Immunocytochemical characterization of viruses and antigenic macromolecules in viral vaccines
-
Upload
independent -
Category
Documents
-
view
0 -
download
0
Transcript of Immunocytochemical characterization of viruses and antigenic macromolecules in viral vaccines
Sciences médicales / Medical sciences
Immunocytochemical characterization of virusesand antigenic macromolecules in viral vaccines
Nguyen Van Mana, Hoang Thuy Nguyenb, Huynh Thi Phuong Lienb, Nguyen Thu Vanb,Nguyen Kim Giaob, Nguyen Minh Lienb, Nguyen Thanh Thuyb, Irene Duniad*, Jean Cohenc,E. Lucio Benedettid
a Poliomyelitis Vaccine Research and Production Center (POLIOVAC) , Hanoi, Viet Namb National Institute of Hygiene and Epidemiology (NIHE), Hanoi, Viet Namc Virologie et immunologie moléculaire, institut national de la recherche agronomique, Jouy-en-Josas, Franced Institut Jacques-Monod–CNRS–universités Paris-6 et Paris-7, FranceReceived 27 March 2001; accepted 30 May 2001
Communicated by Jean-Antoine Lepesant
Abstract – Gold immunolabeling combined with negative staining (GINS) provides avaluable immunocytochemical approach that allows a direct ultrastructural definition ofall viral vaccine constituents that share common antigenic features with pathogenic viralparticles. These results have implications for the development of viral vaccines since ithas been demonstrated that incomplete viral particles such as natural empty capsidesand Rotavirus-like particles lacking the infective genome are potential candidates for theproduction of neutralizing antibodies. Furthermore comparative results of the applica-tion of GINS to either inactivated vaccines or unfixed samples provide direct evidencethat even after inactivation specific antigenic sites are still available for gold immunola-beling. © 2001 Académie des sciences/Éditions scientifiques et médicales Elsevier SAS
gold immunolabeling / negative staining / Polio-virus / Japanese encephalitis virus / hepatitis viruses /Rotavirus (RV) / Rotavirus-like particles (VLPs)
Résumé – Caractérisation Immunocytochimique de virus et de macromoléculesantigéniques dans les vaccins antiviraux. L’application combinée de la colorationnégative et de l’immunomarquage à l’or colloïdal (GINS, pour ‘gold immunolabelingcombined with negative staining’), permet d’identifier à la fois les caractères structurauxet les propriétés antigéniques de plusieurs vaccins à l’aide des anticorps spécifiques.L’application de cette méthode aux vaccins contre les virus de la polio et de l’hépatite A,a mis en évidence la présence de très nombreuses capsides vides dans les préparationsvaccinales. Ces structures ont une antigénicité élevée tout à fait comparable à celle desparticules virales complètes. De même, l’étude de capsides de rotavirus dépourvues degénome, produites par des systèmes recombinants, montre que ces pseudo-particulesvirales ont une antigénicité élevée. La méthode décrite est une technique relativementrapide et simple, adaptée aux moyens des laboratoires de pays en voie de développe-ment. Nos résultats peuvent aussi contribuer au choix d’une stratégie dans la productionde vaccins, fondée sur l’isolement et la production de particules pseudo-virales,dépourvues de génome, mais hautement antigéniques. © 2001 Académie dessciences/Éditions scientifiques et médicales Elsevier SAS
immunomarquage à l’or / coloration négative / virus de la polio / encéphalite japonaise / hépatiteA, hépatite B / virus : poliomyélite, rotavirus (RV) / particules pseudo-virales de rotavirus (VLPs)
*Correspondence and reprints.E-mail address: [email protected] (I. Dunia).
815
C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827© 2001 Académie des sciences/Éditions scientifiques et médicales Elsevier SAS. Tous droits réservésS0764446901013609/FLA
. Version abrégée
L’application conjointe de l’immunomarquage à l’orcolloïdal et de la coloration négative (GINS), à l’étudede plusieurs vaccins par microscopie électronique, apermis d’identifier les caractères structuraux de parti-cules virales et, simultanément, les propriétés antigé-niques de constituants vaccinaux actifs.
Dans les vaccins contre les virus de la polio et del’hépatite A, nous avons pu observer de nombreusescapsides virales dépourvues de matériel génomique.Cependant, ces structures conservent leurs propriétésantigéniques telles qu’on peut les déduire par l’intensitédu marquage obtenu avec la méthode décrite. Demême, les particules pseudo-virales de rotavirus pro-duites dans des systèmes recombinants et constituéespar l’assemblage in vitro de protéines de rotavirus, sontintensément immunomarquées.
L’application de cette méthode a mis en évidencedes différences structurales entre des vaccins del’hépatite B préparés de deux façons différentes : 1) levaccin purifié par l’isolement de l’antigène de surfaceHBsAg à partir de sérum de porteurs sains du virus ;2) le vaccin préparé en produisant l’antigène HBsAgdans un système recombinant. L’antigène HBsAgd’origine humaine est caractérisé par la présence denombreuses particules sphériques de 20 nm dediamètre et la présence de nombreux composantstubulaires. En revanche, l’antigène HBsAg préparédans un système recombinant ne contient que desparticules sphériques de 20 nm de diamètre. Cettedifférence morphologique et
probablement antigénique, est due à la compositionprotéique de chaque préparation. Dans le premier cas(antigène d’origine humaine), la présence d’un com-posant protéique de 44 kDa (L), responsable de laformation de tubules, a été décrite. Ce polypeptide estabsent de la préparation d’antigène du système recom-binant. Il a été également décrit que les particulessphériques du HBsAg obtenues dans un système recom-binant contiennent le récepteur de l’albumine sériquehumaine polymérisée (protéine de 34 kDa). Nous avonspu mettre en évidence, avec nos résultatsd’immunomarquage, que l’antigène HBsAg d’originehumaine contient aussi ce récepteur. En effet, le blo-cage préalable par l’incubation exhaustive de ces pré-parations avec l’albumine sérique humaine, réduit con-sidérablement l’immunomarquage de l’antigèned’origine humaine. Ces résultats indiquent que la méth-ode utilisée est suffisamment sensible pour permettrel’étude qualitative de préparations vaccinales. D’autrepart, l’analyse du nombre de particules d’or associéesaux différents composants peut donner des indicationsutiles sur l’efficacité du marquage et, par conséquent,sur l’immunogénicité de particules virales de chaquevaccin étudié en tenant compte des différents procédésde préparation (inactivation par la chaleur, fixationfaible, etc.). Finalement, l’utilisation de cette méthodepermet un contrôle de qualité constant, économique etrapide des préparations vaccinales, et aussi la possibil-ité d’envisager la production d’un vaccin basé sur lapréparation de particules dépourvues de génome maiscaractérisées par une antigenicité spécifique etsignificative.
1. Introduction
Viral diseases cause major community health problems,particularly in developing countries. Despite great progressin our knowledge of fundamental and applied virology,there are only few identified natural or chemotherapeuticagents that are able to cure viral illnesses. Until recentlyanti-viral vaccines were almost the unique successfulresource for prevention and treatment of many viral syn-dromes [1–3].
At a time when vaccine production and quality assess-ment are to be further developed, it is useful to haveadvanced electron microscopy (EM) methods that can testboth the antigenicity and the ultrastructural features ofvaccine preparations.
Several EM techniques have been developed that couldhelp in the identification of viral particles in a crudesample and in the visualization of interactions betweenviral components and specific antibodies [4–10]. Cryo-EMhas opened a new avenue for high-resolution studies ofthe viral architecture and its interactions with specific
antibodies [11–12]. However, this technique requires fullyspecialized laboratory facilities that are not easily avail-able in developing countries. In our opinion, the com-bined application of gold immunolabeling and negativestaining (GINS), represents a method of more generalapplicability and can provide valuable information con-cerning the relation between ultrastructure and immuno-logical features of viruses and viral vaccines. Viral vac-cines include live attenuated vaccines, killed virusvaccines, antigenic macromolecules produced in livingorganisms by active viral infections and recombinant sub-unit vaccines [2]. The data presented in this report dem-onstrate that the application of GINS to virus and viralvaccines is suitable for the identification of the structuralentities bearing the antigenic determinants which are selec-tively enriched during the purification procedure of thevaccine. Although the purification and quality control of avaccine preparation can be tested by various biochemicaland/or immunological methods, GINS may have theadvantage of providing a direct immunocytochemical esti-
816
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
mation of the antigenic particle population and at thesame time, of the presence of non-immunogenic contami-nants.
2. Materials and methods
2.1. Viral and vaccine preparations– The Japanese encephalitis virus (JEV) vaccine was pre-pared as described [13]. Briefly, viral particles were puri-fied from brain tissue of Swiss mice infected with humanJEV. The virus was then inactivated by treatment with1/4 000 formaldehyde for 40 h.– Poliovirus vaccine was prepared from monkey kidneycell primary cultures infected with poliovirus, accordingto the protocol developed in POLIOVAC-NIHE. The vac-cine preparation was also inactivated by 1/4 000 formal-dehyde for 40 h.– Hepatitis A virus (HAV) vaccine was prepared frommonkey kidney cell primary cultures infected with HAVisolated from human carriers, according to the techniqueof purification of the Laboratory of Hepatitis Virus of theNational Institute of Hygiene and Epidemiology (NIHE),Hanoi, Vietnam. The purified vaccine preparation wasinactivated with by 1/4 000 formaldehyde for 40 h.– Hepatitis B surface antigen (HBsAg) was purified fromasymptomatic human carriers but with a high HBsAg titeras described in [14]. The vaccine preparation was inacti-vated by 1/4 000 formaldehyde for 40 h.– Recombinant HBsAg produced in Chinese hamsterovary transfected cells (CHO) with the hepatitis B virusrecombinant plasmid [15], was a generous gift of Dr. M.L.Michel, who has carried out SDS-polyacrylamide gel elec-trophoresis and immunoblotting experiments to caracter-ize the protein constituents of HBsAg preparations [15],(Laboratory of HBV, Pasteur Institute, Paris).– Rotavirus (RV) and Rotavirus-like particles (VLPs), wereprepared as described previously, [16–18]. Rotaviruspreparations for GINS experiments were at times inacti-vated by 0.2 % formaldehyde treatment for 10 min.– The absence of viral contaminants in polio and HAVvaccines was established by immunological tests on themonkey kidney cell primary cultures before infection withthe purified virus (Elisa essays using specific referenceantibodies raised against SV40, PPLO and foamy virus,provided by WHO).
2.2. Antibodies
Immunolabeling was carried out using specific primaryantibodies:– Affinity purified rabbit antibody raised against purifiedJEV prepared by the Encephalitis Virus Laboratory of NIHE,Hanoi, Vietnam.– Affinity purified rabbit antibodies raised against poliovi-rus prepared in the Laboratory of Virology, POLIOVAC,Hanoi, Vietnam.– Human sera against HAV obtained from the Laboratoryof Hepatitis Virus of NIHE, Hanoi, Vietnam, and also fromthe Laboratory of Virology, Hôpital Saint Antoine, Paris,France.
– Affinity purified rabbit antibodies raised against HB viralenvelope proteins prepared at the Laboratory of HepatitisVirus of NIHE, Hanoi, Vietnam.– Affinity purified rabbit antibodies raised against HBsAgproduced in CHO cells transfected with plasmid contain-ing the S gene and the pre-S region of HBV [15].– Rabbit polyclonal antibodies raised against RV proteinswhich recognize VP2, VP4, VP6, VP7.– Monoclonal antibodies E22 and RV138 raised againstthe RV VP2 and VP6 proteins, respectively [19–20].
The specificity of the antibodies raised against JEV andpoliovirus was established by comparative Elisa essaysusing as reference specific antibodies against JEV providedby Bikan, (Osaka, Japan) and specific antibodies againstpoliovirus provided by WHO.
The specificity of the antibodies raised against Rotavirusproteins was tested by immunoblotting experiments usingpurified Rotavirus capside proteins produced in the recom-binant system [19].
Immunoblotting experiments were also carried out fortesting the specificity of the rabbit antibodies raised eitheragainst human HBsAg or HBsAg from CHO recombinantsystem [15].
2.3. Gold Immunolabeling and Negative Staining
The GINS method that we developed is derived from thesingle drop technique [21] and carried out as follows:
20 mL droplets of viral or vaccine preparations areplaced on a clean parafilm surface. Collodium–carboncoated grids are made hydrophilic by rinsing them with0.01 % Bacitracin in water. The grids still wet are put ontop of sample droplets for 5–10 min to pick up the sample.The grids are washed with 2–3 droplets of PBS and thenallowed to float on a droplet of PBS-2 % bovine serumalbumin (BSA) for 20 min to block non-specific antigenicsites. Some samples of HBsAg were incubated with PBScomplemented with 2 % of human serum albumin (HSA)instead of BSA, as blocking step. The grids were thenreacted with the specific first antibodies, diluted inPBS–BSA 0.5 %, or PBS–HSA 0.5 % for 20 min. Aftercareful washes with PBS–BSA 0.2 %, or PBS–HSA 0.5 %,grids were subsequently incubated for 20 min with proteinA conjugated to 5 or 10 nm gold particles (Dept. CellBiology, University of Utrecht, The Netherlands). This stepwas followed by several washes with PBS and rapid fixa-tion (5 min) with 0.1 % glutaraldehyde in aqueous solu-tion. For monoclonal antibodies, before incubation withgold-labeled protein A we used as bridge antibodies, a15 min incubation with affinity purified rabbit anti-mouseimmunoglobulins, diluted 1:500 in PBS–BSA 0.5 %. Thegrids were then thoroughly washed with a solution of0.1 % ammonium acetate, and negative staining was car-ried out using a 1 % aqueous solution of uranyl acetate oruranyl formate pH 5.4. Ammonium molybdate or phos-photungstic acid both at 1 % and pH 7, were occasionallyused but uranyl salts yielded better results. During theprocedure the grids were not allowed to dry and care was
817
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
taken to maintain them floating on the drop surfaces. Afternegative staining, the grids were dried slowly before observation. All procedures were performed at room tempera-ture. We carried out control experiments testing the speci-ficity of the immunolabeling by treating the samplesdirectly with gold labeled protein A, without previousincubation with specific antibodies. Other control experi-ments were carried out by incubating the samples withnon-specific antibodies.
Specimens were examined with a JEOL EM 1010 and aPhilips EM CM12 both EM working at 80 kV.
2.4. Chemicals
Unless otherwise indicated, all chemicals used for thiswork were purchased from Sigma Chemicals Co. (St LouisMO, USA).
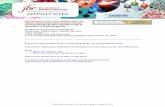
Figure 1. Poliovirus vaccinepreparation immunolabeled usingaffinity purified rabbit antibodiesraised against poliovirus.A. Poliovirus vaccine preparationshowing the presence of 30-nmintact round-shaped virionsmixed with particulate material.Both components are immunola-beled. Arrow points to an emptyviral shell. Arrow heads point to aviral particle displaying featuresof disassembly. Bar: 40 nm.B. Gallery of empty viral shellsalso immunolabeled. Bar: 20 nm.
818
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
3. Results
In our investigation we selected different viral vaccinesthat are commonly used for control and prevention ofserious viral diseases in many developing countries. To testthe general applicability of GINS, we carried out labelingexperiments on viral particles of different sizes and struc-tural organization such as Picornaviridae and Rotavirus. Inthe case of the HBV vaccine we studied by immunolabel-ing the specific viral antigen HBsAg isolated from humancarriers during the various steps of purification. We alsocompared the HBsAg isolated from human carriers withsimilar antigenic molecules constructed by transfection ofrecombinant plasmids. The main issue of our investigationwas to characterize the immunochemical features of viralvaccine preparations using specific antibodies and serafrom human carriers. Furthermore, in view of analysingthe capability of GINS to identify specific viral proteindomains, we studied RV and Rotavirus-like particles (VLPs)using monoclonal antibodies raised against single viralpolypeptides [16–19].
3.1. Poliovirus
Poliovirus, genus Enterovirus, is a member of the Picor-naviridae. It consists of roughly spherical virions 24–30 nmin diameter [12, 22]. The intact particles viewed in thevaccine preparation are intensely gold immunolabeled(figure 1A). In addition, several empty shells (20–30 nm)are found (figure 1B). The empty shells, as well as the viralmaterial at all stages of its degradation, are positivelytagged with gold immunolabeling using antibodies raisedagainst Polio virus particles (figure 1A, arrows and 1B). Nocontaminant viral particles could be detected in any vac-cine preparations.
3.2. HAV vaccine
HAV hepatovirus is a member of the Picornaviridae[23–24]. The diameter of the viral particles ranges between20–30 nm. The viral proteins are assembled according to adodecahedral model in which a capsid surrounds the RNAcore [25]. The vaccine preparation is composed of viralparticles (20–30 nm in diameter, figure 2). The virions aretagged with gold labeled protein A when the sample waspreviously incubated either with specific antibodies raisedagainst HAV or with HAV positive human serum(figure 2B). The HAV vaccine preparation is also charac-terized by many round or oval shells (20–22 nm in diam-eter), that likely correspond to natural empty capsids(NECs; figure 2A), [23]. The empty shells are also goldimmunolabeled. In addition, the vaccine HAV preparationcomprises desintegrated virions displaying a peripheralprotein shell partially stripped away from the core. Thismaterial is also gold immunolabeled and has certain com-mon features with the ‘skullcaps’ (figure 2C), [23].
3.3. JEV vaccine
JEV belongs to the genus Flavivirus (family Flaviviridae).Virions are spherical, 45–55 nm in diameter [22, 26]. They
are characterized by a membranous envelope and a finepeplomer surrounding a spherical nucleocapsid with yetunknown symmetry. The vaccine preparation containsmany intact viral particles 45–50 nm in diameter whichare intensely gold immunolabeled (figures 3A and 3B).Another component is represented by round-shaped emptyshells (30–40 nm) (figure 3C). Furthermore, small particu-late entities which have structural features comparable tofragments of the viral membrane envelope (figure 3Aarrows) are also immunolabeled.
3.4. Hepatitis B viral vaccine (HBsAg from human serumof HBV carriers)
The major constituent of the HB vaccine consists of20–22-nm particles of roughly spherical shape (figure 4A).
Figure 2. HAV vaccine preparation immunolabeled using humansera against HAV.A. HAV vaccine preparation comprises purified 20-nm round-shapedempty viral capsides (arrows) which are immunolabeled using humansera against HAV. Bar : 45 nm.B. Immunolabeled round-shaped 30-nm virion with a distinct outerlayer. Bar: 20 nm.C. The disassembly of a viral particle generates platelets or ‘skullcaps’(arrow head). Bar: 50 nm.
819
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
Tubular-shaped components (20 nm thick) of variablelengths are also observed (figure 4B). All particulate enti-ties are heavily gold immunolabeled by specific HBsAgantibodies (Figures 4A and 4B). Some HBsAg preparationsfrom the sera of human carriers were examined before thelast run of the process of purification by gradient centrifu-gation and found to contain aggregates of round-shaped20-nm particles and tubular components (figure 4C).Occasionally these clusters are associated to granularmaterial that is immunolabeled with antibodies raisedagainst human IGg (figure 4C, arrows).
We have examined an HBsAg preparation produced inCHO cells [15] transfected with a recombinant plasmidcontaining the S gene and the pre-S region of HBV. This
preparation consists of an homogeneous population of20–22-nm spherical particles and no tubular componentscould be detected (figure 5). The particles are intenselygold immunolabeled by anti-HBV antibodies, particularlyrabbit polyclonal antibodies raised against the HBsAgproduced by transfection of the recombinant plasmid (fig-ure 5) (15). When the preparation of HBsAg purified fromhuman serum of HBV carriers is pre-incubated with HSAas blocking agent instead of BSA, the gold immunolabel-ing, using the rabbit polyclonal antibody raised againstHbsAg, is dramatically reduced (figure 6A). This residuallabeling accounts for less than 10 % as compared to morethan 80 % of labeled particles when BSA is used. Reduc-tion of the gold immunolabeling of HBsAg-CHO particleswas also apparent when the preparation is pre-incubatedwith HSA (figure 6B).
3.5. RV and VLPs
Inactivated viral particles [12], purified from RV (family,Reoviridae) infected cultures, are 75 nm in diameter andlook like a wheel with a defined smooth outer rim. Usingpolyclonal antibodies which recognize several viral pro-teins (VP2, VP6 and VP7), gold immunolabeling is detectedeither surrounding the periphery of the virus or at the viralsurface (figure 7A). The VLPs generated by the assembly ofVP2, VP6 and VP7 are 70 nm in diameter and have atriple-layered organization (figure 7B). Gold immuno-labeling with polyclonal antibodies appears as clusters ofgold particles either around the periphery of VLPs or insidethe triple-layered structure (figure 7B). The average num-ber of gold particles labeling the intact formaldehydeinactivated RV counted in > 30 particles was of 13 goldparticles per virion and 5 for triple-layered VLPs. The VLPscomprising only VP2 and VP6 are round-shaped particlesof about 60 nm diameter displaying a double-layeredorganization (figure 8A). The outer layer consists of aregular assembly of subunits (figure 8A). The inner layerappears as a rather uniform stratum (figures 8B–8D). Frag-ments of double-layered VLPs are frequently found inthese preparations and have semi-circular profiles charac-terized by the subunit organization of the uneven outerlayer wrapping the inner stratum (figures 8B–8D). Whenthe monoclonal antibody raised against VP6 is used thesubunits forming the outer layer appear intensely taggedby gold particles (figure 8A). Conversely, gold immunola-beling is mainly restricted to the inner layer when VLPsVP2–VP6 are incubated with the monoclonal antibodyraised against VP2 (figure 8B, arrow). Figure 8C shows onelayered VLP intensely gold labeled with VP2 monoclonalantibody. Figure 8D shows the cup-like aspect of thedouble-layered broken VLP; the VP2 monoclonal antibodyhas access to the VLP inner layer (Figure 8D). The unfixeddouble-layered VLPs (VP2-VP6), immunolabeled with themonoclonal anti-VP6 were tagged by an average numberof 28 gold particles, whereas using the monoclonal anti-body anti-VP2, 7 gold particles were found associated withthe viral inner layer, (the total number of VLPs counted ineach case was 30).
Figure 3. JEV vaccine preparation immunolabeled using affinity puri-fied rabbit antibody raised against JEV.A. The preparation is characterized by the presence of 45–50-nm-immunolabeled intact virions. The JEV sample also comprises par-ticulate entities of different shapes, likely resulting from the degrada-tion of viral particles. This material appears also gold immunolabeled(arrows). Bar: 50 nm.B. Higher magnification of viral particles showing the outer membra-nous shell and the dense core of the viral capsid. The gold particlesare associated to the outer surface of virions. Bar: 30 nm.C. Empty viral shell intensely labeled. Bar: 20 nm.
820
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
4. Discussion
4.1. Ultrastructural features of the viral vaccineconstituents bearing the antigenic activity
In spite of the inherent pitfalls of any technique of directstructural observation of biological specimens, the nega-tive staining method for electron microscopy since itsdevelopment, has provided the most straightforward and
comprehensive information on morphology and designprinciples of viruses [21, cfr.27]. The range of applicabilityof the negative staining technique can be expanded byusing a novel approach that combines gold immunolabel-ing and the electron negative contrast. These complemen-tary techniques have already been successfully applied toseveral problems of fundamental and applied virology[4–8, 21, 28–29]. The application of GINS to HAV, JEV andpoliovirus vaccines provide evidence that in addition to a
Figure 4. HBsAg purified fromhuman sera of HBV carriers.A. The preparation consists ofround-shaped 20–22-nm particleswhich are intensely immunola-beled using antibodies raisedagainst HBsAg-CHO. Bar :50 nm.B. Similar preparation displayingthe pleomorphic organization ofHBsAg characterized by round-shaped particles and tubularstructures both intensely immu-nolabeled using antibodiesdirected against HBsAg fromhuman sera. Bar: 80 nm.C. The sample has been exam-ined before the last run of theprocess of purification by gradi-ent centrifugation. It comprisesclusters of 20-nm particles, tubu-lar structures, mixed with a minoramount of granular material thatappears immunogold labeledusing an antibody directed againsthuman IgG (arrows). Bar: 55 nm.
821
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
major population of intact virions, the preparations con-tain empty shells and structural entities reflecting all stagesof disassembly of the viral particles. The presence ofempty viral shells is a commun feature of purified Picor-naviruses and other viruses (JEV), [30–31]. More recently,these particulate entities were called NECs for ‘naturalempty capsides’ [23]. It is assumed that both infectivevirions as well as empty viral shells are produced when theviruses grow in tissue cultures [32]. Empty shells [30] mayhave different sedimentation coefficients and variable pro-tein compositions in comparison to infective virions [23].It has also been claimed that empty shells can be producedduring the processes of purification and inactivation ofviruses [23]. When Poliovirus inactivation is carried out byharsh heating (56 °C), the RNA genome is released fromthe virion and the protein shells are simultaneously con-verted to a different antigenicity unable to produce neu-tralizing antibodies against the native infective virions [23,33]. However the native antigenicity of empty viral shellscan be preserved when mild conditions of purification and
inactivation are applied, as is the case of our vaccinepreparations which are inactivated by low concentrationsof aldehydes. Intact viruses (HAV, poliovirus and JEV) aswell as the incomplete empty shells of our vaccine prepa-rations appear gold immunolabeled. We may then assumethat the purification and inactivation processes generatedviral structural entities characterized by exposed antigenicsites that are able to interact with specific antibodies raisedagainst the viral antigens. These results need further inves-tigation with complementary immunoassays that couldprovide information on the immunogenicity of a vaccinepreparations.
4.2. Gold immunolabeling and negative staining applied toquality control of HBV vaccine
Previous observations on purified preparations of HBVby negative staining, demonstrated the presence of 3 typesof particulate entities namely, the 42-nm double-shelled‘Dane’ particles corresponding to the infectious HBV, agreat number of 20-nm spherical particles and tubular
Figure 5. HBsAg produced inCHO cells.The sample comprises almostexclusively round-shaped 20-nmparticles which are all immunola-beled with rabbit polyclonal anti-bodies raised against the HBsAg-CHO. Bar: 80 nm.
822
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
components of variable lengths [34, 35]. A common anti-gen, HBsAg, characterizes these different forms. OurHBsAg purified preparations lack Dane particles and con-tain 20-nm spherical particles and tubular assemblies bothheavily immunolabeled using antibodies directed againstHBsAg. The HBsAg 20-nm particles, produced in trans-fected CHO cells [15], have almost the same morphologi-cal features as the HBsAg 20-nm particles of our vaccinepreparation. They appear also heavily immunolabeledwith the rabbit polyclonal antibodies raised against therecombinant HBsAg. However, the recombinant HBsAglacks the tubular forms that we frequently found in thehuman preparation of HBV carriers. The existence ofseveral morphological and immunochemical variants ofHBsAg isolated from different human donors [3, 36], fromsquirrel and duck sera [34] is well documented. Morpho-logical differences are probably related to the variableaminoacid sequence and protein composition of HBsAgsubunits [3]. Gel electrophoresis and immunoblottingexperiments demonstrated that the HBsAg produced fromCHO clones contains polypeptides of 22 kDa (small, S)and 34 kDa (middle, M) but lacks the large polypeptide (L)
of 44 kDa [15]. It is assumed that the presence of the Lpolypeptide is responsible of the assembly of the tubularand filamentous structures [37, 38], therefore it is notsurprising that in the recombinant HBsAg the tubularforms are absent.
HBsAg particles generated by CHO cells carry HSAreceptors associated to the 34-kDa polypeptide [15]. Thepresence of the 34-kDa protein likely confers to HBsAg astrong immunoreactivity in humans.
In our experiments, pre-incubation with HSA as block-ing agent instead of BSA of both HBsAg form human originand HBsAg-CHO, showed that the gold immunolabelingis dramatically reduced. We might then assume that HSAinteracts with a major species-specific antigenic determi-nant – the 34-kDa polypeptide – thus preventing furtherimmunolabeling reaction with the polyclonal antibodiesdirected against human HBsAg. The attraction of thisapproach is that one acquires by the application of GINSan indirect but useful estimate of the presence of specificcomponents of the vaccine structural entites.
Figure 6. A. HBsAg purified fromhuman sera of HBV carriers. Thesample has been pre-incubatedwith HSA as blocking agentinstead of BSA, followed byimmunogold labeling using anti-bodies directed against HBsAgfrom human sera. Immunogoldlabeling of both round-shaped20-nm particles and tubular struc-tures is negligible (arrow). Bar:55 nm.B. HBsAg produced in CHO cells.The sample compring clusters of20-nm particles has been pre-incubated with HSA. The goldimmunolabeling using rabbit anti-bodies directed against HBsAg-CHO is reduced (arrows). Bar:60 nm.
823
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
The use of GINS has also contributed to address theproblem of the presence in the HBsAg isolated from humancarriers of immuno-complexes comprising HBcAg andHBeAg [8]. During the purification procedure of HBsAg,immuno-complexes comprising HBcAg and HBeAg areprogressively eliminated and the application of GINS,using specific anti-IgGs, showed that only in semi-purifiedpreparations could be identified a negligible amount ofhuman immunoglobulins associated to HBsAg. Theseresults suggest that GINS may be a suitable tool to imple-ment the quality control of vaccine samples during purifi-cation procedures.
4.3. Resolution power and labeling efficiency (LE) of GINSapplied to RV, VLPs and viral vaccines
Among icosahedral viruses, the RV structure has beenthoroughly assessed by using cryo-microscopy and 3Dreconstitution methods [12]. Stable VLPs are produced byexpression in insect tissue cultures of Bacculovirus recom-binants with gene coding sequences for RV capsid pro-teins [16–18]. Our data concern VLPs comprising eitherVP2, VP6 and VP7 (triple-layered) or VP2 and VP6 (double-layered). GINS applied to these structures confirms thenotion that VLPs lacking RNA genome are self-assembled
Figure 7. A. Intact RV of 75 nmdiameter, purified from RVinfected cultures. RV are goldimmunolabeled with polyclonalantibodies raised against viral pro-teins VP2–VP7. Bar: 40 nm.B. Triple-layered subunit organi-zation of 75-nm VLP generatedby the assembly of VP2, VP6 andVP7. The three layers are immu-nolabeled by polyclonal antibod-ies raised against VP2-VP7. Bar:35 nm.
824
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
in the appropriate expression system and share commonantigenic features with infective RV. The term of self-assembly is used to indicate the process of association ofviral capside proteins in the absence of viral genome bystereospecific, non-covalent interactions, between identi-cal or quasi-equivalent subunits [12].
The question remains as to whether the results of ourimmunolabeling experiments can provide an indication ofthe antigenic density of the viral vaccines. The problems ofconcentration of the antigens, LE and signal to noise ratiohave been evaluated for gold-immunolabeling of embed-ded tissue sections and non-embedded cryo-sectionswhere the number of antigenic sites for a specific func-tional protein has been previously assessed [39]. The LE isthe proportion of antigen in a preparation that is recog-nized by the labeling antibody. This parameter can bederived by counting the number of immunogold particlesdivided by the known number of antigens which theylabeled [39]. In our experiments we applied GINS topoliovirus, JEV, HAV, and RV previously inactivated byfixation with formaldehyde. In these conditions it is noteasy to estimate the absolute number of antigenic sites stillavailable for gold immunolabeling and in turn, LE. Themore realistic range of LE expected for a known number ofantigenic sites present in fixed biological samples is1–15 % [39]. In our experiments inactivated rotavirionsare labeled with an average of 13 gold particles using thepolyclonal antibodies raised against VP2 to VP7. Assum-ing that the major epitopes of the viral particles are asso-ciated with 260 VP6 trimers, 260 VP7 trimers and 120
subunits of VP2 (1 680 total antigenic sites per virion), theaverage of 13 gold particles tagging a single virion corre-sponds to a LE of 0.8 %. This value is close to the estima-tion for aldehyde-fixed antigens [39]. Using polyclonalantibodies raised against VP2 to VP7 and applied to unfixedtriple-layered VLPs (VP2, VP6, VP7), we observed that thenumber of gold particles tagging the protein subunits isrelatively small (5 gold particles/VLP). This value likelyreflects the topographic distribution of the viral proteinsand the accessibility of specific epitopes. It is assumed thatVP7 is mainly associated with the outer layer of VLPs andin this situation it may hinder the accessibility of VP2 andVP6 which are the major inner constituents of the triple-layered VLPs.
Unfixed and intact double-layered VLPs comprisingVP2 (120 subunits) and VP6 (780 subunits), are labeledrespectively with an average of 28 gold particles using amonoclonal antibody against VP6 and with an average of 7gold particles using a monoclonal antibody against VP2.Hence, LE for VP6 and VP2 are 3.5 % and 5 % respec-tively. This observation is consistent with the notion that inself-assembled double-layered VLPs the VP6 is the majorconstituent of the viral shell exposed to the outer surface ofthe double-layer, whereas VP2 forms the inner core of thisdouble-layered VLP and its immunolabeling is enhancedwhen the inner layer is exposed by artificial removal of theouter layer (cf. figures 8C and 8D).
These data suggest that GINS is a valuable method forthe topographic identification of the various viral constitu
Figure 8. Double-layered VLPsgenerated by the assembly of VP2
and VP6.A. VLP 2/6 tagged by a mono-clonal antibody directed againstVP6. Many gold particles form acrown around the VLP outer layer.Bar: 35 nm.B. VLP 2/6 immunolabeled by amonoclonal antibody directedagainst VP2. The arrow points tothe gold particles associated withthe inner stratum. Bar: 20 nm.C. VLP 2/6 tagged by a mono-clonal antibody directed againstVP2. Single layered VLP sur-rounded by clusters of gold par-ticles. Bar: 35 nm.D. A double-layered VLP 2/6 openat one side, immunolabeled witha monoclonal antibody directedagainst VP2. The gold particlesare associated with the inner layerviewed ‘en face’. Note the circu-lar profile of the fragmented outerlayer. Bar: 40 nm.
825
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
ents but its resolution is limited by the steric hindrancegenerated by the packing density of the antigenic sitesassociated with different layers of the viral capsid.
5. Concluding Remarks
The conjunct application of gold-immunolabeling andnegative staining to selected viral vaccines provides valu-able information both on the ultrastructural features andon the antigenicity of viral particles and other viral macro-molecules. The self-assembled virus-like particles andempty viral shells lacking the infective genome, sharecommun immunocytochemical characters with patho-genic viruses. Therefore these non-infective structures maybe considered as potential candidates for further purifica-tion and vaccine production. We must however admit thatalthough GINS can be considered a reliable technique forthe qualitative analysis of the presence of active antigenicsites and for testing the absence of viral contaminants, itmight requires for quantitation of the antigenic densitymore informations (by immunoassay and other comple-mentary techniques) [3, 33], on the immunochemical andvariable features of the viral preparations.
The resolution and labeling efficiency of GINS is rela-tively high and taking into account a very low backgroundnoise that characterizes our GINS preparations even asmall number of gold particles associated to a viral struc-ture would be highly significant.
Further developments on immunolabeling techniques,particularly the use of high-resolution immuno-probes
and reduced sized electron-dense markers, combined withsite-directed mutagenesis of viral constituents and cryo-electron microscopy are already in progress [12, 40].These approaches might help further topological analysesof the structural and molecular features of viral vaccineconstituents that play a key role in the activation of thehumoral and/or cell mediated protective response and onthe switch of immunological memory.
Acknowledgements. We gratefully acknowledge thegenerous gift of antibodies directed against HBsAg andof recombinant HBsAg by Dr. Marie-Louise Michel,Institut Pasteur, Paris. We wish to thank Dr. CatherineJohanet, Laboratory of Immuno-Hematology, HôpitalSaint-Antoine, Paris, for providing antibodies againstHAV and Dr. Anne-Lise Haenni, Institut Jacques Monod,Paris, for helpful discussion and advice. Drs. E. L.Benedetti, I. Dunia and J. Cohen express their gratitudeto Dr. Hoang Thuy Long, Director of the NationalInstitute of Hygiene and Epidemiology (NIHE) and toDr. Dang Duc Trach, Chairman of the Scientific Councilof NIHE, for their advice and kind hospitality. Thesestudies were supported by the Ministry of Health ofVietnam, the NIHE, the Vaccine Production CenterPOLIOVAC, Hanoi, Vietnam, and by the “Service de laFormation du Centre National de la Recherche Scien-tifique (CNRS) Programme CNRS-UNESCO”, Paris,France.
References
[1] Harrison S.C., Principles of virus structure, in: Fields B.N.,Knipe D.M. (Eds.), Virology, Raven Press, NY, 1990, pp. 37–62.
[2] Levine A.J., The origin of virology, in: Fields B.N., Knipe D.M.(Eds.), Fundamental Virology, Lippin-Cott-Raven Publ. Ltd, NY, 1996,pp. 1–14.
[3] Zuckerman J.N., Zuckerman A.J., Current topics in Hepatitis B,J. Infect. 41 (2000) 130–136.
[4] Beesley J.E., Betts M.P., Virus diagnosis: a novel use for the proteinA-gold probe, Med. Lab. Sci. 42 (1985) 161–165.
[5] Hyatt A.D., Eaton B.T., Lunt R., The grid-cell-culture technique: thedirect examination of virus-infected cells and progeny viruses, J. Microsc.145 (1987) 97–106.
[6] Murti K.G., Brown P.S., Bean W.J., Webster R.G., Composition ofthe helical internal components of influenza virus as revealed by immu-nogold labeling/electron microscopy, Virology 186 (1992) 294–299.
[7] Nermut M.V., Wallengren K., Pager J., Localization of actin inMoloney murine leukemia virus by immunoelectron microscopy, Virol-ogy 260 (1999) 23–34.
[8] Stannard L.M., Lennon M., Hodgkiss M., Smuts H., An electronmicroscopic demonstration of immune complexes of Hepatitis B e-antigenusing colloid gold as a marker, J. Med. Virol. 9 (1982) 165–175.
[9] Hopley J.F., Doane F.W., Development of a sensitive protein A-goldimmunoelectron microscopy method for detecting viral antigens in fluidspecimens, J. Virol Methods. 12 (1985) 135–147.
[10] Biel S.S., Gelderblom H.R., Diagnostic electron microscopy is stilla timely and rewarding method, J. Clin. Virol. 13 (1999) 105–119.
[11] Dubochet J., Adrian M., Chang J.J., Homo J.C., Lepault J., McDow-all A.W., Schultz P., Cryo-electron microscopy of vitrified specimens, Q.Rev. Biophys. 21 (1988) 129–228.
[12] Baker T.S., Olson N.H., Fuller S.D., Adding the third dimension tovirus life cycles: three-dimensional reconstruction of icosahedral virusesfrom cryo-electron micrographs, Microb. Mol. Biol. Rev. 63 (1999)862–922.
[13] Doan Thi Thuy, Huynh Phuonh Lien, Nguyen Hong Hanh, HoangThuy Nguyen, Purification of Japanese Encephalitis virus for vaccineproduction, J. Prevent. Medec. 1 (1991) 19–25.
[14] Hoang Thuy Nguyen, Nguyen Thu Van, Purification of Hepatitis Bsurface antigen for HBsAg-Micro-ELISA kit and Hepatitis B vaccine prepa-ration, Proc. Int. Symp., Immunology and Infection, National Institute ofHygiene and Epidemiology (Hanoi), University of Amsterdam (Holland),1990, Hanoi-Vietnam..
[15] Michel M.L., Pontisso P., Sobczak E., Malpiece Y., Streeck R.E.,Tiollais P., Synthesis in animal cells of hepatitis B surface antigen particlescarrying a receptor for polymerized human serum albumin, Proc. Natl.Acad. Sci. USA. 81 (1984) 7708–7712.
[16] Labbe M., Charpilienne A., Crawford S.E., Estes M.K., Cohen J.,Expression of rotavirus VP2 produces empty corelike particles, J. Virol. 65(1991) 2946–2952.
[17] Crawford S.E., Labbe M., Cohen J., Burroughs, Yong-Jie Zhou,Estes M., Characterization of virus-like particles produced by the expres-sion of Rotavirus capsid proteins in insect cells, J. Virol. 68 (1994)5945–5952.
[18] Crawford S.E., Estes M.K., Ciarlet M., Barone O’Neal C.M.,Cohen J., Conner M.E., Heterotypic protection and induction of a broadheterotypic neutralization response by rotavirus-like particles, J. Virol. 73(1999) 4813–4822.
[19] Pothier P., Kohli E., Drouet E., Ghim S., Analysis of the antigenicsites on the major inner capside protein (VP6) of rotaviruses using mono-clonal antibodies, Ann. Ins. Pasteur/Virol. 138 (1987) 285–295.
[20] Roseto A., Scherrer R., Cohen J., Guillemin M.C., Charpilienne A.,Feynerol C., Peries J., Isolation and characterization of anti-rotavirus
826
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827
immunoglobulins secreted by cloned hybridoma cell lines, J. Gen. Virol.64 (1983) 237–240.
[21] Hayat M.A., Miller S.E., Negative staining, McGraw-Hill Publ. NY,1990, pp. 51–155.
[22] Murphy F.A., Kingsbury D.W., Virus taxonomy, in: Fields B.N.,Knipe D.M. (Eds.), Virology, Raven Press, NY, 1990, pp. 3–36.
[23] Rueckert R.R., Picornaviridae and their replication, in:Fields B.N., Knipe D.M. (Eds.), Virology, Raven Press, NY, 1996,pp. 509–654.
[24] Smit T.J., Baker T.S., Picornaviruses: epitopes, canyons and pock-ets, Adv. Virus Res. 52 (1999) 1–23.
[25] Hollinger F.B., Ticehurst J., Hepatitis A virus, in: Fields B.N.,Knipe D.M. (Eds.), Virology, Raven Press, NY, 1990, pp. 631–670.
[26] Monath T.P., Flaviviruses, in: Fields B.N., Knipe D.M. (Eds.),Virology, Raven Press, NY, 1990, pp. 763–814.
[27] Valentine R.C., Contrast enhancement in the electron microscopyof virus, Adv. Virus Res. 8 (1962) 287–318.
[28] Risco C., Anton I.M., Enjuanes L., Carrascosa J., The transmissiblegastroenteritis Coronavirus contains a spherical core shell consisting of Mand N proteins, J. Virol. 70 (1996) 4773–4777.
[29] Zimmermann W., Breter H., Rudolph M., Ludwig H., Bornadisease virus: immunoelectron microscopic characterization of cell-freevirus and further information about the genome, J. Virol. 68 (1994)6755–6758.
[30] Jacobson M.F., Baltimore D., Morphogenesis of poliovirus. I.Association of the viral RNA with coat protein, J. Mol. Biol. 33 (1968)369–378.
[31] Johnston M.D., Martin S.J., Capsid and procapsid proteins of abovine enterovirus, J Gen Virol. 11 (1971) 71–79.
[32] Putnak J.R., Piticlips B.A., Differences between Poliovirus emptycapsids formed in vivo and those formed in vitro: a role for the morphopoi-etic factor, J. Virol. 122 (1981) 173–183.
[33] Kersten G., Hazendonk T., Beuvery C., Antigenic and immuno-genic properties of inactivated polio vaccine made from Sabin strains,Vaccine 17 (1999) 2059–2066.
[34] Hollinger F.B., Hepatitis B virus, in: Fields B.N., Knipe D.M. (Eds.),Virology, Raven Press, NY, 1990, pp. 2171–2238.
[35] Robinson W.S., Hepadnaviridae and their replication, in:Fields B.N., Knipe D.M. (Eds.), Virology, Raven Press. NY, 1990,pp. 2137–2170.
[36] Stibbe W., Gerlich W.H., Variable protein composition of Hepa-titis B surface antigen from different donors, Virology 123 (1982) 436–442.
[37] Xu Z., Bruss V., Yen B., Formation of intracellular particles byHepatitis B virus large surface protein, J. Virol. 71 (1997) 5487–5494.
[38] Prange R., Werr M., Loffler-Mary H., Chaperones involved inHepatitis B virus morphogenesis, J. Biol. Chem. 380 (1999) 305–314.
[39] Griffith G., Fine Structure Immunocytochemistry, Springer-Verlag,New York, 1993, pp. 417–442.
[40] Zlotnick A., Cheng N., Conway J.F., Booy F.P., Steven A.C.,Stahl S.J., Wingfield P.T., Dimorphism of Hepatitis B virus capsids isstrongly influenced by the C-terminus of the capsid protein, Biochemistry35 (1996) 7412–7421.
827
N. Van Man et al. / C.R. Acad. Sci. Paris, Sciences de la vie / Life Sciences 324 (2001) 815–827