Efficient function and characterization of GH10 xylanase (Xyl10g) from Gloeophyllum trabeum in...
-
Upload
independent -
Category
Documents
-
view
0 -
download
0
Transcript of Efficient function and characterization of GH10 xylanase (Xyl10g) from Gloeophyllum trabeum in...
Ef
HQa
b
c
d
a
ARR1AA
KXGS
1
lrdmbmcmgP
hlT2b
CT
0h
Journal of Biotechnology 172 (2014) 38– 45
Contents lists available at ScienceDirect
Journal of Biotechnology
jou rn al hom ep age: www.elsev ier .com/ locate / jb io tec
fficient function and characterization of GH10 xylanase (Xyl10g)rom Gloeophyllum trabeum in lignocellulose degradation
o Myeong Kima, Kwang Ho Leeb,c, Kyoung Hyoun Kima, Dae-Seok Leeb,d,uynh Anh Nguyena, Hyeun-Jong Baea,b,d,∗
Department of Bioenergy Science and Technology, Chonnam National University, Gwangju 500-757, Republic of KoreaDepartment of Wood Science and Landscape Architecture, Chonnam National University, Gwangju 500-757, Republic of KoreaCenter for Research Facilities, Chonnam National University, Gwangju 500-757, Republic of KoreaBio-Energy Research Center, Chonnam National University, Gwangju 500-757, Republic of Korea
r t i c l e i n f o
rticle history:eceived 2 August 2013eceived in revised form7 December 2013ccepted 20 December 2013
a b s t r a c t
The xylanase gene from Gloeophyllum trabeum was cloned and expressed in Pichia pastoris GS115. Xyl10ghas a molecular weight of approximately 50 kDa, and exhibits maximum specific activity at 70 ◦C and abroad range of pH 4.0–7.0. Purified recombinant Xyl10g efficiently degraded popping-pretreated cornstover and newspaper waste at 50 ◦C and pH 4.0 after 24 h, and showed synergistic effects with Cel5B
vailable online 28 December 2013
eywords:ylanaseloeophyllum trabeumynergistic effect
(endoglucanase) and BglB (�-glucosidase) to increase reduced sugar levels by about 1.71- to 1.88-foldand 2.26- to 2.48-fold, respectively. Although Xyl10g has low specific activity for beechwood xylan, ascompared to XynA, Xyl10g more efficiently degraded corn stover than did XynA. According to immuno-gold labeling analysis, Xyl10g can attack highly substituted, unsubstituted, and low-substituted xylans,whereas XynA cannot efficiently attack highly substituted xylans, which is important for lignocellulosedegradation. These results suggest that GH10 Xyl10g can be used for lignocellulose degradation.
© 2013 Elsevier B.V. All rights reserved.
. Introduction
Plant cell walls are mainly composed of cellulose, hemicellu-oses, and lignin. Lignocellulosic materials, such as agriculturalesidues, have been used as a renewable energy source for the pro-uction of bioethanol because they are an abundant, non-food plantaterial. Also, the carbon savings through the environmental car-
on cycle are very high. The celluloses of plant cell walls are theost abundant polymers and are composed of a long chain of glu-
ose molecules (Ciolacu et al., 2011). Hemicelluloses, the secondost abundant group of heteropolymer, contain xylose, mannose,
lucose, arabinose, and galactose (Beg et al., 2001; Van Dyk andletschke, 2012).
Recently, many studies have explored the development ofydrolytic enzymes, which play an important role in lignocellu-
ose hydrolysis for bioethanol production (Anasontzis et al., 2011;
enkanen et al., 2013; Van Dyk and Pletschke, 2012; Yang et al.,011). In one such study, bioconversion of cellulose was disruptedy the presence of hemicelluloses and lignin in multicomplex∗ Corresponding author at: Department of Bioenergy Science and Technology,honnam National University, Gwangju 500-757, Republic of Korea.el.: +82 62 530 2097; fax: +82 62 530 0029.
E-mail address: [email protected] (H.-J. Bae).
168-1656/$ – see front matter © 2013 Elsevier B.V. All rights reserved.ttp://dx.doi.org/10.1016/j.jbiotec.2013.12.013
lignocellulosic materials (Mussatto et al., 2008). Therefore, anenzyme cocktail for lignocellulose degradation was required, withxylanase as a key enzyme, and the removal of hemicelluloses accel-erated the enzymatic hydrolysis of lignocellulose. Hemicellulosewas degraded by a hydrolytic enzyme complex through a seriesof interactions with compounds such as xylanase (endo-�-1,4-xylanase, EC 3.2.1.8) and �-xylosidase (xylan 1,4-�-xylosidase, EC3.2.1.37). Xylanase attacks the �-1,4-glycosidic bonds of xylan,yielding xylo-oligosaccharides, whereas �-xylosidase releasesxylose from xylo-oligosaccharides. Considerable information isavailable on such enzymes (Ahmed et al., 2009; Lafond et al., 2011).
Numerous microbes have been screened and studied for theproduction of hydrolytic enzymes because the identification of effi-cient hydrolytic enzymes is one of the major challenges in thebioconversion of lignocellulosic substrates. Fungi degrade lignocel-lulosic materials by producing hydrolytic enzymes. For example,the brown rot fungus, Gloeophyllum trabeum, has been studiedbecause of its efficient biodegradation mechanism (Kerem et al.,1999; Gao et al., 2012). Therefore, it is a promising candidate forthe production of hydrolytic enzymes.
Xylanases have mainly been classified into GH10 and GH11
(van Gool et al., 2012). In general, GH10 xylanases have highermolecular weights and lower pI values than GH11 xylanases.Also, GH10 xylanases can cleave up to a non-reducing end sub-stituted xylose, whereas GH11 xylanases cannot hydrolyze highlyBiotec
sTstne
escenX
2
2
hsWg(tuTMa
2
urc
2
d(Wsp(tdca54wd3(uuw3iXEXpf
H.M. Kim et al. / Journal of
ubstituted xylose linkages (Biely et al., 1997; Pollet et al., 2010).he three-dimensional structures of GH10 and GH11 xylanaseshow a (�/�)8 barrel fold and a �-jelly roll, respectively, and glu-amic acid (Glu) residues play an important role as the catalyticucleophile/base or proton donor (Cantarel et al., 2009; Jovanovict al., 2009).
According to a recent report, many xylanases have beenxpressed and purified under various conditions (Table 1). In thistudy, we isolated Xyl10g and expressed it in Pichia pastoris. Weharacterized the enzymatic properties of Xyl10g by assessingnzymatic bioconversion of popping-pretreated corn stover andewspaper waste, and proposed efficient functions of recombinantyl10g.
. Materials and Methods
.1. Strains, vectors, medium, and growth conditions
Escherichia coli TOP10F’ and P. pastoris GS115 were used as theost cell for DNA cloning and the host strain for protein expres-ion, respectively. The pGEM-T Easy vector (Promega, Madison,
I, USA) was used for TA cloning, and pPICZ�A vector (Invitro-en, Carlsbad, CA, USA) was used for protein expression. G. trabeumKACC 43361) inoculated into 100 mL of xylan medium consist ofhe 1% beechwood xylan, 0.42% (NH4)2SO4, 0.2% KH2PO4, 0.02%rea, 0.03% CaCl2, 0.03% MgSO4·7H2O, 0.1% protease peptone, 0.2%ween80, and 0.2% trace element solution (0.5% FeSO4·7H2O, 0.16%nSO4·H2O, 0.14% ZnSO4·7H2O, 0.2% CoCl2) in a 500-mL baffle flask
t 25 ◦C with shaking at 200 rpm for 5 days.
.2. Total RNA extract and synthesis of cDNA
Total RNA was isolated from the filamentous fungus G. trabeumsing Trizol Reagent (Invitrogen, Carlsbad, CA, USA) and then car-ied out long-distance PCR for cDNA synthesis using the SMARTTM
DNA Library Construction kit (Clontech, Mountain View, CA, USA).
.3. Cloning of the full-length Xyl10g
The 3′ rapid amplification of cDNA ends (RACE) PCR was con-ucted to clone the partial Xyl10g using the degenerate primer5′-ARCAKKAWRWRRKRKKMYRCCRMR-3′) (R = A or G; K = G or T;
= A or T; M = A or C; Y = C or T), designed through aligningequences of the GH10 fungal xylanases, and the CDS III/3′ PCRrimer (5′-ATTCTAGAGGCCGAGGCGGCCGACATG-d(T)30N-1N-3′)N = A, G, C, or T; N-1 = A, G, or C) in a 20 �L volume con-aining 2 �L 10× Ex Taq buffer, 1.6 �L of 2.5 mM dNTP, 1 �Legenerate primer, 1 �L CDS III/3′ PCR primer, 3 �L first-strandDNA, and 0.1 �L Ex Taq DNA polymerase (5 U �L−1). The PCRmplification was carried out as follows: 1 cycle of 95 ◦C for
min, 30 cycles of 95 ◦C for 30 s, 60 ◦C for 30 s, and 72 ◦C for5 s, with a final extension for 5 min at 72 ◦C. The PCR productas cloned into the pGEM-T Easy vector. After sequencing, weesigned the 3′ target primer (5′-CTCGTTCACAACATCCCAGCTGTA-′), and 5′ RACE PCR was conducted using the 5′ PCR primer5′-AAGCAGTGGTATCAACGCAGAGT-3′) and the 3′ target primernder the same condition and volume, and then the PCR prod-ct was cloned into the pGEM-T Easy vector. After sequencing,e designed the Xyl10g-F (5′-GGTACCATGTCCTTCAAGACTCTCG-
′) and Xyl10g-R (5′-GTCGCTGGCTTTGCGTCCCTGCAG-3′) primersncluding the restriction enzyme sites, and then the full lengthyl10g gene was amplified in a 20 �L volume containing 2 �L 10×
x Taq buffer, 1.6 �L of 2.5 mM dNTP, 1 �L Xyl10g-F primer, 1 �Lyl10g-R primer, 3 �L first-strand cDNA, and 0.1 �L Ex Taq DNAolymerase (5 U �L−1). The PCR amplification was carried out asollows: 1 cycle at 95 ◦C for 5 min, 30 cycles at 95 ◦C for 30 s, 60 ◦Chnology 172 (2014) 38– 45 39
for 30 s, and 72 ◦C for 1 min, with a final extension for 5 min at72 ◦C. The PCR product was cloned into the pGEM-T Easy vectorand sequenced.
2.4. Expression and purification of xyl10g in Pichia pastoris
After the signal peptide removal of Xyl10g using the PCR, weinserted it into the pGEM-T Easy vector. The target fragment wasinserted into the pPICZ�A vector and then transformed into P.pastoris GS115. The selected colony through western blot analy-sis using anti-His antibody (Abfrontier, Seoul, South Korea) wasinoculated into a 3 mL YPD (1% yeast extract, 2% protease peptone,1% dextrose) medium at 30 ◦C with shaking at 200 rpm for 24 h,and then inoculated into a 100 mL YPG (1% yeast extract, 2% pro-tease peptone, 1% glycerol) medium. After cultivation of 24 h, cellpellets were harvested from the culture medium, and then resus-pended using 100 mL YPM (1% yeast extract, 2% protease peptone,0.5% methanol) medium. After 24 h incubation at 30 ◦C, we har-vested the supernatant by centrifugation for 20 min at 26,000 × g(Avanti J-E, Beckman, Fullerton, CA, USA), and then purified it usinga Ni-NTA matrix (Qiagen, Hilden, Germany).
2.5. Zymographic analysis
The beechwood xylan and wheat arabinoxylan activities wereidentified through zymographic analysis. After the 12% SDS-PAGEcontaining either 500 �L of 1% beechwood xylan or soluble wheatarabinoxylan, gels were incubated in refolding buffer of pH 7.0(20 mM PIPES, 2.5% triton X-100, 2 mM dithiothreitol, 2.5 mMCaCl2) for 40 min at room temperature. The gels were incubatedin a 25 mM sodium phosphate buffer (pH 7.0) at 37 ◦C for 2 h, andthen stained in 0.25% congo red for 10 min. After staining, the gelswere washed with 1 M NaCl.
2.6. Optimal condition, thermal stability, and kinetic parameterassay of Xyl10g
The optimal pH was decided by checking the xylanase activityon beechwood xylan at various pH ranging between 2.0 and 9.0, at50 ◦C, for 0.5 h. The optimal temperature was determined by check-ing activity on beechwood xylan at various ranges of temperature(30–90 ◦C) at pH 7.0 for 0.5 h. The temperature stability was deter-mined by measuring the residual activities at pH 7.0 and 70 ◦C for0.5 h after pre-incubation of Xyl10g for 0, 0.2, 0.5, 1, 1.5, 2, 6, 12,and 24 h without substrate at different temperature. The specificactivities were determined by measuring the reducing sugar on1% beechwood xylan, 1% wheat arabinoxylan, and 1% CMC at pH7.0 and 70 ◦C for 15 min using the dinitrosalicylic-acid reagent anda xylose standard curve (Miller, 1959). The kinetic parameters ofXyl10g were decided using various concentrations of beechwoodxylan, and then values of Km, Vmax and Kcat were calculated usingthe Lineweaver–Burk Plot method.
2.7. Popping pretreatment, chemical composition analysis, andenzymatic hydrolysis
Popping pretreatment of corn stover was conducted in a castiron cylindrical reactor with a gas heater, a hatch, and a mechan-ical rotator at 15 kg f cm−2, 230 ◦C (Wi et al., 2011; Choi et al.,2013). The popping method was used to external heating plate andinternal steam pressure mechanism. The neutral sugar contents ofthe popping-pretreated corn stover and newspaper waste were
analyzed using gas chromatography (GC). The analysis methodwas carried out according to procedure describer by Choi et al.(2012). Enzymatic hydrolysis was performed on a 1% popping-pretreated corn stover and 1% newspaper waste with individual40 H.M. Kim et al. / Journal of Biotechnology 172 (2014) 38– 45
Table 1Comparison of expression system and optimal condition of Xyl10g with other xylanase.
Protein Source Glycosyl hydrolase family (GHF) pH optimum Temperature optimum (◦C) Expression system Refs.
Xyl10g Gloeophyllum trabeum GH10 4.0–7.0 70 P. pastoris This studyXynD Penicillium funiculosum GH10 4.0–5.5 80 P. pastoris Lafond et al. (2011)XynT Bacillus alcalophilus GH10 7.0–9.0 50 E. coli Lee et al. (2012)
.0
.5
.0
oam3(dm(cif
2
2f(igRsA7pa(rwv
2
ox(sbbp
3
3
HtKobs(1a
XynA Schizophyllum commune GH11 5Xylcg Chaetomium globosum GH11 5Thxyn11A Thermobifida halotolerans GH11 9
r mixed enzymes including Xyl10g, Cel5B, and BglB at 50 ◦Cnd pH 4.0 for 24 h (Kim et al., 2012; Jung et al., 2010). Enzy-atic hydrolysis of XynA and XynA + Cel5B was conducted at
7 ◦C and pH 5.0 for 24 h on a 1% popping-pretreated corn stoverSong et al., 2013). The reducing sugar was determined using theinitrosalicylic-acid method, and high-performance liquid chro-atography (HPLC) equipped with a refractive index detector
YoungLin Instruments Anyang, Korea). The Rezex ROA organic acidolumn (Phenomenex, Torrance, CA) was used for soluble sugardentification (300 mm × 7.8 mm) at 65 ◦C, and added 5 mM of sul-uric acid at a flow rate of 0.6 mL min−1.
.8. Immunogold labeling assay of xylan in poplar
The pieces (1 mm × 1 mm × 5 mm) of poplar branches,00 �g mL−1 enzyme-treated for 7 days at 37 ◦C, were fixed at 4 ◦Cor 6 h using 0.1% glutaraldehyde (v/v) and 4% paraformaldehydev/v) in a 50 mM sodium cacodylate buffer (pH 7.2). After washingn a sodium cacodylate buffer, samples were dehydrated in araded ethanol series. The samples were embedded in Londonesin White resin (London resin Co., London, UK). The ultrathinections of 80 nm were mounted on nickel grids of 300 mesh.fter washing the grids using phosphate-buffered saline (PBS, pH.4) and distilled water, they were treated with LM10 and LM11rimary antibody (PlantProbes, Leeds, UK) (McCartney et al., 2005)t 4 ◦C for 2 days, and then labeled with an anti-Rat lgG antibodySigma, St. Louis, MO, USA) conjugated to 10 nm gold particles atoom temperature for 3 h. After staining with 4% uranyl acetate,e observed to sections by JEOL 1010 (TEM) at an acceleration
oltage of 80 kV.
.9. Thin-layer chromatography (TLC) analysis
To analyze enzymatic hydrolysis patterns and products of xylo-ligosaccharides by Xyl10g, we carried out the TLC analysis. Theylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaoseX5), and xylohexaose (X6) were separated on a TLC aluminumheet silica gel 60 plate with a solvent solution consisting of n-utanol:acetic acid:H2O (2:1:1, v/v). The sugars were detectedy a detection solution (100 mL ethanol, 1.23 g p-anisidine, 1.66 ghthalic acid).
. Results and discussion
.1. Gene cloning and amino acid sequence analysis
The full-length Xyl10g gene (GenBank accession numberQ730916) was cloned into the pGEM T-Easy vector, and then
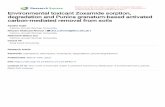
he Xyl10g sequence was identified by Genotech Inc (Daejeon,orea). The Xyl10g gene had an open reading frame (ORF)f 1185 bp encoding a protein of 395 amino acid residues; itelongs to class GH10 (Fig. 1A). According to an analysis of
econdary and three-dimensional structures using ExPASy toolhttp://expasy.org/tools/), Xyl10g is composed of 29.7% helix,2.2% strands, and 58.1% loops, and contained active-site residuest Glu208 and Glu313 (Fig. 1A and E). An NCBI BLAST search50 P. pastoris Song et al. (2013)40 E. coli Singh et al. (2013)70 E. coli Zhang et al. (2012)
showed that Xyl10g (AEJ 35165) exhibits 66%, 70%, and 60%amino acid sequence identity with GH10 endo-1,4-�-xylanases ofPhanerochaete chrysosporium (AAG 44992), Agaricus bisporus (CAB05886), and Postia placenta Mad-698-R (EED 84312), respectively.
3.2. Expression, purification, and characteristics of Xyl10g
For expression and purification of Xyl10g, we inserted the targetfragment into the pPICZ�A vector without a signal peptide due toan unfavorable N-terminal coding sequence, which disturbs recom-binant protein expression in yeast (Kim et al., 2012). When weattempted to express full-length Xyl10g, the target protein was notexpressed.
The recombinant Xyl10g protein had a molecular weight ofapproximately 50 kDa, as determined by SDS-PAGE and zymog-raphy (Fig. 1B–D). According to the zymographic analysis, Xyl10gefficiently degraded beechwood xylan and wheat arabinoxylan(Fig. 1C and D). It efficiently hydrolyzed the xylose linkages nextto residues substituted with arabinose, as well as xylose linkages.However, various debranching enzymes are required to increasethe arabinoxylan degradation efficiency (Lee et al., 2012).
The optimal conditions for maximum specific activity of Xyl10gwere at 70 ◦C and pH 4.0–7.0 (Fig. 2A and B). The range of ther-mal stability of Xyl10g was determined to be 40–70 ◦C at pH 7.0.The residual activity of Xyl10g decreased by about 85% after pre-incubation for 30 min at the optimal temperature. However, Xyl10gwas stable below 50 ◦C, showing an activity of approximately 62.5%after 24 h (Fig. 2C). Therefore, the enzymatic bioconversion of ligno-cellulose and various industrial applications were assessed below50 ◦C for efficient enzymatic hydrolysis.
The specific activities of recombinant Xyl10 on various sub-strates were determined (Table 3). Xyl10g demonstrated a highspecific activity for 1% beechwood xylan, but a low specific activityfor 1% wheat arabinoxylan, because approximately 40% of the sidechains of wheat arabinoxylan are substituted with arabinose (Leeet al., 2012). The kinetic parameters of Xyl10g, determined using aLineweaver–Burk plot, were as follows: Vmax 1.82 mM min−1 mg−1,Km 101.91 mM, and Kcat 1941.43 s−1. These results indicate thatXyl10g is an efficient recombinant enzyme and has high catalyticefficiency for beechwood xylan.
3.3. Enzymatic hydrolytic ability and synergy effects
The monosaccharides of popping-pretreated corn stoverand newspaper waste were mainly comprised of glucose andxylose (Table 2). Purified Xyl10g efficiently hydrolyzed popping-pretreated corn stover and newspaper waste, as well as puresubstrates such as beechwood xylan and wheat arabinoxylan. GH10xylanases exhibit various functions and can easily degrade varioustypes of heteroxylan due to their high catalytic versatility (Bielyet al., 1997; Paës et al., 2012). In the present study, 100 �g mL−1
Xyl10g produced 0.251 mg and 0.183 mg of reducing sugar from
10 mg of popping-pretreated corn stover and newspaper waste,respectively, based on DNS analysis (Figs. 3A and 4A). There-fore, Xyl10g more efficiently degraded popping-pretreated cornstover compared to newspaper waste. Also, synergistic increasesH.M. Kim et al. / Journal of Biotechnology 172 (2014) 38– 45 41
Fig. 1. (A) Amino acid sequence of Xyl10g. The box region shows the signal peptide regions. Red sequences indicate the active site residues. (B) SDS-PAGE analysis of Xyl10gafter Ni-NTA purification. S/M, protein ladder; lane 1 – purified Xyl10g. (C) Zymographic analysis of the effect of Xyl10g on beechwood xylan. S/M, protein ladder; lane 1 –1 �g purified Xyl10g; lane 2 – 1.5 �g purified Xyl10g; lane 3 – 2 �g purified Xyl10g. (D) Zymographic analysis of the effect of Xyl10g on wheat arabinoxylan. S/M, proteinladder; lane 1 – 3 �g purified Xyl10g; lane 2 – 4 �g purified Xyl10g; lane 3 – 5 �g purified Xyl10g. (E) Glu208 and Glu313 were predicted to be active site residues of Xyl10gbased on analysis of the three-dimensional structure.
Table 2Chemical composition of popping-pretreated corn stover and newspaper waste.
(%) Rhamnose Arabinose Xylose Mannose Galactose Glucose Total sugars
0.4.
P
ipra
TC
N
PCS 0.6 ± 0.07 0.4 ± 0.29 8.0 ± 1.06
NW 0.5 ± 0.03 0.1 ± 0.01 6.9 ± 0.15
CS, corn stover; NW, newspaper waste.
n the degradation of popping-pretreated corn stover and news-aper waste of about 1.71- to 1.88-fold and 2.26- to 2.48-fold,espectively, were achieved in the presence of 50 �g mL−1 Cel5Bnd 150 �g mL−1 BglB (Figs. 3B and 4B). High-performance liquid
able 3omparison of specific activities of Xyl10g with XynA on beechwood xylan, wheat arabin
Protein Specific activity (U/mg)
Beechwood Xylan Wheat ArabinoXylan
Xyl10g 727.21 ± 15.75 43.44 ± 2.30
XynA 5768.49 ± 76.50 316.32 ± 32.90
A indicated no activity.
3 ± 0.03 0.3 ± 0.03 47.0 ± 1.82 56.5 ± 2.256 ± 0.36 0.6 ± 0.02 45.9 ± 1.85 58.6 ± 2.21
chromatography (HPLC) showed that various enzyme mixturesproduced different sugars, such as cellobiose, xylobiose, glucose,and xylose (Figs. 3C and 4C; Table 4). This indicated that enzy-matic hydrolysis is most effective when a mixture of various
oxylan, and CMC.
Source Refs.
CMC
NA Gloeophyllum trabeum This studyNA Schizophyllum commune Song et al. (2013)
42 H.M. Kim et al. / Journal of Biotechnology 172 (2014) 38– 45
F optimb ed at
b of Xyl
hlps
TS
N
ig. 2. Optimal pH, optimal temperature, and thermal stability of Xyl10g. (A) The
eechwood xylan for 30 min. (B) The optimal temperature of Xyl10g was determiny measuring the residual activities under optimal conditions after pre-incubation
ydrolytic enzymes is used. In a previous study, commercial cel-ulase, as opposed to other branch enzymes such as xylanase,ectinase, and �-glucosidase, was required for the bioconver-ion of lignocellulosic materials (Hu et al., 2011). In the present
able 4oluble sugar compositions obtained from popping-pretreated corn stover and newspape
Enzyme Substrate Cellobiose (mg/mL) Xylobiose (mg/
ControlPCS ND ND
NW ND ND
Xyl10gPCS 0.017 0.200
NW ND 0.159
Cel5BPCS 0.170 ND
NW 0.029 0.014
Cel5B + BglBPCS 0.022 ND
NW 0.019 0.013
Xyl10g + Cel5BPCS 0.230 0.208
NW 0.055 0.194
Xyl10g + Cel5B + BglBPCS 0.049 0.207
NW ND 0.168
XynA PCS ND 0.028
XynA + Cel5B PCS 0.121 0.028
D indicated no detection. PCS, popping-pretreated corn stover; NW, newspaper waste.
al pH was determined by evaluating activity in the range 2.0–9.0 at 50 ◦C against30–90 ◦C, pH 7.0 for 0.5 h. (C) The temperature stability of Xyl10g was determined10g without substrate at different temperatures (40–70 ◦C).
study, we suggest that the purified xylansase was preferentiallyrequired over other purified enzymes for the hydrolysis of lig-nocellulosic materials, because Cel5B and Cel5B + BglB weaklydegraded newspaper waste. Moreover, cellulose and lignin were
r waste after enzymatic hydrolysis.
mL) Glucose (mg/mL) Xylose (mg/mL) Total sugar (mg/mL)
ND ND NDND ND ND
ND 0.055 0.271ND 0.043 0.202
0.079 ND 0.2480.010 ND 0.053
0.321 ND 0.3430.059 ND 0.091
0.098 0.049 0.5860.010 0.047 0.307
0.377 0.065 0.6980.212 0.129 0.509
ND 0.015 0.0430.069 0.016 0.234
H.M. Kim et al. / Journal of Biotechnology 172 (2014) 38– 45 43
Fig. 3. Enzymatic hydrolysis of popping-pretreated corn stover. (A) Comparison of reduced sugar levels with different concentrations of Xyl10g. (B) Synergistic effects ofmixtures of enzymes, including Xyl10g, Cel5B, and BglB. Enzymatic hydrolysis of XynA and XynA + Cel5B was performed at 37 ◦C and pH 5.0 for 24 h. (C) Soluble sugars wereanalyzed by HPLC. G2, cellobiose; X2, xylobiose; G, glucose; X, xylose.
Fig. 4. Enzymatic hydrolysis of newspaper waste. (A) Comparison of reduced sugar levels with different concentrations of Xyl10g. (B) Synergistic effects of mixtures ofenzyme, including Xyl10g, Cel5B, and BglB. (C) Soluble sugars were analyzed by HPLC. G2, cellobiose; X2, xylobiose; G, glucose; X, xylose.
44 H.M. Kim et al. / Journal of Biotechnology 172 (2014) 38– 45
Fig. 5. Immunogold labeling of poplar branch samples using LM10 and LM11 antibodies. (A) Control. (B) Treatment with enzyme (Xyl10g). (C) Treatment with enzyme( me (X( s. 0.25
ccc
sX5ehcl
3
lPiT
FX
XynA). (D) Control. (E) Treatment with enzyme (Xyl10g). (F) Treatment with enzyG) Quantitative density analysis of gold particles. Data are the mean of 10–15 field
onnected by the cross-linking agent hemicellulose. Therefore,ellulose can be easily hydrolyzed through the removal of hemi-elluloses.
GH11 XynA has very high specific activities for pure substratesuch as beechwood xylan and wheat arabinoxylan compared toyl10g (Table 3) (Song et al., 2013). However, in the present study,0 �g mL−1 XynA degraded popping-pretreated corn stover lessfficiently than Xyl10g (Fig. 3B and C; Table 4) because XynA cannotydrolyze xylose linkages next to residues substituted with side-hains. These results suggested that Xyl10g can efficiently degradeignocellulose.
.4. Immunogold labeling analysis
Monoclonal antibodies LM10 and LM11 were selected to ana-
yze cleavage patterns of poplar xylans by immunogold labeling.oplar wood consists of 24% xylan and 3% glucomanan and xylans the major component of hemicellulose (Zhong and Ye, 2009).herefore, LM10 and LM11 antibody can efficiently bind to theig. 6. Thin-layer chromatography analysis. (A) The preferential cleavage of xylo-oligosacc1, xylose; X2, xylobiose; X3, xylotriose; X4, xylotetraose; X5, xylopentaose; X6, xylohexaose.
ynA). CC, cell corner; ML, middle lamellar; SW, secondary cell wall. Scale bar: 0.5 �m. �m2; 0.5 �m × 0.5 �m.
secondary cell wall of poplar (Kim and Daniel, 2012). Gener-ally, LM10 binds to unsubstituted and low-substituted xylans,whereas LM11 binds to highly substituted and unsubstituted xylans(McCartney et al., 2005; Altaner et al., 2010). After Xyl10g or XynAtreatment of poplar samples, LM10 and LM11 antibodies showedgold labeling in the secondary cell wall (Fig. 5). After labeling withLM10, there was a decrease in the number of gold particles byapproximately 34.9% and 33.9% in Xyl10g or XynA treated samples,respectively, as compared to non-treated samples (Fig. 5B, E andG). Using LM11 gold particles, the number of particles decreased byapproximately 33.9% and 3.7% (Fig. 5C, F and G) in Xyl10g and XynAtreated samples, respectively, as compared to non-treated sam-ples. These results suggested that Xyl10g can efficiently degradehighly substituted xylans by hydrolyzing xylose linkages next toresidues substituted with side-chains, as well as unsubstituted
and low-substituted xylans in poplar secondary cell walls. How-ever, XynA cannot efficiently attack highly substituted xylans.Therefore, Xyl10g can more efficiently hydrolyze lignocellulosicmaterials.harides by Xyl10g and (B) hydrolysis products of Xyl10g from xylo-oligosaccharides.
Biotec
3
adAagte
4
irahMwp1
A
goT
R
A
A
A
B
B
C
C
C
C
G
Cloning, expression, and characterization of an alkaline thermostable GH11
H.M. Kim et al. / Journal of
.5. Hydrolysis patterns and products of xylo-oligosaccharides
Hydrolysis patterns and products of xylo-oligosaccharides werenalyzed by thin-layer chromatography (Fig. 6). Xyl10g did notegrade xylobiose, but weakly hydrolyzed xylotriose to X1 and X2.lso, Xyl10g mainly produced X2 from xylotetraose. Xylopentaosend xylohexaose were mostly degraded to X2 and X3. This sug-ested that Xyl10g was mainly hydrolyzed at the middle part ofhe xylosidic bond and showed the archetypal characterization ofndo-xylansase.
. Conclusion
Xyl10g has many advantages compared to XynA, includ-ng efficient bioconversion of lignocellulosic biomass. Purifiedecombinant Xyl10g attacked highly substituted xylans as wells unsubstituted and low-substituted xylans, and efficientlyydrolyzed popping-pretreated corn stover and newspaper waste.oreover, synergistic effects on the degradation of newspaperaste and popping-pretreated corn stover were achieved in theresence of Cel5B and BglB (increases of approximately 1.71- to.88-fold and 2.26- to 2.48-fold, respectively).
cknowledgements
This work was supported by Priority Research Centers Pro-ram (2010-0020141) through the National Research Foundationf Korea (NRF) funded by the Ministry of Education, Science andechnology to H.-J. Bae.
eferences
hmed, S., Riaz, S., Jamil, A., 2009. Molecular cloning of fungal xylanases: anoverview. Applied Microbiology and Biotechnology 84, 19–35.
ltaner, C.M., Tokareva, E.N., Jarvis, M.C., Harris, P.J., 2010. Distribution of (1 → 4)-�-galactans, arabinogalactan proteins, xylans and (1 → 3)-�-glucans in tracheidcell walls of softwoods. Tree Physiology 30, 782–793.
nasontzis, G.E., Zerva, A., Stathopoulou, P.M., Haralampidis, K., Diallinas, G.,Karagouni, A.D., Hatzinikolaou, D.G., 2011. Homologous overexpression ofxylanase in Fusarium oxysporum increase ethanol productivity during consol-idated bioprocessing (CBP) of lignocellulosics. Journal of Biotechnology 152,16–23.
eg, Q.K., Kapoor, M., Mahajan, L., Hoondal, G.S., 2001. Microbial xylanases and theirindustrial application: a review. Applied Microbiology and Biotechnology 56,326–338.
iely, P., Vrsanská, M., Tenkanen, M., Kluepfel, D., 1997. Endo-beta-1,4-xylanase fam-ilies: differences in catalytic properties. Journal of Biotechnology 57, 151–166.
antarel, B.L., Coutinho, P.M., Rancurel, C., Bernard, T., Lombard, V., Henrissat, B.,2009. The carbohydrate-active enzymes database (CAZy): an expert resourcefor glycogenomics. Nucleic Acids Research 37, 233–238.
hoi, I.S., Kim, J.H., Wi, S.G., Kim, K.H., Bae, H.J., 2013. Bioethanol productionfrom mandarin (Citrus unshiu) peel waste using popping pretreatment. AppliedEnergy 102, 204–210.
hoi, I.S., Wi, S.G., Kim, S.B., Bae, H.J., 2012. Conversion of coffee residues wasteinto bioethanol with using popping pretreatment. Bioresource Technology 125,
132–137.iolacu, D., Gorgieva, S., Tampu, D., Kokol, V., 2011. Enzymatic hydrolysis of differentallomorphic forms of microcrystalline cellulose. Cellulose 18, 1527–1541.
ao, Z., Mori, T., Kondo, T., 2012. The pretreatment of corn stover with Gloeophyllumtrabeum KU-41 for enzymatic hydrolysis. Biotechnology for Biofuels 5, 28.
hnology 172 (2014) 38– 45 45
Hu, J., Arantes, V., Saddler, J.N., 2011. The enhancement of enzymatic hydrolysis oflignocellulosic substrates by the addition of accessory enzymes such as xylanase:is it an additive or synergistic effect? Biotechnology for Biofuels 4, 36.
Jovanovic, I., Magnuson, J.K., Collart, F., Robbertse, B., Adney, W.S., Himmel, M.E.,Baker, S.E., 2009. Fungal glycoside hydrolases for saccharification of lignocellu-loses: outlook for new discoveries fueled by genomics and functional studies.Cellulose 16, 687–689.
Jung, S., Kim, S., Bae, H., Lim, H.S., Bae, H.J., 2010. Expression of thermostable bacterialbeta-glucosidase (BglB) in transgenic tobacco plants. Bioresource Technology101, 7155–7161.
Kim, H.M., Lee, Y.G., Patel, D.H., Lee, K.H., Lee, D.S., Bae, H.J., 2012. Characteristics ofbifunctional acidic endoglucanase (Cel5B) from Gloeophyllum trabeum. Journalof Industrial Microbiology and Biotechnology 39, 1081–1089.
Kim, J.S., Daniel, G., 2012. Distribution of glucomannans and xylans in poplar xylemand their changes under tension stress. Planta 236, 35–50.
Kerem, Z., Jensen, K.A., Hammel, K.E., 1999. Biodegradative mechanism of thebrown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellularhydroquinone-driven Fenton reaction. FEBS Letters 446, 49–54.
Lafond, M., Tauzin, A., Desseaux, V., Bonnin, E., Ajandouz, E.-H., Giardina, T.,2011. GH10 xylanase D from Penicillium funiculisum: biochemical studies andxylooligosacharide production. Microbial Cell Factories 10, 20.
Lee, D.S., Lee, K.H., Cho, E.J., Kim, H.M., Kim, C.S., Bae, H.J., 2012. Characteri-zation and pH-dependent substrate specificity of alkalophilic xylanase fromBacillus alcalohilus. Journal of Industrial Microbiology and Biotechnology 39,1465–1475.
McCartney, L., Marcus, S.E., Knox, J.P., 2005. Monoclinal antibodies to plant wallxylans and arabinoxylans. Journal of Histochemistry and Cytochemistry 53,543–546.
Miller, G.L., 1959. Use of dinitrosalicylic acid reagent for determination of reducingsugar. Analytical Chemistry 31, 658–666.
Mussatto, S.I., Fernandes, M., Milagres, A.M.F., Roberto, I.C., 2008. Effect of hemicel-lulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain.Enzyme and Microbial Technology 43, 124–129.
Paës, G., Berrin, J.G., Beaugrand, J., 2012. GH11 xylanases: struc-ture/function/properties relationships and applications. BiotechnologyAdvances 30, 564–592.
Pollet, A., Delcour, J.A., Courtin, C.M., 2010. Structural determinants of the substratespecificties of xylanases from different glycoside hydrolase families. CriticalReviews in Biotechnology 30, 176–191.
Singh, R.K., Tiwari, M.K., Kim, D., Kang, Y.C., Ramachandran, P., Lee, J.K., 2013. Molec-ular cloning and characterization of a GH11 endoxylanase from Chaetomiumglobosum, and its use in enzymatic pretreatment of biomass. Applied Microbi-ology and Biotechnology 97, 7205–7214.
Song, Y., Lee, Y.G., Choi, I.S., Lee, K.H., Bae, H.J., 2013. Heterologous expression ofendo-1,4-�-xylanase A from Schizophyllum commune in Pichia pastoris andfunctional characterization of the recombinant enzyme. Enzyme and MicrobialTechnology 52, 170–176.
Tenkanen, M., Vrsanská, M., Siika-aho, M., Wong, D.W., Puchart, V., Penttilä, M., Salo-heimo, M., Biely, P., 2013. Xylanase XYN IV from Trichoderma reesei showing exo-and endo-xylanase activity. FEBS Journal 208, 285–301.
Van Dyk, J.S., Pletschke, B.I., 2012. A review of lignocellulose bioconversion usingenzymatic hydrolysis and synergistic cooperation between enzymes – fac-tors affecting enzymes, conversion and synergy. Biotechnology Advances 30,1458–1480.
van Gool, M.P., van Muiswinkel, G.C., Hinz, S.W., Schols, H.A., Sinitsyn, A.P., Grup-pen, H., 2012. Two GH10 endo-xylanases from Myceliophthora thermophila C1with and without cellulose binding module act differently towards soluble andinsoluble xylans. Bioresource Technology 119, 123–132.
Wi, S.G., Chung, B.Y., Lee, Y.G., Yang, D.J., Bae, H.J., 2011. Enhanced enzymatic hydrol-ysis of rapeseed straw by popping pretreatment for bioethanol production.Bioresource Technology 102, 5788–5793.
Yang, B., Dai, Z., Ding, S.Y., Wyman, C.E., 2011. Enzymatic hydrolysis of cellulosicbiomass. Biofuels 2, 421–450.
Zhang, F., Chen, J.J., Ren, W.Z., Lin, L.B., Zhou, Y., Zhi, X.Y., Tang, S.K., Li, W.J., 2012.
xylanase from Thermobifida halotolerans YIM90462T. Journal of Industrial Micro-biology and Biotechnology 39, 1109–1116.
Zhong, R., Ye, Z.H., 2009. Secondary cell walls. In: Encyclopedia of Life Science (ELS).John Wiley & Sons, Ltd., Chichester.