Ovarian dual oxidase (Duox) activity is essential for insect eggshell hardening and waterproofing
Biochemical and functional characterisation of eggshell matrix proteins in hens
-
Upload
independent -
Category
Documents
-
view
0 -
download
0
Transcript of Biochemical and functional characterisation of eggshell matrix proteins in hens
[CANCER RESEARCH 55, 4855-4864, November 1, 1995]
Characterization of the Receptor Protein Tyrosine Phosphatase Gene Product PTPy:Binding and Activation by Triphosphorylated Nucleosides1
Claudio Sorio, Jeannine Menarola, Zhuangwei Lou, Sal LaForgia, Carlo M. Croce, and Kay Huebner2
Jefferson Cancer Institute, Jefferson Medical College. Philadelphia, Pennsylvania 19107
ABSTRACT
A full-length cDNA for a novel isoform of the human receptor tyrosinephosphatase y gene d'I l'I«,\ was overexpressed in Sf9 insect cells, andthe gene product. PTPy, was purified and characterized. The protein wasexpressed as a Mr ~185,000 protein accompanied by a Mr —¿ 120,000putative cleavage product on SDS-PAGE analysis. The protein undergoesjV-linked glycosylation and constitutive phosphorylation of serine residues. When assayed for tyrosine-specific phosphatase activity, PTPy de-phosphorylatcd myelin basic protein at a pH optimum of 7.5 and a A,„of12.6 UM; reduced carboxyamidomethylated and maleylated lysozyme(RCM-lysozyme) at a pH optimum of 6.0 and a k,,, of 12 UM; andp-nitrophenylphosphate with a pH optimum of 5.5 and a A,,, of 3.5 HIM.Phosphatase activity was inhibited by ZnCU and sodium orthovanadate;Mg2*, Mn2% and Ca2* ions were ineffective. The partially purified form of
the enzyme was allosterically activated by triphosphorylated nucleosides,with a preference for purines. This activation was prevented by Mg2*
addition and did not occur when a purified form of the enzyme wasutilized, suggesting that its activation depends on specific activating factors or conformational constraints. Interestingly, PTPy protein was specifically bound by an ATP-agarose matrix through its intracellular domain, suggesting a link between binding of nucleotides and activation ofthe phosphatase.
INTRODUCTION
The demonstration of tyrosine phosphorylation of signaling proteins has had a unique impact on the understanding of processes suchas cellular activation, differentiation, and proliferation. In this context,the role of tyrosine kinases, their mechanisms of action, and theirregulatory pathways have been reviewed extensively (1, 2). Becausemany oncogenes have been shown to possess deregulated kinaseactivity, it has been speculated that enzymes able to antagonize suchactivity could also play an important role in cellular behavior. In thelast several years, a growing number of gene products possessingspecific phosphatase activities has been identified and cloned. As forthe better characterized families of kinases, the phosphatases could beclassified into subfamilies according to phosphoamino acid specificityand the presence or absence of a transmembrane and extracellulardomain (3). PTPy is a member of the receptor-like family of Tyr-specific phosphatases, originally cloned from human brain stem andplacental cDNA libraries by using probes derived from the intracellular domain of CD45 (4) or Drosophila PTP (5), respectively. Withthe exception of PTPß(5) and the Drosophila enzyme, DPTP10D (6),this family of tyrosine-specific phosphatases is characterized by awell-conserved, tandemly repeated, intracellular catalytic domain anda highly variable extracellular region separated by a Hydrophobie
Received 8/17/95; accepted 9/5/95.The costs (if publication of this article were defrayed in part hy the payment of page
charges. This article must therefore be hereby marked advertisement in accordance with18 U.S.C. Section 1734 solely to indicate this fact.' This work was supported in part by National Cancer Institute Grants CA51083 and
CA39860 and by a gift from R. R. M. Carpenter III and Mary K. Carpenter. J. M. wassupported hy National Cancer Institute Training Grant T32-CA09662. C. S. is a recipientof a Dottorato di Ricerca in Biologia e Patologia Molecolare e Cellulare from theUniversity of Verona. Verona. Italy.
2To whom requests for reprints should be addressed, at Jefferson Cancer Institute,Jefferson Medical College, BLSB, Room 1008, 233 South 10th Street, Philadelphia. PA19107.
stretch of amino acids. The "receptor-like" extracellular domains
imply ligand binding capability, which may modulate the enzymaticactivity. Indeed, interesting homologies among selected regions of thereceptor phosphatase extracellular domains and cell adhesion molecules have been documented (3). Recently, two members of thereceptor protein tyrosine phosphatase family, PTPju. and PTP/< havebeen shown to possess homophilic binding capacity in vitro and alsowhen expressed in eukaryotic cells (7-9). This interaction did notseem to alter the enzymatic activity of the catalytic domain of PTP/xwhen assayed in in vitro phosphatase assays.The extracellular domain of PTPy shares a high degree of homol-
ogy with the "CAH'-like" and FNIII domains of PTPf/RPTPß (10-
12); on the basis of these similarities, PTPy and PTP£/RPTPßare theprototype members of a subfamily of receptor tyrosine phosphatases.Interestingly, 3F8, a major rat brain proteoglycan (13), has beenshown to be a variant of PTP£/RPTPß(13-15), suggesting that someform of PTPy may also function as a proteoglycan. The PTPy genemaps to chromosome region 3pl4.2, which is involved in allelic lossin 85% of renal cell carcinomas. The common region of loss encompasses the breakpoint of the constitutional translocation t(3;8) (pl4.2;q24.1), associated with the development of hereditary renal cell carcinoma in a large Italian-American family (16). Furthermore, we havecharacterized a homozygous deletion within the CAH-like domain ofPTPy (17) in a murine tumor cell line, another hallmark of tumorsuppressor genes.To provide reagents and a biochemical background for under
standing PTPy function, we have characterized a variant full-length PTPy cDNA lacking the intracellular juxtamembrane exonof the reported PTPy cDNA, which is expressed as the dominantisoform in most tissues.4 We have overexpressed this PTPyisoform in insect cells, generated and characterized specific poly-clonal antibodies, and have purified and characterized the recombinant protein. Data are presented describing posttranslationalprocessing of PTPy in Sf9 insect cells, a unique pattern of bindingand activation by NTPs and localization of an ATP-binding sitewithin the first catalytic domain of the phosphatase.
MATERIALS AND METHODS
Production of Polyclonal Antibodies. Synthetic peptides P3 (CRNQTEP-SPTPSSPNRT, amino acids 618-633 of human sequence), PU (CQKGN-PKGRQN, amino acids 1005-1014) and P4 (CGSDPKRPEMPSKKPMSRG-DRFSED, amino acids 687-710) were produced by the in-house peptidesynthesis facility and coupled, through an NH2-terminal cystcine, toKLH (Sigma Chemical Co., St. Louis, MO) utilizing the hcterobifunctionalreagent N-succinimidyl 3-(2-pyridyldithio)propionate (Pierce Chemical Co.,
' The abbreviations used are: CAH, carbonic 50 anhydrasc; KLH. keyhole limpet
hemocyanin; FBS, fetal bovine serum; CLAP, 10 mg/ml chymostatin, leupeptin, aprotinin,pepstatin in DMSO; PMSF, phenylmethylsulfonyl fluoride; TCA, trichloroacctic acid;MBP, myelin basic protein; MES, 4-morpholine propanesulfonic acid; RCM-lysozyme,reduced carboxyamidomethylated and maleylated lysozyme; RIPA, radioimmuno-precip-itation assay buffer; NTP. triphosphorylated nucleosides; PAP, potato acid phosphatase;EGF, epidermal growth factor; EGFR, EGF receptor; RSB, reducing sample buffer [62.5mM Tris-HCL (pH 6.8), 1% SDS, 5% glycerol. 0.7% 2-ß-mercaptoethanol].4 Z. Lou, T. Druck, C. Sorio, P. Melotti, A. Nayak, K. Wary, K. Kastury, S. LaForgia,
J. Mendrola, J. Rothslein, and K. Huebner. Regulated expression of a novel receptortyrosine phosphatase gamma isoform, manuscript in preparation.
4855
PTP-y: BINDING AND ACTIVATION BY TRIPHOSPHORYLATED NUCLEOSIDES
Rockford, IL), following the manufacturer's instructions. The KLH-conjugatedpeptides (0.5 mg) were injected into New Zealand White rabbits in Freund's
complete adjuvant for the first s.c. injection, followed by at least three boosts inFreund's incomplete adjuvant. Rabbit sera were subjected to ammonium sulfate
precipitation and dialysis versus PBS, essentially as described in Ref. 18. Portionsof the partially purified immunoglobulin fractions were affinity purified by usingthe specific peptides covalently coupled to CNBr-Sepharose (Pharmacia. Uppsala,Sweden), following the manufacturer's instructions.
Isolation of the Recombinant Baculovirus. A 5.3-kb £coRI fragment,containing a full-length PTPy cDNA from a human kidney tumor-derived cellline, ACHN (American Type Culture Collection, Rockville, MD), was clonedin the appropriate site of a pVL1393 plasmid (Invitrogen, La Jolla, CA). Thenucleotide sequence of the cDNA revealed absence of 87 nucleotides immediately downstream of the transmembrane coding region, relative to the published PTPy sequence. Thus, the predicted protein would be missing the 29juxtamembrane amino acids of the reported sequence (10, 12). Reverse tran-scriptase-PCR and sequencing of the products revealed that this form of PTPymRNA was the majority form in many human and mouse cell types.4 After
cotransfection of the PTPy construct with linearized baculovirus (19) DNA byusing cationic liposomes (Insectin liposomes; Invitrogen), the recombinantbaculovirus was purified by following the manufacturer's instructions.
Propagation of Sf9 Cells. Sf9 cells were grown as suspension cultures inGrace's insect medium (supplied as a powder by GIBCO-BRL, Gaithersburg,
MD), supplemented with lactalbumin hydrolysate, yeastolate, and 10% FBS(insect cell tested grade, Sigma). Titering experiments and infections wereperformed according to standard protocols.Western Blotting and Immunofluorescence. For Western blotting anal
ysis, 2 X 10" cells were directly lysed in SDS-PAGE |RSB] or at 20-30 X IO6
cells/ml, in 1% NP40, 25 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA,10 fig/ml CLAP, 1 mM PMSF, and 1 mM DTT (LB), incubated for 10 min at4°C, and centrifuged 10 min at 15,000 x g. The supernatant and pellet(NP40-insoluble fraction) were treated with RSB to obtain a final Ix concentration, boiled for 5 min, and subjected to SDS-PAGE under reducing conditions. The gel was blotted, and the membrane was blocked in 5% dry milk inPBS for 1-2 h at room temperature. The membrane was rinsed and incubatedovernight at 4°Cin 2 ng/ml affinity-purified anti-P4 IgG in PBS, 1% BSA, and0.1% Tween 20 (PBS-BSA). After three washes of 5 min in PBS, 0.1% Tween20 at room temperature, incubation for l h at room temperature in horse radishperoxidase-conjugated goat anti-rabbit immunoglobulin (diluted 1:15,000 inPBS-BSA), and additional washing, the signal was detected utilizing theenhanced chemiluminescence system (Amersham, Buckinghamshire, UnitedKingdom) exactly as described by the manufacturer.For immunofluorescence, cells were harvested at different time points after
infection, allowed to adhere to 8-well chamber slides (Nunc, Naperville, IL),washed twice with PBS, and fixed for 10 min in methanol at -20°C. The cellswere reequilibraled in PBS, blocked for 15 min at 37°Cin 5% goat serumin PBS, and incubated for 30 min at 37°Cin 5 fxg/ml affinity-purified anti-P4
IgG in the same solution. After extensive washes, the samples were reactedwith a 1:100 dilution of FITC-conjugated goat antirabbit F(ab'), fragment
(Boehringer Mannheim, Mannheim. Germany), and the reaction was prolonged for 30 min at room temperature. After three washes with PBS and threewashes with deionized water, the sample was mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and subjected to confocalimage analysis by using a Zeiss Axiovert 100 microscope coupled to a Bio-RadMRC600 Kr/Ar Laser system (Bio-Rad Laboratories).Tunicamycin Treatment. PTPy-infected cells (2 x IO6) were harvested
from a suspension culture at 48-h postinfection and plated into a 60-mm dishin Grace's insect medium containing 5 pig/ml of tunicamycin (Boehringer
Mannheim). The cells were allowed to incubate for 4 h at room temperature ina humid chamber. The medium was removed, and the cells were washed oncein methionine-deficient Grace's medium and then resuspended in 1 ml of freshmethioninc-free medium supplemented with 10% dialyzed FBS plus tunicamycin. After 1 additional h of incubation, 50 fiCi of [15S]methionine (10
mCi/ml, >37 TBq/mmol Amersham) were added, and the cells were allowed tolabel for 2 h. The cells were then washed three times in PBS and lysed in LBat a concentration of 4 X IO6 cells/ml. The clarified lysate was immunopre-
cipitated by the addition of 10 ¡¿Iof ImmunoPure immobilized protein A(Pierce) prebound with saturating amounts of anti-P4 affinity-purified IgG.The immunoprecipitate was washed three times with LB and once with TBS
[25 mM Tris-HCl (pH 7.5) 150 mM NaCl, and 1 mM DTT]. RSB (50 ¿il)wasadded, the samples were boiled for 5 min, and the supernatants were run on 6%SDS-PAGE. The gel was treated with PPO (22% in DMSO), washed, dried,and exposed to X-ray film.PAP Treatment. The immunoprecipates described above were resus
pended in 48 /il 0.1 M citrate buffer (pH 5.6), and 2 /mlof a PAP (grade II:Boehringer Manneheim) solution, prepared by a 1:150 dilution of a 10 mg/mlstock into 6% BSA, were added and incubated at room temperature for 15 min.The reaction was stopped by addition of 10 /¿Iof 5 x RSB. After boiling, thesamples were fractionated on SDS-polyacrylamide gels and treated with PPOas described.Orthophosphate Labeling and Phosphoamino Acid Analysis. I'll'-.
infected cells (20 X I0h) were harvested at 48 h, washed once in PBS, andincubated in 5 ml of phosphate-free Grace's insect medium supplemented with
10% dialyzed FBS and incubated for 1 h at room temperature in a humidchamber. The medium was replaced with fresh medium containing 0.5-1.0mCi/ml 32P (370 MBq/ml) in dilute HO (Amersham). After 5-8 h, the cellswere lysed and immunoprecipitated as described above. After 7.5% SDS-PAGE, the gel was washed in excess dH2O, dried onto 1 mM Whatman paper,and exposed overnight. The bands corresponding to both the upper Mr~ 185,000 and lower Mr ~ 120,000 products were cut out and processedindependently. The gel slices were rehydrated in 500 jxl of freshly preparedrehydration buffer (50 mM NH4HCO„0.1% SDS, 5% ß-mercaploethanol) for10 min at room temperature, homogenized and boiled for 5 min, and proteinswere extracted on a rotating wheel for 2 h at room temperature. The soliddebris was removed by passing through glass wool, and the eluate was clarifiedby centrifugation. The proteins were TCA (10% final concentration)-precipi-tated overnight at 4°Cwith BSA (50 fig/ml) as a carrier. The precipitate was
washed extensively with acetone and air dried. Two hundred /xl of 5.7 MHC1were added and incubated at 110°Cfor 90 min to hydrolyze the polypeptide
chains. After hydrolysis, the samples were dried in a vacuum centrifuge, and10 fig of phosphorylated cold Ser, Thr, and Tyr ( 1 mg/ml in dH2O) were addedto the samples, followed by drying. The pellets were resuspended in 5 n\ of pH3.5 buffer (pyridine:glacial acetic acid:water, 4:40:756) and spotted onto aKodak chromagram sheet (microcrystalline cellulose adsorbent without fluorescent semi indicator, Eastman Kodak Co.). The plate was electrophoresed ona Hunter thin layer electrophoresis system (model no. HTLE-7000) for 40 minat 1.3 kV. The spots were visualized after spraying with ninhydrin-ethanolsolution and baking at 80°C.The cellulose plate was exposed by using NENDuPont's REFLECTION Intensifying screen, NEF-491, and REFLECTIONNEF-496 autoradiographic film for 21 days.Phosphatase Assay. MBP and RCM-lysozyme (GIBCO-BRL) were resus
pended at 10 mg/ml in H2O, and 2 mg aliquots were resuspended in kinasebuffer [20 mM HEPES (pH 7.5) 2 mM DTT, K) UM ATP, 10 mM MgCI, and100 ;j.Ci [3:P)ATP] to a final volume of 400 >il. The reaction was started withthe addition of 20 units of p60srt (Oncogene Science, Cambridge, MA) andincubated for 12-16 h at 25°C.MBP was separated from free nucleotides byTCA precipitation (10% final concentration, 1-3 h at 4°C), followed by
extensive washing with 10% cold TCA and acetone. After air drying, theresulting pellet was solubilized to the original volume (0.2 ml) in 20 mMTris-HCl. pH 7.5. RCM-lysozyme was separated from free nucleotides by gelfiltration by using a PD10 column (Pharmacia) and equilibrated in 20 mMMES, pH 6.0. PNPP (Sigma) was diluted in 20 mM MES (pH 5.5), 10 mM DTTto the appropriate concentration, and pH from a 1 M solution in H,0.The membrane fraction of Sf9 cells was prepared from 48-h haculovirus-
infected cells, harvested, and washed twice in PBS. The pellet was resuspendedin 10 mM HEPES-NaOH (pH 7.9), 1.5 mM MgCI2, 10 mM KC1, 2 mM DTT, 1mM PMSF, 10 /j.g/ml CLAP, and lysed by several strokes with a Douncehomogenizer. The lysate was centrifuged for 10 min at 500 x ¡>to removenuclei and the insoluble cytoskeleton matrix. The supernatant was centrifuged(100,000 X g, for 1 h), the pellet fraction was resuspended in 20 mMTris HC1(pH 7.5), 150 mM NaCl, 1 mM EDTA, 2 mM DTT, 1 mM PMSF. 10 ng/mlCLAP, and 0.2% Triton X-100 and stored at -70°C after aliquoting. WhenMBP and RCM-lysozyme served as substrates, the assay was performed in afinal volume of 80 fil. Briefly, the sample containing either the purifiedphosphatase or the membrane fraction was resuspended at the appropriateconcentration (0.5—2¿tgof protein for the membrane fraction) in 30 fxl of theappropriate assay buffer [20 mM Tris-HCl (pH 7.5) 10 mM DTT for MBP, and20 mM MES (pH 6.0), and 10 mM DTT for RCM-lysozyme], in the presence
4856
PTPy: BINDING AND ACTIVATION BY TRIPHOSPHORYLATED NUCLEOSIDES
or absence of the indicated effectors. The assay was initiated by the additionof 50 (xlof substrate at the appropriate concentration (3.2-13 JIM)and stoppedat the appropriate time by addition of 10 /xl of 100%TCA with 10 jxl of 1%BSA as the carrier protein. After at least a 2-h incubation at 4°C,the sampleswere centrifuged for 10-15 min at 15,000 X g, and 70 til of supernatant wasadded to 4 ml of Scintiverse (Fisher Scientific, Springfield, New Jersey). Theradioactivity was quantified in a Rackbeta 1209 liquid scintillation counter(Wallac, Inc., Gaithersburg, MD). When PNPP was the substrate, 10 /xg oflight membrane fraction was resuspended in 50 fil of 20 mMMES (pH 5.5), 10mMDTT. The reaction was initiated by addition of 100 fil of the appropriateamount of substrate diluted in the same buffer. The assay was stopped after 20min at 25°Cby the addition of 100 ¿xlof a 1 Msolution of Na,CO, and the96-well plate was read at 405 nm in a Bio-Rad model 3550 multiplate reader.The specific phosphate release associated with the light membrane fraction ofPTP-ybaculovirus-infected cells was arbitrarily set to 100%. The correspondent fraction from the control cells (Sf9 cells, infected under the sameconditions with the recombinant baculovirus expressing either the extracellulardomain of murine Ptpy or wild-type baculovirus) gave no phosphate releasefor PNPP and a 15-37% release for MBP and RCM-lysozyme. This resultindicates the presence of endogenous light membrane-associated tyrosinephosphatase activity in insect cells.In Vitro Transcription—Translation and ATP/ADP Agarose Binding.
Full-length mouse PTPy cDNA (LO)and the constructs LI, L2, L3, and L7(see Fig. 6 for description of the cDNA fragments) were cloned in BluescriptSK- vector (LO) and SK+ (all others). A c-myc epitope (EQKLISEEDL) wasinserted at the 5' end of the constructs L1-L7. One fig of DNA was in vitrotranscribed and translated by using a rabbit reticulocyte lysate-based system(troponin T coupled reticulocyte lysate system, Promcga, Madison, Wl),following the manufacturer's instructions. One /xl of the reaction (total) wasadded directly to a tube containing 1X RSB and treated at 95°Cfor 5 h beforebeing subjected to SDS-PAGE in a 7.5% gel. The same amount of material wasadded to a tube containing 150 |xl of 25 mMsodium-acetate buffer, pH 6.0 (25mMNaCl, 1% BSA, 0.5% Triton X-100, 1mMDTT, 1mMPMSF, and 10 fxlof a 1:1 slurry of ATP- or ADP-agarose; Sigma). After 1-2 h of incubation ona rotating wheel, the pelleted material was washed once with the pH 6.0 bufferand three times with RIPAbuffer. After SDS-PAGE, the gels were treated withAmplify (Amersham), following the manufacturer's instructions.ATP-Agarose Binding. Baculovirus-infected lysates (-0.5 ml, 20 X IO6
cells/ml/reaction), prepared as for immunoprecipitation, were incubated in thepresence of 30 >xlof a 1:1 slurry of ATP- or ADP-agarose/H2O(Sigma) for 1h at 4°Cand treated exactly as for the immunoprecipitation experiments. Formetal ion competition experiments, 10 mm MgCU, MnCI:, and CaCl2 wereadded either to the lysate at the beginning of the matrix binding incubation orafter the finalwashing step and then incubated for 15-30 min at 4°C.These lastsamples were then washed twice with RIPA buffer, and (after the addition of10 fxlRSB) all samples were subjected to SDS-PAGE analysis and blotting ofthe protein to the membrane; the nitrocellulose filter was reacted with theanti-P4 antibody as described above, and the antibody binding was detected byusing the enhanced chemiluminescence system (Amersham), following themanufacturer's instructions.
RESULTS
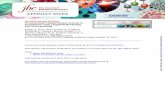
Polyclonal Antibodies against Human and Mouse I' I l'y. Wegenerated polyclonal antibodies against the synthetic peptides P3, P4,and PU, matching amino acids 618-633, 687-710 of the extracellularregion, and 1005-1014 of the intracellular region of the cDNA-derived PTP-y amino acid sequence. The peptides, which containedNH2-terminal cysteines, were coupled to KLH with the bifunctionalreagent SPDP. Sera were collected from rabbits and titered in ELISAassays against serial dilutions of specific peptides. When titers weremaximal, the immunoglobulin fractions were purified through ammonium sulfate precipitation and affinity chromatography. The antibodies reacted specifically against PTP-y expressed from recombinantbaculovirus, as demonstrated for anti-P4 in Fig. 1. We have not beenable, thus far, to demonstrate unequivocably the detection of endogenous PTPy in mammalian cells by these antibodies. Our interpreta-
V B12 123
24 48 721 24 48 72h 24 48 72 | 24 48 72 | 24 48 72hkDa
- 200-
- 97 -
f -
Fig. 1. Expression of recombinant PTPy in insect cells. A. time course of expressionof PTP-y: wild-type baculovirus-infecled cells (2 x 10" cells/lane) [Lanes I, 24-72 h (left3 lanes of A)] or PTPy recombinant baculovirus-infected cells (2 x 10" cells/lane) [Lanes2, 24-72 h (right 3 lanes of A)] at different time points postinfection were directly lysedin RSB and fractionated by SDS-PAGE, followed by Western blotting and proteindetection by using anti-P4 peptidc antibody. A specifically reacting band at M, ~ 185,000appears between the 24- and 4fi-h time points (Lane 2, compare 48 h lane to Lane I, 48h) in the recombinant baculovirus-infected Sf9 cells. By 72 h, proteolytic cleavageproducts of M, 120,000 and M, 110,000/114,000 are abundant (Lane 2, 72 h). B. timecourse of appearance of PTPy in NP40-insolublc and NP40-soluble fractions: wild-type orPTP-y recombinant baculovirus-infected cells were lysed in 1% NP40, and the NP40-soluble (supernatant) and NP40-insoluble (pellet) fractions were separated by centrifuga-tion, resuspended to 1 X RSB, and boiled and fractionated by SDS-PAGE, followed byWestern blotting and PTP-y detection by anti-P4 antibody. Lanes I, 24-72 h, containedNP40-insoluble protein from wild-type baculovirus-infected cells at (he three time pointspostinfection. Lanes 2, 24-72 h, contained NP4()-insolublc protein from PTP-y recombinant infected cells at the three time points postinfection and ÕMnes3, 24-72 h, containedNP40-soluble protein from the three time points postinfection. The 48- and 72-h timepoints under Lane 2 show that most of the PTPy protein expressed in insect cells isNP40-insoluble; compare the Lunes 2, 48 and 72-h NP40-inso!uhle fractions to Lanes 3,48 and 72 h NP40-soluble fractions. Note that the M, - 185,000 full-length PTP-y productis present in the soluble fraction (Lane 3, 72 h) but it appears earlier and in larger amountsin the insoluble fractions (Lanes 2, 48 and 72 h). Similar experiments sometimes showedpresence of the full-length M, -185,000 PTP-y band in the soluble fraction by 48-hpostinfection. C indirect immunofluorescent detection of PTPy in insect cells from0-48-h postinfection. Cells were harvested at the 0-, 24-, 36-, and 4K-h time points, andPTPy expression was delected by immunofluorescence analysis with the anti-P4 peptideantibody. Surface staining was visible at the 36-h time point. Cylosolic localization wasapparent at the later time point, probably due to saturation of the processing machinery.kDa. kilodalton.
tion, supported by observations from immunoprecipitations of meta-bolically labeled Sf9 cells infected with recombinant baculovirus, isthat the antibodies are characterized by a relatively low affinity for theprotein, requiring overexpression to enable detection.Generation of Recombinant Baculovirus and Expression of
Recombinant Protein in Sf9 Cells. Sf9 cells were infected with ahigh multiplicity (5-10 viral particles/cell) of recombinant baculovirus containing the full-length, alternatively spliced PTPy cDNA (Fig.1), under the control of the polyhedrin promoter; at 24-, 48-, and 72-hpostinfection, cells were harvested and analyzed by Western blottingor immunofluorescence staining by using the anti-P4 antibody. Themolecular weight for the PTPy protein estimated from the predicted
4857
PTP-y: BINDING AND ACTIVATION BY TRIPHOSPHORYLATED NUCLEOSIDES
amino acid sequence is —¿ 162,000.Fig. 1 illustrates the time course ofprotein production: proteins with relative molecular weights-185,000, 120,000, and 110,000/114,000 were detected at the 72-htime points (Fig. \A, Lane 2). In a number of similar experiments, theM, —¿ 120,000protein was the only one consistently present (data notshown) and might, therefore, represent a specific processing product.When anti-P4-treated membranes were stripped and reacted withanti-P3 or anti-PU, the same pattern was observed (data not shown),indicating that the putative proteolytic process preserves the regionbetween amino acids 618 and 1014. Because this region is predictedto have a molecular weight of —¿ 45,300,putative cleavage site(s) mustbe located 5' or 3' to these positions.
Fig. 1, panel B, shows the relative resistance of the protein productto extraction with nonionic detergent, with most of the protein retained in the NP40 insoluble fraction (Lane 2), particularly at the 48-htime point. Soluble protein begins to appear between the 48 and 72-htime points. This relative insolubility is probably due to intracellularaggregation, as suggested by the confocal image of immunofluores-cence at the 48-h time point (Fig. 1C). When Triton X-100, N-octylglucoside, Briji 35, and 3-[(3-cholamidopropyl)dimethylamino]-1-propanesulfonate were tested, essentially the same degree ofextraction was observed. Fig. 1C shows that the recombinant proteinwas expressed on the cell surface, as expected from its receptor-likestructure. Specific staining at the cell surface was most clearly observed at 36 h, the earliest time point for which expression wasobserved by immunofluorescence and a time when the protein synthesis and processing apparatus of the cells was probably not saturated. At the 48-h time point, intracellular aggregates began to appear(Fig. 1C).Purification of PTPy. Full-length PTP-y, overexpressed in the
baculovirus system, was only partially soluble in nonionic detergents,as shown above in Fig. Iß.Denaturating reagents caused irreversibleloss of enzymatic activity. The protein had a tendency to aggregateand stick to column filters during ionic exchange chromatography,decreasing the already low yield. Therefore, we used high performance affinity beads, conjugating a saturating amount of affinity-purified anti-P4 antibody to protein A-Sepharose beads. These beadswere used to immunoprecipitate orthophosphate-labeled PTPy proteinas illustrated in Fig. 2. Fig. 2, Lane 2, shows the Coomassie blue-stained anti-P4 precipitate. The control immunoprecipitation performed with a nonspecific rabbit IgG fraction did not show thespecific bands (Fig. 2, Lane 1). The M, —¿ 50,000band, visible inLanes 1 and 2, represents the heavy chain of rabbit IgG utilized forimmunoprecipitation. Lanes 3 and 4 show the autoradiogram of Lanes1 and 2. Although this immunoprecipitation procedure could not bereadily scaled up, because of the limited availability of the affinity-purified antibody, small scale purification was used in all of thefollowing experiments. This procedure consistently provided enzy-matically active PTPy protein with an estimated purity exceeding90%.Posttranslational Processing of PTPy. Because newly synthe
sized proteins are often posttranslationally modified and such modifications can dramatically affect the fate and function of the proteinproduct, we sought evidence for posttranslational processing of PTPyby AMinked glycosylation and phosphorylation. We pretreated PTPyinfected Sf9 cells with tunicamycin, an inhibitor of the TV-linkedglycosylation process. After 4 h of incubation, 50 /xCi/ml of [35S]me-thionine were added, the incubation was prolonged for an additional2 h, and the cells were washed and lysed. The lysates, from cellstreated and untreated with the drug (Fig. 3A, Lanes 2 and /, respectively), were immunoprecipitated with anti-P4 antibody covalentlyconjugated to CNBr-Sepharose beads. Fig. 3A, Lane 2, shows anestimated M, 10,000 decrease in the size of the PTPy protein from
1 2 3 4kDa
—¿ 200
—¿ 97
—¿ 46Fig. 2. Affinity purification of phosphorylatcd rccombinant PTPy. Immunoprecipita
tion from 4 X 10" ("PJ-labeled PTPy expressing Sf<)cells at the 48-h lime point by usingprotein A-Sepharose beads coupled with nonimmune rabbit IgG (Lane I) or specificanti-P4 (Lune 2) antibody. Left panel, Coomassie blue-stained gel with visible protein atM, ~185.(XK) and 120.000 in the anti-P4 antibody precipitated lane. The protein has beenpurified an estimated >90% based on Coomassie staining (Ltine 2). Limes 3 and 4, the gelafter exposure to X-ray film overnight. Two hands, corresponding to the Coomassieblue-stained bands present in Lane 2, were phosphorylated, as seen in Lane 4. kDa,kilodalton.
tunicamycin-treated cells, indicating the occurrence of /V-glycosyla-tion. A similar decrease in molecular weight was evident in immu-noprecipitates of PTPy treated with endoglycosidase F, which specifically hydrolyzes /V-glycans from glycoproteins (data not shown).
Phosphorylation of polypeptides at serine-threoninc or tyrosineresidues is another widely occurring modification involved in enzymatic activation and protein-protein association. Treatment of theimmunoprecipitates with the enzyme PAP resulted in a M, decrease of8,000-10,000 of PTPy from both tunicamycin-treated and untreatedcells (Fig. 3A, Lanes 4 and 3, respectively), indicating that the immunoprecipitated PTPy that was used as PAP substrate had beenphosphorylated in the Sf9 cells. Endogenous phosphorylation ofPTPy protein in Sf9 cells was already demonstrated by [32P]-]abelingof the recombinant baculovirus-infected cells, followed by immunoprecipitation with control and anti-P4 antibodies as shown above (Fig.2, Lanes 3 and 4). To determine whether Ser/Thr or Tyr residues werethe target of endogenous phosphorylation, the [•12P]-labeledimmuno
precipitated M, 185,000 and 120,000 bands were cut from a gelsimilar to that shown in Fig. 2, Lane 4, and the protein was hydrolyzedand analyzed for the phosphoamino acid content. Fig. 3ßshows thatphosphoserine was the only phosphoamino acid detected in the A/r185,000 protein, and the same results were obtained for the 120,000protein (data not shown). The same pattern of phosphorylation wasobserved when the murine cDNA of the full-length phosphatase wasexogenously expressed in embryonic stem cells.5Enzymatic Characterization of PTPy. The PTPy gene product
was predicted from the cDNA sequence to be a Tyr-specific phosphatase. To demonstrate Tyr phosphatase activity, we incubated affinity-purified recombinant protein in the presence of either [32P]-labeled Tyr or [32P]-labeled Ser[12P]-labeled Thr-phosphorylated
? Unpublished observation.
4858
PTPy BINDING AND ACTIVATION BY TRIPHOSPHORYLATED NUCLEOSIDES
Fig. 3. Posttrunslational processing of PTPy. A,PTPy recombinant haculovirus-infected Sf"9 cells(2 X 10fl cells, 4K h postinfection) were treated inthe presence (+) (Lanes 2 and 3) or absence (-)(Limes I and 4) of tunicamycin. An estimatedMt 10,000 shift in molecular weight was observedas a consequence of the treatment. When the sameimmunoprecipitates were treated with PAP (Lemcs3 and 4), a further shift of M, 8,000-10,000 wasevident, suggesting that phosphorylation occurredin this protein. Noie that the prolein was expressedas a doublet in the tunicmamycin-untreated samples due to incomplete ^-linked glycosylation,probably because of saturation of the enzymaticmachinery as a consequence of the protein overex-pression. B. phosphoumino acid (/*) analysis of theM, ~-185,000 band, from a sample equivalent tothat shown in Fig. 2. Lune 4, showing that Ser wasthe only phosphorylated amino acid in this protein.The same result was obtained with the Mf-120,000 band (data not shown). kDti, kilodalton.
Tunicamycin -PAP
B
200 kDa-
1234
1*^P-Ser—P-Thr*-
P-Tyr*«—
Partiallyhydrolyzedpeptide
MBP. Under the same experimental conditions, the Tyr-phosphoryl-ated substrate gave a ~24% specific 12P release, whereas the SerAThrphosphorylated substrate gave only —¿ 1.4%specific release (data notshown). We also determined the pH optimum for activity toward thesubstrates MBP, RCM-lysozyme, and PNPP. With MBP as a substrate, the optimal pH was 7.5, shifting to a more acidic 6.0 forRCM-lysozyme and 5.5 for PNPP (data not shown). All additionalexperiments were performed at the pH optimum for the respectivesubstrate. The Km for MBP was calculated to be 12.6 JU.M(;i = 3), forRCM-lysozyme 12 (n = 2), and for PNPP 3.5 rnw (n = 2). We thenanalyzed the response of PTPy to a variety of effectors known to havedifferent impacts on various members of the receptor tyrosine phos-phatase families. Table 1 summarizes the effects of these chemicals onthe activity of various PTPy preparations. Most of the compoundstested had no effect on PTPy activity, ZnCK and sodium orthovana-
Tahle 1 Effect of various compounds on PTPy activityPhosphatase activity was measured with MBP and RCM-lysozyme for the membrane
fraction and with MBP for the affinity-purified fraction of PTPy infected Sf9 cells (andcontrol cells in the first row), as described in "Materials and Methods." Negative control,wild-type baculovirus-infected cells, membrane fraction; untreated, recombinant PTPybaculovirus-infected Sf9 cells, membrane fraction. Values ±SD are given as a percentageof the control activity measured without additions.
MembranefractionNegative
controlUntreated0.5
mMCaCl20.5mMMgC'i,0.5
mMMnCU0.5mMZnCl/O.ImMZnClî0.01
mmZnCI2i.omMEDTA"0.5mMSpermidine0.1mMsodium-orthovanadate1.0mMsodium-orthovanadate0.2mMATP0.2mMADP0.2mMAMP0.2muadenosine0.2mM ATP/1 mM MgCI2MBP37
±10 (n -10)°100103±5(n
=3)103±8 (n «3)101±5(n
=5)<10(n=2)118±
10(n =2)102±8 (n =2)105±7 (n =2)103±3 (n =3)<10
(n =3)<10(n =3)180±27(n
=10)122+14(n =6)100
±13 (n =4)95±12(n =3)103±14(n =3)"
Parentheses, number of experimentsperformed''nd. not determined.RCM-lysozyme25
±7 (n =3)10088±9(n
=2)«6±M(n =2)92±8 (n =2)<10
(n =2)<10(n =2)75
±6 (n =2)ndnd<20
(n =2)<20(n =2)213±19(n
=2)ndndndndfor
each conditionAffinity
PurifiedMBP<7
(n =3)100I14±20(n
=2)H0±23(n=2)85
±5 (n =2)<7(n =2)ndndnd118±28(n
=3)<7(n =2)<7(n =2)117
±31(n =3)118±10(n =2)90
±5 (n =2)87±7 (n =2)100±9(n
= 2)
date had a powerful inhibitory effect, although ZnCK was more activewhen RCM-lysozyme was the substrate. No compound tested had theability to activate the purified enzyme. When the 1(K),(KM)X gmembrane fraction of recombinant PTPy-infected Sf9 cells was thesource of the enzyme, all the effectors showed results comparable tothose observed with the affinity-purified PTPy, with the notableexception of ATP, which stimulated the phosphatase activity towardboth MBP and RCM-lysozyme substrates (see Table 1). We examinedthis phenomenon in detail, and in the course of experimentation,noticed variability in the response to ATP as a consequence of slightdifferences in the ATP preparations (possibly due to variable rates ofspontaneous hydrolysis) and variation in storage time of the PTPymembrane preparations. To minimize the variability in the study ofeffects of the phosphorylated nucleosides, the experiments shown inFig. 4 were performed under established optimal conditions: a singlemembrane preparation, a new stock of the four NTPs of the highestpurity grade, purchased from the same company (10 mM concentrationstocks) and not subjected to multiple freeze-thawing cycles. Thephosphatase activities obtained under the optimized conditions were amean of duplicate measurements that did not vary >5%. Results of thedetailed analysis of effects of various nucleoside compounds on PTPyactivity in membrane fractions are summarized in the set of experiments shown in Fig. 4. Briefly, the experiments demonstrated thefollowing points: (a) the maximal activation by NTPs in this set ofexperiments reached 4.4 times that of the control, as illustrated in Fig.4£;(/;) a comparison between the membrane fraction and the affinity-purified form of PTPy demonstrated the requirement for the membrane form of the enzyme to detect the ATP-mediateil activation (Kig.4/1); (c) a strict dose dependence of the effect of ATP on enzymeactivity was observed (Fig. 4B); in the range from 0.2 to 0.8 mM therewas no additional increase of activity (data not shown). The lack ofactivation by 0.2 mM ADP and AMP confirms the specificity of theeffect; (d) both triphosphorylated purines and pyrmidines possessedactivating capability (with a preference for the former) and the com-plexing of Mg"+ to the NTPs had a powerful inhibitory effect on the
activation, without affecting the basal activity, as illustrated in Fig.4D; in addition to ADP, GDP had a marginal activating effect compared to GTP (Fig. 4C); (e) the activating effect was independent ofATP hydrolysis because the nonhydrolyzable analogue, ATPyS, was
4859
PTP-y: BINDING AND ACTIVATION BY TRIPHOSPHORYLATED NUCLEOS1DES
0.2 mM ADP
Mg/ATP
0.2 mM ATP
1mM MgCI
D Ip FTP
0 |p ECDQ.
® membr. PTP <SQ membr. ECD E
100 200 300 400
% INCREASE
0 100 200 300 400 500
% INCREASE
0.2 mM GDP
0.2 mM QTP
0.2 mM ADP
0.2 mM ATP
pyr ¡midinns
purines
0.2 mM CTP
D - UgCI2
M » MgCI2
100 200 300 400 500
% INCREASE
100 200 300 400 500
% INCREASE
0.2 mM ATP-y-S -
0.2 mM ATP -
•¿ 491
12.5
7.5-
1 /V
(y = 0.042«+ 3.030;
—¿ I—100
D l/ls] - ATP
O l/[s] + ATP
200 300O 200 400 600
% INCREASEFig. 4. Effect of NTPs on PTPy phosphatase activity. All the figures are representative of at least two experiments, performed in duplicate, with 8 JJ.MMBP as substrate at 30°C
for 3 min. The values given are averages, and the duplicate values did not vary from each other >5%. The basal activity was set at 100%, with the effect of NTPs expressed as apercentage of increase relative to the untreated sample. A, the effect on the phosphatase activity was specific for the membrane preparation and not explained by effects on theendogenous tyrosine phosphatase activity: Ip PTP, anti-P4 peptide immunoprecipitated from lysate of cells infected with PTPy recombinant baculovirus; Ip ECD, immunoprecipitatedfrom cells infected with a recombinant baculovirus expressing the extracellular domain of murine PTPy, as a negative control; membr. PTP. 100,000 x g fraction of PTPy infectedSf9 cells prepared as described; membr. ECD, 100,000 X g fraction from Sf9 cells infected with the extracellular domain recombinant baculovirus. Note the lack of activation by ADPand the inhibitory effect of Mg2* ions, which by themselves do not alter the basal activity, ß,dose-response curve in presence of increasing amounts of ATP (0.025-0.2 mM). Noincrease in activity was observed when ADP and AMP (both at 0.2 mw) were used as effectors. C, comparison of the effect of triphosphorylated and diphosphorylated nucleosides,indicating the importance of the third phosphate group for the response (see A and B as well). D, effect of 0.2 mM triphosphorylated purines and pyrimidines in presence (+) or absence
4860
PTPr BINDING AND ACTIVATION BY TRIPHOSPHORYI.ATCD NUCLEOSIDES
12345678—¿ 220
—¿ 97—¿ 69
—¿ 50Fig. 5. Specific binding of PTP-y to ATP-agarose matrix and competition by metal ions.
PTP*y recombinant baculovirus-infected Sf9 lysale was affinity purified with ADP-agarose (Lane I), ATP-agarose (Lane 2) or in the presence of 10 mM MgCl2 (Lane .Î),MnCI, (Lane 4). or CaCl, (Lane 5). The affinity-purified material used in ¡Mne2 wasrcsuspcnded in RIPA buffer containing a 10 imi final concentration of the metal ions ofLanes 3-5. After 15-30 min of incubation and two additional washes, all the samples wereblotted and reacted with an affinity-purified anti-PTPy (anti-P4 peptide) antibody. Themetal ions prevented PTP-y binding to the matrix (Lanes 3-5) but did not release thecomplex when added after binding of the protein to ATP had occurred (Lines 6, 7, and8: Mg2*, Mn2*, and Ca2* ions, respectively).
as powerful as ATP in eliciting the response (Fig. 4E): and (/') analysisof the kinetics of the ATP effect showed that ATP induced a —¿ 2-foldincrease of Vmax without significantly affecting the Km', Fig. 4F,illustrates a representative kinetic experiment (of the three performed), a Lineweaver-Burk plot of phosphatase activity in the presence, (+) or absence (-) of 0.2 ITIMATP, with MBP as a substrate. Thelines linking the experimental values (±ATP) intercept the abscissa(I/A",,,) very close to each other but intercept the ordinate (l/Vnlax) far
apart. The values of activation upon ATP treatment were; Km,1.18 ±0.19-fold activation over control; Vmax2.0 ±0.26-fold activation over control (average ±SD from three independent experiments).PTPy Binds Specifically to an ATP-Agarose Matrix. After the
observation of the stimulation of enzymatic activity by NTPs, weinvestigated PTPy binding to a representative nucleotide by performing an affinity purification experiment. We also investigated the effectof metal cations, which have been shown to prevent ATP-inducedactivation, on PTPy binding to the ATP-agarose matrix. As describedin "Materials and Methods," we utilized lysates from wild-type bacu-lovirus and PTPy recombinant baculovirus-infected cells harvested atthe 60-h time point. Briefly, cellular lysates (0.5 ml, 20 X 1()Kcells/mlin LB) were incubated in the presence of either ATP-agarose orADP-agarose matrix. ATP-agarose samples were incubated in thepresence of 5-10 HIM Mg2+, Mn2+, or Ca2+ ions or incubated for15-30 min ¡nthe presence of 10 HIM concentrations of the samecompounds after matrix binding and RIPA washes. After incubationand washes, agarose pellets containing bound proteins were electro-phoresed, blotted, and PTPy protein was detected by using anti-P4antibody. As shown in Fig. 5, anti-P4 antibody detected the presenceof PTPy protein in the sample derived from incubation with ATP-agarose (Fig. 5. ¡¿me2) but not in the sample derived from incubationof the PTPy lysates with ADP-agarose (Fig. 5, Lane I). Incubation inthe presence of the metal ions prevented PTPy from binding to thematrix (Fig. 5, Lanes 3-5). Treatment with the same metal ions afterbinding did not induce PTPy release, indicating that, under these
experimental conditions, the ATP-binding site(s) are no longer accessible to these compounds (Fig. 5, Lanes 6-8). Samples derived fromthe incubation of ATP-agarose with wild-type infected lysates did notshow any reactivity (data not shown).The Dl Domain of PTPy Binds Specifically to an ATP-Agarose
Matrix. To localize the ATP-binding region, we first demonstratedthat murine PTPy protein was able to bind ATP-agarose when thefull-length construct LO was expressed in vitro utilizing a reticulocytelysate system; we then performed similar experiments by using constructs containing various domains derived from the full-length murine PTPy cDNA. The constructs are illustrated in Fig. 6ß,and resultsof the binding experiments are shown in Fig. f>A,which demonstratesthat: (a) the full-length form of murine PTPy, translated from the LOconstruct, specifically binds to ATP agarose; (b) polypeptides translated from all constructs encoding intracellular region segments, LI,L2, and L3, specifically bind to ATP-agarose matrix; and (r) thepolypeptide encoded by construct L7, including nearly the entireextracellular region, does not bind to the matrix under the sameexperimental conditions.
The results of these binding experiments allowed localization of aminimal binding region that encompasses the first catalytic domain I.We can not exclude, with the available constructs, the presence ofanother binding site in the homologous domain II.
DISCUSSION
Transmembrane receptor tyrosine phosphatascs represent a growing family of enzymes the structural features of which suggest a rolein the control of cellular phosphotyrosine balance. The involvement ofthe extracellular domains in the regulation of the enzymatic activity,the identification of physiological substrates, as well as additionalinteracting molecules, are critical areas of investigation for establishing functions for this class of Tyr phosphatases.
PTPy represents a particularly interesting subject of investigation: (a) it is a broadly expressed enzyme, according to mRNAanalyses (10, 20), unlike the closely related PTP£/RPTPß,whichhas been reported to be expressed solely in specific regions of thebrain (11); such a broadly expressed enzyme could affect themetabolism of many different cell types, possibly through interactions with unique classes of ligands; (h) the chromosomal locationof the PTPRG gene (20, 21) and studies showing loss of heterozy-gosity of the gene in kidney tumors (16) have suggested PTPy asa candidate tumor suppressor gene in the chromosome region3pl4.2; and (c) a unique extracellular domain distinguishes thisenzyme from the majority of other transmembrune phosphatases,suggesting a possible interaction with a distinct class of ligands;this suggestion is supported by the recently reported interaction ofthe extracellular domain of the related PTPf/RPTPß with tenascinand contactin (13-15, 22).
To establish a basis for investigation of a function for the PTPyprotein, we have characterized the baculovirus-cxpressed PTPy protein encoded by a full-length PTPy cDNA isoform. In parallel withthese studies, we have observed that with oligonucleotide primersflanking the transmembrane region, two reverse transcriptase-PCRproducts were obtained from RNA of most murine and human cell
(-) of 1 mM Mg2* ions, which form complexes with these compounds. All the compounds activate the phosphatase, with purines more effective than pyrimidines (activation byATP/GTP = 4.16 ±0.27-fold increase versus untreated control, n = 4; activation by TTP/CTP = 2.37 ±0.05-fold increase WT.VH.Vuntreated control, n = 2). The presence in the assaybuffer of Mg2* ions prevented the activation by these compounds. £.activation occurs in the presence of the ATP analogue. ATPyS. f-'. kinetic analysis of ATP effect on phosphataseactivity (Lineweaver-Burk plot). Membrane fractions (0.5 /ig) from PTPy-expressing cells were incubated in the presence (+) or absence (-) of 0.2 mM ATP and reacted with theindicated amount of substrate (from 2.5 to SO /AMMBP) for 3 min. ATP induced a 2.0 ±0.19-fold (n = 3. independent experiments) increase of V[1]11X,compared It) the untreated sample,without significantly affecting the Knl (1.1 S ±0.19, n = 3). [.v],substrate concentration in mM; v, pmole of substrate hydrolyzed/min. The assay was performed using a different ATPstock than the one used for the experiments in A-E.
4861
PTP-y: BINDING AND ACTIVATION BY TRIPHOSPHORYLATED NUCLEOSIDES
L1 L2 L3 L7 LO
11 LO
D T D T-220
-97-69
-50
L2 L3 L7
-220
-97-69
D T D T DT
-220
-97-69
-50
B 239ATG —¿
CAH TM D1 D2 4606•¿ -TGA LO
2815
239ATG
ATG
ATGl
2210
n
ATG
2217
4606»-TGA L1
, 2217
•¿ TGA
2815
3709•¿ TGA L2
4606•¿ —TGA L3
3709
L7
MBRFig. 6. Localization of the PTP-y ATP-binding domain. A, left, 1 /il of total in vitra transcribed and translated material from each construct, illustrated in H, was loaded to demonstrate
the presence and estimate the amount of labeled protein; center and righi, ADP-agarose (D) and ATP-agarose (7~)binding of 1 ¿il(~ M' for construct L7) of the material shown in the
left panel. B. structure of the constructs utilized for the experiments oÃ-A. Nucleotide numbers are taken from the Genhank mouse cDNA sequence. CAH. carbonic anhydrase domain;TM, transmembrane domain: DI and D2, catalytic domains I and II. respectively; MBR. minimal binding region.
lines and tissues.4 Indeed, all the cDNAs cloned from the kidney
umor and human fibroblast cDNA libraries represented the smallerisoform, which is the major form in many cell types.4 This isoform is
missing the 29 intracellular juxtamembrane amino acids of the published sequence (Genbank accession no. L09562). Variant isoforms ofother receptor protein tyrosine phosphatases have been described(23-25). Another receptor protein tyrosine phosphatase gene, LAR,has been shown recently to exhibit alternatively spliced exons in thejuxtamembrane region, which occur preferentially in neural tissue andare developmentally regulated (26).It has been observed that an artificially deleted variant of PTPo, the
deletion of which falls immediately NH2-terminal to either the catalytic domain I or domain II, abolished the activity of the individuallyexpressed domains. When the NH2-terminal sequence of domain Iwas deleted from the tandem catalytic domain-containing protein, allphosphatase activity was lost, in spite of the presence of an intact,potentially active domain II, possibly due to interference in proteinfolding (27). Although the region missing in this PTPy isoform ismore NH2-terminal relative to the catalytic domain, a role for thealternative exon in modulation of phosphatase activity toward selectedsubstrates may be envisioned.Other groups have shown the occurrence of homophilic binding in
receptor phosphatase family members that exhibit unique subsets ofimmunoglobulin and FNIII-like domains (7-9). We have not detectedthis phenomenon with baculovirus expressed PTPy. This is perhaps
not surprising because there is only one FNIII domain and no identified immunoglobulin-like domains in the PTPy extracellular domain. Several lines of evidence suggest a link between the process ofcell adhesion and tyrosine phosphorylation, including the identification of a receptor Tyr kinase that displays homology to the cadherinfamily of adhesion molecules in its extracellular domain (28), thepresence of extracellular segments comprising two immunoglobulin-like and two FNIII-like domains in some kinases (29, 30). TheDrosophila gene D-lrk bears structural homology to neural cell adhesion molecule in its extracellular domain and undergoes ho-mophilic-binding interactions, which stimulate its protein kinase activity (31). A link between cell adhesion molecules of the integrinfamily and a member of the .vrc family of Tyr kinases, c-fgr, has beendescribed recently (32). Moreover, the recent description of the binding of the extracellular domain of PTPf/RPTPß to the extracellularmatrix protein tenascin and the glycosylphosphatidylinositol-anchored glycoprotein contactin, together with the identification of areceptor tyrosine phosphatase (DEP-1) the expression of which isdependent on variations in cell density, further strengthens this connection (14, 22,33).Insect cells have been reported to possess most of the posttransla-
tional machinery of higher eukaryotes (19), together with the advantage of baculovirus-mediated high-level expression, making this system ideal for an initial model for complex glycoproteincharacterization. Therefore, we sought evidence for typical posttrans-
PTP-y: BINDING AND ACTIVATION BY TRIPHOSPHORYI.ATED NUCLEOSIDES
lational processing of the PT?7 protein, which affects most receptorproteins in this system, and verified the results, whenever possible, inthe mammalian counterpart. Our demonstration of the occurrence ofAMinked glycosylation of the insect cell-expressed protein, togetherwith the observation that PTPy-transfected mammalian cells overex-press a product of the same molecular weight as the PTP7 expressedby insect cells,6 validates the Sf9 insect system and allows the
speculation that posttranslational modification may be involved inligand-binding specificity. We have also shown the occurrence ofconstitutive Ser phosphorylation in insect cell-expressed PTP7, suggesting that quantitative or qualitative modulation of phosphorylationstates could be involved in enzyme activation, determination of substrate specificity, or association with other molecules. The significance of this observation is strengthened by the finding that embryonic stem cells, constitutively overexpressing murine PTP7, showphosphorylation only of Ser residues,7 as shown for the Sf9/baculo-virus-expressed protein.The demonstration of specific modulation of PT?7 enzymatic ac
tivity by NTPs, possibly mediated by NTP binding to the phosphatase,represents a novel finding. To summarize, PTP7 was activated byNTPs only in a partially purified form, indicating the presence ofspecific NTP-dependent activating factors or conformational restraints (see Fig. 44); the 7 phosphate group was essential for thebinding and activating effects because di-, mono-, and unphosphoryl-ated purine derivatives were ineffective or poorly effective (Fig. 4, Band C and Fig. 5), and Mg2+ ions, known to complex with NTPs
through the phosphate groups, completely abolished the effect (Fig. 4,A and D and Fig. 5). Addition of metal ions after PT?7 binding did notresult in dissociation of the complex, at least under the experimentalconditions used. The activation process did not significantly alter theKm of the enzyme for the substrate (1.18 ±0.19-fold activation byATP), but significantly altered the Vmax(2 ±0.26-fold activation byATP), indicating interaction of the effector in a region distinct fromthe catalytic center in the first phosphatase domain (Fig. 4F). We can,therefore, consider these compounds allosteric activators of the enzyme that alter the catalytic rate constant but not the substrate bindingconstant; activation by NTPs was accompanied by the tight binding ofATP to the protein, as indicated by the ability of ATP-agarose, but notADP-agarose, to bind PTP7 under relatively stringent conditions(extensive 1% Triton X-100 or RIPA washes; Fig. 5).The lack of response of the purified form of PT?7 to NTPs might
suggest that membrane anchoring is required for activation. Indeed,Niklinska et al. (34) recently demonstrated that membrane anchoringof CD45, in addition to its tyrosine phosphatase activity, is requiredfor T-cell antigen receptor signaling. It is also possible that theobserved activation might be linked to the presence of specific activating factors in the lysates but not in the purified PTP7 preparations,or an NTP-mediated dimerization effect; this last possibility seemsless likely because Desai et al. (35) showed that EGF-mediateddimerization of a EGFR/CD45 chimera caused ligand-dependent negative regulation of phosphatase activity and not enhancement, asexpected by the effect of the same ligand on EGFR tyrosine kinaseactivity.Because of the high specific activity of Tyr phosphatase en
zymes, compared to the tyrosine kinase counterparts, it has beensuggested that the effects of phosphatases on cellular metabolismmight be mediated by negative regulation of phosphatase activity.Support for this possibility comes from the results obtained in theaforementioned EGFR/CD45 chimera experiments. Our experiments with NTPs indicate the potential for PTP7 enzymatic activ-
6 C. Sorio, unpublished results.7 J. Menarola and C. Sorio, unpublished results.
ity to be positively modulated upon interaction with physiologically relevant effectors.The observation of NTP binding to, and stimulation of, phosphatase
activity requires comment. The biological function of ATP is probablythe best understood of all the NTPs. Extracellular adenine nucleotidesinteract with a variety of receptor subtypes to elicit various biologicaleffects (36, 37).Very little is known regarding the regulation of enzymes by intra-
cellular ATP. Even data regarding intracellular concentration of nucleotides are given with large approximations. The available data (38)shows the concentration in cultured cells as follows: ATP,2.537 ±1.217 HIM;GTP, 0.232 ±0.202 mM; UTP, 0.227 ±0.230 IHM;and CTP, 0.083 ±0.133mM. Most authors, based on the calculated Kmof many kinases versus ATP, estimate the concentration of thisnucleotide to be around 1 mM. Only a few examples of enzymesregulated by nucleotides have been reported: for example, ATP regulated the activity of inositol-l,4,5-triphosphate to its receptor andpotentiated inositol-l,4,5-triphosphate-stimulated Ca2+ fluxes, where
as at higher concentrations (>500 /xmol/liter) ATP inhibited botheffects. This effect is linked to the binding of the adenine moiety ofadenine nucleotides to the enzyme (39). Another enzyme known to beactivated by ATP is the aspartate carbamoyl-transferase regulatorysubunit (40).Assuming a direct interaction between ATP and PT?7, we looked
for potential NTP-binding sites in the amino acid sequence of theprotein by seeking appropriate consensus sequences. By using thenomenclature of Traut (40), we could identify, in both human andmouse PTP7, four "kinase-2" motifs in the intracellular domain, threeof which are localized within the identified ATP-binding region,encompassing the first catalytic domain (Dl domain). These motifsare localized within nucleotides 2874-2885, 2913-2927, 3435-3449and 4062-4076 of the published mouse sequence. The motif has beenconsidered to be a binding site for the phosphates of ATP, making itparticularly suitable as a candidate PTP7-binding site, considering theimportance of the phosphate groups for enzyme activation and binding. The number of sites also fits the reported requirement (40) ofmultiple peptide segments (at least two and up to five) in order to formthe ligand-binding pocket. Although this kind of evidence is suggestive, the determination of the actual binding sequences will require adirect experimental approach. Although interpretation of the possiblephysiological significance of ATP binding would be speculative, wewish to draw attention to a recent report by Dent et al. (41), whichdescribed a decrease in Raf-1 activity upon incubation with a membrane fraction and GTP, a decrease that was sensitive to tyrosinephosphatase inhibitors. The authors speculated on the possible presence of a GTP-sensitive/GTP-dependent tyrosine phosphatase activitythat may be able to regulate Raf-1 kinase.We are currently evaluating the use of selective ATP binding for
PTP7 purification from cell lysates. As suggested by the experimentshown in Fig. 5, it is possible to prcabsorb nuclcotide-binding proteinsto an ADP-agarose column and run the eluate on an ATP-agarosecolumn to obtain enrichment of PTP7.A number of questions concerning PT?7 arise as a result of the
experiments reported: is there a difference in the response of the shortversus the long form of PTP7 to various effectors or substrates?, whatis the mechanism of NTP-mediated activation and binding?; and isthis phenomenon physiologically relevant? Answers to some of thesequestions might contribute to the understanding of mechanisms ofregulation of protein tyrosine phosphatase activity, still an underdeveloped area of investigation, and perhaps, unveil novel physiologicalroles for NTPs.
4863
PTP-y: BINDING AND ACTIVATION BY TRIPHOSPHORYLATED NUCLEOSIDES
ACKNOWLEDGMENTS
We thank Teresa Druck and Ashwini Nayak for sequencing of cDNAs andRT-PCR products and Almeta Mathis for preparation of the manuscript.We also thank Dr. Emad Alnemri of the Jefferson Cancer Institute for help inestablishing the baculovirus system technology.
REFERENCES
1. Hanks, S. K.. Ouinn, A. M., and Hunter, T. The protein kinase family: conservedfeatures and deduced phylogeny of the catalytic domains. Science (Washington DC),241: 41-42, 1988.
2. Schlessinger, J., and Ullrich, A. Growth factor signaling by receptor tyrosinc kinases.Neuron, 9: 383-391, 1992.
3. Saito. H. Structural diversity of eukaryotic protein tyrosine phosphatascs: functionaland evolutionary implications. Semin. Cell Biol.. 4: 379-387, 1993.
4. Kaplan, R.. Morse. B.. Hucbner. K., Croce, C., Ravcra, M., Ricca. G., Jaye, M., andSchlessinger, J. Cloning of three novel human tyrosine phosphatases reveals theexistence of a multigene family of receptor-linked protein tyrosine phosphatases.Proc. Nati. Acad. Sci. USA, 87: 7000-7004, 199(1.
5. Krueger, N. X., Streuli, M., and Saito, H. Structural diversity and evolution of humanreceptor-like protein tyrosine phosphatases. EMBO J., 9: 3241-3252, 1990.
6. Tian, S. S., Tsoulfas, P., and Zinn, K. Three receptor-linked protein-lyrosine phosphatases are selectively expressed on central nervous system axons in the Drosophilaembryo. Cell, 67: 675-68», 1991.
7. Gebbink, M. F. B. G., Zondag, G. C. M., Wubbolls, R. W., Beijersbergen, R. L., vanEtten. 1., and Moulcnar, W. H. Cell-cell adhesion mediated by a receptor-like proteintyrosine phosphatase. J. Biol. Chem., 268: 16101-16104, 1993.
8. Brady-Kalnay, S. M.. Flint, A. J.. and Tonks, N. E. Homophilic binding of PTPn. areceptor-type protein tyrosine phosphatase, can mediate cell-cell aggregation. J. CellBiol.. 122: 961-972. 1993.
9. Sap. J., Jiang, Y-P., Friedlander, D., Grumet, M., and Schlessinger. J. Receptortyrosine phosphatase R-PTP-K mediates homophilic binding. Mol. Cell. Biol., 14:1-9, 1994.
10. Barnea, G.. Silvennoinen, O., Shaanan. B., Honegger, A. M., Canoll, P. D.,D'Euslachio. P., Morse, B., Levy, J. B., LaForgia, S., Huebner, K.. Musacchio, J. M.,Sap, J., and Schlessinger, J. Identification of a carbonic anhydrase-like domain in theextracellular region of RPTPy defines a new subfamily of receptor tyrosine phosphatases. Mol. Cell. Biol., 13: 1497-1506, 1993.
11. Levy, J. B., Canoll, P. D., Silvennoinen, O., Barnea, G., Morse, B., Honegger, A. M.,Huang, J-T.. Cannizzaro. L. A.. Park, S-H., Druck, T., Huebner, K., Sap, J., Erhlich,M., Musacchio, J. M., and Schlessinger. J. The cloning of a receptor-type proteintyrosine phosphatase expressed in the central nervous system. J. Biol. Chem., 268:10573-10581. 1993.
12. Krueger, N. X.. and Saito. H. A human transmemhrane protein-tyrosinc phosphatase,PTP£,is expressed in brain and has an ^-terminal receptor domain homologous tocarbonic anhydrases. Proc. Nati. Acad. Sci. USA, 89: 7414-7421. 1993.
13. Maurel, P., Rauch, U., Fiad. M., Margolis, R. K., and Margolis, R. U. Phosphacan, aehoruiroitin sulfate protcoglycan of hntin that interacts with neurons and neuralcell-adhesion molecules, is an extracellular variant of a receptor type protein tyrosinephosphatase. Proc. Nati. Acad. Sci. USA, 91: 2512-2516, 1994.
14. Barnea, G., Grumet, M.. Milev. P., Silvennoinen, O., Levy, J. B.. Sap. J., andSchlessinger, J. Receptor tyrosine phosphatase ßis expressed in the form of protcoglycan and hinds to the extracellular matrix protein tenascin. J. Biol. Chem., 269:14349-14352, 1994.
15. Barnea, G., Grument, M., Sap, J., Margolis. R. U., and Schlessinger, J. Closesimilarity between receptor-linked tyrosine phosphatase and rat brain proteoglycan.Cell. 76: 205, 1994.
16. Lubinski, J., Hadaczek, P., Podolski, J., Toloczko, A., Sikorski, A., McCue, P.,Druck, T., and Huebner, K. Common regions of deletion in chromosome regions3pl2 and 3pl4.2 in primary clear cell renal carcinomas. Cancer Res., 54:3710-3713, 1994.
17. Wary. K. K.. Lou, Z., Buchberg, A. M., Siracusa, L. D., Druck, T., LaForgia. S., andHuehner. K. A homozygous deletion within the carbonic anhydrase-like domain ofthe Ptprg gene in murine L-cells. Cancer Res., S3: 1498-1502, 1993.
18. Harlow, E., and Lane. D. Antibodies, pp. 298-315. Cold Spring Harbor. NY: ColdSpring Harbor Laboratory, 1988.
19. O.Rcilly, D. R., Miller, L. K., and Luckow. V. A. Baculovirus expression vectors. W.H. Freeman and Co., 1992.
20. LaForgia. S. K., Morse, B., Levy, J., Barnea, G., Li, F., Cannizarro. L. A., Nowell.P. C., Click, J., Boghosian-Scll. L., Weston, A., Harris, C C., Drabkin, H., Patterson,D., Croce, C. M., Schlessinger. J.. and Huebner, K. Receptor protein-tyrosine phosphatase y is a candidate tumor suppressor gene at human chromosome region 3p21.Proc. Nati. Acad. Sci. USA, 81: 5036-5040, 1991.
21. LaForgia, S., Lasota, J., Latif, F., Boghosian-Sell, L., Kastury, K., Ohta. M.. Druck.T.. Atchison, L., Cannizzaro, L., Barnea, G.. Schlessinger. J.. Modi. W.. Kuzmin, !..Tory, K„Zbar, B., Croce. C. M., Lcrman, M., and Huebner. K. Detailed genetic andphysical map of the 3p chromosome region surrounding the familial RCC chromosome translocation, t(3;8)(pl4.2;q24.1). Cancer Res., 53: 3118-3124, 1993.
22. Peles, E.. Nativ. M.. Campbell. P. L., Sakurai, T., Martinez, R.. Lev, S., Clary, D. O.,Schilling. J., Barnea, G.. Plowman, G. D., Grumet, M., and Schlessinger, J. Thecarbonic anhydrase domain of receptor tyrosine phosphatase ßis a functional ligandfor the axonal cell regulation molecule contactin. Cell. 82: 251-260, 1995.
23. Yang, X. H., Seow, K. T., Bahri, S. M., Don, S. H., and Chia, W. Two Drosophilareceptor-like tyrosine phosphatase genes are expressed in a subset of developingaxons and pioneer neurons in the embryonic CNS. Cell, 67: 661-673. 1991.
24. McLaughlin, S., and Dixon, J. E. Alternative splicing gives rise to a nuclear proteintyrosinc phosphatase in Drosophila. J. Biol. Chem., 268: 6839-6842, 1993.
25. Matthews. J., Cahir, E. D.. and Thomas, M. L. Identification of an additional memberof the protein-tyrosine-phosphatase family: evidence for alternative splicing in thetyrosine phosphatase domain. Proc. Nati. Acad. Sci. USA, 87: 4444-4448, 1990.
26. Zhang, J. S., and Longo, F. M. LAR tyrosine phosphatase receptor: alternativesplicing is preferential to nervous system, coordinated with cell growth and generatesnovel isoforms containing extensive CAG repeats. J. Cell Biol., 128: 415-431, 1995.
27. Wang, Y.. and Pallen. C. J. The receptor-like protein tyrosine phosphatase HPTPa hastwo active catalytic domains with distinct substrate specificities. EMBO J.. 10:3231-3237, 1991.
28. Takahashi, M., Buma, Y., Iwamoto, T., Inaguma, Y., Ikeda. H., and Hiai, H. Cloningand expression of the ret proto-oncogene encoding a tyrosine kinase with twopotential transmembrane domains. Oncogene. 3: 571-578, 1988.
29. Rescigno, J., Mansukhani, A., and Basilico, C. A putative receptor tyrosine kinasewith unique structural topology. Oncogene. 6: 1909-1913, 1991.
30. O'Bryan. J. P., Frye, R. A., Cogswell, P. C., Neubauer, A., and Kitch, B. Axl, a
transforming gene isolated from primary human myeloid leukemia cells, encodes anovel receptor tyrosine kinase. Mol. Cell. Biol.. //: 5016-5031, 1991.
31. Pulido, D., Campuzano, S., Koda, T., Modotell. J.. and Barbacid, M. Dirk, aDrosophila gene related to the Irk family of neurotrophin receptors, encodes a novelclass of neural cell adhesion molecule. EMBO J.. //: 391-404, 1992.
32. Berton. G.. Fumagalli. L. Laudanna, C.. and Sorio, C. (32 integrin-dependent proteintyrosinc phosphorylation and activation of the FGR protein tyrosine kinase in humanneutrophils. J. Cell. Biol., 126: 1111-1121, 1994.
33. Ostman, A.. Yang, Q., and Tonks. N. K. Expression of DEP-1. a receptor-likeprotein-tyrosine-phosphatase. is enhanced with increasing cell density. Proc. Nati.Acad. Sci. USA, 91: 9680-9684, 1994.
34. Niklinska, B. B., Hou, D, June. C., Weissman, A. W., and Ashwell, J. D. CD45tyrosinc phosphatase activity and membrane anchoring are required forT-ccll antigenreceptor signaling. Mol. Cell. Biol.. 14: 8078-8084. 1994.
35. Desai, D. M., Sap, J., Schlessinger, J., and Weiss, A. Ligand-mediated negativeregulation of a ertimene transmembrane receptor tyrosine phosphatase. Cell, 73:541-554, 1993.
36. Brunstock, G. Purines as cotransmitters in adrencrgic and cholincrgic neurones. In: T.Hokfelt, K. Fuxe, and B. Pernow (Eds.). Progress in Brain Research, Vol. 68, pp.193-203. Amsterdam: Elsevier, 1986.
37. El-Motassim. C., Dornand. J., and Mani, J-C. Extracellular ATP and cell signalling.Biochim. Biophys. Acta, I134: 31-35, 1992.
38. Traut, T. W. Physiological concentrations of purines and pyrimidines. Mol. Cell.Biochem., 140: 1-22, 1994.
39. Marshall, I. C. B., and Taylor, C. W. Regulation of inositol 1,4,5-triphosphatereceptors. J. Exp. Biol., 184: 161-182, 1993.
40. Traut. T. W. The functions and consensus motifs of nine types of peptide segmentsthat form different types of nucleotide-hinding sites. Eur. J. Biochem., 222; 9-19,1994.
41. Dent, P., Jelinek, T., Morrison, D. K.. Weber, M. J.. and Slurgill, T. W. Reversal ofraf-1 activation of purified and membrane-associated protein phosphatascs. Science(Washington DC), 26«.-1902-1906, 1995.
4864