Lifelogging memory appliance for people with episodic memory impairment
Verbal Episodic Memory Impairment in Schizophrenia: A Comparison with Frontal Lobe Lesion Patients
-
Upload
mcleanhospital -
Category
Documents
-
view
1 -
download
0
Transcript of Verbal Episodic Memory Impairment in Schizophrenia: A Comparison with Frontal Lobe Lesion Patients
This article was downloaded by: [24.141.56.161]On: 26 June 2013, At: 12:11Publisher: RoutledgeInforma Ltd Registered in England and Wales Registered Number: 1072954 Registeredoffice: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK
The Clinical NeuropsychologistPublication details, including instructions for authors andsubscription information:http://www.tandfonline.com/loi/ntcn20
CE Verbal Episodic Memory Impairmentin Schizophrenia: A Comparison withFrontal Lobe Lesion PatientsBruce K. Christensen a d , Regan E. Patrick a d , Donald T. Stuss b c e
, Susan Gillingham b & Robert B. Zipursky aa Department of Psychiatry & Behavioural Neuroscience , McMasterUniversity, Hamilton , Ontario , Canadab Rotman Research Institute at Baycrest , University of Toronto ,Toronto , Ontario , Canadac Departments of Medicine and Psychology , University of Toronto ,Toronto , Ontario , Canadad McMaster Integrative Neuroscience Discovery & Study (MiNDS) ,McMaster University , Hamilton , Ontario , Canadae Ontario Brain Institute , Toronto , Ontario , CanadaPublished online: 01 May 2013.
To cite this article: Bruce K. Christensen , Regan E. Patrick , Donald T. Stuss , Susan Gillingham &Robert B. Zipursky (2013): CE Verbal Episodic Memory Impairment in Schizophrenia: A Comparisonwith Frontal Lobe Lesion Patients, The Clinical Neuropsychologist, 27:4, 647-666
To link to this article: http://dx.doi.org/10.1080/13854046.2013.780640
PLEASE SCROLL DOWN FOR ARTICLE
Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching, and private study purposes. Anysubstantial or systematic reproduction, redistribution, reselling, loan, sub-licensing,systematic supply, or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representationthat the contents will be complete or accurate or up to date. The accuracy of anyinstructions, formulae, and drug doses should be independently verified with primarysources. The publisher shall not be liable for any loss, actions, claims, proceedings,demand, or costs or damages whatsoever or howsoever caused arising directly orindirectly in connection with or arising out of the use of this material.
CE Verbal Episodic Memory Impairment in Schizophrenia:A Comparison with Frontal Lobe Lesion Patients
Bruce K. Christensen1,4, Regan E. Patrick1,4, Donald T. Stuss2,3,5,Susan Gillingham2, and Robert B. Zipursky1
1Department of Psychiatry & Behavioural Neuroscience, McMaster University, Hamilton,Ontario, Canada2Rotman Research Institute at Baycrest, University of Toronto, Toronto, Ontario, Canada3Departments of Medicine and Psychology, University of Toronto, Toronto, Ontario, Canada4McMaster Integrative Neuroscience Discovery & Study (MiNDS), McMaster University,Hamilton, Ontario, Canada5Ontario Brain Institute, Toronto, Ontario, Canada
Schizophrenia (SCZ)-related verbal memory impairment is hypothesized to be mediated, inpart, by frontal lobe (FTL) dysfunction. However, little research has contrasted the performanceof SCZ patients with that of patients exhibiting circumscribed frontal lesions. The current studycompared verbal episodic memory in patients with SCZ and focal FTL lesions (left frontal, LF;right frontal, RF; and bi-frontal, BF) on a four-trial list learning task consisting of three lists ofvarying semantic organizational structure. Each dependent variable was examined at two levels:scores collapsed across all four trials and learning scores (i.e., trial 4–trial 1). Performancedeficits were observed in each patient group across most dependent measures at both levels.Regarding patient group differences, SCZ patients outperformed LF/BF patients (i.e., eitherlearning scores or scores collapsed across trial) on free recall, primacy, primary memory,secondary memory, and subjective organization, whereas they only outperformed RF patientson the semantically blocked list on recency and primary memory. Collectively, these resultsindicate that the pattern of memory performance is largely similar between patients with SCZand those with RF lesions. These data support tentative arguments that verbal episodic memorydeficits in SCZ may be mediated by frontal dysfunction in the right hemisphere.
Keywords: Schizophrenia; Frontal lobes; Learning; Verbal memory; Free recall; Lesion.
INTRODUCTION
Memory impairment is one of the more severe and well-documented cognitivedeficits associated with schizophrenia (SCZ). A meta-analysis conducted by Heinrichsand Zakzanis (1998) revealed that deficits in global verbal memory produced the larg-est mean effect size (d = 1.53) relative to more than 20 other cognitive measures,including tests of attention, general intelligence, spatial ability, executive function, andlanguage. Using structural equation modeling, Dickinson, Ragland, Gold, and Gur(2008) further demonstrated that, while much of cognitive impairment in SCZ can beattributed to a generalized cognitive deficit, only verbal memory (and to a lesser extent
Address correspondence to: Bruce Christensen, Ph.D., C.Psych., Department of Psychiatry andBehavioural Neuroscience, McMaster University, 100 West 5th Street, Hamilton, ON, Canada L8N 3K7.Email: [email protected]
Accepted for publication: February 22, 2013. First published online: April 25, 2013
The Clinical Neuropsychologist, 2013Vol. 27, No. 4, 647–666, http://dx.doi.org/10.1080/13854046.2013.780640
� 2013 Taylor & Francis
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
processing speed) directly and uniquely accounted for cognitive dysfunction. Thus,research clearly demonstrates that verbal memory is reliably and robustly impaired inSCZ and, therefore, an important target for investigations of aberrant cognition.
Previous research has sought to clarify the specific pattern of verbal memorydeficits observed in SCZ. In this context, meta-analytic studies indicate that the greatestimpairment emerges on tasks designed to assess free recall (Aleman, Hijman, de Haan,& Kahn, 1999). One important facet of impoverished recall memory in SCZ is theirreduced ability to spontaneously apply organizational strategies to aid recall (e.g., Chris-tensen, Girard, Benjamin, & Vidailhet, 2006). Gold, Randolph, Carpenter, Goldberg, andWeinberger (1992) observed that patients with SCZ demonstrated persistent impairmentin recall memory regardless of the organizational structure of the wordlists. They alsoobserved a performance plateau in the SCZ group across trials indicative of a bluntedlearning effect. Patients were, however, able to benefit from wordlists in which thesemantic relatedness of the words was concretely highlighted (i.e., blocked) upon presen-tation, though not to the level of healthy controls. This was interpreted as evidence for asemantic system that is most effective when no self-initiated strategic recall is necessary(Gold et al., 1992). This effect has since been replicated using experimental methodolo-gies that account for difficulty confounds (Christensen et al., 2006) and illness chronicity(Chan et al., 2000).
Although the exact brain regions that mediate recall impairment in SCZ areunknown, some researchers have suggested that the configuration of deficits reflects anunderlying frontal lobe (FTL) dysfunction (Beatty, Jocic, Monson, & Stanton, 1993;Goldberg, Weinberger, Plishkin, Berman, & Podd, 1989; Sanz de la Torre, Barrios, &Junque, 2005). Previous list-learning studies with FTL lesion patients have revealed asimilar pattern of deficits to those observed in SCZ (e.g., Albuquerque, Loureiro, &Martins, 2008; Alexander, Stuss, & Fansabedian, 2003; Gershberg and Shimamura,1995; Kopelman and Stanhope, 1998; Turner, Cipolotti, Yousry, & Shallice, 2007;Stuss et al., 1994; Vilkki, Servo, & Surma-aho, 1998). For example, FTL lesionpatients who were grouped according to the anatomical position of their lesion (i.e.,right frontal, RF; left frontal, LF; and bi-frontal, BF) showed significant deficits in freerecall and organization strategy that were more pronounced when the left hemispherewas involved (Stuss et al., 1994). Like individuals with SCZ (e.g., Chan et al., 2000;Gold et al., 1992), all lesion groups in this study performed better on blocked word-lists, implying that explicit semantic organization enhanced performance. Alexanderet al. (2003) extended these findings using the California Verbal Learning Test (CVLT)by demonstrating that recall impairment was most pronounced when the left dorsal lat-eral and posterior medial regions were involved. However, a more recent list-learningstudy demonstrated that only medial and right lateral lesion patients were significantlyimpaired in free recall (Turner et al., 2007). Thus, it appears that there is some resem-blance between memory deficits exhibited by FTL lesion patients and those observedin SCZ; however, the exact pattern is uncertain.
Given the similarities between SCZ and FTL lesion patients, a more directmethod of establishing a link between SCZ-related memory impairment and FTL dys-function would be to directly compare the performance of patients with SCZ topatients with documented lesions of FTL brain tissue using identical experimentaltasks. To date, a limited number of studies have made such a comparison (e.g.,Ornstein, Sahakian, & McKenna, 2008; Pantelis et al., 1997). Unfortunately, these
648 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
studies did not investigate if or how FTL lesion location differentially affected memoryperformance. This is important since the frontal lobes are not anatomically or cytoar-chitectonically homogenous (see Stuss and Alexander, 2007; Stuss et al., 2002) anddirect comparison using more defined lesion groups may further elucidate FTL pathol-ogy in SCZ.
The primary objective of the current study was to elucidate the neuroanatomicalsubstrates associated with verbal episodic memory impairment in persons with SCZ bycomparing their memory performance to FTL lesion patients with well-defined, cir-cumscribed lesions. This was accomplished by examining recall performance on afour-trial list-learning task comprised of three different lists of varying semantic struc-ture (see Stuss et al., 1994). No unidirectional predictions were made regarding whichspecific FTL lesion group would most closely resemble individuals with SCZ becauseprevious research lacks the consistency needed to formulate meaningful a priorihypotheses.
METHODS
Participants
SCZ patients (n = 35) were recruited through the Schizophrenia Program at theCentre for Addiction and Mental Health (Toronto, Canada) via poster advertisementsand referrals from health professionals. Psychiatric diagnoses were confirmed using theStructured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I; First, Spitzer,Gibbon, & Williams, 2001) and symptom were assessed using the Positive and Nega-tive Syndrome Scale (PANSS; Kay, Fiszbein, & Opler, 1987). Exclusion criteria forSCZ patients were as follows: (a) history of non-psychotic Axis I psychiatric disorder;(b) history of neurological illness/disease; (c) recent change (<2 weeks) in use of anti-psychotic medication; and (d) prescribed medications with known deleterious cognitiveeffects (i.e., tricyclic antidepressants, benzodiazapines, anticonvulsants, and anticholin-ergics).
Frontal lobe lesion patients (n = 29) were divided into three separate groupsaccording to lesion location: left frontal (LF; n = 10), right frontal (RF; n = 7), and bi-frontal (BF; n = 12). Inclusion criteria were as follows: (a) lesions resulted from anacute event (e.g., infarct, resection of a benign tumor); (b) stable clinical condition(i.e., median 14 months post-onset); and (c) CT- or MRI-confirmed lesion site. Exclu-sion criteria included: (a) untreated hydrocephalus (revealed by MRI or CT); (b) activeseizure disorder; (c) history of alcoholism or symptomatic depression (revealed throughclinical interview); and (e) self-reported prior neurological illness. Clinical and demo-graphic characteristics of the all patient groups are provided in Table 1.
Healthy control (HC) participants (n = 64) were recruited from the communityvia newspaper advertisements. Screening for the presence of any previously unidenti-fied psychiatric illness was achieved using the SCID-I. Inclusion criteria for HCincluded: (a) the ability to provide informed consent, (b) age between 18 and 60 yearsold, (c) English as primary the language, and (d) normal or corrected-to-normal vision.Exclusion criteria included: (a) any self-reported history of psychiatric illness or neuro-logical disease/disorder, (b) having a first degree relative with a psychotic disorder, (c)recent use of any psychotropic drug (<2 weeks), and (c) prescribed medications with
VERBAL EPISODIC MEMORY IMPAIRMENT IN SCHIZOPHRENIA 649
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
known deleterious cognitive side effects (see those listed above). Demographic infor-mation for the HC sample is provided in Table 1. The study was approved by theResearch Ethics Board at the University of Toronto. Participants provided voluntarywritten consent to participate, and they were compensated $10/hour.
Experimental measures and procedure
Three 16-item word-lists of varying organizational structure were used (catego-rized-blocked, categorized-unblocked, and unrelated). The three word-lists were pre-sented orally in counterbalanced order across participants. Each list was presented fourtimes, with the order of presentation of items on each word-list remaining the same foreach trial. Participants were then asked to freely recall as many items as possible
Table 1. Demographic and clinical characteristics of HC, SCZ, and FTL lesion groups
HC SCZ LF RF BF
Agea 38.72 ± 15.65 27.80 ± 6.67 50.90 ± 10.02 52.00 ± 9.66 52.42 ± 13.85Etiology of
lesionb
Infarct – – 4 4 1Hemorrhage – – 3 1 0Subarachnoidhemorrhage
– – 1 2 5
Trauma – – 2 1 5Tumour – – 0 1 2
Time post-onsetof lesionb
2–3 months – – 1 2 23–12 months – – 2 4 3>12 months – – 7 3 8
Seizure disorderb 0 0 1 1 2Antipsychotic
medicationb
2nd Generation – 27 (17 olanzapine,6 clozapine,3 risperidone,1 quetiapine)
– – –
1st Generation – 8 (3 haloperidol,2 loxapine,1 fluanxol)
– – –
PANSS-Positivescorea
– 14.17 ± 5.74 – – –
PANSS-Negativescorea
– 14.11 ± 5.58 – – –
PANSS-Generalscorea
– 26.71 ± 7.28 – – –
Number of hospitalizationsa – 2.26 ± 2.52 –– –
aMeans ± Standard Deviations (M ± SD).bFrequencies (ns) of patient characteristics.Abbreviations: BF = bi-frontal; HC = healthy control; LF = left frontal; PANSS = Positive and NegativeSyndrome Scale (Kay et al., 1987); RF = right frontal; SCZ = schizophrenia.
650 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
following each presentation. After four trials of one list, the same procedure wasrepeated for the other two list types. Participants also completed a brief baseline neuro-psychological assessment aimed at assessing language competency. This included theNational Adult Reading Test—R (NART; Blair and Spreen, 1989) and the BostonNaming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983).
Data preparation and analyses
Four measures related to memory performance were analyzed: immediate freerecall, primacy/recency, primary/secondary memory, and organizational strategy. Imme-diate free recall was defined as the number of correctly reproduced words following alist presentation. As per Stuss et al. (1994), free recall was further characterized accord-ing to primacy (i.e., recall of any of the first four words in the presented list) andrecency (the last four words) effects. Free recall was also characterized as either primarymemory (PM) or secondary memory (SM). A recalled item was considered PM if sevenor fewer words intervened between its presentation and subsequent recall. A recalleditem which had greater than seven intervening words was classified as SM (Tulving andColotla, 1970). PM is a measure of one’s ability to maintain a limited number of activerepresentations in mind for on-going processing (Unsworth and Engle, 2007), whereasSM measures retrieval capacity for information displaced from PM (Rao, Leo, & St.Aubin-Faubert, 1989; Unsworth and Engle, 2007). Strategic organization was analyzedusing three separate measures. Semantic categorization was defined as the number ofwords in the same category that were recalled consecutively for the blocked andunblocked lists. These data were used to calculate a ratio of repetition (Frender andDoubilet, 1974). Serial ordering was defined as the number of words recalled in thesame order as presented for each trial. This measure was transferred for analysis of vari-ance (ANOVA) by dividing the tabulated order of clusters by the theoretically maximalnumber of possible order clusters for the actual number of words recalled in a given list(Stuss et al., 1994). Subjective organization was defined as the number of word pairsrecalled together in list n that were also recalled together in list n + 1, regardless oforder (e.g., if words A, B, C, and D are recalled in List 1, and A, B, E, C, and D arerecalled in List 2, the pair frequency score would be 2: A&B, and C&D). Each of thesedependent measures was examined at two levels: (1) total score collapsed across allfour trials and (2) learning scores (i.e., trial 4–trial 1). The latter measure was includedto examine the effects of repeated list exposure on each measure.
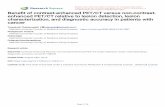
Main effects and interactions for all memory parameters were analyzed using a3 � 4 mixed repeated-measures ANOVA, with List (blocked, unblocked, and unre-lated) as the within-subjects independent variable and Group (SCZ, RF, LF, and BF)as the between-subjects independent variable. The HC group was not included in theANOVAs because our primary research question concerned similarities and differencesbetween SCZ and FTL lesion groups. Previous research has contrasted these patientgroups with HC samples using very similar list learning paradigms and found reliabledeficits (e.g., Gold et al., 1992; Stuss et al., 1994). That said, because a neurologicallyintact sample provides a useful benchmark to facilitate qualitative comparisonsbetween groups, the HC group data served as the normative sample for calculating thez-scores that are depicted in Figures 1 to 3. In addition, Cohen’s d effect sizes fordifferences between HC and patient groups are provided in Tables 2–5.
VERBAL EPISODIC MEMORY IMPAIRMENT IN SCHIZOPHRENIA 651
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
Post-hoc comparisons were only performed for significant omnibus effectsinvolving Group (i.e., main effect of Group or Group � List interaction).Between-group comparisons were only performed between the SCZ group and each ofthe FTL lesion groups (i.e., no between frontal group comparisons). Within-group com-parisons were performed for each patient group. In addition, a one-way ANOVA wasperformed to determine if between-patient group differences were present for the twobaseline measures—the BNT and NART (Note: No significant differences wereobserved). Effect sizes are reported as partial eta squared (ηp
2) and Cohen’s d (d). Thesmall sample size and associated low power within the FTL lesion groups meant thatvery large effect sizes would likely be needed to achieve statistical significance forcomparisons involving the FTL groups. Accordingly, differences were deemed to bestatistically meaningful if either the alpha value exceeded the p < .05 threshold or theeffect size exceeded d = 0.83 (Barnette and MacLean, 1999).1
RESULTS AND DISCUSSION
Given the large number of statistical procedures that were performed, omnibusand post-hoc analyses for each DV have been summarized in Table 6 (collapsed across
Collapsed Across Trials - Overall
-5
-4
-3
-2
-1
0
1
2
3
4
5
Z Sc
ores
rela
tive
to H
C g
roup
Learning Scores (T4 - T1) - Overall
-5
-4
-3
-2
-1
0
1
2
3
4
5
Z Sc
ores
rela
tive
to H
C g
roup
FR Pr Re PM SM SO SC SubO
FR Pr Re PM SM SO SC SubO
SCZ
RF
LF
BF
SCZ
RF
LF
BF
(a)
(b)
Figure 1. Graphical depiction of z-scores for each dependent variable collapsed across list type for (a)scores collapsed across trials and (b) learning scores. HC group data served as the normative sample whencalculating z-scores (dashed line). Abbreviations: BF = bi-frontal; FR = free recall; HC = healthy control; LF= left frontal; PM = primary memory; Pr = primacy; Re = recency; RF = right frontal; SC = semanticcategorization; SCZ = schizophrenia; SM = secondary memory; SO = serial ordering; SubO = subjectiveorganization.
652 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
trial) and Table 7 (learning scores). Figures1 to 3 depict patient group z-scores relativeto the HC group for each dependent measure. Figure 1a shows z-scores for each mea-sure collapsed across list type and trial, and Figure 1b shows learning z-scores of eachmeasure collapsed across list type. Figures 2 and 3 depict the same data broken downaccording to list type.
Blocked List - Collapsed Across Trials
-5
-4
-3
-2
-1
0
1
2
3
4
Z Sc
ores
rela
tive
to H
C g
roup
SCZ
RF
LF
BF
Unblocked List - Collapsed Across Trials
-4
-3
-2
-1
0
1
2
3
4
Z Sc
ores
rela
tive
to H
C g
roup
SCZ
RF
LF
BF
Unrelated List - Collapsed Across Trials
-4
-3
-2
-1
0
1
2
3
4
Z Sc
ores
rela
tive
to H
C g
roup
SCZ
RF
LF
BF
FR Pr Re PM SM SO SC SubO
FR Pr Re PM SM SO SC SubO
FR Pr Re PM SM SO SubO
(a)
(b)
(c)
Figure 2. Graphical depiction of z-scores for each dependent variable collapsed across all four learningtrials in the (a) blocked list, (b) unblocked list, and (c) unrelated list. HC group data served as normativesample when calculating z-scores (dashed line). See Fig. 1 for list of abbreviations.
VERBAL EPISODIC MEMORY IMPAIRMENT IN SCHIZOPHRENIA 653
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
Memory processes in SCZ were assessed by directly comparing theirperformance to FTL lesion patients on a list-learning task. In an effort to examine thecontributions of regional FTL pathology to recall impairment in SCZ, FTL lesionpatients were separated according to their lateralized lesion location. The current study
Blocked List - Learning Scores
-4
-3
-2
-1
0
1
2
3
4
Z Sc
ores
rela
tive
to H
C g
roup
SCZ
RF
LF
BF
Unblocked List - Learning Scores
-4
-3
-2
-1
0
1
2
3
4
Z Sc
ores
rela
tive
to H
C g
roup
SCZ
RF
LF
BF
Unrelated List - Learning Scores
-4
-3
-2
-1
0
1
2
3
4
Z Sc
ores
rela
tive
to H
C g
roup
SCZ
RF
LF
BF
FR Pr Re PM SM SO SC SubO
FR Pr Re PM SM SO SC SubO
FR Pr Re PM SM SO SubO
(a)
(b)
(c)
Figure 3. Graphical depiction of learning score z-scores for each dependent variable in the (a) blocked list,(b) unblocked list, and (c) unrelated list. HC group data served as normative sample when calculating z-scores (dashed line). See Figure 1 for list of abbreviations.
654 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
demonstrated that even a relatively crude division of the frontal cortex based on lesionlateralization resulted in interesting between patient-group differences. The ensuing dis-cussion focuses primarily on results relevant to SCZ-FTL group differences as theprincipal goal of the current study was to determine the extent to which specific FTLpathology produces recall impairment commensurate to that associated with SCZ.
Immediate free recall
Each patient group demonstrated reduced absolute recall collapsed across trialsrelative to the HC group, with effect sizes ranging from 1.23 to 1.99 (see Figure 1a andTable 2). Learning scores were also smaller in each patient group, though the magnitudeof this difference was not as large (effect sizes ranged from 0.25 to 1.07, see Figure 1band Table 3). The current study, therefore, supports previous research in terms ofshowing deficient verbal episodic recall in both SCZ and FTL samples. Regardingbetween-patient group comparisons, the SCZ group demonstrated greater absolute recall(i.e., collapsed across trials; Figure 1a) and benefited more from repeated list exposure(i.e., learning scores; Figure 1b) relative to the LF group. The lack of List � Groupinteractions indicates that the SCZ-LF difference did not vary significantly as a functionof the semantic structure of the word lists (Figures. 2 and 3). That is, regardless of howsalient the semantic relationship was between items (i.e., blocked, unblocked, or unre-lated), SCZ patients exhibited superior verbal episodic recall overall compared to indi-viduals with LF damage. A qualitatively similar pattern of results was observedbetween the SCZ and BF groups, who are also characterized by partial left-frontal lobe
Table 2. Summary of Cohen’s d effect sizes for differences between the HC group and each patient groupcollapsed across list type (scores collapsed across trial)
HCvs.
Freerecall Primacy Recency
Primarymemory
Secondarymemory
Serialpairs
Semanticclusters
Subjectiveorganization
SCZ 1.51 0.30 –1.28 –0.50 1.63 0.64 1.23 1.12RF 1.23 –0.17 –0.63 0.01 1.08 0.63 1.09 1.07LF 1.92 0.73 –1.26 0.15 1.78 1.68 1.31 2.21BF 1.99 0.60 –1.04 0.37 1.78 1.02 1.62 1.63
Negative effect sizes indicate that the HC group was smaller.Abbreviations: SCZ = schizophrenia; HC = healthy control; LF = left frontal; RF = right frontal; BF = bi-frontal.
Table 3. Summary of Cohen’s d effect sizes for differences between the HC group and each patient groupcollapsed across list type (trial 4–trial 1 difference scores)
HCvs.
Freerecall Primacy Recency
Primarymemory
Secondarymemory
Serialpairs
Semanticclusters
Subjectiveorganization
SCZ 0.25 –0.34 0.74 –0.20 0.33 0.52 0.50 0.70RF 1.06 0.16 0.90 –0.15 1.08 0.90 1.41 1.34LF 1.07 0.65 0.61 –0.13 0.92 0.99 1.07 1.34BF 0.69 0.29 0.13 –0.14 0.67 0.54 0.82 0.83
Negative effect sizes indicate that the HC group was smaller.Abbreviations: SCZ = schizophrenia; HC = healthy control; LF = left frontal; RF = right frontal; BF = bi-frontal.
VERBAL EPISODIC MEMORY IMPAIRMENT IN SCHIZOPHRENIA 655
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
damage. Taken together, these findings suggest that: (1) LF damage is associated withthe most severe impairment in list learning, (2) individuals with SCZ demonstrate sig-nificantly better verbal episodic recall than patients with macroscopic LF damage, bothin terms of absolute ability and trial-to-trial learning, and (3) SCZ and RF patients wereequivalent in terms of absolute recall and learning scores. The disproportionate impair-ment among LF patients may have been due to the linguistic nature of the stimuli—a
Table 4. Summary of Cohen’s d effect sizes for differences between the HC group and each patient groupat each level of list (collapsed across trial)
ListHCvs.
Freerecall Primacy Recency
Primarymemory
Secondarymemory
Serialpairs
Semanticclusters
Subjectiveorganization
Blocked SCZ 1.61 0.02 –1.52 –0.53 1.69 0.73 1.39 1.03RF 0.86 –0.44 –0.32 0.33 0.55 0.60 0.74 0.60LF 1.85 0.03 –0.81 0.19 1.65 1.51 1.82 2.11BF 1.83 0.24 –0.34 0.64 1.33 1.35 1.89 1.58
Unblocked SCZ 1.20 0.17 –1.06 –0.49 1.30 0.21 0.59 0.83RF 1.07 –0.65 –0.54 –0.29 1.16 0.16 0.91 0.86LF 1.54 0.76 –1.21 –0.18 1.54 0.69 0.41 1.23BF 1.93 0.26 –1.26 –0.37 2.13 0.04 0.67 0.96
Unrelated SCZ 1.18 0.33 –0.81 –0.15 1.16 0.43 – 0.68RF 1.29 0.25 –0.63 –0.11 1.26 0.78 – 1.23LF 1.98 0.81 –1.14 0.31 1.72 1.30 – 1.46BF 1.55 0.86 –0.85 0.57 1.24 0.88 – 1.18
Negative effect sizes indicate that the HC group was smaller.Abbreviations: SCZ = schizophrenia; HC = healthy control; LF = left frontal; RF = right frontal; BF =bi-frontal.
Table 5. Summary of Cohen’s d effect sizes for differences between the HC group and each patient groupat each level of list (trial 4–trial 1 difference scores)
ListHCvs.
Freerecall Primacy Recency
Primarymemory
Secondarymemory
Serialpairs
Semanticclusters
Subjectiveorganization
Blocked SCZ –0.21 –0.47 0.46 –0.14 –0.11 0.36 0.34 0.49RF 1.02 0.99 0.90 0.46 0.54 0.88 1.05 1.04LF 0.43 –0.25 0.40 –0.05 0.38 0.86 0.92 1.06BF 0.23 0.08 0.40 0.43 –0.04 0.37 0.48 0.42
Unblocked SCZ 0.54 –0.21 0.58 –0.05 0.52 0.29 0.37 0.73RF 0.98 –0.29 0.99 –0.38 1.08 0.16 0.84 0.96LF 1.59 1.03 0.75 –0.08 1.24 0.36 0.72 0.90BF 0.75 –0.16 0.33 –0.47 1.00 0.01 0.77 0.81
Unrelated SCZ 0.33 0.11 0.22 –0.19 0.38 0.49 – 0.16RF 0.49 –0.20 0.05 –0.46 0.70 0.83 – 0.68LF 0.85 0.48 0.23 –0.16 0.83 0.72 – 0.64BF 0.64 0.72 –0.54 –0.40 0.83 0.79 – 0.53
Negative effect sizes indicate that the HC group was smaller.Abbreviations: SCZ = schizophrenia; HC =healthy control; LF = left frontal; RF = right frontal; BF = bi-frontal
656 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
Tab
le6.
Omnibusandpost-hoc
results
foreach
dependentvariable
collapsed
across
trial(H
Cgroupexcluded)
Group
List�
Group
Omnibus
Post-ho
cOmnibus
Post-hoc
Freerecall
F=3.13
SCZ>LF(p
=.02,
d=0.72
)F=1.02
n/a
p=.03
p=.41
η p2=.14
η p2=.05
Primacy
F=1.47
n/a
F=0.77
n/a
p=.23
p=.59
η p2=.04
η p2=.07
Recency
F=1.62
n/a
F=2.15
Blocked:
SCZ>RF(p
=.24,
d=1.12
)p=.20
p=.05
SCZ:
Bl<Unr
(p<.01,
d=0.59)
η p2=.08
η p2=.10
RF:
Bl<Unr
(p=.04,
d=1.01)
LF:
Bl<Unr
(p=.04,
d=0.92)
BF:
Unb
<Unr
(p=.05,
d=0.41)
Bl<Unb
(p=.01,
d=0.94
)Bl<Unr
(p=.01,
d=0.94
Primarymem
ory
F=4.11
SCZ>LF(p
=.04,
d=0.64
)F=5.22
Blocked:
SCZ>RF(p
=.37,
d=1.06
)p=.01
SCZ>BF(p
=.002
,d=0.84)
p<.001
Unrelated:
SCZ>LF(p
=.43,
d=0.88)
η p2=.17
η p2=.21
SCZ:
SCZ>BF(p
=.02,
d=1.55
)SCZ>BF(p
=.29,
d=0.86
)RF:
Bl>Unb
(p=.04,
d=0.40
)BF:
Bl>Unr
(p=.02,
d=0.45)
Bl<Unb
(p=.05,
d=0.57
)Bl<Unr
(p=.04,
d=0.69)
Bl<Unb
(p<.01,
d=1.05
)Unb
>Unr
(p<.01,
d=0.72)
Secon
dary
mem
ory
F=1.82
n/a
F=2.76
SCZ:
Bl>Unb
(p<.01,
d=0.51
)
(Con
tinued)
VERBAL EPISODIC MEMORY IMPAIRMENT IN SCHIZOPHRENIA 657
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
Tab
le6.
(Con
tinued).
Group
List�
Group
Omnibus
Post-hoc
Omnibu
sPost-ho
c
p=.15
p=.02
RF:
Bl>Unr
(p<.01,
d=0.71)
η p2=.08
η p2=.12
LF:
Bl>Unb
(p=.02,
d=1.07)
Bl>Unr
(p<.01,
d=1.43)
BF:
Unb
>Unr
(p=.01,
d=0.66
)Bl>Unb
(p=.01,
d=0.37)
Bl>Unr
(p<.01,
d=0.62)
Bl>Unb
(p<.01,
d=1.02)
B>Unr
(p=.01,
d=0.71)
Serialordering
F=1.74
n/a
F=1.31
n/a
p=.17
p=.27
η p2=.08
η p2=.06
Sem
antic
categorizatio
nF=0.66
n/a
F=2.88
SCZ:
Bl>Unb
(p<.01,
d=2.41)
p=.58
p=.04
RF:
Bl>Unb
(p<.01,
d=3.06)
η p2=.03
η p2=.13
LF:
Bl>Unb
(p<.01,
d=1.52)
BF:
Bl>Unb
(p<.01,
d=2.09)
Sub
jectiveorganizatio
nF=2.56
SCZ>LF(p
=.01,
d=0.85)
F=1.48
n/a
p=.06
p=.22
η p2=.11
η p2=.07
Onlystatistically
meaningful(i.e.,p<.05or
d>0.83)post-hoc
results
areshow
n.Effectsizesarereported
aspartialetasquared(ηp2
)andCohen’s
d(d).
⁄ Denotes
Geisser–G
reenho
usecorrectio
n.Abb
reviations:BF=bi-frontal;LF=leftfrontal;RF=rightfrontal;SCZ=schizophrenia;
Bl=blockedlist;Unb
=unblockedlist;Unr
=Unrelated
list;n/a=post-hoc
comparisons
notperformed
dueto
non-significant
omnibu
seffects.
658 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
Tab
le7.
Omnibusandpost-hoc
results
forlearning
scores
(i.e.,trial4–trial1)
foreach
dependentvariable
(HCgrou
pexclud
ed)
Group
List�
Group
Omnibus
Post-ho
cOmnibus
Post-hoc
Freerecall
F=2.76
SCZ>LF(p
=.01,
d=0.71
)F=.79
n/a
p=.05
p=.58
η p2=.12
η p2=.04
Primacy
F=3.20
SCZ>LF(p
=.008,d=0.59
)F=2.45
RF:
Bl<Unb
(p=.12,
d=1.18
)p=.03
SCZ>BF(p
=.05,
d=0.39)
p=.03
LF:
Bl<Unr
(p=.01,
d=1.88)
η p2=.14
η p2=.11
Bl>Unb
(p=.02,
d=1.26
)Recency
F=1.15
n/a
F=.79
n/a
p=.34
p=.58
η p2=.06
η p2=.04
Primarymem
ory
F=.03
n/a
F=1.80
n/a
p=1.00
p=.10
η p2=.001
η p2=.08
Secondary
mem
ory
F=2.52p=.07
SCZ>LF(p
=.02,
d=0.62
)F=.41
n/a
p=.87
η p2=.11
η p2=.02
Serialordering
F=.63
n/a
F=.71
n/a
p=.60
p=.64
η p2=.03
η p2=.03
Sem
antic
categorizatio
nF=1.82
n/a
F=.18
n/a
p=.15
p=.91
η p2=.08
η p2=.01
Sub
jectiveorganizatio
nF=1.16
n/a
F=.39
n/a
p=.33
p=.87
η p2=.06
η p2=.02
Onlystatistically
meaningful(i.e.,p<.05or
d>0.83)po
st-hoc
results
areshow
n.Effectsizesarerepo
rted
aspartialetasquared(ηp2
)andCoh
en’s
d(d).⁄D
enotes
Geisser–G
reenhousecorrectio
n.Abb
reviations:BF=bi-frontal;LF=leftfrontal;RF=rightfrontal;SCZ=schizophrenia;
Bl=blockedlist;Unb
=unblockedlist;Unr
=Unrelated
list;n/a=post-hoc
comparisons
notperformed
dueto
non-significant
omnibu
seffects
VERBAL EPISODIC MEMORY IMPAIRMENT IN SCHIZOPHRENIA 659
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
result that is well documented in the literature (e.g., Baldo, Shimamura, Delis, Kramer,& Kaplan, 2001; Floel et al., 2004; Kelley et al., 1998).
Another interesting pattern emerged between the SCZ and HC groups thatwarrants further discussion. Learning scores in the SCZ group were within one stan-dard deviation of the HC group at each level of list type (see Figure 3). This suggeststhat, despite exhibiting an expected deficit in absolute recall ability relative to HCs(see Figure 1a), the SCZ group may not have differed substantially from HCs in mag-nitude of recall improvement across trials. Indeed, the overall mean (SD) free recalldifference score for HCs was 5.79 (1.49) versus 5.38 (1.82) in the SCZ group, withdifference scores actually larger in the SCZ group on the blocked list (see Figure 3a).This finding is contrary to previous data reported by Gold et al. (1992) who observedblunted learning curves in SCZ patients relative to HCs on a similar list learning para-digm. The reason for this discrepancy is not readily apparent, though it may reflect dif-ferences in the number of learning trials and/or items per list used in each study.Specifically, Gold et al. (1992) utilized wordlists with 20 items and presented partici-pants with three learning trials, whereas the current study employed lists with 16 itemsand four learning trials. The combination of a reduced number of items and increasedlearning trials may have facilitated recall in our SCZ sample, thus resulting in a similarmagnitude of improvement across trials relative to HCs. It should also be noted, how-ever, that HC group participants in the current study were more frequently at or nearceiling on trial 1, particularly on the blocked list. As such, the small difference in freerecall learning scores between HC and SCZ groups may simply be a reflection of thehigher absolute recall performance in the HC group and, by extension, more limitedpossible trial-to-trial improvement.2
Primacy and recency
SCZ-FTL group differences for primacy were only apparent for learning scores,with the SCZ group demonstrating increased primacy recall across trials compared tothe LF and BF groups (Figure 1b). This suggests that repeated list exposure led to lar-ger incremental increases in primacy recall in SCZ patients relative to patients withleft-lateralized (or partial left-lateralized—BF) damage. Unlike the RF and LF groups,the SCZ group did not exhibit differential primacy learning scores as a function ofsemantic list structure. Stuss et al. (1994) observed that primacy recall in HCs did notimprove across trials in either the blocked or unblocked lists, but did improve acrosstrials on the unrelated list. The current study replicated this finding using trial 4–trial 1learning scores; primacy learning scores were greater in the unrelated list relative tothe other two list types (ps < .05). This suggests that, in the absence of semantic cuesto facilitate recall, serial position effects, namely primacy, are an important moderatingfactor in improving recall performance across trials in healthy individuals. The onlypatient group in the current study to show a similar pattern of performance was the RFgroup (unrelated > blocked for primacy learning score).
Concerning recency effects, the only SCZ-FTL group difference was on theblocked list between SCZ and RF groups for recency scores collapsed across trial,with SCZ group demonstrating a greater percentage of recall from the recency positionthan RF patients. No differences were observed for recency learning scores. The SCZand FTL groups all showed a similar within-group pattern, whereby recency recall was
660 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
greater in the blocked versus unrelated lists. This finding highlights an importantcaveat of the current experimental design (and all list-learning paradigms that use listsof varying semantic organizational structure). Items in the recency position on theblocked list are also explicitly organized according to semantic category. Therefore,the greater recency recall observed across patients groups in the blocked list could bea reflection of recency effects and/or semantic organization effects. The same issuearises for recall from the primacy position. As such, the purest between-group compar-ison of primacy and recency likely comes from the unrelated word lists because suchcomparisons are not contaminated by semantic activation. In this vein, no patientgroup differences were observed on primacy or recency recall on the unrelated list.
Another interesting finding concerns the patient groups’ primacy and recencyrecall (collapsed across trials) relative to the HC group. Figures 1a and 2a–c show thateach patient group recalled more items from the recency position than the HC group.If we assume that the unrelated list provides the purest measure of recency recall (seeearlier), then this between-group difference is present at medium-to-large effect sizesranging from –0.63 to –1.14 (see Table 4). With the exception of RF patients, theopposite pattern of results was observed for primacy recall, albeit with generallysmaller effect sizes. This indicates that both SCZ and FTL lesion groups relied primar-ily on recently presented items when semantic cues were not provided. A qualitativelysimilar pattern of results is seen for PM versus SM recall (see later). This suggeststhat, in the absence of any semantic activation, SCZ and FTL lesions groups employ asimilar approach to verbal episodic recall that draws heavily on active memory repre-sentations (i.e., recency and PM). As with free recall scores, the SCZ and RF groupsperformed more similarly in this regard (see Figure 2c).
Primary and secondary memory
Group differences were only observed for PM scores collapsed across trial. SCZpatients demonstrated greater recall from PM than the LF and BF groups. When exam-ined at each level of list, the SCZ group exhibited greater PM recall than each FTLgroup on the blocked list, and the BF group on the unrelated list. Only the SCZ groupdemonstrated greater PM recall on the blocked list relative to other list types. This pat-tern of results is complementary to what was observed for free recall in that SCZpatients outperformed the LF and BF groups overall. However, unlike for free recall,the PM recall advantage in the SCZ group was greatest when semantically relateditems were blocked together. This suggests that, as with primacy/recency recall, greatersemantic activation may have been central to the enhanced PM recall in the SCZgroup. Unsworth and Engle (2007) have suggested that PM serves to maintain a lim-ited number of active representations in mind for on-going processing and is, therefore,an important component of working memory. Within this framework, the current datasuggests that individuals with SCZ are either better able to actively maintain bits ofinformation online for future recall or they have a larger PM capacity than FTL lesionpatients, namely those with LF and BF damage. Importantly, this effect appears tohinge on wordlists being organized with explicit semantic structure. This suggests that,for PM recall, the semantic system of SCZ patients is activated to a greater extent thanFTL lesion patients when the salience of category information is concretely high-lighted.
VERBAL EPISODIC MEMORY IMPAIRMENT IN SCHIZOPHRENIA 661
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
Regarding SM recall, HC-patient group differences closely resembled free recalldata, with both absolute recall (Table 2) and learning scores (Table 3) being reduced inall patient groups (effect sizes ranging from 0.33 to 1.78). SCZ-FTL group differenceswere only observed for learning scores, with SCZ patients again outperforming LFpatients. In addition, each patient group demonstrated better SM recall (collapsedacross trial) on the blocked list than each the other two lists. This pattern of resultscomplements free recall learning data and suggests that the learning advantage thatSCZ patients demonstrated in free recall relative to LF patients may have been partlydue to increased SM recall across trials. SM recall is thought to be a cue-dependentretrieval process for items that have been displaced from PM (Rao et al., 1989;Unsworth and Engle, 2007). It is reasonable to suggest that, relative to LF patients,repeated list exposure enhanced this retrieval process in SCZ patients for items thatwere no longer held online in PM. Given that each patient group demonstrated thehighest SM scores on the blocked list, it appears that both SCZ and FTL lesionpatients were able to capitalize on the salient semantic list structure to improve SMretrieval processes.
Organizational strategy
Relative to HCs, patient group deficits were evident across all measures of strat-egy for both absolute and learning scores, with effect sizes ranging from 0.50 to 2.21(see Tables 2 and 3). No learning score differences were observed between SCZ andFTL groups for serial ordering, semantic categorization, or subjective organization.This suggests that, regardless of list structure, repeated list exposure did not translateinto differential implementation of these organizational strategies across patient groupsas trials progressed. When strategy variables were collapsed across trial, no patientgroup differences were observed for serial ordering or semantic categorization, andeach patient group demonstrated a qualitatively similar pattern of semantic categoriza-tion across list type (i.e., blocked > unblocked). By contrast, subjective organizationscores collapsed across trial were greater in the SCZ versus LF group. This parallelswhat was observed for PM recall and free recall. Given this parallel, it stands to reasonthat the SCZ over LF advantage in PM and free recall may have been facilitated bygreater use of subjective organization. It is important to note that the term “strategy”in this context is not meant to imply a volitional cognitive process. Although partici-pants may have purposefully adopted a specific strategy to facilitate recall, the currentparadigm is not designed to differentiate volitional implementation of organizationalstrategy from implicit semantic activation associated with the degree of relatednessbetween list items.
CONCLUSIONS
The primary objective of this investigation was to further elucidate the contribu-tion of frontal lobe dysfunction to verbal episodic memory impairment in SCZ.Relative to the HC group, performance deficits were observed in each patient groupacross most measures when collapsed across trial, with the exception of recency recalland PM recall. The same is true of learning scores, albeit at a reduced level of
662 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
severity. This latter result may have reflected ceiling effects in trial 1 among some HCparticipants in certain measures, which would have limited the amount of possibleimprovement across trials and, by extension, reduced their learning scores.
SCZ-FTL group differences in absolute performance (i.e., collapsed across trialand list) were observed on free recall, primary memory (namely on the blocked list),and subjective organization, with these group differences reflecting the SCZ group out-performing the LF or BF groups. Learning score differences were observed for freerecall, primacy, and secondary memory, with these differences also in the direction ofSCZ patients outperforming LF or BF patients. By contrast, the SCZ and FTL groupsdemonstrated equivalent absolute performance on primacy, secondary memory, serialordering, and semantic categorization, as well as equivalent learning scores on recency,primary memory, serial ordering, semantic categorization, and subjective organization.Collectively, these results indicate a pattern of memory impairment that is qualitativelysimilar between patients with SCZ and those with FTL lesions, and, therefore, tenta-tively support the notion that SCZ-related deficits in verbal episodic memory may beassociated with frontal lobe dysfunction. Importantly, the magnitude of impairmentwas more severe in patients with left frontal cortex damage relative to SCZ. The quali-tative and statistical similarities between the RF and SCZ groups support tentativearguments that verbal episodic memory deficits in SCZ may be mediated by frontalcortical dysfunction in the right hemisphere. These findings are inconsistent withprevious research showing SCZ-related frontal impairment that is disproportionatelyleft-lateralized for verbal episodic memory (e.g., Achim and Lepage, 2005). Thereason for this discrepancy is not readily apparent. The disproportionate impairment inpatients with LF lesions may have been related to the linguistic nature of the memorystimuli used in this study and the sensitivity of language processing to disruption byleft frontal cortex damage. The macroscopic nature of their brain damage may haverendered the LF/BF patients more sensitive to perturbed language processing, therebyexacerbating their verbal memory deficits relative to SCZ patients.
LIMITATIONS AND FUTURE DIRECTIONS
The aforementioned conclusions must be qualified with the following limitationsin mind. First, the current sample size was relatively small and had reduced powerwithin our separate FTL lesion groups. Although efforts were made to account forpower-related issues, this remains an important caveat that must be taken into accountwhen interpreting the data. Nevertheless, it should be recognized that the FTL patientsin the current study were well characterized with circumscribed lesions, providinganother advantage for focal comparisons not available in other studies. Second, a sepa-rate comparison group comprised of only temporal lobe lesion patients was notincluded. Given the widely accepted involvement of structures such as the hippocam-pus in cognitive impairment in SCZ, this would be a highly suitable comparison groupfor exploring the contributions of different components of the fronto-temporal networkin SCZ pathology. Recent data strongly indicates that the mechanisms underlyingmemory impairment in SCZ likely reflect a compromised medial temporal system,rather than impaired IQ or executive functioning (Kopald, Mirra, Egan, Weinberger, &Goldberg, 2012). Third, measures of delayed verbal memory were not collected.Therefore, the current data set can only speak to SCZ-FTL comparisons in immediate
VERBAL EPISODIC MEMORY IMPAIRMENT IN SCHIZOPHRENIA 663
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
verbal memory processes. Examining how these patient groups differ or resemble oneanother in terms of delayed memory processes, such as recognition rate, sensitivity,response bias, and decay rate, is an interesting avenue of investigation for futureexperiments as this may further elucidate frontal contributions to SCZ verbal memoryimpairment. Finally, the division of our FTL lesion population was simplistic giventhe growing knowledge of a more complex frontal cortex anatomy. While lateralizedeffects likely play an important role in moderating qualitative differences in recallimpairment, more refined lesion groups separated on the basis of inter- andintra-hemispheric lesion sites (Alexander et al., 2003; Alexander, Stuss, & Gillingham,2009) would in all likelihood be more effective in discerning FTL contributions torecall impairment in SCZ.
ACKNOWLEDGEMENT
The authors gratefully acknowledge Rae Dolman, Jenny Chao, and Dan LaPortefor their assistance with subject recruitment, data collection and data management.This research was supported by an operating grant to RBZ from the Medical ResearchCouncil of Canada. The authors have no conflict of interest (financial or otherwise) toreport with regards to the research reported in this manuscript.
Notes
1. Barnette and MacLean (1999) have utilized Monte Carlo simulations in order to model how standardeffect size measures vary systematically as a function of sample size (n) and number of groups (K).From these, they have provided reliable estimations of mean effect size values that could be expectedon the basis of chance alone given a particular combination of n and K. In our study, comparisons werealways made between two groups (i.e., K = 2), and the lowest sample sizes were in the frontal lesiongroups (RF: n = 7, LF: n = 10, BF: n = 12). Therefore, using conservative parameter estimates of K = 2and n = 10, it can be seen from the data provided by Barnette and MacLean (1999) that a mean effectsize value of 0.38 (SD = 0.30) can be expected by chance alone. With this data in mind, we reasonedthat a mean effect size that is greater than 1.5 SDs above this chance cut-off could be considered statisti-cally meaningful. Accordingly, we set our meaningful difference cut-off at Cohen’s d > 0.83. As aresult, we interpreted group differences that exceeded thresholds of either p < .05 or d > 0.83 as beingstatistically meaningful. This allowed us to account for the small sample size in the frontal lobe lesiongroups and, by extension, minimize the possibility of Type II error.
2. The issue of ceiling effects applies to learning scores for each dependent measure. As such, we exam-ined each dependent variable to determine if the trial 1 group means at any level of list were within20% of ceiling. We found that only the RF group’s trial 1 recency recall on the blocked list was within20% of ceiling (14.3%). Importantly, the HC group means were not within 20% of ceiling on trial 1 forany dependent measure at any level of list. However, some individual HC participants were occasionallyat ceiling on trial 1 for some measures, particularly on the blocked list. For these participants, there wasno way to achieve a learning score above zero. This would have artificially deflated their learning scoreson these measures and, by extension, reduced the magnitude of HC-patient group differences.
REFERENCES
Achim, A. M., & Lepage, M. (2005). Episodic memory-related activation in schizophrenia: Ameta analysis. British Journal of Psychiatry, 187, 500–509.
664 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
Albuquerque, L., Loureiro, C., & Martins, I. P. (2008). Effect of lesion site on serial positionduring list learning: A study with the CVLT. International Journal of Neuroscience, 118,917–933.
Aleman, A., Hijman, R., de Haan, E. H. F., & Kahn, R. S. (1999). Memory impairment inschizophrenia: A meta-analysis. American Journal of Psychiarty, 156, 1358–1366.
Alexander, M. P., Stuss, D. T., & Fansabedian, N. (2003). California Verbal Learning Test: Per-formance by patients with focal frontal and non-frontal lesions. Brain, 126, 1493–1503.
Alexander, M. P., Stuss, D. T., & Gillingham, S. (2009). Impaired list learning is not a generalproperty of frontal lesions. Journal of Cognitive Neuroscience, 21, 1422–1434.
Baldo, J. V., Shimamura, A. P., Delis, D. C., Kramer, J., & Kaplan, E. (2001). Verbal and designfluency in patients with frontal lobe lesions. Journal of the International NeuropsychologicalSociety, 7, 586–596.
Barnette, J. J., & MacLean, J. E. (1999, November). Empirically based criteria for determiningmeaningful effect size. Paper presented at the Annual Meeting of the Mid-South Educa-tional Research Association, Point Clear, AL.
Beatty, W. W., Jocic, Z., Monson, N., & Staton, D. (1993). Memory and frontal lobe dysfunctionin schizophrenia and schizoaffective disorder. The Journal of Nervous and Mental Disease,181, 448–453.
Blair, J. R., & Spreen, O. (1989). Predicting premorbid IQ: A revision of the National AdultReading Test. The Clinical Neuropsychologist, 3, 129–136.
Chan, A. S., Kwok, I. C., Chiu, H., Lam, L., Pang, A., & Chow, L. (2000). Memory and organi-zational strategies in chronic and acute schizophrenic patients. Schizophrenia Research, 41,431–445.
Christensen, B. K., Girard, T. A., Benjamin, A. S., & Vidailhet, P. (2006). Evidence for impairedmnemonic strategy use among patients with schizophrenia using the part-list cuing para-digm. Schizophrenia Research, 85, 1–11.
Dickinson, D., Ragland, J. D., Gold, J. M., & Gur, R. C. (2008). General and specific cogni-tive deficits in schizophrenia: Goliath defeats David? Society of Biological Psychiatry, 64,823–827.
First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. V. (2001). Structured Clinical Inter-view for DSM-IV-TR Axis I Disorders, Research Version. New York, NY: BiometricResearch, New York State Psychiatric Institute.
Floel, A., Poeppel, D., Buffalo, E. A., Braun, A., Wu, C. W., Seo, H. J., & Cohen, L. G.(2004). Prefrontal cortex asymmetry for memory encoding of words and abstract shapes.Cerebral Cortex, 14, 404–409.
Frender, R., & Doubilet, P. (1974). More on measures of category clustering in free recall—Although probably not the last word. Psychological Bulletin, 81, 64–66.
Gershberg, F. B., & Shimamura, A. P. (1995). Impaired use of organizational strategies in freerecall following frontal lobe damage. Neuropsychologia, 13, 1305–1333.
Gold, J. M., Randolph, C., Carpenter, C. J., Goldberg, T. E., & Weinberger, D. R. (1992). Formsof memory failure in schizophrenia. Journal of Abnormal Psychology, 101, 487–494.
Goldberg, T. E., Weinberger, D. R., Pliskin, N. H., Berman, K. F., & Podd, M. H. (1989). Recallmemory deficit in schizophrenia: A possible manifestation of prefrontal dysfunction. Schizo-phrenia Research, 2, 251–257.
Heinrichs, R. W., & Zakzanis, K. K. (1998). Neurocognitive deficit in schizophrenia: A quantita-tive review of evidence. Neuropsychology, 12, 426–445.
Kaplan, E. F., Goodglass, H., & Weintraub, S. (1983). The Boston Naming Test (2nd ed.). Phila-delphia, PA: Lea & Febiger.
Kay, S., Fiszbein, A., & Opler, L. (1987). The Positive and Negative Syndrome Scale (PANSS)for Schizophrenia. Schizophrenia Bulletin, 13, 261–276.
VERBAL EPISODIC MEMORY IMPAIRMENT IN SCHIZOPHRENIA 665
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013
Kelley, W. M., Miezin, M., McDermott, K. B., Buckner, R. L., Raichle, M. E., Cohen, N. J., &Petersen, S. E. (1998). Hemispheric specialization in human dorsal frontal cortex and med-ial temporal lobe for verbal and nonverbal memory encoding. Neuron, 20, 927–936.
Kopald, B. E., Mirra, K. M., Egan, M. F., Weinberger, D. R., & Goldberg, T. E. (2012).Magnitude of impact of executive functioning and IQ on episodic memory in schizophrenia.Biological Psychiatry, 71, 545–551.
Kopelman, M. D., & Stanhope, N. (1998). Recall and recognition memory in patients with focalfrontal, temporal lobe and diencephalic lesions. Neuropsychologia, 36, 785–796.
Ornstein, T. J., Sahakian, B. J., & McKenna, P. J. (2008). Memory and executive impairment inschizophrenia: Comparison with frontal and temporal brain damage. PsychologicalMedicine, 38, 833–842.
Pantelis, C., Barnes, T. R. E., Nelson, H. E., Tanner, S., Weatherley, L., Owen, A. M., &Robbins, T. W. (1997). Frontal-striatal cognitive deficits in patients with chronic schizophre-nia. Brain, 120, 1823–1843.
Rao, S. M., Leo, G. J., & St. Aubin-Faubert, P. (1989). On the nature of memory disturbance inmultiple sclerosis. Journal of Clinical and Experimental Neuropsychology, 11, 699–712.
Sanz de la Torre, J. C., Barrios, M., & Junqué, C. (2005). Frontal lobe alterations in schizophre-nia: Neuroimaging and neuropsychological findings. European Archives of Psychiatry andClinical Neuroscience, 255, 236–244.
Stuss, D. T., & Alexander, M. P. (2007). Is there a dysexecutive syndrome? Philosophical Trans-actions of the Royal Society of London, Series B: Biological Sciences, 362, 901–915.
Stuss, D. T., Alexander, M. P., Floden, D., Binns, M. A., Levine, B., McIntosh, A. R., &Hevenor, S. J. (2002). Fractionation and localization of distinct frontal lobe processes: Evi-dence from focal lesions in humans. In D. T. Stuss & R. T. Knight (Eds.), Principles offrontal lobe function (pp. 392–407). New York, NY: Oxford University Press.
Stuss, D. T., Alexander, M. P., Palumbo, C. L., Buckle, L., Saver, L., & Pogue, J. (1994). Orga-nizational strategies of patients with unilateral or bilateral frontal lobe injury in word listlearning tasks. Neuropsychology, 8, 355–373.
Tulving, E., & Colotla, V. A. (1970). Free recall of trilingual lists. Cognitive Psychology, 1,86–98.
Turner, M. S., Cipolotti, L., Yousry, T., & Shallice, T. (2007). Qualitatively different memoryimpairments across frontal lobe subgroups. Neuropsychologia, 45, 1540–1552.
Unsworth, N., & Engle, R. W. (2007). The nature of individual differences in working memorycapacity: Active maintenance in primary memory and controlled search from secondarymemory. Psychological Review, 114, 104–132.
Vilkki, J., Servo, A., & Surma-aho, O. (1998). Word list learning and prediction of recall afterfrontal lobe lesions. Neuropsychology, 12, 268–277.
666 BRUCE K. CHRISTENSEN ET AL.
Dow
nloa
ded
by [
24.1
41.5
6.16
1] a
t 12:
11 2
6 Ju
ne 2
013