Effect of 17 beta-estradiol on gene expression in lumbar spinal cord following sciatic nerve crush...
Transcript of Effect of 17 beta-estradiol on gene expression in lumbar spinal cord following sciatic nerve crush...
Brain Research 966 (2003) 65–75www.elsevier.com/ locate/brainres
Research report
E ffect of 17b-estradiol on gene expression in lumbar spinal cordfollowing sciatic nerve crush injury in ovariectomized mice
Rustem R. Islamov, Wesley A. Hendricks, Laxmansa C. Katwa, Roger J. McMurray,*Elena S. Pak, Nicole S. Spanier, Alexander K. Murashov
Department of Physiology, The Brody School of Medicine at East Carolina University, 600 Moye Blvd., Greenville, NC 27858,USA
Accepted 11 December 2002
Abstract
Previously, we observed that estrogen treatment enhances regeneration of the sciatic nerve after crush injury [Brain Res. 943 (2002)283]. In this research, we studied expression of estrogen receptors and effects of estrogen on gene expression in the lumbar spinal cord,following sciatic nerve crush injury. Using the Atlas Mouse 1.2 Array, changes in the expression of 267 of 1176 genes were registered 4days after nerve injury. Those genes that exhibited a change in signal intensity ratios of 2-fold or greater were selected as up-regulated(42) or down-regulated (21). In estrogen treated mice, we have observed up-regulation of the genes known to control apoptosis, cellproliferation, and growth, which might account for the positive effects of estrogen on the regeneration of motor neurons. Immuno-histochemical staining revealed estrogen receptor-a and estrogen receptor-b localized in the nucleus and cytoplasm of lumbar motorneurons, and in the regenerating neurites of the sciatic nerve. Expression of estrogen receptor-a and estrogen receptor-b mRNA in lumbarspinal cord was shown by traditional RT-PCR. Using real-time quantitative RT-PCR, we demonstrated increased expression of estrogenreceptors-a and -b mRNA on the injured side of the lumbar spinal cord. Western blot analysis showed the accumulation of ERs inregenerating sciatic nerve, and revealed a 40% increase of activated ERK1/2 in estrogen treated mice, compared to placebo. Our findingsindicate that: (i) axotomized motor neurons increase expression of estrogen receptors-a and -b mRNA, (ii) estrogen mediates theexpression of genes which accelerate the growth and maturation of axons, and (iii) estrogen receptors are transported from the perikaryoninto regenerating neurites, and estrogen promotes regeneration locally through the non-genomic ERK-activated signaling pathway. 2002 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Regeneration
Keywords: Spinal cord; Sciatic nerve; Motor neuron; Estrogen; Estrogen receptor; Gene expression; Regeneration
1 . Introduction effects of estrogen are not restricted to the reproductivesystem; the lipophilic hormone may pass through the
The estrogen-receptor complex is a well-known tran- plasma membrane of any mammalian cells and affect cellscription factor which binds to the estrogen-responsive function.elements of target genes and modulates their expression In the nervous system, estrogen plays an important role[14]. The alternative genomic and non-genomic pathways in the function of different cell types in distinct regions. Itof estrogen action may also modulate cell function by is well established that estrogen has a neuroprotectiveinteraction of activated estrogen receptors (ER) with effect; it increases survival of neurons in vitro [7,26,39]membrane or cytosolic targets [6,18,25]. The biological and protects neurons against ischemia and apoptosis in
vivo [4,43]. Estrogen treatment in postmenopausal womenis thought to prevent development of Alzheimer’s disease
*Corresponding author. Tel.:11-252-816-3111; fax:11-252-816-[36], and to improve motor disability in Parkinson’s3460.disease [40]. Localization of estrogen receptors in en-E-mail addresses: http: / /www.ecu.edu/physio / labakm/ (A.K.
Murashov),[email protected](A.K. Murashov). kephalinergic neurons of dorsal horns [1] and increased
0006-8993/02/$ – see front matter 2002 Elsevier Science B.V. All rights reserved.doi:10.1016/S0006-8993(02)04191-4
66 R.R. Islamov et al. / Brain Research 966 (2003) 65–75
transcription of the enkephalin gene by estrogen [45] experimentation. After ovariectomy in the experimentalsuggest that estrogen is involved in pain sensitivity [20]. In group of mice, silastic capsules containing 24mg 17b-our laboratory, we study the effects of estrogen on estradiol (Sigma) were implanted s.c. according to ourperipheral nerve regeneration. We have demonstrated that a laboratory protocol [11]. Control mice received capsuleshigh-dose estrogen treatment accelerates growth and matu- with vehicle (sesame oil). As we showed previously [11] inration of regenerating nerve fibers and enhances functional estrogen treated mice, serum concentration of estradiol wasrecovery, following crush injury of the sciatic nerve in at a supraphysiological level throughout the first 3 weeksovariectomized mice [11]. and slowly decreased on week 4 after implantation of the
Regeneration of peripheral nerves is based on the estrogen capsules. In placebo mice, serum concentration ofinformational intercellular interactions between different estrogen corresponded to the lowest level of hormone intypes of cells in the central and peripheral nervous system proestrus.(motor- and interneurons, sensory neurons, glial cells, Then 1 week after implantation of the capsules, in bothmacrophages, fibroblasts). During regeneration of spinal groups of mice, right sciatic nerves were crushed at themotor neurons, axons face the task of growing a long level of the sciatic notch for 15 s with a fine hemostat. Indistance to reach denervated muscle fibers. Axonal growth sham operations right sciatic nerves were briefly exposed,and path finding at such a long distance depends on the wounds closed, and the mice were allowed to recover.various extracellular signals. Neurotrophins, cytokines,extracellular matrix components produced by Schwann 2 .2. Immunohistochemistrycells [16,46], skeletal muscle cells [31], macrophages[10,32] and fibroblasts [29,34], are the essential molecules For immunohistochemical studies, mice were sacrificedpromoting regeneration. In this research, we hypothesized on days 4, 7, 14, and 21 after nerve injury (three mice perthat estrogen could be one of the critical factors in the time point in each group). The euthanized mice wereregeneration of peripheral nerves. perfused first with cold PBS and then with cold 4%
Two isoforms of ERs (ERa and ERb) have been found paraformaldehyde in PBS (pH 7.4). Lumbar spinal cordin sensory neurons of dorsal root ganglia (DRG) [28,38] and parts of sciatic nerves distal from the crush site wereand Schwann cells [13,37]. In the spinal cord, estrogen removed and postfixed in 4% paraformaldehyde on areceptors have been shown to be expressed in interneurons rocker table. Tissue was cryoprotected in 30% sucrose andof dorsal horns [33], astrocytes [9], and ependymal cells embedded in TBS tissue freezing medium (Triangle Bio-[3]. There is evidence that motor neurons can accumulate medical Science, Durham, NC). Frozen free-floating (40estrogen [2,42] and that estrogen promotes survival of mm) and slide mounted (10mm) coronal serial sections ofmotor neurons in primary culture [26]. At the same time, it lumbar spinal cord and slide mounted (10mm) longitudinalis still unclear whether motor neurons may possess es- sections of sciatic nerves were prepared. Rabbit anti-ERa
trogen receptors. antibodies (Upstate Biotechnology) were applied at 1:2000In the present study on the model of sciatic nerve crush dilution. Rabbit anti-ERb polyclonal antibodies (Upstate
injury we investigated the expression ofERa and ERb Biotechnology and Chemicon) were applied at 1:200 andmRNA in motor neurons. The effects of 17b-estradiol on 1:800 dilutions, respectively. Rabbit anti-Hsp25 polyclonalthe profile of gene expression in lumbar spinal cord were antibodies (StressGen Biotechnology) were applied atanalyzed using the Atlas Mouse 1.2 Array. We also 1:5000 dilution. Sections were stained using the Elite ABCexamined the role of estrogen in the activation of the Kit (Vector), and tissue antigens were visualized with aERK-signaling pathway in regenerating sciatic nerve. DAB Substrate Kit for Peroxidase (Vector).
2 .3. Western blot analysis2 . Material and methods
At 1 week after crush injury of the sciatic nerve, placebo2 .1. Animals and treatments and estrogen treated mice (five in each group) were
euthanized, and sciatic nerves (distal part from the crushMature 8-week-old female mice (Charles River outbred site) were removed, frozen in liquid nitrogen and stored at
ICR strain) were employed in this study. Mice were 280 8C. Tissue was homogenized in ice cold lysis buffer:housed with unlimited access to food and water and a 12-h 10 mM Tris pH 7.4, 1 mM EDTA, 1 mM EGTA pH 8.0,light /dark schedule. Animal protocol was approved by the 150 mM NaCl, 1% Triton X-100, 0.5% NP-40, 0.2 mMEast Carolina University Animal Care and Use Committee. sodium orthovanadate, and protease inhibitor cocktail
All surgical procedures were performed under ketamine- (Sigma). Protein levels were quantified using a Bio-Radxylazine anesthesia (1.5 mg/100 g, i.p.). To regulate the Protein Assay Kit (Bio-Rad, CA). Proteins (40mg perblood level of estrogen, bilateral ovariectomy was per- well) were separated by SDS–PAGE electrophoresis informed as described previously [22] and animals were 12% gel and transferred to Immobilon P membraneallowed to recover from surgery for 7 days before further (Millipore, Bedford, MA). Membranes were incubated
R.R. Islamov et al. / Brain Research 966 (2003) 65–75 67
overnight with rabbit polyclonal antibodies against ERa (Molecular Probes, Eugene, OR) was used for cDNA(Upstate Biotechnology) at 1:5000, ERb (Chemicon) at quantitation. The PCR mixture (25ml final volume per1:2000, Hsp25 (StressGen Biotechnology) at 1:2000 dilu- reaction) was prepared according to the manufacturer’stion and phospho-ERK1/2 (Cell Signaling) at 1:1000 protocol. The cDNA synthesis was performed for 30 mindilutions. Secondary anti-rabbit horseradish peroxidase at 508C using 200hg of total RNA per each reaction. PCRconjugated antibodies (Amersham Life Science, UK) were amplification was done in 45 cycles of denaturation atused at 1:10,000 dilution. Proteins bands were visualized 958C for 15 s followed by 45 s of annealing andusing a chemiluminescence detection system (ECL1plus, elongation at 588C for ERa, at 568C for ERb, and atAmersham, UK). Membranes were exposed to autoradiog- 558C for GAPDH. Mouse ERa specific primers wereraphy film, scanned on an Epson Perfection 1200 flatbed ERaS2 sense: 59-GGC-TGG-AGA-TTC-TGA-TGA-TTG-scanner, and analyzed using Kodak Digital Science 1D G-39 and ERaS2 antisense: 59-GGG-TAT-GTA-GTA-Image Analysis software (Eastman Kodak, Rochester, GGT-TTG-TAA-GG-39 (608 bp) [8] and mouseERbNY). specific primers were ERbD1 sense: 59-CTT-GCC-TGT-
AAA-CAG-AGA-GAC-C-39 and ERbD1 antisense: 59-2 .4. RT-PCR assay GAC-GGC-TCA-CTA-GCA-CAT-TGG-39 (507 bp) [8].
The primer pairs were synthesized by Invitrogen.To study expression ofERa and ERb in spinal cord, Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
mice were sacrificed on days 4, 7, 14, and 21 after nerve was used as an internal control.GAPDH oligonucleotideinjury (three mice per each time point in each group). The primers sense: 59-AGA-TCC-ACA-ACG-GAT-ACA-TT-lumbar spinal cords were removed, frozen in liquid 39 and GAPDH antisense: 59-TCC-CTC-AAG-ATT-GTC-nitrogen and stored at280 8C. Thawed tissue was AGC-AA-39 (195 bp) were synthesized at the DNA Corehomogenized in 1 ml TRIzol reagent (Gibco BRL, Facility at the University of Missouri Health SciencesGaithersburg, MD) and total RNA was isolated according Center, Columbia, MO.to the manufacturer’s instructions.
The SuperScript One-Step RT-PCR with PlatinumTaq2 .6. cDNA array hybridization
(Invitrogen, Life Technologies) was carried out in PCRSPRINT system (Hybaid, Middlesex, UK). cDNA syn-
The Atlas Mouse 1.2 Array (Clontech, Palo Alto, CA)thesis was performed in 30-min incubation at 508C using 1
was used to study gene expression in lumbar spinal cordmg of RNA per each reaction. PCR amplification was done
on the 4th day after crush injury of the sciatic nerve inin 35 cycles of denaturation at 958C for 1 min, annealing
placebo and estrogen treated mice (ten mice per eachfor 1 min and extension at 728C for 1 min. The annealing
group). Total RNA was isolated as described above and 40temperatures forERa and ERb were 58 and 498C,
mg of RNA was used in cDNA preparation. Labeling ofrespectively. MouseERa specific primers were ERaS2 32RNA was performed with [a- P]dATP (3000 Ci /mmol,sense: 59-GGC-TGG-AGA-TTC-TGA-TGA-TTG-G-39and 3210 mCi/ml; Perkin-Elmer). P-labeled cDNA probes wereERaS2 antisense: 59-GGG-TAT-GTA-GTA-GGT-TTG-
hybridized to Atlas array membranes according to theTAA-GG-39 (608 bp) [8] and mouseERb specific primers
manufacturer’s instructions. After hybridization Atlas arraywere ERBST sense: 59-CTA-TGA-CAT-TCT-ACA-GTC-
membranes were exposed to phosphorimaging screens,C-39 and ERB3 antisense: 59-GTA-ATG-ATA-CCC-AGA-
scanned on the Molecular Dynamics Phosphor Imager andGCA-39 (351 bp) [21]. The primer pairs were synthesized
analyzed using Atlas Image 2.01 software (Clontech, Paloby Invitrogen. PCR products were displayed on 2% Tris
Alto, CA).borate–EDTA agarose gels containing ethidium bromide.
2 .5. Real-time quantitative RT-PCR3 . Results
Estrogen treated and placebo mice were sacrificed 1week after nerve injury (six mice in each group). The 3 .1. Studying of gene expression in lumbar spinal cordlumbar parts of spinal cords were removed, divided into on day 4 after sciatic nerve crush injuryright (ipsilateral) and left (contralateral) parts, frozen inliquid nitrogen and stored at280 8C. Thawed tissue was The time point selected to study gene expression washomogenized in 1 ml TRIzol reagent (Gibco BRL, based on our previous research [23], which showed theGaithersburg, MD) and total RNA was isolated according first significant increase in the level of Hsp25, phospho-to the manufacturer’s instructions. p38, and phospho-Akt proteins in sciatic nerve on day 4
PCR reactions were performed using Platinum qRT- after injury. Hybridization results are presented in Fig. 1.PCR ThermoScript One-Step System (Invitrogen, Life Only 267 of 1176 genes were reported. Those genes thatTechnologies, CA) in the Cepheid Real Time PCR ther- exhibited signal intensity ratios of 2-fold or greater weremocycler (Sunnyvale, CA). Fluorescent dye SYBR Green I selected as up-regulated (42) or down-regulated (21) and
68 R.R. Islamov et al. / Brain Research 966 (2003) 65–75
ing with antibodies to ERs was used. In the present studywe found ERa and ERb immunopositive motor neurons inlumbar spinal cord. In motor neurons, immunohistochemi-cal precipitate was localized in nucleus, perikaryon andneurites (Fig. 2). ERb stained motor neurons were re-vealed using antibodies from both Chemicon and UpstateBiotechnology. ERa and ERb immunopositive inter-neurons in dorsal horns and ependymal cells were detectedas well. Light microscopy analysis did not show a notabledifference in the pattern of ERa and ERb immunostainingin either placebo or estrogen treated mice.
It is known that after axotomy, motor neurons noticeab-ly increase expression of Hsp25 [23]. In the present study,we used antibodies against Hsp25 as a marker of theinjured side of the spinal cord. On serial sections weobserved a slight increase in the ERa and ERb immuno-reactivity at the injured side of spinal cord in both placeboand estrogen treated mice throughout the experiment.
Immunohistochemical staining has also revealed bothisoforms of ERs in sciatic nerves. Immunopositive ERa
and ERb nerve fibers were observed in the regeneratingsciatic nerves from placebo and estrogen treated mice (Fig.3).
3 .3. RT-PCR and real-time PCR analysis of ERa andERb mRNA expression in lumbar spinal cord
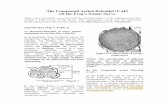
Fig. 1. The Atlas Mouse 1.2 Array images of cDNA hybridization. TheRT-PCR was performed to investigate the expression ofupper panel (placebo mice), and the lower panel (estrogen treated mice)
ERa and ERb in spinal cord after crushing the sciaticrepresent gene expression in the lumbar spinal cord 4 days after crushnerve in placebo and estrogen treated mice at differentinjury of sciatic nerve.
time points during nerve regeneration. Total RNA wasisolated from the lumbar part of spinal cord 4, 7, 14 and 21
are listed in Table 1. The order of genes in the table days after crushing of sciatic nerve.corresponds to their location on the membranes. The presence of both ERs in the spinal cord was shown
In both placebo and estrogen treated groups, on the 4th (Fig. 4). Primer pairs amplifiedERa (608 bp) andERbday after crushing of sciatic nerve, we registered expres- (351 bp) mRNA. Notable differences in expression ofERssion of genes known to regulate apoptosis, cell prolifer- mRNA in placebo and estrogen treated mice at any timeation and growth. In estrogen treated mice we have point during regeneration of sciatic nerve were not ob-observed up-regulation of several genes, which could be served.responsible for the enhanced regeneration of the sciatic While we have detected expression ofER mRNA innerve. Moreover, it should be mentioned that in estrogen lumbar spinal cord by RT-PCR and our immunohistoch-treated mice, among the genes which had signal intensity emical observation indicated that motor neurons at theratios ranging from 1.4- to 2-fold increase, there were injured side had more intensive staining (more prominent 1documented genes promoting axon growth, such as bdnf / week after injury), we proposed that motor neuronsnt-3 growth factors tyrosine kinase receptor (TRK-B), increase expression ofERs mRNA after injury. To test thisneuromodulin (GAP-43), kinesin motor protein C2 hypothesis we used real-time quantitative RT-PCR to(KIFC2), neuronal kinesin heavy chain (NKHC), Rac1, analyze expression ofERs genes in injured and contralater-cortactin, microtubule-associated protein 2 kinase (ex- al parts of lumbar spinal cord, 1 week after crushing sciatictracellular signal-regulated kinase 1;ERK1) and ERK nerves in placebo and estrogen treated mice.activator kinase 1 (MEK1). In all samples, we have detected expression ofERa and
ERb genes. As expected, the amplification plots reached3 .2. Immunohistochemical detection of ERa and ERb in the plateau after 35 cycles. However, the threshold cyclelumbar spinal cord and sciatic nerve (Ct) values of duplicate RT-PCR reactions were lower for
ERa and ERb in the ipsilateral (injured) side of spinalTo study cellular localization of the ERs in lumbar cord in both placebo and estrogen treated mice (Table 2).
spinal cord and sciatic nerve immunohistochemical stain- The higher level of expression ofERa and ERb genes in
R.R. Islamov et al. / Brain Research 966 (2003) 65–75 69
Table 1The list of up- and down-regulated genes in the lumbar part of spinal cord in estrogen treated mice versus placebo on the 4th day after crush injury ofsciatic nerve
GenBank Accession No. Estrogen/placebo Gene/protein name
M63801 Up Gap junction alpha 1 protein (GJA1); connexin 43 (CXN43; CX43); gap junction43-kDa heart protein
U70674 Up m-numb (m-NB)S94664 Up Homeobox protein Hox-D10 (Hox-4.5) (Hox-5.3)U59496 Up Hypoxia-inducible factor 1 alpha (HIF-1 alpha) (Arnt interacting protein)U42554 Down Sim transcription factorM20157 Down Early growth response protein 1 (EGR1); KROX-24 protein; ZIF/268U19617 Down ets-Related transcription factor; E74-like factor 1 (ELF1)M17192 Down Homeobox protein 1.1 (Hox-1.1)X07439 Up Homeobox protein 3.1 (Hox-3.1)D50621 Down PSD-95/SAP90AX60831 Up Transcription factor UBFX72310 Up Transcription factor E2F dimerization partner 1 (TFDP1); DRTF1 polypeptide 1L21027 Up D-3-Phosphoglycerate dehydrogenase (PGDH); transcription factor A10X14951 Down Cell surface adhesion glycoproteins LFA-1/CR3/p150,95 beta subunit precursor;
integrin beta 2 (ITGB2); CD18 antigen; complement receptor C3 beta subunitAJ011739 Down Integrase interactor 1A protein (INI1A)M63903 Down Max protein. Max protein (MYN protein)X76654 Down Ear-2; v-erbA related proto-oncogeneX67812 Up ret proto-oncogene precursor; c-retM17031 Down c-Src proto-oncogeneM13071 Down A-Raf proto-oncogeneM64429 Down B-raf proto-oncogeneU14173 Up ski proto-oncogeneAF029874 Up Heme oxygenase 2 (HO-2)U20107 Down Synaptotagmin VIII (fragment)AF058055 Up Monocarboxylate transporter MCT1X56518 Up Acetylcholinesterase precursor (AChE)L28177 Up Growth arrest and DNA-damage-inducible protein 45 (GADD45); DNA-damage-
inducible transcript 1 (DDIT1)U05671 Up Adenosine A1M receptorAF041054 Up NIP3; Bcl-2 and adenoviral E1B-interacting proteinZ31663 Up Activin type I receptorAF028242 Up Calcitonin gene-related peptide-receptor component
protein (CGRP-receptor component protein)S49542 Down 5-Hydroxytryptamine receptor; serotonin receptor type 2 (5HT2)X72230 Down 5-Hydroxytryptamine (serotonin) receptor 1cD82866 Up Nociceptin precursor; ORL1 receptor agonist precursor (endogenous agonist of
opioid receptor-like ORL1 receptor; orphanin FQ; PPNOCM97017 Down Bone morphogenetic protein 8a (BMP-8a) (TGF-beta family)M93428 Up Endothelial ligand for L-selectin (GLYCAM 1)D49921 Up Glial cell line-derived neurotrophic factorM13926 Up Granulocyte colony-stimulating factor (GCSF; CSFG); colony stimulating factor
3 (CSF3)M30642 Down Fibroblast growth factor 4 precursor (FGF4); KFGF; HBGF4M92415 Down Fibroblast growth factor 6 precursor (FGF6); heparin-binding growth factor 6
(HBGF6)U10115 Up Segment polarity protein dishevelled homolog 1 (DVL1); DSH homolog 1AF080215 Down PAR4; protease-activated receptor 4 G protein-coupled receptor; thrombin receptorM28489 Down ribosomal protein S6 kinase II alpha 1 (S6KII-alpha; RPS6KA1); ribosomal S6
kinase 1 (RSK1)X76850 Up MAPKAPK-2; MAP kinase-activated protein kinase; MAPKAP kinase 2U18310 Up MAPKK4; MAP kinase kinase 4; Jnk activating kinase 1 (JNKK1; SEK1; MKK4)L35236 Up Stress-activated c-jun N-terminal kinase 3 (JNK3); MAP kinase p49 3F12;
PRKM10; SERK2U20238 Up GapIII; GTPase-activating proteinZ71173 Up Inositol 1,4,5-trisphosphate receptor type 2U03279 Up PI3-K p110; phosphatidylinositol 3-kinase catalytic subunitAF020339 Up Guanylate cyclase soluble beta-1 subunit; guanylate cyclase 70-kDa subunitM21531 Up Calbindin-28KAJ001700 Up Neuroserpin precursor (protease inhibitor 17)
70 R.R. Islamov et al. / Brain Research 966 (2003) 65–75
Table 1. Continued
GenBank Accession No. Estrogen/placebo Gene/protein name
M64086 Up Serine protease inhibitor 2-2 (SPI2-2); SPI2 proteinase inhibitor; SPI2/EB4L19622 Up Tissue inhibitor of metalloproteinases 3 (TIMP3); SUNAF013116 Up Kinesin motor protein 3C (KIF3C)U72520 Up Mena protein; enabled homolog (ENAH)S69407 Up Endoglin precursor (EDG; ENG); cell surface MJ7/18 antigenD26077 Up Kinesin like protein KIF 3BX53068 Up Proliferating cell nuclear antigen (PCNA); cyclinD86726 Down MCM6 DNA replication licensing factor (P105MCM)AF033188 Up WSB2 proteinU77039 Up Four and a half LIM domains 1 (FLH1); skeletal muscle LIM protein 1 (SLIM1)U24233 Up huntingtin; Huntington disease homolog (HDH)
the ipsilateral side versus contralateral side was confirmed lack of estrogen induces higher stress in regeneratingwhen the expression was normalized forGAPDH. neurons.
3 .4. Western blot analysis4 . Discussion
Our findings that axotomized motor neurons increasedexpression ofERs, the up-regulation ofERK1and MEK1 The major components forming sciatic nerve are pro-expression in spinal cord of estrogen treated mice, and the cesses of motor neurons, processes of sensory neurons ofpresence of ERs in regenerating neurites, raised the DRG, and Schwann cells. ERs have been shown in DRGquestion whether ERs were transported from perikaryon neurons [28,38] and Schwann cells [13,37], although thereinto the neurites to promote regeneration locally through was no evidence that motor neurons express any type ofthe alternative non-genomic mechanism. ERs. Meanwhile, there is accumulating data indicating that
Changes in the level of ERa and ERb, Hsp25 and after injury of the sciatic nerve, estrogen suppresses thephospho-ERK1/2 proteins were studied to investigate scar reaction [24], accelerates regeneration of nerve fibersactivation of the ERK-signaling pathway in regenerating and functional recovery [11], and stimulates muscle rein-sciatic nerve. Based on our previous data, indicating that 1 nervation [41]. These positive effects of estrogen onweek after crush injury of the sciatic nerve there was a regeneration of the sciatic nerve could be explained by themarked increase in sciatic functional index, and in the total action of estrogen on motor neurons, sensory neurons,number of regenerating nerve fibers, we chose this time astrocytes, Schwann cells, and macrophages.point for protein profiling. Estrogen can affect the function of macrophages [8], and
Accumulation of ERa and ERb in sciatic nerve was stimulate proliferation of Schwann cells [37]. Intercellularconfirmed by immunoblot analysis. Protein lysates ob- informational interaction between Schwann cells andtained from intact and regenerating placebo or estrogen macrophages plays a key role in growth of regeneratingtreated sciatic nerves contained both types of ERs (Fig. 5). nerve fibers. The neuroprotective effects of estrogen onDensitometric analysis of duplicate immunoblot examina- sensory neurons might stimulate the regenerating capacitytions revealed a slight increase of ERa level intensity in of these cells [28]. The fact that astrocytes are in intimateregenerating placebo and estrogen treated nerves, relative contact with motor neurons and possess ERs indicates ato intact sciatic nerve (Fig. 5A). A pronounced 3-fold possible role of astrocytes in estrogen-mediated regenera-increase in the level of ERb intensity was detected in tion of motor neurons. Thus, the positive effect of estrogenregenerating nerves from both placebo and estrogen treated on regeneration of the peripheral nerve may be the resultmice (Fig. 5B). of summation of estrogen actions on different types of cell
The level of activated ERK1/2 was elevated 1.8-fold in involved in regeneration.placebo and 2.2-fold in estrogen treated mice, comparing Although previous studies demonstrated that motorwith intact sciatic nerve (Fig. 5C). It is worthwhile noting neurons could up-take estrogen [2] and that estrogen mightthat the increase in the estrogen group was|40% higher prevent glutamate- and nitric oxide induced motor neuronthan in placebo. death in primary culture [26], ERs have never been
Our previous observation has shown accumulation of detected in spinal motor neurons. To the best of ourHsp25 in injured motor neuron axons [23]. In the present knowledge, we provide the first evidence of the presenceresearch a 2.5-fold increase in Hsp25 level was observed in of ERa and ERb in motor neurons of lumbar spinal cord.regenerating placebo nerves and a 1.9-fold increase in Immunohistochemically, ERa and ERb positive motorestrogen nerves relative to intact sciatic nerve (Fig. 5D). neurons have been observed in the lumbar spinal cord.The higher level of Hsp25 in placebo animals suggests that Nuclear and cytoplasmic staining was detected in the
R.R. Islamov et al. / Brain Research 966 (2003) 65–75 71
Fig. 2. Immunohistochemical staining of the spinal cord with antibodies to ERa (A, B), ERb (C, D) and Hsp25 (E, F). The left panel (placebo mice) andthe right panel (estrogen treated mice) represent sections of the injured side of the lumbar spinal cord 1 week after sciatic nerve injury. Arrows indicatemotor neurons. Inserts show motor neurons at higher magnification.
motor neurons of both estrogen treated and placebo mice. genomic mechanisms [18,19]. The genomic mechanismOur RT-PCR data have confirmed expression ofERa and involves binding of the estrogen-receptor complex toERb mRNA in lumbar spinal cord in estrogen treated and estrogen responsive elements, with subsequent initiation ofplacebo mice. The finding of increased expression ofER gene expression [14]. A wide array of different genes aregenes on the injured side of the lumbar spinal cord, and involved in cell regeneration. Our study of gene expressionincreased immunoreactivity in axotomized motor neurons, in the lumbar spinal cord of estrogen treated mice hasmay suggest that regenerating motor neurons require an revealed up-regulation of numerous genes known to beadditional number of ERs. responsible for cell survival and regeneration. Increased
Estrogen may act on neurons via genomic and non- expression of glial cell line-derived neurotrophic factor and
72 R.R. Islamov et al. / Brain Research 966 (2003) 65–75
Fig. 3. Immunohistochemical staining of the sciatic nerve with antibodies to ERa (A) and ERb (B) 1 week after crush injury of the nerve in estrogentreated mice. Inserts show individual nerve fibers.
Fig. 4. PCR products representing mRNA ofERa (upper panel) andERb (lower panel) in the lumbar spinal cord at different time points after sciatic nervecrush, in estrogen treated (E) and placebo (P) mice. Primer pairs amplifiedERa andERb mRNA of 608 and 351 base pairs, respectively. The marker is aladder of progressive length of 100-bp DNA.
Table 2Quantitation of the target genes expression in the right (injured) and in the left (contralateral) parts of lumbar spinal cords from estrogen treated(E) andplacebo (P) mice
Target Ct value of Normalization Ct value of Normalizationgenes target genes coefficient target genes coefficient
E right E left E right E left P right P left P right P left
GAPDH 15.29 15.65 15.52 15.56ERa 15.28 17.77 0.99 1.14 16.71 18.35 0.96 1.03ERb 14.66 16.1 1.08 1.18 16.1 18.09 1.04 1.16
Ct, mean of duplicate threshold cycles of the target genes; normalization coefficient, the ratio of CtERa and ERb to Ct GAPDH.
R.R. Islamov et al. / Brain Research 966 (2003) 65–75 73
Fig. 5. Immunoblot representation and densitometric analysis of the relative intensities of ERa (panel A), ERb (panel B), phospho-ERK1/2 (panel C), andHsp25 (panel D) proteins in sciatic nerves from intact mice (I), and a week after crush injury from placebo (P) and estrogen treated (E) mice (n55 in eachgroup). Protein extract from ovaries (O) was used as a positive control.
its receptor, calcitonin gene-related peptide-receptor com- Taken together, our results and the recent evidence thatponent protein, stress-activated c-jun N-terminal kinase 3, ERK can be transported in the anterograde direction in theski proto-oncogene, transcription factor UBF, hypoxia- sciatic nerve [30], and that estrogen enhances ERK phos-inducible factor 1 alpha, heme oxygenase 2, kinesin motor phorylation [35], indicates that ERs may play a critical roleprotein, mena protein, connexin 43, and nociceptin pre- in regenerating axons via the local ERK-activated mecha-cursor, among others may account for the positive effect of nism.estrogen on the regeneration of motor neurons (Table 1). We propose that: (i) axotomized motor neurons increaseThese data suggest that estrogen could be one of the expression ofERa andERb mRNA, (ii) estrogen mediatescritical factors regulating transcription of genes involved in expression of genes which accelerate growth and matura-regeneration. tion of the axons, and (iii) ERs are transported from the
Recent investigations of alternative non-genomic mecha- perikaryon into the regenerating neurites, where estrogennisms of estrogen action have revealed involvement of a promotes regeneration locally through the non-genomicvariety of different intracellular signaling cascades ERK-activated signaling pathway.[15,35,44]. The non-genomic mechanism of estrogen ac-tion implies interaction of estrogen and activated ERs withothers membrane and/or cytosolic targets such as G-A cknowledgementsprotein-coupled receptors, mitogen-activated protein ki-nases (MAP), adenylyl cyclase, and protein kinase C [6]. We thank Dr Abdel A. Abdel-Rahman, Dr Gregory S.MAP-kinases (ERK, JNK and p38) have been shown to be Iams and Dr Bruce McEwen for helpful advice andinvolved in transduction of the estrogen initiated intracellu- comments on the manuscript. We are grateful to Michaellar signals. Estrogen also increases PI3-kinase and AktSmith and Robert Jones for technical assistance. We alsoactivities in MCF-7 cells [5], and activates ERK, JNK and would like to acknowledge Dr Vishnu Chintalgattu for hisp38 in rat cardiomyocytes [27]. In neurons, estrogen is help with real-time PCR assay.believed to activate PI3-kinase and MAPK pathways[12,17,35].
In our experiments we have observed the increasedR eferencesexpression ofERK1 andMEK1 in spinal cord, accumula-tion of ERs in the sciatic nerve, and increased level of [1] A. Amandusson, O. Hermanson, A. Blomqvist, Colocalization ofphosphorylated ERK along the regenerating nerve fibers. oestrogen receptor immunoreactivity and preproenkephalin mRNA
74 R.R. Islamov et al. / Brain Research 966 (2003) 65–75
expression to neurons in the superficial laminae of the spinal and [20] V.M. Medina, L. Wang, A.R. Gintzler, Spinal cord dynorphin:medullary dorsal horn of rats, Eur. J. Neurosci. 8 (1996) 2440– positive region-specific modulation during pregnancy and parturi-2445. tion, Brain Res. 623 (1993) 41–46.
[2] S.M. Breedlove, A.P. Arnold, Sex differences in the pattern of [21] C.A. Moffatt, E.F. Rissman, M.A. Shupnik, J.D. Blaustein, Induc-steroid accumulation by motoneurons of the rat lumbar spinal cord, tion of progestin receptors by estradiol in the forebrain of estrogenJ. Comp. Neurol. 215 (1983) 211–216. receptor-alpha gene-disrupted mice, J. Neurosci. 18 (1998) 9556–
9563.[3] K.A. Burke, D.M. Schroeder, R.A. Abel, S.C. Richardson, R.M.Bigsby, K.P. Nephew, Immunohistochemical detection of estrogen [22] M.K. Mohamed, A.A. Abdel-Rahman, Effect of long-term ovariec-receptor alpha in male rat spinal cord during development, J. tomy and estrogen replacement on the expression of estrogenNeurosci. Res. 61 (2000) 329–337. receptor gene in female rats, Eur. J. Endocrinol. 142 (2000) 307–
314.[4] G.P. Cardona-Gomez, P. Mendez, L.L. DonCarlos, I. Azcoitia, L.M.Garcia-Segura, Interactions of estrogens and insulin-like growth [23] A.K. Murashov, I. Ul Haq, C. Hill, E. Park, M. Smith, X. Wang, D.J.factor-I in the brain: implications for neuroprotection, Brain Res. Goldberg, D.J. Wolgemuth, Crosstalk between p38, Hsp25 and AktBrain Res. Rev. 37 (2001) 320–334. in spinal motor neurons after sciatic nerve injury, Brain Res. Mol.
Brain Res. 93 (2001) 199–208.[5] G. Castoria, A. Migliaccio, A. Bilancio, M. Di Domenico, A. deFalco, M. Lombardi, R. Fiorentino, L. Varricchio, M.V. Barone, F. [24] A.K. Nachemson, G. Lundborg, R. Myrhage, F. Rank, NerveAuricchio, PI3-kinase in concert with Src promotes the S-phase regeneration and pharmacological suppression of the scar reaction atentry of oestradiol-stimulated MCF-7 cells, EMBO J. 20 (2001) the suture site. An experimental study on the effect of estrogen-6050–6059. progesterone, methylprednisolone-acetate and cis-hydroxyproline in
rat sciatic nerve, Scand. J. Plast. Reconstr. Surg. 19 (1985) 255–[6] A.C. Cato, A. Nestl, S. Mink, Rapid actions of steroid receptors in260.cellular signaling pathways, Sci. STKE 2002 (2002) RE9.
[25] A. Nadal, M. Diaz, M.A. Valverde, The estrogen trinity: membrane,[7] P.S. Green, J. Bishop, J.W. Simpkins, 17 alpha-estradiol exertscytosolic, and nuclear effects, News Physiol. Sci. 16 (2001) 251–neuroprotective effects on SK-N-SH cells, J. Neurosci. 17 (1997)255.511–515.
[26] T. Nakamizo, M. Urushitani, R. Inoue, A. Shinohara, H. Sawada, K.[8] Z. Guo, J. Krucken, W.P. Benten, F. Wunderlich, Estradiol-inducedHonda, T. Kihara, A. Akaike, S. Shimohama, Protection of culturednon-genomic calcium signaling regulates genotropic signaling inspinal motor neurons by estradiol, Neuroreport 11 (2000) 3493–macrophages, J. Biol. Chem. 277 (2002) 7044–7050.3497.[9] E. Hosli, W. Ruhl, L. Hosli, Histochemical and electrophysiological
[27] S. Nuedling, S. Kahlert, K. Loebbert, R. Meyer, H.Vetter, C. Grohe,evidence for estrogen receptors on cultured astrocytes: colocaliza-Differential effects of 17beta-estradiol on mitogen-activated proteintion with cholinergic receptors, Int. J. Dev. Neurosci. 18 (2000)kinase pathways in rat cardiomyocytes, FEBS Lett. 454 (1999)101–111.271–276.[10] P.M. Hughes, G.M. Wells, V.H. Perry, M.C. Brown, K.M. Miller,
Comparison of matrix metalloproteinase expression during Wallerian [28] C. Patrone, S. Andersson, L. Korhonen, D. Lindholm, Estrogendegeneration in the central and peripheral nervous systems, Neuro- receptor-dependent regulation of sensory neuron survival in de-science 113 (2002) 273–287. veloping dorsal root ganglion, Proc. Natl. Acad. Sci. USA 96 (1999)
[11] R.R. Islamov, W.A. Hendricks, R.J. Jones, G.J. Lyall, N.S. Spanier, 10905–10910.A.K. Murashov, 17beta-Estradiol stimulates regeneration of sciatic [29] S.F. Pu, H.X. Zhuang, D.J. Marsh, D.N. Ishii, Time-dependentnerve in female mice, Brain Res. 943 (2002) 283–286. alteration of insulin-like growth factor gene expression during nerve
[12] T. Ivanova, P. Mendez, L.M. Garcia-Segura, C. Beyer, Rapid regeneration in regions of muscle enriched with neuromuscularstimulation of the PI3-kinase/Akt signalling pathway in developing junctions, Brain Res. Mol. Brain Res. 63 (1999) 207–216.midbrain neurones by oestrogen, J. Neuroendocrinol. 14 (2002) [30] A.J. Reynolds, I.A. Hendry, S.E. Bartlett, Anterograde and retro-73–79. grade transport of active extracellular signal-related kinase 1
[13] I. Jung-Testas, M. Schumacher, P. Robel, E.E. Baulieu, Actions of (ERK1) in the ligated rat sciatic nerve, Neuroscience 105 (2001)steroid hormones and growth factors on glial cells of the central and 761–771.peripheral nervous system, J. Steroid Biochem. Mol. Biol. 48 (1994) [31] K. Sakuma, K. Watanabe, M. Sano, I. Uramoto, H. Nakano, Y.J. Li,145–154. S. Kaneda, Y. Sorimachi, K. Yoshimoto, M. Yasuhara, T. Totsuka, A
[14] C.M. Klinge, Estrogen receptor interaction with estrogen response possible role for BDNF, NT-4 and TrkB in the spinal cord andelements, Nucleic Acids Res. 29 (2001) 2905–2919. muscle of rat subjected to mechanical overload, bupivacaine in-
[15] S. Kousteni, T. Bellido, L.I. Plotkin, C.A. O’Brien, D.L. Bodenner, jection and axotomy, Brain Res. 907 (2001) 1–19.L. Han, K. Han, G.B. DiGregorio, J.A. Katzenellenbogen, B.S. [32] S. Shamash, F. Reichert, S. Rotshenker, The cytokine network ofKatzenellenbogen, P.K. Roberson, R.S. Weinstein, R.L. Jilka, S.C. Wallerian degeneration: tumor necrosis factor-alpha, interleukin-Manolagas, Non-genotropic, sex-non-specific signaling through the 1alpha, and interleukin-1beta, J. Neurosci. 22 (2002) 3052–3060.estrogen or androgen receptors: dissociation from transcriptional [33] P.J. Shughrue, M.V. Lane, I. Merchenthaler, Comparative distributionactivity, Cell 104 (2001) 719–730. of estrogen receptor-alpha and -beta mRNA in the rat central
[16] T. Kubo, T. Yamashita, A. Yamaguchi, K. Hosokawa, M. Tohyama, nervous system, J. Comp. Neurol. 388 (1997) 507–525.Analysis of genes induced in peripheral nerve after axotomy using [34] J. Siironen, E.Vuorio, M. Sandberg, M. Roytta, Expression of type IcDNA microarrays, J. Neurochem. 82 (2002) 1129–1136. and III collagen and laminin beta1 after rat sciatic nerve crush
[17] H. Lee, W. Bai, Regulation of estrogen receptor nuclear export by injury, J. Peripher. Nerv. Syst. 1 (1996) 209–221.ligand-induced and p38-mediated receptor phosphorylation, Mol. [35] M. Singh, G. Setalo Jr., X. Guan, D.E. Frail, C.D. Toran-Allerand,Cell. Biol. 22 (2002) 5835–5845. Estrogen-induced activation of the mitogen-activated protein kinase
[18] B.S. McEwen, Invited review: estrogens effects on the brain: cascade in the cerebral cortex of estrogen receptor-alpha knock-outmultiple sites and molecular mechanisms, J. Appl. Physiol. 91 mice, J. Neurosci. 20 (2000) 1694–1700.(2001) 2785–2801. [36] A.J. Slooter, J. Bronzova, J.C. Witteman, C. Van Broeckhoven, A.
[19] B. McEwen, K. Akama, S. Alves, W.G. Brake, K. Bulloch, S. Lee, Hofman, C.M. van Duijn, Estrogen use and early onset Alzheimer’sC. Li, G. Yuen, T.A. Milner, Tracking the estrogen receptor in disease: a population-based study, J. Neurol. Neurosurg. Psychiatryneurons: implications for estrogen-induced synapse formation, Proc. 67 (1999) 779–781.Natl. Acad. Sci. USA 98 (2001) 7093–7100. [37] A.F. Svenningsen, M. Kanje, Estrogen and progesterone stimulate
R.R. Islamov et al. / Brain Research 966 (2003) 65–75 75
Schwann cell proliferation in a sex- and age-dependent manner, J. target cells in the spinal cord of the armadillo and baboon: aNeurosci. Res. 57 (1999) 124–130. comparative study, Histol. Histopathol. 2 (1987) 143–145.
[38] N. Taleghany, S. Sarajari, L.L. DonCarlos, L. Gollapudi, M.M. [43] P.M. Wise, D.B. Dubal, M.E. Wilson, S.W. Rau, M. Bottner, K.L.Oblinger, Differential expression of estrogen receptor alpha and beta Rosewell, Estradiol is a protective factor in the adult and agingin rat dorsal root ganglion neurons, J. Neurosci. Res. 57 (1999) brain: understanding of mechanisms derived from in vivo and in603–615. vitro studies, Brain Res. Brain Res. Rev. 37 (2001) 313–319.
[39] C.D. Toran-Allerand, L. Ellis, K.H. Pfenninger, Estrogen and insulin [44] C.C. Zhang, D.J. Shapiro, Activation of the p38 mitogen-activatedsynergism in neurite growth enhancement in vitro: mediation of protein kinase pathway by estrogen or by 4-hydroxytamoxifen issteroid effects by interactions with growth factors?, Brain Res. 469 coupled to estrogen receptor-induced apoptosis, J. Biol. Chem. 275(1988) 87–100. (2000) 479–486.
[40] K.L. Tsang, S.L. Ho, S.K. Lo, Estrogen improves motor disability in [45] Y.S. Zhu, D.W. Pfaff, DNA binding of hypothalamic nuclear proteinsparkinsonian postmenopausal women with motor fluctuations, on estrogen response element and preproenkephalin promoter:Neurology 54 (2000) 2292–2298. modification by estrogen, Neuroendocrinology 62 (1995) 454–466.
[41] G. Vita, R. Dattola, P. Girlanda, G. Oteri, F. Lo Presti, C. Messina, [46] D.W. Zochodne, C. Cheng, Neurotrophins and other growth factorsEffects of steroid hormones on muscle reinnervation after nerve in the regenerative milieu of proximal nerve stump tips, J. Anat. 196crush in rabbit, Exp. Neurol. 80 (1983) 279–287. (Pt 2) (2000) 279–283.
[42] F.J. Weaker, P.J. Sheridan, Autoradiographic localization of estrogen