Thermodynamic modelling of wetting at silicon nitride/NiCrSi alloy interfaces
-
Upload
independent -
Category
Documents
-
view
2 -
download
0
Transcript of Thermodynamic modelling of wetting at silicon nitride/NiCrSi alloy interfaces
Materials Science and Engineering, A189 (1994) 209-217 209
Thermodynamic modelling of wetting at silicon nitride/Ni-Cr-Si alloy interfaces
Ali M. H a d i a n and R o b i n A. L. D r e w
Department of Mining and Metallurgical Engineering, McGill Universi~, Montreal, H3A 2A 7 (Canada)
(Received December 2(I, 1993; in revised form February 22, 1994)
Abstract
Through thermodynamic modelling of the reaction at ceramic-metal interface, wetting of a sintered silicon nitfide was investigated with a series of Ni-based brazing alloys without addition of active elements. The thermodynamic calculations showed that Ni-Cr-Si alloys with Ni/Cr ratio of 3.5 and Xs~ < 0.25 will react with and wet Si3N 4 ceramics at 1220 °C (1493 K) and Py~ = 15 Pa. Environmental verifications using sessile drop method confirmed the validity of the calcula- tions and the assumptions made in the wetting model.
1. Introduction
Silicon nitride, because of its superior mechanical properties, is under active consideration for advanced structural applications such as gas turbines. Joining of Si3N 4 is a necessary processing step to form complex shapes or to incorporate this material with metallic components to produce a good structural integrity.
Of the techniques being evaluated for joining ceram- ics, brazing has received extensive attention. A key factor in ceramic brazing is wetting of ceramics by filler metals. However, due to the chemical stability of Si3N 4 most of the pure metals and alloys do not wet Si3N 4 readily. The two commonly employed methods to improve wetting are (i) a two-step active metal coating on the ceramic surface or a metallizing process and (ii) addition of such elements as Ti or Zr to the filler metal to activate wetting.
Direct brazing of ceramics without prior surface treatment or addition of active elements is advan- tageous through the reduction of extra processing costs and time. However, designing suitable braze composi- tions by which the ceramic can be wetted and bonded strongly is of great importance. It has been shown that the achievement of a strong bond at a ceramic-filler metal interface requires some chemical reactions between the joining materials [1, 2].
Several attempts have been made to calculate wet- tability in terms of bonding interactions in various metal-ceramic systems. Kingery and Humenik [3] suggested a correlation of wetting behaviour for oxide ceramics with ceramic-melt reactivity. McDonald &
Eberhart [4] performed wetting experiments between alumina and several kinds of pure metals and reported a linear relationship between the work of adhesion and the free energy change due to the formation of the metal oxides.
2. Thermodynamics
Wetting of ceramics by liquid metals is essentially possible through partial or complete dissociation of interatomic bonding in the solid bodies [5, 6]. In the absence of any reaction or mass transport of atoms across a solid-liquid interface, the attractive energy required for wetting is provided by reversible physical interaction such as van der Waals forces. Under these conditions, wetting is defined by a steady state contact angle (0) represented by the well known Young's equation:
?'~,. - 7~1 = 7~v cos 0 ( 1 )
where: 7~,. is the solid surface energy, ),~ is the solid-liquid interfacial energy, and 7~v is the liquid surface tension.
Under nonreactive conditions, wetting always occurs when 7~v > 7~ > V~v, and nonwetting is when 7~v < 7~J < ?'iv. As wetting proceeds, the liquid spreads over the substrate, resulting in a reduction in the affec- rive area to which V~v is applied. Therefore, the driving force for wetting can be defined as ( V~,, - ~l) [ 1 ].
When a reaction occurs between a solid and a liquid, the free energy of reaction enhances the driving force
0921-5093/94/$7.00 © 1994 Elsevier Sequoia. All rights reserved SSDI 0921-5093(94)09556-C
210 A. M. Hadian, R. A. L. Drew / Wetting at silicon nitride/Ni-Cr-Si alloy interfaces
for wetting as indicated by the inequality [1]:
( + d Tt] cos 0 (2)
In this case the driving force of wetting is enhanced by the free energy change of the interfacial reaction (d G r ). Thus, it can be stated that the chemical interaction at the ceramic-liquid metal interface can determine the degree by which a ceramic can be wetted by a molten metal.
The simplest reaction at a ceramic-liquid metal interface is the solution of one phase by the other to form an equilibrium saturation at the interface and then its continuation into the bulk in order to reach the lowest free energy state. In a solution reaction when the liquid phase is unsaturated with respect to the ceramic, its composition will change while the ceramic composi- tion will remain unchanged [7]. The free energy change for this solution reaction can then be used to predict wetting affinity. This approach was used by McDermid and Drew [8] for designing suitable filler metals for brazing SiC ceramics.
The objective of this study was to develop a tech- nique based on solution thermodynamics for predict- ing wetting at Si3N4/Ni-Cr-Si alloy interfaces and to experimentally verify the results of the calculations using the sessile drop method.
For the purpose of Gibbs energy evaluation in the system of Si3N 4 in contact with a liquid Ni-Cr-Si alloy, it was assumed that a given quantity of the alloy reacts with Si3N 4 at 1220 °C (1493 K) to achieve a state of thermodynamic equilibrium. This temperature allows for the Ni-Cr-Si alloy compositions to be in the liquid state [9-11]. The assumptions made in the calculations can be summarized as:
(i) The Ni/Cr ratio of the reactant alloys was taken to be 3.5, i.e. the same as the commercial AWS BNi-5, Ni-18Cr-19Si, used as the braze alloy. This assump- tion was made because there is no sink or source for Ni and Cr in the system. Thus all of the starting Ni-Cr-Si alloys react with Si3N 4 and lie within the ternary Ni-Cr-Si composition triangle along the line shown in Fig. 1. This line represents all the compositions having a fixed Ni/Cr ratio of 3.5.
(ii) The Si liberated as a result of decomposition of Si3N 4 dissolves in the liquid alloy and N 2 escapes into the environment.
(iii) The reaction between Si3N 4 and a liquid Ni-Cr-Si alloy takes place under isobaric and isother- mal conditions at 1220 °C (1493 K) and PN~ = 15 Pa.
(iv) The liquid alloy reacts with the surface of Si3N 4 instead of the bulk. This assumption was first intro- duced by Chidambaraham et al. [12].
Si A • s10
/ / / \ * BNi-5 (S19) / / \ ° sz5
Ni[ i \ Cr
Fig. 1. The path of the alloy compositions within the Ni-Cr-Si ternary system used in thermodynamic calculations.
The appropriate chemical equation, for which the Gibbs energy must be found is as follows:
(Si3N4) + {Ni-Cr-Si}L,--' {Ni-Cr-Si}L2 + (N2) (3)
This unbalanced equation indicates that the reactant alloy with composition L 1 reacts with solid Si3N 4 to produce a new liquid of composition L 2 and N 2 gas. The reasons for assuming that the liberated N 2 will escape into the environment without any reaction with the Ni and Cr are:
(a) N 2 is insoluble in and does not react with Ni at temperatures up to 1400 °C to form a stable nitride [ 13] (the free energy of formation of Ni3N is about + 10 kJ mol-1 at 1473 K).
(b) In the Cr-N system in the absence of external N 2 pressure chromium nitride will not form above 1000 °C [14].
In order to obtain the corresponding Gibbs energy equation, the chemical equation (eqn. (3)) should be broken up as follows:
y(Si3N4)--' 3y(Si) + 2y(N2) AG, (4)
3y(Si)--> 3y{Si} A G 2 (5)
x-x i/1-X r/x i + z [1.28 / 4.5 / ]L, (6)
z ( 1 - x ) N i / Z ( 1 - x ) c r / Z X + 3 y } 1.28(z+3y) /4~-(z+3-y) / z + 3 y S i L 2 AG3
Equation 4 is the decomposition of Si3N 4 into solid Si and N2 gas and eqn. (5) represents the fusion of solid Si. Equation (6) shows dissolution of Si into the initial Ni-Cr-Si alloy with composition L~ to form a new composition L 2.
The corresponding Gibbs energy equations for the system will be the sum of AG~, AG2, and AG3:
A G r = A G 1 + A G 2 + A G 3 (7)
The free energy change AGr, represents the energy released or required when Si3N 4 reac ts with the liquid
A. M. Hadian, R. A. L. Drew / Wetting at silicon nitride/Ni-Cr-Si alloy interfaces 211
alloy. A negative AG r indicates a spontaneous reaction and, hence, wetting. The reaction will continue until thermodynamic equilibrium is reached, i.e. AGr = 0.
In order to predict the sign and magnitude of A Gr, the numerical values of AG~, AG 2 and AG 3 need to be calculated. AG~ and AG2 are readily available, but A G3 which is the difference between the free energy of two ternary Ni-Cr-Si solutions (i.e. L~ and L2) has to be calculated. Because of the lack of solution thermo- dynamic data for this ternary system, the Toop and Kohler equations were employed to compute the ter- nary thermodynamic functions from the relevant binary solution thermodynamic data [15, 16] and consisted of the Ni-Cr [17, 18], Cr-Si [19, 20] and Ni-Si [21] binary systems (Appendix I).
The corresponding Gibbs free energy change for eqn. 6 as a function of Xs~ in the reactant alloy is pre- sented in Fig. 2. An examination of the Fig. 2 shows that A G r = 0 for Xs~=0.255 in the curve generated using Toop's equation and a corresponding value of Xs~=0.24 results for Kohler's equation. Thus, the Toop equation predicted that a Ni-Cr-Si alloy with a composition of 57.95 Ni-16.55 Cr-25.5 Si will be in thermodynamic equilibrium with Si3N 4 and the Kohler equation predicted that the equilibrium composition would be 59.l Ni-16.9 Cr-24 Si.
3. Experimental verification
In order to confirm the prediction of the model, wetting experiments were performed using the sessile-
drop technique. The furnace used was essentially a horizontal tube furnace, consisting of a 60 cm long alumina tube having 26 mm inner diameter (Fig. 3). The hot zone inside the tube was almost 10 cm long. The furnace could be heated up to 1250 °C and a vacuum of 2 Pa could be obtained by a rotary pump. A water-cooled quartz window was fitted to one end of the alumina tube for direct viewing of the substrate. An optical system was arranged in front of the window to take photographs of the drops at temperature. The furnace configuration allowed for rapid or slow heating and cooling. In the case of rapid heating, the sample holder along with the Si3N 4 substrate was pushed by a vacuum tight push rod from the cold zone inside the tube to the hot zone, and vice versa for fast cooling.
The experimental silicon nitride substrates were fabricated in house using slip casting, followed by pressureless sintering [22]. All the substrate surfaces were mechanically ground and polished to a 1 ~m surface finish and degreased ultrasonically with acetone prior to testing. Four different alloy composi- tions having 10, 19, 25, and 30 at.°/,, Si with identical Ni/Cr ratio of 3.5 were prepared for the wetting experiments (Table 1). The alloys were made through the addition of either Si powder (in the case of fabrica- ting alloys having > 19 at.% Si) or a Ni-20wt.%Cr alloy to the commercial $19 in the required quantity followed by intimate mixing to obtain a uniform powder mixture with an average particle size of 100 ktm. A small amount of the alloy ( =0.05 g) was mixed with distilled water to form a paste and placed on the Si3N 4 surface. The 6 × 6 mm 2 substrate was then slowly pushed into the cold zone of the tube furnace, and after
~ 1 2 0 <1
8O
~ 0 . . . . . . . . . . . .
~ :i i
~.m ii -1~ !i
, i • 2 ~ , i l
, i i
0.1 0.2 0.3 0.4 0.$ 0.6 0.7 0.8 0.9 Mole fraction of Si in starting alloy (Xsi)
Fig. 2. Variation of A G, vs. Xsi in the starting alloy.
Water Furnace Sample Water cooled cooled \\ / Camera ~[~ ,. : ° .,o//~. :
T o vacuum pump
Fig. 3. Experimental sessile drop furnace.
TABLE 1. Chemical analysis of the experimental alloys
Designation* Composition (at.%)
Ni Cr Si
SI0 69.61 20.35 10.04 S19 (BNi-5) 62.65 18.4 18.95 $25 58.34 16.66 25.0 $30 54.45 15.55 30.0
*The alloy designations refer to their Si contents.
212 A. M. Hadian, R. A. L. Drew / Wetting at silicon nitride/Ni-Cr-Si alloy interfaces
Fig. 4. Photographs of liquid Ni-Cr-Si alloys after 10 rain contact with Si3N 4 substrates at 1220 °C, (a) S10, (b) S19, (c) $25 and (d) $30 alloy.
evacuation, the sample was slowly pushed the hot zone. The heating rate was estimated to be about 100 °C rain -1 to 500 °C and about 200 °C min -~ to the experimental temperature (1220 °C). After the alloy started to melt, photographs of the drop were taken every minute for wetting angle measurements.
4. Results and discussion
Figure 4 shows the different compositions of liquid Ni-Cr-Si alloy drops on the Si3N 4 substrate at 1220 °C. These photographs were taken after 10 rain contact between the liquid alloys and the ceramic under a N2 partial pressure of 15 Pa, i.e. the same conditions as used in the thermodynamic calculations. From Fig. 4, it is quite obvious that the alloys contain- ing Xsi < 0.3 wet Si3N 4 and so conforms to the thermo- dynamic predictions, particularly using the Toop equation (see Fig. 2). Figure 5 demonstrates the effect of the Gibbs excess energy due to the surface. Curve 1 shows A G r vs. Xsi without taking into account the excess surface energy and curve 2 is the same as that
illustrated in Fig. 2 (Toop equation). Curve 1 in Fig. 5 indicates that a Ni-Cr-Si alloy of Xsi = 0.22 will be in thermodynamic equilibrium with Si3N4, whereas, curve 2 predicts,a value of Xsi = 0.25. Comparison with Fig. 4 indicates that the later value is more appropriate and confirms that the surface excess energy should be accounted for in the thermodynamic calculations,
4.1. Effect of time on wetting angle The variation of contact angle (0) with time from the
sessile drop experiments for different alloy composi- tions are presented in Fig. 6. The points on the graphs result from measurements made at 5 min intervals.
The change of wetting angle with time may be explained in terms of the reaction kinetics in the ceramic-metal system. For highly reactive systems, the interfacial reactions proceed quickly after physical contact between the ceramic and the molten metal. In such instances, the contact angle drops rapidly in a short time. Further reductions of wetting angle will proceed at a slower rate until equilibrium wetting is achieved [23]. Figure 6 shows a rapid reduction of wetting angle for the Si3N4/$10 system from ~-160 ° to
A. M. Hadian, R. A. L. Drew / Wetting at silicon nitride/Ni-Cr-Si alloy interfaces 213
3 2 0 .
"1 1,0
8O
O 0
O -40
-80
~ -120
-160
-200
t . t . O ° ~ - Q
Curve 1 ~ ~ ' " *
/~.¢' "Curve 2
..............
/ i
S,~ i i
,' I , I , I , l , l , I , i , I ,
0.1 0.2 0.3 0.4 0.8 0.6 0.7 0.8 0.9
Mole fraction of Si in starting alloy (Xsi)
Fig. 5. Variation of AG~ vs. Xs~ , showing the effect of surface excess energy on equilibrium Si content.
200
160
7140 ~a~ 120
,oo
40
20
, I i I L I , I , 10 20 30 40
Time (rain.) 5 0
Fig. 6. Time dependence of the wetting of silicon nitride by Ni-Cr-Si alloys at 1220 °C.
40 ° after 5 min and results from the high interracial reactivity due to the low concentration of Si in the alloy. For less reactive ceramic-metal systems, wetta- bility is generally poorer and the wetting process will advance at a slower rate and takes longer to reach equilibrium wetting [23, 25]. This is observed for the Si3N4/$25 system and is largely due to the near- equilibrium composition of the $25 alloy with Si3N 4.
Figure 7, which shows the variation of the Si contents of the alloys can provide additional informa- tion on kinetics of the interfacial reactions. From the
30
25
~ 2 0
y - -
[ s~ s19 s~o ]
0 10 20 30 40 50
Time (mill.)
Fig. 7. Variation of Xs~ in the product alloy vs. the contact time.
figure, it can be seen that all of the reactant alloys have moved, with different rates, towards the equilibrium compositions in terms of their Si contents. However, the S10 alloy shows a higher rate compared to the other alloys. Figure 7, again would tend to emphasize the higher reactivity of the S10 alloy with Si3N 4. From eqn. 2, a high reactivity at the ceramic-metal interface can enhance the driving force for wetting as was the case for the Si3N4/S10 system.
4.2. Effect o f N 2 partial pressure and temperature Another important factor in determining the degree
to which liquid Ni-Cr-Si alloys wet Si3N 4 and react chemically is the N 2 partial pressure (PN2) in the furnace environment. The PN2 dependence of AG r is illustrated in Fig. 8. From Fig. 8 it can be seen that a wetting-to-nonwetting transition occurs when the PN2 is increased. For instance, at PN2 = 10 5 Pa for an alloy of Xs~=0.19, the value of AG r is + 160 mJ, thus, this alloy is not expected to react with and wet Si3N 4. The change of wettability with local atmospheric conditions around the drop was verified experimentally. The results demonstrated that S 19 alloy, which was able to adequately wet Si3N 4 at a PN~ of 15 Pa (Fig. 4(b)), did not wet the ceramic when the P~, was raised to atmospheric pressure (Fig. 9).
Variations of A Gr vs. Xs~ at two different tempera- tures, 1200 °C and 1300 °C, are shown in Fig. 10. From Fig. 10, it can be seen that the composition of Xsi = 0.3 will be in thermodynamic equilibrium with Si3N 4 at 1300 °C. This indicates that, increasing the temperature causes A Gr=0 to be shifted towards higher values of Xsi.
The experimental results showed that Si3N 4 was wetted by an alloy of Xs~=0.3 at 1300 °C (Fig. 11),
214 A. M. Hadian, R. A. L. Drew / Wetting at silicon nitride/Ni-Cr-Si alloy interfaces
440 p N2_=15 Pa
P N 2 ~ E 360
320 ~3 <1 280 _ . .
• . o . t _ o . 4 e- • 2 4 0
. l i t " e.0 200 ~ . '~ 'm' ° ' ' t - ° ' 4 - ' e - e ~,9"
"~ 160 e.~ i e" C,..) 120 i .."
: .s" o 80 i ""
[ dd 4 0 , • 0 0
-40 ~"
"~ -80 / i o i l [- .lZ0 .' i
-160 • i / !
-200 ,' ! ,' i I a i i , I , I , [ , I h I r f ,
0.1 0.2 0.3 0A 0.5 0.6 0.7 0.8 0.9 Mole fraction of Si in starting alloy (Xsi)
Fig. 8. Variation of AG~ vs. Xs~ , showing the effect of N 2 partial pressure on equilibrium Si content.
28O
24O
200
16o
120 ,el
o ~ .4o
.so -~ 420
~.160
.200
.240
/ ,' i
• i n ' a ~ l l : l n l n l n
0 0.1 0.2 0.3 0,4 0.5 0,,6 0.7 0.8 0.9 1 Mole fraction of Si in starting alloy (Xst)
Fig. 10. Variation of AG r vs. Xst , showing the effect of tempera- ture on equilibrium Si content.
Fig. 9. A photoghraph of S 19 alloy on Si3N 4 substrate at 1220 °C and PN~ = 105 Pa.
whereas, no wetting was observed at 1220 °C (Fig. 4(d)).
5. Conclusions
1. A thermodynamic method was developed to predict the wetting of Si3N 4 by Ni-Cr-Si alloys. The technique was based on the free energy change of the system as a result of decomposition of Si3N 4 and the resulting change of composition of the candidate alloys. The alloys studied were based on the AWS BNi-5 with the same Ni/Cr ratio, but different Si contents. The
11811&
Fig. 11. A photograph of $30 alloy on Si3N 4 substrate at 1300 °C and PN, = 15 Pa.
solution thermodynamic properties of the ternary alloys were calculated from the data of the binary systems using the Toop and Kohler equations.
2. The method showed that, using the Toop equation, under the conditions of 1220 °C (1493 K) and PN2 = 15 Pa, an Ni-Cr-Si alloy with Ni/Cr ratio of 3.5 and S s i = 0.25 would be in thermodynamic equili- brium with Si3N 4. The equilibrium Si content using the Kohler equation were found to be 0.24.
3. Experimental verification confirmed the validity of the calculations (particularly using the Toop equa- tion), as it showed that the alloys with Si content of less than the equilibrium concentration reacted with and wetted the Si3N 4 ceramic. The degree of wetting increased as the Si content decreased. The results of
A. M. Hadian, R. A. L. Drew / Wetting at silicon nitride/Ni-Cr-Si alloy interfaces 215
wetting experiments showed that no wetting occurred between Si3N 4 and an alloy with Xsi = 0.3 under the above conditions.
4. Both the thermodynamic calculations and experi- mental verifications showed that the degree to which Si3N 4 will react with the alloys is strongly influenced by the environmental N 2 partial pressure and tempera- ture, An increase of t empera ture to 1300 °C resulted in an increase of the equilibrium Si content to about 0.3 Under a N2 partial pressure of 105 Pa, nonwetting was observed between the ceramic and the experimental N i - C r - S i alloys.
5. The study showed the validity of treating wetting (from a thermodynamic viewpoint) as a reaction between a liquid metal and the surface rather than the bulk of a ceramic.
R e f e r e n c e s
l J.A. Pask, From technology to the science of glass/metal and ceramic/metal sealing, Am. Ceram. Soc. Bull., 66 (1987) 1587-1592.
2 M. M. Schwartz, Ceramic Joining, American Society for Metals, Metals Park, OH, 1990, pp. 1-16.
3 W. D. Kingery and M. Humenik, Jr., Surface tension at elevated temperatures. I. Furnace and method for use of the sessile drop method; surface tension of silicon, iron, and nickel, J. Phys. Chem., 57(1953) 359-363.
4 J. E. McDonald and J. G. Eberhart, Adhesion in aluminum oxide-metal systems, Trans. Metal. Soc. A1ME, 233 (1965) 512-517.
5 J. V. Naidich, The wettability of solids by liquid metals, in D. A. Cadenhead and J. E Danielli (eds.), Progress in Surface and Membrane Science, Vol. 14, Academic Press, New York, 1981, pp. 353-458.
6 M. G. Nicholas, Interaction at oxide-metal interfaces, Mater. Sci. Forum, 29(1988) 127-150.
7 1. A. Aksay, C. E. Hoge and J. A. Pask, Wetting under chemi- cal equilibrium and nonequilibrium conditions, J. Phys. ('hem., 78(1974) 1178-1183.
8 J.R. McDermid and R. A. L. Drew, Thermodynamic brazing alloy design for brazing silicon carbide, J. Am. Ceram. Soc., 74(1991) 1855-1860.
9 E. Lugscheider, O. Knotek and K. Klohn, Melting behaviour of nickel-chromium-silicon system, Thermochem. Acta., 29 (1979) 323-329.
10 I. Ansara, T. G. Chart, R Y. Chevalier, K. Hack, G. McHugh, M. H. Rand and P. J. Spenser, Phase Diagrams for Fe-Cr-Ni Based Alloys, Part 1: Phase Diagrams for Binary and Ternary Alloys o[ Fe, Cr, and Ni with Si and C, Commission of the European Communities Report No. EUR 9657/I/EN (1985).
11 R. W. Guard and E. A. smith, Constitution of nickel-based ternary alloys: V Nickel-chromium-silicon system, J. Inst. Met., 88 ( 1959-196(/) 369-374.
12 R R. Chidambaram, G. R. Edwards and D. L. Olson, A ther- modynamic criterion to predict wettability at metal-alumina interfaces, Metall. Trans., 23B (1992) 215-222.
13 P. M. Hansen, Constitution of Binary Alloys, 2nd edn., McGraw-Hill Book Co., New York, 1958.
14 J. C. Schuster, Silicon nitride-metal joints: phase equilibria in the systems Si3N4-Cr, Mo, W and Re, J. Mater. Sci., 23 (1988) 2792-2796.
15 G. W. Toop, Predicting ternary activities using binary data, Trans. Met. Soc. AIME, 233(1965) 850-855.
16 O. Kubaschewski and C. B. Alcock, Metallurgical Thermo- chemistry, 5th edn., Pergamon Press, Toronto, 1979, pp. 57-64.
17 P. Nash, The Cr-Ni (chromium-nickel) system, Bull, Alloy Phase Diagram, 7 (1986) 466-476.
18 L. Kaufman and H. Nesor, Calculation of the binary phase diagrams of iron, chromium, nickel and cobalt, Z. Metall., 64 (1983)249-257.
19 J. R Riegert, A. Vermande and I. Ansara, Thermodynamic properties of Cr-Si alloys at 1900 °C, High Temp.-ttigh Pressure, 5(1973) 231-235 (in French).
20 A. B. Gokhale and G. L. Abbaschian, The Cr-Si (chro- mium-silicon) system, Bull. Alloy Phase' Diagram, 8 (1987) 474-484.
21 R Nash and A. Nash, The Ni-Si (nickel-silicon) system, Bull. Alloy Phase Diagram, 8 (1987) 6-14.
22 M. D. Pugh and R. A. L. Drew, Enhanced processing of sintered silicon nitride, J. Can. Ceram. Sot., 58 (1989) 45-49.
23 S.-Y. Oh, J. A. Cornie and K. C. Russell, Wetting of ceramic particulates with liquid aluminum alloys: Part II. Study of wettability, Metall. Trans., 20/t (1989) 533-541.
24 X. M. Xue, J. T. Wang and M. X. Quan, Wettability and spreading kinetics of liquid aluminium on boron nitride, J. Mater. Sci., 26 ( 1991 ) 6391-6395.
A p p e n d i x I
A sample o f the calculations
The following calculations will determine the total free energy change of the reaction between Si3N4 and a N i - 2 0 C r - 1 0 Si at.% (i.e. S10) alloy at 1493 K and PN2 = 15 Pa ( ~- 1.5 × 10 -4 atm). The following assump- tions were made for the calculations and scaling up the reaction between the ceramic and the liquid alloy:
- - 0.001 mol of the alloy will react with 10 -~' mol of the ceramic. Selection of this value was based on the assumption that the reaction will occur between the alloy and a thickness of 2 /~m of the ceramic over an area of 0.2 cm 2. With respect to molar weight and density of Si3N4 (140 g mol - J and 3.27 g cm-3 respectively) this value was calculated to be 9.37 × 10 -v m o l ( = 10 -6 mol).
- - T h e standard state was taken to be the pure elemental liquid in the computa t ion of Gibbs energy of N i - C r - S i ternary alloys.
To calculate the AGr, one must calculate AGI , AG2, and A G 3 in eqn. (7). AGj which is the free energy change of decomposi t ion of 10 -6 mol of Si3N 4 on the surface, can be calculated from:
216 A. M. Hadian, R. A. L. Drew / Wetting at silicon nitride/Ni-Cr-Si alloy interfaces
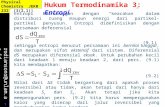
el 2 3 A G = ( A G O _ G X S ) + R T l n Na as~ (A1)
aSi3N4
where A G ° is the standard Gibbs free energy change for decomposition of Si3N 4 in the bulk and G xs is the Gibbs surface excess energy.
Gxs is defined as:
G x~ =A), (A2)
where 7 is the surface energy and:
A = m N 1/3 Vm 2/3 (A3)
where V m is the molar volume, N is the Avogadro's number, and m is the fraction of nearest-neighbour atoms lying in an adjacent layer. The Si3N 4 structure consists of SiN 4 tetrahedra joined by sharing N corners so that each N is common to three tetrahedra. There- fore, each N atom is shared between three tetrahedra so that each Si atom has four N atom nearest neigh- bours and each N has three Si atoms nearest neigh- bours. If we assume that the Si3N54 crystal cleaves along the plane where Si atoms are located (this assumption compares well with alumina ceramic with an h.c.p, structure in which cleavage occurs along the basal plane where the oxygen ions are situated), one out of four Si-N bonds will break. Therefore, a value of 1/4 was used for m in the calculations. Assuming:
aSi3N" = 0.9
as i = 1 xs = Gsi,N, 29698 Jmol-1
(calculated from: V, nSi,N, = 140/3.27=42.8 and m = 1/4) thus:
AG 1 = 6.24 mJ
A G2, which represents the free energy change of fusion of 3 x 10 -6 mol Si (from decomposition of 10 -6 mol of Si3N 4 ) was determined to be:
A G2 = (50530 - 29.99 T ) × 3 x 10-6 = 17.26 mJ
The last term to be calculated in eqn. 7 for deter- mining the total free energy change of the reaction is AG 3 which is a free energy change fo 10 -3 mol for the liquid Ni-18 Cr-20 Si at.% alloy as a result of the addition of 3 × 10 6 mol of liquid Si. The addition of Si will change the composition of the starting alloy. Thus, A G 3 will be the free energy change of the following reaction:
( 10- 3)Ni-20 Cr-10 Si + (3 x 10-6)Si
=(10 -3 + 3 x 10-6)Ni-19.94 Cr-10.27 Si
The free energy change of the above reaction can be calculated by knowing the Gibbs energy of the ternary
Ni-Cr-Si solutions with the above compositions. As mentioned previously, Toop and Kohler equations was used for determining the Gibbs energy of ternary Ni-Cr-Si systems from the data of binary Ni-Cr, Ni-Si, and Cr-Si systems. The Toop and Kohler equa- tions are given respectively as [16]:
xs FXA ] GABc = / + / [ 1 - X B 1--XB Ix ,
+(1- 2 xs XB) [ G AC]XA/Xc (A4)
GABC=( 1 2 xs x~ - X c ) [GAB]XA/X.+(1--XB) 2 xs 2 xs X[G Ac]XA/XC+(1--XA) [GBc]X./X,. ( A S )
where G ×S is the molar excess Gibbs free energy and is defined as:
G XS = G - G id (A6)
The definition and location of the terms in eqns. (A4) and (A5) are given in Fig. A1.
The following formula was used to compute the binary system compositions from the ternary composi- tion for the "Toop" equation (see Fig. Al(a); taking A= Ni, B = Si, and C = Cr):
B
(a) ~ p o ~ o n XAXBX C
x s C A [GA.c] XA
XC
B (b)
xs C A [GA.c] XA
XC
Fig. A.1. Illustration of the binary terms used in application of the (a) Toop, and (b) the Kohler equations.
A. M. Hadian, R. A. L. Drew / Wetting at silicon nitride/Ni-Cr-Si alloy interfaces
TABLE A1. Thermodynamic data used for the calculation of AG M of ternary Ni-Cr-Si solutions
217
System H M (J tool l) S xs (J mol - ~ K- ~) G xs (j mol- i)
Cr-Ni -- -- - XNiXcr8368 Cr-Si xc,xs~(177842Xs~ 2 - 223000Xs~- 36400) xoxs~(28.73 - 26.56Xs~) - Ni-Si - XNiXsi(XNi259994 + Xsi133888) - XNiXsi(XNi62.01 + Xsi22.01 ) - -
in N i - S i and C r - S i sys tems,
Xsi, binary = Xsi, ternary
XNi, binary=( ]--Xsi . binary) and
in N i - C r sys tem,
XNi, binary -- XNi, ternary XNi, ternary "]- Xcr, ternary
Xcr , binary = (1 -- XNi, binary)
XCr. binary= (1 -- XSi, binary )
U s i n g the T o o p e q u a t i o n and with r e spe c t to the a b o v e f o r m u l a s and the da ta g iven in Tab le A l , A G~
was ca l cu la t ed to be:
A G 3 = - 1 8 2 . 1 mJ
Fina l ly , the to ta l f ree ene rgy of the r eac t ion (AGr) b e t w e e n SisN 4 and the N i - 2 0 C r - 1 0 Si a l loy a r e the sum of A G I , AG2, and A G ~ , i.e.:
A G r = A G I + A G 2 + A G 3 = 6 .24 + 17.26 - 182.1
= - 1 5 8 . 6 mJ