifaunal bivalves or vice
versa.
Therefore, in this study the abundance of epifaunal bivalves and
coverage of marine macrophytes will be estimated. The relationship
between these two groups of organisms will be explored. Epifaunal
bivalve richness and diversity for each station along the shoal will be
determined, while the coverage and species composition of seagrass and
macroalga, Ulva spp., will be taken into account. Ulva spp. is normally
found loose among seagrasses (Japar Sidik et al. 2001). Ulva is a genus
of thin-leaf green macroalgae which is also known as sea lettuce. Unlike
the seagrasses which have root system that would limits the seagrasses
movement to its leaves, Ulva spp. often hang loose between seagrasses
blades and could cover a wide area with its flexible sheet-like thallus. It
might play an important role in affecting the abundance of epifaunal
bivalve by providing protection against predations.
MATERIALS AND METHODS
Monthly field trips for sample collection were conducted during low
spring tides between May 2008 and July 2009. Due to the highly turbid
water in this area (which exposed to muddy mangrove soils in the north
43
and Singapore industrial zone in the east), research activities were
limited to low tides when tide is lower than 0.2 m and the shoal is
exposed during receded tide. Sampling trip was ceased from September
2008 to April 2009 because of unsuitable tides and resumed by May
2009. Eighteen transect belts (three transects per station) with 50m in
length and 5m in width were laid perpendicular to the length of
Merambong Shoal in Pulai River Estuary. For each transect belt, five
0.5m x 0.5m quadrats were placed along the belt for macrophytes
coverage estimation using the phytosociological technique of Saito and
Atobe (1970) as described in English et al. (1994). All epifaunal found
aboveground by visual and touch among macrophytes were collected and
preserved in 5% buffered formalin before transported back to the
laboratory in Institute of Bioscience, Universiti Putra Malaysia.
Bivalve specimens were identified based on the references of
Poutiers (1998), Lamprell and Healy (1998) and Okutani (2000) while
macrophytes were identified with the aid of Seagrass-Watch (McKenzie
et al. 2009). The relationship between bivalves and macrophytes were
explored using Spearman’s Rank correlation coefficient (r2) in SPSS
statistical analysis programme while bivalves diversity were determined
with the Shannon-Wiener diversity index in PRIMER 5.
RESULTS
There are 15 species from 10 families of epifaunal bivalves found in the
seagrass ecosystem in this study (Table 1). Family Veneridae is the most
diverse group with four species found within transects followed by
families Mytilidae and Pinnidae with two species each. Anadara
gubernaculum is widely distributed and could be found in almost all
transects with total number of specimens collected up to 647 (216±35
ind./transect) in Station 3 (Table 1). However, low abundance were
observed in Stations 4 and 6 with only 94 individuals (31±16
ind./transect) and 14 individuals (5±5 ind./transect) sampled.
Though lower in epifaunal bivalves abundance, Station 4 (21
ind./100m2) and Station 6 (25 ind./100m
2) have higher species richness
compared to the other stations (Table 2). Shannon-Wiener diversity
indices for both Station 4 (H’= 1.2137; J=0.5271; 1-D=0.5807) and
Station 6 (H’=1.5279; J=0.5957; 1-D=0.6696) revealed higher epifaunal
bivalve diversity and the species were more heterogeneous and evenly
distributed in comparison to other stations (Table 2).
Meanwhile, a total of eight species of macrophytes were identified in
this study. These are Enhalus acoroides, Ulva spp., Halophila ovalis,
Halophila minor, Halophila spinulosa, Halodule pinifolia, Cymodocea
serrulata and Thalassia hemprichii. Among these macrophytes, E.
acoroides, Ulva spp. and H. minor were found in all stations (Table 3).
44
The highest number of macrophytes present were observed in Stations 5
and 6, whereas the lowest number of species presence where found in
both Stations 1 and 3 with five species in total (Table 3).
Among the eight species of macrophytes, Ulva spp. was found to be
the most dominant. In fact it was found to dominate in all the stations,
taking up more than 50% of the total macrophytes populations in Stations
1 and 3, 41.8% in Station 4 and more than 33% in Stations 5 and 6
(Figure 2). Whereas, Halodule pinifolia was found to be the least
dominant species with the highest percentage contribution of 2.37% in
Station 4. Likewise Halodule pinifolia was present only in three stations
(Stations 4, 5 and 6).
Overall, Spearman’s Rank correlation coefficient (r2) between the
abundance of epifaunal bivalves and macrophytes coverage are weak and
not significant (Table 4). However, significant negative correlations
between the abundance of C. scripta and Ulva spp. coverage (r2= -0.829,
P=0.042) was observed (Table 4).
DISCUSSION
Seagrasses habitat generally formed along the mainland coastal areas
between mangroves and corals from low tide level to coral reef fringe
(Japar Sidik et al. 2006). Merambong Shoal seagrass bed creates a unique
ecosystem unlike any other seagrass beds in Malaysia. Surrounded by
various ecosystems and influenced by heavy human impacts, it is under
greater threat than any other seagrass ecosystem along the coastal area.
The complexity of the environment might contribute to the lack of
uniformity in biodiversity throughout the shoal.
Anadara gubernaculum was found in distinctively high density in
most stations compared to other epifaunal species. Interestingly, this
species is a burrowing infaunal in later stage of their life and only their
youngs could be found attach to macrophytes with byssus. Most of the
specimens collected were found attached on blades of E. acoroides or
thallus of Ulva spp. Though there is no significant correlation between A.
gubernaculum and coverage of macrophytes E. acoroides and Ulva spp.,
it is interesting to note that there exist a negative correlations between
this bivalve species and coverage of two seagrass species, H. minor and
H. ovalis (Table 4.). Most A. gubernaculum specimens found have length
ranging from 10 to 23 mm. Perhaps, the size and mass of A.
gubernaculum may be too much for the fragile stem and leaf of these two
relatively small seagrass species. Hence, contributed to the negative
relationship.
In contrary, negative correlations were found between Modiolus
philippinacum and macrophytes E. acoroides and Ulva spp. (Table 4).
Again, although the relationship was not statistically significant, M.
45
philippinacum apparently present in area with less shed and better
exposure. This was further proven during other trips where M.
philippinacum was collected for other research purposes. Most
specimens of this species were found among small seagrass species such
as H. ovalis and H. minor. Enhalus acoroides and Ulva spp. were found
throughout the shoal (Table 3) with the long blades of E. acoroides
providing calmer water columns for the free-floating Ulva spp. Ulva spp.
is a group of well-known macroalgae which are effective biofilter for
treatment of effluents from aquaculture farm (Vandermeulen and Gordin
1990; Cohen and Neori 1991; Neori et al. 1991, 2003; Jimenez et al.
1996; Msuya and Neori 2002; Msuya et al. 2006). This group of green
algae has been tested for their ability to control harmful algal bloom and
Ulva lactuca was successfully tested for successfully reducing the cell
density of common harmful algal bloom species via allelopathy (Tang
and Gobler 2011).
The increase in abundance of Ulva spp. might be closely related to
other environmental factors. Sousa-Dias and Melo (2008) found that the
coverage of Ulva spp. shows a significant positive correlation with
temperature and concluded that algae such as Ulva spp. are excellent
environmental indicators. Increase in air and sea surface temperature
could provide a positive influence in flourishing of Ulva spp. and
therefore, as suggested by Sousa-Dias and Melo (2008), the current
global warming scenarios might increase the dominance of Ulva spp.
Using microcosm experiment, Bartoli et al. (2003) has proved that the
presense of clam increases the growth rate and production of Ulva spp.
thallus segments. However, thick macroalgae mats could create
unfavourable environment for the clams. Thick Ulva spp. mats on the
surface of sediment could reduce the dissolved oxygen level under the
macroalgal mats (Marsden and Bressington, 2009) and causing the clams
to move away from this harsh living condition. This could explain the
negative correlations between the highly abundant Ulva spp. and
epifaunal bivalves, C. scripta, P. sella and M. philippinacum (Table 4).
Local fishermen have commented on the decrease of seagrass
density and the appearance of patchiness on the shoal over the last tenth
of years. With the rapid development on the coastal area along Johor
Straits, heavy silt carried by the turbid river outflow may have caused
sedimentation on the northern shoal over years and threaten the survival
of smaller seagrasses species (Figure 2).
In a study conducted by Peterson and Heck Jr. (2001), seagrass
assemblages significantly affect the survival of suspension feeding
bivalve and shows a strong mutualistic relationship between Modiolus
americanus and Thalassia testudinum. The study also suggests that
seagrass meadows may exist as a nutrient and productivity ‘hot spots’
with the presence of suspension feeding organisms to provide essential
46
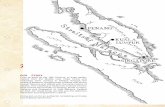
Figure 1. Arrows show the location of Merambong Shoal seagrass bed
between Peninsular Malaysia and Singapore. (Lower inserted image
shows sampling stations along the shoal.)
Table 1. Total number of epifaunal bivalves collected from each station
(750m2) on Merambong Shoal, Johor.
Family Species St.1 St.2 St.3 St.4 St.5 St.6
Arcidae Anadara gubernaculum
(Reeve, 1844) 281 537 647 94 506 14
Veneridae Circe scripta Linnaeus,
1758 1 16 13 44 23 101
Placamen isabellina
(Philippi, 1849) 0 0 0 3 4 6
Anomalocardia squamosa
Linnaeus, 1758 0 1 0 5 1 0
Gafrarium divaricatum
(Gmelin, 1791) 0 1 0 1 0 2
Placunidae Placuna sella
(Philipsson, 1788) 0 5 5 7 1 15
Mytilidae Modiolus philippinarum
Hanley, 1844 0 0 0 2 0 37
Perna viridis
(Linnaeus, 1758) 0 0 1 0 0 1
Pinnidae Pinna bicolor Chemnitz in 0 3 3 0 0 2
47
Gmelin, 1790
Atrina vexillum Born, 1778 0 0 0 0 0 4
Mactridae Mactra mera (Reeve, 1853) 0 0 3 2 1 1
Pteridae Pinctada fucata
(Gould, 1850) 0 0 0 2 0 4
Solenidae Solen sp. 0 0 0 1 0 0
Pectinidae Chlamys sp. 0 0 0 0 0 1
Placutilidae Plicatula sp. 0 0 0 0 0 1
Total: 10 15 282 563 672 161 536 189
Table 2. Ecological indices calculated for epifaunal bivalve from each
station at Merambong Shoal, Johor.
St.1 St.2 St.3 St.4 St.5 St.6
Shannon (H') 0.0235 0.2386 0.2073 1.2137 0.2612 1.5660
Species Richness
(S) 2 6 6 10 6 13
Evenness (J’) 0.0340 0.1332 0.1157 0.5271 0.1458 0.6105
Table 3. Marine macrophytes recorded from each sampling station in
Merambong Shoal, Johor.
Species\Site St.1 St.2 St.3 St.4 St.5 St.6
Enhalus acoroides (L.f.) Royle + + + + + +
Ulva spp. + + + + + +
Halophila ovalis (R.Br.) Hook. f. + +
+ + +
Halophila minor (Zoll.) den Hartog + + + + + +
Halophila spinulosa Aschers.
+
+ +
Halodule pinifolia (Miki) den Hartog
+ + +
Cymodocea serrulata (R. Br.) Aschers.
and Magnus
+ + + +
Thalassia hemprichii (Ehrenb.)
Aschers.
+ + + + +
Total: 5 6 5 6 8 8
48
Table 4. Spearman’s Rank correlation coefficient (r2) of four most
abundance bivalve and macrophytes species in Merambong Shoal
seagrass ecosystem.
nutrients for floral productivity. There are numerous possibilities when
exploring the truth behind the decreasing of epifaunal molluscs
population which are collected by the local fishermen as food product.
Some might suggest that the decline in seagrass density might be the
reason of the falling mollusc population. If the theory of Peterson and
Heck Jr. (2001) is taken into account, one might need to explore the
condition in an opposite direction which suggest that the decline in
suspension-feeding mollusc could be the reason for the lost of seagrasses.
Further studies are necessary to pinpoint the threats behind the decrease
of certain bivalve population.
Bivalve abundance
(total number per
transect)
Coverage (%)
Enhalus
acoroides Ulva spp.
Halophil
a minor
Halophila
ovalis
Anadara
gubernaculum
r2 0.371 0.429 -0.486 -0.543
P 0.468 0.397 0.329 0.266
Circe scripta r2 0.029 -0.829* 0.657 -0.029
P 0.957 0.042 0.156 0.957
Placuna sella r2 0.174 -0.522 0.551 -0.319
P 0.742 0.288 0.257 0.538
Modiolus
philippinacum
r2 -0.068 -0.541 0.541 0.101
P 0.899 0.268 0.268 0.848
*Correlation is significant at the 0.05 level.
49
Figure 2. Species composition of macrophytes in each station in
Merambong Shoal, Straits of Malacca.
50
REFERENCES
Bartoli, M., Naldi, M., Nizzoli, D., Roubaix, V. and Viaroli, P. 2003. Influence of clam
farming on macroalgal growth: a microcosm experiment. Chemistry and
Ecology 19(2-3): 147-160.
Cohen, I. and Neori, A. 1991. Ulva lactuca biofilters for marine fishpond effluents:
I.Ammonia uptake kinetics and nitrogen content. Botanica Marina 34: 475-
482. English, S., Wilkinson, C. and Baker, V. 1994. Survey Manual for Tropical Marine
Resources. ASEAN-Australia Marine Science Project. Townsville: Australian
Institute of Marine Science.
Japar Sidik, B., Muta Harah, Z. and Arshad, A. 2006. Distribution and significance of
seagrass ecosystems in Malaysia. Aquatic Ecocsystem Health and
Management 9(2): 203-214.
Japar Sidik, B., Muta Harah, Z., Kanamoto, Z. and Mohd. Pauzi, A. 2001. Seagrass
Communities of the Straits of Malacca. In Aquatic Resource and
Environmental Studies of the Straits of Malacca: Current Research and
Reviews, ed. by Japar Sidik B., Arshad A., Tan S.G., Daud S.K., Jambari
H.A., Sugiyama S. pp. 81-98 Malacca Straits Research and Development
Centre (MASDEC), Universiti Putra Malaysia, Malaysia.
Jimenez del Rio, M., Ramazanov, Z. and Garcia-Reina, G. 1996. Ulva rigida (Ulvales,
Chlorophyta) tank culture as biofilters for dissolved inorganics nitrogen from
fishpond effluents. Hydrobiologia 326/327: 61-66.
Lamprell, K. and Healy, J. 1998. Bivalves of Australia. Volume 2. Leiden: Backhuys
Publishers.
Marsden, I. D. and Bressington, M. J. 2009. Effects of macroalgal mats and hypoxia on
burrowing depth of the New Zealand cockle (Austrovenus stutchburyi).
Estuarine, Coastal and Shelf Science 81: 438-444.
McKenzie, L. J., Yoshida, R. L. and Coles, R. G. 2009. Tropical Seagrass Identificaion.
Seagrass-Watch.Accessed 20 Dec.
http://www.seagrasswatch.org/id_seagrass.html.
MPP-EAS. 1999. Total Economic valuation: Coastal and marine resources in the
Straits of Malacca. MPP-EAS Technical report No. 24/PEMSEA Technical
Report No. 2. Global Environmental Facility/United Nations Development
Programme/International Maritime Organization Regional Programme for the
Prevention and Management of Marine Pollution in the East Asian Seas (MPP-
EAS)/Partnerships in Environmental Management for the Seas of East Asia
(PEMSEA), Quezon City, Phillippines.
Msuya, F. E. and Neori, A. 2002. Ulva reticulate and Gracilaria crassa: macroalgae
that can be biofilter effluent from tidal fishponds in Tanzania. Western Indian
Ocean Journal of Marine Science 1: 117-126.
Msuya, F. E., Kyewalyanga, M. S. and Salum, D. 2006. The performance of the
seaweed Ulva reticulate as a biofilter in a low-tech, low cost, gravity generated
water flow regime in Zanzibar, Tanzania. Aquaculture 254: 284-292.
Nakaoka, M. 2005. Plant-animal interactions in seagrass beds: ongoing and future
challenges for understanding population and community dynamics. Population
Ecology 47: 167-177.
Neori, A., Cohen, I. and Gordin, H. 1991. Ulva lactuca biofilters for marine fishpond
effluents: II. Growth rate, yield and C:N ratio. Botanica Marina 34: 483-489.
Neori, A., Msuya, F. E., Shauli, L., Schuenhoff, A., Fidi, K. and Shpigel, M. 2003. A
novel three-stage seaweed (Ulva lactuca) biofilter design for integrated
mariculture. Journal of Applied Phycology 15: 543-553.
51
Okutani, T. 2000. Marine Mollusks in Japan. Japan: Tokai University Press. Peterson,
B. J. and Heck, Jr. K. L. 2001. Positive interactions between suspension-
feeding bivalves and seagrass – a facultative mutualism. Marine Ecology
Progress Series 213: 143-155.
Poutiers, J. M. 1998. Bivalves (Acephala, Lamellibranchia, Pelecypoda). In The living
marine resources of the Western Central Pacific. Volume 1, Seaweeds, corals,
bivalves and gastropods, edited by K.E., Carpenter, V.H., Niem. pp. 123-362
FAO, Rome.
Saito, Y. and Atobe, S. 1970. Phytosociological study of intertidal marine algae, I.
Usujiri Benten-Jima, Hokkaido. Bulletin of the Faculty of Fisheries, Hokkaido
University 21(2): 37-69.
Sousa-Dias, A. and Melo, R. A. 2008. Long-term abundance of macroalgae in relation
to environmental variables in the Tagus Estuary (Portugal). Estuarine, Coastal
and Shelf Science 76: 21-28.
Tang, Y. Z and Gobler, C. J. 2011. The green macroalga, Ulva lactuca, inhibits the
growth of seven common harmful algal bloom species via allelopathy.
Harmful Algae 10: 480-488.
Vandermeulen, H. and Gordin, H. 1990. Ammonia uptake using Ulva (Chlorophyta) in
intensive fishpond system: mass culture and treatment of effluent.
Journal of Applied Phycology 2: 363-374.