The distribution of putative neurosecretory cells in the central nervous system of the North...
-
Upload
independent -
Category
Documents
-
view
2 -
download
0
Transcript of The distribution of putative neurosecretory cells in the central nervous system of the North...
The distribution of putative neurosecretory cells in the central nervous system of the North American medicinal leech Macrobdella decora
RODNEY A. W E B B AND I. ORCHARD Dcptrrtment of Biology, York Uniac~rsity, Dolt 'nsuie~t~, O n f . , Ctrnodrr M3J IPS
Received February 28, 1979
WEBB, R. A , , and I. ORCHARD. 1979. The distribution of putative neurosecretory cells in the central nervous system of the North American medicinal leech Mrrc,rohdrllrr dc>cor.rr. Can. J. Zool. 57: 1905-1914.
Nerve cells that displayed a distinct Tyndall blue-white effect were observed in the supra- and sub-oesophageal ganglia, the ventral nerve cord ganglia. and the posterior ganglion of the Gnathobdellid leech Macrobdella decoru. These cells formed four anatomically distinct groups in the supraoesophageal ganglion and occupied characteristic and analogous positions in the other ganglia. Light microscopy of these blue-white cells revealed histochemical properties not seen in other nerve cells. The perikarya of the four groups of blue-white cells in the supraoesophageal ganglion were examined with the electron microscope and found to contain large membrane- bounded electron-densegranules similar to those observed in proven neurosecretory cells. Tracts from two of these cell groups possibly form primitive neurohemal organs. A neurosecretory role for the blue-white cells is suggested.
WEBB, R. A., et I. ORCHARD. 1979. The distribution ofputative neurosecretory cells in the central nervous system of the North American medicinal leech Macrobdella decora. Can. J . Zool. 57: 1905-1914.
Chez le gnathobdellide Macrobdella decora, les ganglions supraoesophagien et suboesopha- gien, les ganglions du cordon nerveux ventral et le ganglion posterieur comportent des cellules nerveuses qui manifestent distinctement un effet Tyndall bleu-blanc. Ces cellules forment quatre groupes distincts dans le ganglion supraoesophagien et occupent des positions caracteristiques analogues dans les autres ganglions. Vues au microscope photonique, ces cellules ont des proprietes histochimiques tres particulieres. Le pericaryon a ete examine au microscope elec- tronique dans les quatre groupes de cellules bleu-blanc du ganglion supraoesophagien: ces cellules contiennent de gros granules opaques, entoures d'une membrane et semblables a ceux des cellules reconnues comme neurosecretrices. Les prolongements de deux de ces groupes de cellules forment probablement des organes neurohemaux prirnitifs. I1 est possible que ces cellules bleu-blanc aient une fonction neurosecretrice.
[Traduit par le journal]
Introduction The central nervous system of the leech ha5
proved a useful system in which to study identifiable nerve cells (see Nicholls and Baylor 1968: Nicholls and van Essen 1974; Lent 1977; Wallace cgt NI. 1977). The ganglia contain a relatively small number of nerve cells (-350 per segmental gan- glion) and individual identified cells can be recog- nized by their characteristic shapes, sizes. po4- tion, branching patterns, and electrical properties. In spite of this, there have been no studies involving individually identified neurosecretory cel t s. While the neurosecretory systems of the brains of several leeches have been investigated (Hagadurn 1958: Nambudiri and Vijayakrishnan 1958: Uzcchowicz 1963 ; Ionescu-varo and Grigoriu 1465; Tumpling 1965; Malecha 1970), only the rhynchobdellid leech Theromyzon rude has been extensively studied with respect to neuronal compartmenta- tion, number of cells, staining properties, and ultra- structure (Hagadorn 1958; Hagadorn et (11. 1963).
However, none of these studies related these cells in any detailed way to individual cells which could he identified in living preparations. Because of the prt~bahle involvement of neurohormones in a vari- ety d hody functions, including the control of spermatogenesis in leeches (Hagadorn 1966), it is important ro know the locations of the neurosec- retory cells which may perform these functions. In order to provide a firm basis for future studies of identifiable neurosecretory cells, we have examined living preparations of the central nervous system (CNS) of the gnathobdellid leech Macrob- dirilrr cli~r-or(r and correlated certain individual identifiable cells to their positions within the neuronal compartments. Furthermore, we provide hislological and ultrastructural evidence for their neurosecretory nature.
Materials and Methods Juvenile, sexually mature, and postbreeding specimens of the
leech Mtrt robdclln dccor.o were collected from the swamp at Beaver Meadows, Haliburton, Ont., from July to October 1977
0008-4301/79/101905- 10$01,00/0 GI979 National Research Council of Canada/Conseil nat~onal de recherches du Canada
1906 CAN. J . ZOOL. VOL. 57. 1979
and May to September 1978. Specimens were maintained in the laboratory in aerated, dechlorinated tap water at 15'C or. to simulate winter conditions in the field. at 6°C in the dark. Leeches were cooled to 5°C. and dissected under leech saline (Nicholls and Baylor 1968). The CNS was viewed under a dis- secting microscope with either indirect incandescent lighting or Volpi fiber optics, and examined for the presence of cells dis- playing a white or Tyndall blue-white effect.
Various ganglia of the CNS were pinned out, using cactus needles, on dishes coated with Sylgard resin and fixed in Bouins-trichloroacetic acid (TCA) for 30 min. The compart- ments and the positions of the Tyndall blue-white cells (which continue to show this characteristic in fixed tissues) were examined and drawn. The tissues were then fixed for a further 24 h and prepared for light microscopy. Paraffin sections (7 pm) were stained in paraldehyde fuchsin (PAF) followed by Halmi's counterstain (Meola 1970). During fixation some Tyndall blue-white cells or groups of cells were dissected from the ganglia, and the ganglia were subsequently sectioned and stained and compared with the sections from intact ganglia.
Single cells or groups of cells of the supraoesophageal ganglia displaying a Tyndall blue-white effect, and located in specific compartments, were dissected free hand from tissues and im- mersed in 2% glutaraldehyde in 0.1 M cacodylate buffer. pH 7.4. Because of the difficulty in removal of group IV cells, whole pieces of compartment 8 were fixed. Following fixation for 2 h, the cells were postfixed in I%osmium tetroxide, blockstained in uranyl acetate, dehydrated in ice cold ethanol. and embedded in either Epon-Araldite or Spurr resin. Silver-gray sections were cut on a Sorval UM2 ultramicrotome, picked up on formvar- coated copper grids. contrasted in uranyl acetate and lead cit- rate, and examined on a Phillips EM 201. The membrane-bound electron-dense granules of the neuronal perikarya were meas- ured in their maximum and minimum diameters with a magnify- ing micrometer scale. Only those granules displaying a distinct membrane were measured. Granule sizes from the different cell groups were analysed using the t-test.
Results For the purpose of this study, the CNS may
conveniently be divided into three parts; the brain, the ventral nerve cord, and the posterior ganglion.
The Brain The brain consists of the supraoesophageal
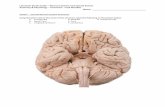
ganglion overlying the oesophagus, and connected to the ventral suboesophageal ganglion by the perioesophageal connectives (Fig. I).
Suprcroesophageal Grrnglion The nerve cell bodies of the supraoesophageal
ganglion are normally contained within eight pairs of compartments. Occasionally the compartments may be further divided. For example, compartment 4 has been observed to be divided into two, as has compartment 5. This subdivision, however, occurs in less than 10% of the specimens examined. In living specimens certain cells within these com- partments displayed a white appearance or Tyndall blue-white effect. These cells were readilv dis- cernible into four anatomically distinct groups lying on each side of the supraoesophageal gan- glion.
Group I-Four white cells, often displaying a Tyndall blue-white effect, were observed in com- partment I of the supraoesophageal ganglion (Fig. 1). In sectioned material these cells measured -50 pm in diameter and always stained very in- tensely with Orange G (Fig. I). These cells, how- ever, did not stain with PAF. Orange G tracts from these cells were observed occasionally in the neuropile and abutting the neural lamella of the outer capsule of the medioposterior face of the supraoesophageal ganglion, but could not be traced with certainty to their terminations. In cells sec- tioned for electron microscopy, the perikarya were filled with large, electron-dense, membrane- bounded granules, round to oval in shape (Fig. 13), measuring 156 f 3.1 nm (SD) and 123 f 2.5 nm in their maximum and minimum diameters respec- tively.
Group IZ-Two cells displaying a Tyndall blue-white effect were observed in compartment 2 (Figs. 2, 3). Their position within the compartment was variable; sometimes the cells were adjacent to each other, other times the cells were widely sepa- rated. In histological sections these cells were pyriform in shape, and measured -50pm at the level of the nucleus. Each cell possessed a pro- nounced Orange G staining "cap," while the re- mainder of the cytoplasm stained a pale blue (Fig. 3). At no time throughout the year did these cells display fuchsinophilia. Because of the lack of tinctoral properties, the axons could not be traced beyond their entry into the neuropile. Electron micrographs of these cells revealed the presence of large islands of membrane-bounded electron-dense granules. The granules measured 13 1 f 1.5 nm (SD) and 118 f 1.5 nm in their maximum and minimum diameters respectively (Fig. 14).
Group IZI-Five, or more rarely, six cells showing a white-blue coloration -30 pm diameter were observed in compartment 7. Occasionally, one or more cells in this group were found in com- partment 8, immediately adjacent to the other cells of the group. All of these pyriform cells showed histological properties identical to those of the group I1 cells (Fig. 4). When viewed with the elec- tron microscope the neuronal perikarya were seen to be filled with islands of membrane-bound electron-dense granules (Fig. 15). These granules measured 132 f 2.3 nm (SD) and 117 f 1.9 nm in their maximum and minimum diameters respec- tively, and were not significantly different (P > 0.2) in size from those of the group I1 cells.
Group ZV-A pair of white cells were often ob- served in each pair of compartment 8. In sectioned material these cells were oval, measuring 45 by 20 pm at the level of the nucleus. Following PAF
WEBB A N D ORCHAKD
ant
V 0
ant
ant
FIG. I. Diagrammatic views of the CNS of M. decorn. Dorsal (D) and ventral (V) views are shown in each diagram. (A) The supra- and sub-oesophageal ganglion. For simplicity, the supraoesophageal ganglion has been moved anteriad, through90", to the same plane as the suboesophageal ganglion. The positions of all the neurosecretory cells of the supraoesophageal ganglia are shown as black circles. Only the Orange G positive cells are figured in the other ganglia. The compartments of the supraoesophageal ganglia are numbered 1-8. (B) Ventral nerve cord ganglion. ( C ) Posterior ganglion. ant, anterior; post, posterior.
staining, the cytoplasm was almost filled with a fuchsinophilia, some PAF tracts were observed in dense deep-purple granulation (Fig. 5). Indeed, the neural lamella of the outer capsule (Coggeshall these were the only cells in the entire CNS that and Fawcett 1964) on the medioposterior and displayed a prominent fuchsinophilia. While the medioventral face of the supraoesophageal gan- initial axonal tracts did not reveal a distinct glion (Figs. 5 , 6). On the other hand, the axonal
1908 CAN. J . ZOOL. VOL. 57. 1979
FIG. 2. Section through compartments I and 2 of the supraoesophageal ganglion revealing three of the four group I cells (large arrow) and a single group I1 cell (small arrow) respectively. x 350. FIG. 3. Group I1 cells in compartment 2. Note the distinctive Orange G cell cap (arrow). x 600. FIG. 4. Group 111 cell in compartment 7 of the supraoesophageal ganglion. Note the Orange G cap (arrow). x 450. FIG. 5. Group IV fuchsinophilic cell (arrow head) in compartment 8. Note thefuchsinophilic tracts (arrow) in the neurilemma of the medioventral face of the supraoesophageal ganglion. x 720. FIG. 6. Fuchsinophilic tracts (arrows) in the neurilemma of the medioposterior face of the supraoesophageal ganglion. x 1800. FIG. 7. Nerve cell with a distinctive Orange G staining cap in the lateral aspect of the ventral compartment of the suboesophageal ganglion. x 450. FIG. 8. Medial PAF cells of the ventral compartments of the suboesophageal ganglion showing small fuchsinophilic granulation (arrows). x 450.
WEBB AND ORCHARD 1909
FIG. 9. Nerve cell with the characteristic Orange G staining cap in the posterior laterodorsal compartment of the ventral nerve cord ganglia. x 450. FIG. 10. Fuchsinophilic cell (arrow) in the posterior laterodorsal compartment of the ventral nerve cord ganglia. x 450. FIG. I I . Nerve cells (arrows) displaying the distinctive Orange G stainingcap in the lateral aspects of the ventral compartments of the posterior ganglion. x 230. FIG. 12. Fuchsinophilic cell observed in compartment 5 of the supraoesophageal ganglion. x 600.
tracts were in close proximity to the fuchsinophilic tracts.
Because no other cells stained intensely with PAF in the CNS it is possible that these fuchsinophilic tracts are the terminations of the group 1V cells.
Step-serial sectioning of compartment 8 for electron microscopy revealed only one cell that contained large numbers of inclusions likely to stain with PAF. The perikaryon was virtually filled with membrane-bound inclusions containing mate- rial of variable electron density, often suggesting a subgranular structure (Fig. 16). These membrane- bound granules measured 137 k 2.2 nm (SD) and 124 k 1.8 nm in their maximum and minimum diameters respectively.
Several other small nerve cells, showing small
numbers of fine granules staining with PAF, have been observed in the supraoesophageal ganglia, most frequently in compartment 5 (Fig. 12). How- ever, their occurrence was not regular and their appearance showed no relationship to the time of year. On the other hand, the histological staining reactions of groups I to IV was identical in over- wintering, immature and sexually mature, and postbreeding leeches.
Suhoesophrrgccrl Ganglion The nerve cell bodies of the suboesophageal
ganglion are contained within six pairs of dorsal and six pairs of ventral compartments (see Fig. 1A). In living preparations, a single Tyndall blue-white cell was observed occasionally in the lateropos- terior aspect of each ventral compartment. In sec-
1910 CAN. J . ZOOL. VOL. 57. 1979
tioned material. a single cell measuring -45 pm and possessing a cap that stained intensely with Orange G was observed in the position corresponding to the white cells (Fig. 7). These cells did not stain with PAF. On the other hand, a small (-25 pm diameter) cell was observed, occupying the medial aspect of each ventral compartment that displayed small numbers of fine granules staining with PAF (Fig. 8). The staining character of this cell or the Orange G cap cell did not vary in specimens fixed at different times of the year.
Ventrrrl Nerve Cord The paired ventral nerve cord is interrupted in
each corporeal segment by a ganglion. The ganglionic cortex consists of six compartments of neuronal perikarya; two paired laterodorsal (one anterior and one posterior) and two unpaired ven- tromedial compartments (one anterior and one posterior) (see Fig. IB). This is in agreement with the compartmentation of the ventral nerve cord ganglia of M. decorn described by Fernandez (1978). Two pairs (one pair in the last ganglion) of lateral nerves emanate from each ganglion between the anterior and posterior laterodorsal compart- ments. In living specimens, a single pair of cells displaying a distinct Tyndall blue-white effect were observed, one cell at the posterior edge of each posterior laterodorsal compartment. These cells possessed a prominent Orange G cap, meas- ured -45 pm in diameter, but the cytoplasm did not stain with PAF (Fig. 9). The axonal process was devoid of differentially stained material and could not be traced into the neuropile. A single pair of cells containing small numbers of fine granules staining with PAF were observed, one each in the posterior laterodorsal compartments, often adja- cent to the Orange G cell (Fig. 10). Removal of the Tyndall blue-white cells and subsequent section- ing and staining of the ventral ganglia proved that these cells were those staining with Orange G.
Posterior Gcrnglion The neuronal perikarya of the posterior ganglia
are contained within seven paired dorsal and seven paired ventral compartments (Fig. IC). A lateral nerve emanates from each ventral compartment and a small single nerve emerges between the last pair of compartments. Occasionally, cells display- ing a faint Tyndall blue-white effect were observed in the posterior-lateral aspect of each ventral com- partment. These cells also display a prominent Orange G cap and measure -45 pm at the level of the nucleus (Fig. 11). A small cell (-25 pm diame- ter) containing a few fine fuchsinophilic granules
was observed close to the medial edge of each ventral compartment.
Discussion Cytological criteria have generally been used to
identify neurosecretory cells (see Finlayson and Osborne 1975; Rowell 1976; Gabe 1966), although the concept of neurosecretion also requires physiological evidence for the release of a neurohormone (see Bern and Knowles 1966; Gabe 1966). While it is difficult to attribute a neuro- secretory function on purely morphological grounds, the body of evidence suggests the corre- lation is indeed a good one (Rowell 1976). Neuro- secretory cells usually possess electron-dense granules, 100-300 nm in diameter, have an affinity for histochemical stains such as PAF and Orange G (see Hagadorn 1958; Rowell 1976) and show evi- dence of forming neurohemal organs. Many cells showing some of these properties can be identified in living preparations, for example, the nervous tissue of molluscs (see Chase and Goodman 1977). crustacea (Iwasaki and Satow 1971; Maynard 1961). and insects (Berlind and Maddrell 1979; Hinks 1975) by virtue of their blue-white colora- tion. This coloration, the Tyndall effect, is believed to result from the presence of numerous vesicles in the size range of 100-500 nm (see Hinks 1975).
Several of the morphological features associated with known neurosecretory cells are present in some of the nerve cells of M. decor-a. A number of Tyndall blue-white cells were observed in the CNS of living specimens of M. decor-a. These cells formed four anatomically distinct groups in the supraoesophageal ganglion and occurred in characteristic positions in the suboesophageal, ventral nerve cord, and posterior ganglia. The perikarya of these cells, but not the other nerve cells showed a distinct affinity for either PAF or Orange G. In particular, the group I and IV cells which stained with Orange G and PAF, respec- tively, contained numerous membrane-bound electron-dense granules larger than 100 nm and, because of the PAF and Orange G staining tracts at or in the neural lamella, possibly form neurohemal organs. Group I1 and I11 cells also stained with Orange G and contained numerous membrane- bound electron-dense granules larger than 100 nm but could not be traced to any possible neurohemal structures. In addition, preliminary electrical re- cordings from the groups I to IV cells of M. decorcr (unpublished data) demonstrate action potentials of long duration, consistent with the data of Yagi et a / . (1963) from the opaque white cells of Theromyzon.
WEBB A N D ORCHARD 1911
FIGS. 13- 15. Electron micrograph of the dense granules in the perikaryaof the group I, 11, and I11 Tyndall blue-white cells respectively of the supraoesophageal ganglia. Note the dense homogenous nature of the granules. All x 31 500. FIG. 16. Electron micrograph of the perikaryon of the group IV cells of the supraoesophageal ganglia. Note the variation in granular contents, as compared with Figs. 13-15. x 3 1 500.
1912 CAN. J. ZOOL. VOL. 57, 1979
Long duration action potentials are believed to be characteristic of neurosecretory cells (see reviews by Yagi and lwasaki 1977 and Finlayson and Os- borne 1975). Overall, the data obtained from the group I to IV cells lead us to conclude that these are probable neurosecretory cells.
The cells in the suboesophageal, ventral nerve cord, and posterior ganglia, because of their white appearance and histochemical staining reactions, are similar to known neurosecretory cells and, therefore, are also likely to be neurosecretory. However, further elucidation of the nature of these cells is required.
Previous histochemical studies on the CNS of leeches have revealed cells which show affinities for PAF and others for Orange G (Hagadorn 1958, 1966; Czechowicz 1963; Tumpling 1965; Malecha 1970). There are, however, certain differences between the species studied. Clearly the staining affinities of the group I Orange G staining cells of M. dectorrr correspond to the P cells of Theromyzon rude but the size of the granules appear different, with those of M. decorcr significantly larger than those of T. rude (see Hagadorn et crl. 1963). Group I1 and 111 cells, of M. dec-orrr which display a dis- tinct Orange G cap, but whose perikarya do not take up PAF or Orange G, do not appear to corre- spond to the cells described in the other species. Interestingly, however, the size and morphology of their granules is remarkably similar to the probable p cells of T. rude (see Hagadorn et a/. 1963). The significance of the "cap" is not clear. A previous report of a distinct cap, although this time stainable with PAF, has been described in the protocerebral neurosecretory cells of the cricket Achetn domes- ticus (Geldiay and Edwards 1973). This cap corre- sponds to a concentric layer of rough endoplasmic reticulum but the nature of the cap in M. decora is not yet known.
The group IV cells stain with PAF and corre- spond in tinctoral properties to the cl cells of T. rude and other species (Hagadorn 1958; Czechowicz 1963 ; Tumpling 1965; Malecha 1970). In agreement, also, is the appearance of the granules of the group IV cells which are very similar in size and morphol- ogy to those postulated as belonging to the perikarya and terminals of the fuchsinophilic cr cells of T. rude (Hagadorn et nl. 1963).
An interesting observation from the work on leech neurosecretory cells lies in the numbers of cells described. According to previous studies, as many as 50% of the nerve cells of the CNS of various leeches stain with PAF or chrome-hema- toxylin-phloxin (Hagadorn 1958; Nambudiri and
Vijayakrishnan 1958; Czechowicz 1963 ; Ionescu- varo and Grigoriu 1965; Tumpling 1965; Malecha 1970), although i t is possible that in some cases many cells were staining in a nonspecific manner (see Golding and Whittle 1978). All of these studies do, however, consistently show the presence of more than one pair of fuchsinophilic cells in the supraoesophageal ganglion. In contrast, the only consistent appearance of any fuchsinophilic cells in M. decorcr was confined to a single pair, one cell in each of compartment 8. In T. rude the a and P cells of the supraoesophageal ganglia occur in a ratio of about 5:3 while in M. decorrr the ratio of cl to P cells is 1: 10. These differences are not simply due to the different staining techniques because the PAF technique, as used in the present study, reveals the numerous PAF cells known to occur in the brain of E~pobdellr octoculata (Webb, unpublished; Czechowicz 1963; Tumpling 1965). This study thus suggests that the differences in number of neuro- secretory cells observed in different genera of leeches may be a real phenomenon and raises some important questions concerning cell homologies. Indeed while neurons that perform a similar func- tion and possess identical electrical properties may occupy a similar position in a species from one preparation to another (see Nicholls and Baylor 1968) or between closely related species (Keyser and Lent 1977) the same may not hold true in com- parisons between more distantly related species. Clearly there are large differences between the neurosecretory cell population of the gnathobdellid M. decora and the rhynchobdellid and pharyngob- dellid leeches.
The compartmentation of the neuronal perikarya in the brain of M. decora also requires discussion as it shows distinct differences from that seen in other leeches. For example, the supraoesophageal gan- glia of the rhynchobdellid leeches Ther-ornyzon rude (Hagadorn 1958), T. tessulatum, Glossiphonia complanata , and Piscicola geometra (Tumpling 1965) and!the pharyngobdellid leech Herpohdella (= Erpobdella) octoculata (Tumpling 1965) all contain six pairs of compartments while the gnathobdellid leech M. decora contains eight pairs. The homologies between the compartments and the neurosecretory cells contained within is not clear. On the other hand, both the suboesophageal and posterior ganglia of M. decora have the same numbers of compartments as the other gnathobdel- lids (Hirudo medicinalis and Haemopis sangisuga (Sanchez 1909, 1912; Rubin 1978)).
The presence of six pairs of Orange G staining cells in the suboesophageal ganglion raises the
WEBB A N D ORCHARD 1913
question as to their origin, because the sub- oesophageal ganglion is believed to be derived from the embryonic fusion of four segmental ganglia. Thus, if these Orange G staining cells are homolo- gous to those observed in the segmental ganglia and the posterior ganglion (which is suggested by the histological staining pattern and the appearance of a single PAF cell in each compartment), then one would expect four pairs to appear in the sub- oesophageal ganglion. Similar anomalies were ob- tained by Rude (1968) in finding eight monoamine cells lying at the anterior region of the sub- oesophageal ganglion of H. medicinalis. Further- more, the supraoesophageal ganglia contain fewer monoamine cells than would be expected if, as postulated (see Rude 1968), the supraoesophageal ganglia are formed from the fusion of two segmental ganglia. It is possible, therefore, that some groups of cells, originally associated with the first two segmental ganglia, have migrated to form part of the suboesophageal ganglion. The association of some groups of nerve cells of the first two em- bryological ganglia with the suboesophageal gan- glia is not unexpected in view of the confluence of the supra- and sub-oesophageal ganglia to form a peripharyngeal mass in BI-anchrllion and Ponrob- della (Francois 1886, as quoted in Grasse 1959).
In conclusion, we have described probable neurosecretory cells in the leech Mao-obdrlla de- c o r ~ and shown that the majority of these cells can be identified in living preparations by their color and position, and in histological sections by virtue of their size, position, and tinctoral properties. This now enables physiological studies to be performed on individually identified neurosecretory cells.
Acknowledgements We thank Sylvia Gilchrist for the use of facilities
at Beaver Meadows, and Pam Melling and Vivian Tsang for technical assistance. This work was sup- ported by a NRCC negotiated development grant to York University and NRCC grant in aid of research A65508 to R.A.W.
BERLIND, A,. and S. H. P. MADDRELL. 1979. Changes in hor- mone activity of single neurosecretory cell bodies during a physiological secretion cycle. Brain Res. 161: 459-467.
BERN, H. A., and F. G. W. KNOWLES. 1966. Neurosecretion. In Neuroendocrinology. Vol. I . Edited by L. Martini and F. W. Ganong. Academic Press, New York. pp. 139-186.
CHASE, R., and H. E. GOODMAN. 1977. Homologous neuro- secretory cell groups in the land snail Achntina fulica and the sea slug Aplysia californiccr. Cell Tissue Res. 176: 109-120.
COGGESHALL, R. E., and D. W. FAWCETT. 1964. The fine structure of the central nervous system of the leech, Hirudo medicinalis. J . Neurophysiol. 27: 229-289.
CZECHOWICZ, K. 1963. Neurosecretion in leeches and the an- nual cycle of its changes. Zool. Pol. 13: 163-184.
FERNANDEZ, J . 1978. Structure of the leech nerve cord: dis- tribution of neurons and organization of fiber pathways. J . Comp. Neurol. 180: 165- 192.
FINL.AYSON, L. H.. and M. P. OSBORNE. 1975. Secretory activ- ity of neurons and related electrical activity. In Advances in comparative physiology and biochemistry. Vol. 6. Edited by 0 . Lowenstein. Academic Press, New York. pp. 165-258.
GABE. M. 1966. Neurosecretion. Pergamon, Oxford. G ~ L D I A Y . S.. and J . S. EDWARDS. 1973. The protocerebral
neurosecretory system and associated neurohemal area of Achetrr domesficus. Z. Zellforsch. Mikrosk. Anat. 145: 1-22.
GOLDING, D. W., and A . C. WHITTLE. 1978. Neurosecretion and related phenomena in annelids. Int. Rev. Cytol. 50 (Suppl.): 189-302.
GRASSE, P. P. (Editor). 1959. Traite de Zoologie. Tome V. Mas- son et Cie, Paris.
HAGADORN, 1. R. 1958. Neurosecretion and the brain of the rhynchobdellid leech, Theromyzon rude (Baird, 1869). J . Morphol. 102: 55-90.
1966. Neurosecretion in the Hirudinea and its possible role in reproduction. Am. Zool. 6: 251-261.
HAGADORN, J. R.. H. A. BERN, and R. S. NISHIOKA. 1963. The fine structure of the supraoesophageal ganglion of the rhyn- chobdellid leech, Theromyzon rude, with special reference to neurosecretion. Z. Zellforsch. Mikrosk. Anat. 58: 714-758.
HINKS, C. F. 1975. Peripheral neurosecretory cells in some Lepidoptera. Can. J . Zool. 53: 1035-1038.
lo~kscu-VARO. M., and A. GRICORIU. 1965. La neurosecretion chez Trvchettr bukouskii Gedroye (Glossiphoniidae). Trav. Mus. Hist. Nat. "Grigore Antipa." 5: 99-105.
IWASAKI. S. , and Y. SATOW. 1971. Sodium- and calcium- dependent spike potentials in the secretory neuron soma of the X-organ. J . Physiol. (London), 57: 216-238.
K ~ Y S E R , K. T., and C. M. LENT. 1977. Onneuronal homologies within the nervous system of leeches. Comp. Biochem. Physiol. 58A: 285-297.
LENT. C. M. 1977. The Retzius cells within the central nervous system of leeches. Prog. Neurobiol. (N.Y.) 8: 8 I - 1 17.
MALECHA. J . 1970. Influence de la temperature sur la sper- matogentse et I'activite neurosecretrice d'Hirudo medicinalis L. (Hirudinee Gnathobdelliforme). Gen. Comp. Endocrinol. 14: 368-380.
MAYNARD, D. M. 1961. Thoracic neurosecretory structures in Brachyura. 11. Secretory neurons. Gen. Comp. Endocrinol. 1: 237-263.
MEOLA. S. M. 1970. Sensitive PF technique for neurosecretory system of mosquitoes. Trans. Am. Microsc. Soc. 89: 66-71,
NAMBUDIRI, P. N.. and K. P. VIJAYAKRISHNAN. 1958. Neurosecretory cells of the brain of the leech Hirudinrrria granulvsa (sav.). Curr. Sci. 27: 350-351.
NICHOLLS, J. G.. and D. A. BAYLOR. 1968. Specific modalities and receptive fields of sensory neurons in the CNS of the leech. J . Neurophysiol. 31: 740-756.
NICHOLLS, J . G., and D. VON ESSEN. 1974. The nervous system of the leech. Sci. Am. 230: 38-48.
ROWELL, H. F. 1976. The cells of the insect neurosecretory system: constancy, variability, and the concept ofthe unique identifiable neuron. Adv. Insect Physiol. 12: 63- 123.
RLJBIN. E. 1978. The caudal ganglion of the leech, with particu- lar reference to homologues of segmental touch receptors. J . Neurobiol. 9: 393-405.
RUDE, S. 1968. Monoamine-containing neurons in the central nervous system and peripheral nerves of the leech Hirudo medicinalis. J . Comp. Neurol. 136: 349-372.
1914 CAN. J. ZOOL. VOL. 57. 1979
S ~ N C H E Z , D. 1909. El sistema newiosa de 10s hirudineos. Trab. leech ganglia maintained in culture. Proc. R. Soc. London Lab. Invest. Biol. Univ. Madrid, 7: 31-199. Ser. B, 199: 567-585.
1912. EI sistema nerviosa de 10s hirudineos. Trab. Lab. YAGI, K., H. A. BERN, and I. R. HAGADURN, 1963. Action Invest. Biol. Univ. Madrid, 10: 1-143. potentials of neurosecretory neurons in the leech Therornyzon
TUMPLING, W., VON. 1965. Untersuchungen iiber neurosekre- rude. Gen. Comp. Endocrinol. 3: 490-495. tion bei Hirudineen. Z. Wiss. Zool. Abt. A, MI: 1-43. YAGI, K., and S. IWASAKI. 1977. Electrophysiology of the
WALLACE, B. G., M. N. ADAL, and J. G. NICHOLLS. 1977'. neurosecretory cell. Int. Rev. Cytol. 48: 141-186. Regeneration of synaptic connections by sensory neurons in