Flying under the radar: figurative language impairments in focal lesion patients
Amyloid precursor protein accumulates in white matter at the margin of a focal ischaemic lesion
Transcript of Amyloid precursor protein accumulates in white matter at the margin of a focal ischaemic lesion
Ž .Brain Research 760 1997 150–157
Research report
Amyloid precursor protein accumulates in white matter at the margin of afocal ischaemic lesion
P.S. Yam a,) , T. Takasago a, D. Dewar a, D.I. Graham b, J. McCulloch a
a Wellcome Surgical Institute and Hugh Fraser Neuroscience Laboratories, UniÕersity of Glasgow, Garscube Estate, Bearsden Road, Glasgow G61 1QH,UK
b Department of Neuropathology, UniÕersity of Glasgow, Glasgow, UK
Accepted 25 February 1997
Abstract
Ž .Amyloid precursor protein APP is transported by fast anterograde axonal transport. Since disruption of this transport results in APPaccumulation, APP has been proposed as a sensitive marker of axonal injury. In the present study, axonal injury in subcortical whitematter and myelinated fibre tracts permeating the striatum, 24 h after permanent middle cerebral artery occlusion in the rat, has beenexamined by assessing the location and extent of APP immunoreactivity. Increased APP immunoreactivity was present in both areas. Thiswas localised to a circumscribed zone immediately adjacent to the boundary of the ischaemic lesion in grey matter. The amount of APPimmunoreactivity was associated with the volume of the ischaemic lesion in individual animals. Increased APP immunoreactivity insubcortical white matter and myelinated fibre tracts at the margin of the ischaemic zone may prove to be a valuable marker for assessingstrategies to protect axons after an ischaemic insult.
Keywords: Amyloid precursor protein; Focal cerebral ischaemia; Rat; Axon
1. Introduction
Classically white matter has been considered less vul-w xnerable than grey matter to ischaemic injury 19 . How-
ever, it is becoming increasingly recognised that whitew xmatter is highly vulnerable to focal ischaemia 7,27 . Since
white matter does not contain neuronal cell bodies orsynapses, it is likely that the mechanisms of injury andstrategies for its protection are different from those in greymatter. Waxman et al., using an in vitro model of isolated
w xoptic nerve 39,40 , demonstrated that myelinated axonsare susceptible to anoxia in the absence of their perikaryaand that functional and structural impairment occurs withinthe axon itself and not only as a consequence of impairedperikaryal function. In addition, ischaemia is suggested toplay a role in the pathogenesis of leuko-araiosis in mandue to its frequency among patients who have risk factors
w xassociated with vascular disease 26 . Thus it appears thatwhite matter is susceptible to the effects of ischaemia and
) Ž .Corresponding author. Fax: q44 141 943 0215; e-mail:[email protected]
its protection should be considered alongside that of greymatter.
White matter injury may be a major determinant ofclinical outcome. The study of traumatic brain injury inboth man and in a non-human primate model of experi-mental injury suggests a direct link between the extent ofdiffuse axonal injury and the ensuing patient morbidityw x1,8 . In order to define the responses of axons to injury avariety of markers have been investigated. Amyloid pre-
Ž . w xcursor protein APP 9,10,31 , neurofilament subunitsw x w x6,13,22 and ubiquitin 14 have been used to investigateaxonal injury after head injury and these immunocyto-chemical labels suggest that it is more widespread than
w xoriginally indicated by traditional silver stains 10 . APPhas attracted particular interest in head injury as it hasbeen found to be a sensitive marker of axonal damagew x4,10,20,31 , although this has not been systematicallyexamined in the ischaemic rat model. APP is a transmem-brane glycoprotein from which b-APP, the main compo-nent of amyloid plaques in Alzheimer’s disease, is derivedw x11,12 . APP is ubiquitously expressed in the nervoussystem from early embryogenesis throughout adulthoodw x w x2 . It is associated with the cytoskeleton 30 and under-
0006-8993r97r$17.00 Copyright q 1997 Elsevier Science B.V. All rights reserved.Ž .PII S0006-8993 97 00290-4
( )P.S. Yam et al.rBrain Research 760 1997 150–157 151
goes fast anterograde axonal transport in the peripheralw xnervous system 18 . The interruption of axonal transport,
w x w xwhether it be due to traumatic 25 , ischaemic 15,36 orw xchemical insults 16,21,33 , has been shown to result in the
accumulation of APP, indicative of dysfunction and per-haps eventual discontinuity of the axon.
To allow future assessments of protection of whitematter by therapeutic intervention, a method by whichdamage to axons can be visualised and quantified is re-quired. The current study was undertaken to investigateAPP as a suitable marker of white matter injury in re-sponse to middle cerebral artery occlusion in the rat. The
Ž .aims of the study were to determine 1 whether increasedAPP immunoreactivity is a consistent observation after
Ž .focal ischaemia, 2 the topography of increased APPimmunoreactivity in relation to the boundary of ischaemic
Ž .lesions, and 3 whether there is a quantitative relationship
between increased APP immunoreactivity and volume ofthe focal ischaemic lesion.
2. Materials and methods
2.1. General preparation
Anaesthesia was induced in 14 adult male Fischer ratsŽ .Harlan Olac, Bicester, UK by placing them in a perspexchamber into which 70% nitrous oxide, 30% oxygen and5% halothane were flowing. The rats were intubated usinga 45 mm, 16-gauge catheter, and maintained on 1–1.5%halothane by positive pressure ventilation. A femoral arteryand vein were cannulated using polyethylene catheters toallow the continuous monitoring of mean arterial bloodpressure, the repeated sampling of blood and the adminis-
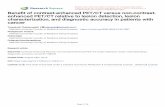
Ž w x.Fig. 1. A: line diagram of coronal level 3 anterior coordinate of 7.19 mm 17 showing the location of the following photomicrographs. B: increase inAPP immunoreactivity in corpus callosum immediately adjacent to the ischaemic lesion. C: no increase in APP immunoreactivity evident in thenon-ischaemic corpus callosum. D: no increase in APP immunoreactivity evident in the densely ischaemic corpus callosum. Bars50 mm for B, C and D.
( )P.S. Yam et al.rBrain Research 760 1997 150–157152
tration of fluids when appropriate. Rectal temperature wasmeasured and the rats were maintained normothermic byheating lamp.
2.2. Middle cerebral artery occlusion
The left middle cerebral artery was exposed using aw xsubtemporal approach 38 , leaving the zygomatic arch
w xintact 35 . The animals were placed in lateral recumbencyand a 1 cm vertical skin incision was made between theleft orbit and the external auditory canal. The underlyingfascia was removed and the exposed temporalis musclebluntly dissected and retracted to expose the inferior partof the temporal fossa. A small craniectomy was madeusing a dental drill at the junction between the medial walland the roof of the temporal fossa, approximately 1 mmdorsal to the foramen ovale. The dura was opened using a30-gauge dental needle and then removed using a finehook to reveal the middle cerebral artery. The main trunkof the artery was exposed proximal to the olfactory tract towhere it crosses the inferior cerebral vein. The exposedartery was occluded by micropolar coagulation from itsorigin to the point where it crosses the inferior cerebralvein and then severed to prevent recanalisation. The
w Ž .craniectomy was covered by Gelfilm Upjohn . The mus-cle and skin were sutured using 2r0 braided silk. Follow-ing middle cerebral artery occlusion, anaesthesia was dis-continued and, under strict observation, the animals wereallowed to regain consciousness. Saline was injected sub-cutaneously to minimise dehydration. The animals werehoused individually and had access to softened food andwater. There was minimal interanimal variability in mea-sured physiological parameters which may influence the
Ž .extent of infarction Table 1 . Blood pressure was lower inthe operative period than in the post-operative periodreflecting the halothane anaesthesia. p O was higher ata 2
this time, reflecting the higher level of oxygen in theinspired gas mixture than air.
2.3. Histology and immunocytochemistry
Twenty-four hours after middle cerebral artery occlu-sion, the rats were re-anaesthetised and maintained with
Ž .Fig. 2. Representative line diagrams from three animals A, B and CŽ .showing areas of neuronal necrosis left column and the location of
Ž .increased APP immunoreactivity right column . The APP score for theseanimals was 3, 2 and 1 respectively.
3% halothane by face mask. The animals were killed bytranscardiac perfusion. In brief, a thoracotomy was per-formed and a cannula inserted into the ascending aorta viathe left ventricle, the right atrium was incised, and 200 mlof heparinised saline infused at the animals mean arterial
Žblood pressure, followed by 200 ml of fixative 40%formaldehyderglacial acetic acidrabsolute methanol in
.the ratio 1:1:8, vrvrv at the same pressure. The head wasstored in fixative for at least 24 h at 48C before beingremoved and transferred to a 70% solution of methanol.
After detaching the hindbrain, the forebrain was cut intofour equally spaced coronal blocks, embedded in paraffin
Ž .wax, and sectioned nominally 10 mm thick at multiplelevels. The sections were stained by haematoxylin andeosin, and a method that combined Luxol Fast blue and
Table 1Physiological variables prior to and following middle cerebral artery occlusion
Ž . Ž . Ž . Ž . Ž .MABP mmHg pH p CO mmHg p O mmHg Glucose mM Rectal temp. 8Ca 2 a 2
apre-MCAO 87.6"3.9 7.42"0.01 38.7"0.7 159"6 8.0"0.3 37.2"0.0aMCAO 90.6"3.3 7.39"0.01 42.3"0.9 147"6 7.7"0.3 37.0"0.1
1 h 116.1"3.1 7.41"0.00 40.2"0.9 76"2 6.4"0.3 37.5"0.124 h 120.2"1.8 7.49"0.01 34.7"0.6 97"2 6.1"0.3 38.1"0.1
MABP: mean arterial blood pressure; Rectal temp.: rectal temperature.Data are presented as mean"S.E.M. ns14.a Ž .Animals were anaesthetised for surgical occlusion of the middle cerebral artey MCAO .At 1 and 24 h the animals were conscious.
( )P.S. Yam et al.rBrain Research 760 1997 150–157 153
Fig.
3.In
cide
nce
and
topo
grap
hyof
the
isch
aem
icle
sion
and
APP
imm
unor
eact
ivity
for
the
eigh
tpr
esel
ecte
dco
rona
lle
vels
inth
e14
rats
afte
rm
iddl
ece
rebr
alar
tery
occl
usio
n.T
oga
inan
impr
essi
onof
the
Žto
pogr
aphy
and
inci
denc
eof
isch
aem
icda
mag
e,th
ear
eaof
the
isch
aem
icle
sion
ofin
divi
dual
coro
nal
slic
es,a
tgi
ven
coor
dina
tes,
was
supe
rim
pose
dus
ing
NIH
Imag
eco
lour
scal
eba
rre
pres
ents
num
ber
of.
anim
als
with
isch
aem
icda
mag
e.
To
gain
anov
eral
lim
pres
sion
ofdi
stri
butio
nan
dre
latio
nshi
pto
the
area
ofth
eis
chae
mic
lesi
on,i
ncre
ased
APP
imm
unor
eact
ivity
for
the
14an
imal
sw
asm
appe
don
tolin
eŽ
.di
agra
ms
whi
chw
ere
subs
eque
ntly
supe
rim
pose
dre
pres
ente
das
dots
onip
sila
tera
lhe
mis
pher
e.
( )P.S. Yam et al.rBrain Research 760 1997 150–157154
Cresyl violet, and were examined by conventional lightmicroscopy by an investigator ‘‘blind’’ to the immuno-
w xcytochemistry results. Areas of infarction 5 were delin-eated on paraffin sections at the eight preselected stereo-taxically determined coronal levels and transcribed onto
Ž .scale diagrams =4 actual size of forebrain drawn fromw x Ž .the atlas of Konig and Klippel 17,24 Fig. 2 . An approx-¨
imation to total volume of ischaemic damage was achievedby integration of area with the distance between each level.In order to obtain a diagram showing total area of is-chaemia for the 14 animals, the areas of infarction drawnon the scale diagrams were superimposed using NIH Im-
Ž .age for each coronal level Fig. 3 .Sections adjacent to those used for mapping areas of
infarction were prepared for immunocytochemistry so thatthe topography of increased APP immunoreactivity couldbe compared to that of infarction. Sections were mounted
Ž .on 3-aminopropyltriethoxy-saline APES coated slides anddried at 378C overnight. In order to remove the wax, thesections were placed in xylene. The sections were dehy-drated by placing them in absolute alcohol for 2 min andrinsed in methanol. They were left in methanol and hydro-
Ž .gen peroxide solution 0.5% for 30 min. The sectionswere then rinsed in tap water and placed in 0.1% trypsinfor 10 min followed by a further rinse in tap water and
Ž .finally phosphate-buffered saline PBS for 2 min. BovineŽ .serum albumin BSA in PBS, at a concentration of 1:50
Ž .2% , was applied for 30 min to block non-specific bindingsites. The excess BSA was wiped off and optimally dilutedprimary antibody, which had been made up in 1% BSA,
Žwas applied. In this case the antibody 22C11, Boehringer.Mannheim was at a concentration of 1:500 and was left
overnight at 48C. The sections were washed in PBS forŽ3=10 min. Diluted biotinylated antibody solution Vecta-
.stain Elite ABC Kit, Vector Labs was applied for 30 minand sections washed again in PBS for 3=10 min. Theavidinrbiotinylated horseradish peroxidase solutionŽ .Vectastain Elite ABC Kit, Vector Labs , which had beenprepared 30 min prior to use, was applied for 30 min. Thewashing procedure with PBS was repeated and then thesections allowed to develop in 3,3X-diaminobenzidine solu-
Ž .tion Sigma for 8–10 min. Finally, the sections werewashed in tap water before being lightly counterstained inhaematoxylin. The sections were rinsed with water, dehy-drated, cleared and mounted in DPX mountant.
Since diaminobenzidine was used as a chromagen in theimmunocytochemistry, the increase in APP immuno-
Ž .reactivity within the white matter was brown Fig. 1 .Equivalent coronal sections from each animal, at defined
w xcoronal planes 17 , were viewed by conventional lightmicroscopy and the distribution of increased APP immuno-reactivity was mapped onto line diagrams by an observer‘‘blind’’ to the topography of the ischaemic lesion in grey
Ž .matter Fig. 2 . To determine the topography of APPaccumulation for the 14 animals, the line diagrams onwhich the increased APP immunoreactivity had been
mapped, at predetermined coordinates, were superimposedŽ .Fig. 3 .
In order to assess the APP immunoreactivity further,each coronal section was then analysed for APP by lightmicroscopy and a semi-quantitative rating was given, viz.,0 for no APP, 1 for some APP, 2 for a moderate amount of
Ž .APP, and 3 for large amounts of APP Fig. 2 . The totalAPP immunoreactivity score for each animal, out of amaximum of 24, was the sum of the scores for the eightcoronal levels.
3. Results
3.1. APP immunocytochemistry after midddle cerebralartery occlusion
In the non-ischaemic contralateral hemisphere, in allanimals, APP immunoreactivity was faint. In contrast, inthe ipsilateral ischaemic hemisphere, all animals hadanatomically circumscribed zones of increased APP im-munoreactivity in the subcortical white matter and in themyelinated fibre tracts permeating the striatum. The in-creased axonal immunoreactivity was ‘‘bulbous’’ in ap-
Ž .pearance Fig. 1 , reminiscent of axonal swellings whichoccur following traumatic brain injury and which have
w xbeen suggested as an early indicator of axotomy 28 .Increased APP immunoreactivity was present in both themedial caudate and dorsal subcortical white matter andwas confined to a zone immediately adjacent to the bound-
Fig. 4. The volume of ischaemic damage was compared to total accumu-lation of APP for each animal. The total APP accumulation was the scoresum for the eight coronal levels. Total APP accumulation score increasedwith increasing volume of infarction. Each point represents data from asingle animal.
( )P.S. Yam et al.rBrain Research 760 1997 150–157 155
ary of ischaemia. This could be readily seen when zones ofischaemic damage and points of APP immunoreactivitywere visualised either at a single level within an animalŽ .Fig. 2 or in the composite diagram for the 14 animalsŽ .Fig. 3 . Increased APP immunoreactivity was detected incell bodies in the ischaemic side, as has been reported in
w xprevious studies 36 . Increased APP immunoreactivitywas not detected in the subcortical white matter or inmyelinated fibre tracts in the lateral caudate deep within
Ž .the areas of ischaemia Fig. 3 .
3.2. Relationship between ischaemia and APP accumula-tion
w xZones of ischaemic damage 5 were restricted to thoseareas of the brain normally supplied by the middle cerebral
w xartery 41 . There was ischaemia in all the animals at levelŽ . Ž2 anterior coordinate of 8.92 mm . Levels 1 and 3 ante-
w x.rior coordinates of 10.50 and 7.19 mm respectively 17were affected in the majority of animals and fewer animals
Fig. 5. Mean APP accumulation score and mean area of the ischaemiclesion for the 14 animals at each coronal level were calculated. MeanAPP accumulation score was highest in the coronal levels with the largest
Ž .area of ischaemic damage anterior coordinates 7.19 and 8.92 mm . DataŽ .are presented as mean"S.E.M. ns14 .
had evidence of ischaemia in the more caudal coronalŽ .sections anterior coordinates of 2.18 and 1.02 mm . Is-
chaemia was not seen in the contralateral hemisphere inany case. The volume of ischaemia in the cerebral hemi-
3 Ž .sphere was 136"10 mm mean"S.E., ns14 . Thevolume of cortical necrosis was 105"9 mm3 and thevolume of striatal necrosis was 24"1 mm3. There was anassociation between the volume of ischaemic damage andthe amount of increased APP immunoreactivity as assessed
Ž .by semi-quantitative analysis Fig. 4 , the increase in APPimmunoreactivity being greatest in rats with the largestvolumes of ischaemic damage. A similar association couldbe discerned between the area of ischaemic damage andthe extent of increased APP immunoreactivity at individual
Ž .coronal planes Fig. 5 .
4. Discussion
In this study we have demonstrated that increased APPimmunoreactivity is a consistent outcome in white matterafter focal cerebral ischaemia in the rat. Increased APPimmunoreactivity is likely to reflect APP accumulationw x33 . The topographical increase in immunoreactivity ofAPP in white matter tracts is correlated with the distribu-tion of infarction. Furthermore, the larger the volume ofthe ischaemic lesion, the greater the extent of increasedAPP immunoreactivity. APP is transported by fast antero-
w xgrade axonal transport 18 along the microtubules byw xkinesin 3 and therefore the presence of APP within axons
at the site of injury is thought to be due to its accumulationw xafter inhibition of axoplasmic flow 33 . Disruption of
axonal microtubules has recently been shown to occurw xafter 2 h in a rat model of ischaemia 27 and such
disruption could result in APP accumulation as seen in ourstudy. The APP accumulations were generally found in thecorpus callosum immediately ventral to the anterior cingu-late cortex, a region in which pallor in haematoxylin andeosin stained sections was not present after middle cerebralartery occlusion. In addition increased APP staining wasalso present in white matter tracts permeating the caudatenucleus but only at the margin of the zones of the is-
Ž .chaemic lesion in grey matter Fig. 3 . These findings lendcredence to the possibility that focal ischaemia results inaccumulation of APP at the periphery of the ischaemiclesion due to disturbances in axoplasmic flow. AxonalAPP accumulation has also been reported to occur aftercytoskeletal disruption incurred after needle stab injuryw x25 and injections of excitotoxins such as kainic acidw x w x16,34 or ibotenic acid 21 .
In man, APP has been used to detect axonal injury inw xthe brains of patients who died after head injury 4,9,32
where it is found to accumulate within axonal swellings,further substantiating its use as a good marker of axonal
w xdisruption 9 . The use of APP as a marker of axonal injuryhas significant advantages over traditional silver impregna-
( )P.S. Yam et al.rBrain Research 760 1997 150–157156
w xtion techniques 29 , in that axonal damage can be detectedin head-injured patients who survive as little as 3 hw x10,20,31,32 . As has been previously mentioned, APPimmunoreactive swellings are not specific for traumaticinjury and occur where axons are damaged, such as at the
w xmargins of infarcts 4 . In the human brain APP accumula-tion has been identified at the margins of cerebral infarc-
w x w xtion in white matter 23 and in ischaemic lesions 37 .w xSuenaga et al. 37 reported that in humans, APP-like
immunoreactivity was present in mild white matter lesionsbut not in moderate or severe cases of white matter injuryŽ .Binswanger’s disease and multi-infarct encephalopathy ,suggesting that APP-like immunoreactive bundles tend tooccur in the early stage of white matter lesions and are notdetectable in the latter stage. This is concordant with other
w xreports of insults to the CNS 16,25 where APP immuno-reactivity occurs in the early stage after damage anddecreases in number in the chronic stage. In the present
w xstudy, as in others 23 , immunoreactivity was not ob-served in the core of the infarct. Ischaemic brain injurieshave been shown previously to induce accumulations ofAPP in neuronal perikarya and damaged axons of the ratw x15,36 but quantification was not performed.
In our study, the interpretation that axonal APPimmunoreactivity represents damage to axons is supported
Ž .by the fact that it was found within swollen axons Fig. 1and it occurred in the areas bordering the ischaemic lesion
Žwhere axonal disruption is likely to have taken place Fig..3 . Since APP is transported by fast anterograde axonal
w xtransport 18 , 24 h survival post middle cerebral arteryocclusion is sufficient time for the protein, which is syn-thesised in the cell bodies, to be delivered to and accumu-late at the site of axonal injury in sufficient quantities for itto be detected. The axons in which APP accumulationoccurs may thus have viable cell bodies in regions notlocated within boundaries of the ischaemic lesion. Theabsence of APP staining within the zone of ischaemicnecrosis reflects the intense ischaemia affecting both axonsand cell bodies; transport and subsequent accumulation ofAPP do not occur due to total energy failure. One couldspeculate that within the first few hours of middle cerebralartery occlusion there exists a ‘‘therapeutic window’’ dur-ing which axons could be saved, however, no consensus ofopinion on this matter has yet been reached. APP ‘‘swell-ings’’, where continuity of the axon is still maintained,may reflect a temporary disturbance in axoplasmic flowwhich has the potential for recovery. However, the devel-opment of APP-positive bulbs, whereby secondary axo-tomy has occurred, may indicate the evolution of irre-versible brain damage. The time course of axonal bulbformation in the ischaemic rat model has yet to be estab-lished. In human head injury, there appears to be a poten-tial for recovery which is time-dependent, in that withinthe first 10 h it may be possible in just under 35% of theaxons whereas from 10 h onwards, there is potential for
w xrecovery in less than 10% of the axons 20 .
There is clearly little point in salvaging grey matterresulting from an ischaemic insult if the connecting axonsare non-functional. This is the first study where an attempthas been made to map and quantify the accumulation ofAPP and relate this to the region and extent of ischaemicdamage. We have shown that the amount of APP accumu-lation is determined by lesion size. This study providesgood evidence that APP is an appropriate marker of dam-age to axons and that there is disruption of axonal trans-port in white matter tracts bordering the ischaemic lesion.In the future APP may be a useful marker of axonal injuryand for assessments of therapeutic interventions in theprotection of white matter after an ischaemic insult.
Acknowledgements
The authors thank Dr. Jim Patterson, Department ofClinical Physics, University of Glasgow for his expertisein image analysis. This work was supported by the BBSRCand MRC.
References
w x1 J.H. Adams, D. Doyle, I. Ford, D.I. Graham, D. McLellan, Diffuseaxonal injury in head injury; definition, diagnosis and grading,
Ž .Histopathology 15 1989 49–59.w x2 B. Allinquant, K.L. Moya, C. Bouillot, A. Prochiantz, Amyloid
precursor protein in cortical neurons: coexistence of two poolsdifferentially distributed in axons and dendrites and association with
Ž .cytoskeleton, J. Neurosci. 14 1994 6842–6854.w x3 A. Amaratunga, S.E. Leeman, K.S. Kosik, R.E. Fine, Inhibition of
kinesin synthesis in vivo inhibits the rapid transport of representativeproteins for three transport vesicle classes into the axon, J. Neu-
Ž .rochem. 64 1995 2374–2376.w x4 P.C. Blumbergs, G. Scott, J. Manavis, H. Wainwright, D.A. Simp-
son, A.J. McLean, Staining of amyloid precursor protein to studyŽ .axonal damage in mild head injury, Lancet 344 1994 1055–1056.
w x Ž .5 D.I. Graham, J.H. Adams, L.W. Duchen Eds. , Greenfield’s Neu-ropathology, 5th ed., Edward Arnold, London, 1992, pp. 153–268.
w x6 C.W. Christman, M.S. Grady, S.A. Walker, K.L. Holloway, J.T.Povlishock, Ultrastructural studies of diffuse axonal injury in hu-
Ž .mans, J. Neurotrauma 11 1994 173–186.w x7 D. Dewar, D.A. Dawson, Changes of cytoskeletal protein immuno-
staining in myelinated fibre tracts after focal cerebral ischaemia inŽ . Ž .the rat, Acta Neuropathol. Berl. 93 1997 71–77.
w x8 T.A. Gennarelli, L.E. Thibault, J.H. Adams, D.I. Graham, C.J.Thompson, R.P. Marcincin, Diffuse axonal injury and traumatic
Ž .coma in the primate, Ann. Neurol. 12 1982 564–574.w x9 S.M. Gentleman, M.J. Nash, C.J. Sweeting, D.I. Graham, G.W.
Ž .Roberts, Beta-amyloid precursor protein b-APP as a marker forŽ .axonal injury after head injury, Neurosci. Lett. 160 1993 139–144.
w x10 S.M. Gentleman, G.W. Roberts, T.A. Gennarelli, W.L. Maxwell,J.H. Adams, S. Kerr, D.I. Graham, Axonal injury: a universal
Ž .consequence of fatal closed head injury?, Acta Neuropathol. Berl.Ž .89 1995 537–543.
w x11 G.G. Glenner, C.W. Wong, Alzheimer’s disease: initial report of thepurification and characterization of a novel cerebrovascular amyloid
Ž .peptide, Biochem. Biophys. Res. Commun. 120 1984 885–890.w x12 G.G. Glenner, C.W. Wong, Alzheimer’s disease and Down’s syn-
drome: sharing of a unique cerebrovascular amyloid fibril protein,Ž .Biochem. Biophys. Res. Commun. 122 1984 1131–1135.
( )P.S. Yam et al.rBrain Research 760 1997 150–157 157
w x13 M.S. Grady, M.R. McLaughlin, C.W. Christman, A.B. Valadka,C.L. Fligner, J.T. Povlishock, The use of antibodies targeted againstthe neurofilament subunits for the detection of diffuse axonal injury
Ž .in humans, J. Neuropathol. Exp. Neurol. 52 1993 143–152.w x14 S.H. Gultekin, T.W. Smith, Diffuse axonal injury in craniocerebral
trauma. A comparative histologic and immunohistochemical study,Ž .Arch. Pathol. Lab. Med. 118 1994 168–171.
w x15 R.N. Kalaria, S.U. Bhatti, E.A. Palatinsky, D.H. Pennington, E.R.Shelton, H.W. Chan, G. Perry, W.D. Lust, Accumulation of the betaamyloid precursor protein at sites of ischemic injury in rat brain,
Ž .NeuroReport 4 1993 211–214.w x16 T. Kawarabayashi, M. Shoji, Y. Harigaya, H. Yamaguchi, S. Hirai,
Expression of APP in the early stage of brain damage, Brain Res.Ž .563 1991 334–338.
w x17 J.F.R. Konig, R.A. Klippel, The Rat Brain: a Stereotaxic Atlas of the¨Forebrain and Lower Parts of the Brain Stem, Krieger, New York,1963.
w x18 E.H. Koo, S.S. Sisodia, D.R. Archer, L.J. Martin, A. Weidemann, K.Beyreuther, P. Fischer, C.L. Masters, D.L. Price, Precursor ofamyloid protein in Alzheimer disease undergoes fast anterograde
Ž .axonal transport, Proc. Natl. Acad. Sci. USA 87 1990 1561–1565.w x19 F.W. Marcoux, R.B. Morawetz, R.M. Crowell, U. De Girolami, J.R.
Halsey, Differential regional vulnerability in transient focal cerebralŽ .ischemia, Stroke 13 1982 339–346.
w x20 K.J. McKenzie, D.R. McLellan, S.M. Gentleman, W.L. Maxwell,T.A. Gennarelli, D.I. Graham, Is b-APP a marker of axonal damage
Ž . Ž .in short-surviving head injury?, Acta Neuropathol. Berl. 92 1996608–613.
w x21 Y. Nakamura, M. Takeda, H. Niigawa, S. Hariguchi, T. Nishimura,Amyloid beta-protein precursor deposition in rat hippocampus le-
Ž .sioned by ibotenic acid injection, Neurosci. Lett. 136 1992 95–98.w x22 H.K. Ng, R.D. Mahaliyana, W.S. Poon, The pathological spectrum
of diffuse axonal injury in blunt head trauma: assessment with axonŽ .and myelin stains, Clin. Neurol. Neurosurg. 96 1994 24–31.
w x23 T. Ohgami, T. Kitamoto, J. Tateishi, Alzheimer’s amyloid precursorprotein accumulates within axonal swellings in human brain lesions,
Ž .Neurosci. Lett. 136 1992 75–78.w x24 K.A. Osborne, T. Shigeno, A.M. Balarsky, I. Ford, J. McCulloch,
G.M. Teasdale, D.I. Graham, Quantitative assessment of early braindamage in a rat model of focal cerebral ischaemia, J. Neurol.
Ž .Neurosurg. Psychiatry, 50 1987 402–410.w x25 N. Otsuka, M. Tomonaga, K. Ikeda, Rapid appearance of beta-
amyloid precursor protein immunoreactivity in damaged axons andreactive glial cells in rat brain following needle stab injury, Brain
Ž .Res. 568 1991 335–338.w x26 L. Pantoni, J.H. Garcia, The significance of cerebral white matter
abnormalities 100 years after Binswanger’s report. A review, StrokeŽ .26 1995 1293–1301.
w x27 L. Pantoni, J.H. Garcia, J.A. Gutierrez, Cerebral white matter isŽ .highly vulnerable to ischemia, Stroke 27 1996 1641–1647.
w x28 J.T. Povlishock, Traumatically induced axonal injury: pathogenesisŽ .and pathobiological implications, Brain Pathol. 2 1992 1–12.
w x29 J.T. Povlishock, D.P. Becker, C.L. Cheng, G.W. Vaughan, AxonalŽ .changes in minor head injury, J. Neuropathol. Neurobiol. 42 1983
225–242.w x30 L.M. Refolo, I.S. Wittenberg, V.L. Friedrich Jr., N.K. Robakis, The
Alzheimer amyloid precursor is associated with the detergent-insolu-Ž .ble cytoskeleton, J. Neurosci. 11 1991 3888–3897.
w x31 F.E. Sherriff, L.R. Bridges, S.M. Gentleman, S. Sivaloganathan, S.Wilson, Markers of axonal injury in post mortem human brain, Acta
Ž . Ž .Neuropathol. Berl. 88 1994 433–439.w x32 F.E. Sherriff, L.R. Bridges, S. Sivaloganathan, Early detection of
axonal injury after human head trauma using immunocytochemistryŽ .for beta-amyloid precursor protein, Acta Neuropathol. Berl. 87
Ž .1994 55–62.w x33 K. Shigematsu, P.L. McGeer, Accumulation of amyloid precursor
protein in neurons after intraventricular injection of colchicine, Am.Ž .J. Pathol. 140 1992 787–794.
w x34 K. Shigematsu, P.L. McGeer, D.G. Walker, T. Ishii, E.G. McGeer,Reactive microgliarmacrophages phagocytose amyloid precursorprotein produced by neurons following neural damage, J. Neurosci.
Ž .Res. 31 1992 443–453.w x35 T. Shigeno, J. McCulloch, D.I. Graham, A.D. Mendelow, G. Teas-
dale, Pure cortical ischemia versus striatal ischemia, Surg. Neurol.Ž .24 1985 47–51.
w x36 D.T. Stephenson, K. Rash, J.A. Clemens, Amyloid precursor proteinaccumulates in regions of neurodegeneration following focal cere-
Ž .bral ischemia in the rat, Brain Res. 593 1992 128–135.w x37 T. Suenaga, K. Ohnishi, M. Nishimura, S. Nakamura, I. Akiguchi, J.
Kimura, Bundles of amyloid precursor protein-immunoreactive ax-ons in human cerebrovascular white matter lesions, Acta Neu-
Ž . Ž .ropathol. Berl. 87 1994 450–455.w x38 A. Tamura, D.I. Graham, J. McCulloch, G.M. Teasdale, Focal
cerebral ischaemia in the rat. I. Description of technique and earlyneuropathological consequences following middle cerebral artery
Ž .occlusion, J. Cereb. Blood Flow Metab. 1 1981 53–60.w x39 S.G. Waxman, J.A. Black, P.K. Stys, B.R. Ransom, Ultrastructural
concomitants of anoxic injury and early post-anoxic recovery in ratŽ .optic nerve, Brain Res. 574 1992 105–119.
w x40 S.G. Waxman, B.R. Ransom, P.K. Stys, Non-synaptic mechanismsof Ca2q-mediated injury in CNS white matter, Trends Neurosci. 14Ž .1991 461–468.
w x41 Y. Yamori, R. Horie, H. Handa, M. Sato, M. Fukase, Pathogeneticsimilarity of strokes in stroke-prone spontaneously hypertensive rats
Ž .and humans, Stroke 7 1976 46–53.