Paromomycin-loaded albumin microspheres: Efficacy and stability studies
-
Upload
independent -
Category
Documents
-
view
1 -
download
0
Transcript of Paromomycin-loaded albumin microspheres: Efficacy and stability studies
Paromomycin-loaded albumin microspheres:Efficacy and stability studiesWahid Khan,a Rajendra Kumar,b Sukhvinder Singh,b
Sunil Kumar Arorab and Neeraj Kumara*
In theQ4 present work, paromomycin-loaded albumin microspheres (PM-MS) have been formulated for passive targeting ofparomomycin (PM) to macrophages, for the treatment of visceral leishmaniasis (VL). PM-MS were prepared by spray-dryingmethod with a mean particle size of� 3mm. Thermal and chemical cross-linking methods were used for controlling drugrelease from the prepared microspheres (MS). PM-MS were then tested for efficacy and stability studies. In efficacy study,in vitro promastigote assay was carried out to assess the susceptibility of promastigote to PM in the concentration range of5.0–150mg/ml; cytotoxicity assay was performed to determine possible toxicity of PM for the host cells (peritoneal macro-phages) and intracellular amastigote assay was carried out to determine the efficacy of free PM (PM solution) and encapsu-lated PM (PM-MS). Results obtained indicated a significant increase in efficacy of PM-MS in comparison to PM solution atequivalent concentration. Subsequently, stability studies of prepared formulation was carried out at various temperatureand humidity conditions, these studies provided stability of formulation at all tested conditions including accelerated condi-tions. Thus, it can be concluded that present work provides an optimized formulation with stability and enhanced efficacy.Copyright © 2011 John Wiley & Sons, Ltd.
Keywords: leishmaniasis; paromomycin; microspheres; amastigote; promastigote
Introduction
Visceral leishmaniasis (VL) is a communicable disease that isendemic and causes high morbidity and mortality worldwide.[1]
Treatment for leishmaniasis has remained unsatisfactory due tovarious unresolved issues such as poverty, malnutrition, inade-quate vector control, insensitive diagnostic tools, and unaffordabil-ity of treatment. Leishmania parasite is an obligate intracellularparasite that replicates and cause infection exclusively within thephagolysosomes of the host macrophages.[2] Biopharmaceuticalissues get more complicated in VL where the response of anti-parasitic drugs is mainly governed by the drug concentrationachieved in microenvironment of intracellular location of parasiterather than the availability in host systemic circulation. As no vac-cine is available to date for leishmaniasis, the management ofleishmania relies primarily on chemotherapy.[3] Major frontlinedrugs available for the treatment of leishmaniasis are eithertoxic or ineffective against the parasite due to emergence ofresistance.[4] Thus, there is a need for a better delivery system todeliver this drug. Among the available delivery options, the besttreatment available today for VL is AmBisome (liposomal productof amphotericin B) which is a good trade-off between activityand toxicity, but is very expensive and unaffordable.[5]
In 2006, paromomycin (PM) intramuscular injection was ap-proved in India for treatment of VL and in 2007, it was includedin 15th edition of the WHO Model List of Essential Medicines fortreatment of VL.[6,7] PM is an efficacious, but one of the leastexploited molecules, due to its low permeability which resultsin poor bioavailability and dose-dependent adverse effects in-cluding local toxicity at the site of injection, ototoxicity, nephro-toxicity, and tetany.[8] To address these problems, biodegradablealbumin microspheres have been designed as a specific carrier
system for passive targeting of PM to the macrophages wherethe leishmania parasites reside. This delivery system would resultin increased efficacy of PM by reducing the dose required fortreatment and subsequently reducing associated toxicities. Thepre-formulation,[9] formulation development and targeting,[10] andpharmacokinetics and toxicity[11] studies of prepared formulationhave already been reported elsewhere. In the present work, PM-MS were evaluated mainly for efficacy and stability of the preparedformulation. Stability studies were carried out to optimize theformulation, so that it can be stored at room temperature and canbe used in the remote areas where VL is most prevalent.
Experimental
Reagents and materials
Paromomycin sulfate (labelled potency 675mg/mg) was a giftsample by Gland Pharma Ltd (Hyderabad, India). Bovine serumalbumin (Bovine Fraction V) and Trypsin (2000 units/mg) wasobtained from sd fine-chem. Ltd. (Mumbai, India). RPMI-1640medium and foetal calf serum (FCS) was purchased from Sigma(St Louis, MO, USA). Penicillin and streptomycin was purchasedfrom HiMedia Laboratories Pvt. Ltd (Mumbai, India). Ultrapure
* Correspondence to: Neeraj Kumar, Department of Pharmaceutics, National Instituteof Pharmaceutical Education&Research (NIPER), Sector 67, S.A.S. Nagar-160062, India.E-mail: [email protected]
a National Institute of Pharmaceutical Education & Research (NIPER), Nagar, India
b Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh,India
Drug Test. Analysis (2011) Copyright © 2011 John Wiley & Sons, Ltd.
Research ArticleDrug Testing
and Analysis
Received: 15 September 2011 Revised: 20 October 2011 Accepted: 21 October 2011 Published online in Wiley Online Library
(wileyonlinelibrary.com) DOI 10.1002/dta.389
1Journal Code Article ID Dispatch: 12.11.11 CE:
D T A 3 8 9 No. of Pages: 6 ME:
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
water was obtained using the Elgastat water purification system(Bucks, UK). Other chemicals and reagents used in the experi-ments were purchased from local Indian suppliers.
Parasites
The experiment was performed on Leishmania parasites namelyLeishmania donovani (MHOM/IN/80/Dd8). L. donovani promasti-gotes were grown at 25 �C and cultured in RPMI-1640 mediumsupplemented with 10% (v/v) heat inactivated fetal calf serum,100 IU/ml penicillin and 100mg/ml streptomycin. Metacyclic-stage parasites were isolated from 6-day-old parasite cultures,centrifuged, washed twice with 0.02M phosphate buffered saline(PBS) pH 7.2 and resuspended in RPMI-medium. The strain wasmaintained in Syrian golden hamster (Mesocricetus auratus).
Preparation of PM-loaded albumin microspheres
A spray-drying method was used for preparation of PM-MS.Briefly, an aqueous solution of PM and albumin was sprayed ina spray dryer to form MS. Particles of desired size (≤5 mm) witha good yield were obtained by selecting low feed flow rate(2ml/min), 5% total solid content of sprayed solution, inlet tem-perature 120 �C, and by selecting other optimized instrumentparameters.[10] MS obtained were kept at 60 �C for 3 h to get adried product. Thereafter, particles were cross-linked by chemicaland thermal methods.
Thermal/chemical cross-linking of microspheres
MS prepared by spray drying need to be cross-linked for obtainingthe optimum release profile and for extended shelf life of theproduct. Two methods of cross-linking – (1) chemical cross-linkingusing glutaraldehyde, and (2) thermal cross-linking using heattreatment at high temperatures – were used in present experi-ments.[12–14] Thermal cross-linking was carried out at 120 º C fordifferent time periods (12 and 24h).[10] Whereas, in chemicalcross-linking, four sets of 100mg of MS were suspended in 5mlof 1-butanolat room temperature and MS were stabilized by add-ing glutaraldehyde (25% glutaraldehyde solution) to attain 1%,2%, 3%, and 4% glutaraldehyde concentration. This suspensionwas then stirred at 700 rpm for 90min to allow the cross-linkingof MS. Thereafter, cross-linked MS were filtered under vacuumusing 0.2mm nylon filter and washed with 1-butanol. Finally, MSwere vacuum dried overnight at room temperature.
In vitro drug release studies
In vitro drug release studies were carried for PM-MS, both ther-mally cross-linked (heated at 120 �C, for 12 and 24 h) and chem-ically cross-linked (with 1, 2, 3, and 4% glutaraldehyde). PMreleased from these MS was studied over a period of 9 h usingdialysis bag method. Briefly, 10mg MS (equivalent to approxi-mately 1mg of PM) were transferred to dialysis tubing (M.W.cut off 10 000 Da) containing 3ml of 0.2% trypsin in PBS (pH7.4), and bag was immersed in a vial containing 17ml of PBS(pH 7.4). Vial was placed on a reciprocal shaker water bath main-tained at 37 �C.[10] One ml aliquot was withdrawn at a presettime point and was simultaneously replenished with the samevolume of release media. Samples were assayed for PM releaseusing developed high performance liquid chromatography(HPLC) method.[9]
In vitro antileishmanial activity of formulation
In vitro antileishmanial activity of both thermally and chemicallycross-linked PM-MS formulations was investigated and comparedwith the PM solution. In these experiments, promastigote assay(to determine IC50 value for parasites) was initially performed fol-lowed by cell cytotoxicity assay (to determine drug concentrationrange for intracellular amastigote assay) and lastly intracellularamastigote assay was performed (to determine activity of devel-oped formulation against intracellular amastigotes).
Promastigote assay
The susceptibility of promastigote to PM was assessed using milte-fosine (MF) as a standard drug. Promastigotes (105 parasites/well) intheir logarithmic growth phase were cultured in 96 well plates.[15]
Various dilutions of the drugs were added in each well (n= 3) tomake final concentration of MF (2.5, 5, 10, and 20mg/ml) and PM(5, 10, 50, 100, and 150mg/ml) and incubated at 26 �C for 48 h. Afterthis incubation, 20ml of Alamar-Blue reagent was added and keptfor 5 h. Thereafter, absorbance was measured at 570 and600nm.[16] The concentration that produced 50% reduction ingrowth (IC50) of promastigotes as compared to control (untreated)was determined for both PM and MF.
Cytotoxicity assay for the peritoneal macrophages
Peritoneal macrophages were harvested from Balb/C mice andincubated overnight in a 96 well plate (104 cells/well) in RPMI-1640 medium at 37 �C in 5% CO2. Thereafter, macrophages wereincubated with different concentrations (0.5, 5, 50, 100, and150 mg/ml) of PM solution, PM-MS (both thermally and chemicallycross-linked) and equivalent amount of blank MS (n = 3), threewells were left as untreated control wells. PM solution and MSformulations were removed after 6 h, and then macrophageswere washed and placed in medium for an additional 42 h. Afterthis time period, medium was aspirated and fresh medium sup-plemented with Alamar-Blue reagent was added, incubated forfurther 5 h and absorbance at 570nm and 600nm were deter-mined. Results were expressed as percentagemacrophage viability,compared with untreated control wells.[16–18]
Intracellular amastigote assay
In this experiment, in vitro antileishmanial activity of PM-MSformulation (thermally and chemically cross-linked) was investi-gated by intracellular amastigote assay. Peritoneal macrophageswere harvested from Balb/C mice and 5 x 105 macrophagecells/well were cultured in 6-well plates. On subsequent Q3days,these macrophages were exposed to promastigotes (L. donovani,MHOM/IN/80/Dd8) at multiplicity of infection of 10:1 (parasites:macrophage) and incubated at 37 �C for 12 h. Non-internalizedpromastigotes were removed, whereas internalized promasti-gotes were allowed to convert into amastigotes. These cells werethen incubated with 1ml of different concentrations (0.5, 5, 50,and 100mg/ml) of PM solution and with the same concentrationof PM-MS; two wells of untreated infected macrophages wereused as control. These formulations (PM solution and PM-MS)were removed after 6 h by washing and then macrophages werecultured in complete culture media for an additional 42 h. Afterthat, cells were fixed with methanol and stained with Giemsastain. The intracellular amastigotes were counted to determinenumber of amastigotes per macrophages.[19]
W. Khan et al.
Drug Testing
and Analysis
wileyonlinelibrary.com/journal/dta Copyright © 2011 John Wiley & Sons, Ltd. Drug Test. Analysis (2011)
2
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
Stability studies of formulation
To perform stability study of PM-MS (thermally cross-linked)samples were divided into three sets and were stored at 4 �C(refrigerator), ambient temperature/humidity and at 40 �C/75%RH (stability chamber) for 1 and 3months.[20] Samples in triplicatewere kept for specified condition and for each time point. At1month and 3months time period, samples were withdrawn andanalyzed for physical change, drug content, and release profileusing developed HPLC method.[9]
Statistical analysis
Statistical analysis for the efficacy of PM in solution and PM-MSwas performed. Comparison of mean values between variousgroups was performed by one-way ANOVA, followed by pair wisemultiple comparison by Bonferroni t-test.
Result and discussion
Preparation of PM loaded albumin microspheres
PM-MS of desired size (≤5mm) were prepared by a spray-dryingmethod. Various factors affecting size and product yield wereoptimized in step wise manner by varying one parameter at atime while keeping other parameters constant and vice versa.
PM-MS with a mean particle size of� 3 mm were obtained witha reasonable good product yield (45%).
Cross-linking of microspheres and in vitro drug releasestudies
Cross-linking of these prepared MS was carried out to change thesurface property of MS from hydrophilic to hydrophobic, therebyaltering drug release profile. The higher the cross-linking, thelower would be the solubility of albumin hence the slower wouldbe the drug release from MS. Both chemical cross-linking usingglutaraldehyde and thermal cross-linking using heat treatmentwere used in the present experiment. Glutaraldehyde has a widespectrum of industrial and biomedical applications as a cross-linking agent. The aldehyde group of glutaraldehyde reacts witha primary amine of albumin to form a Schiff base, altering watersolubility of albumin. In thermal cross-linking (at temperatureabove 80 �C) albumin undergoes intermolecular aggregationand irreversible structural changes i.e. denaturation. The aggre-gation is due to the formation of intermolecular S-S bonds,whereas, denaturation depends on the temperature, exposuretime, pH, and salt concentration. Typical denaturation tempera-tures range from 80 to 160 �C used to stabilize albumin MS withdifferent exposure time according to thermal stability of thedrug.[21] PM was found to be stable at 120 �C for 24 h.[9] Therefore,120 �C was selected as thermal cross-linking temperature.
0
20
40
60
80
100
0 2 4 6 8 10
% C
umul
ativ
e R
elea
se
Time (h)
1% Glutraldehyde Crosslinking
2% Glutraldehyde Crosslinking
3% Glutraldehyde Crosslinking
4% Glutraldehyde Crosslinking
(B)(A)
0
20
40
60
80
100
0 2 4 6 8 10
% C
umul
ativ
e R
elea
se
Time (h)
12 h Thermally crosslinked
24 h Thermally crosslinked
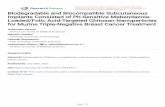
Figure 1. Drug release behaviour of PM-loaded microspheres. Drug release studies were conducted for two types of microspheres (A) thermally cross-linked at 120 �C for 12 h and 24 h and (B) chemically cross-linked with different concentration of glutaraldehyde for 90min. Release study was carried inPBS (pH 7.4) with 0.2% trypsin at 37 �C (n = 3).
Colou
ron
line,
B&W
inprint
0
20
40
60
80
100
5 10 50 100 150
% R
edu
ctio
n i
n P
rom
asti
go
tes
Concentration (µg/ml)
Paromomycin
0
20
40
60
80
100
2.5 5 10 20
Concentration (µg/ml)
Miltefosine
IC50 value
Figure 2. % Reduction in promastigotes observed when treated with different concentrations of PM and MF.
Colou
ron
line,
B&W
inprint
Paromomycin loaded albumin microspheres: Efficacy and stability studies
Drug Testing
and Analysis
Drug Test. Analysis (2011) Copyright © 2011 John Wiley & Sons, Ltd. wileyonlinelibrary.com/journal/dta
3
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
In vitro drug release profiles of PM loaded albumin MS stabilizedat 120 �C for different time periods are shown in FigureF1 1A. MSexhibited a biphasic release pattern, where an initial burst releasewas followed by a slower release. These release results for ther-mally cross-linked MS were previously reported,[10] but given againin this manuscript to obtain better comparison with chemicalcross-linking method. MS cross-linked by chemical method isshown in Figure 1B. PM release from albumin MS cross-linked withdifferent glutaraldehyde concentrations (1, 2, 3, and 4%) showedthe dependence of drug release on the amount of cross-linkingagent. Higher the amount of cross-linking agent, lower would bethe amount and rate of PM release obtained from PM-MS. Theseresults indicate that time taken during thermal cross-linking andconcentration of cross-linking agent in chemical cross-linkingsignificantly affect the release profile of drug from albumin MS.The objective of this formulation is to deliver the drug intracellu-
lar to macrophages where leishmania parasites reside. Dependingon the size and surface properties of MS, in vivo uptake of MS inmacrophages occurs maximum within minutes of intravenousinjection.[22]Therefore, MS stabilized at 120 �C for 24 h (thermallycross-linked) and with 3% glutaraldehyde (chemically cross-linked)were used for further experiments. It is also pertinent to mention
that these MS were able to hold the higher amount of drug forlonger period and could release the drug in controlled mannerthereby more drug could be targeted to the macrophages.
In vitro antileishmanial activity of formulation
Promastigote assay
In vitro antileishmanial activity assay was performed on the extra-cellular promastigote form of L. donovani, Dd8 strain. In thisassay, activity of different concentrations of PM (5–150mg/ml)against parasites was determined. Miltefosine in different con-centrations (2.5–20mg/ml) was used as a standard drug. As ob-served from Figure F22, when drug concentration increased, it ledto reduced numbers of viable promastigotes. The concentrationthat produced 50% reduction in viability of promastigotes(IC50) was determined and found to be 119mg/ml for PM and11.4 mg/ml for MF.
Cytotoxicity assay for the peritoneal macrophages
This experiment was carried out to determine possible toxicity ofPM for the host cells (peritoneal macrophages) to determine theeffect of used doses on intracellular amastigotes on the survivalof macrophage cell itself. There is only one report availabledescribing that there was no cytotoxicity observed with PM upto27 mg/ml of PM.[23] In this experiment, macrophages were incu-bated with different concentrations (up to 150mg/ml) of PMsolution, PM-MS (both chemically and thermally cross-linked) andblank MS. It was found that both PM solution and encapsulated
Figure 3. Cytotoxicity behaviour of PM solution and different PM-MSformulations. PM neither in solution form nor as PM-MS produced anycytotoxicity in the given concentration range.
0
1
2
3
4
5
6
7
8
Control 0.5µg/ml 5µg/ml 50µg/ml 100µg/ml
Am
astg
ote
co
un
t (P
er m
acro
ph
age)
Control PM Solution PM-MS (Thermal Crosslinking)
##
##
#
#
#*
*
*
Figure 4.Q1 Effect of PM solution and PM-MS (thermally cross-linked) atequivalent drug concentration on intracellular amastigotes of leishmaniaparasites. Results are expressed as number of amastigotes/macrophage.#P value <0.05, Vs. control for all samples, *P value <0.05 Vs. PM solutionof respective group. Experimental values are expressed as mean� SD.Comparison of mean values between various groups was performed byone way ANOVA, followed by pair wise multiple comparison by Bonferronit-test.
Colouronlin
e,B&W
inprint
0
1
2
3
4
5
Control 0.5µg/ml 5µg/ml 50µg/ml
Am
astg
ote
co
un
t (P
er m
acro
ph
age)
Control PM Solution PM-MS (Chemical Crosslinking)
#
#
#
#
#
**
*
Figure 5. Effect of PM solution and PM-MS (chemically cross-linked byglutaraldehyde) at equivalent drug concentration on intracellular amasti-gotes of leishmania parasites. Results are expressed as number of amasti-gotes/macrophage. #P value <0.05, Vs. control for all samples, *P value<0.05 Vs. PM solution of respective group. Experimental values areexpressed as mean� SD. Comparison of mean values between variousgroups was performed by one way ANOVA, followed by pair wise multiplecomparison by Bonferroni t-test.
Colour
online,B&W
inprint
Table 1. Observations of stability studies on % drug content in dif-ferent storage conditions.
Storage conditions % Drug content (�SD)
1month 3months
Refrigerator 99.73 (4.11) 98.40 (2.19)
Ambient T/H 100.40 (3.65) 98.73(0.12)
Stability chamber 98.70 (0.91) 99.17 (0.35)
W. Khan et al.
Drug Testing
and Analysis
wileyonlinelibrary.com/journal/dta Copyright © 2011 John Wiley & Sons, Ltd. Drug Test. Analysis (2011)
4
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
MS (0.5–150mg/ml) did not produced any toxicity to macrophages(FigureF3 3). Therefore, it can be concluded that tested concentrationrange 0.5–150mg/ml can be used for in vitro amastigote assaywithout affecting cell viability.
Intracellular amastigote assay
In vitro antileishmanial activity of new PM-MS formulations wasinvestigated on intracellular amastigotes of leishmania. Since, onlyone report describing in vitro activity of PM in different strains ofleishmania parasites was available in literature,[24] therefore drugconcentrations were selected on the basis of promastigote assay,keeping cell cytotoxicity data in consideration. The activity at dif-ferent concentrations of PM in various formulations against intra-cellular L. donovani (Dd8 strain) was assessed in macrophages. Itwas observed that equivalent amount of PM encapsulated in albu-min MS gave a significantly higher efficacy of PM in comparison to
PM solution. PM solution showed 5, 29, 35, and 41% reduction inintracellular parasite number at the doses of 0.5, 5, 50, and100mg/ml, respectively. Whereas, PM-MS formulations showedbetter efficacy as compared to PM solution with thermally cross-linked formulation causing 33, 41, 66, and 75% parasite reductionat 0.5, 5, 50 and 100mg/ml, respectively (Figure F44) and chemicallycross-linked formulation was equally efficacious showing 41, 46,and 67% parasite inhibition at similar doses (Figure F55). Therefore,it can be concluded that PM-MS have better efficacy of PM inLeishmania amastigote clearance.
Stability studies of formulation
Stability of any formulation is an important parameter for prod-uct development. It is necessary that the product should not losethe specification during storage and thus a stability study of PM-MS (thermally cross-linked) samples was carried out. Samples
0
20
40
60
80
100
0 2 4 6 8 10 12
% C
um
ula
tive
Rel
ease
Time (h)
Control
Ambient temperature/humidityRefrigerator
Stability chamber
0
20
40
60
80
100
0 2 4 6 8 10 12
% C
um
ula
tive
Rel
ease
Time (h)
Control
Ambient T/H
Refrigerator
Stability chamber
(B)(A)
Figure 6. Release profile of stability samples kept in different conditions for (A) 1month and (B) 3months.
Colou
ron
line,
B&W
inprint
Control Refrigerator
Stability Chamber Ambient T/H
Figure 7. SEM images of MS after keeping samples for 3months in different storage conditions.
Paromomycin loaded albumin microspheres: Efficacy and stability studies
Drug Testing
and Analysis
Drug Test. Analysis (2011) Copyright © 2011 John Wiley & Sons, Ltd. wileyonlinelibrary.com/journal/dta
5
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
were stored at 4 �C (refrigerator), ambient temperature/humidityand at 40 �C/75% RH (stability chamber). At predetermined timepoints, samples were withdrawn and analyzed for drug content,physical change, and for release profile. Drug content of allformulations (TableT1 1) as determined by the developed HPLCmethod was found to be within acceptable limit of 100(�5%).In vitro release studies revealed that all samples exhibits samerelease pattern comparable to control (FigureF6 6). In all samplesafter 1 and 3months, no physical changes were observed bySEM (FigureF7 7). Therefore, it can be concluded that developedformulation was stable in the tested storage conditions.
Conclusions
PM-loaded albumin MS for parenteral (intravenous) administra-tion were prepared by spray-drying method. Thermal and chem-ical cross-linking method was successfully used for cross-linkingof MS and to achieve the desired in vitro drug release pattern.In vitro efficacy studies carried out in macrophage cell showedsignificantly increased efficacy of PM-MS, when compared withPM solution. Stability studies also showed the stability of formu-lation in accelerated conditions. Present experiment providespassive targeting of PM to macrophages with increased efficacycompared to free PM.
Acknowledgements
WK is thankful to NIPER for the PhD fellowship. Financial assis-tance from NIPER grant (C1111-NJK) to carry out this researchwork is duly acknowledged.
REFERENCES
Q2 [1] S. Das, W. Khan, S. Mohsin, N. Kumar. Miltefosine loaded albuminmicroparticles for treatment of visceral leishmaniasis: formulation de-velopment and in vitro evaluation. Polym. Adv. Technol. 2011, 22, 172.
[2] H.W. Murray, J.D. Berman, C.R. Davies, N.G. Saravia. Advances in leish-maniasis Lancet 2005, 366, 1561.
[3] J. Mishra, A. Saxena, S. Singh. Chemotherapy of leishmaniasis: past,present and future. Curr. Med. Chem. 2007, 14, 1153.
[4] S.L. Croft, S. Sundar, A.H. Fairlamb. Drug resistance in leishmaniasis.Clin. Microbiol. Rev. 2006, 19, 111.
[5] E.L. Romero, M.J. Morilla. Drug delivery systems against leishmania-sis? Still an open question. Expert Opin. Drug Del. 2008, 5, 805.
[6] S. Sundar, T.K. Jha, C.P. Thakur, P.K. Sinha, S.K. Bhattacharya. Inject-able paromomycin for visceral leishmaniasis in India. N. Engl. J.Med. 2007, 356, 2571.
[7] R.N. Davidson, M. den Boer, K. Ritmeijer. Paromomycin. Trans. R. Soc.Trop. Med. Hyg. 2008, 103, 653.
[8] C.P. Thakur. Tetany in kala azar patients treated with paromomycin.Indian J. Med. Res. 2008, 127, 489.
[9] W. Khan, N. Kumar. Characterization, thermal stability studies, andanalytical method development of Paromomycin for formulationdevelopment. Drug Test. Anal. 2011, 3, 363.
[10] W. Khan, N. Kumar. Drug targeting to macrophages using paromo-mycin-loaded albumin microspheres for treatment of visceral leish-maniasis: an in vitro evaluation. J. Drug Target. 2011, 19, 239.
[11] W. Khan, S.S. Sharma, N. Kumar. Bioanalytical method develop-ment, pharmacokinetics and toxicity studies of paromomycin andparomomycin loaded in albumin microspheres. Drug Test. Anal.(in press) 2011.
[12] A.F. Yapel Jr. Albumin microspheres: heat and chemical stabilization.Method Enzymol. 1985, 112, 1.
[13] F.Q. Li, J.H. Hu, B. Lu, H. Yao, W.G. Zhang. Ciprofloxacin-loaded bovineserum albumin microspheres: preparation and drug-release in vitro.J. Microencapsul. 2001, 18, 825.
[14] H. Nettey, D. Haswani, C.W. Oettinger, M.J. D’Souza. Formulation andtesting of vancomycin loaded albumin microspheres prepared byspray-drying. J. Microencapsul. 2006, 23, 632.
[15] I.P. Singh, S.K. Jain, A. Kaur, S. Singh, R. Kumar, P. Garg, S.S. Sharma, S.K. Arora. Synthesis and antileishmanial activity of piperoyl-aminoacid conjugates. Eur. J. Med. Chem. 2010, 45, 3439.
[16] L. Ordonez-Gutierrez, R. Espada-Fernandez, M.A. Dea-Ayuela, J.J. Torrado,F. Bolas-Fernandez, J.M. Alunda. In vitro effect of new formulations ofamphotericin B on amastigote and promastigote forms of Leishmaniainfantum. Int. J. Antimicrob. Ag. 2007, 30, 325.
[17] A. Nan, S.L. Croft, V. Yardley, H. Ghandehari. Targetable water-solublepolymer-drug conjugates for the treatment of visceral leishmaniasis.J. Control. Release 2004, 94, 115.
[18] K.D. Manandhar, T.P. Yadav, V.K. Prajapati, S. Kumar, M. Rai, A. Dube,O.N. Srivastava, S. Sundar. Antileishmanial activity of nano-amphotericinB deoxycholate. J. Antimicrob. Chemother. 2008, 62, 376.
[19] G. Pujals, J.M. Sune-Negre, P. Perez, E. Garcia, M. Portus, J.R. Tico,M. Minarro, J. Carrio. In vitro evaluation of the effectiveness andcytotoxicity of meglumine antimoniate microspheres producedby spray drying against Leishmania infantum. Parasitol. Res. 2008,102, 1243.
[20] P.M. Dandagi, V.S. Mastiholimath, M.B. Patil, M.K. Gupta. Biode-gradable microparticulate system of captopril. Int. J. Pharm.2006, 307, 83.
[21] A.G.A. Coombes, V. Breeze, W. Lin, T. Gray, K.G. Parker, T. Parker.Lactic acid-stabilised albumin for microsphere formulation andbiomedical coatings. Biomaterials 2001, 22, 1.
[22] F. Chellat, Y. Merhi, A. Moreau, L. Yahia. Therapeutic potential ofnanoparticulate systems for macrophage targeting. Biomaterials2005, 26, 7260.
[23] R.A. Neal, S.L. Croft. An in vitro system for determining the activity ofcompounds against the intracellular amastigote form of Leishmaniadonovani. J. Antimicrob. Chemother. 1984, 14, 463.
[24] R.A. Neal, S. Allen, N. McCoy, P. Olliaro, S.L. Croft. The sensitivity ofLeishmania species to aminosidine. J. Antimicrob. Chemother. 1995,35, 577.
W. Khan et al.
Drug Testing
and Analysis
wileyonlinelibrary.com/journal/dta Copyright © 2011 John Wiley & Sons, Ltd. Drug Test. Analysis (2011)
6
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
Author Query Form
Journal: Drug Testing and Analysis
Article: dta_389
Dear Author,
During the copyediting of your paper, the following queries arose. Please respond to these by annotating your proofs with thenecessary changes/additions.• If you intend to annotate your proof electronically, please refer to the E-annotation guidelines.• If you intend to annotate your proof by means of hard-copy mark-up, please refer to the proof mark-up symbols guidelines. Ifmanually writing corrections on your proof and returning it by fax, do not write too close to the edge of the paper. Pleaseremember that illegible mark-ups may delay publication.
Whether you opt for hard-copy or electronic annotation of your proofs, we recommend that you provide additional clarificationof answers to queries by entering your answers on the query sheet, in addition to the text mark-up.
Query No. Query Remark
Q1 AUTHOR: Figures 4 and 5 contains moire pattern. Please correct and resupply.
Q2 AUTHOR: Please provide updated publication details for ref. 11 if available.
Q3 AUTHOR: Please check that meaning has been retained.
Q4 AUTHOR: Please provide a suitable figure (abstract diagram or illustration selected fromthe manuscript or an additional eye-catching figure) and a short ‘GTOC’ abstract(maximum 80 words or 3 sentences) summarising the key findings presented in thepaper for Table of Content (TOC) entry.
USING e-ANNOTATION TOOLS FOR ELECTRONIC PROOF CORRECTION
Required software to e-Annotate PDFs: Adobe Acrobat Professional or Adobe Reader (version 8.0 or
above). (Note that this document uses screenshots from Adobe Reader X)
The latest version of Acrobat Reader can be downloaded for free at: http://get.adobe.com/reader/
Once you have Acrobat Reader open on your computer, click on the Comment tab at the right of the toolbar:
1. Replace (Ins) Tool – for replacing text.
Strikes a line through text and opens up a text
box where replacement text can be entered.
How to use it
Highlight a word or sentence.
Click on the Replace (Ins) icon in the Annotations
section.
Type the replacement text into the blue box that
appears.
This will open up a panel down the right side of the document. The majority of
tools you will use for annotating your proof will be in the Annotations section,
pictured opposite. We’ve picked out some of these tools below:
2. Strikethrough (Del) Tool – for deleting text.
Strikes a red line through text that is to be
deleted.
How to use it
Highlight a word or sentence.
Click on the Strikethrough (Del) icon in the
Annotations section.
3. Add note to text Tool – for highlighting a section
to be changed to bold or italic.
Highlights text in yellow and opens up a text
box where comments can be entered.
How to use it
Highlight the relevant section of text.
Click on the Add note to text icon in the
Annotations section.
Type instruction on what should be changed
regarding the text into the yellow box that
appears.
4. Add sticky note Tool – for making notes at
specific points in the text.
Marks a point in the proof where a comment
needs to be highlighted.
How to use it
Click on the Add sticky note icon in the
Annotations section.
Click at the point in the proof where the comment
should be inserted.
Type the comment into the yellow box that
appears.
USING e-ANNOTATION TOOLS FOR ELECTRONIC PROOF CORRECTION
For further information on how to annotate proofs, click on the Help menu to reveal a list of further options:
5. Attach File Tool – for inserting large amounts of
text or replacement figures.
Inserts an icon linking to the attached file in the
appropriate pace in the text.
How to use it
Click on the Attach File icon in the Annotations
section.
Click on the proof to where you’d like the attached
file to be linked.
Select the file to be attached from your computer
or network.
Select the colour and type of icon that will appear
in the proof. Click OK.
6. Add stamp Tool – for approving a proof if no
corrections are required.
Inserts a selected stamp onto an appropriate
place in the proof.
How to use it
Click on the Add stamp icon in the Annotations
section.
Select the stamp you want to use. (The Approved
stamp is usually available directly in the menu that
appears).
Click on the proof where you’d like the stamp to
appear. (Where a proof is to be approved as it is,
this would normally be on the first page).
7. Drawing Markups Tools – for drawing shapes, lines and freeform
annotations on proofs and commenting on these marks.
Allows shapes, lines and freeform annotations to be drawn on proofs and for
comment to be made on these marks..
How to use it
Click on one of the shapes in the Drawing
Markups section.
Click on the proof at the relevant point and
draw the selected shape with the cursor.
To add a comment to the drawn shape,
move the cursor over the shape until an
arrowhead appears.
Double click on the shape and type any
text in the red box that appears.
UNCORRECTED PROOFS
WILEY AUTHOR DISCOUNT CLUB
We would like to show our appreciation to you, a highly valued contributor to Wiley’s publications, by offering a unique 25% discount off the published price of any of our
books*.
All you need to do is apply for the Wiley Author Discount Card by completing the
attached form and returning it to us at the following address:
The Database Group (Author Club)
John Wiley & Sons Ltd The Atrium Southern Gate Chichester
PO19 8SQ UK
Alternatively, you can register online at www.wileyeurope.com/go/authordiscount
Please pass on details of this offer to any co-authors or fellow contributors.
After registering you will receive your Wiley Author Discount Card with a special promotion
code, which you will need to quote whenever you order books direct from us.
The quickest way to order your books from us is via our European website at:
http://www.wileyeurope.com
Key benefits to using the site and ordering online include: Real-time SECURE on-line ordering Easy catalogue browsing
Dedicated Author resource centre Opportunity to sign up for subject-orientated e-mail alerts
Alternatively, you can order direct through Customer Services at:
[email protected], or call +44 (0)1243 843294, fax +44 (0)1243 843303
So take advantage of this great offer and return your completed form today.
Yours sincerely,
Verity Leaver Group Marketing Manager [email protected]
*TERMS AND CONDITIONS This offer is exclusive to Wiley Authors, Editors, Contributors and Editorial Board Members in acquiring books for their personal use. There must be no resale through any channel. The offer is subject to stock availability and cannot be applied retrospectively. This
entitlement cannot be used in conjunction with any other special offer. Wiley reserves the right to amend the terms of the offer at any time.
UNCORRECTED PROOFS
To enjoy your 25% discount, tell us your areas of interest and you will receive relevant catalogues or leaflets
from which to select your books. Please indicate your specific subject areas below.
AccountingPublic
Corporate
[ ] [ ]
[ ]
Architecture
Business/Management
[ ]
[ ]
Chemistry
Analytical Industrial/Safety Organic
Inorganic Polymer Spectroscopy
[ ]
[ ] [ ] [ ]
[ ] [ ] [ ]
Computer Science
Database/Data Warehouse Internet Business Networking
Programming/Software Development Object Technology
[ ]
[ ] [ ] [ ]
[ ]
[ ]
Encyclopedia/Reference Business/FinanceLife Sciences
Medical Sciences Physical Sciences Technology
[ ] [ ] [ ]
[ ] [ ] [ ]
EngineeringCivilCommunications Technology
Electronic EnvironmentalIndustrial
Mechanical
[ ] [ ] [ ]
[ ][ ] [ ]
[ ]
Earth & Environmental Science
Hospitality
[ ]
[ ]
Finance/InvestingEconomics
Institutional Personal Finance
[ ] [ ]
[ ] [ ]
GeneticsBioinformatics/
Computational Biology
Proteomics Genomics Gene Mapping Clinical Genetics
[ ] [ ]
[ ] [ ] [ ] [ ]
Life Science
Landscape Architecture
MathematicsStatistics
Manufacturing
Materials Science
[ ]
[ ]
[ ]
[ ]
[ ]
[ ] Medical Science
Cardiovascular
Diabetes Endocrinology Imaging Obstetrics/Gynaecology
Oncology Pharmacology Psychiatry
[ ] [ ]
[ ] [ ] [ ] [ ]
[ ] [ ] [ ]
PsychologyClinical
Forensic Social & Personality Health & Sport Cognitive
Organizational Developmental & Special Ed Child Welfare
Self-Help
[ ] [ ]
[ ] [ ] [ ] [ ]
[ ] [ ][ ]
[ ]
Non-Profit [ ] Physics/Physical Science [ ]
Please complete the next page /
REGISTRATION FORM
For Wiley Author Club Discount Card
UNCORRECTED PROOFS
I confirm that I am (*delete where not applicable):
a Wiley Book Author/Editor/Contributor* of the following book(s):
ISBN: ISBN:
a Wiley Journal Editor/Contributor/Editorial Board Member* of the following journal(s):
SIGNATURE: …………………………………………………………………………………… Date: ………………………………………
PLEASE COMPLETE THE FOLLOWING DETAILS IN BLOCK CAPITALS:
TITLE: (e.g. Mr, Mrs, Dr) …………………… FULL NAME: …………………………………………………………………………….…
JOB TITLE (or Occupation): ..…………………………………………………………………………………………………………………
DEPARTMENT: ……………………………………………………………………………………………………………………………………………..
COMPANY/INSTITUTION: ……………………………………………………………………………………………………………………………
ADDRESS: ……………………………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………………………………………………………………
TOWN/CITY: …………………………………………………………………………………………………………………………………………………
COUNTY/STATE: ………………………………………………………………………………………………………………………………………….
COUNTRY: …………………………………………………………………………………………………………………………………………………….
POSTCODE/ZIP CODE: …………………………………………………………………………………………………………………………………
DAYTIME TEL: ………………………………………………………………………………………………………………………………………………
FAX: ………………………………………………………………………………………………………………………………………………………………
E-MAIL: …………………………………………………………………………………………………………………………………………………………
YOUR PERSONAL DATA
We, John Wiley & Sons Ltd, will use the information you have provided to fulfil your request. In addition, we would like to:
1. Use your information to keep you informed by post of titles and offers of interest to you and available from us or other
Wiley Group companies worldwide, and may supply your details to members of the Wiley Group for this purpose.
[ ] Please tick the box if you do NOT wish to receive this information
2. Share your information with other carefully selected companies so that they may contact you by post with details of
titles and offers that may be of interest to you.
[ ] Please tick the box if you do NOT wish to receive this information.
E-MAIL ALERTING SERVICE
We also offer an alerting service to our author base via e-mail, with regular special offers and competitions. If you DO wish to
receive these, please opt in by ticking the box [ ].
If, at any time, you wish to stop receiving information, please contact the Database Group ([email protected]) at John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, PO19 8SQ, UK.
TERMS & CONDITIONS This offer is exclusive to Wiley Authors, Editors, Contributors and Editorial Board Members in acquiring books for their personal use. There should be no resale through any channel. The offer is subject to stock availability and may not be applied retrospectively. This entitlement cannot be used in conjunction with any other special offer. Wiley reserves the right to vary the terms of the offer at any time.
PLEASE RETURN THIS FORM TO:
Database Group (Author Club), John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, PO19 8SQ, UK [email protected]
Fax: +44 (0)1243 770154