Evaluating diagnostic tests for bovine tuberculosis in ... - PLOS
Expression of bone morphogenetic protein2 (BMP2), BMP4 and BMP receptors in the bovine ovary but...
-
Upload
independent -
Category
Documents
-
view
2 -
download
0
Transcript of Expression of bone morphogenetic protein2 (BMP2), BMP4 and BMP receptors in the bovine ovary but...
Expression of bone morphogenetic protein2
(BMP2), BMP4 and BMP receptors in the bovine
ovary but absence of effects of BMP2 and BMP4
during IVM on bovine oocyte nuclear maturation
and subsequent embryo development
A.N. Fatehia,*, R. van den Hurka, B. Colenbrandera,A.J.J.M. Daemena, H.T.A. van Tola, R.M. Monteirob,
B.A.J. Roelena, M.M. Beversa
aDepartment of Farm Animal Health, Faculty of Veterinary Medicine,
Yalelaan 7, 3584 CL Utrecht, The NetherlandsbHubrecht Laboratory, Netherlands Institute for Developmental Biology, Uppsalalaan 8,
3584 CT Utrecht, The Netherlands
Received 21 March 2004; received in revised form 7 May 2004; accepted 12 May 2004
Abstract
Bone morphogenetic proteins (BMPs) have been implicated in the regulation of ovarian
follicular development and are promising candidates to apply in IVM and IVF protocols. We
investigated the expression of BMP2, BMP4 and BMP receptors in bovine ovaries and the effects
of BMP2 and BMP4 during oocyte maturation on bovine IVM. Reverse transcription polymerase
chain reaction studies with antral follicles showed the expression of BMPR-IA, BMPR-IB,
ActR-IA, ActR-IIB, BMPR-II and BMP4 mRNA in all follicular compartments, while BMP2
mRNA was generally restricted to theca and cumulus tissue. Immunohistochemistry demonstrated
the presence of BMPR-II in oocytes and granulosa cells of preantral follicles but only in oocytes of
antral follicles. The immunostaining of BMP2 and BMP4 was limited to theca interna and
approximately 25% of oocytes of antral follicles. Exogenously added BMP2 or BMP4 to IVM
medium did not affect oocyte nuclear maturation, cumulus cell expansion, nor blastocyst
formation following IVF. It is concluded that a BMP-signaling system, consisting of BMP2,
BMP4, type II and I receptors, is present in bovine antral follicles and that this system plays a role
Theriogenology 63 (2005) 872–889
* Corresponding author. Tel.: þ31-30-253-1135; fax: þ31-30-253-4811.
E-mail address: [email protected] (A.N. Fatehi).
0093-691X/$ – see front matter # 2004 Published by Elsevier Inc.
doi:10.1016/j.theriogenology.2004.05.013
in development and functioning of these follicles rather than in final oocyte maturation and
cumulus expansion.
# 2004 Published by Elsevier Inc.
Keywords: BMP; BMP receptors; Bovine; Oocyte maturation; Embryo development
1. Introduction
The transforming growth factor ß (TGF-ß) superfamily figures prominently in the
regulatory events of morphogenesis, organogenesis and cytodifferentiation including
ovarian folliculogenesis [1]. An increasing body of evidence indicates that various peptide
growth factors, including members of the TGF-ß superfamily, are expressed by oocytes,
granulosa and theca cells in a developmental stage-related manner and function as
intraovarian regulatory molecules involved in follicle recruitment, granulosa and theca
cell proliferation/atresia, steroidogenesis, oocyte maturation, ovulation, and luteinization
[2]. Within the TGF-ß superfamily, bone morphogenetic proteins (BMPs) comprise the
largest subgroup of ligands with 20 BMPs being described [3]. BMPs interact with two
classes of transmembrane serine-threonine kinase receptors, BMP receptor types I and type
II. In mammals, three type I receptors (BMPR-IA/Alk3, BMPR-IB/Alk6 and ActRI/Alk2)
and three type II receptors (BMPR-II, ActR-II, and ActR-IIB) have been identified [2].
Individually, type I and II BMP receptors are able to bind ligand but efficient ligand binding
and subsequent signal transduction by BMP receptors requires the formation of a
heteromeric complex between both receptor types [4,5]. Once the ligand-receptor complex
is formed, the type II receptor, which has constitutive kinase activity, phosphorylates and
activates the type I receptor that triggers downstream events in the BMP signaling pathway
[6,7].
Several BMP genes are expressed in the mammalian ovary. The mRNA encoding BMP2,
3, 3b, 4, 6, 7 and 15 has been identified in ovaries of various mammalian species [8–13].
Normal cyclic rats express mRNA for BMP4 and BMP7 in the theca layer of most ovarian
follicles, while mRNA for BMPR-IA, BMPR-IB and BMPR-II is expressed in oocytes and
granulosa cells [12]. In sheep, BMP receptors are localized in the oocytes, granulosa cells
(in both primary and antral follicles), theca cells (antral follicles), and the ovarian surface
epithelium [14,15].
Though BMPs have been implicated in the paracrine regulation of ovarian follicular
development, their precise role in reproduction is not clearly understood. A functional role
for BMPs in the ovary is suggested by the observation that in vitro cultured rat granulosa
cells treated with BMP4 or BMP7 show increased oestradiol production and a reduction in
progesterone secretion [12]. Similarly BMP2, added to sheep granulosa cell culture
resulted in enhanced oestradiol production [15]. In rat, BMP7 can stimulate oestradiol
production, decreases serum progesterone levels and may act to facilitate the transition of
follicles from the primordial stage to the later stages, though it lowers the ovulation rate
[16]. Furthermore, BMP7 [16] and BMP15 [17] were found to stimulate proliferation of in
vitro cultured rat granulosa cells. In cultured rat granulosa cells, BMP6 [18] and BMP15
[19] are important determinants of FSH action through the ability to down-regulate
A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889 873
adenylate cyclase activity and to inhibit FSH receptor expression, respectively. The
importance of this putative regulatory system has been confirmed by the observation that
naturally occurring mutations in the BMP signaling pathways have resulted in marked
perturbation of ovarian function in a dosage-sensitive manner [20]. In sheep, mutations in
the gene coding for BMP15 can cause increased ovulation rate and multiple births in
heterozygotes but primary ovarian failure in homozygotes [21]. Likewise, mice lacking
BMP15 are fertile but have reduced litter size [22]. In contrast mice lacking BMP6 appear
to have normal fertility [23]. On the other hand, mutations in BMPR-IB can result in a
greatly increased ovulation rate and multiple births in sheep [14,20,24], while mice that are
genetically deficient for BMPR-IB are infertile, hypo-oestrogenic and show defective
cumulus cell expansion [25].
Since BMPs may play an important role in follicular growth and differentiation, cumulus
expansion and ovulation, they are promising candidates for addition in assisted fertility and
IVF protocols. In this study, we examined the significance of BMP2 and BMP4 for in vitro
production of bovine embryos. To that end, we studied the mRNA and protein expression
of BMP2, BMP4 and their receptors in the bovine ovary and the effects of these BMPs
during IVM on oocyte maturation, cumulus cell expansion, IVF, blastocyst formation and
blastocyst quality.
2. Materials and methods
2.1. Collection of ovaries and COCs
Bovine ovaries were collected at a slaughterhouse in a thermo flask and transported to
the laboratory within 1 h. Part of the total number of excised ovaries were fixed overnight at
4 8C in 4% (w:v) paraformaldehyde in phosphate-buffered saline (PBS) and subsequently
dehydrated and embedded in paraffin wax (Histoplast, Shandon Scientific, Pittsburgh, PA,
USA). The rest of the collected ovaries were rinsed in physiological saline (0.9% NaCl)
containing antibiotics (100 IU penicillin and 100 mg streptomycin per mL) and dried with
paper towel. Cumulus–oocyte complexes (COCs) were aspirated from small antral follicles
(2–8 mm diameter) using an 18-ga needle attached to a tube in line with a vacuum
pump. COCs were selected on the presence of a multilayered compact cumulus investment
and homogeneous ooplasm and randomly assigned to the various treatments.
2.2. Immunohistochemistry
Serial 5 mm sections of ovaries were mounted on poly-L-lysine coated slides and dried
overnight at 37 8C. After removing the paraffin and washing the slides in TBS (0.05 M
Tris/0.15 M NaCl; pH 7.4), endogenous peroxidase was blocked by a 30 min incubation in
methanol with 0.75% (v/v) H2O2. The slides were then rinsed twice for 5 min in TBS and
incubated in TBS/glycin 0.75% for 30 min. For detection of BMPR-II, sections were boiled
in citrate buffer (0.01 M; pH 6.0) for 10 min to activate epitopes. The slides were washed
three times for 5 min in TBS/Tween 0.05% (Merck, Darmstadt, Germany) and covered
with blocking buffer (10% normal horse serum in TBS) for 15 min, incubated for 1 h at
874 A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889
room temperature in a moist chamber and subsequently overnight at 4 8C with a primary
goat anti-human BMP2, goat anti-human BMP4 or goat anti-human BMPR-II antibody (all
from Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:20 in blocking buffer.
The following day slides were rinsed three times for 10 min in TBS/Tween, incubated with
biotinylated horse anti-goat antibody (Vector Labs, Burlingame, CA, USA) (1:200 diluted
in 1% horse serum in TBS) for 1 h at room temperature. Afterwards, the slides were rinsed
three times for 5 min in TBS/Tween and incubated with avidin–biotin complex 1:600
(Vectastain Elite ABC kit, Vector Labs, Burlingame, CA, USA) for 1 h. Finally, the
presence of antibody was visualised using 3,30-diaminobenzidine tetrachloride (DAB,
Sigma Chemicals, St Louis, MO, USA) in TBS (pH 7.6) containing 0.03% H2O2 either or
not followed by addition of 0.2% nickel ammonium sulphate to intensify the reaction or by
counterstaining for 15 s in Mayer’s haematoxylin. When counterstaining was omitted,
slides were rinsed in distilled water and after dehydration, mounted in DEPEX (EMS,
Hatfield, PA, USA). When counterstaining was carried out, slides were washed for 10 min
in running tap water before dehydration and mounting. The specificity of the immunos-
taining was confirmed by (i) replacing the primary antibody by goat IgG antiserum in the
same concentration as the first antibody and (ii) preabsorbing the primary antibody
overnight at 4 8C with its blocking peptide (Santa Cruz Biotechnology, Santa Cruz,
CA, USA) at five-fold weight excess.
2.3. Classification and measurement of follicles
Ovarian follicles were classified as primordial (with one layer of flat granulosa cells),
primary (with one layer of cuboidal granulosa cells), secondary (with multiple granulosa
cells) and antral (with multiple layer of granulosa cells enclosing an antrum). The diameter
of the follicles was calculated as described [26].
2.4. Collection of follicular cells, extraction of total RNA and reverse transcription
From the follicular content, collected as described above, COCs with a compact cumulus
investment and pieces of compact mural granulosa were selected. Using a narrow-bore
Pasteur pipette, the cumulus was separated from the oocytes. Denuded oocytes, cumulus
cells and mural granulosa were washed four times in PBS and stored at �80 8C until RNA
extraction. Ten denuded oocytes, cumulus cells from 10 COCs or three pieces of mural
granulosa were collected per sample.
To collect theca cell tissue, follicles between 2 and 8 mm in diameter were isolated from
the ovary and dissected free of stromal tissue with forceps. The follicles were cut in halves
and the granulosa cells were scraped off with a scalpel blade. The theca cell layers were
vortexed 1 min in 1 mL HEPES buffered M199 (GibcoBRL, Paisley, UK) transferred to a
fresh mL of buffer, vortexed for another min, washed twice in 2 mL HEPES buffered
M199, collected separately and stored at �80 8C until RNA extraction. Five replicates of
each tissue sample were made. Isolation of total RNA combined with on-column DNAse
digestion was performed using the RNeasy Mini Kit and the RNAse-free DNAse Set
(Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions after which the
RNA was eluted in 30 vL RNAse-free water.
A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889 875
Prior to the reverse transcription reaction, the RNA sample was incubated for 5 min at
70 8C, vortexed for 5 s and chilled on ice. Reverse transcription was done with 10 vL RNA
in a total volume of 20 vL containing reverse transcriptase buffer (GibcoBRL, Breda, The
Netherlands), 8 units RNAsin (Promega, Madison, WI, USA), 150 units Superscript II
reverse transcriptase (GibcoBRL, Paisley, UK), 0.036 U random primers (Life Technol-
ogies BV, Leiden, The Netherlands) and final concentrations of 10 mM DTT and 0.5 mM
of each dNTP. The mixture was incubated for 1 h at 42 8C, for 5 min at 95 8C, and stored at
�20 8C. Minus RT blanks were prepared under the same conditions but without reverse
transcriptase.
2.5. Polymerase chain reaction (PCR)
PCR reactions were carried out using 1 vL cDNA as template in 25 vL containing final
concentrations of 2 mM MgCl2, 200 mM of each dNTP, 0.5 mM each of primers and
0.625 units Taq DNA polymerase (HotStarTaq, Qiagen, Valencia, CA, USA). Primers used
for amplification are presented in Table 1.
The thermal cycling profile for the first round was: 15 min at 94 8C, followed by 40
cycles of 15 s at 94 8C, 30 s at 55 8C and 45 s at 72 8C. Final extension was for 10 min at
72 8C. Heminesting was used for the amplification of BMP2 and BMP4 cDNA to increase
the sensitivity. For heminesting 1 vL of the first round product was transferred to 24 vL
PCR mixture as above, and amplified for 30 cycles according to the same profile. All
reactions were performed in a 2400 thermocycler (Perkin-Elmer, Gouda, The Nether-
lands). Visualization of the products was done by electrophoresis in 1% agarose gels
containing 0.4 mg/mL ethidium bromide, and illumination by UV light. A 100 base pair
(bp) DNA ladder (GibcoBRL, Paisley, UK) was included as a reference for fragment size.
An image of the gel was taken using a digital camera (Olympos C-4040, New York, NY,
USA). A standard sequencing procedure (ABI PRISM 310 Genetic analyzer, Applied
Biosystems, Foster city, CA, USA) was used to verify the specificity of the amplified
products.
2.6. IVM, IVF and IVC
COCs collected as described before, were rinsed in HEPES buffered M199 (Gibco,
Paisley, UK) and in groups of 35 randomly allocated to each well of a 4-well culture plate
(Nunc A/S, Roskilde, Denmark) containing 500 vL of maturation medium per well and
then cultured for 23 h. The maturation medium consisted of M199 (Gibco, Life Tech-
nologies, Breda, The Netherlands) with penicillin/streptomycin and 100 ng/mL of recom-
binant human BMP2 or recombinant human BMP4 (Genetics Institute, Cambridge, MA,
USA) in the presence or absence of 0.05 units/mL recombinant human FSH (Organon, Oss,
The Netherlands).
Frozen/thawed spermatozoa used for IVF were centrifuged over a percoll gradient for
30 min at 700 � g at 25 8C. The sperm sample was collected by removing the gradient
except for the last 150 vL containing the sperm pellet. Thirty-five COCs were transferred to
430 vL fertilization medium (Fert-TALP) as described by Parrish et al. [27] without
glucose and 10 mg/mL penicillin/streptomycin instead of gentamycin. Twenty vL of sperm
876 A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889
suspension (final concentration 0:5 � 106 spermatozoa/mL), 20 vL heparin (final con-
centration 10 mg/mL) and 20 vL PHE (consisting of 20 mM D-penicillamin, 10 mM
hypotaurine, and 1 mM epinephrine) were added to each well. After 18–20 h of incubation,
the presumptive zygotes were freed from cumulus cells by vortexing for 3 min and in
groups of 35 randomly placed in a co-culture system of 0.5 mL M199 supplemented with
10% FCS on a monolayer of Buffalo rat liver (BRL) cells. On day 4 and 8 of culture,
embryos were transferred to fresh co-culture wells. IVM and IVF as well as in vitro embryo
culture (IVC) took place at 39 8C in a humidified atmosphere of 5% CO2 in air.
2.7. BRL-cell culture
BRL cells separated from the BRL cell line from the American Type Culture Collection
(ATCC) were cultured routinely in a 1:1 mixture of Ham’s F12 medium and Dulbecco’s
modified Eagle’s medium (GibcoBRL, Paisley, UK) supplemented with 7.5% FCS
(GibcoBRL, Paisley, UK) and antibiotics.
2.8. BMP reporter embryonic stem cells
Mouse embryonic stem (ES) cells stably transfected with a gene construct consisting of a
BMP-specific reporter element in front of the lacZ gene (BRE-lac1, [28]) were used to
control for BMP activity. Culture medium to which BMP2 or BMP4 was added (100 ng/ml)
was split into two, half was used for maturation of the oocytes, the other half was used to
measure BMP activity with the ES reporter cells. ES cells were cultured in BRL
Table 1
Primers used for PCR analysis of cDNA in oocytes, cumulus cells, theca tissue and mural granulosa
Target
gene
Primer sequence (50 ! 30) Sense Position GenBank accession
number
BMP2 CTGTCTTCTAGCGTTGCTGC s 341–360 GI: 4557368 (2003)
GTCCTGAGCGAGTTCGAGTT s 468–487 Homo sapiens BMP2
CAGCTGTGTTCATCTTGGTG as 1074–1093
BMP4 GACTTCGAGGCGACACTTCT s 379–398 GI: 18088238 (2002)
CCTTGAGGTAACGATCGGCT as 795–814 Homo sapiens BMP4
CCAGTAGTCGTGTGATGAGG as 976–995
BMPR-II GATATGCAGGTTCTGGTGTC s 20–39 GI: 26985548 (2002)
AGTTCAGCCATCCTCTCTTC as 170–189 Bos taurus BMPRII
ActR-IIB CAACTTCCAGAGAGACGCCT s 1280–1299 GI: 31341841 (2003)
ACACTCGCTCCTCCACACAG as 1574–1593 Bos taurus ActR-IIB
ActR-IA/ AGATGAGAAGCCCAAGGTAAA s 193–213 GI: 31341405 (2003)
ALK2 CCAGAGATGTGGAATATGGCC as 603–624 Bos taurus ACVR1
BMPR-IA/ TCGTCGTTGTATTACAGGAG s 1603–1632 GI: 6753193 (2003)
ALK3 TTACATCCTGGGATTCAACC as 1952–1971 Mus musculus Bmpr1a
BMPR-IB/ ATGGAGCAGTGATGAGTGTCT s 1554–1574 GI: 6680801 (2003)
ALK6 GTCCCAGGACATTAAACTCTG as 1674–1694 Mus musculus Bmpr1b
A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889 877
conditioned medium (BRL CM), DMEM medium conditioned in BRL cells, supplemented
with 2 mM L-glutamine and non-essential aminoacids (1:100) and 20% FCS) as described
previously [29]. To investigate the BMP activity in the maturation media, these ES cells
were cultured in BRL CM in the absence of feeders, supplemented with 0.1 mM
b-mercaptoethanol and either 20% or 5% FCS. On day 1, 2:5 � 104 cells/cm2 were seeded
in gelatinized 12-well plates and allowed to grow for 24 h. On day 2 cells were washed once
with PBS and fresh maturation medium with 100 ng/mL of recombinant BMP2 or BMP4 was
added. On day 3, BRE-lac1 ES cells were lysed with 100 vL of RIPA buffer (150 mM NaCl,
50 mM Tris–HCl pH 8, 1% NP-40, 0.5% deoxycholate, 2 mM EDTA, 25 mMb-glycenopho-
sphate, 1 mM Na3VO4, 100 mM NaF and 20 mg/mL aprotinin, 40 mg/mL leupeptin,
0.75 mM PMSF). Reporter activity was measured in 10 vL of whole cell lysates for
b-galactosidase. b-galactosidase activity was measured with the Galacto Plus kit
(Applied Biosystems, Foster City, CA, USA) and Packard luminometer (Long Island
Scientific, East Setauket, NY, USA), following the manufacturer’s instructions.
2.9. Assessment of nuclear maturation and cumulus expansion
After culture the nuclear status of the oocyte was determined after 4,6-diamino-2-
phenyl-indole (DAPI) staining as described by Mori et al. [30]. Briefly, COCs were
denuded by vortexing for 3–7 min and fixed for 15 min in 2.5 % (w:v) glutaraldhyde, then
washed with PBS, stained with 2.5 % (w:v), DAPI (Sigma Chemical Co, St. Louis, MO,
USA) and mounted on slides. The nuclear state of the stained oocytes was assessed under a
fluorescence microscope (490–500 nm). Oocytes in which diffuse or slightly condensed
chromatin could be identified were classified as being in the germinal vesicle (GV) stage.
Oocytes that possessed clumped or strongly condensed chromatin that formed an irregular
network of individual bivalents (prometaphase) or a metaphase plate but no polar body
were classified as being in Metaphase I (MI) stage and oocytes with either a polar body or
two shiny chromatin spots were classified as being in Metaphase II (MII) stage of the
maturation process.
Measuring the diameter of the COCs, using a calibrated stage micrometer, assessed
cumulus expansion, at the onset and the end of the incubation period. Diameter measure-
ment for each COC was done in two perpendicular directions.
2.10. Assessment of embryo development
Embryos were examined on the basis of morphology and the efficiency of the culture
system was evaluated as (i) the percentage of cleaved embryos 4 days after fertilization and
(ii) the percentage of blastocysts on day 7 and 9 expressed on basis of the number of
oocytes at the onset of culture.
2.11. Assessment of embryo quality by ethidium staining and TUNEL assay
Embryos were rinsed in PBS (Gibco, Paisley, UK) with 0.1% poly-vinyl alcohol (PVA,
Sigma Chemical Co., St. Louis, MO, USA) incubated for 5 min in 4 mM Ethidium
homodimer-1 (Molecular Probes, Eugene, OR, USA) in PBS, rinsed in PBS–0.1%
878 A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889
PVA and fixed overnight with 4% (w:v) paraformaldeyde (Merck, Darmstadt, Germany) in
PBS. After fixation, an in-situ cell death detection kit using fluorescein-conjugated dUTP
(terminal deoxynucleotidyl transferase mediated dUTP nick end labeling, TUNEL)
(Roche, Mannheim, Germany) was used for labeling the apoptotic cells [31]. Briefly,
embryos were rinsed twice in PBS–0.1% PVA and permeabilised in PBS with 0.1% Triton
X-100 plus 0.1% sodium citrate for 5 min on ice. Embryos were rinsed two times in PBS–
0.1% PVA and incubated in groups of five embryos per 30 vL of TUNEL reaction mixture for
1 h at 37 8C in a dark and humidified atmosphere. After incubation, the oocytes were rinsed in
PBS–BSA and stained with 10 mM Hoechst 33342 (Sigma, Aldrich Chemie bv., Zwijndrecht,
The Netherlands) in PBS for 10 min. After rinsing in PBS–0.1% PVA and in PBS the embryos
were mounted on a glass slide in Vectashield (Vector Laboratories Inc., Burlingame, USA)
and examined using a fluorescence microscope. Using different filters, the total number of
cells of each embryo (Hoechst positive, 465 nm), the number of dead cells (ethidium stained,
580 nm) and the number of TUNEL positive cells (525 nm) were counted.
2.12. Statistical analysis
The distribution of oocytes at different stages and the distribution of embryos at different
stages were analyzed by a w2-test and P values <0.05 were considered to be significant.
3. Results
3.1. BMPs and BMP receptors are expressed in follicles
The ovarian sections contained primordial, primary, secondary and antral follicles. The
latter type of follicles reached a maximum diameter of 8 mm. Immunohistochemical
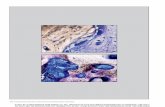
analysis demonstrated BMP2 and BMP4 deposits in cells of the theca interna of antral
follicles (Fig. 1a–c) and occasionally (i.e. in about 25% of antral follicles) in their oocytes
(Fig. 1d). Approximately 75% of antral follicles showed a positive BMPR-II staining in
their oocytes (Fig. 2a). Expression of BMPs and BMPR-II, however, was not mutually
exclusive, oocytes that expressed BMP2 and BMP4 also expressed BMPR-II. A positive
BMPR-II reaction was also found in primordial, primary and secondary follicles both in
their granulosa cells and oocytes (Fig. 3a–c). In contrast, BMP2 (Fig. 3d) and BMP4
(Fig. 3e) staining was absent at these sites. The collective data of BMP2, BMP4 and
BMPR-II immunostaining in different follicular stages is presented in Table 2. No staining
was observed at the immunoreacting sites described above, when the BMP antibodies were
replaced by normal goat IgG in the same concentration as the first antibody (Fig. 1e).
Preincubation of antibodies with their respective blocking peptide also resulted in absence
of staining (Figs. 2b and 3f).
3.2. Expression of BMP2, BMP4 and BMP receptor mRNA in antral follicles
Amplification of cDNA from oocytes, cumulus cells, theca tissue and mural granulosa
cells primed with specific primers for BMP4, BMPR-II, ActR-IIB, ActR-IA, BMPR-IA,
A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889 879
Fig. 1. BMP2 and BMP4 immunostaining in bovine antral follicles. The immunoreactions shown were obtained
after enhancement of formed DAB-percipitate with nickel ammonium sulphate. (a) BMP2 staining in the oocyte
and cells of the theca interna. (b) Higher magnification of the same follicle as presented in (a) showing BMP2
localization in the cytoplasm of theca interna cells. (c) BMP4 immunostaining in theca interna cells. (d) BMP4
immunostaining in an oocyte. (e) Absence of immunostaining in the antral follicle wall and (f) absence of
880 A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889
and BMPR-IB resulted in all replicates in a PCR product after one round of amplification
except for identification of BMP4 expression that required two rounds of amplification
(Fig. 4). PCR analysis also demonstrated BMP2 expression in all five replicates generated
from theca tissue, but in one and two of the five replicates generated from respectively
oocytes and cumulus cells. No BMP2 expression was detected in cDNA originating from
mural granulosa cells (Fig. 4). These data corroborate with the data obtained by immu-
nohistochemistry and suggest the existence of a functional BMP signaling system in
developing follicles.
Sequence analysis of the amplified BMP2 and BMP4 products showed respectively 87%
and 93% homology with human BMP2 and BMP4 mRNA, and it demonstrated the presence
of three nucleotides (positions 99–101) in the bovine BMP4 sequence which are lacking in
the published human sequence (Fig. 5). Compared to the human BMP4 protein, these extra
nucleotides will result in an extra glutamic acid at a stretch of glutamic acids in the prodomain
of the bovine BMP4, similar to the protein sequence of the mouse BMP4 [32]. Since this
prodomain is cleaved off during activation of the protein, the extra glutamic acid will not give
rise to differences in the structure of the active bovine BMP4 compared to the human BMP4.
3.3. IVM, IVF and IVC of COCs and denuded oocytes
Culture of intact COCs for 23 h in M199 resulted in 3% GV, 20% MI, 75% MII, and 2%
degenerated stages. Adding BMP2 or BMP4 to the culture medium did not significantly
change these percentages (Table 3).
The presence of FSH (0.05 units/mL) in the culture medium during maturation of COCs
for 23 h resulted in decrease of the percentage of MI (12%) stage and an increase in the
percentage of oocytes in MII stage (84%) compared to the oocytes matured in medium
Fig. 2. BMPR-II immunostaining in the cumulus–oocyte complex of a bovine antral follicle. (a) Specific spot
like reaction in the oocyte. (b) Negative control showing the absence of immunostaining after preabsorbtion of
the BMPR-II antibody with excess blocking peptide. Bars: 50 mm. Abbreviations: oc, oocyte; gc, granulosa
cells; cc, cumulus cells.
immunostaining in the cumulus–oocyte complex of an antral follicle after replacement of (primary) BMP
antibody with homologues IgG-fraction. Bars: (a) 50 mm; (b, d, f) 25 mm; (c, e) 100 mm. Abbreviations: oc,
oocyte; tc, theca interna; gc, granulosa cells; gv, germinal vesicle; cc, cumulus cells.
A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889 881
Fig. 3. BMPs and BMPR-II immunostaining in preantral follicles. Positive reaction of BMPR-II in primordial
follicles (a) in a primary follicle (b) and in a secondary follicle (c). Absence of BMP2 and BMP4 staining in
primary follicles (d) and absence of BMP4 staining in an early secondary follicle (e). Absence of
immunostaining in an advanced secondary follicle (f) after preabsorbation of the BMPR-II antibody with the
peptide used for immunization. Bars 20 mm. Abbreviations: oc, oocyte; tc, theca interna; gc, granulosa cells.
Table 2
Presence or absence of BMP2, BMP4 and BMPR-II immunoreactivity during folliculogenesis
Follicles Oocyte Granulosa cells Theca cells
Primordial
BMP2 � � ND
BMP4 � � ND
BMPR-II þ þ ND
Primary
BMP2 � � ND
BMP4 � � ND
BMPR-II þ þ ND
Secondary
BMP2 � � �BMP4 � � �BMPR-II þ þ �
Antral
BMP2 þ (25%)a � þBMP4 þ (25%)a � þBMPR-II þ (75%)a � �
�, absence; þ, presence; ND, not developed.a
Approximate percentage of oocytes showing a positive reaction.
882 A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889
Fig. 4. Expression of bovine BMPs and BMP receptors mRNA in follicular cells as detected by RT-PCR.
Samples are indicated at the bottom and a 100 bp ladder is indicated as the marker for fragment size.
Fig. 5. Nucleotide sequence of the amplified product of bovine BMP4 (bBMP4) cDNA and its alignment with
the corresponding human homologue (hBMP4).
A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889 883
without FSH. Administration of BMP2 or BMP4 to this medium did not alter these
percentages (Table 3).
Activity of the BMPs was however checked with BMP reporter ES cells [28] and these
experiments confirmed specific activity of the BMPs (data not shown).
The presence of BMP2 or BMP4 with or without FSH in the maturation medium of
COCs did not influence the fertilization rate nor the blastocyst formation rate following
IVF (Table 4).
3.4. Embryo quality
In order to further test the developmental potential of oocytes that had been in vitro
maturated in the presence of BMPs, the numbers of apoptotic (TUNEL positive) and dead
(Ethidium homodimer reacting) cells were determined in day 9 blastocysts yielded from
IVF of those oocytes. No differences were observed in these numbers when comparing
embryos derived from oocytes that had been matured in the presence or absence of BMP2
or BMP4 (Table 5) suggesting that maturation in the presence of these BMPs had no effect
on embryo quality.
3.5. Cumulus expansion
When administered to the maturation medium of intact COCs, either in the presence or
absence of FSH, neither BMP2 nor BMP4 significantly influenced the subsequent cumulus
Table 3
The effects of BMP2 or BMP4 (each 100 ng/mL) on the progression of meiosis and degeneration rate of intact
bovine COCs after 23 h of culture in medium M199 supplemented with 0.05 units/mL of FSH
COCs (n) GV (%) MI (%) MII (%) Degenerated (%)
M199 354 3 a 20 a 75 a 2 a
M199 þ BMP2 343 1 a 20 a 76 a 3 a
M199 þ BMP4 324 3 a 19 a 76 a 2 a
M199 þ FSH 294 2 a 12 b 84 b 2 a
M199 þ FSH þ BMP2 322 1 a 15 b 83 b 1 a
M199 þ FSH þ BMP4 267 1 a 18 ab 79 ab 2 a
a and b differ significantly (P < 0.05).
Table 4
The effect of BMP2 or BMP4 (each 100 ng/mL) during maturation of intact COCs in a M199 culture medium
with or without FSH on subsequent fertilization, embryo cleavage and blastocyst formation rates
COCs
(n)
Cleavage
day 4 (%)
Blastocyst
day 7 (%)
Blastocyst
day 9 (%)
M199 540 63 2 16
M199 þ BMP2 511 54 2 14
M199 þ BMP4 491 63 3 15
M199 þ FSH 332 78 11 25
M199 þ FSH þ BMP2 353 75 10 25
M199 þ FSH þ BMP4 353 77 9 22
884 A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889
expansion after 23 h of culture. Mean COC diameters were not different after maturation in
M199 (380 � 25 mm), M199 þ BMP2 (394 � 37 mm) or M199 þ BMP4 (375 � 33 mm).
Adding FSH to the culture medium resulted in a significant increase in mean COC diameter
(730 � 69 mm) but no differences were observed in the presence of BMP2 (716 � 85 mm)
or BMP4 (680 � 77 mm).
4. Discussion
In the present study, the expression of BMPR-IA, BMPR-IB, ActR-IA, ActR-IIB and
BMPR-II mRNA could be detected in all follicular compartments of bovine antral follicles
suggesting that cells in these compartments have the capacity to respond to BMP signals.
However, BMPR-II protein was found only in oocytes of antral follicles. Disparity in
localization of BMPR-II protein and its gene expression in our study may be due to absence
of translation of the transcript or differences in sensitivity of the used RT-PCR method and
immunohistochemical procedure to demonstrate the objected compounds.
The observed sites of BMPR-II mRNA and protein within bovine follicles only partly
correspond with that previously found in sheep and rat. In sheep, BMPR-II mRNA was
demonstrated both in oocytes and granulosa cells of antral follicles [14], while BMPR-II
protein was observed in all follicular compartments [15]. Similarly, in rats, BMPR-II
mRNA was detected in oocytes and granulosa cells of antral follicles [12,33]. Recently,
Glister et al., by using a different antibody could demonstrate the expression of BMPR-II
protein in bovine granulosa and theca cells [34]. In their work however, granulosa and theca
cells were cultured for 6 days before the immunohistochemical study. Here we demonstrate
that different type I BMP receptors are expressed by follicular cells which is compatible
with findings of Glister et al. [34]. These results suggest that together with type II receptors,
BMPR-II [this study and [34]] and ActR-II [34,35], different receptor complexes can be
assembled to properly respond to various BMP signals.
Our present study also showed the expression of BMP4 mRNA in all compartments of
bovine antral follicles and that of BMP2 mRNA in theca and occasionally in oocyte and
cumulus cells, while no BMP2 mRNA could be detected in mural granulosa cells,
indicating the possibility of autocrine/paracrine functions for BMPs in follicles. In rats,
BMP2 mRNA was detected in both granulosa and theca cells, but the BMP4 mRNA was
found exclusively in theca cells [33]. However, Jaatinen et al. [10] found no expression of
BMP4 or BMP2 mRNA in isolated human granulosa cells, which were obtained at oocyte
retrieval for IVF.
Table 5
The effect of the presence of BMP2 or BMP4 during maturation of COCs on embryo quality parameters on day
9 IVP blastocysts (n ¼ 15 in each group)
Cells per embryo
(n � S.E.M.)
Dead (ethidium-positive)
cells (% � S.E.M.)
Apoptotic (TUNEL-positive)
cells (% � S.E.M.)
M199 102 � 36 7 � 4.8 12 � 5.9
M199 þ BMP2 97 � 33 8 � 4.1 11 � 4.7
M199 þ BMP4 94 � 44 7 � 6.2 9 � 6.5
A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889 885
In our study, BMP2 and BMP4 proteins were demonstrated in internal theca cells of
antral follicles and in approximately 25% of their oocytes. The absence of expression of
BMP2 mRNA in all oocytes from antral follicles and the presence of BMP2 and BMP4
proteins in about 25% of these oocytes is intriguing and could mean that this BMP system
is not available continuously during antral follicle development or that its presence is
associated with survival or degeneration of oocytes. Further studies on the viability of
follicles with or without a functional BMP/receptor signaling complex in their oocytes
could clarify this matter. In this respect, it is interesting to note the suggestion of Shimasaki
et al. [12] that in antral follicles, BMPs like BMP4 and BMP7, might be the long sought
‘luteinizing inhibitor’ during their growth and development. A similar role for another
oocyte-derived TGF-ß family member, GDF-9, was demonstrated by Elvin et al. [36] in
experiments with mice genetically deficient for GDF-9.
Absence of BMP2 and BMP4 proteins in primordial, primary and secondary bovine
follicles in our study, contrasts with findings on their gene expression in sheep [14,15] and
rat [12,33]. On the other hand, the currently demonstrated presence of BMPR-II in small
bovine follicles in the absence of BMP2 and BMP4 protein may indicate that the receptor
binds other TGF-ß family members like GDF9 or BMP15, which previously were found to
be expressed in early follicle stages [36–39] and to bind BMPR-II [40,41]. Despite the fact
that BMP2 and BMP4 have been identified as regulators of primordial germ cell generation
from the epiblast [42,43], to our knowledge, there are no publications that suggest a role of
BMP2 and BMP4 in early follicular processes. Interestingly, BMP-15 has recently been
demonstrated to stimulate granulosa cell proliferation in early follicles of rats [17–19,37].
The expression of mRNA and protein for BMP2, BMP4 and their receptor in bovine
antral follicles are indicative for the presence of a BMP signaling system in these
structures. These findings are illustrative for a possible regulatory role of BMP2 and
BMP4 in the functioning or the differentiation of bovine follicle cells. TGF-ß family
members like BMPs and activin, have been reported to regulate mammalian folliculogen-
esis by influencing granulosa cell proliferation, steroidogenesis and inhibin production
[44,45] or to act as modulators of FSH action on the expression of specific steroidogenic
enzymes in the mammalian ovarian follicle wall resulting in increased oestradiol,
decreased progesterone synthesis [12] or enhanced inhibin production [46].
Moreover, various autocrine and paracrine in vitro effects of several BMPs have been
described. In the rat ovary, for example, BMP4 and BMP7 of theca cell origin appeared to
modulate FSH-stimulated steroidogenesis in cultured granulosa cells [12], while BMP4
affected androgen production in cultured human theca tumor cells [47]. Likewise, BMP2
enhanced oestradiol production in cultured sheep granulosa cells [15] and BMP6 derived
from the oocyte stimulated inhibin-ßB subunit mRNA expression in cultured human
granulosa cells [44].
The results of our present in vitro experiments with BMP2 and BMP4 do not support a
role of these proteins in oocyte maturation and subsequent blastocyst formation, since their
addition to the various maturation media did not seem to affect progression of meiosis nor
the percentage or quality of blastocysts developed after IVF of cultured COCs. The
ineffectiveness of exogenously added BMPs could, however, have resulted from release of
autocrine BMPs during IVM, leading to adequate concentration of these proteins in the
vicinity of the cultured oocytes and masking the effect of any additional BMPs. In this
886 A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889
respect, in vitro studies with BMP-binding agents like Noggin, Gremlin, Cerberus and
DAN [48] could give additional information on the role of BMPs in oocyte maturation.
Apart from their ineffectiveness in oocyte maturation, exogenously administered BMP2
and BMP4 also did not affect cumulus expansion in in vitro cultured bovine COCs.
Similarly, BMP6 and BMP15 were unable to induce cumulus expansion in mice [38].
Under in vitro circumstances, exogenously added BMP2 and BMP4 during maturation
did not affect cumulus expansion, oocyte maturation, blastocyst formation nor blastocyst
quality and they thus possibly are involved in processes preceding follicle maturation, such
as the formation of adequate steroids and inhibins as has been demonstrated by other
authors for a restricted number of mammalian species. Further studies on the effects of
BMPs or their blockers on in vitro cultured bovine oocytes, granulosa cells and theca cells
may give more information about their significance for the various follicular compartments.
Acknowledgements
Dr. R. Hanssen (Organon, Oss, The Netherlands) is thanked for providing recombinant
FSH. BMPs were a generous gift from the Genetics Institute, Cambridge, MA, USA.
Thanks are also due to the members of the Department of Light-Optical Registration
(Faculty of Biology) for helping with photography.
References
[1] Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes
Dev 1996;10:1580–94.
[2] Knight PG, Glister C. Local roles of TGF-beta superfamily members in the control of ovarian follicle
development. Anim Reprod Sci 2003;78:165–83.
[3] Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 2000;
19:1745–54.
[4] Liu F, Ventura F, Doody J, Massague J. Human type II receptor for bone morphogenic proteins (BMPs):
extension of the two-kinase receptor model to the BMPs. Mol Cell Biol 1995;15:3479–86.
[5] Findlay JK, Drummond AE, Dyson ML, Baillie AJ, Robertson DM, Ethier JF. Recruitment and
development of the follicle the roles of the transforming growth factor-beta superfamily. Mol Cell
Endocrinol 2002;191:35–43.
[6] Massague J. TGF-beta signal transduction. Annu Rev Biochem 1998;67:753–91.
[7] Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell
Physiol 2001;187:265–76.
[8] Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene
is X-linked and expressed in oocytes. Mol Endocrinol 1998;12:1809–17.
[9] Hino J, Takao M, Takeshita N, Konno Y, Nishizawa T, Matsuo H, et al. cDNA cloning and genomic structure
of human bone morphogenetic protein-3B (BMP-3b). Biochem Biophys Res Commun 1996;223:304–10.
[10] Jaatinen R, Rosen V, Tuuri T, Ritvos O. Identification of ovarian granulosa cells as a novel site of
expression for bone morphogenetic protein-3 (BMP-3/osteogenin) and regulation of BMP-3 messenger
ribonucleic acids by chorionic gonadotropin in cultured human granulosa-luteal cells. J Clin Endocrinol
Metab 1996;81:3877–82.
[11] Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that
transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development.
Genes Dev 1989;3:1657–68.
A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889 887
[12] Shimasaki S, Zachow RJ, Li D, Kim H, Iemura S, Ueno N, et al. A functional bone morphogenetic protein
system in the ovary. Proc Natl Acad Sci USA 1999;96:7282–7.
[13] Takao M, Hino J, Takeshita N, Konno Y, Nishizawa T, Matsuo H, et al. Identification of rat bone
morphogenetic protein-3b (BMP-3b), a new member of BMP-3. Biochem Biophys Res Commun 1996;
219:656–62.
[14] Wilson T, Wu XY, Juengel JL, Ross IK, Lumsden JM, Lord EA, et al. Highly prolific Booroola sheep have
a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is
expressed in both oocytes and granulosa cells. Biol Reprod 2001;64:1225–35.
[15] Souza CJ, Campbell BK, McNeilly AS, Baird DT. Effect of bone morphogenetic protein2 (BMP2) on
oestradiol and inhibin A production by sheep granulosa cells, and localization of BMP receptors in the
ovary by immunohistochemistry. Reproduction 2002;123:363–9.
[16] Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis
and ovulation in the rat. Biol Reprod 2001;65:994–9.
[17] Otsuka F, Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and
granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc Natl Acad Sci USA
2002;99:8060–5.
[18] Otsuka F, Moore RK, Shimasaki S. Biological function and cellular mechanism of bone morphogenetic
protein-6 in the ovary. J Biol Chem 2001;276:32889–95.
[19] Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-
stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem 2001;276:
11387–92.
[20] Souza CJ, MacDougall C, Campbell BK, McNeilly AS, Baird DT. The Booroola (FecB) phenotype is
associated with a mutation in the bone morphogenetic receptor type 1 B (BMPR1B) gene. J Endocrinol
2001;169:R1–6.
[21] Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, et al. Mutations in an
oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-
sensitive manner. Nat Genet 2000;25:279–83.
[22] Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, et al. Synergistic roles of bone morphogenetic
protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 2001;15:854–66.
[23] Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function.
Dev Genet 1998;22:321–39.
[24] Mulsant P, Lecerf F, Fabre S, Schibler L, Monget P, Lanneluc I, et al. Mutation in bone morphogenetic
protein receptor-IB is associated with increased ovulation rate in Booroola Merino ewes. Proc Natl Acad
Sci USA 2001;98:5104–9.
[25] Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, Lyons KM. The type I BMP receptor BmprIB is essential for
female reproductive function. Proc Natl Acad Sci USA 2001;98:7994–9.
[26] van den Hurk R, Dijkstra G, Hulshof SC, Vos PL. Micromorphology of antral follicles in cattle after
prostaglandin-induced luteolysis, with particular reference to atypical granulosa cells. J Reprod Fertil
1994;100:137–42.
[27] Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod
1988;38:1171–80.
[28] Monterio RM, Chuva de Sousa Lopes SM, Korchynskyi O, ten Diyke P, Mummery CL. Spatio-temporal
activation of Smad1 and Smad5 in vivo: monitoring transcriptional activity of Smad proteins. J Cell Sci
2004; in press.
[29] Mummery CL, Feyen A, Freund E, Shen S. Characteristics of embryonic stem cell differentiation: a
comparison with two embryonal carcinoma cell lines. Cell Differ Dev 1990;30:195–206.
[30] Mori C, Hashimoto H, Hoshino K. Fluorescence microscopy of nuclear DNA in oocytes and zygotes
during in vitro fertilization and development of early embryos in mice. Biol Reprod 1988;39:737–42.
[31] Watson AJ, De Sousa P, Caveney A, Barcroft LC, Natale D, Urquhart J, et al. Impact of bovine oocyte
maturation media on oocyte transcript levels, blastocyst development, cell number, and apoptosis. Biol
Reprod 2000;62:355–64.
[32] Kurihara T, Kitamura K, Takaoka K, Nakazato H. Murine bone morphogenetic protein-4 gene: existence
of multiple promoters and exons for the 50-untranslated region. Biochem Biophys Res Commun 1993;192:
1049–56.
888 A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889
[33] Erickson GF, Shimasaki S. The spatiotemporal expression pattern of the bone morphogenetic protein
family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrinol 2003;1:9.
[34] Glister C, Kemp CF, Knight PG. Bone morphogenetic protein (BMP) ligands and receptors in bovine
ovarian follicle cells: actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-
1 phosphorylation by follistatin. Reproduction 2004;127:239–54.
[35] Izadyar F, Dijkstra G, Van Tol HT, Van den Eijnden-van Raaij AJ, Van den Hurk R, Colenbrander B, et al.
Immunohistochemical localization and mRNA expression of activin, inhibin, follistatin, and activin
receptor in bovine cumulus–oocyte complexes during in vitro maturation. Mol Reprod Dev 1998;49:
186–95.
[36] Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in
the growth differentiation factor 9-deficient ovary. Mol Endocrinol 1999;13:1018–34.
[37] Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15.
Identification of target cells and biological functions. J Biol Chem 2000;275:39523–8.
[38] Elvin JA, Yan C, Matzuk MM. Oocyte-expressed TGF-beta superfamily members in female fertility. Mol
Cell Endocrinol 2000;159:1–5.
[39] Bodensteiner KJ, Clay CM, Moeller CL, Sawyer HR. Molecular cloning of the ovine Growth/
Differentiation factor-9 gene and expression of growth/differentiation factor-9 in ovine and bovine ovaries.
Biol Reprod 1999;60:381–6.
[40] Vitt UA, Mazerbourg S, Klein C, Hsueh AJ. Bone morphogenetic protein receptor type II is a receptor for
growth differentiation factor-9. Biol Reprod 2002;67:473–80.
[41] Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O. Growth differentiation
factor-9 (GDF-9) signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol
Endocrinol 2004;18:653–65.
[42] Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, et al. Bmp4 is required for the
generation of primordial germ cells in the mouse embryo. Genes Dev 1999;13:424–36.
[43] Ying Y, Qi X, Zhao GQ. Induction of primordial germ cells from murine epiblasts by synergistic action of
BMP4 and BMP8B signaling pathways. Proc Natl Acad Sci USA 2001;98:7858–62.
[44] Jaatinen R, Bondestam J, Raivio T, Hilden K, Dunkel L, Groome N, et al. Activation of the bone
morphogenetic protein signaling pathway induces inhibin beta(B)-subunit mRNA and secreted inhibin B
levels in cultured human granulosa-luteal cells. J Clin Endocrinol Metab 2002;87:1254–61.
[45] Bondestam J, Kaivo-Oja N, Kallio J, Groome N, Hyden-Granskog C, Fujii M, et al. Engagement of activin
and bone morphogenetic protein signaling pathway Smad proteins in the induction of inhibin B production
in ovarian granulosa cells. Mol Cell Endocrinol 2002;195:79–88.
[46] Roh JS, Bondestam J, Mazerbourg S, Kaivo-Oja N, Groome N, Ritvos O, et al. Growth differentiation
factor-9 stimulates inhibin production and activates Smad2 in cultured rat granulosa cells. Endocrinology
2003;144:172–8.
[47] Dooley CA, Attia GR, Rainey WE, Moore DR, Carr BR. Bone morphogenetic protein inhibits ovarian
androgen production. J Clin Endocrinol Metab 2000;85:3331–7.
[48] Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin
identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell 1998;1:673–83.
A.N. Fatehi et al. / Theriogenology 63 (2005) 872–889 889