Molecular Therapeutic Strategies for Spinal Muscular Atrophies
-
Upload
khangminh22 -
Category
Documents
-
view
1 -
download
0
Transcript of Molecular Therapeutic Strategies for Spinal Muscular Atrophies
Clinical Therapeutics/Volume 36, Number 1, 2014
Molecular Therapeutic Strategies for Spinal MuscularAtrophies: Current and Future Clinical Trials
Chiara Zanetta, MD; Monica Nizzardo, PhD; Chiara Simone, PhD; Erika Monguzzi, PhD;Nereo Bresolin, MD; Giacomo P. Comi, MD; and Stefania Corti, MD, PhD
Dino Ferrari Centre, Neuroscience Section, Department of Pathophysiology and Transplantation (DEPT),University of Milan, Neurology Unit, IRCCS Foundation Ca’ Granda Ospedale Maggiore Policlinico,Milan, Italy
Accepted for publication November 22, 2013.http://dx.doi.org/10.1016/j.clinthera.2013.11.0060149-2918/$ - see front matter
& 2014 Elsevier HS Journals, Inc. All rights reserved.
ABSTRACT
Background: Spinal muscular atrophy (SMA) is anautosomal recessive motor neuron disease caused bymutations in the survival motor neuron gene (SMN1)and the leading genetic cause of infant mortality.Currently, there is no effective treatment other thansupportive care.
Objective: This article provides a general overviewof the main aspects that need to be taken into accountto design a more efficient clinical trial and to summa-rize the most promising molecular trials that arecurrently in development or are being planned forthe treatment of SMA.
Methods: A systematic review of the literature wasperformed, identifying key clinical trials involvingnovel molecular therapies in SMA. In addition, ab-stracts presented at the meetings of the Families ofSpinal Muscular Atrophy were searched and theFamilies of Spinal Muscular Atrophy Web site wascarefully analyzed. Finally, a selection of SMA clinicaltrials registered at clinical- trials.gov has been includedin the article.
Results: The past decade has seen a markedadvancement in the understanding of both SMAgenetics and molecular mechanisms. New moleculestargeting SMN have shown promise in preclinicalstudies, and various clinical trials have started to testthe drugs that were discovered through basic research.
Conclusions: Both preclinical and early clinical trialresults involving novel molecular therapies suggestthat the clinical care paradigm in SMA will soonchange. (Clin Ther. 2014;36:128–140) & 2014 Elsev-ier HS Journals, Inc. All rights reserved.
Key words: clinical trials, gene therapy, ISIS-SMNRx, olesoxime, oligonucleotides, small molecules,spinal muscular atrophy.
128
INTRODUCTIONSpinal muscular atrophies (SMAs) are a group ofhereditary autosomal recessive neuromuscular dis-eases that are characterized by the degeneration ofmotor neurons in the spinal cord and brainstem,resulting in progressive proximal muscle weakness,hyposthenia, and paralysis, which are usually sym-metrical. SMA is the most frequent genetic cause ofinfant mortality, with an estimated incidence of 1 in6000 to 1 in 10,000 live births and a carrier frequencyof 1 in 40 to 1 in 60.1,2 The classical form of thedisorder is caused by a genetic mutation3 in the5q11.2-q13.3 locus, which affects the survival motorneuron (SMN) gene4 and leads to the reduction ofSMN protein. SMA is clinically conventionallyclassified into 4 phenotypes (I, II, III, and IV) on thebasis of age of onset and highest motor functionachieved, with an additional phenotype (type 0) todescribe the severe forms with an antenatal onset.5
Prognosis depends on the phenotypic severity, rangingfrom high mortality within the first year for SMA typeI to no mortality for the chronic and later-onset forms.The increased attention to early diagnosis and to severalaspects of management of SMA has stimulated thedevelopment of clinical guidelines and standards ofcare.6,7 In the past decade, many promising newtherapeutic approaches have been tested in clinical trialsof patients with SMA8–11 but with limited or no success.At the present, no effective therapy is available for SMA,besides supportive care. Consequently, the developmentof novel therapies in SMA now has strong academic,government, and industry involvement, in addition to
Volume 36 Number 1
C. Zanetta et al.
the interest of several parental organizations and foun-dations. The goal of the present article was to summa-rize the literature on the emerging molecular therapeuticapproaches that are currently being investigated orplanned to be tested in clinical trials of SMA (Figure 1).
METHODSA systematic review of the English-language articleslisted in PubMed over the past 10 years was per-formed by using the following key words: spinalmuscular atrophy, SMA, oligonucleotides, gene ther-apy, molecular therapy, and small molecules. Allarticles found were systematically analyzed and takeninto consideration when preparing the review. Inaddition, abstracts presented at the annual 2010 to2013 meetings of the Families of Spinal MuscularAtrophy were searched by using the same key words,and the Families of Spinal Muscular Atrophy Web site(www.fsma.org) was carefully analyzed. Finally, aselection of SMA clinical trials registered at clinical-trials.gov has been included in the article.
RESULTSDesigning a Reasonable Clinical Trial for SMA:Issues to Address
This section analyzes some of the issues andconcerns that need to be taken into account to design
Spinal Muscular Atrophy D
Trophos/olesoxime
Identification Optimization Safety and manufa
ISIS - Biogen/ASO
Pfizer/quinazoline
Genzyme, Sanofi/genetherapy
PTC/Roche/smallmolecule
NW/gene therapy
Basicresearch
seedsideas
Preclinical: discovery
Figure 1. List of the main clinical trials based on moleongoing, illustrated by their stage of developmFood and Drug Administration; Trophos ¼ TBiogen ¼ ISIS Pharmaceuticals/Biogen Idec; NHoffmann-La Roche AG/PTC Therapeutics, In
January 2014
a reasonable clinical trial for the treatment of SMA.The increasing expansion of clinical trials planninghas raised several concerns, including the following:are there enough patients identified to enroll easily?What is the relative power of the study that can beobtained? In addition, it is imperative to discern ifthere are reliable, valid, and sensitive outcome meas-ures available.12
Many difficulties exist in undertaking randomized,double-blind, placebo-controlled studies of rare disor-ders such as SMA. These difficulties include the processof enrollment and stratification, even though interna-tional registries for patients have increased the chancesof identifying and recruiting patients for clinical trials. Interms of stratification, SMN2 copy number could beused as a stratification criterion because the number ofcopies of SMN2 correlates with motor functionachieved.13 Nevertheless, even in a relatively homo-geneous group of SMA type I patients, 2 cohorts ofsubjects can be identified: 1 characterized by an earlypresentation (genetic diagnosis o6 months) and anothercohort with a relatively late onset of symptoms (geneticdiagnosis46 months). Furthermore, another aspect thatmakes the analysis of the data even more complicated isthat the intensity of care can profoundly affectsurvival.14 Moreover, common standards of care, aswell as consistent assessment methods and outcome
rug Pipeline: Current
cturing Phase I Phase II Phase III
IND
Clinical deveopmentFDA
approval
cular strategies that have been conducted and areent. IND ¼ Investigational New Drug; FDA ¼ US
rophos SA; ASO ¼ antisense oligonucleotide; ISIS-W ¼ Nationwide Children’s Hospital; PTC/Roche ¼c/SMA Foundation.
129
Clinical Therapeutics
measures to make data from different centerscomparable, are needed when designing a clinical trial.15
Drug selection process and study design are factorsthat also need to be taken into consideration. Drugselection based on preclinical data may not be a reliablepredictor of a therapeutic effect in patients. Thus, it isimportant to define how preclinical models parallel thedisease in humans and which of the most predictiveoutcomes are for human SMA. Another important pointto address is the correct evaluation of drug pharmaco-kinetics in humans. For instance, it is fundamental todemonstrate that the target compound induces SMNprotein expression in the required cell types in vivo inhumans, following what it does in preclinical models.
Furthermore, the majority of Phase I and II trialshave enrolled patients with SMA types II and III;Phase III trial design is underdeveloped. Trial imple-mentation has to contend with various challenges:information on SMA populations is not complete,natural history data are not always reliable, and thedifferences in incidence and survival between coun-tries are unknown.
Treatment in late stages of the disease could partlyexplain past trial failures. Mouse studies found thatneonatal treatment is fundamental for efficacy, sug-gesting that a limited therapeutic window exists, atleast in animal models.16 It has been suggested that thefirst few months of life are the most critical time ofdenervation in SMA types I and II, leading to the ideathat implementation of neonatal trials and thedevelopment of strategies for identifying patients atpresymptomatic or early symptomatic stages would bethe best option for developing an efficient clinical trialfor SMA. Therefore, the timing of SMN rescue can bedifferent in patients with SMA type I compared withSMA type II or III patients.
Another difficulty is the absence of validatedmarkers of disease progression. For SMA type I,electrophysiologic measures such as motor unit num-ber estimation (MUNE) and compound muscle actionpotential (CMAP) may be better indicators of efficacythan survival.17 Furthermore, biomarkers of treatmenteffects are urgently needed. Currently, numerousoverlapping motor functional scales serve as clinicalend points. These should be tailored for presym-ptomatic, early symptomatic, and chronic stages foreach SMA subtype.
Overall, the main recommendations for conductinga reasonable clinical trial can be summarized as
130
follows: (1) testing of the preclinical effectiveness ofdrugs in SMA animal models; (2) planning of thefeasibility of each phase of the trials; (3) harmonizationof the European regulatory processes for drug approval;(4) targeting of the optimal therapeutic window forSMA; and (5) analysis of biomarkers (measures ofupper limb function for nonambulant patients, musclebiomarkers [MUNE/CMAP, muscle MRI, EIM] and theSMA Foundation’s biomarker panel) (Table).
The balance of this review provides information onthe main clinical trials based on molecular strategiesthat have been conducted and are ongoing (Figure 1).
Neuroprotection Strategy for SMA: Olesoxime(TRO19622)
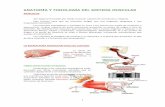
Olesoxime (TRO19622) is a small molecule with acholesterol-like structure that displays strong neuro-protective properties.18 It has demonstrated ef-fectiveness in keeping motor neurons alive in culture.Preclinical in vitro studies have shown that thecompound promotes the function and survival ofneurons and other cell types under disease-relevantstress conditions when interacting with the mitochon-drial permeability transition pore (Figure 2A).
In October 2010, Trophos SA initiated a pivotalefficacy and safety study of olesoxime, in �150 SMApatients, in a program funded by the AssociationFrancaise contre les Myopathies in France. Thisefficacy and safety study is a 24-month, Phase II,multicenter, randomized, adaptive, double-blind,placebo-controlled study in nonambulant patientsaged 3 to 25 years with SMA type II and type III.19
Recruitment of 165 patients with SMA was completedin September 2011; no further patients can beenrolled. Patients in the study are treated for 2years, and results are expected before the end of2013. Trophos has been granted orphan drugdesignation for olesoxime for the treatment of SMAby the US Food and Drug Administration (FDA). Inthe European Union, olesoxime has been grantedOrphan Medicinal Product designation for SMA bythe European Commission.20 Randomization was 2:1for olesoxime or matching placebo. The subjectsenrolled in this study received either a liquidsuspension formulation of olexosime (100 of 150patients; 100 mg/mL at a dose of 10 mg/kgadministered orally once a day with food at dinner)or a liquid suspension formulation of placebo (50 of150) always administered once a day with food at
Volume 36 Number 1
Table. Description of biomarkers, functional scales, and electromyography measures conventionally used in the spinal muscular atrophy (SMA)field. SMA-MAP biomarkers: list of top 13 SMA motor function regressor markers, called SMA-MAP, in 2 SMA populations. Twenty-sevenanalytes were selected for inclusion into a new biomarker panel, called SMA-MAP. The 13 analytes that regressed to motor outcomes ofSMA in both the BforSMA (Biomarker for SMA) study and PNCR NHS (Pediatric Neuromuscular Clinical Research Network HistoryStudy) were included in the panel and are shown in the table. A description of the main functional scales and electromyography measuresconventionally used is provided.
Variable Full Name Function
CorrelatedOutcomeMeasures
Correlation to ModifiedHFMS
PNCRNHS P
BforSMAP
SMA-MAP biomarkersCOMP Cartilage oligomeric
matrix proteinCell proliferation, apoptosis, cell movement andattachment; binds strongly to calcium
Motor o0.001 o0.001MUNEStrength
AXL Receptor tyrosinekinase
Transduces signals from the extracellular matrixby binding growth factor GAS6 regulating: cellsurvival, cell proliferation, migration, anddifferentiation
FVC o0.001 o0.001MotorPQP
CD93 Cluster ofdifferentiation 93
Cell to cell adhesion, clearance of apoptotic cells Motor o0.001 o0.001MUNEPQCStrength
PEPD Peptidase D Splits dipeptides with a prolyl or hydroxyprolylresidue in the C-terminal position; plays animportant role in collagen metabolism
CMAP o0.001 o0.001MotorMUNEStrength
THBS4 Thrombospondin-4 Adhesive glycoprotein, mediates cell-to-cell andcell-to-matrix interactions
Motor o0.001 o0.001Mune
LUM Lumican Regulates: collagen fibril organization andcircumferential growth, corneal transparency,epithelial cell migration, and tissue repair
CMAP o0.001 o0.001MotorMUNEStrength
MB Myoglobin Myoglobin is the primary oxygen-carrying pigment of muscles
FVC 0.001 o0.001MotorStrength
C.Zan
ettaet
al.
January
2014
131
Table (continued).
Variable Full Name Function
CorrelatedOutcomeMeasures
Correlation to ModifiedHFMS
PNCRNHS P
BforSMAP
DPP4 Dipeptidylpeptidase-4
Antigenic enzyme expressed on the surface ofmost cell types associated with: immuneregulation, signal transduction, and apoptosis
Motor 0.001 o0.001MUNEPQPStrength
SPP1 Secretedphosphoprotein 1,also known asosteopontin
Has a role in: biomineralization, boneremodeling, immune response, chemotaxis,apoptosis, and cell activation
Motor 0.002 o0.001
CHI3L1 Chitinase-3–likeprotein 1
Catalyzes the hydrolysis of chitin; has a role in:Inflammation and tissue remodeling
Motor 0.01 o0.001
CDH13 Cadherin 13, alsoknown as H-cadherin (heart)
Roles: mediation of intracellular signaling invascular cells, regulation of cell growth, guidingmolecule in vascular and nervous system
Motor 0.039 0.001
APCS Amyloid P component Interacts with DNA and histones, scavengesnuclear material released from damagedcirculating cells
Motor 0.041 o0.001
LEP Leptin Inhibits appetite, acts as adiposity signal,interacts with amylin, mediates the feeling ofsatiety, promotes angiogenesis, promotes theactivity of lung surfactant, acts on bonemetabolism
Motor 0.058 o0.001
Functional scalesMFM Motor function
measureQuantitative scale that makes it possible tomeasure the functional motor abilities ofpatients affected by neuromuscular diseases;whatever the diagnosis and the extent of motordeficiencies, MFM allows us to: specify the
— —
(continued)
Clin
icalTherap
eutics
132
Volume36Number
1
Table (continued).
Variable Full Name Function
CorrelatedOutcomeMeasures
Correlation to ModifiedHFMS
PNCRNHS P
BforSMAP
symptoms and the evolution of neuromusculardiseases; objectivize the repercussion of thetherapeutic measures; direct the rehabilitationand adaptation measures; facilitatecommunication between the various persons incharge of care; and select homogeneousgroups of patients in view of therapeutic trials
HFMS HammersmithFunctional MotorScale
Functional motor scale that can be used to:assess gross motor abilities of nonambulantchildren with SMA, monitor progression andgross motor abilities over time
— —
Electromyographymeasures
CMAP Compound muscleaction potential
The size of the evoked CMAP reflects the numberof muscle fibers accessible from the stimulatednerve and will be reduced by atrophy (fromwhatever cause) by conduction block in theperipheral nerve or at the neuromuscularjunction
— —
MUNE Motor unit numberestimation
They have been used to study the rate of motorunit loss in patients affected by neuromusculardisorders
— —
FVC ¼ forced vital capacity; PQP ¼ PedQL quality of life, parent score; PQC ¼ PedQL quality of life, child score.
C.Zan
ettaet
al.
January
2014
133
Quinazolines
876876
Exons
6 8 6 7 8
With quinazolines
Dcp2Dcp1
Exon 7 inclusion
Olesoxime
Environment
a
C
Membrane
ATP Cytoplasm
ADP + P
A Bα δ
αβ αβ
H H H
F
H
b
F
Y
Increased:• SMN protein levels• Size and strength of muscle fibers• Number of spinal cord motor neurons• Median survival
7 86
Injection into thecerebral ventriclesand spinal cord ofan SMA mouse
Generation of antisenseoligonucleotides ormorpholino (base pairwith an intronic splicingsilencer), allowing incorporation of exon 7 intoSMN2-nRNA
No drugIntrons
SMN2 DNA
SMN2mRNA
SMN2Pre-mRNA
C
Figure 2. Mechanisms of action of main molecular therapeutic strategies that are currently tested in clinicaltrials for spinal muscular atrophy (SMA). (A) Olesoxime targets proteins of the outer mitochondrialmembrane and prevents permeability transition pore opening mediated by, among other things,oxidative stress. (B) Antisense oligonucleotides (ASOs), with different chemical structures includingmorpholino chemistry, designed to target and block intronic splicing silencer number one (ISSN-1)are able to promote exon 7 inclusion in the majority of survival motor neuron gene (SMN2) mRNAtranscripts and therefore lead to the production of full-length and functional SMN protein. (C)Quinazolines are a family of compounds that inhibits RNA decapping enzymes (DcpS; Dcp 1 and 2are represented in the figure) involved in RNA turnover. This inhibition can consequently increasefull-length SMN2 transcript and SMN protein.
Clinical Therapeutics
dinner. The primary end point of the study is thechange from baseline in the Motor Function Measure(MFM) functional scale. Secondary end points includethe Hammersmith Functional Motor Scale (HFMS)and electromyography (CMAP and MUNE) as well asmeasures of safety, tolerance, and quality of life. Thestudy is being conducted at 22 centers in 7 Europeancountries. Inclusion criteria include weakness andhypotonia consistent with a clinical diagnosis of
134
SMA type II or III and laboratory documentation ofhomozygous absence of SMN1 exon 7 and/or deletionand mutation on other alleles. To be included, subjectshad to have an MFM relative score (percentage of themaximum sum of both dimensions) Z15% (D1 þ D2score) and HFMS score at baseline Z3. The studyenrolled nonambulant patients, defined as patientswith an HFMS score r38, aged 3 to 26 years attime of enrollment. The patients’ age at onset of
Volume 36 Number 1
C. Zanetta et al.
symptoms had to be r3 years. Additional inclusioncriteria were normal laboratory test results and theability to take the study treatment. Exclusion criteriawere significant comorbidities and administration ofother medications intended for SMA treatment. Theongoing study period was divided into 3 stages: stage1, three-month safety assessment to confirm theadequate dose after 1 month of treatment (an inde-pendent data monitoring committee [DMC] has thegoal of assessing the safety of olesoxime every 3months); stage 2, efficacy/futility analyses at 1 year(a first-interim efficacy analysis was planned; afterthat, all patients will be treated for 1 year [52 weeks]to assess the need to continue the study to reach theplanned objectives); and stage 3, efficacy and safetyanalysis at 2 years.
Trophos announced the completion of the interimanalysis of the pivotal efficacy study of olesoxime. Theindependent DMC has reviewed the treatment effectsat 1 year, taking into account the primary outcomemeasures of efficacy and changes in motor function(MFM scale), along with the latest safety reportincluding ECG tracings, periodic laboratory findings,hemostatic parameters, and serious adverse eventlistings for all participants. Based on the trial-stopping criteria as defined in the protocol, as wellas the absence of safety concerns related to olesoximetreatment, the DMC recommendation is to continuethe study as planned. Following the recommendationsof the DMC, the study will continue until all partic-ipants are treated for 2 years, with finalization of thelast patient scheduled for September 2013. Top-lineresults are expected by the end of 2013.20,21
Oligonucleotides Therapy for SMA: ISIS-SMNRx
SMA is caused by the loss of the SMN1 gene,leading to reduced SMN protein levels. The humangenome harbors an additional SMN2 gene (or genes)that produces low levels of full-length SMN butcannot adequately compensate for the loss of SMN1due to an aberrant splicing. The majority of SMN2gene transcript lacks exon 7 and the resultant SMNΔ7mRNA is translated into an unstable but partiallyfunctional protein. Therapeutic strategies with thegoal of promoting exon 7 retention, thus increasingfull-length SMN2 transcript, are promising treatmentsfor patients with SMA. Different splice-silencing se-quences in SMN2 have been identified as potentialtargets for splice modifications directed by antisense
January 2014
oligonucleotides. A particularly strong splice silenceris found in SMN2 intron 7 downstream of exon 7.Antisense oligonucleotides designed against this motifpromote SMN2 exon 7 retention in the mature SMN2transcripts, increasing SMN protein expression inSMA cells (Figure 2B).22
Isis Pharmaceuticals is developing a specific oligo-nucleotide called ISIS-SMNRx designed to act againstthis strong splicing silencer to promote the inclusion ofthe missing exon (exon 7); the goal is to create anmRNA that codes for the production of the full-lengthfunctional SMN protein for proper motor neuronfunction. The FDA granted orphan drug status andfast track designation to ISIS-SMNRx for the treatmentof patients with SMA. Isis is currently in collaborationwith Biogen Idec to develop and potentially commerci-alize this compound to treat all types of SMA. Underthe terms of the January 2012 agreement, Isis isresponsible for global development and Biogen Idechas the option to license the compound until com-pletion of the first successful Phase II/III study. Severalnonprofit organizations (including Families of SpinalMuscular Atrophy) also support this study. ISIS-SMNRx is a uniformly 2′-O-methoxyethyl modifiedantisense drug that corrects the splicing disorder inSMN2, resulting in the production of fully functionalSMN protein in model systems. In mild and severeSMA mouse models, it is able to provide both aphenotypic and a pathologic benefit when deliveredcentrally23,24 and has a long half-life in centralnervous system tissue (46 months in animal models).The results in preclinical studies were very promising,with rescue of the SMA phenotype with early systemictreatment.25
Isis has completed a Phase I, open-label, safety,tolerability, and dose range–finding study with thepurpose of testing the safety, tolerability, and phar-macokinetics of a single dose of ISIS-SMNRx admin-istered into the cerebrospinal fluid (CSF) as a singleinjection in patients with SMA.26 This study wasstarted in November 2011 and was completed inJanuary 2013. A total of 28 subjects were enrolledin this trial, and all of them completed the study; nohealthy volunteers were accepted. The inclusion criteriaof this study were: a documented SMN1 homozygousgene deletion and clinical signs attributable to SMA, age2 to 14 years at time of screening, the ability to completeall study procedures, and the demonstration thatparents/patients have adequate supportive psychosocial
135
Clinical Therapeutics
circumstances. Moreover, the estimated life expectancyhad to be 42 years from the time of screening. Themajor exclusion criteria were: respiratory insufficiencydefined by the need for invasive or noninvasiveventilation during a 24-hour period, presence of a gastricfeeding tube, previous scoliosis surgery or scoliosissurgery planned during the duration of the study thatwould interfere with the lumbar puncture injectionprocedure, conditions that interfere with intrathecaladministration of the drugs, severe comorbidities, ortaking medications aimed at treating SMA. Four doselevels have been sequentially evaluated. Each dose levelwas studied in a cohort of 6 or 10 patients; all patientsreceived active drug. Primary end points of the studywere safety/tolerability and CSF and plasma drug levelpharmacokinetics. Safety and tolerability assessmentswere obtained through analysis of adverse events,neurologic examinations, CSF laboratory tests, vitalsigns, clinical laboratory tests, physical examinationsand weight reports, ECGs, and the analysis of concom-itant medication use. Pharmacokinetic measures usedwere plasma levels of the drug (over 24 hours’ postdos-ing and at 7 days’ postdose) and CSF levels of drug (at 7days’ postdose).
Exploratory end points of this study of ISIS-SMNRx
were HFMS and neuromuscular electrophysiologyCMAP/MUNE.26 Safety and tolerability results canbe summarized as follows: (1) ISIS-SMNRx was welltolerated, with no significant safety finding whengiven as a single dose up to 9 mg; (2) the injectionprocedure was well tolerated and was shown to befeasible; and (3) data suggest that the tolerability ofthe injection procedure is improved with the use of asmaller needle (ie, 24 or 25 gauge). In terms of drugconcentrations, CSF and plasma drug levels weremeasurable, and they were dose dependent and onlymoderately variable. Through the use of HFMS, itwas evaluated that at day 85, there was a meanchange in motor function from baseline of 3.1 points(P ¼ 0.02) and a percentage of change of 17.6%.Moreover, 6 of 10 subjects had a change from baselinemajor of 4 points from their basal level score at theHFMS (3 of 6 of these subjects were aged 45 years).CMAP and multipoint incremental MUNE were per-formed in the highest-dose group at baseline andat day 85, with the primary purpose of determine thefeasibility of using this method in patients with SMA.These examinations were performed on the rightulnar nerve that innervates the abductor digiti minimi.
136
Electrophysiology measurements demonstrated stableCMAP, with a potential increase in MUNE. Theconclusions and implications of this Phase I study arethat ISIS-SMNRx was well tolerated at all dose levels,and no safety or tolerability concerns were identified.The injection procedure used was shown to be feasiblein children with SMA. CSF and plasma drug concen-trations observed were dose dependent and consistentwith preclinical data, supporting infrequent administra-tion (ie, every 6–9 months). An improvement in HFMSscores was observed in the group injected with thehighest dose. Electrophysiology measurements in thehighest-dose cohort at 3 months demonstrated anincrease in MUNE with stable CMAP.
In September 2013, the preliminary results of theopen-label Phase I study of ISIS-SMNRx in childrenwith SMA (24 subjects) were reported; they high-lighted that the majority of children with SMAreceiving the 2 highest doses of the drug (6 and 9mg) continued to show improvements in musclefunction tests (mean HFMS improvement with a doseof 9 mg of ISIS-SMNRx : 5.75) up to 14 months after asingle injection of the drug.21,27 The amelioration wasdose dependent, and no decline was observed.Although the data are encouraging, it must be empha-sized that no placebo was included in the study. Basedon this evidence, the investigators have amended theinfant study to increase the dose from 9 to 12 mg.28 Inaddition to the infant pilot study, Isis is alsocompleting a multiple-dose, dose-escalating Phase Ib/IIa study of ISIS-SMNRx in children with SMA type IIand III. Isis has completed dosing in all 3 dose cohorts(3, 6, and 9 mg) and is now considering adding a 12-mg dose cohort to this study.29
ISIS-SMNRx is now being studied in a multiple-dose Phase II study27,30 that is designed with the goalof testing the safety, tolerability, and pharmacoki-netics of multiple doses of the drug administered intothe spinal fluid 3 times over the duration of the trial inpatients with infantile-onset SMA. Two dose levelswill be evaluated sequentially. Each dose level will bestudied in a cohort of 4 patients; all patients willreceive active drug.30 The estimated number ofsubjects enrolled in the study is 8, and the eligibleage is up to 210 days. No healthy volunteers havebeen accepted. The inclusion criteria are: geneticdocumentation of 5q SMA (homozygous gene dele-tion or mutation), onset of clinical signs and symptomsconsistent with SMA at Z21 days and o6 months
Volume 36 Number 1
C. Zanetta et al.
(180 days) of age at study entry, receiving adequatenutrition and hydration, body weight 45th percentilefor age according to Centers for Disease Control andPrevention guidelines, medical care that meets and isexpected to continue meeting guidelines set out in theConsensus Statement for Standard of Care in SpinalMuscular Atrophy6 in the opinion of the site investi-gator, gestational age of 35 to 42 weeks, and gesta-tional body weight Z2 kg. Moreover, patients need toreside within �9 hours’ ground-travel distance from aparticipating study center for the duration of the study(residence 42 hours’ ground-travel distance from astudy center can require special clearance).30
Major exclusion criteria are: hypoxemia (oxygensaturation awake o96% or oxygen saturation asleepo96%, without ventilation support) and significantcomorbidities, in particular those interfering with CSFadministration, or treatment with another investiga-tional drug. The main primary outcome measure ofthe study is the number of participants with adverseevents (participants will be followed up for theduration of the study). Secondary outcome measuresare: (1) plasma pharmacokinetics (assessments at 1, 2,4, and 24 hours after dosing); (2) Cmax; (3) Tmax; and(4) the AUC from the time of the intrathecal dose tothe last collected sample (20 hours after dosing).30
The infantile-onset study might provide importantinformation related to the therapeutic windows ofSMN rescue.
Small Molecules for SMA: QuinazolinesQuinazolines are a family of compounds that
inhibits RNA decapping enzyme DcpS (involved inRNA turnover). This inhibition can consequentlyincrease SMN2 expression (Figure 2C).31 A high-throughput screening identified a few quinazolines aslead compounds that increase full-length SMN2 tran-script and SMN protein.32 These compounds hadpoor blood–brain barrier (BBB) penetration andrequired high doses to achieve a sufficient upregula-tion of SMN. Based on a lead quinazoline compound,derivatives were developed to overcome the obstaclesassociated with the low BBB penetration. Furtheroptimization of this compound led to the identifi-cation of D157495, also called RG3039, whichameliorates the phenotype of SMA mice.16,33
Currently, RG3039 is undergoing a Phase I clinicaltrial in which the safety and efficacy of various doseswill be evaluated.21 This compound is included in
January 2014
the Pfizer SMA Drug Development Programs asPF-06687859. A Phase I, first-in-man, double-blind,placebo-controlled, ascending single-dose, safety andpharmacokinetics study in healthy volunteers has beenconducted. Two of 4 cohorts of a multidose Phase Istudy in 16 healthy volunteers have been completed,with no further patient enrollment. A biomarker andoptimal dosing plan is being generated, as well asbackup compounds, and enabling technologies havebeen developed.
Planned Clinical TrialsGene Therapy
Gene therapy promises a permanent solution forSMA through viral delivery and insertion of the entireSMN1 gene or cDNA sequence into the genome ofpatients with SMA.31 This process is generallyirreversible, and there are apparent risks if theexogenously delivered gene is inserted at a wronglocation and/or overexpressed. Overall, the results ofgene therapy aiming at SMN1 delivery by scAAV9 inSMA mice and large animals seem promising and mayoffer one of the best therapeutic alternatives to aspecific group of SMA patients who are too weak toreceive frequent invasive treatments.
After submission of an Investigational New Drugapplication, the FDA has given its approval tophysician/scientists at Nationwide Children’s Hospitalin Columbus, Ohio, to begin a Phase I clinical trial ofa systemic AAV9-delivered human SMN gene.21 Theclinical trial is expected to begin in early 2014 and willbe limited to patients with type I SMA, with an agerange of 0 to 9 months. Previous research fromNationwide Children’s Hospital Principal Investiga-tor Brian Kaspar, PhD, demonstrated that the AAV9viral vector is able to cross the BBB. Based on thesefindings and additional preclinical studies, the SMNgene will be delivered by injection into the blood-stream as part of this Phase I trial. Neurologist JerryMendell, MD, director of the Center for GeneTherapy at Nationwide Children’s Hospital, willlead the study. They plan to recruit 9 subjects, withan age of r6 months with proven SMN mutation andretaining 2 copies of SMN2. Exclusion criteria are theuse of ventilatory support or pulse oximetry satura-tion o95% and high levels of antibody directedagainst AAV9.
Physician/scientists are also planning a dose esca-lation study (low and high dose). The improvement
137
Clinical Therapeutics
function will be determined by SAFETY/CHOP-IN-TEND (Children's Hospital of Philadelphia InfantTest of Neuromuscular Disorders). The plannedfollow-up is at 1, 2, 4, 8, and 12 weeks and thenevery 3 months until 2 years of age. An additionalstudy is planned using the AAV9-delivered SMN gene,with the aim of examining a different route ofadministration (injecting the virus into the CSF). Thesafety study has been completed in large mammals,and the study is proceeding toward the pre–Investiga-tional New Drug stage at the FDA.
Moreover, Genzyme/Sanofi are planning a SMN1gene replacement therapy program. They are prepar-ing to nominate a vector candidate for preclinicaltoxicology studies and for studies in nonhumanprimates to optimize delivery protocol and dose.Overall, gene therapy is one of the most promisingnew approaches, given its potential effectiveness inresolving the SMA molecular defect.21
Small MoleculesSmall molecule compounds aimed at increasing
SMN levels offer several advantages, including aneasy transport across biological barriers. Consideringthat SMA is a neurodegenerative disease, compoundsthat are transported across the BBB would be bestsuited for an effective therapy.
Small molecule SMN2 splicing modifiers, whichincrease full-length SMN mRNA and protein in cellsisolated from SMA patients, are being developed byHoffmann-La Roche AG/PTC Therapeutics, Inc/SMAFoundation.21 In August 2013, PTC Therapeuticsannounced the selection of a development candidate.This approach could be advantageous if an easyadministration route (eg, oral administration) ispossible. However, this strategy may be limited bythe possibility of off-target effects.
CONCLUSIONSCurrently, no effective treatment is available for SMAand other connected motor neuron disorders. Themain therapeutic strategies that are now in use arebased on symptomatic treatment and supportive care.At a preclinical level, many therapeutic strategies havebeen investigated, and some of them have been testedand continue to be tested in clinical trials both inEurope and in the United States. This review outlinedthe principal aspects that should be taken into accountwhen designing a reasonable clinical trial. Moreover,
138
we also provided a description of the most promisingclinical trials that are currently ongoing and/or areplanned for the near future.
The overall evaluation is that an increasing num-ber of clinical studies over the next few years areexpected for SMA. One issue that is noteworthy andunique for SMA is that the phenotype of patientsvaries significantly among different SMA types (I, II,and III). Moreover, even in a relatively homogenousgroup of SMA type I patients, 2 cohorts of sub-jects can be identified: 1 characterized by an earlypresentation (genetic diagnosis o6 months) andanother 1 with a relatively late onset of symptoms(genetic diagnosis 46 months). This aspect cannotbe ignored when planning a reasonable clinical trialfor SMA. SMN2 copy number directly correlateswith both clinical phenotype and highest motorfunction achieved by patients and could therefore beused as a stratification criterion. Therefore, a power-ful strategy could be to design different clinical trialsaccording to each SMA subtype. Although manytherapeutic strategies for SMA (eg, stem cell therapy)are being developed, what now seems certain is thatmore preclinical studies are needed before thosestrategies could be investigated in clinical trials andtherefore used for the treatment of SMA in humanpatients.
Overall, the results of both preclinical and earlyclinical trials involving novel molecular therapiessuggest that the clinical care paradigm in SMA willsoon change.
ACKNOWLEDGEMENTSThis work was supported by the Ministry of Health(GR-2009-1483560) and MIUR (FIRB RBFR08RV86)to Dr. Corti. The authors also thank the “AssociazioneAmici del Centro Dino Ferrari” for their support. Dr.Zanetta was responsible for the study design, literaturesearch, figure creation, writing, main revision. Dr.Monguzzi was responsible for the literature searchand revision. Dr. Corti was responsible for the studydesign, literature search and writing. All remainingauthors contributed equally in the literature searchand revision processes.
CONFLICTS OF INTERESTThe authors have indicated that they have no conflictsof interest regarding the content of this article.
Volume 36 Number 1
C. Zanetta et al.
REFERENCES1. Ogino S, Leonard DG, Rennert H,
et al. Genetic risk assessment incarrier testing for spinal muscularatrophy. Am J Med Genet.2002;110:301–307.
2. Prior TW. Spinal muscular atrophy:a time for screening. Curr Opin
Pediatr. 2010;22:696–702.3. Brzustowicz LM, Lehner T, Castilla
LH, et al. Genetic mapping of chronicchildhood-onset spinal muscularatrophy to chromosome 5q11.2-13.3. Nature. 1990;344:540–541.
4. Lefebvre S, Burglen L, Reboullet S,et al. Identification and character-ization of a spinal muscularatrophy-determining gene. Cell.1995;80:155–165.
5. Munsat TL, Davies KE. InternationalSMA consortium meeting (26-28June 1992, Bonn, Germany). Neuro-muscul Disord. 1992;2:423–428.
6. Wang CH, Finkel RS, Bertini ES,et al. Consensus statement forstandard of care in spinal muscularatrophy. J Child Neurol. 2007;22:1027–1049.
7. D’Amico A, Mercuri E, Tiziano FD,et al. Spinal muscular atrophy. Or-phanet J Rare Dis. 2011;6:71.
8. Swoboda KJ, Scott CB, Reyna SP,et al. Phase II open label study ofvalproic acid in spinal muscularatrophy. PLoS One. 2009;4:e5268.
9. Swoboda KJ, Scott CB, CrawfordTO, et al. Project Cure Spinal Mus-cular Atrophy Investigators Network.SMA CARNI-VAL trial part I: dou-ble-blind, randomized, placebo-controlled trial of L-carnitine andvalproic acid in spinal muscularatrophy. PLoS One. 2010;5:e12140.
10. Tiziano FD, Lomastro R, Pinto AM,et al. Salbutamol increases survivalmotor neuron (SMN) transcript lev-els in leucocytes of spinal muscularatrophy (SMA) patients: relevancefor clinical trial design. J Med Genet.2010;47:856–858.
11. Kissel JT, Scott CB, Reyna SP, et al.Project Cure Spinal Muscular Atro-phy Investigators’ Network. SMA
January 2014
CARNIVAL TRIAL PART II: a pro-spective, single-armed trial of L-carnitine and valproic acid in ambu-latory children with spinal muscularatrophy. PLoS One. 2011;6:e21296.
12. Cano SJ, Mayhew A, Glanzman AM,et al. Rasch analysis of clinical out-come measures in spinal muscularatrophy. Muscle Nerve. 2013 Jul 8[Epub ahead of print].
13. Tiziano FD, Bertini E, Messina S,et al. The Hammersmith functionalscore correlates with the SMN2 copynumber: a multicentric study. Neuro-muscul Disord. 2007;17:400–403.
14. Rudnik-Schöneborn S, Berg C,Zerres K, et al. Genotype-phenotypestudies in infantile spinal muscularatrophy (SMA) type I in Germany:implications for clinical trials andgenetic counselling. Clin Genet. 2009;76:168–178.
15. Mercuri E, Bertini E, Iannaccone ST.Childhood spinal muscular atrophy:controversies and challenges. LancetNeurol. 2012;11:443–452.
16. Lutz CM, Kariya S, Patruni S, et al.Postsymptomatic restoration ofSMN rescues the disease phenotypein a mouse model of severe spinalmuscular atrophy. J Clin Invest.2011;121:3029–3041.
17. Swoboda KJ, Prior TW, Scott CB,et al. Natural history of denervationin SMA: relation to age, SMN2 copynumber, and function. Ann Neurol.2005;57:704–712.
18. Bordet T, Buisson B, Michaud M,et al. Identification and character-ization of cholest-4-en-3-one, oxime(TRO19622), a novel drug candi-date for amyotrophic lateral sclero-sis. J Pharmacol Exp Ther. 2007;322:709–720.
19. ClinicalTrial.gov. Safety and efficacyof olesoxime (TRO19622) in 3-25years SMA patients. http://clinicaltrials.gov/show/NCT01302600. Ac-cessed October 8, 2013.
20. Trophos. Therapeutic programs, spi-nal muscular atrophy. http://www.trophos.com/research/spinal.htm.Accessed June 15, 2013.
21. Families of Spinal Muscular Atrophy(FSMA). http://www.fsma.org. Ac-cessed October 8, 2013.
22. Porensky PN, Burghes AH. Antisenseoligonucleotides for the treatmentof spinal muscular atrophy. Hum
Gene Ther. 2013;24:489–498.23. Hua Y, Sahashi K, Hung G, et al.
Antisense correction of SMN2 splic-ing in the CNS rescues necrosis in atype III SMA mouse model. Genes
Dev. 2010;24:1634–1644.24. Passini MA, Bu J, Richards AM, et al.
Antisense oligonucleotides deliveredto the mouse CNS ameliorate symp-toms of severe spinal muscular atro-phy. Sci Transl Med. 2011;3:72ra18.
25. Hua Y, Sahashi K, Rigo F, et al.Peripheral SMN restoration is essen-tial for long-term rescue of a severespinal muscular atrophy mousemodel. Nature. 2011;478:123–126.
26. ClinicalTrial.gov. An open-label safety,tolerability, and dose-range findingstudy of ISIS SMNRx in patients withspinal muscular atrophy. http://clinicaltrials.gov/ct2/show/NCT01494701.Accessed October 7, 2013.
27. Isis Pharmaceuticals. http://www.isispharm.com/index.htm. AccessedOctober 7, 2013.
28. ClinicalTrial.gov. An open-labelsafety and tolerability study of ISISSMNRx in patients with spinal mus-cular atrophy who previously par-ticipated in ISIS 396443-CS1.http://clinicaltrials.gov/ct2/show/NCT01780246. Accessed October 7,2013.
29. ClinicalTrial.gov. An open-labelsafety, tolerability and dose-rangefinding study of multiple doses ofISIS SMNRx in patient with spinalmuscular atrophy (SMNRx-CS2).(http://clinicaltrials.gov/ct2/show/NCT01703988. Accessed September15, 2013.
30. ClinicalTrial.gov. A study to assessthe safety and pharmacokinetics ofISIS SMNRx in infants with spinalmuscular atrophy. http://www.clinicaltrials.gov/ct2/show/NCT01839656.Accessed October 6, 2013.
139
Clinical Therapeutics
31. Singh J, Salcius M, Liu SW, et al.DcpS as a therapeutic target forspinal muscular atrophy. ACS Chem
Biol. 2008;3:711–722.32. Jarecki J, Chen X, Bernardino A,
et al. Diverse small-molecule modu-lators of SMN expression found byhigh-throughput compound screen-ing: early leads towards a therapeu-tic for spinal muscular atrophyHum. Mol Genet. 2005;14:2003–2018.
33. Thurmond J, Butchbach ME, Pal-omo M, et al. Synthesis and bio-logical evaluation of novel 2,4-diaminoquinazoline derivatives asSMN2 promoter activators for thepotential treatment of spinal mus-cular atrophy. J Med Chem. 2008;51:449–469.
140
Address correspondence to: Stefania Corti, MD, PhD, IRCCS FoundationCa’ Granda Ospedale Maggiore Policlinico, via Francesco Sforza 35,20122 Milan, Italy. E-mail: [email protected]
Volume 36 Number 1