Measurement of Fecal Testosterone Metabolites in Mice - MDPI
Interactions among impulsiveness, testosterone, sex hormone binding globulin and androgen receptor...
Transcript of Interactions among impulsiveness, testosterone, sex hormone binding globulin and androgen receptor...
Physiology & Behavior 147 (2015) 91–96
Contents lists available at ScienceDirect
Physiology & Behavior
j ourna l homepage: www.e lsev ie r .com/ locate /phb
Interactions among impulsiveness, testosterone, sex hormone bindingglobulin and androgen receptor gene CAG repeat length
Anton Aluja a,b,⁎, Luís F. García b,c, Maite Martí-Guiu a,b, Eduardo Blanco a,b, Oscar García d,J. Fibla a,b, Àngel Blanch a,b
a University of Lleida, Spainb Institute of Biomedical Research of Lleida (IRB Lleida), Spainc Autonomous University of Madrid, Spaind European University of Madrid, Spain
⁎ Corresponding author at: Department of Psychology,(IRB Lleida), Avda. Estudi General, 4, University of Lleida,
E-mail address: [email protected] (A. Aluja).
http://dx.doi.org/10.1016/j.physbeh.2015.04.0220031-9384/© 2015 Elsevier Inc. All rights reserved.
a b s t r a c t
a r t i c l e i n f oArticle history:Received 22 December 2014Received in revised form 7 April 2015Accepted 10 April 2015Available online 16 April 2015
Keywords:AR CAG repeat lengthTestosteroneSex hormone binding globulinPersonality
Impulsive personality phenotype has been extensively relatedwith genetic and hormonal factors. This study hastwo objectives: a) to analyse the interactions between testosterone levels and CAG repeat length polymorphismas a modulator of androgen receptor (AR) sensitivity with regard to impulsiveness traits, and b) to evaluate thecontribution of other biological variables as Luteinising Hormone, Follicle Stimulating Hormone, Sex HormoneBindingGlobulin (LF, FSH, SHBG) and albumin in the relationship between testosterone levels and AR CAG lengthpolymorphism with impulsiveness. A sample of 105 healthy males (mean age 26.71 ± 9.68 SEM) was analysedresulting in three groups of subjects according to CAG repeat lengths. Impulsiveness was measured through theBarratt's Impulsiveness Personality Scale, including three components: Motor Impulsiveness, Cognitive Impul-siveness and Non-Planning Impulsiveness. A series of ANOVAS and linear regression models predicting impul-siveness scales were conducted. Age, hormones, CAG repeat length and hormone × CAG repeat interactionswere included in the regression models as independent variables. Results show that subjects with short or me-dium CAG repeat length tended to show higher impulsiveness phenotypes compared to long CAG repeat. The in-teraction between Free Testosterone and CAG, and between SHBG and CAG accounted for differences onimpulsiveness (R: .47, R2: .22 and R: .43; R2: .18, respectively).This pattern was especially observed for theshort CAG repeat group and Motor Impulsiveness.
© 2015 Elsevier Inc. All rights reserved.
1. Introduction
The human androgen receptor (AR) gene is located on the long arm(Xq11–12) of the X chromosome. In a normal population, this triplet isrepeated 8–31 times (average ≃21) and tends to be normally distribut-ed. It contains a CAG (glutamine) repeat polymorphism in exon 1whichis inversely associated with transcriptional activity of the AR [1]. Conse-quently, differences in the number of CAG repeats could affect the po-tency of androgenic action [2] with lower number of CAG repeatsassociated with a greater sensitivity to androgens and increased tran-scriptional activity. The trinucleotide (CAG) repeat polymorphism isthought to regulate AR activity, with longer alleles conferring reducedreceptor activity. The polyglutamine tract length is inversely correlatedto the transcriptional competence of the receptor, with longer tractsbeing associated with lower levels of AR [3].
Institute of Biomedical Research25001 Lleida, Catalonia, Spain.
Therefore, shorter CAG repeat length would produce larger pheno-typic effects of androgens [4,5]. Most empirical studies support this neg-ative relationship between testosterone and CAG repeat length. Thus,lower levels of total testosterone in individuals with long CAG se-quences were reported in a sample of Swedish women [6]. Also, thetestosterone-related increase of white-matter volume was stronger inshort than in long CAG repeat of male adolescents [7]. However, otherstudies have found the opposite pattern, suggesting that subjects withlarger CAG repeat length would have higher testosterone concentra-tions [8,9].
On the other hand, the hypothesis that greater androgen receptortranscriptional activity is related to shorter CAG alleles [10] is stronglycongruent with many physical, psychological and behavioural manifes-tations of androgenity associated with short CAG repeat length. For in-stance, shortened CAG repeats have been associated with an increasedrisk of prostate cancer and symptomatic benign prostatic hyperplasia[11], alopecia [12], lower ratio of the length of the index finger to thelength of the ring finger [13], physical strength [14], Alzheimer's diseaseinmen [15], blood pressure [16], risk of coronary artery disease [17] andhigher sperm concentrations [18]. On the other hand, longer CAG
92 A. Aluja et al. / Physiology & Behavior 147 (2015) 91–96
repeats were associated with significantly greater total fat-free mass,spinal bulbar muscular atrophy or infertility in men [19].
Shorter CAG repeats have also been associated with conduct disor-ders and impulsive-disinhibited syndromes, including attentiondeficit/hyperactivity and oppositional defiant disorders or Tourette'ssyndrome [20]. In criminal and forensic psychiatric populations, includ-ing rapists andmurderers, the same phenomenonwas observed [21,22].Impulsive-disinhibited personality traits such as dominance, aggression,sensation seeking, psychoticism or impulsivenessmeasured by differentquestionnaires have also been relatedwith shorter CAG repeats [23–25],and more recently, the relationship between impulsive-disinhibitedpersonality traits and the androgen receptor CAG and GGN repeatpolymorphisms has been analysed in two samples of inmates andcontrol subjects [26]. Following the recommendations for genetic asso-ciation studies in the personality field [27], the latter work measuredthe impulsive-disinhibited personality construct using an index formedby several phenotypic measures (Impulsive Sensation Seeking,Psychoticism, Sensitivity to Reward, Novelty Seeking and others). Re-sults indicate that inmates carrying CAG short and GGN long haplotypegroup (short–long haplotype) obtained higher scores on all personalityscales. Besides, inmates with the short–long haplotype weremore prev-alent in the high extreme impulsive-disinhibited group than in the con-trol group. Authors discussed the usefulness of including a serumtestosterone measure to clarify the role played by AR polymorphismsand testosterone in the differences on psychological variables.
In recent decades, therehas been an increasing interest in the relation-ships between testosterone levels, social dominance and aggressive be-haviours. Some personality traits, such as impulsiveness and sensationseeking, have been consistently associated with testosterone levels[28–30]. However, a few studies do not support this relationship [31] orsuggest a low effect size [32]. Some methodological difficulties may ac-count for these results. Testosterone is segregated in the blood streamin a pulsatile and irregular way and active testosterone is Free Testoster-one (FT) which represents only a 2% of Total Testosterone (TT). Noticethat FT is not directly available. TT is bound to the Sex Hormone-Binding Globulin (SHGB) and albumin, and its production is controlledinmenby the LuteinisingHormone (LH) and the Follicle-StimulatingHor-mone (FSH) function. Inmen, LH stimulates testosterone production fromthe interstitial cells of the testes (Leydig cells). FSH stimulates testiculargrowth and enhances the production of an androgen-binding protein bythe Sertoli cells, which are necessary components of the testicular tubuleandnecessary for sustaining thematuring spermcell. The SHBG is synthe-sized by liver cells and has a 7-day half-life in circulation. In both sexes,SHBG concentration increases sharply just after birth and gradually de-clines until puberty. Liver damage caused by alcohol or diet can increaseSHBG levels affecting the concentration of TT [28,30]. In addition, SHBGlevels are high in hyperthyroidism and unregulated thyroid SHBG hor-monal levels [33]. Individuals with hypogonadism are characterized bylow testosterone, low or normal FSH/LH, and impaired spermatogenesis.
Testosterone concentrationsmay vary depending on the age and theeffects of other biological variables. Therefore, other hormones (such asLH and FSH) and transporter proteins (such as SHBG and albumin)should be taken into account to understand differences in testosteronelevels. This study has two objectives: a) to analyse the interactions be-tween testosterone levels and CAG repeat length polymorphism as amoderator of AR sensitivity with regard to impulsiveness traits, andb) to analyse the role of other biological variables (LH, FSH, SHBG and al-bumin) in the relationship between testosterone levels, and CAG lengthpolymorphism with impulsiveness.
2. Method
2.1. Sample
Participantswere 105male Caucasian volunteers recruited from stu-dents and university staff. The age rangewas from 17 to 53 years (mean
26.71 ± 9.68 SEM). All measures were anonymously collected. Allsubjects signed an informed consent and received a monetary reward(30 Euros) for participating in the study. This study was approved bythe ethical committee of the University of Lleida.
2.2. Laboratory methods
2.2.1. Hormone assaysBlood samples were drawn three times (10 ml × 3) from the
antecubital vein at intervals of 20 min between 8.00 and 9.00 a.m., inorder to minimize the pulsatile effects of the hormone levels. Theywere then pooled and transferred to heparinized tubes and then centri-fuged. Supernatant plasma was withdrawn and stored in a freezer(−20 °C) before proceeding with each determination. These sampleswere analysed bymeans of radioimmunoassay for luteinizing hormone(LH), Follicle-Stimulating Hormone (FSH), Total Testosterone (TT,Immunotech, Marseille, France), Sex Hormone Binding Globulin(SHBG), and were measured using the IRMA method (FarmosDiagnostica, Oulunsalo, Finland). Free Testosterone (FT) and Bioavail-able Testosterone (BT)were calculated after the TT values, SHBG and al-bumin values, using a calculator developed at the HormonologyDepartment, University Hospital of Ghent, Belgium by Dr. Tom Fiersand Prof. Dr. J.M. Kaufman (http://www.issam.ch/freetesto.htm).
2.2.2. CAG repeat lengthGenomic DNAwas obtained frombuccal swabs using the BuccalAmp
DNA Extraction Kit (Epicentre, Madison, USA). Fluorescent polymerasechain reaction (PCR) protocols were followed to detect repeat lengthpolymorphisms (CAG trinucleotide repeat) AR gene located in codingexon 1 sequence at positions +171 from translation start site. CAG re-peat primer sequences were: forward labelled primer VIC — AGAATCTGTTCCAGAGCGTG, and reverse primer AAGGTTGCTGTTCCTCATCC.PCR reactions were performed in a 50 μl incubation mixture containing50 ng DNA as well as 0.5 and 0.3 μmol of flanking specific primers forCAG repeats. CAG repeat alleles were amplified by the Roche GC-RichPCR System under the following programme: an initial denaturationphase of 3′ at 95 °C; 10 cycles of denaturation 30″/95 °C, annealing30″/57 °C and extension 45″/72 °C, followed by 25 cycles of 30″/95 °C,30″/57 °C and 45″/72 °C, increasing the extension time 5″ in eachcycle from 45″ to 2′ 40″. A final extension step of 7′ at 72 °C wasperformed to ensure completion of sequence synthesis. Amplifiedfragments were resolved in a 3730XL sequence analyser (AppliedBiosystems).
2.3. Measures
2.3.1. Barratt's Impulsiveness Scale (BIS-10)TheBIS-10measures three components of impulsiveness:Motor Im-
pulsiveness (Imp-M), Cognitive Impulsiveness (Imp-C) and Non-Planning Impulsiveness (Imp-NP), which implies acting without think-ing, making quick cognitive decisions on the spur of the moment, and“present orientation” or lack of “futuring”, respectively [34]. It is formedby 34 items with an answer format of 4-point Likert-type scale. Barrattreported internal consistency coefficients between .89 and .92 for theBIS-10 components. The Spanish adaptation was administered in thepresent study [35].
2.4. Statistical analysis
Descriptive statistics and mean differences in personality and hor-monal variables were obtained for short, medium and high CAG repeatlength groups. CAG repeat length is taken as a continuous variable but ithas been categorized by taking the mean repeat length (22.11) S.D.(3.02). This allowed us to classify the sample into short (≤20; n =35), medium (N20 and ≤23; n = 34) and high (N23; n = 36) CAG re-peat length groups. Normal distribution frequency of variables was
93A. Aluja et al. / Physiology & Behavior 147 (2015) 91–96
tested assessing kurtosis, skewness and the Kolmogorov–Smirnov test.Pearson correlations between CAG and hormonal and personality vari-ables were computed. Besides, a one-way ANOVAwas conducted to de-tect significant differences between the three groups. ANOVA and theScheffe significance post-hoc test after the Bonferroni adjustment testwere also performed. In addition, a series of linear regression modelswere performed using the “enter” method, which enters all the vari-ables at the same time. Equationswere developed taking ImpulsivenessBIS-10 scales as dependent variables, considering age, biological vari-ables (FT, SHBG, LH, FSH) and CAG repeat length as predictors (ModelI). Then, we incorporated the interaction among FT × CAG repeat length(Model II), and finally, the interaction between SHBG × CAG repeatlengths (Model III)was considered as an independent variable. The sim-ple slope analysis shows the relationship between FT/SHBG and CAG re-peat at different levels (±1 standard deviation) of the impulsive motortrait [36,37].
Biological and psychological variables may be considered normallydistributed. It is well known that when data is non-normally distribut-ed, a test of the significance of Pearson's r may increase type I errorand reduce its power [38]. Besides, it should be also remarked that hor-monal variables were obtained at three twenty-minute intervals at thesame moment in the morning in order to minimize pulsatile effects.Both, normal distribution of variables and hormone assays allow us torely on reported results, since mathematical artefacts or hormonalbiases are minimized [39,40].
3. Results
Table 1 displays the descriptive statistics and correlations betweenbiological and personality variables, and CAG repeat length groups.Age correlated −.23 (p b .02), −.33 (p b .001) and −.36 (p b .001)with total TT, FT and BT, respectively. The SHBG correlated negativelywith FT and BT (r: −.35; p b .001, and r: −.35; p b .010). FT and BT, asexpected, obtained a high correlation (r: .95; p b .001).
Alphas ranged between .50 and .82 for personality variables (Imp-C:.50; Imp-M: .75; Imp-NP: .77 and BIS-10: .82), being similar to the orig-inal Spanish validation [35]. Low reliability for Imp-C almost precludesthe possibility of finding significant associations with biological vari-ables for this scale. Hormone level means in the present study were
Table 1Pearson correlations (r), descriptive, alpha reliability and mean differences (ANOVA & post-hogroups.
Unit CAG b20a
(N = 35)
r S K M SD
Age .06 1.3 .17 25.77 9.76HormonesTT (ng/dL) −.05 .34 −.51 648.62 ±198.30FT (ng/dL) .04 .41 −.43 13.13 ±4.52BT (ng/dL) .06 .27 −.51 314.25 ±113.04LH (mU/mL) −.01 .78 .77 3.61 ±1.87FSH (mU/mL) −.23 2.45 8.55 4.35 ±2.75ProteinsAlbumin (ng/mL) .08 −1.05 2.32 43.94 ±5.10SHBG (nmol/L) −.15 .92 .63 39.70 ±17.36Scales AlphaBIS-10 .82 −.11 .28 −.43 47.48 ±14.87Imp-NP .77 −.11 .38 −.68 15.18 ±7.87Imp-M .75 −.11 .46 −.37 14.03 ±6.42Imp-C .50 −.02 .03 −.37 18.27 ±6.03
Note: TT: Total Testosterone; FT: Free Testosterone; BT: Bioavailable Testosterone; LH: LuteinisS: skewness; K: kurtosis.
a Short CAG repeat length group.b Medium CAG repeat length group.c Long CAG repeat length group.d After Bonferroni adjustment test.
normative for healthy men [41]. Almost all skewness and kurtosisvalues were within the ±1 range, with the exception of albuminand FSH. One-sample Kolmogorov–Smirnov (KS) test showed thatall personality and hormonal variables were normally distributedexcept in the case of age and albumin. Fig. 1 shows the distribution ofAR CAG in our sample. The distribution is normal according to the KStest and also similar to previous studies with Caucasian populationsamples.
The correlation between hormones and CAG repeats was near zeroand correlations between impulsiveness scales and CAG were, as ex-pected, negative but not significant. We found significant mean differ-ences by CAG groups for Imp-NP (p b .023), with higher scores for theshort CAG group. As expected, short or medium CAG groups presentedhigher levels of TT, FSH and SHBG compared to the long CAG groups.As a complementary analysis, the short group presented higher scoreson the BIS-10 total scale and Imp-M when total sample was dividedinto two groups (short CAG group, n = 55, and long CAG group, n =46, assuming a cut-off of 23). For BIS-10 short group, there was amean of 14.82 (SD: 7.50), and for long CAG group, a mean of 11.83(SD: 6.27); t-test: 2.18, p b .031. For Imp-M short CAG group, therewas a mean of 51.09 (SD: 16.65), and long CAG group, a mean of44.30 (SD: 14.54); t-test: 2.19, p b .031, respectively.
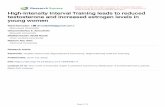
Table 2 displays the results of the linear regression analysis for thetotal BIS-10 scale and the three components, with the standardized co-efficients of the estimated regression model, t statistics and the p valueassociated with the corresponding t. In Model I, age was negatively re-lated with Imp-M (β: −2.14; p b .035) and Imp-NP (β: −2.13;p b .035). The FSH was associated with Imp-NP (β: −2.22; p b .028)but CAG repeat length was associated with no dependent variable. TheR ranged between .34 and .42. InModel II, all independent variables, ex-cluding FSH, were significantly related to Imp-M (R: .47). A significantpositive effect was found for CAG repeat length (β: 2.81; p b .006),and a negative one for the FT × CAG repeat length interaction (β:−3.28; p b .001), indicating that the effect of FT on Imp-M was morepronounced in subjects with shorter CAG repeat lengths (Fig. 2). InModel III, we tested whether SHBG and CAG repeat length moderatedthe relationship of SHBG with personality variables. There was a signif-icant effect for the interaction between SHBG × CAG repeat length andImp-M (β: 2.46; p b .016). This indicates that the effect of SHBG on
c test) of hormones, and personality scales for short, medium and long CAG repeat length
20–23b
(N = 34)N23c
(N = 36)F Sig. Scheffed
M SD M SD
26.71 8.96 26.97 9.77 .17 ns
623.62 ±168.33 609.62 ±177.46 .378 ns13.29 ±4.21 13.28 ±4.91 .021 ns
320.79 ±5.30 321.35 ±111.84 .043 ns3.36 ±1.03 3.40 ±1.50 .268 .0733.49 ±2.20 3.10 ±1.53 2.689 ns
44.79 ±5.46 44.85 ±3.87 .353 ns35.32 ± 4.00 34.28 ±11.97 1.245 ns
52.82 ±17.51 43.58 ±14.58 2.905 .05918.53 ±7.46 13.59 ±7.14 3.850 .025 2 N 3 (.023)14.38 ±8.06 11.97 ±6.63 1.147 ns19.91 ±5.95 18.12 ±5.30 1.007 ns
ing Hormone; FSH: Follicle-Stimulating Hormone; SHBG: Sex Hormone Binding Globulin;
Fig. 1. Distribution of AR CAG in our sample. Stan
dard
ized
pre
dict
ed v
alue
(Im
puls
iven
ess
mot
or)
Free Testosterone
Fig. 2. Interactions between Free Testosterone (FT) withMotor Impulsiveness in the threegenetic groups (short: b20, medium: N20 and b23, long: N23 CAG repeat lengths).
94 A. Aluja et al. / Physiology & Behavior 147 (2015) 91–96
Imp-Mwas less pronounced for subjects with short CAG repeat lengths(Fig. 3).
Table 3 displays the simple slopes for the interactions terms for FTand SHBG with CAG repeat. For the FT by CAG repeat interaction, theslopes were always significant for those with half or one standard devi-ation below the mean in CAG repeat (8.46, p b .001; 5.23, p b .001; 2,p b .05; −1.23, ns; −4.46, p b .001), and one standard deviationabove the mean in the CAG repeat length groups. For the SHBG byCAG repeat interaction, the slopes were only significant in thosebelow and 1 SD above the mean in CAG repeat (−7.22, p b .001;−4.45, p b .001;−1.69, ns; 1.07, ns; 3.84, p b .001).
Table 2Linear regressionmodels predicting BIS-10 impulsiveness scales (dependent variables) after agerepeats (Model III).
Total impulsiveness Non-Planning Impulsiven
β t Sig. β t
Model I(Constant) 5.13 .000 4.02Age −.22 −2.08 .040 −.06 −.62FT .10 .93 .354 .06 .55SHBG .14 1.34 .184 .02 .20LH −.19 −1.81 .073 −.08 −.78FSH −.17 −1.59 .113 −.24 −2.22CAG_repeats −.15 −1.47 .144 −.18 −1.74
R: .41; R2: .17 R: .34; R2: .12
Model II(Constant) .98 .325 1.76Age −.24 −2.26 .026 −.06 −.60FT .97 1.44 .153 .00 .00SHBG .16 1.47 .144 .02 .19LH −.20 −1.93 .056 −.08 −.76FSH −.14 −1.38 .169 −.24 −2.20CAG_repeats .21 .71 .475 −.20 −.67FT × CAG repeats −.96 −1.30 .194 .06 .08
R: .43; R2: .19 R: .33; R2: .11
Model III(Constant) 3.00 .003 1.20Age −.21 −2.05 .043 −.07 −.66FT .10 .98 .325 .05 .44SHBG −.26 −.34 .731 .71 .92LH −.18 −1.77 .079 −.09 −.82FSH −.16 −1.54 .126 −.25 −2.28CAG repeats −.28 −1.06 .292 .04 .17SHBG × CAG rep. .41 .54 .588 −.70 −.89
R: .42; R2: .17 R: .35; R2: .12
Note: TT. Total Testosterone; FT: Free Testosterone; BT: Bioavailable Testosterone; LH: Luteinis
4. Discussion
The relationship between AR sensitivity, sexual hormones and im-pulsiveness was analysed in a sample of healthy Caucasian men. Agewas negatively correlated with testosterone and, as expected, TT waspositively correlated with the SHBG [28,42]. Pearson correlations be-tween the CAG polymorphism and testosterone (Total, Free and
, hormones and CAG repeats (Model I), adding FT × CAG repeats (Model II) or SHBG×CAG
ess Motor Impulsiveness Cognitive Impulsiveness
Sig. β t Sig. β t Sig.
.000 2.844 .005 4.90 .000
.531 −.23 −2.14 .035 −.23 −2.13 .035
.579 .13 1.24 .217 .02 .21 .832
.836 .20 1.81 .073 .12 1.10 .274
.437 −.20 −1.94 .055 −.15 −1.44 .153
.028 −.00 −.07 .941 −.13 −1.18 .238
.085 −.07 −.74 .458 −.07 −.67 .501R: .36; R2: .13 R: .34; R2: .12
.082 −1.71 .090 2.27 .025
.546 −.28 −2.72 .008 −.23 −2.06 .042
.994 2.28 3.44 .001 −.14 −.20 .840
.844 .23 2.23 .028 .12 1.06 .290
.445 −.24 −2.33 .022 −.15 −1.40 .163
.030 .04 .42 .675 −.13 −1.20 .232
.502 .80 2.81 .006 −.14 −.45 .649
.936 −2.36 −3.28 .001 .18 .23 .811R: .47; R2: .22 R: .32; R2: .11
.231 3.58 .001 2.18 .031
.507 −.22 −2.09 .039 −.23 −2.13 .035
.661 .17 1.57 .119 .02 .17 .859
.360 −1.62 −2.16 .033 .32 .41 .676
.412 −.19 −1.87 .064 −.15 −1.44 .152
.025 .01 .11 .913 −.13 −1.19 .234
.863 −.68 −2.56 .012 −.00 −.01 .991
.371 1.85 2.46 .016 −.20 −.26 .793R: .43; R2: .18 R: .34; R2: .12
ing Hormone; FSH: Follicle-Stimulating Hormone; SHBG: Sex Hormone Binding Globulin.
Stan
dard
ized
pre
dict
ed v
alue
(Im
puls
iven
ess
mot
or)
Sex Hormone Binding Globulin (SHBG)
Fig. 3. Interactions between SHBG with Motor Impulsiveness in the three genetic groups(short: b20, medium: N20 and b23, long: N23 CAG repeat lengths).
95A. Aluja et al. / Physiology & Behavior 147 (2015) 91–96
Bioavailable) were near zero, showing no association between the ARsensitivity and testosterone concentrations. This pattern of results wasconsistent with some studies [43,44] but not with others [8,9]. In anycase, our data showed that CAG repeat length and testosterone concen-trations were not necessary related.
On the other hand, CAG repeat length had low negative correlationswith impulsiveness and its components. Although this pattern was notstatistically significant, it was in linewith other studies considering nor-mal or forensic samples. For instance, an association between short CAGrepeat length and high scores on impulsiveness and Monotony Avoid-ance scales of the Karolinska Personality Scales has been reported inpast research [45]. It should be remarked that short and long CAGgroups presented higher and lower levels, respectively, on both hor-mones and impulsiveness.
With regard to SHBG, short CAG subjects had the tendency of pre-senting more SHBG concentration. In this sense, other authors have re-ported an inverse association of SHBG and the 5-HT1A receptor, showingthat more aggressive subjects were also characterized by lower SHBGlevels [46]. It was argued that higher SHBG plasma levels correspondedto lower postsynaptic 5-HT1A receptor affinities or densities in the pre-frontal cortex, and more pronounced ones in the orbitofrontal cortexand posterior cingulate cortex and in the amygdala. With regard tothese previous results, serotonin seems to regulate impulsiveness andthe inhibitory control of aggression [47,48]. Normally, the common var-iance between impulsiveness (BIS-10) and aggressiveness was around42% [49]. Aggression and impulsiveness are modified by sex hormones,which exert influence on serotonergic neurotransmission. Serotoninlevels have been frequently linked to higher levels of aggression in ro-dents, nonhuman primates, and humans [50]. In this way, fluoxetine(selective serotonin reuptake inhibitor) has aggression-lowering
Table 3Simple slopes for interaction of FT and SHBG by CAG repeats.
CAG repeats (SD) FT × CAG repeats SHBG × CAG repeats
−1 8.46⁎⁎⁎ −7.22⁎⁎⁎
−1/2 5.23⁎⁎⁎ −4.45⁎⁎⁎
0 2 −.1691/2 −1.23 1.071 −4.46⁎⁎⁎ 3.84⁎⁎⁎
⁎⁎⁎ p b 0.001.
effects, as it has been demonstrated by Coccaro and collaborators in sev-eral studies [51–53]. Congruent with this rationale, subjects with shortCAG repeat lengths in the present study tended to be more impulsiveand showed lower SHBG levels. Therefore, these subjectswere expectedto be more aggressive, in line with previous findings [46].
There were main effects of FT and CAG repeat on Motor Impulsive-ness. These results support that free active androgens may contributeto individual differences in impulsiveness [45], in agreement with pre-vious studies relating several impulsive-disinhibited personality traitssuch as extraversion, sensation seeking or other externalization behav-iours in normal and inmates samples [21,23–25,45]. Considering thatthe negative interaction effect (FT × CAG repeats) is larger than themain effect, and the slopes observed for the three genetic groups, itcan be concluded that the effect of FT onMotor Impulsiveness is greaterin subjects with shorter CAG repeat lengths.
Similarly, the positive interaction term between SHBG and CAG re-peat length was larger than the main negative effects observed forSHBG and CAG repeat on Motor Impulsiveness. The SHBG presents anegative β coefficient (low SHBG is associated with high Motor Impul-siveness), but the interaction term was positive, suggesting that the re-lationship varies by CAG group. Impulsiveness was associated with lowSHBG in the short CAG group,whereas high impulsivenesswas associat-edwith high SHBG in the long CAG group. Notice that SHBG is negative-ly correlatedwith FT and BT. The genetic configurationmight contributeto modify the observed relationships between gonadal hormones andsome personality variables.
The present study has some limitations. First, a small number ofhealthy participants were analysed. The nature of the sample (studentsand university staff) was somewhat restricted. The inclusion of a groupof high impulsive or aggressive subjects would be advisable in futurestudies. Second, the design of the study was cross-sectional, so nocause–effect relationships can be established. Besides, only one poly-morphism was considered. Other authors have shown that thepolyglycine tract encoded by a polymorphic GGN repeat in exon 1 oftheARmight have somemodulatory effects [20]. Future research shouldalso try to control for lifestyle-related factors such as smoking, diet andphysical activity.
4.1. Conclusion
The role of AR CAG repeat length, several Testosterone variants(Total, Free and Bioavailable), LH, FSH, SHBG and several Impulsivenesspersonality traits (motor, cognitive and non-planning) were conjointlyanalysed. Results suggest that: a) there is nodirect relationship betweentestosterone levels and CAG repeats lengths, b) the presence of shortCAG repeats length tend to be associatedwith high scores on Impulsive-ness, and c) there is some evidence to support interactions betweenFree Testosterone and Impulsiveness, and for SHBG and Impulsivenessin short CAG repeats length subjects.
Declaration of interests
We declare that we have no financial and personal relationshipswith other people or organizations.
Acknowledgements
This research was supported by the Spanish Ministry of Educationand Science through the “Ramon y Cajal” Programme (second author).Also, necessary financial support was given to the first author by grantsfrom the “Fondo de Investigaciones Sanitarias” (PI-050303)” and theSpanish Ministry of Economy and Competitiveness (PSI2011-24789).This research was performed within the framework of DURSI Consoli-dated Group 2014 SGR 111.
96 A. Aluja et al. / Physiology & Behavior 147 (2015) 91–96
References
[1] S. Walsh, J.M. Zmuda, J.A. Cauley, P.R. Shea, E.J. Metter, B.F. Hurley, et al., Androgenreceptor CAG repeat polymorphism is associated with fat-free mass in men,J. Appl. Physiol. 98 (2005) 132–137.
[2] H.P. Gerber, K. Seipel, O. Georgiev, M. Hofferer, M. Hug, S. Rusconi, et al., Transcrip-tional activation modulated by homopolymeric glutamine and proline stretches,Science 263 (1994) 808–811.
[3] J. Beilin, E.M. Ball, J.M. Favaloro, J.D. Zajac, Effect of the androgen receptor CAG re-peat polymorphism on transcriptional activity: specificity in prostate and non-prostate cell lines, J. Mol. Endocrinol. 25 (2000) 85–96.
[4] N.L. Chamberlain, E.D. Driver, R.L. Miesfeld, The length and location of CAG trinucle-otide repeats in the androgen receptor N-terminal domain affect transactivationfunction, Nucleic Acids Res. 22 (1994) 3181–3186.
[5] C.S. Choong, J.A. Kemppainen, Z.X. Zhou, E.M. Wilson, Reduced androgen receptorgene expression with first exon CAG repeat expansion, Mol. Endocrinol. 10 (1996)1527–1535.
[6] L. Westberg, F. Baghaei, R. Rosmond, M. Hellstrand, M. Landen, M. Jansson, et al.,Polymorphisms of the androgen receptor gene and the estrogen receptor betagene are associated with androgen levels in women, J. Clin. Endocrinol. Metab. 86(2001) 2562–2568.
[7] J.S. Perrin, P.Y. Herve, G. Leonard, M. Perron, G.B. Pike, A. Pitiot, et al., Growth ofwhite matter in the adolescent brain: role of testosterone and androgen receptor,J. Neurosci. 28 (2008) 9519–9524.
[8] S.B. Manuck, A.L. Marsland, J.D. Flory, A. Gorka, R.E. Ferrell, A.R. Hariri, Salivary tes-tosterone and a trinucleotide (CAG) length polymorphism in the androgen receptorgene predict amygdala reactivity in men, Psychoneuroendocrinology 35 (2010)94–104.
[9] R.D. Stanworth, D. Kapoor, K.S. Channer, T.H. Jones, Androgen receptor CAG repeatpolymorphism is associated with serum testosterone levels, obesity and serum lep-tin in men with type 2 diabetes, Eur. J. Endocrinol. 159 (2008) 739–746.
[10] C.S. Choong, E.M. Wilson, Trinucleotide repeats in the human androgen receptor: amolecular basis for disease, J. Mol. Endocrinol. 21 (1998) 235–257.
[11] P.E. Clark, R.A. Irvine, G.A. Coetzee, The androgen receptor CAG repeat and prostatecancer risk, Methods Mol. Med. 81 (2003) 255–266.
[12] M.E. Sawaya, A.R. Shalita, Androgen receptor polymorphisms (CAG repeat lengths)in androgenetic alopecia, hirsutism, and acne, J. Cutan. Med. Surg. 3 (1998) 9–15.
[13] J.T. Manning, P.E. Bundred, D.J. Newton, B.F. Flanagan, The second to fourth digitratio and variation in the androgen receptor gene, Evol. Hum. Behav. 24 (2003)399–405.
[14] A.W. Lukaszewski, J.R. Roney, The origins of extraversion: joint effects of facultativecalibration and genetic polymorphism, Personal. Soc. Psychol. Bull. 37 (2011)409–421.
[15] D.J. Lehmann, H.T. Butler, D.R. Warden, M. Combrinck, E. King, J.A. Nicoll, et al., As-sociation of the androgen receptor CAG repeat polymorphism with Alzheimer's dis-ease in men, Neurosci. Lett. 340 (2003) 87–90.
[16] Z. Pausova, M. Abrahamowicz, A. Mahboubi, C. Syme, G.T. Leonard, M. Perron, et al.,Functional variation in the androgen-receptor gene is associated with visceral adi-posity and blood pressure in male adolescents, Hypertension 55 (2010) 706–714.
[17] K. Saltiki, A. Cimponeriu, M. Garofalaki, L. Sarika, A. Papathoma, K. Stamatelopoulos,et al., Severity of coronary artery disease in postmenopausal women: associationwith the androgen receptor gene (CAG)n repeat polymorphism, Menopause 18(2011) 1225–1231.
[18] S. von Eckardstein, A. Syska, J. Gromoll, A. Kamischke, M. Simoni, E. Nieschlag, In-verse correlation between sperm concentration and number of androgen receptorCAG repeats in normal men, J. Clin. Endocrinol. Metab. 86 (2001) 2585–2590.
[19] C.A. Davis-Dao, E.D. Tuazon, R.Z. Sokol, V.K. Cortessis, Male infertility and variationin CAG repeat length in the androgen receptor gene: a meta-analysis, J. Clin.Endocrinol. Metab. 92 (2007) 4319–4326.
[20] D.E. Comings, C. Chen, S. Wu, D. Muhleman, Association of the androgen receptorgene (AR) with ADHD and conduct disorder, Neuroreport 10 (1999) 1589–1592.
[21] D. Cheng, C.J. Hong, D.L. Liao, S.J. Tsai, Association study of androgen receptor CAG re-peat polymorphism and male violent criminal activity, Psychoneuroendocrinology31 (2006) 548–552.
[22] S. Rajender, G. Pandu, J.D. Sharma, K.P. Gandhi, L. Singh, K. Thangaraj, Reduced CAGrepeats length in androgen receptor gene is associated with violent criminal behav-ior, Int. J. Legal Med. 122 (2008) 367–372.
[23] E.G. Jonsson, C. von Gertten, J.P. Gustavsson, Q.P. Yuan, K. Lindblad-Toh, K. Forslund,et al., Androgen receptor trinucleotide repeat polymorphism and personality traits,Psychiatr. Genet. 11 (2001) 19–23.
[24] J.C. Loehlin, S.E. Medland, G.W. Montgomery, N.G. Martin, Eysenck's psychoticismand the X-linked androgen receptor gene CAG polymorphism in additionalAustralian samples, Personal. Individ. Differ. 39 (2005) 661–667.
[25] R. Turakulov, A.F. Jorm, P.A. Jacomb, X. Tan, S. Easteal, Association of dopamine-p-hydroxylase and androgen receptor gene polymorphisms with Eysenck's P andother personality traits, Personal. Individ. Differ. 37 (2004) 191–202.
[26] A. Aluja, L.F. Garcia, A. Blanch, J. Fibla, Association of androgen receptor gene, CAGand GGN repeat length polymorphism and impulsive-disinhibited personality traitsin inmates: the role of short-long haplotype, Psychiatr. Genet. 21 (2011) 229–239.
[27] M.R. Munafo, T.G. Clark, L.R. Moore, E. Payne, R. Walton, J. Flint, Genetic polymor-phisms and personality in healthy adults: a systematic review and meta-analysis,Mol. Psychiatry 8 (2003) 471–484.
[28] A. Aluja, L.F. Garcia, Sensation seeking, sexual curiosity and testosterone in inmates,Neuropsychobiology 51 (2005) 28–33.
[29] R.J. Daitzman, M. Zuckerman, P. Sammelwitz, V. Ganjam, Sensation seeking and go-nadal hormones, J. Biosoc. Sci. 10 (1978) 401–408.
[30] M. Zuckerman, Psychobiology of Personality, 2nd ed. Cambridge University Press,Cambridge, 2005.
[31] J.C. Rosenblitt, H. Soler, S.E. Johnson, D.M. Quadagno, Sensation seeking and hor-mones in men and women: exploring the link, Horm. Behav. 40 (2001) 396–402.
[32] J. Archer, N. Graham-Kevan, M. Davies, Testosterone and aggression: a reanalysis ofbook, Starzyk, and Quinsey's (2001) study, Aggress. Violent Behav. 10 (2005)241–261.
[33] D.C. Anderson, Sex-hormone-binding globulin, Clin. Endocrinol. 3 (1974) 69–96.[34] E.S. Barratt, Impulsiveness subtraits: arousal and information processing, in: J.T.
Spence, C.E. Izard (Eds.), Motivation, Emotion and Personality, Elsevier Science,New York 1985, pp. 137–146.
[35] M.A. Luengo, M.T. Carrillodelapena, J.M. Otero, The components of impulsiveness —a comparison of the I.7 Impulsiveness Questionnaire and the Barratt ImpulsivenessScale, Personal. Individ. Differ. 12 (1991) 657–667.
[36] A. Blanch, A. Aluja, Work, family and personality: a study of work-family conflict,Personal. Individ. Differ. 46 (2009) 520–524.
[37] L.E. Toothmaker, Multiple-regression — testing and interpreting interactions —
Aiken, LS, West, SG, J. Oper. Res. Soc. 45 (1994) 119–120.[38] A.F. Hayes, Permutation test is not distribution-free: testing H-0: rho = 0, Psychol.
Methods 1 (1996) 184–198.[39] A.J. Bishara, J.B. Hittner, Testing the significance of a correlation with nonnormal
data: comparison of Pearson, Spearman, transformation, and resampling ap-proaches, Psychol. Methods 17 (2012) 399–417.
[40] J.D. Veldhuis, D.M. Keenan, S.M. Pincus, Motivations and methods for analyzing pul-satile hormone secretion, Endocr. Rev. 29 (2008) 823–864.
[41] B.A. Mohr, A.T. Guay, A.B. O'Donnell, J.B. McKinlay, Normal, bound and nonboundtestosterone levels in normally ageing men: results from the Massachusetts MaleAgeing Study, Clin. Endocrinolo. 62 (2005) 64–73.
[42] A. Aluja, L.F. Garcia, Role of sex hormone-binding globulin in the relationship be-tween sex hormones and antisocial and aggressive personality in inmates, Psychia-try Res. 152 (2007) 189–196.
[43] M. Goutou, C. Sakka, N. Stakias, I. Stefanidis, G.N. Koukoulis, AR CAG repeat lengthis not associated with serum gonadal steroids and lipid levels in healthy men,Int. J. Androl. 32 (2009) 616–622.
[44] H. Vermeersch, G. T'Sjoen, J.M. Kaufman, J. Vincke, M. Van Houtte, Testosterone, an-drogen receptor gene CAG repeat length, mood and behaviour in adolescent males,Eur. J. Endocrinol. 163 (2010) 319–328.
[45] L. Westberg, S. Henningsson, M. Landen, K. Annerbrink, J. Melke, S. Nilsson, et al.,Influence of androgen receptor repeat polymorphisms on personality traits inmen, J. Psychiatry Neurosci. 34 (2009) 205–213.
[46] A.V. Witte, A. Floel, P. Stein, M. Savli, L.K. Mien, W. Wadsak, et al., Aggression is re-lated to frontal serotonin-1A receptor distribution as revealed by PET in healthysubjects, Hum. Brain Mapp. 30 (2009) 2558–2570.
[47] P.F. Ferrari, P. Palanza, S. Parmigiani, R.M. de Almeida, K.A. Miczek, Serotonin and ag-gressive behavior in rodents and nonhuman primates: predispositions and plastici-ty, Eur. J. Pharmacol. 526 (2005) 259–273.
[48] R.J. Nelson, B.C. Trainor, Neural mechanisms of aggression, Nat. Rev. Neurosci. 8(2007) 536–546.
[49] C. Garcia-Forero, D. Gallardo-Pujol, A. Maydeu-Olivares, A. Andres-Pueyo,Disentangling impulsiveness, aggressiveness and impulsive aggression: an empiri-cal approach using self-report measures, Psychiatry Res. 168 (2009) 40–49.
[50] S. Chiavegatto, V.L. Dawson, L.A. Mamounas, V.E. Koliatsos, T.M. Dawson, R.J. Nelson,Brain serotonin dysfunction accounts for aggression in male mice lacking neuronalnitric oxide synthase, Proc. Natl. Acad. Sci. U. S. A. 98 (2001) 1277–1281.
[51] E.F. Coccaro, R.J. Kavoussi, Fluoxetine and impulsive aggressive behavior inpersonality-disordered subjects, Arch. Gen. Psychiatry 54 (1997) 1081–1088.
[52] E.F. Coccaro, R.J. Kavoussi, R.L. Hauger, Serotonin function and antiaggressive re-sponse to fluoxetine: a pilot study, Biol. Psychiatry 42 (1997) 546–552.
[53] R.J. Kavoussi, E.F. Coccaro, Divalproex sodium for impulsive aggressive behavior inpatients with personality disorder, J. Clin. Psychiatry 59 (1998) 676–680.