Serum testosterone and electroencephalography spectra in developmental male rhesus monkeys
Effects of testosterone and 2-hydroxyflutamide on progesterone receptor expression in porcine...
Transcript of Effects of testosterone and 2-hydroxyflutamide on progesterone receptor expression in porcine...
r e p r o d u c t i v e b i o l o g y 1 2 ( 2 0 1 2 ) 3 3 3 – 3 4 0
Available online at www.sciencedirect.com
journal homepage: http://www.elsevier.com/locate/repbio
Original Research Article
Effects of testosterone and 2-hydroxyflutamide onprogesterone receptor expression in porcine ovarianfollicles in vitro
Malgorzata Duda a,*, Malgorzata Durlej-Grzesiak a, Zbigniew Tabarowski b,Maria Slomczynska a
aDepartment of Endocrinology, Institute of Zoology, Jagiellonian University, Cracow, PolandbDepartment of Experimental Hematology, Institute of Zoology, Jagiellonian University, Cracow, Poland
a r t i c l e i n f o
Article history:
Received 12 May 2012
Accepted 10 September 2012
Keywords:
2-Hydroxyflutamide
Progesterone receptor
Granulosa cells
Ovarian follicles
Pig
a b s t r a c t
The purpose of the study was to test the possible role of the androgen receptor (AR)
agonist (testosterone; T), an AR antagonist (2-hydroxyflutamide; 2-Hf) or combination of both
(T + 2-Hf) on progesterone receptor (PGR) expression in cultured porcine granulosa cells (GCs)
or whole follicles. GCs isolated from mature pig follicles (6–8 mmin diameter) were cultured for
48 h. Experimental cultures were carried out with the addition of T (10�7 M), 2-Hf (1.7 � 10�4 M)
or both T and 2-Hf for the last 24 h of culture. To better imitate in vivoconditions, isolated whole
porcine follicles (6–8 mm in diameter) were cultured for 24 h in an organ culture system, with
the addition of the same factors. The cells or sections obtained from cultured follicles were
processed for PGR immunocytochemical or immuno-histochemical staining. In addition,
expression of PGR protein was determined by Western blot and progesterone (P4) concentra-
tions in the culture media weremeasuredby a radioimmunoassay. Wefound that isoform A of
PGR is expressed in both granulosal and follicular cultures. The 2-Hf in the presence of
T increased PGR protein expression in porcine GCs and whole follicles. In both granulosal
and follicular cultures, 2-Hf or T alone inhibited P4 secretion, but simultaneous addition of 2-Hf
and T increased P4 secretion.Our results indicatethatandrogensmay be involved in the control
of PGR expression in porcine GCs in vitro. Moreover, we suggest a potential auto/paracrine
regulation of the follicular function by androgen-dependent signaling pathway.
# 2012 Society for Biology of Reproduction & the Institute of Animal Reproduction and
lish Academy of Sciences in Olsztyn. Published by Elsevier Urban &
Partner Sp. z o.o. All rights reserved.
Food Research of Po
1. Introduction
Reproduction in mammals is controlled by the hypothalamic–
pituitary–gonadal axis through a cascade of hormones whose
* Corresponding author at: Department of Endocrinology, Institute ofPoland.
E-mail address: [email protected] (M. Duda).
1642-431X/$ – see front matter # 2012 Society for Biology of ReproducPolish Academy of Sciences in Olsztyn. Published by Elsevier Urban &http://dx.doi.org/10.1016/j.repbio.2012.10.006
concentrations are tightly regulated by feedback control
mechanisms [1]. Disruption of normal reproductive function,
either transient or permanent, has been linked to endocrine
disrupting compounds (EDCs) which interact directly to
activate or antagonize the sex hormone receptors such as
Zoology, Jagiellonian University, Gronostajowa 9, 30-387 Cracow,
tion & the Institute of Animal Reproduction and Food Research ofPartner Sp. z o.o. All rights reserved.
r e p r o d u c t i v e b i o l o g y 1 2 ( 2 0 1 2 ) 3 3 3 – 3 4 0334
the androgen receptor (AR; [2]). These compounds constitute a
useful tool to study the involvement of signaling pathways
they block or alter in the physiology of cells equipped with
appropriate receptors. Porcine granulosa cells (GCs) are
considered to be the main site of AR- mediated androgen
action in the follicle because of their strongest expression,
which was demonstrated in whole follicles [3] and GCs in vitro
[4]. The presence of AR within GCs provides a basis for the
interactions with AR agonists and antagonists. The agonist- or
antagonist-bound steroid hormone receptors regulate gene
expression by binding to the regulatory elements in promoters
of susceptible genes, in a tissue-dependent manner. In this
work we focused on 2-hydroxyflutamide (2-Hf) which is
known to interact with the AR. Flutamide (FLU) was the first
AR blocker to achieve a wide-spread use. It is metabolized into
2-Hf, the biologically active form of the drug. FLU was used to
treat prostate cancer, where it competes with testosterone (T)
for binding to ARs, thereby reducing the growth of cancer cells
[5]. It has shown its ability to reduce fecundity in the fathead
minnow by decreasing oocyte maturation in the ovaries and
causing spermatocyte degeneration and necrosis in the testis
[6]. The anti-androgenic activity of 2-Hf and FLU has been
well established [7,8]; it also was demonstrated that 2-Hf
is approximately 1–5 times more potent than its parental
compound [9].
Progesterone (P4) is an essential regulator of female
reproductive functions, such as the control of ovulation,
regulation of the function of corpus luteum or initiation of
decidualization [10,11]. These abundant physiological effects
of P4 are mediated through the intracellular progesterone
receptor (PGR; [12]). PGR is a ligand-activated transcription
factor whose biological activity is affected by hormone-
dependent phosphorylation [13]. In the absence of P4, PGR is
maintained in an inactive complex that contains heat shock
proteins in the nuclei of target cells. After P4 binding, PGR
starts to change distinctly, including dissociation of the heat
shock proteins, phosphorylation, and dimerization. Binding of
PGR to progesterone response elements (PGREs) promotes the
formation of a stable initiation complex, resulting in gene
transcription [14]. PGR is expressed as two isoforms termed
PGRA and PGRB [15] arising from a single gene, each under the
control of a separate promoter [16]. The biological effects of P4
are dependent on the activation of both PGR isoforms [17].
PGRs are widespread in an animal organism. In the reproduc-
tive system they have been found in the ovary, fallopian tube,
muscle and mucous membrane of the uterus, its cervix, vagina
and the nipple [18,19]. Immunolocalization and immunoex-
pression of PGRs in female ovaries still arouses great interest
of researchers. This is mainly because of the need of diagnosis
and treatment of infertility, safety of hormone therapy in the
perimenopausal period, as well as the influence on oncogene-
sis in a nipple, endometrium and ovary. PGR expression in GCs
is induced mainly by luteinizing hormone (LH) but other
signaling pathways can contribute to its regulation [20,21].
On the basis of findings that the anti-androgen 2-Hf
suppresses PGR expression in the uterus, in a manner akin
to down-regulation [22], the aim of the present study was to
test the possible role of the AR agonist: T, an antagonist: 2-Hf
or combination of both on PGR expression in cultured porcine
GCs or whole follicles. We hypothesize that, the exposure of
porcine GCs or whole follicles to these substances would alter
PGR expression and that a mixture of these two compounds
would abrogate their individual effects.
2. Materials and methods
2.1. Sample collection
Porcine ovaries were obtained from Polish Landrace sows at a
local slaughterhouse and placed in cold phosphate-buffered
saline (PBS; pH 7.4, PAA The Cell Culture Company, Dart-
mouth, MA, USA) containing Antibiotic/Antimycotic Solution
(AAS 10 ml/ml; PAA The Cell Culture Company, Dartmouth,
MA, USA). Ovaries were transported to the laboratory within
30 min and rinsed twice with sterile PBS supplemented with
antibiotics. In each experiment, ten ovaries from five animals
were selected for cell isolation. Each ovary yielded 3–5 follicles,
thus the total number of follicles varied from 30 to 50. The
phase of the estrous cycle was determined according to the
established morphological criteria [23]. Medium follicles
(6–8 mm in diameter), classified by morphometric criteria as
healthy [24], were selected for cell and organ cultures. Briefly,
follicles were dissected free from the ovarian stroma and
separately classified under a microscope. Healthy follicles
were characterized by a well-vascularized follicular wall and
the clarity of the follicular fluid. Early atretic and atretic
follicles were traversed by few or no blood vessels and the
surface of the follicles was opaque with the progression of
atresia. This procedure has been chosen to minimize the
variability between tissues and animals.
2.2. Granulosa cell isolation and culture
Granulosa cells were scraped from the follicular wall with
round-tip ophthalmologic tweezers. After collection, GCs were
washed several times in PBS and recovered by low speed
centrifugation (90 � g for 10 min; [25]). Cell viability was tested
by the trypan blue exclusion test (mean � SEM: 92% � 1%). The
cells were seeded in 24-well culture plates equipped with a
round coverslip (Nunc, Kalmstrup, Denmark) at an initial
density of 3 � 105 cells/ml (for immunocytochemical analysis)
or in 6-well culture plates (Nunc) at an initial density of
6 � 105 cells/ml (for Western blot). Control cultures were
carried in McCoy’s 5A medium (HyClone Laboratories Inc.,
W Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS, PAA The Cell Culture Company) for 48 h. Experimental
cultures (48 h) were carried out in McCoy’s 5A medium with
the addition of T (10�7 M; Sigma–Aldrich, St. Louis, MO, USA),
2-Hf (1.7 � 10�4 M; Sigma–Aldrich) or T and 2-Hf for the last
24 h of culture. After termination of culture, all media were
collected and stored at �20 8C for P4 radioimmunoassay. All
experiments were performed in quadruplicate in five separate
experiments (n = 5).
2.3. Follicle culture and preparation forimmunohistochemical analysis
Whole follicles (n = 36, 6–8 mm in diameter) isolated from
porcine ovaries were cultured on a filter disk on a triangular
r e p r o d u c t i v e b i o l o g y 1 2 ( 2 0 1 2 ) 3 3 3 – 3 4 0 335
stainless steel grid over a well of McCoy’s 5A medium
supplemented with 10% FBS and AAS (5 m/ml; [26]). Follicles
during organ culture were randomly assigned to treatment
groups with the addition of T (10�7 M), 2-Hf (1.7 � 10�4 M) or T
and 2-Hf (T + 2-Hf). All culture media were collected after 24 h
and stored at �20 8C for further P4 analysis, whereas follicles
(n = 3/each group) were fixed in 4% paraformaldehyde,
subsequently dehydrated in an increasing gradient of ethanol
and embedded in paraplast (Sigma–Aldrich). Sections of 5 mm
in thickness were mounted on slides coated with 3-amino-
propyltriethoxysilane (Sigma–Aldrich), deparaffinized, and
rehydrated through a series of decreasing alcoholic solutions.
2.4. Immunocytochemstry and immunohistochemistry
The cells were fixed in 2% paraformaldehyde and permeabi-
lized with 0.1% TritonX-100 (Sigma–Aldrich) in Tris-buffered
saline (TBS; 0.05 M Tris–HCl plus 0.15 M NaCl, pH 7.6). To
quench endogenous peroxidase activity, cells were treated for
30 min with 0.1% H2O2. Non-specific binding was blocked by
incubation for 30 min with 5% normal horse serum (Sigma–
Aldrich). Then, cells were incubated overnight at 4 8C with a
mouse monoclonal antibody anti-PGR (NCL-L-PGR-312, clone
16, Novocastra, Leica Biosystems Newcastle Ltd., UK) at a
dilution 1:100. Afterwards, the cells were intensively washed
in TBST (TBS plus 0.1% Tween 20) and incubated for 1.5 h at
room temperature (RT) with biotynylated horse anti-mouse
antibody (1:300; Vector Laboratories, Burlingame CA, USA)
followed by washing with TBST and incubated at RT for 1 h
with avidin–biotin–peroxidase complex (1:1:100; Strept ABC
complex/HRP, DAKO/AS, Glostrup, Denmark). The color
reaction was performed using Stable DAB solution (Research
Genetics, Inc., Huntsville AL, USA) for 4 min. For negative
control the primary antibody was omitted.
Sections of follicles were subjected to a microwave oven
3� for 4 min, in 0.01 M citric acid buffer (pH 6.0), to retrieve
antigens. Endogenous peroxidase activity was blocked by
incubation with 0.3% H2O2 in TBS (Tris-buffered saline, pH
7.4), and non-specific binding was blocked by incubation with
5% normal horse serum (Sigma–Aldrich). Immunohisto-
chemical reactions were performed using the antibody used
in immunocytochemistry and incubation was carried out at
4 8C overnight. Control sections were incubated with 5%
normal horse serum instead of the primary antibody. The
antigens were visualized using biotynylated secondary
antibody – horse anti-mouse antibody (1:300, 1.5 h at RT;
Vector Laboratories, Burlingame, CA, USA), avidin–biotin–
peroxidase complex (1:100, 40 min at RT; StreptABComplex-
HRP, DAKO/AS), and 3,30-diaminobenzidine as the substrate.
Slides were dehydrated and mounted in DPX (Sigma–Aldrich).
The intensity of immunoreaction was analyzed on each
coverslip and section.
2.5. Quantitative evaluation of staining intensity andstatistical analysis
The cells and sections were photographed using the Nikon
Eclipse E200 microscope with the Coolpix 5400 digital camera
(Nikon, Tokyo, Japan) and corresponding software. To esti-
mate quantitatively the intensity of immunoreaction, image
processing and analyses were performed on each coverslip
where at least 500 cells and six different sections from each
examined follicle were analyzed using a public domain ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
The source images were converted to 8-bit grayscale images
and the intensity of PGR staining was measured detachedly
using point selection tool. The background was also analyzed.
Results from each sample were saved as individual means and
interpolated to the following formula:
ROD ¼ODspecimen
ODbackground¼
logðGLblank=GLspecimenÞlogðGLblank=GLbackgroundÞ
!
where GL means gray level for stained area (specimen) and
unstained area (background), and blank is described as a gray
level measured after removing the slide from the light path.
The intensity of immunoreaction was expressed as relative
optical density (ROD).
2.6. Western blot
After termination of culture, GCs were washed twice with ice-
cold saline, and then proteins were extracted with 50 ml of
radioimmunoprecipitation assay buffer (RIPA; Thermo Sci-
entific, Inc., Rockford IL, USA) in the presence of protease
inhibitor cocktail (Sigma–Aldrich). Cultured porcine follicles
were homogenized on ice with a cold Tris/EDTA buffer
(50 mM Tris, 1 mM EDTA, pH 7.5), sonicated and centrifuged at
10,000 � g for 20 min at 4 8C. Supernatant was collected and
stored at �20 8C. Protein concentration was determined with
Bradford reagent (Bio-Rad Protein Assay; Bio-Rad Laborato-
ries GmbH, Munchen, Germany) using bovine serum albumin
(BSA) as a standard. Aliquots (50 mg protein) of cell lysates and
follicle homogenates containing 20 mg of protein were
solubilized in a sample buffer consisting of 62.5 mM Tris–
HCl pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, 5%
b-mercaptoethanol (Bio-Rad Laboratories) and heated for
3 min at 99.9 8C. After denaturation, samples were separated
via 10% SDS-polyacrylamide gel electrophoresis under re-
ducing conditions [27]. Separated proteins were transferred
onto a nitrocellulose membrane using a wet blotter in the
Genie Transfer Buffer (20 mM Tris, 150 mM glycine in 20%
methanol, pH 8.4) for 90 min at a constant voltage of 135 V.
After overnight blocking with 5% non-fat milk in TBS, 0.1%
Tween 20 (dilution buffer) at 4 8C with gentle shaking, the
membranes were treated with the primary antibody (for
details see immunohistochemistry subchapter: anti PGR,
dilution 1:1000) for 1.5 h at RT. b-Actin was used as an internal
control (1:2000; Sigma–Aldrich). The membranes were
washed and incubated with a secondary antibody conjugated
with the horseradish–peroxidase labeled horse anti-mouse
IgG (Vector Laboratories; dilution 1:1000) for 1 h at RT. The
signals were detected by chemiluminescence using Western
blotting Luminol Reagent (Santa Cruz Biotechnology). The
blots were visualized using the ChemiDocTM and all bands
were quantified using the Image LabTM 2.0 Software (BioRad
Laboratories). The bands were densitometrically scanned,
and the data obtained for PGR were normalized against
b-actin. The protein level within the control group was
arbitrarily set as 1, against which statistical significance was
analyzed.
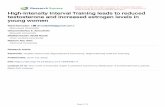
[(Fig._1)TD$FIG]
Fig. 1 – The effects of testosterone (T) and antiandrogen (2-hydroxyflutamide, 2-Hf) treatment on progesterone receptor (PGR)
immunostaining in cultures of porcine granulosa cells (A–D) and whole follicles (E–H). (A and E) Control cultures; (B and F)
T-treated cells/follicles; (C and G) 2-Hf-treated cells/follicles; (D) H: T + 2-Hf-treated cells/follicles. Nuclear localization of PGR
in granulosa cells (black arrows), cytoplasmic localization of PGR in granulosa cells (white arrowheads), nuclear staining in
theca cells (white arrows). (I and J) Semiquantitative analysis of PGR staining intensity (means W SEM) in granulosa cell (I)
and follicle (J) cultures exposed to androgen receptor agonist (T), antagonist (2-Hf) or both (T + 2-Hf), expressed as relative
optical density (ROD) of diaminobenzidine brown reaction products. Different letters indicate statistically significant
differences at *p < 0.05 or **p < 0.01; bar = 50 mm.
r e p r o d u c t i v e b i o l o g y 1 2 ( 2 0 1 2 ) 3 3 3 – 3 4 0336
r e p r o d u c t i v e b i o l o g y 1 2 ( 2 0 1 2 ) 3 3 3 – 3 4 0 337
2.7. Radioimmunoassay
Samples of the culture media were analyzed for P4 content
using the radioimmunoassay [28]. [1,2,6,7-3H] progesterone
(specific activity 96 Ci/mmol; Hartmann Analytic GmbH,
Braunschweig) was used as a tracer and an antibody induced
in sheep against 11a-hydroxyprogesterone succinyl: BSA
(a gift from Professor Brian Cook, University of Glasgow,
Glasgow, Scotland). The sensitivity of the assay was 20 pg.
Coefficients of variation within and between assays were
below 5.0% and 9.8%, respectively.
2.8. Statistical analysis
Statistical analysis was performed using Statistica 5.1 program
(StatSoft Inc., Tulsa, OK, USA). Immunocyto- and immunohis-
tochemical data were examined by one-way analysis of
variance followed by Tukey’s test. Radioimmunological and
Western blot data are expressed as means � SEM (n = 3). Each
experiment was performed in quadruplicate. Significant
differences in steroid medium concentration between the
control and experimental cultures were analyzed by Student’s
t-test. Statistical significances were set at *p < 0.05, **p < 0.01
or ***p < 0.001.
3. Results
3.1. PGR immunolocalization and Western blot analysis
The anti-PGR antibody used in this study for immunohis-
tochemistry detects only the isoform A of PGR. The PGR
immunostaining was detected in the nuclei of control and
experimental GCs. Control and T-treated GCs showed similar
PGR staining (Fig. 1A and B), whereas treatment with 2-Hf
resulted in a reduced staining (Fig. 1C). The most intensive
reaction for PGR was observed in GCs treated with T + 2-Hf
(Fig. 1D).
PGR immunostaining was observed in various cell types of
control and experimental follicles (Fig. 1E–H). A weak TC
staining was observed in control follicles (Fig. 1E) and follicles
treated with T or 2-Hf (Fig. 1F and G). In theca cells (TC), PGR
[(Fig._2)TD$FIG]Fig. 2 – Representative Western blots of PGR protein expression
homogenates (B) from control, testosterone (T), 2-hydroxyflutam
n = 3/per treatment). Different letters indicate statistically signif
was always detected in nuclei and the intensity of TC staining
slightly increased after addition of T + 2-Hf (Fig. 1H). PGR
expression in the GCs was consistently higher than in other
cell types. In follicles cultured with T + 2-Hf, the intensity of
nuclear staining in GCs was the strongest (Fig. 1H), whereas
the intensity of immunoreaction dramatically decreased in
follicles cultured with the addition of 2-Hf (Fig. 1G). A decrease
in the staining intensity of GCs was accompanied with
cytoplasmic localization. Semiquantitative evaluation of the
intensity of the immunocyto- and immunohistochemical
reaction in the GCs and whole follicles expressed as relative
optical density (ROD) confirmed generally the qualitative data
(Fig. 1I and J).
To provide further evidence that GCs and whole follicles
express PGR protein and to confirm the specificity of the
antibody, Western blot analysis was performed. The antibody
used for Western blot detects both A and B isoform of PGR, but
only a single band of 94 kDa (PGRA) was detected in this study.
Densitometric analysis of relative protein level revealed a
distinct decrease in PGR level, in both granulosal and follicular
cultures after 2-Hf treatment. In addition, an increase in
PGR level was observed in GCs and whole follicles exposed to
T + 2-Hf (Fig. 2A and B).
3.2. Progesterone medium concentration
Administration of T and 2-Hf alone decreased granulosal P4
secretion (1.3-fold and 4.4-fold, respectively; p < 0.05; Fig. 3A),
whereas T + 2-Hf significantly increased P4 secretion (1.4-fold;
p < 0.05; Fig. 3A). Similarly, T and 2-Hf significantly inhibited P4
production by porcine follicles (1.4-fold and 2.2-fold, respec-
tively; p < 0.05; Fig. 3B), while T + 2-Hf significantly stimulated
follicular P4 production (1.6-fold; p < 0.05; Fig. 3B).
4. Discussion
The study was focused on the possible involvement of AR
signaling on the induction and localization of PGR by using
specific AR antagonist, 2-hydroxyflutamide and AR agonist,
testosterone. Our results indicated that the AR antagonist
exerts a negative effect on PGR protein expression in both
(mean W SEM) in granulosa cell lysates (A) and follicular
ide (2-Hf) and T + 2-Hf- treated cells (48 h) or follicles (24 h;
icant difference at *p < 0.05 or ***p < 0.001.
[(Fig._3)TD$FIG]
Fig. 3 – Progesterone secretion (mean W SEM) by granulosa cells (A) and whole follicles (B) cultured for 48 and 24 h,
respectively (n = 3 independent experiments). Different letters indicate statistically significant difference at *p < 0.05 and
**p < 0.01; (C) control, T: testosterone, 2-Hf: 2-hydroxyflutamide, T + 2-Hf: testosterone + 2-hydroxyflutamide.
r e p r o d u c t i v e b i o l o g y 1 2 ( 2 0 1 2 ) 3 3 3 – 3 4 0338
cultured porcine GCs and whole ovarian follicles. Additionally,
2-Hf induced perinuclear rather than nuclear distribution of
PGR in granulosa cells after whole follicle culture.
2-Hydroxyflutamide is considered to be a potent, nonste-
roidal antiandrogen which has, as yet, no other known
agonistic or antagonistic hormonal properties. It binds to
the AR and competitively inhibits the binding of T and
dihydrotestosterone (DHT; [29]). However, similar to the
results of the current study, findings of Chandrasekhar and
Armstrong [22] suggested an anti-progestational activity of
2-Hf, which hitherto has been claimed to lack other agonistic
or antagonistic activities [30]. It has been observed that 2-Hf
blocks ovulation and the preovulatory LH surge in hCG-primed
prepubertal rats [31], and these effects are reversed by an
injection of LHRH or P4 on the day of proestrus [32]. 2-Hf also
delays the initiation of implantation, fetal development, and
parturition in pregnant rats and suppresses decidualization
after artificial stimulation of the sensitized uterus of ovariec-
tomized steroid-treated pseudopregnant rats [33].
In the present study we demonstrated that PGR protein
expression in granulosa cells and whole follicles of pigs is
decreased after the addition of 2-Hf to the culture medium.
Interestingly, simultaneous addition of T and 2-Hf significant-
ly increased the PGR staining intensity. Thorough analysis of
PGR distribution within the cultured follicles revealed quali-
tative differences in the localization and staining intensity. In
control follicular cultures, PGR staining was observed in
granulosa and theca layers, while in follicles treated with
2-Hf, the theca layer had almost no PGR immunoreaction. This
is consistent with the report by Durlej et al. [34] who
demonstrated the differential expression of both PGR isoforms
in the porcine ovary during the estrous cycle, and suggested
cell-specific influence of progesterone.
It was reported that the ability of antiandrogens to
antagonize certain androgenic effects and to agonize others
may result from its unique interactions with the AR [35]. 2-Hf
can bind to the AR and induce conformational changes of the
receptor responsible for conveying the androgen signal on
cytoplasmic transduction pathways, but is unable to induce
the conformational changes necessary for DNA binding and
gene regulation [36]. Furthermore, Hickey et al. [37] indicated
the existence of a non-genomic pathway of androgen action
leading to modulation of the transcriptional activity of target
genes. The authors found that FSH-stimulated P4 secretion
was suppressed by DHT and 2-Hf in porcine granulosa. We
hypothesize that androgens may affect PGR protein expres-
sion via AR during follicular development, however to reveal
the actual mechanism it requires additional examination.
2-Hf is known to inhibit expression of AR-responsive genes
such as 5-a reductase, which metabolizes T into DHT in rats
[38], caspase-8 or FADD-like apoptosis regulator [39]. 2-Hf can
also block androgen-regulated expression by indirect modes
of action, e.g.by inhibition the AR action via the recruitment of
corepressors [40]. Antiandrogens can also act as weak
androgens when acting alone and as antagonists when
acting in combination with androgens [41]. In addition,
2-Hf may activate other receptor pathways such as the aryl
hydrocarbon receptor [42]. It may also induce feedback
regulation at the level of gonadotropins [43]. Thus, it is
possible that 2-Hf employs multiple action modes involved in
receptor activation.
Our recent studies [44] revealed that androgens play a
critical role in selective apoptosis of granulosa cells during
porcine follicular atresia. It has been shown that 2-Hf, in the
presence of a high T level, attenuates induction of granulosal
cell apoptosis. The response of the ovarian follicular cells to
the combined effects of survival and death promoting signals
determines their ultimate fate: atresia or ovulation. Proges-
terone is one of the factors that are induced by LH, and it has
been reported to act as an antiapoptotic factor in luteinized rat
and human granulosa cells [45,46]. In our experiment,
simultaneous addition of T and 2-Hf to GC culture media of
granulosa cells and intact follicles caused a significant
increase in P4 production. Such increase is typical for cells
undergoing luteinization [47].
We found that intact follicles or GCs treated with T or 2-Hf
alone produced less P4 than controls. This is in agreement with
Henderson et al. [48] who demonstrated that bovine GCs
treated with T secreted lower amounts of P4 than untreated
cells. Moreover, Tanabe et al. [49] reported that P4 production
by porcine granulosa cells, in the presence of gonadotropins, is
modulated in a paracrine or an autocrine fashion by andro-
gens. These authors also found that androgens may exert their
actions partially by altering the activity of cholesterol side
chain cleavage enzymes. In turn, the inhibitory effect of
T antagonist, FLU on P4 secretion was demonstrated by Rangel
et al. [50] who showed that the absence of an increased P4
release resulted in a lack of preovulatory LH surge.
Our medium P4 data corresponded well with the granulosal
PGR expression. The positive P4 effects on the expression of its
r e p r o d u c t i v e b i o l o g y 1 2 ( 2 0 1 2 ) 3 3 3 – 3 4 0 339
own receptors is well known. The presence of PGR in the
granulosa and theca layer of preovulatory follicles marks the
cells as targets for P4 action. Results of studies performed on
PR-A knockout (PRAKO) mice clearly indicated that the
presence of PGRA in granulosa cells of preovulatory follicles
was critical for ovulation [51]. The 2-Hf-induced changes in
PGR expression and P4 level may help to understand the
granulosa cell apoptosis and follicular atresia.
In the present study, we also demonstrated that some
granulosa cells of follicles treated with 2-Hf showed both
nuclear and perinuclear/cytoplasmic PGR immunostainning.
According to D’Haeseleer et al. [52] this type of staining may
reflect the cycle-dependent localization of PGR. A negative
correlation was found between the PGR staining intensity and
the plasma P4 concentration. It is not clear whether in the cells
producing low amounts of P4, the receptor was unbound and
inactive or was ready to be ubiquitinated.
In summary, the present study revealed for the first time
that testosterone antagonist 2-Hf can affect PGR protein
expression and progesterone secretion by porcine granulosa
cells and whole ovarian follicles in culture, indicating that
androgens are involved in normal follicular development. The
down-regulation of PGR expression after exposure to 2-Hf
confirms its antiprogestational activity and demonstrate a key
role of androgens in physiological granulosa cell apoptosis and
porcine follicular atresia.
Acknowledgments
The study was supported by the Ministry of Science and
Higher Education (Grant no. N303 538838). M. Durlej-Grzesiak
is a scholar of the Foundation for Polish Science (START
Programme 2011).
r e f e r e n c e s
[1] Palermo R. Differential actions of FSH and LH duringfolliculogenesis. Reproductive BioMedicine Online2007;15:326–37.
[2] Tabb MM, Blumberg B. New models of action for endocrine-disrupting chemicals. Molecular Endocrinology2006;20:475–82.
[3] Slomczynska M, Tabarowski Z. Localization of androgenreceptor and cytochrome P450 aromatase in the follicle andcorpus luteum of the porcine ovary. Animal ReproductionScience 2001;65:127–34.
[4] Duda M, Slomczynska M. Immunohistochemicallocalization of androgen receptor in two subpopulations ofporcine granulosa cells in vitro. Reproduction in DomesticAnimals 2007;42:22–5.
[5] Singh SM, Gauthier S, Labrie F. Androgen receptorantagonists (antiandrogens): structure–activityrelationships. Current Medicinal Chemistry 2000;7:211–47.
[6] Jensen KM, Kahl MD, Makynen EA, Korte JJ, Leino RI,Butterworth BC, et al. Characterization of responses to theantiandrogen flutamide in a short-term reproductionassay with the fathead minnow. Aquatic Toxicology2004;70:99–110.
[7] Clark CR, Nowell NW. The effect of the non-steroidal anti-androgen flutamide on neural receptor binding of
testosterone and intermale aggressive behaviour of mice.Psychoneuroendocrinology 1980;5:39–45.
[8] Neri RO. Antiandrogens. In: Sighal RL, Thomas JA, editors.Cellular mechanisms modulating gonadal hormone action.Baltimore: University Park Press; 1976. p. 233–62.
[9] Neri RO, Peets E, Watnick A. Antiandrogenicity of flutamideand its metabolite Sch 16423. Biochemical SocietyTransaction 1979;1:565–9.
[10] Espey LL, Lipner H. Ovulation. In: Knobil E, Neill JD, editors.The physiology of reproduction. New York: Reven Press;1994. p. 725–80.
[11] Evans AC. Characteristics of ovarian follicle developmentin domestic animals. Reproduction in Domestic Animals2003;38:240–6.
[12] Leonhardt SA, Boonyaratanakornkit V, Edwards DP.Progesterone receptor transcription and non-transcriptionsignaling mechanism. Steroids 2003;68:761–70.
[13] Migliaccio A, Didomenico M, Green S, Defalco A,Kajtaniak EL, Blasi F, et al. Phosphorylation on tyrosine ofin vitro synthesized human estrogen receptor activatesits hormone binding. Molecular Endocrinolology1989;3:1061–9.
[14] Tsai MJ, O’Malley BW. Molecular mechanisms of action ofsteroid/thyroid receptor superfamily members. AnnualReview of Biochemistry 1994;63:451–86.
[15] Conneely OM, Mulac-Jericevic B, Lydon JP. Progesteronedependent regulation of female reproductive activity bytwo distinct progesterone receptor isoforms. Steroids2003;68:771–8.
[16] Kastner P, Krust A, Turcotte B, Stropp U, Tora L,Gronemeyer H, et al. Two distinct estrogen-regulatedpromoters generate transcripts encoding the twofunctionally different human progesterone receptor formsA and B. EMBO Journal 1990;9:1603–14.
[17] Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ.Reproductive functions of the progesterone receptorisoforms: lessons from knock-out mice. Molecular andCellular Endocrinology 2001;179:97–103.
[18] Slomczynska M, Krok M, Pierscinski A. Localization of theprogesterone receptor in the porcine ovary. ActaHistochemica 2000;102:183–91.
[19] Starczewski A, Brodowska A, Laszczynska M, Piasecka M.The presence and role of progesterone receptor in theovaries of postmenopausal women who have not appliedhormone replacement therapy. Folia Histochemica etCytobiologica 2008;46:277–82.
[20] Clemens JW, Robker RL, Kraus WL, Katzenellenbogen BS,Richards JS. Hormone induction of progesterone receptor(PR) messenger ribonucleic acid and activation of PRpromoter regions in ovarian granulosa cells: evidence for arole of cyclic adenosine 30,50-monophosphate but notestradiol. Molecular Endocrinology 1998;12:1201–14.
[21] Iwai M, Yasuda K, Fukuoka M, Iwai T, Takakura K, Taii S,et al. Luteinizing hormone induces progesterone receptorgene expression in cultured porcine granulosa cells.Endocrinology 1991;129:1621–7.
[22] Chandrasekhar Y, Armstrong DT. Ability of progesterone toreverse anti-androgen (hydroxyflutamide) inducedinterference with preovulatory LH surge and ovulation inPMSG-primed immature rats. Journal of Reproduction andFertility 1989;85:309–16.
[23] Gregoraszczuk E, Wojtusiak A. Histochemical evaluation ofD5,3b-hydroxysteroid dehydrogenase activity in two typesof porcine corpora lutea and granulosa cells in tissueculture. Acta Histochemica 1982;70:22–30.
[24] Lin P, Rui R. Effects of follicular size and FSH ongranulosa cell apoptosis and atresia in porcine antralfollicles. Molecular Reproduction and Development2010;77:670–8.
r e p r o d u c t i v e b i o l o g y 1 2 ( 2 0 1 2 ) 3 3 3 – 3 4 0340
[25] Duda M, Knet M, Tabarowski Z, Slomczynska M. Lutealmacrophage conditioned medium affects steroidogenesisin porcine granulosa cells. Reproductive Biology2011;11:117–43.
[26] Freshney RI. Culture of animal cells. In: Freshney RI, editor.A manual of basic technique. New York: John Wiley andSons; 2005. p. 481–95.
[27] Laemmli UK. Cleavage of structural proteins during theassembly of the head of bacteriophage T4. Nature1970;227:680–5.
[28] Abraham GE, Swerdloff R, Tuchlinsky D, Odell WD.Radioimmunoassay of plasma progesterone. Journal ofClinical Endocrinology and Metabolism 1971;32:619–24.
[29] Brogden R, Chrisp P. Flutamide: a review of itspharmacokinetic properties and therapeutic usein advanced prostatic cancer. Drugs and Aging1991;1:104–15.
[30] Neri R, Florance K, Koziol P, Van Cleave S. A biologicalprofile of a nonsteroidal antiandrogen, SCH 13521 (40-nitro-30-trifluoromethylisobutyoanilide). Endocrinology1972;91:427–37.
[31] Opavsky MA, Chandrasekhar Y, Roe M, Armstrong DT.Interference with the preovulatory luteinizing hormonesurge and blockage of ovulation in immature pregnantmare’s serum gonadotropin-primed rats with anti-androgenic drug, hydroxyflutamide. Biology ofReproduction 1987;36:636–42.
[32] Chandrasekhar Y, Armstrong DT. Human chorionicgonadotropin (hCG) and luteinizing hormone-releasinghormone (LHRH) reverse the blockage of ovulation inpregnant mare’s serum gonadotropin (PMSG)-primedimmature rats by the anti-androgenic drug,hydroxyflutamide. Canadian Journal of Physiology andPharmacology 1988;66:83–787.
[33] Chandrasekhar Y, Armstrong DT, Kennedy IG.Implantation delay and anti-deciduogenic activity in therat by the anti-androgen, hvdroxyflutamide. Biology ofReproduction 1990;42:120–5.
[34] Durlej M, Tabarowski Z, Slomczynska M.Immunohistochemical study on differential distribution ofprogesterone receptor A and progesterone receptor Bwithin the porcine ovary. Animal Reproduction Science2010;121:167–73.
[35] Zhu X, Li H, Liu JP, Fonder JW. Androgen stimulatesmitogen-activated protein kinase in human breastcancer cells. Molecular and Cellular Endocrinology1999;152:199–206.
[36] Kemppainen JA, Lane MV, Sar M, Wilson EM. Androgenreceptor phosphorylation, turnover, nuclear transport, andtranscriptional activation. Specificity for steroids andantihormones. Journal of Biological Chemistry1992;267:968–74.
[37] Hickey TE, Marrocco DL, Gilchrist RB, Norman RJ,Armstrong DT. Interactions between androgen and growthfactors in granulosa cell subtypes of porcine antral follicles.Biology of Reproduction 2004;71:45–52.
[38] Nguyen TV, Yao M, Pike CJ. Flutamide and cyproteroneacetate exert agonist effects: induction of androgenreceptor-dependent neuroprotection. Endocrinology2007;148:2936–43.
[39] Garcia-Reyero N, Villeneuve DL, Kroll KJ, Liu L, Orlando EF,Watanabe KH, et al. Expression signatures for model
androgen and antiandrogen in the feathead Minnow(Pimephales promelas) ovary. Environmental Science andTechnology 2009;43:2614–9.
[40] Rahman MM, Miyamoto H, Lardy H, Chang C. Inactivationof androgen receptor coregulator ARA55 inhibits androgenreceptor activity and agonist effect of antiandrogens inprostate cancer cells. Proceedings of the NationalAcademy of Sciences of the United States of America2003;100:5124–9.
[41] Wong C, Kelce WR, Sar M, Wilson EM. Androgen receptorantagonist versus agonist activities of the fungicidevinclozolin relative to hydroxyflutamide. Journal ofBiological Chemistry 1995;270:19998–20003.
[42] Coe KJ, Nelson SD, Urlich RG, He Y, Dai X, Cheng O, et al.Profiling the hepatic effects of flutamide in rats: amicroarray comparison with classical aryl hydrocarbonreceptor ligands and atypical CYP1A inducers. DrugMetabolism and Disposition 2006;34:1266–75.
[43] Thackray VG, McGillvray SM, Mellon PL. Androgens,progestins and glucocorticoids induce FSH beta-subunitgene expression at the level of the gonadotrope. MolecularEndocrinology 2006;20:2062–79.
[44] Duda M, Durlej M, Knet M, Knapczyk-Stwora K,Tabarowski Z, Slomczynska M. Does 2-hydroxyflutamideinhibit apoptosis in porcine granulosa cells? An in vitrostudy. Journal of Reproduction and Development2012;58:438–44.
[45] Makrigiannakis A, Coukos G, Christofidou-Solomidou M,Montas S, Coutifaris C. Progesterone is an autocrine/paracrine regulator of human granulosa cell survivalin vitro. Annals of the New York Academy of Sciences2000;900:16–25.
[46] Yacobi K, Tsafriri A, Gross A. Luteinizing hormone-inducedcaspase activation in rat preovulatory follicles is coupled tomitochondrial steroidogenesis. Endocrinology2007;148:1717–26.
[47] Channing CP, Ledwitz-Rigby F. Methods for assessinghormone mediated differentiation of ovarian cells inculture and short-term incubations. In: Hardman JG,O’Malley BW, editors. Methods in enzymology. New York:Academic Press; 1975. p. 183–230.
[48] Henderson KM, McNatty KP, Smith P, Gibb M, O’Keeffe LE,Lun S, et al. Influence of follicular health on thesteroidogenic and morphological characteristics of bovinegranulosa cells in vitro. Journal of Reproduction andFertility 1987;79:185–93.
[49] Tanabe K, Tamura T, Saijo A, Kikuchi M, Sho R, Mashu A,et al. Modulation of progesterone secretion by androgens inporcine granulosa cell cultures. Hormone Research1992;37(1):25–31.
[50] Rangel PL, Sharp PJ, Gutierrez CG. Testosterone antagonist(flutamide) blocks ovulation and preovulatory surges ofprogesterone, luteinizing hormone and oestradiol in layinghens. Reproduction 2006;131:1109–14.
[51] Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP,Conneely OM. Subgroup of reproductive functions ofprogesterone mediated by progesterone receptor-Bisoform. Science 2000;289:1751–4.
[52] D’Haeseleer M, Simoens P, Van den Broeck W. Cell-specificlocalization of progesterone receptors in bovine ovary atdifferent stages of the oestrous cycle. Animal ReproductionScience 2007;98:271–81.