Neurologic diseases in HIV-infected patients
-
Upload
facultaddemedicina -
Category
Documents
-
view
3 -
download
0
Transcript of Neurologic diseases in HIV-infected patients
Handbook of Clinical Neurology, Vol. 121 (3rd series)Neurologic Aspects of Systemic Disease Part IIIJose Biller and Jose M. Ferro, Editors© 2014 Elsevier B.V. All rights reserved
Chapter 90
Neurologic diseases in HIV-infected patients
MOHAMMED BILGRAMI AND PAUL O’KEEFE*
Department of Medicine, Loyola University Medical Center, Maywood, IL, USA
INTRODUCTION
Since the introduction of highly active antiretroviraltherapy (HAART) there has been an improvement inthe quality of life of people with human immunodefi-ciency virus (HIV) infection. Despite this progress,about 70% of HIV-infected patients develop neurologiccomplications. They originate either in the central or theperipheral nervous system (Sacktor, 2002) and aredivided into primary and secondary disorders. The pri-mary disorders result from the direct effects of thevirus and include HIV-associated neurocognitive disor-der (HAND), HIV-associated vacuolar myelopathy(VM), and distal symmetric polyneuropathy (DSP),which are prevalent at all levels of immune function(CD4þ T cell counts). Secondary disorders resultfrom marked immunosuppression and include opportu-nistic infections and primary central nervous systemlymphoma (PCNSL). It is challenging to evaluate andmanage HIV-infected patients who present with neuro-logic signs and symptoms. Diagnostic testing is morevaluable when a prioritized differential diagnosis is keptin mind. It can be accomplished by detailed history, neu-rologic examination, and having a good understand-ing of the role of HIV in various neurologic disorders.A broad, unfocused diagnostic evaluation will result inunnecessary testing and possibly confuse the clinicalpicture.
ETIOLOGYANDDIAGNOSTIC APPROACH
A recommended initial approach is to attempt to narrowthe focus to one of the broad categories outlined inTable 90.1.
*Correspondence to: Paul O Keefe, M.D., Loyola University Med
Maywood, IL 60153, USA. Tel: þ1-708-216-9453, E-mail: pokeefe@
PATHOGENESISOF NEUROLOGICCOMPLICATIONSOFHIV
Neurologic complications of HIVmay result from directeffects of HIV on the central nervous system. They mayalso result from HIV-related immune dysfunction withCD4 cell depletion and its associated increased host sus-ceptibility to neurologic opportunistic infections, dis-eases, and neoplasms. HIV infection of the centralnervous system (CNS) leads to immune activation andoverexpression of inflammatory cytokines, resulting incellular and tissue dysfunction with damage to theblood–brain barrier (BBB), surrounding glial cells andneurons. The primary HIV- associated neurologic disor-der of the brain is HIV-associated dementia whereasHIV infection involving the spinal cord causes HIV-associated myelopathy. With the widespread use ofantiretroviral therapy, the incidence and prevalence ofneurologic opportunistic infections has declined(Maschke et al., 2000). Conditions due to direct effectsof HIV itself on the CNS, such as HIV-associateddementia or sensory neuropathies, have also declinedbut remain a source of considerable morbidity.
HIV uses at least two surface receptors to enter thetarget cell. The primary receptor is the CD4 moleculewhich is expressed on the surface of CD4 lymphocytes,dendritic cells, and monocytes/macrophages. After thevirus binds to its primary receptor, it binds to one oftwo co-receptors, either CCR5 or CXCR4. These co-recetptors are chemokine receptors. HIV that utilizeCCR5 are also called R5 or M tropic whereas those uti-lizing CXCR4 are called X4 or T tropic (Albright et al.,2003). CCR5-tropic virus is commonly transmitted dur-ing primary infection whereas CXCR4-tropic virus is
ical Center, 2160 S. First Avenue, Building 102, Room 7604
lumc.edu
,
Table 90.1
Neurologic manifestations of HIV infection
Clinical syndrome Etiologic agent or disease
Cerebrovascular disease CytomegalovirusHIVInfective endocarditis with
emboliMycobacterium tuberculosisTreponema pallidumVaricella zoster virus
Dementia HIVTreponema pallidum
Encephalitis HIV (acute HIV infection)
CytomegalovirusHerpes simplexWest Nile virus
Mass lesion in brain Toxoplasma gondiiJC virus (the cause ofprogressive multifocal
leukoencephalopathy)Primary central nervoussystem lymphoma
Meningitis Cryptococcus neoformansHIVMycobacterium tuberculosisTreponema pallidum
Myelopathy/radiculopathy CytomegalovirusHIVVaricella zoster virus
Peripheral neuropathy HIVMedications (didanosine,stavudine)
Toxins (alcohol)
Vitamin deficiencies(B12, folate)
(Reproduced with permission from Clinical Care Options: CCO HIV,
inPractice, 2011, available at www.inpractice.com)
Table 90.2
CNS cell types involved in HIV neuropathogenesis
Cell typeHIVinfection Nature of infection
Astrocytes Yes Restricted, nonproductiveEndothelial cells Yes Possibly productive
Microglia Yes ProductiveMonocytes/macrophages
Yes Productive
Neurons No
Oligodendrocytes No
(Reproduced with permission from Clinical Care Options: CCO HIV,
inPractice, 2011, available at www.inpractice.com)
1322 M. BILGRAMI AND P. O’KEEFE
transmitted less often. As HIV infection progresses inthe individual, the R5 phenotype may be replaced byX4 due to selective pressure. The more pathogenic X4phenotype induces syncytial formation among HIV-infected and uninfected cells. These multinucleate giantcells are dysfunctional and rapidly die. Fusion of micro-glia and brain macrophages, resulting in multinucleatedgiant cells in the CNS, is a distinct feature of HIV-associated encephalitis (Rock et al., 2004).
Macrophages are derived from peripheral monocyteswhich enter the CNS and differentiate into perivascularmicroglia as well as perivascular, meningeal, and cho-roid plexus macrophages. Microglia are phagocytic cellsin the CNS, which, like monocytes in the periphery,remove pathogens and debris from the local site. Thesecells exhibit bidirectional BBB passage.
Table 90.2 shows the various cell types in the centralnervous system. Microglia express only the CCR5 co-receptor and are the primary target cells of HIV. Mono-cytes and macrophages in the CNS express both CXCR4and CCR5 co-receptors and thus bind both R5 and X4virus. Similar to microglia they support HIV growthand replication (Gartner and Liu, 2002).
Astrocytes, well represented in the CNS, are capable ofbeing infected but do not support growth and replicationof the HIV. This restricted infection may contribute toneuronal dysfunction (Brack-Werner, 1999). Endothelialcells express both co-receptors and are capable of beinginfected. Whether they support HIV replication or notis still unclear. Neurons and oligodendrocytes are notknown to be infected by HIV. CNS cell types involvedin HIV neuropathogenesis are depicted in Table 90.2.
Brain parenchyma is separated from the systemic circu-lation by the BBB which is composed of tightly boundendothelial cells. Passage of proteins and some drugs,including certain antiretrovirals, into the CNS is limitedby the BBB. Immune cells which develop and differentiatein the periphery exhibit bidirectional movement betweenthe systemic circulation and the CNS. HIV enters theCNS by infecting monocytes which can cross the BBB(the Trojan horse model) (Haase, 1986). Cytokine expres-sion aswell as toxic products ofHIV infection can damageneurons and glial cells resulting in a more porous BBB.This may allow entry of cell free HIV virions into the CNS.
Once within the CNS, HIV can damage neurons. Onemodel suggests that HIV-infected microglia and macro-phages release various neurotoxic viral proteins such asHIV envelope glycoprotein 160 (gp 160) and its cleavageproducts gp 120 and gp 41 or regulatory proteins includingTat, Nef, and Vpr (Li et al., 2005). Other studies haveshown that inflammatory cytokines fromglial cells ormac-rophages or other soluble products are released in responseto HIV infection. Most importantly these includetumor necrosis factor-a (TNF-a) and interleukin 1 (IL-1).
N
Glial cells and macrophages also release other cytokinesand other factors including b-chemokines such as MIP(macrophage inflammatory protein)-1a, MIP-1b, andRANTES (regulated on activation normal T cell expressedand secreted); the a-chemokine interferon-g-inducibleprotein(IP)-10; arachidonic acid; platelet-activating factor;quinolinic acid; nitric oxide; and superoxide anions. Signif-icantly elevated concentrations of all of these factors havebeen found in the brain or cerebrospinal fluid (CSF) ofHIV-infected persons (Smith et al., 2001).
NEUROLOGIC DISEASES I
OPPORTUNISTIC INFECTIONS
Toxoplasmosis
Toxoplasmosis is caused by an intracellular protozoan,Toxoplasma gondii, and has a worldwide distribution.Seroprevalence in the US is about 15% but reaches50–75% in some European and resource-limitedcountries. Its prevalence is increased in areas of theworld that have hot, humid climates and lower altitudes.Toxoplasmosis is the most common parasiticopportunistic infection (OI) involving the CNS in per-sons with AIDS with the frequency progressivelyincreasing as CD4 counts reach 100 and lower.Nearly all episodes of toxoplasmosis occur as a resultof reactivation of latent infection acquired some timeearlier. Toxoplasmosis is not transmitted from personto person, except in instances ofmother to child (congen-ital) transmission, blood transfusion, or organ transplan-tation. Most human infections occur by one of tworoutes: (1) consumption of undercooked, contaminatedmeat (especially pork, lamb, and venison) containingencysted organisms, or (2) accidental ingestion ofoocysts shed in cat feces through such activities as clean-ing a litter box or ingestion of oocysts in contaminatedsoil or water.
TOXOPLASMA GONDII: HUMAN INFECTION
After ingestion, T. gondii are carried by monocytes tothe liver and then can spread throughout the body.Among HIV-infected persons, latent infection in thebrain can reactivate with severe immunodeficiency andcause toxoplasma encephalitis. In recent years, the inci-dence of toxoplasma encephalitis has declined substan-tially due both to the widespread availability ofantiretroviral therapy and the common practice ofemploying the drug trimethoprim/sulfamethoxazole(TMP-SMX) for the prevention of pneumonia due toPneumocystis jiroveci. In a study that tracked opportu-nistic infections during the years 1994–2007, the inci-dence of CNS toxoplasmosis decreased from 4.1 per1000 person-years in the years 1994–1997 to 1.3 per
1000 person-years in 1998–2002. It declined further to0.5 per 1000 person-years in 2003–2007 with the wide-spread use of highly active antiretroviral therapy(HAART) (Buchacz et al., 2010).
Among HIV-infected patients the seroprevalenceof toxoplasmosis mirrors the rate of seropositivityin the general population. Among 2525 women in theUS the seroprevalence was 15% and was notdifferent in HIV-positive versus HIV-negative womenin the sample (Falusi et al., 2002). Age over 50 orbeing born outside of the US were both associatedwith a greater likelihood of seropositivity (Falusi et al.,2002). In patients with AIDS, cat ownership was notassociated with greater risk of toxoplasmosis (Wallaceet al., 1993). Because the parasite can reactivate andcause disease in persons with AIDS, all persons withHIV should be screened for toxoplasma antibodies(IgG). Seropositive persons have an approximately30% chance of reactivation in the CNS if not on effectiveprophylaxis (Luft and Remington, 1992).
HIV-INFECTED PATIENTS 1323
CLINICAL MANIFESTATIONS
In immunocompetent people, infection with T. gondii isusually asymptomatic. Some individuals develop amononucleosis-like illness with enlarged, tender lymphnodes, muscle aches and fever that lasts for severalweeks. Others may develop chorioretinitis. Once a personis infected, the parasite remains in an inactive state andcan reactivate if the person becomes immunosuppressede.g., HIV with CD4 counts less than 100 cells/mm3.
Toxoplasmosis in immunocompromised patients isusually acute, generalized, and commonly involves thebrain in the form of toxoplasma encephalitis (TE). TE isthe most common AIDS-defining disease affecting thecentral nervous system (Montoya and Liesenfeld, 2004).Other less common but important sites include the lungs(pulmonary toxoplasmosis) and the eye (retinitis), andsome patients develop multiorgan disease. Patients withTE typically present with fever, headache, and confusion.Focal neurologic deficits or seizures are also common. Inone retrospective review of 115 cases, 55%, 52%, and 47%had headache, confusion, and fever, respectively (Porterand Sande, 1992). In recent studies conducted duringthe HAART era, reported inpatient mortality has beenaround 15% (Miro and Murray, 2008).
Clinical signs of toxoplasmosis are nonspecific andmimic other CNS diseases including lymphoma, progres-sive multifocal leukoencephalopathy, mycobacterialinfection, or cryptococcosis. Diagnosis is establishedby (1) compatible clinical findings, (2) neuroimaging,(3) specific diagnostic testing including serology, biopsyfindings, or molecular (DNA) methods, and (4) responseto therapy
1324 M. BILGRAMI AN
NEUROIMAGING
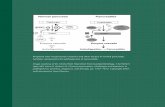
Contrast MRI is more sensitive than contrast CT scan-ning and is recommended as the study of choice for ini-tial assessment and for following response to therapy. Infollowing response to therapy, clinicians should not com-pare MRI to CT scans as CT can miss lesions which areseen with MRI. The most common MRI findings withCNS toxoplasmosis, seen in 90% of patients, are multipleenhancing lesions that often involve basal ganglia and thegray–white junction (Fig. 90.1). Mass effect secondary totoxoplasmosis is not as commonly seen as it is in lym-phoma. In order to differentiate toxoplasmosis from lym-phoma, clinicians have utilized other imaging techniques.Magnetic resonance spectroscopy, both single-photonemission computed tomography (SPECT) using thallium201 and positron emission tomography (PET) usinglabeled substrates such as 2-fluorodeoxyglucose, appearsuseful. In patients with mass lesions on CT or MRI, theabsence of increaseduptake on thallium201 single-photonemission computed tomography scanning, or decreasedactivity on positron emission tomography scans (“cold”or hypometabolic lesions) are characteristic of infection.By contrast, lymphoma is almost invariably associatedwith increased uptake using these two scanning tech-niques (Skiest et al., 2000).
LABORATORY DIAGNOSIS
About 95% of patients with acute toxoplasma encepha-litis will have antitoxoplasma IgG antibodies usingELISA and about 85% have detectable antibodies usingan IFA assay. Antitoxoplasma IgM has minimal value in
Fig. 90.1. Brain MRI: T2-weighed image of a patient with
cerebral toxoplasmosis showing numerous hyperintense signal
abnormalities predominately in the subcortical gray–white
junction and basal ganglia.
diagnosis. Lumbar puncture can provide useful informa-tion to rule out other diagnoses. The cell count andEpstein–Barr virus (EBV) polymerase chain reaction(PCR) is helpful in ruling out CNS lymphoma whichmay mimic toxoplasma encephalitis clinically and radio-graphically. EBV PCR inCSF is very sensitive and specificfor CNS lymphoma. PCR testing forT. gondii is available,but the test lacks sensitivity (Nogui et al., 2009).
APPROACH WITH SUSPECTED TOXOPLASMA
ENCEPHALITIS
Factors that suggest toxoplasma encephalitis include apositive IgG serology for T. gondii, non-use of toxo-plasma prophylaxis, the presences of multiple enhancingbrain lesions, and a positive CSF PCR for T. gondii.Mostexperts would recommend a trial of empiric therapy fortoxoplasma encephalitis in patients who are toxoplasmaseropositive and have multiple enhancing brain lesions.Brain biopsy carries significant risk and should generallybe reserved for patients who do not demonstrate clinicalor radiographic improvement after 10–14 days of anti-toxoplasma therapy. This delayed biopsy approach isnot appropriate in cases in which clinical, laboratory,and radiographic indicators strongly point to a diagnosisof lymphoma (Antinori et al., 1997).
TREATMENT
Guidelines for the treatment of toxoplasmosis in HIV-infected persons have been published by the US Centersfor Disease Control and Prevention (CDC), NationalInstitutes of Health (NIH), and the HIV Medicine Asso-ciation of the Infectious Diseases Society of America(Kaplan et al., 2009). The treatment of choice forpatients with toxoplasmosis is a combination of sulfadi-azine plus pyrimethamine plus leucovorin (folinic acid)for 6–8 weeks (Table 90.3). Leucovorin is given to avoidhematologic toxicity. If intracranial hypertension is pre-sent, corticosteroids are recommended, as are anticon-vulsants if the patient experiences seizures. Valproicacid is the recommended antiepileptic for patientsreceiving antiretroviral therapy with non-nucleosidereverse-transcriptase inhibitors (NNRTIs) or proteaseinhibitors (PIs) to avoid the possible drug interactionswith other antiepileptic agents. In case of sulfadiazineallergy or intolerance, clindamycin plus pyrimethamineplus leucovorin is the recommended regimen. Otheralternatives are available. Patients receiving antitoxo-plasma treatment should be monitored for toxicitybecause adverse effects are common with all regimens.If treatment failure is observed after 10–14 days, a brainbiopsy is recommended in order to confirm toxoplasmaencephalitis or to diagnose another AIDS-defining centralnervous system disease. If the biopsy confirms the diag-nosis of toxoplasma encephalitis after the 10–14 day trial
D P. O’KEEFE
Table 90.3
Treatment of CNS toxoplasmosis*
Initial treatment
6 weeks; may be longer if clinical or radiologic diseaseis extensive or response is incomplete at 6 weeks
Preferred therapy:
Pyrimethamine 200mg po� 1 dose, then 50mg (<60kg) or75mg (>60kg) po dailyþ sulfadiazine 1000mg (60kg)or 1500mg (>60kg) po q 6hþ leucovorin (folinic acid)10–25mg po daily
Alternative therapy regimens:Pyrimethamine/leucovorin (above doses)þclindamycin600mg IV or po q 6h
TMP-SMX (5mg/kg TMP) IV or po bidPyrimethamine/leucovorin (above doses)þatovaquone1500mg po bid with food
Atovaquone 1500mg po bid with foodAtovaquone 1500mg po bid with foodþ sulfadiazine1000–1500mg po q 6h
Pyrimethamine/leucovorin (above doses)þazithromycin900–1200mg po daily
Maintenance
Preferred therapy:
Pyrimethamine 25–50mg po dailyþsulfadiazine 2–4g po qd(in 2–4 divided doses)þ leucovorin 10–25mg po daily
Alternative therapy regimens:
Pyrimethamine/leucovorin (above doses)þclindamycin600mg po q 8hAtovaquone 750mg po q 6–12h�either (pyrimethamine25mg poþ leucovorin 10mg po daily) or (sulfadiazine 2–4g
po daily)
*(2009 CDC/IDSA/NIH Guidelines for Prevention and Treatment of
Opportunistic Infections in Adults with HIV Infection; Kaplan et al.,
2009).
po, oral; IV, intravenous; q, every; qd, daily; bid, twice daily; TMP-
SMX, trimethoprim/sulfamethoxazole.
Table 90.4
Primary prophylaxis of CNS toxoplasmosis
Pathogen Indication First choice
Toxoplasma gondii Toxoplasma IgG-positive withCD4< 100 cells/
mm3
Trimethoprim-sulfamethoxSMX) 1 DS
(2009 CDC/IDSA/NIH Guidelines for Prevention and Treatment of Oppo
PO, oral; DS, dose; single strength.
NEUROLOGIC DISEASES IN
some experts recommend switching to an alternative anti-toxoplasma regimen (Kaplan et al., 2009).
Antiretroviral therapy-induced immune reconstitu-tion inflammatory syndrome (IRIS) associated withtoxoplasma encephalitis has been reported but appearsto be rare. There are not enough cases to provide recom-mendations on how to manage toxoplasma encephalitis-associated IRIS.
RESPONSE TO INITIAL THERAPY
More than 70% of patients show both clinical and radio-graphic improvement to initial therapy. In a retrospec-tive study involving 35 patients, 86% showedimprovement by day 7 and 91% showed improvementby day 14 (Luft et al., 1993).
PRIMARY PROPHYLAXIS
Allof theagencies thatpublish treatmentrecommendationsalso recommend that prophylaxis be prescribed for alltoxoplasma-seropositive HIV-infected patients withCD4þ cell counts less than100 cells/mm3. Trimethoprim-sulfamethoxazole, one double-strength tablet daily, usedto preventPneumocystis jirovici pneumonia, also preventstoxoplasmosis. Toxoplasma-seropositive patients who arereceiving pneumocystis pneumonia prophylaxis that isnot active against toxoplasmosis, such as inhaled pentami-dine, should either modify the prophylaxis or receive addi-tional medication that will prevent toxoplasmosis if theCD4þ cell count declines below 100 cells/mm3 (seeTable 90.4).
SECONDARY PROPHYLAXIS
Secondary prophylaxis should be administered to allpatients who have completed initial therapy for toxo-plasma encephalitis. However, it can be discontinuedif the patient’s CD4þ cell count increases to over
HIV-INFECTED PATIENTS 1325
Alternative
azole (TMP-PO daily
TMP-SMX 1 DS PO three times weeklyTMP-SMX 1 SS PO dailyDapsone 50mg PO dailyþPyrimethamine
50mg PO weeklyþ leucovorin (folinicacid) 25mg PO weekly
Dapsone 200mgþpyrimethamine
75mgþ leucovorin 25mg) PO weeklyAtovaquone 1500mg�pyrimethamine25mgþ leucovorin 10mg) PO daily
rtunistic Infections in Adults with HIV Infection; Kaplan et al., 2009)
AN
200 cells/mm3 for more than 6 months and if the HIV-1RNA level remains undetectable with the use of antire-troviral therapy. Prophylaxis should be reintroduced ifthe CD4þ cell count falls below 200 cells/mm3.
Primary central nervous system lymphoma
Primary CNS lymphoma (PCNSL) is defined as non-Hodgkin’s lymphoma (NHL) limited to the CNS. It isto be differentiated from systemic lymphoma withtumor spread to CNS.
PCNSL is the second most common intracranial masslesion seen in HIV patients besides CNS toxoplasmosis.It is usually seenwith advancedHIVdisease (CD4þ countless than 50 cells/mm3). It is an aggressive malignancyaccounting for 15% of all NHL in HIV-infected patients.CNS lymphoma is about 1000 times more common inHIV-infected persons than in the general population.Although it is restricted to the CNS, it may spread to theleptomeninges, spinal cord, and eye. Before the HAARTera PCNSLwas seen in about 2—6%ofHIV-infected per-sons (MacMahon et al., 1991). The incidence has declinedin the HAART era but at a rate less than that seen withother HIV-related complications. CDC data have docu-mented a decline from 8 per 1000 person-years in 1994to 2.3 per 1000 person-years in 1997 (Kaplan et al., 2000).
PATHOGENESIS
Virtually all cases of PCNSL are Epstein–Barr virus-related. EBV-infected B cells may undergo monoclonalproliferation in the presence of immune dysregulationand a severely immunosuppressed state (CD4þ countless than 50 cells/mm3).
CLINICAL PRESENTATION
PCNSL usually presents within the fourth decade of life.It is more common in men than women (Surawicz et al.,1999). Signs and symptoms evolve over days to months.The presentation may be nonspecific with focal and/ornonfocal signs and symptoms including B symptomsin 80% of cases. Symptoms and signs include confusion,headache (30–40%), memory loss, aphasia, hemiparesisand/or seizures. Fever is notably absent in most patients.The CD4 count is generally less than 50 cells/mm3.
Diagnosis of PNCSL involves neuroimaging, CSFanalysis, and biopsy.
NEUROIMAGING
MRI is more sensitive and more accurate in characteriz-ing focal brain lesions such as PCNSL.MRIwith contrastusually shows a single, irregular, contrast-enhancinglesion, but there can be multiple lesions in up to 50%of cases (Johnson et al., 1997). Contrast enhancement
1326 M. BILGRAMI
can be in a ring or homogeneous pattern. These lesionsusually involve the periventricular area, corpus callo-sum, or periependymal area; however, they can be any-where. These lesions are generally 2–6 cm in diameterand are usually associated with impressive mass effect.Single photon emission computed tomography (SPECT)is useful (see section on toxoplasmosis). If lumbar punc-ture can be safely performed, then it is advised in orderto facilitate the diagnosis of lymphoma and rule outother infectious complications. In addition to routinetesting, CSF should be sent for cytology, and PCR assaysfor EBV (PCNSL), JC virus (progressive multifocal leu-koencephalopathy (PML)), CMV (cytomegalovirusencephalitis) and Mycobacterium (CNS tuberculosis).PCR for EBV in CSF is a very helpful diagnostic testand carries a sensitivity of about 80% and a specificityof 94% (Skiest, 2002).
BRAIN BIOPSY
Definitive diagnosis can be made by stereotactic brainbiopsy either when CSF analysis is not possible or unre-vealing or when patients have not responded to empiricantitoxoplasma treatment within 2 weeks.
MANAGEMENT
There is no curative treatment at present. In the pre-HAART era, median survival for untreated PCNSLwas 1–3 months and for treated PCNSL was up to3.5 months (Fine and Mayer, 1993). Mortality is usuallydue to advanced immunodeficiency or to other opportu-nistic infections. Longer survival appears to be associatedwith HAART. In a retrospective analysis with a medianfollow-up of 667 days, six of seven HAART-treatedpatients with PCNSL were alive versus none of 18 patientsnot receivingHAART (Skiest andCrosby, 2003). Becauseof the diffuse nature of PCNSL aggressive surgicaldecompression is of no benefit. Until recently, high-doseradiation therapy was considered the standard treatment,but prospective studies done by the Radiation TherapyOncology Group (RTOG) have shown that the diseaserecurs in the brain in 92% of patients (Nelson et al., 1992).
All patients with PCNSL should be offered chemo-therapy as first-line treatment when their performancestatus is adequate and if they have relatively high CD4counts, i.e., greater than 100 and preferably greater than200. Chemotherapy regimens should include high dosemethotrexate often in combination with cytarabine.A retrospective review of 226 patients described betterresults with the use of high-dose methotrexate or cytar-abine combined with radiation therapy than the resultswith other combination chemotherapy regimens. TheCHOP regimen failed to show any benefit over radiationtherapy alone. Currently multiple trials are ongoing
D P. O’KEEFE
Fig. 90.2. Brain MRI: T2 FLAIR image of a patient with pro-
gressive multifocal leukoencephalopathy.
N
employing various combination chemotherapies withgood CNS penetration. Recent studies documenting bet-ter responses with combinations such as methotrexateand ifosfamide (Fischer et al., 2009) or with intra-arterialmethotrexate plus intravenous etoposide and cyclophos-phamide (MacNealy et al., 2008) are being reported. Cor-ticosteroids can provide substantial but, unfortunately,short- lived remissions. Steroids should be avoidedbefore brain biopsy as they may alter the histology andprevent adequate diagnosis (Johnson et al., 1997).
Progressive multifocal leukoencephalopathy
EPIDEMIOLOGY
Progressive multifocal leukoencephalopathy (PML) is aprogressive, demyelinating disease caused by a humanpolyoma virus called John Cunningham virus (JCV).PML is the most common infiltrative disease of the brainseen in persons with HIV/AIDS. JC virus is ubiquitous;about 85% of healthy people are seropositive for JCvirus. In the pre-HAART era the prevalence of PMLwas 1–10% among patients with AIDS. Once diagnosed,it was relentlessly progressive, usually resulting in deathwithin a few months. Now, after introduction ofHAART, the incidence of PML has decreased and sur-vival has improved (Sacktor, 2002).
PATHOGENESIS
About 85% of the normal population is asymptomati-cally infected with JCV in childhood or early adulthood(Kaplan et al., 2009). The virus remains latent in lym-phoid tissue and the kidney. Latent JC virus reactivatesin states of immunosuppression. It circulates in B lym-phocytes and infects oligodendrocytes as well as astro-cytes causing cell lysis and demyelination.
CLINICAL PRESENTATION
Clinical features are nonspecific and vary according tothe area of the brain involved. PML should be suspectedin HIV patients with focal neurologic symptoms andsigns, especially with a CD4 count of less than100 cells/mm3. However, in one reported series, 7–25%of HIV patients with PML had CD4 counts greater than200 cells/mm3 (Skiest, 2002). JC virus causes white mat-ter demyelination resulting in the classic clinical pictureof focal neurologic deficits and cognitive impairment.The disease is characterized by progressive dementiawith eventual coma and death. Symptoms are insidiousin onset and progress over weeks tomonths as comparedto other major opportunistic focal brain disorders inwhich symptoms progress in hours to days. Less com-monly, patients with PML have a waxing and waningclinical course which may extend for years. Fever and
NEUROLOGIC DISEASES I
headache are usually not seen with PML. When JC virusinvolves the cerebellum without white matter involve-ment on MRI and without classic PML histopathology,it is referred to as the cerebellar variant (Koralniket al., 2005). Common presenting signs and symptomsin PML include motor weakness often with hemiparesis,dementia, speech disturbances including aphasia anddysarthria, vision abnormalities, and gait instability.
HIV-INFECTED PATIENTS 1327
DIAGNOSIS
The presumptive diagnosis can often bemade by the clin-ical presentation along with neuroimaging studies andlumbar puncture results. CT scan typically reveals hypo-dense lesions of the white matter without enhancementor mass effect, but it can be normal. MRI is more sen-sitive and typically shows hypointensity on T1-weightedimages and increased intensity on T2-weighted images(Fig. 90.2). Lesions are usually bilateral and multiple,but some patients may show a single focal lesion. Thelesions may have a scalloped appearance on MRIbecause of involvement of subcortical white matter.
Routine testing of CSF is usually not helpful, and itmay be normal in many cases. Cell count may be normalor mildly elevated (less than 20 cells/mm3) and protein isnormal or mildly elevated. PCR for JCV in CSF is a veryhelpful diagnostic test and carries a sensitivity of70–80% and a specificity of 95–100% (Simona et al.,2005). Definite diagnosis is made by obtaining a brainbiopsy showing the characteristic features consisting
AN
of loss of myelin with enlarged, bizarre astrocytes andlipid-laden macrophages. Brain biopsy is not necessaryin all patients with a classic presentation that includescognitive deficits, focal neurologic findings, multiplenonenhancing white matter lesions and CSF PCR posi-tive for JCV.
PROGNOSIS
Prognosis of PML is poor with a median survival of 1–6months after diagnosis. However, about 8% of patientswith PML will have a benign course and even spontane-ously recover (Skiest, 2002). Factors associated with lon-ger survival include PML as the initial AIDS diagnosis,high CD4 count at diagnosis, patients on HAART,increase of CD4 count by greater than 100 from nadir,low HIV viral load, low levels of JCV in CSF, and lackof neurologic progression in the first 2 months afterdiagnosis.
TREATMENT
There is no effective treatment for PML. HAARTappears to prolong survival and improve neurologic def-icits when immune reconstitution is achieved. AlthoughHAART is considered the treatment of choice, somepatients have progressed despite receiving HAARTand others have even developed PML while receivingHAART (Cinque et al., 2001). Reports of treatment withprednisone, cytarabine, aciclovir, vidarabine, amanta-dine, interferon-a, cidofovir, topotecan, mirtazapine,or mefloquine did not benefit patients with PML(Kaplan et al., 2009). There have been reports of wors-ening of PML associated with immune reconstitutionin patients placed on HAART. This is thought to resultfrom enhanced inflammatory responses and is calledthe immune reconstitution inflammatory syndrome orIRIS (Cinque et al., 2001).
As JC virus is ubiquitous and exposure to the viruscannot be prevented, initiation of HAART in personswith HIV infection will greatly lessen the likelihood ofdeveloping PML.
Cryptococcal meningitis
EPIDEMIOLOGY
Cryptococcus neoformans is a ubiquitous environmentalencapsulated fungus found in abundant quantities in soiland bird feces. Transmission occurs via inhalation.C. neoformans is the most common cause of meningitisin HIV patients, occurring when CD4þ counts declinebelow 100 cells/mm3. Because signs and symptoms ofmeningitis can be subtle in HIV-infected persons, isola-tion of Cryptococcus from any site must be followed bya lumbar puncture to rule out meningitis. Although seen
1328 M. BILGRAMI
worldwide, the prevalence of cryptococcal disease variesin different regions. It is rare in Europe, occurs in 5–8%of AIDS patients in the US and in 20–30% of personswith HIV infection in sub-Saharan Africa and SouthAsia (Kaplan et al., 2009; Park et al., 2009). Since theintroduction of antiretroviral therapy and widespreaduse of fluconazole for candidiasis, the incidence of cryp-tococcosis has decreased (Mirza et al., 2003). There arefour different serotypes of Cryptococcus which can bedifferentiated based on serology. Serotypes A and Dinclude the more common Cryptococcus neoformansvar neoformans, whereas serotypes B and C includeCryptococcus neoformans var gattii. In recent yearsC. gattii has emerged in the Pacific Northwest, but themajority of cases did not have underlying HIV disease.Thus, C. neoformans causes the great majority of infec-tions in persons with HIV/AIDS.
PATHOGENESIS
Cryptococcus enters the body via inhalation. In the lungsit results in subclinical infection or overt pulmonarycryptococcosis. It can disseminate to other organsincluding blood, skin, meninges (the most common site),brain, eye, bone, prostate, and adrenals. Like otherencapsulated organisms, C. neoformans infects themeninges where immunoglobulin and complement arelacking. Persons with HIV manifest infection with C.neoformans when CD4þ counts decline to below 100/mm3 and particularly below 50/mm3 (Darras-Jolyet al., 1996).
CLINICAL FEATURES
In HIV patients, cryptococcosis most commonly occursas a subacutemeningitis or meningoencephalitis. Presen-tation is indolent often with symptoms present for 2–4weeks before the diagnosis is made. Common symptomsinclude fever, malaise, and headache. Some patientsdemonstrate encephalopathic symptoms such as leth-argy, altered behavior, personality changes, or memoryloss which result from elevated intracranial pressure.Classic meningeal symptoms and signs of neck stiffnessand photophobia are seen in only 25–33% of patientswith cryptococcal meningitis (Darras-Joly et al., 1996).Most cases are associated with cryptococcemia, i.e.,cryptococcus in the blood. Cryptococcal pneumoniamay be the initial presentation manifesting as fever,cough, and shortness of breath with abnormal chestimaging. Features on chest imaging are indistinguishablefrom other infections, and sometimes Cryptococcus isisolated in respiratory cultures when the chest X-ray isnormal. Interstitial and alveolar infiltrates are commonin HIV patients with low CD4 counts and may mimicinfection with Pneumocystis jiroveci. In one study,
D P. O’KEEFE
N
78% of HIV patients with cryptococcal meningitis hadpulmonary symptoms in the months before and/or atthe time their cryptococcal infection was diagnosed(Batungwanayo et al., 1994). All patents with HIV andpulmonary cryptococcosis should undergo lumbar punc-ture to rule out CNS dissemination. Skin involvement isseen in about 10% of patients, and there are several dif-ferent skin lesions described. The most common typeresemblesMolluscum contagiosum, but the lesions oftenprogress to central necrosis or ulceration in cutaneouscryptococccosis, a finding not seen withMolluscum con-tagiosum (Murakawa et al., 1996).
NEUROLOGIC DISEASES I
DIAGNOSIS
Cryptococcal antigen in serum and CSF can be detectedby latex agglutination or other similar tests that identifycryptococcal polysaccharide. When there is a high clini-cal suspicion of cryptococcal infection, the serum cryp-tococcal antigen level is a very helpful initial screeningtest. It is sensitive and specific. The serum antigen is pos-itive in over 95% of patients with cryptococcal meningi-tis whereas it is often negative in patients having onlylung involvement (Feldmesser et al., 1996). Approxi-mately 20% of patients with cryptococcal meningitis willhave a normal CSF formula including cell count, protein,and glucose. Therefore a normal CSF formula does notrule out cryptococcal meningitis. CSF should be testedfor cryptococcal antigen and cultured for fungus. TheCSF cryptococcal antigen is positive in virtually allpatients with cryptococcal meningitis. CSF opening pres-sure should be measured as it is elevated in the majorityof patients with cryptococcal meningitis. Patients withelevated pressure may require specific treatment to
Table 90.5
Treatment of cryptococcal meningitis
Preferred regimen
Induction ( for at least 2 weeks)Amphotericin B 0.7 mg/kg IV dailyþflucytosine 100 mg/kg
PO daily in four divided doses or lipid amphotericin B4–6 mg/kg IV dailyþflucytosine
Consolidation (start after significant clinical improvement and
negative CSF culture)Fluconazole 400 mg PO daily for 8 weeksMaintenance therapy: continue fluconazole 200 mg PO dailyuntil patient is asymptomatic, has completed induction and
consolidation therapy, and has a CD count over 200 for 6months on antiretroviral therapy (Vibhagool et al., 2003)
*For persons unable to tolerate or unresponsive to amphotericin B
(2009 CDC/IDSA/NIH Guidelines for Prevention and Treatment of Oppo
PO, oral; bid, twice daily; IV, intravenous; CSF, cerebrospinal fluid.
lower the pressure and more frequent follow-up lumbarpunctures (Perfect et al., 2010). Cryptococcus grows inblood cultures in 50–70% of patients with meningitis.A positive blood culture for cryptococcus in a patientwith HIV infection should prompt a lumbar punctureto rule out meningitis.
IMAGING
In order to rule out space-occupying mass lesions, CT orMRI should be obtained before performing a lumbarpuncture in patients presenting with focal deficits orsigns and symptoms suggestive of raised intracranialpressure.
TREATMENT
Guidelines for the treatment of cryptococcal meningitisin HIV-infected persons have been published by theCDC, NIH, and the HIV Medicine Association of theInfectious Diseases Society of America (Kaplan et al.,2009). The principles of treatment for patients with cryp-tococcal meningitis include: (1) antifungal therapy, (2)lowering elevated intracranial pressure, (3) initiating oroptimizing antiretroviral therapy to improve immunefunction, and (4) management of immune reconstitutionif it develops.
Antifungal therapy is divided into induction, consol-idation, and maintenance phases as shown in Table 90.5.In general, amphotericin B can clear cryptococcus morerapidly from CSF than fluconazole (Bicanic et al., 2007)and amphotericin B plus flucytosine more rapidlythan amphotericin B alone (Brouwer et al., 2004). Atthe present time, most centers use lipid formulationsof amphotericin B in lieu of the older, more toxic
HIV-INFECTED PATIENTS 1329
Alternative therapy
1. Amphotericinþfluconazole 400 mg PO or IV daily2. Amphotericin or lipid amphotericin alone
3. Fluconazole 400–800 mg daily IV or POþflucytosinefor 4–6 weeks*
Itraconazole 200 mg PO bid for 8 weeks
Continue itraconazole 200 mg PO daily (consider for personsunable to tolerate or unresponsive to fluconazole)
rtunistic Infections in Adults with HIV Infection; Kaplan et al., 2009)
AN
deoxycholate form (Hamill et al., 2010). Additionally,fluconazole plus flucytosine is less effective thanamphotericin B (Bicanic et al., 2007). Induction therapy,usually with amphotericin B and flucytosine, is contin-ued for at least 2 weeks in patients showing improve-ment, but longer in those remaining seriously ill. Whenpatients improve, consolidation therapy followed bylong-term maintenance therapy is continued, usuallywith fluconazole. During all three phases of therapy,patients should be monitored for side-effects. Withregard to the newer azole antifungals, including vorico-nazole and posaconazole, limited data documentingtheir efficacy in treatment of cryptococcal meningitisexist. Finally, echinocandins are not active against C.neoformans.
MANAGEMENT OF ELEVATED INTRACRANIAL PRESSURE
All patients with cryptococcal meningitis should have lum-bar puncture with measurement of opening pressure in thelateral decubitus position. Elevated opening pressure(greater than 25 cm H2O) is associated with more clinicalsigns and higher mortality. More than 90% of reporteddeaths within first 2 weeks and 40% of deaths in weeks3–10 were due to raised intracranial pressure (Graybillet al., 2000). Thus clinicians should manage elevated pres-sure very aggressively with daily lumbar punctures. Highopening pressure can be reduced to half by removal of20–30 mL of CSF. If repeated lumbar punctures cannotbe accomplished or fail to reduce symptoms or cerebraledema, placement of a lumbar drain, ventricolostomy, orventriculoperitoneal shunt should be considered.
ANTIRETROVIRAL THERAPY
Antiretroviral therapy should be included in the treat-ment of all patients diagnosed with cryptococcal menin-gitis; however, the timing of initiation of antiretroviraltherapy remains unclear. Concern about immune recon-stitution inflammatory syndrome (IRIS) has led manyexperts to recommend deferring antiretroviral therapyfor 2-10 weeks (until CSF cultures become negative)after initiating antifungal therapy. The incidence of IRISafter starting antiretroviral therapy in patients with cryp-tococcal meningitis is between 20% and 40%. Themajor-ity of patients who develop IRIS are antiretroviral naıveand have higher HIVRNA levels (Shelburne et al., 2005).In addition, IRIS is associated with pre-ART increases inTh17 and Th2 responses (Boulware et al., 2010). Symp-toms of IRIS resemble the initial presentation of crypto-coccal meningitis and may suggest treatment failure.Lumbar puncture should be obtained and negative fun-gal cultures from the CSF along with rapid decline inHIV RNA levels and a marked improvement in CD4counts all favor IRIS as the cause of the ambiguous
1330 M. BILGRAMI
clinical picture. Appropriate management of IRIS is tocontinue both antiretroviral therapy and antifungaltherapy. Some experts recommend corticosteroids forsevere symptomatic cases of IRIS (Venkataramanaet al., 2006).
PROPHYLAXIS
Although controlled clinical trials have shown that flu-conazole or itraconazole can reduce the frequency ofprimary cryptococcal infection in patients with CD4þcell counts of less than 50/mm3, primary prevention ofcryptococcal infection in HIV-infected persons is notrecommended due to the infrequency of cryptococcaldisease, lack of survival benefit with prophylaxis, druginteractions, drug resistance and cost (Perfect et al.,2010). Before HAART was available, lifelong treatmentwith oral fluconazole, 200 mg daily, was used for sec-ondary prophylaxis after control of the meningitis withinduction and consolidation therapy. With HAART, sec-ondary prophylaxis can be discontinued after the patientis asymptomatic, has completed induction and consoli-dation therapy (at least 10 weeks), and has a CD4þ cellcount over 200 for 6 months on ART (Vibhagool et al.,2003) (Table 90.5).
Cytomegalovirus encephalitis
Cytomegalovirus (CMV) is a double-stranded DNAvirus belonging to the herpesvirus family. Amongpatients with advanced immunosuppression, the viruscan cause disseminated or localized end organ disease.Patients with CMV encephalitis may present with onlyencephalitis or may have associated CMV infection atother sites including the retina, GI tract (esophagus tocolon), adrenals, or other less commonly involved sites.Risk factors for CMV include CD4 count less than 50/mm3 (not onART or not responding to current regimen),HIV RNA level over 100000 copies/mL, history of pre-vious opportunistic infection, or evidence or prior CMVinfection, i.e., CMV IgG positive. Virtually all CMVinfections in AIDS represent reactivation of latent infec-tion. Since the introduction of highly active antiretroviraltherapy (HAART), the incidence of new cases of CMVend organ disease has declined by 75–80%. Incidence isnow estimated to be fewer than six cases per 100 person-years (Jabs et al., 2007). Mortality associated with CMVdisease has also decreased (Palella et al., 1998).
CLINICAL FEATURES OF NEUROLOGIC
CYTOMEGALOVIRUS DISEASE
CMV is responsible for three distinct neurologic syn-dromes. These include dementia, encephalitis with ventri-culoencephalitis, and ascending polyradiculomyelopathy.
D P. O’KEEFE
N
Dementia presents as lethargy, confusion, and fevermimicking HIV dementia. CSF shows a lymphocyticpleocytosis, normal to elevated protein, and low to nor-mal glucose.
Ventriculoencephalitis has an acute onset with confu-sion, focal deficits, cranial nerve abnormalities, nystag-mus, ataxia, and rapid progression to death. CSFtypically demonstrates a lymphocytic pleocytosis andan elevated protein. However, the spinal fluid formulacan be normal with CMV encephalitis and thus, normalCSF does not rule out CMVencephalitis. CT orMRIwithcontrast may demonstrate perivetricular enhancementwhich supports the diagnosis of CMV encephalitisbut is not specific for the disease. Ascending polyradicu-lomyelopathy due to CMV results in a Guillain–Barre-like syndrome with ascending bilateral leg weaknessalong with bladder/bowel incontinence, radicular pain,and sensory deficits. CSF demonstrates neutrophilicpleocytosis.
NEUROLOGIC DISEASES I
DIAGNOSIS
CMV viremia can be detected by PCR or by antigenassays. Viremia is usually observed with end organ dis-ease, but it may also be present in the absence of endorgan disease.
CMV antibodies are not useful in diagnosis althougha negative anti-CMV IgG makes CMV infectionunlikely. Spinal fluid cultures are positive in less than50% of cases with CMV encephalitis. Detection ofCMV DNA by PCR in the CSF is positive in 80%. Thespecificity of spinal fluid PCR is 90% in CMV neuro-logic disease (Skiest, 2002). Definitive diagnosis, then,is based on a compatible clinical syndrome and demon-stration of CMV in CSF or brain tissue after biopsy.
TREATMENT
Anti-CMV therapy may be effective if given early in thecourse of disease although clinical response to therapy isunpredictable. As recommended in the treatment guide-lines from the CDC, NIH and the HIVMedicine Associ-ation of the IDSA 2009, combination therapy withintravenous ganciclovir 5 mg/kg twice a day and intrave-nous foscarnet 90 mg/kg twice a day should be used tostabilize disease and maximize response. It should becontinued until improvement is demonstrated, whichmight take weeks to months depending on the severityof neurologic disease. After symptomatic improvementoral valganciclovir 900 mg twice daily along with intra-venous foscarnet once daily should be used for lifeunless there is evidence of immune recovery. Becauseof rare fatal cases of IRIS attributed to CMV infectionof the CNS, initiation of Highly active antiretroviral
therapy (HART) can be delayed until there is symptom-atic improvement (French et al., 2000).
PREVENTION
Antiretroviral therapy with control of HIV replicationand improved immune function will prevent CMV dis-ease. Primary prophylaxis with anti-CMV medicationsis not recommended.
SPINAL CORDDISORDERS INHIVPATIENTS/VACUOLARMYELOPATHY
EPIDEMIOLOGY
Spinal cord disorders are less common than peripheralnervous systemdisorders inHIVpatients. HIV-associatedmyelopathy occurs as a result ofHIV infection and is alsocalled vacuolar myelopathy (VM). VM can occur at anystage during the course ofHIV infection but ismore com-mon with advanced immunosuppression.
INCIDENCE AND PREVALENCE
Reliable data on incidence and prevalence ofmyelopathyin HIV patients is not available. This is because of under-diagnosis or the lack of symptoms and signs in affectedpatients. One autopsy-based case control study docu-mented VM in 46% of persons with AIDS (Dal Panet al., 1994).
PATHOLOGY
Vacuolar myelopathy is a pathologic diagnosis in whichvacuolation of myelin in the posterior and lateral col-umns of the spinal cord, with or without inflammation,is seen. The upper thoracic levels of the spinal cord arecommonly involved. The HIV virus does not directlyinvolve the spinal cord. It facilitates the developmentof disease by stimulating macrophages to produceinflammatory cytokines. Clinically and pathologically,HIV myelopathy often resembles subacute combineddegeneration of spinal cord as is seen with vitamin B12
deficiency (Petito et al., 1985).
CLINICAL FEATURES
Vacuolar myelopathy is characterized by a subacuteonset of clinical signs and symptoms, developing overweeks to months. Common symptoms include bilaterallower extremity weakness with spasticity. Patients alsoexperience bowel, bladder, and erectile dysfunction withvariable sensory disturbances. Deep tendon reflexes arehyperactive with an extensor plantar response (Babinskisign). Upper extremities are normal. Ambulatorypatients may have unsteadiness of gait. When paresthe-sias or numbness are present, VM can be differentiated
HIV-INFECTED PATIENTS 1331
AN
from peripheral neuropathy by noting the brisk reflexeswith VM as opposed to depressed reflexes with periph-eral neuropathy.
DIAGNOSIS
Diagnosis of VM is by exclusion and requires ruling outother causes bymeasuring serum vitamin B12 levels, cop-per levels, rapid plasma reagin (RPR), and humanT-lymphotrophic virus-1 (HTLV-1) antibodies beforemaking the diagnosis. Lumbar puncture should be per-formed to rule out infection with herpes simplex virus,varicella zoster virus, CMV, and neurosyphilis. In VMthe CSF may be normal, or it may show mild pleocytosiswith mild elevation in protein. Similar CSF findings maybe seen in asymptomatic HIV persons. Therefore, CSF isnot helpful in making the diagnosis but remains impor-tant in excluding the above noted infections. The spinalMRI may be normal, or it may show spinal atrophy orpatchy abnormalities on T2-weighted images. Somato-sensory evoked potentials (SEP) help to confirmmyelop-athy, whereas nerve conduction studies can detectcoexistent neuropathy if present.
TREATMENT
There is no effective therapy that will reverse or elimi-nate HIV-associated vacuolarmyelopathy. Symptomatictreatment with antispasticity agents such as baclofen,tizanidine, or botulinum toxin can relieve spasms. Rou-tine care such asmaintenance of skin integrity or preven-tion of urinary retention is essential. The role ofantiretroviral therapy in improving VM has not beenestablished (Geraci and Di Rocco, 2000). Trials withmethionine supplements and corticosteroids have notshown benefit (Di Rocco et al., 2004). Trials with intra-venous immunoglobulin (IVIG) are ongoing.
NEUROMUSCULAR DISORDERS
Peripheral nervous system disorders causing pain, sen-sory disturbance, or motor weakness occur commonlyin persons with HIV infection. They may be caused byHIV itself or may occur secondary to opportunistic infec-tions or certain antiretroviral drugs. These disorders arefurther classified into four categories: (1) distal symmetricpolyneuropathy (DSP), (2) inflammatory demyelinatingpolyneuropathy (IDP), (3) mononeuropathy and mono-neuropathy multiplex, and (4) radiculopathies.
Distal symmetric polyneuropathy
Distal symmetric polyneuropathy (DSP) is themost com-mon form of neuropathy seen in HIV-infected patients.Although it can develop at any stage of HIV disease it ismost commonly observed in advanced stages with
1332 M. BILGRAMI
immunosuppression. Pathogenesis is complex, multifac-torial and incompletely understood. DSP may be associ-ated with other primary HIV-related neurologicdisorders such as dementia or vacuolar myelopathy.Dideoxynucleoside reverse transcriptase inhibitors, suchas stavudine and didanosine, are also implicated ascauses of DSP. Once the mainstay of treatment, stavu-dine and didanosine are no longer recommended aspreferred agents for the treatment of HIV infection(DHHS HIV Treatment Guidelines, 2012). Attributedto advanced immunosuppression in the pre-HAARTera, DSP is now observed in patients with controlledinfection and intact immune function suggesting thatother causes have assumed a more significant role(Evans et al., 2011). In a study of 2141 patients whostarted antiretroviral therapy and were followed for atleast 3 years between 2000 and 2007, the AIDS ClinicalTrials Group demonstrated rates of peripheral neuropa-thy and symptomatic peripheral neuropathy of 32% and8.6% respectively. Some 87% of the patients in the studyhad HIV RNA levels less than 400 and 70% had CD4counts over 350 cells/mm3 (Evans et al., 2011). Risk fac-tors for symptomatic peripheral neuropathy were olderage, diabetes, and use of neurotoxic antiretrovirals includ-ing stavudine, didanosine, zalcitibine, and nevirapine.
D P. O’KEEFE
CLINICAL FEATURES
Symptoms are primarily sensory, beginning in the feet.Specific symptoms include symmetric numbness, tight-ness, pain, burning, and hyperalgesia. With progression,symptoms proceed proximally to involve ankles, calves,and finally the hands. Motor weakness is not seen. Phys-ical examination demonstrates decreased vibratory andtemperature sensation with either decreased or hyperal-gesic pin-prick sensation. Deep tendon reflexes aredecreased at the ankles compared to the knees.
DIAGNOSIS
Diagnosis is clinical, but electrophysiologic testing suchas nerve conduction studies and electromyography canhelp to confirm the diagnosis. Clinical signs and electro-physiologic evidence of DSP occur in the absence ofsymptoms in 25% of cases (Skopelitis et al., 2006).
TREATMENT
Implicated reverse transcriptase inhibitors (stavudine,didanosine, and zalcitabine) should be stopped and alter-native antiretrovirals substituted. Symptoms may persistfor several weeks ormayworsen after stopping the drugs.Otherwise, treatment is symptomatic aiming to reduceneuropathic pain. Nonsteroidal anti-inflammatory agents
N
have been tried, but some patients require opioids. Gaba-pentin can be used, and it showed benefit in one placebocontrolled trial (Hahn et al., 2004). Lamotrigine 25 mgtwice daily increasing to 300 mg/day was found to beeffective in clinical trials (Simpson et al., 2000). Pregaba-lin can also be used, though only US Food and DrugAdministration (FDA) approved for diabetic neuropathy(Simpson et al., 2010). Antidepressants including nortrip-tyline, amytriptyline, imipramine, desipramine, andduloxetine are also used with benefit in some patients.Topical agents such as lidocaine and capsaicin have beentried but were not effective in clinical trials (Simpsonet al., 2008).
NEUROLOGIC DISEASES I
Inflammatory demyelinatingpolyneuropathy
Inflammatory demyelinating polyneuropathy (IDP) is anuncommon form of neuropathy seen in HIV-infectedpersons. It occurs in two forms: acute inflammatorydemyelinating polyneuropathy (AIDP) or Guillain–Barre syndrome (GBS), and chronic inflammatorydemyelinating polyneuropathy (CIDP).
Acute inflammatory demyelinating polyneuropathyAIDP/GBS occurs early in the course of HIV infectionparticularly at the time of seroconversion, either as a partof the acute retroviral syndrome or in otherwise asymp-tomatic persons (Piette et al., 1986). Its incidence isunknown. Pathogenesis is complex and the role of HIVin the pathogenesis is not completely understood. It is pos-tulated thatHIV can trigger an immune-mediated or auto-immune process resulting in demyelination. Antibodiesdirected against peripheral nerve myelin have beendescribed (Petratos et al., 1998). Clinically it manifestsas rapid, symmetric, ascendingmotor weakness with gen-eralized areflexia and relative sparing of sensation. Facialweakness and ophthalmoparesis can also be seen(Cornblath et al., 1987). Symptoms usually peak at 4weeksafter onset. Serious complications include respiratorycompromise and autonomic dysfunction (Cornblathet al., 1987).
Chronic inflammatory demyelinating polyneuropa-thy/CIDP can develop in early or in late stages of HIVinfection. If symptoms and signs ofAIDP persist beyond8 weeks the condition is called chronic inflammatorydemyelinating polyneuropathy. The course can bemono-phasic or relapsing.
Diagnosis of AIDP is based on clinical presentation,CSF analysis, and electrophysiologic studies. The CSFtypically shows an elevated protein andmild lymphocyticpleocytosis, usually 10–50 cells/mm3. In non-HIV-infected patients with AIDP or CIDP, the CSF is usuallywithout cells. Nerve conduction studies show a conduc-tion block with decreased nerve conduction velocitydue to demyelination. Electromyography shows reduced
action potential amplitudes in affected muscles (Alportand Sander, 2012).
TREATMENT
Acute inflammatory demyelinating polyneuropathyis treated with IVIG 400 mg/kg/day for 5 days. Plasmaexchange is also used (Kiprov et al., 1988). Chronicinflammatory demyelinating polyneuropathy is alsotreated with IVIG for 5 days. This may be followedup with maintenance IVIG every 3 weeks dependingon the patient response (Malamut et al., 1992).Plasma exchange or prednisone 1 mg/kg/day,continued until therapeutic response, may also be tried(Leger et al., 1989).
Mononeuropathy andmononeuropathy multiplex
Mononeuropathies are rarely seen in HIV-infected per-sons. They can be seen involving either cranial or periph-eral nerves. The cause of these mononeuropathies is notalways clear, but HIV itself, other infections, immuno-logic processes or compression from external structureshave all been implicated (Verma et al., 2004). A commonearly presentation is unilateral or bilateral facial nervepalsy which is most commonly seen in acute HIV infec-tion (Wechsler andHo, 1989). In late stages ofHIV infec-tion, mononeuropathies are caused by other infectionsor processes such as varicella zoster virus (VZV) infec-tion, syphilis, tuberculosis, or meningeal lymphoma(Verma et al., 2004). Examples of clinical presentationsinclude wrist drop, foot drop, sensorineural hearing loss,and diaphragmatic paralysis (Piliero et al., 2004). Diag-nosis is based on clinical presentation and electrophysi-ologic testing. Regardless of HIV status, treatment ofmononeuropathies is the same. Corticosteroids may beeffective, but antivirals have not been shown to providebenefit (Sullivan et al., 2007).
Mononeuritis multiplex is classically associated withvasculitis. It is rare but when seen in HIV-infected per-sons it occurs either in early stages, due to immunologicfactors, or in late stages, due to infections such as CMV,hepatitis B, hepatitis C, or VZV (Verma et al., 2004).Mononeuritis multiplex develops slowly, typicallyinvolving sensory, motor, and autonomic functions. Itmost commonly presents as a painful, asymmetric poly-neuropathy affecting multiple nerves in a stepwise fash-ion (Verma et al., 2004). Diagnosis is established by theclinical presentation and electrophysiologic testing. Inpatients with low CD4 counts, CMV should be ruledout with CSF CMV PCR or nerve biopsy. If CMV isdocumented or in patients with CD4 counts less than200 cells/mm3 where CMV is likely, either directed orempiric treatment for CMV infection (see section in thischapter on CMV infection) with ganciclovir should be
HIV-INFECTED PATIENTS 1333
AN
started (Kaplan et al., 2009). In patients with CD4 countsover 200 cells/mm3 supportive treatment is recom-mended for this often self-limited condition. If anerve biopsy discloses vasculitis, treatment with IVIG,plasma exchange, or corticosteroids is recommended(Brew, 2001).
1334 M. BILGRAMI
Radiculopathies
PROGRESSIVE POLYRADICULOPATHY
Polyradiculopathy is defined as inflammation and necro-sis of nerve roots at the site of exit from the spinal cord.Progressive polyradiculopathy typically affects lumbo-sacral nerve roots and is characterized by radicular painand sensorimotor deficits. In the pre-HAART era, CMVwas the most common cause of progressive polyradicu-lopathy. It was typically seen in patients with advancedimmunosuppression (CD4 counts less than 50 cells/mm3) and evidence of CMV at other sites. In one report,CMV radiculopathy was found in 2% of patients withAIDS (Gans et al., 1990). Since the introduction of anti-retrovirals the incidence of radiculopathy has decreased.Other causes of progressive polyradiculopathy areherpes simplex virus, varicella zoster virus, syphilis,tuberculous meningitis, and lymphomatous meningitis.The classic clinical presentation is that of a rapidly evolv-ing cauda equina syndrome with weakness and numb-ness in the lower extremities, bowel and bladdersphincter dysfunction, radicular pain in the lowerextremities in a cauda equina distribution, and saddleanesthesia (perineal area). Involvement of upper extrem-ities and cranial nerves may occur in later stages. Promptdiagnosis is imperative in order to avoid irreversiblenerve root necrosis. Lumbar puncture must be per-formed and CSF sent to rule out other etiologies by test-ing for CMV, HSV, and VZV by PCR, RPR, AFBculture, and cytology. The CSF in CMV polyradiculitisis characterized by a neutrophilic pleocytosis with ele-vated protein, normal to low glucose, and positivePCR. Viral cultures may not be helpful. Nerve conduc-tion studies and electromyography will show multilevelnerve root involvement. MRI should be done to ruleout compressive or space-occupying lesions of the caudaequina or lower thoracic spinal cord. On the MRI onemay see thickening or enhancement of lumbosacralnerve roots. While awaiting results treatment shouldbe started promptly as CMV polyradiculitis can be fatal.Combination therapy with intravenous ganciclovir plusfoscarnet is recommended. Clinical trials with CMVpolyradiculitis have not been done. Therefore benefitsof single versus combination therapy have not beenestablished and the optimal duration of therapy is notknown. Antiretrovirals should be initiated or optimizedseveral weeks after starting anti-CMV therapy to
prevent immune reconstitution (French et al., 2000).The neuropathies and radiculopathies associated withHIV infection are summarized in Table 90.6.
D P. O’KEEFE
NEUROSYPHILIS
Epidemiology and clinical manifestations
Neurosyphilis refers to infection of the central nervoussystem caused by Treponema pallidum. It is a sexuallytransmitted infection in which the bacteria enter the bodythrough skin abrasions or mucous membranes. Data areconflicting regarding the incidence, symptoms, severity,and treatment response in HIV-infected patients (Rolfset al., 1997; Collis and Celum, 2001). HIV-infectedpatients with syphilis may present with atypical andmoreaggressive disease. Serologically they respond less wellto therapy when compared to HIV-negative patients(Kingston et al., 2005). Based on small case series, theincidence of neurosyphilis was about 9% before intro-duction of HAART. Data on the incidence afterHAART are lacking. Syphilis is classified as early,including primary and secondary stages, and late, whichincludes latent and tertiary stages. Classically, neurosy-philis is a late manifestation, but neurologic complica-tions of early secondary syphilis including meningitisand stroke are seen more frequently in HIV-infectedpatients (Marra et al., 2004). The stages of neurosyphilisand associated clinical syndromes are depicted inTables 90.7 and 90.8.
DIAGNOSIS
Diagnosis of neurosyphilis is based on clinical presenta-tion as well as CSF findings. Lymphocytic pleocytosis(WBC greater than 5/mm3) and elevated protein arethe most common CSF abnormalities seen with neurosy-philis. T. pallidum cannot be cultured so the fluorescenttreponemal antibody absorption test (FTA-ABS) andtreponema particle agglutination tests (TPPA) andnon-treponemal rapid plasma reagin (RPR) and Vene-real Disease Research Laboratory (VDRL) serologictests are used. The nontreponemal tests are quantitativeand are used for screening and for post-treatmentfollow-up. Treponemal tests are used to confirm thescreening nontreponemal test. They are also occasionallyused for diagnosis of neurosyphilis when the nontrepo-nemal CSF test is negative in a patient with symptomssuggestive of neurosyphilis. VDRL in CSF is specificbut less sensitive whereas the CSF FTA-ABS test is verysensitive but less specific. Because false-negative CSFVDRL tests are not uncommon and pleocytosis andelevated protein are seen in many other conditions, diag-nosis of neurosyphilis can be difficult. The indicationsfor lumbar puncture in HIV-infected patients with
Table 90.6
Neuropathies and radiculopathies associated with HIV infection
Disease Symptom progressionEtiology andpathogenesis Classic presentation Management
Distal symmetricpolyneuropathy
Months Unknown, watch forneurotoxicantiretrovirals
Symmetric pain, numbness, burningof toes/feet
No weakness
Absent/decreased ankle reflex
NCV/EMG: axonal neuropathySymptomatic (analgesics, anticonvulsants,antidepressants)
Inflammatorydemyelinating
polyneuropathy
Acute: peak in 4 weeksChronic: >8 weeks
Immunologic andautoimmune process
Rapid ascending weakness withgeneralized areflexia, relative sparing
of sensation
NCV/EMG: demyelinating polyneuropathyCSF: pleocytosis, increased protein, cultures
negativeIVIG, plasma exchange
Mononeuritismultiplex
Variable, over weeks tomonths
Rule out CMV andvasculitis
Asymmetric motor and sensory deficits NCV/EMG: asymmetric multifocal defectsRule out CMV (CSF/nerve biopsy)
Treat for CMV if CD4 count<50Progressive
polyradiculopathyDays to weeks Rule out CMV Rapid weakness and numbness in legs,
perineal area with reduced or absent
knee and ankle reflexes
NCV/EMG: multilevel nerve root involvement.CSF to rule out CMV. Ganciclovir�foscarnet
for CMV polyradiculitis
CMV, cytomegalovirus; CSF, cerebrospinal fluid; NCV, nerve conduction velocities; EMG, electromyogram; IVIG, intravenous immunoglobulin.
Table 90.7
Stages of neurosyphilis
Neurosyphilis stage Presentation Sites affected in CNS Clinical syndrome
Early stage Within weeks to years butcommon< 1 year afterinitial infection
CSFMeningesVasculature
Asymptomatic meningitisSymptomatic meningitisCranial neuropathies
StrokeGummas (mass lesions)Optic disease
Late stage Years to decades after
initial infection
Brain
Spinal cord
General paresis of insane
Tabes dorsalis (posteriorcolumn)
CNS, central nervous system; CSF, cerebrospinal fluid.
Table 90.8
Clinical features and laboratory findings in neurosyphilis
Early stage neurosyphilis Clinical features
CSF: cell count (lymphocytes), protein,
VDRL
Asymptomatic meningitis Absent WBC< 100, protein< 100, � VDRL
Symptomatic meningitisCranial neuropathiesOcular disease
Headache, confusion and meningealsigns, hydrocephalus, seizures,syphilitic gumma as mass lesions
Optic, facial, auditory nerves arecommonly involved
Posterior uveitis, chorioretinits, opticneuritis, etc.
WBC 200–400, protein 100–200, VDRLpositive
Meningovascular disease/stroke Ischemic stroke in young person(thrombosis, ischemia, infarction ofcerebral vessel)
WBC< 100, protein 100–200, � VDRL
Late stage neurosyphilisBrain parenchyma General paresis (dementia, personality
changes)CSF: WBC< 100, protein< 100,generallyþVDRL
Imaging: cerebral atrophySpinal cord parenchyma Tabes dorsalis (ataxia, lancinating pains,
papillary abnormalitiesCSF may be normal or show mildlymphocytic pleocytois and mild
increased protein, 25% of cases willbe VDRL negative
VDRL, Venereal Diseases Research Laboratory test; CSF, cerebrospinal fluid; WBC, white blood cell count.
1336 M. BILGRAMI AND P. O’KEEFE
syphilis are neurologic, ocular, or auditory signs andsymptoms, evidence of tertiary syphilis (aortitis orgumma), and treatment failure. Treatment failureincludes continued presence of signs or symptoms, ora fourfold rise in serum RPR or a failure of the serumRPR to decrease by fourfold within 2 years of treatment.Some studies have shown that development of neurosy-philis is more likely in HIV patients if the CD4 count is
less than 350/mm3 or if the RPR titer exceeds 1:32(Ghanem et al., 2009).
TREATMENT
Parenteral penicillin is the drug of choice for treatmentof neurosyphilis. Recommended treatments from theCDC are shown in Table 90.9. According to the CDC
Table 90.9
Treatment of neurosyphilis
Disease Preferred therapy Alternate therapy
NeurosyphilisSome experts recommend benzathinebenzylpenicillin (penicillin G) 2.4million units IM weekly for 3 weeksafter completion of recommended IVtherapy
Aqueous crystalline benzylpenicillin18–24 million units IV daily individed doses given every 4 hours, or
given by continuous infusion for10–14 days
Procaine benzylpenicillin (penicillin G)2.4 million units IM QD for 10–14daysþprobenecid 500 mg PO QID
for 10–14 daysorCeftriaxone 2 g IV/IM QD for 10–14days*
*Limited data on ceftriaxone in neurosyphilis.
(2010 CDC STD Treatment Guidelines; Workowski and Berman, 2010)
IM, itramuscular; IV, intravenous; PO, oral; qd, daily; qid, four times a day.
NEUROLOGIC DISEASES IN HIV-INFECTED PATIENTS 1337
guidelines, CSF protein and glucose should be normal by2 years. The cell count should show evidence of declineby 6 months.
TUBERCULOSISOF THE CENTRALNERVOUS SYSTEM
Both in immunocompetent and immunocompromisedindividuals, Mycobacterium tuberculosis occurs withinthe central nervous system either by reactivation or bydissemination, usually from a primary focus in thelungs. Infections with HIV and M. tuberculosis tend tooccur with greater frequency in areas of the world wheretuberculosis is endemic. Some reports describing themanifestations of CNS tuberculosis in patients withand without HIV coinfection do not describe differ-ences, whereas other reports describe distinct clinical,pathologic, and radiographic features in HIV-infectedpersons (Berenguer et al., 1992; Katrak et al., 2000).CNS tuberculosis generally manifests in advanced HIVdisease with CD4 counts less than 200/mm3, but thereare reports of the disease in patients with higher CD4counts as well.
PATHOGENESIS
Mycobacterium tuberculosis protein antigens spill intothe subarachnoid space and cause intense inflammatoryresponses. This commonly occurs at the base of the brainleading to proliferative arachnoiditis and vasculitis.Hydrocephalus develops if the inflammatory exudateextends to the basilar cisterns. Varied clinical manifesta-tions are seen depending on the location and extent ofthese changes.
CLINICAL FEATURES
Clinical manifestations include meningitis, stroke, cere-bral abscess, tuberculoma, or involvement of the spinal
cord. The most common signs of TB meningitis areheadache and low grade fever. Meningitis is progressivewith development of personality changes, followed bymeningismus, lethargy, cranial nerve palsies, and hemi-paresis. Late findings include delirium, stupor, coma,multiple cranial nerve palsies, and hemiplegia. Strokesresult from vasospasm and thrombosis and typicallyinvolve the basal ganglia. Cerebral abscess is character-ized by fever, headache, delirium, cranial nerve palsies,seizures, and hemiparesis. Tuberculomas may or maynot have clinical manifestations. When present, clinicalmanifestations consist of focal symptoms and signs ofan intracranial space-occupying mass lesion generallywithout systemic illness ormeningeal inflammation. Spi-nal tuberculosis results in arachnoiditis and presents asmeningitis or cord compression (Rock et al., 2008).
DIAGNOSIS
Diagnosis of CNS tuberculosis is difficult and challeng-ing. With high clinical suspicion antituberculosis therapyshould be started empirically while waiting for CSF cul-ture results. Delay in diagnosis is associated with highmortality. The CSF typically shows a lymphocytic pleo-cytosis with elevated protein and low glucose. A positiveculture forMycobacterium tuberculosis is the gold stan-dard for diagnosis but takes 2–8 weeks. In addition theculture is not uniformly sensitive and, in patients inwhom there is high clinical suspicion, some experts rec-ommend a minimum of three lumbar punctures per-formed daily in order to improve the yield. Acid-fastsmears from CSF are positive in only 25% of cases.PCR can be used to detect DNA of M. tuberculosis inAFB smear negative cases. The PCR is 56% sensitiveand 98% specific (Pai et al., 2003). Therefore, when pos-itive the test is very helpful in the diagnosis but, if neg-ative, it does not rule out tuberculous meningitis. The
Table 90.11
Initiation of HAART in the setting of HIV/TB coinfection
CD4 count in HIV patient withTB
Timing of initiation ofHAART after starting
TB therapy
CD4 count<200 cells/mm3 Within 2–4 weeks
CD4 count 200–500 cells/mm3 2–4 weeks or at least8 weeks
CD4 count>500 cells/mm3 Within 8 weeks
(DHHS HIV Management Guidelines 2012)
HAART, highly active antiretroviral therapy; HIV, human immunode-
ficiency virus; TB, tuberculosis.
AND P. O’KEEFE
chest X-ray is abnormal in about 50% of HIV-infectedpatients with CNS tuberculosis.
NEUROIMAGING
Because of its greater sensitivity in detecting meningealenhancement, MRI is preferred over CT. Other MRIfindings with nervous system tuberculosis include small,enhancing lesions in the basal ganglia, brainstem, andspinal cord (Offenbacher et al., 1991). Tuberculomasappear as small, enhancing, space-occupying lesionswithout significant mass effect, whereas tuberculouscerebral abscesses are larger ring-enhancing lesions.
TREATMENT
Treatment, depicted in Table 90.10, combines recom-mendations from the American and British ThoracicSocieties, the CDC and the Infectious Diseases Societyof America (ATS/CDC/IDSA Treatment Guidelinesfor Tuberculosis, 2003; Blumberg et al., 2003). Treat-ment should be initiated whenever there is strong clinicalsuspicion of CNS tuberculosis and should not be delayeduntil the infection is proven. It consists of an initial2 month period of intensive therapy using four drugswhich is followed by a prolonged continuation phaseusually with two drugs lasting 7–10 months dependingon clinical response and the established drug sensitivityof the isolate. Duration of therapy should be extended to18 months in patients with tuberculoma whereas 18–24months is recommended for drug-resistant organisms.Corticosteroids during the early phase of treatment oftuberculous meningitis have been shown to improve
1338 M. BILGRAMI
Table 90.10
Treatment of CNS tuberculosis
Initial phase
Use 4 drugs for 2 months
Isoniazid, rifampicin,
ethambutol andpyrizinamide
Continuation phase
Use 2 drugs for 7–10 monthsIsoniazid with rifampicin(only if susceptible)
Duration
Drug-susceptible TBDrug-resistant TB (modify
regimen accordingly)Tuberculoma
9–12 months dependingon response
18–24 months
18 months
Corticosteroids
Dexamethasone, orPrednisone
0.3–0.4 mg/kg, taper over
6–8 weeks1 mg/kg for 3 weeks andtaper over 3–5 weeks
(2003 ATS/CDC/IDSA Guidelines; Blumberg et al., 2003)
CNS, central nervous system; TB, tunerculosis.
outcomes and decrease mortality (Thwaites et al.,2004). Factors associated with poor prognosis of CNSTB in HIV-infected patients include more severe illnessat presentation, CD4 count less than 50/mm3, and thepresence of multidrug-resistant strains (Thwaiteset al., 2005; Cecchini et al., 2007).
Initiation of HAART in patients with CNS tuberculo-sis may be associated with worsening due to the frequentoccurrence of the immune reconstitution inflammatorysyndrome (IRIS). With CNS tuberculosis, IRIS mani-fests as worsening of meningeal symptoms or expand-ing/new intracranial lesions (Lawn et al., 2005). TheDepartment of Health and Human Services has pub-lished recommendations concerning timing of initiationof HAART in patients who are being treated for activetuberculosis (Table 90.11).
If an HIV patient on HAART develops CNS tubercu-losis,HAARTshouldbe continuedbut the dosesmodifiedin order to avoid drug–drug interactions. Interactionsbetween rifampicin and the protease inhibitors arecommon, most often resulting in increased levels ofrifampicin due to a decrease in its rate of metabolism.Levels of the protease inhibitors are decreased due toaccelerated metabolism by rifampicin. Rifabutin isassociated with reduced interactions and is often used inplace of rifampicin in patients receiving protease inhibi-tors (Burman et al., 1999).
HIV-ASSOCIATEDNEUROCOGNITIVEDISORDER
HIV-associated neurocognitive disorder (HAND) is theresult of neural damage caused by HIV replicationand immune activation. HIV entry into the CNS and pro-posed mechanisms by which HIV promotes inflamma-tion have been discussed in the introductory section of
Table 90.12
Frascati classification of HIV-associated neurocognitive disorders
HIV-associated neurocognitivedisorder (HAND) subgroup Neuropsychological test Daily functions
Asymptomatic neurocognitiveimpairment (ANI)
Mild impairment in test No impairment in daily functions
Mild neurocognitive disorder (MND) Mild impairment in test Mild functional impairmentHIV-associated dementia (HAD) Marked cognitive impairment Marked functional impairment
(Antinori et al., 2007)
NEUROLOGIC DISEASES IN HIV-INFECTED PATIENTS 1339
this chapter. Frascati has classified HAND into the threesubgroups listed in Table 90.12.
PREVALENCE
In the pre-HAART era, HIV-associated dementia(HAD) was seen in approximately 7% of persons withHIV/AIDS. The condition was seen with CD4þ cellcounts of less than 200 cells/mm3 and was characterizedby rapid progression to death, usually within 6 months.With the introduction of antiretroviral drugs and thenHAART, there have been changes in the epidemiologyof HAD. Data from the CASCADE cohort (ConcertedAction on Seroconversion to AIDS andDeath in Europe)described a decline in the incidence of HAD from20–30% to 10–15% in patients with advanced HIV dis-ease (Bhaskaran et al., 2008). In the Multicenter AIDSCohort study the incidence of HAD declined by 50%between the 1990–1992period and the 1996–1998period–again attributed to the introduction of antiretrovirals(Sacktor, 2002). The prevalence of severe HAD hasclearly decreased but the prevalence of mild to moderateHAND has not changed during this same time period.
Risk factors associated with development of HANDare as follows: low CD4 count, high HIV RNA levels inserum and CSF, older age at seroconversion, duration ofHIV infection, anemia, pre-existing neurocognitive dys-function, coexisting hepatitis C, and female sex (Sternet al., 2001).
In the pre-HAART era higher CSF to serum ratios ofHIV RNA levels correlated with the severity of cognitiveimpairment (Cinque et al., 1998) but after the introduc-tion of HAART the correlation was no longer seen.Whereas in the pre-HAART era, HAD was driven byHIV, in the post-HAART era the driver for HAND isnot as clear. Comorbid conditions including syphilis,CMV infection, hepatitis, drugs, trauma, or psychiatricconditions and their treatment have been proposed toplay a role. Another possible cause is the virus itself.
HIV replication in the CNS occurs independent of repli-cation in plasma. CNS replication may generate resis-tance mutations even with suppression of viralreplication in the serum. Measurement of HIV replica-tion in CSF may be indicated when a new neurologicproblem occurs. However, the finding of discordant rep-lication is rare and does not explain the frequency withwhich HAND is encountered. Inflammation, as identi-fied by elevated levels of inflammatory markers in theCSF or by visualization of active microglial cells(Garvey, 2012), despite control of viral replication hasalso been proposed. Other suggested and studied possi-ble causes include aging and vascular disease.
Mild neurocognitive disorder and depression are themost accurate predictors of severity of HAD (Sternet al., 2001). Progression of neurocognitive disorders isvariable; patients may have mild dysfunction over a longperiod of time or rapid progression with severe impair-ment. Studies done by Letendre (2004, 2010) showed thatpoor CNS penetration of antiretroviral regimens is asso-ciated with ongoing viral replication. This lower penetra-tion is associated with increased risk of HAD although ithas yet to be proven that use of antiretroviral agents withbetter CNS penetration leads to decreased risk of HAD.
CLINICAL FEATURES
Mild neurocognitive disorder is subtle and often not rec-ognized without testing. HAD is a subcortical dementiacharacterized by decreased concentration,motor slowing,and behavioral changes. Some of the subtle early, as wellas the overt late symptoms are listed in Table 90.13.
DIAGNOSIS
Diagnosis of HIV neurocognitive disorder is based onhistory, physical examination, and neuropsychologicaltests to assess neurocognitive function. Common screen-ing tests include the HIV dementia scale (Diesing et al.,2002); the International HIV dementia scale (naming
Table 90.13
Symptoms of HIV-associated dementia
Early symptoms
Subtle
Memory loss, unsteady gait,
limb weakness, tremor,apathy, depression,anhedonia
Late symptoms
Marked
Global dementia,
bradyphrenia,bradykinesia, saccadic eyemovements,
hyperreflexia,dysdiadokinesis, frontalrelease signs
(Antinori et al., 2007)
1340 M. BILGRAMI AN
four objects, fingertapping, “Laura” psychomotor learn-ing task (learning a sequence of hand positions), andrecall of names (Berger and Brew, 2005; Sacktoret al., 2005); and the Montreal cognitive assessment(MoCA©), which takes 5–10minutes to perform in clinic(©Z. Nasreddine 2003-2012. www.mocatest.org). Neuro-imaging, preferably with MRI, should be done to ruleout other etiologies. Common MRI findings withHAD are cerebral atrophy with diffuse periventricularwhite matter abnormalities.
TREATMENT
HAART is the mainstay of treatment for HAND as itreduces viral load both in peripheral circulation and
Table 90.14
Antiretoviral agents and CNS penetration effectiveness scores
Drug class4High penetration
3Intermedpenetrati
Nucleoside reversetranscriptaseinhibitors
Zidovudine AbacavirEmtricita
Non-nucleoside reversetranscriptaseinhibitors (NNRTI)
Nevirapine DelavirdiEfavirenz
Protease inhibitors Indinavir/r DarunaviFosempreLopinavir
Entry/fusion inhibitors MaraviroIntegrase inhibitors Raltegrav
(Reproduced with permission from IAS–USA; Letendre et al., 2010.)
r denotes Ritonavir.
the CNS thereby preventing widespread CNS HIVinfection. There is debate over which agents are the mosteffective. In the pre-HAART era zidovudine was themost effective antiretroviral for reducing impairmentscaused by HAD (Sidtis et al., 1993). Subsequentlyother drugs such as protease inhibitors have beenshown to be good options as well. Recent work byLetendre has shown that antiretrovirals with betterCNS penetration are associated with lower CSF viralloads. He also developed the central nervous systempenetration effectiveness scores (CPE scores) forindividual antiretroviral drugs (Table 90.14). Using a1 to 4 scale, the drugs in column 4 have the highestpenetration. Adding the scores of all the drugs in a mul-tiple drug regimen, a score greater than 7 was associatedwith CSF viral loads less than 2 copies/mL. This scoringsystem has been criticized as being simplistic and notproven. It needs to be evaluated prospectively to berecommended (Letendre et al., 2010).
Many patients with HAND have associated psychiat-ric disorders, most commonly depression. These disor-ders should be appropriately treated.
In summary, cognitive function may be impaired intreated HIV patients. Optimizing management byavoiding low CD4þ counts, maintaining undetectableHIV RNA levels, minimizing chronic immune activa-tion, and optimizing cerebral perfusion are allimportant goals. Finally, a healthy lifestyle, along withHIV control, should enhance neurologic function.(Clifford, 2012).
D P. O’KEEFE
iateon
2Intermediatepenetration
1Low penetration
bineDidanosineLamivudineStavudine
TenofovirZalcitabine
ne Etravirine
r/rnavir/r/r
AtazanavirAtazanavir/rFosamprenavir
c Enfuvirtideir
NEUROLOGIC DISEASES IN HIV-INFECTED PATIENTS 1341
REFERENCES
Albright A, Soldan S, Gonzalez-Scarano F et al. (2003).
Pathogenesis of human immunodeficiency virus induced
neurological disease. J Neurovirol, 9: 222–227.
Antinori A, Ammassari A, De Luca A et al. (1997). Diagnosis
of AIDS-related focal brain lesions: a decision-making
analysis based on clinical and neuroradiologic characteris-
tics combined with polymerase chain reaction assays in
CSF. Neurology 48: 687–694.Alport A, Sander H (2012). Clinical approach to peripheral
neuropathy. Anatomic localization and diagnostic testing.
Continuum Lifelong Learning Neurol 18: 13–38.Antinori A, Arendt G, Becker JT et al. (2007). Updated
research nosology for HIV-associated neurocognitive dis-
orders. Neurology 69: 1789–1799.Batungwanayo J,TaelmanH,Bogaerts J et al. (1994). Pulmonary
cryptococcosis associatedwithHIV-1 infection inRwanda: a
retrospective study of 37 cases. AIDS 8: 1271–1276.Berger J, Brew B (2005). An international screening tool for
HIV dementia. AIDS 19: 2165–2166.Berenguer J, Moreno S, Laguna F et al. (1992). Tuberculous
meningitis in patients infected with the human immunode-
ficiency virus. N Engl J Med 326: 668.Bhaskaran K, Mussini C, Antinori A et al. (2008). Changes in
the incidence and predictors of human immunodeficiency
virus-associated dementia in the era of highly active antire-
troviral therapy. Ann Neurol 63: 213–221.
Bicanic T, Meintjes G, Wood R et al. (2007). Fungal burden,
early fungicidal activity, and outcome in cryptococcal
meningitis in antiretroviral-naive or antiretroviral-
experienced patients treatedwith amphotericin B or flucon-
azole. Clin Infect Dis 45: 76–80.Blumberg H, Burman W, Chaisson R et al. (2003). American
Thoracic Society/Centers for Disease Control and
Prevention/InfectiousDiseasesSocietyofAmerica: treatment
of tuberculosis. Am J Respir Crit Care Med 167: 603–662.Boulware D, Meya D, Bergemann T et al. (2010). Clinical fea-
tures and serum biomarkers in HIV immune reconstitution
inflammatory syndrome after cryptococcal meningitis: a
prospective cohort study. PLoS Med 7: e1000384.
Brack-Werner R (1999). Astrocytes: HIV cellular reservoirs
and important participants in neuropathogenesis. AIDS
131: 1–22.Brew B (2001). AIDS dementia complex. In: HIV Neurology,
Contemporary Neurology Series, Vol. 61. OxfordUniversity Press, New York, pp. 62–81.
Brouwer A, Rajanuwong A, Chierakul W et al. (2004).
Combination antifungal therapies for HIV-associated cryp-
tococcal meningitis: a randomized trial. Lancet 363:1764–1767.
Buchacz K, Baker R, Palella F Jr et al. (2010). AIDS-defining
opportunistic illnesses in US patients, 1994–2007. AIDS
24: 1549–1559.
Burman W, Gallicano K, Peloquin C (1999). Therapeutic
implications of drug interactions in the treatment of human
immunodeficiency virus-related tuberculosis. Clin Infect
Dis 28: 419–429.
Cecchini D, Ambrosioni J, Brezzo C et al. (2007). Tuberculous
meningitis in HIV-infected patients: drug susceptibility
and clinical outcome. AIDS 21: 373–374.Cinque P, Vago L, Ceresa D et al. (1998). Cerebrospinal fluid
HIV-1 RNA levels: correlation with HIV encephalitis.
AIDS 12: 389–394.Cinque P, Pierotti C, Vigano MG et al. (2001). The good and
evil of HAART in HIV-related progressive multifocal leu-
koencephalopathy. J Neurovirol 7: 358–363.Clifford D (2012). IAS-USA. Improving the Management of
HIV Disease. Chicago, IL, http://www.iasusa.org/cme/
2012-s/chicago/syllabus.
CollisT,CelumC(2001).Theclinicalmanifestations and treat-
ment of sexually transmitted diseases in human immunode-
ficiency virus-positive men. Clin Infect Dis 32: 611–622.Cornblath D, McArthur J, Kennedy P et al. (1987).
Inflammatory demyelinating peripheral neuropathies asso-
ciated with human T-cell lymphotropic virus type III infec-
tion. Ann Neurol 21: 32–40.Dal PanG,Glass J,McArthur J et al. (1994). Clinicopathologic
correlations of HIV associated vacuolar myelopathy.
Neurology 44: 2159.Darras-Joly C, Chevret S,WolffM et al. (1996).Cryptococcus
neoformans infection in France: epidemiologic features of
and early prognostic parameters for 76 patients who were
infected with human immunodeficiency virus. Clin Infect
Dis 23: 369–376.
Di Rocco A,Werner P, Bottiglieri T et al. (2004). Treatment of
AIDS-associated myelopathy with L-methionine.
Neurology 63: 1270–1275.
Diesing T, Swindells S, Gelbard H et al. (2002). HIV-1 asso-
ciated dementia: a basic science and clinical perspective.
AIDS Read 12: 358–368.
Evans S, Ellis R, ChenH et al. (2011). Peripheral neuropathy in
HIV: prevalence and risk factors. AIDS 25: 919–928.Falusi O, French A, Seaberg E et al. (2002). Prevalence and
predictors of Toxoplasma seropositivity in women with
and at risk for human immunodeficiency virus infection.
Clin Infect Dis 35: 1414–1417.Feldmesser M, Harris C, Reichberg S et al. (1996). Serum
cryptococcal antigen in patients with AIDS. Clin Infect
Dis 23: 827–830.Fine H, Mayer R (1993). Primary central system lymphoma.
Ann Intern Med 119: 1093–1104.Fischer L, Korfel A, Kiewe P et al. (2009). Systemic high dose
methotrexate plus ifosfamide is highly effective for central
nervous system involvement of lymphoma. Ann Hematol
88: 133–139.French M, Lenzo N, John M et al. (2000). Immune restoration
disease after the treatment of immunodeficient HIV-
infected patients with highly active antiretroviral therapy.
HIV Med 1: 107–115.Gans J, Tiessens G, Portegies P et al. (1990). Predominance of
polymorphonuclear leucocytes in cerebrospinal fluid of
AIDS patients with cytomegalovirus polyradiculomyelitis.
J AIDS 3: 1155–1158.
Gartner S, Liu Y (2002). Insights into the role of immune
activation in HIV neuropathogenesis. J Neurovirol 8: 69–75.
1342 M. BILGRAMI AND P. O’KEEFE
Garvey L, Pavese N, Politis M et al. (2012). Microglial
cell activation is visualized with [11C]-PK11195 posi-
tron emission tomography in neurologically asymptomatic
HIV-infected subjects on suppressive ART. 19th
Conference on Retroviruses and Opportunistic Infections,
Abstract #78LB.
Geraci AP, Di Rocco A (2000). Anti-HIV therapy. AIDS 14:
2059–2061.Ghanem K, Moore R, Rompalo A et al. (2009). Lumbar punc-
ture in HIV-infected patients with syphilis and no neuro-
logic symptoms. Clin Infect Dis 48: 816–821.Graybill J, Sobel J, SaagMet al. (2000).Diagnosis andmanage-
ment of increased intracranial pressure in patientswithAIDS
and cryptococcal meningitis. Clin Infect Dis 30: 47–54.
Haase A (1986). Pathogenesis of lentivirus infections. Nature
322: 130–136.Hahn K, Arendt G, Braun J et al. (2004). A placebo-controlled
trial of gabapentin for painful HIV-associated sensory neu-
ropathies. J Neurol 251: 1260–1266.HamillR,Sobel J,El-SadrWetal. (2010).Comparisonof2doses
of liposomal amphotericin B and conventional amphotericin
B deoxycholate for treatment of AIDS-associated acute cryp-
tococcal meningitis: a randomized, double-blind clinical trial
of efficacy and safety. Clin Infect Dis 51: 225–232.Jabs D, Van Natta M, Holbrook J et al. (2007). Longitudinal
study of the ocular complications of AIDS: 1. Ocular diag-
noses at enrollment. Ophthalmology 114: 780–786.
Johnson B, Fram E, Johnson P et al. (1997). The variable MR
appearance of primary lymphoma of the central nervous
system: comparison with histopathologic features. AJNR
Am J Neuroradiol 18: 563–572.Kaplan J, Handson D, Dworkin MS et al. (2000).
Epidemiology of human immunodeficiency virus-
associated opportunistic infections in the United States in
the era of highly active antiretroviral therapy. Clin Infect
Dis 30 (Suppl 1): S5–S14.
Kaplan J, Benson C, Holmes K et al. (2009). Guidelines for
prevention and treatment of opportunistic infections in
HIV-infected adults and adolescents: recommendations
from CDC, the National Institutes of Health, and the
HIV Medicine Association of the Infectious Diseases
Society of America. MMWR Recomm Rep 58: 1–207.Katrak S, Shembalkar P, Bijwe S et al. (2000). The clinical,
radiological and pathological profile of tuberculous menin-
gitis in patients with andwithout human immunodeficiency
virus infection. J Neurol Sci 181: 118–126.
Kingston A, Vujevich J, Shapiro M et al. (2005). Seronegative
secondary syphilis in 2 patients coinfected with human
immunodeficiency virus. Arch Dermatol 141: 431–433.Kiprov D, Pfaeffl W, Parry G et al. (1988). Antibody-mediated
peripheral neuropathiesassociatedwithARCandAIDS: suc-
cessful treatment with plasmapheresis. J Clin Apher 4: 3–7.Koralnik I, W€uthrich C, Dang X et al. (2005). JC virus granule
cell neuronopathy: a novel clinical syndrome distinct from
progressive multifocal leukoencephalopathy. Ann Neurol
57: 576–580.
Lawn S, Bekker L, Miller R (2005). Immune reconstitution
disease associated with mycobacterial infections in
HIV-infected individuals receiving antiretrovirals. Lancet
Infect Dis 5: 361–373.Leger J, Bouche P, Bolgert F et al. (1989). The spectrum of
polyneuropathies in patients infected with HIV. J Neurol
Neurosurg Psychiatry 52: 1369–1374.Letendre S, McCutchan J, Childers M et al. (2004). Enhancing
antiretroviral therapy for human immunodeficiency virus
cognitive disorders. Ann Neurol 56: 416–423.Letendre S, Ellis R, Ances B et al. (2010). Neurologic compli-
cations of HIV disease and their treatment. Top HIV Med
18: 45–55.LiW,GaleyD,MattsonMet al. (2005).Molecular and cellular
mechanisms of neuronal cell death in HIV dementia. AIDS
19: 1367.
Luft B, Remington J (1992). Toxoplasmic encephalitis in
AIDS. Clin Infect Dis 15: 211–222.Luft B, Hafner R, Korzun A et al. (1993). Toxoplasmic
encephalitis in patients with the acquired immunodefi-
ciency syndrome. Members of the ACTG 077p/ANRS
009 Study Team. N Engl J Med 329: 995–1000.
MacMahon E, Glass J, Hayward S et al. (1991). Epstein–Barr
virus in AIDS-related primary central nervous system lym-
phoma. Lancet 338: 969–973.
MacNealy M, Newton H, McGregor J et al. (2008). Primary
meningeal CNS lymphoma treated with intra-arterial che-
motherapy. J Neurooncol 90: 329–333.Malamut R, Leopold N, Chester P et al. (1992). The treatment
of HIV-associated chronic inflammatory demyelinating
polyneuropathy (HIV-CIDP) with intravenous immuno-
globulin (IVIG). Neurology 42: 335.
Marra C, Maxwell C, Smith S et al. (2004). Cerebrospinal
fluid abnormalities in patients with syphilis: association
with clinical and laboratory features. J Infect Dis 189:
369–376.Maschke M, Kastrup O, Esser S et al. (2000). Incidence and
prevalence of neurological disorders with HIV. J Neurol
Neurosurg Psychiatry 69: 376–380.Miro J, Murray H (2008). Toxoplasmosis. In: R Dolin,
H Masur, M Saag (Eds.), AIDS Therapy. 3rd edn.
Churchill Livingstone, Philadelphia, pp. 659–681.
Mirza S, PhelanM, Rimland D (2003). The changing epidemi-
ology of cryptococcosis: an update from population-based
active surveillance in 2 large metropolitan areas, 1992–
2000. Clin Infect Dis 36: 789–794.Montoya J, Liesenfeld O (2004). Toxoplasmosis. Lancet 363:
1965–1976.
Murakawa G, Kerschmann R, Berger T (1996). Cutaneous
Cryptococcus infection and AIDS. Report of 12 cases and
review of the literature. Arch Dermatol 132: 545–548.Nelson D, Martz K, Bonner H et al. (1992). Non-Hodgkin’s
lymphoma of the brain: can high dose, large volume radia-
tion therapy improve survival survival? Report on a
prospective trial by the Radiation Therapy Oncology
Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol
Phys 23: 9–17.Nogui F, Mattas S, Junior G et al. (2009). Neurotoxoplasmosis
diagnosis for HIV-1 patients by real-time PCR of cerebro-
spinal fluid. Braz J Infect Dis 13: 18.
NEUROLOGIC DISEASES IN HIV-INFECTED PATIENTS 1343
Offenbacher H, Fazekas F, Schmidt R et al. (1991). MRI
in tuberculous meningoencephalitis: report of four cases
and review of the neuroimaging literature. J Neurol 238:340.
Pai M, Flores L, Pai N et al. (2003). Diagnostic accuracy of
nucleic acid amplification tests for tuberculous meningitis:
a systematic review and meta-analysis. Lancet Infect Dis 3:
633–643.Palella F, Kathleen M, Moorman A et al. (1998). Declining
morbidity and mortality among patients with advanced
human immunodeficiency virus infection. N Engl J Med
338: 853–860.Park B, Wannemuehler K, Marston B et al. (2009).
Estimation of the current global burden of cryptococcal
meningitis among persons living with HIV/AIDS. AIDS
23: 525–530.Perfect J, Dismukes W, Dromer F et al. (2010). Clinical prac-
tice guidelines for the management of cryptococcal dis-
ease: 2010 update by the infectious diseases society of
America. Clin Infect Dis 50: 291–322.
Petito C, Navia B, Cho E et al. (1985). Vacuolar myelopathy
pathologically resembling subacute combined degenera-
tion in patients with the acquired immunodeficiency syn-
drome. N Engl J Med 312: 874–879.Petratos S, Turnbull V, Papadopoulos R et al. (1998).
Antibodies against peripheral myelin glycolipids in
people with HIV infection. Immunol Cell Biol 76:
535–541.Piette A, Tusseau F, Vignon D et al. (1986). Acute neuropathy
coincident with seroconversion for anti-LAV/HTLV-III.
Lancet 1: 852.Piliero P, Estanislao L, Simpson D (2004). Diaphragmatic
paralysis due to isolated phrenic neuropathy in an HIV-
infected man. Neurology 62: 154–155.Porter S, Sande M (1992). Toxoplasmosis of the central ner-
vous system in the acquired immunodeficiency syndrome.
N Engl J Med 327: 1643–1648.Rock R, Gekker G, Hu S et al. (2004). Role of microglia in
central nervous system infections. Clin Microbiol Rev 17:942–964.
Rock R, OlinM, Baker C et al. (2008). Central nervous system
tuberculosis: pathogenesis and clinical aspects. Clin
Microbiol Rev 21: 243–261.
Rolfs R, Joesoef R, Hendershot E et al. (1997). A randomized
trial of enhanced therapy for early syphilis in patients with
and without human immunodeficiency virus infection.
N Engl J Med 337: 307–314.Sacktor N (2002). The epidemiology of human immunodefi-
ciency virus-associated neurological disease in the era of
highly active antiretroviral therapy. J Neurovirol
8 (Suppl 2): 115–121.Sacktor N, Wong M, Nakasujja N et al. (2005). The interna-
tional HIV dementia scale: a new rapid screening test for
HIV dementia. AIDS 19: 1367–1374.Shelburne S, Darcourt J, White C et al. (2005). The role of
immune reconstitution inflammatory syndrome in AIDS
related Cryptococcus neoformans disease in the era of
highly active antiretroviral therapy. Clin Infect Dis 40:1049–1052.
Sidtis J, Gatsonis C, Price R et al. (1993). Zidovudine treat-
ment of the AIDS dementia complex: results of a
placebo-controlled trial. Ann Neurol 33: 343–349.Simona B, Giliola C, Francesca M et al. (2005). Prognostic
significance of JC virus DNA levels in cerebrospinal fluid
of patients with HIV-associated progressivemultifocal leu-
koencephalopathy. Clin Infect Dis 40: 738–744.
Simpson D, Olney R, McArthur J et al. (2000). A placebo-
controlled trial of lamotrigine for painful HIV-associated
neuropathy. Neurology 54: 2115–2119.Simpson D, Brown S, Tobias J et al. (2008). Controlled trial of
high-concentration capsaicin patch for treatment of painful
HIV neuropathy. Neurology 70: 2305–2313.SimpsonD, Schifitto G, Clifford D et al. (2010). Pregabalin for
painful HIV neuropathy: a randomized, double-blind,
placebo-controlled trial. Neurology 74: 413–420.Skiest D (2002). Focal neurological disease in patients with
acquired immunodeficiency syndrome. Clin Infect Dis
34: 103–115.Skiest D, ErdmanW, ChangW et al. (2000). SPECT thallium-
201 combined with Toxoplasma serology for the presump-
tive diagnosis of focal central nervous system mass lesions
in patients with AIDS. J Infect 40: 274–281.Skiest D, Crosby C (2003). Survival is prolonged by highly
active antiretroviral therapy in AIDS patients with primary
central system lymphoma. AIDS 17: 1787–1793.Skopelitis E, Kokotis P, Kontos A et al. (2006). Distal sensory
polyneuropathy in HIV-positive patients in the HAART
era: an entity underestimated by clinical examination. Int
J STD AIDS 17: 467–472.
Smith D, Guillemin G, Pemberton L et al. (2001). Quinolinic
acid is produced by macrophages stimulated by
platelet activation factor,Nef, andTat. JNeurovirol 7: 56–60.
Stern Y, McDermott M, Albert S et al. (2001). Factors associ-
ated with incident human immunodeficiency virus-demen-
tia. Arch Neurol 58: 473–479.Sullivan F, Swan I, Donnan P et al. (2007). Early treatment
with prednisolone or acyclovir in Bell’s palsy. N Engl J
Med 357: 1598–1607.Surawicz T,McCarthyB,KupelianV et al. (1999). Descriptive
epidemiology of primary brain and CNS tumors: results
from the Central Brain Tumor Registry of the United
States, 1990–1994. Neuro-oncol 1: 14–25.
ThwaitesG,NguyenD,NguyenHet al. (2004).Dexamethasone
for the treatmentof tuberculousmeningitis inadolescentsand
adults. N Engl J Med 351: 1741–1751.Thwaites G, Duc BangN, HuyDung N et al. (2005). The influ-
ence of HIV infection on clinical presentation, response to
treatment, and outcome in adults with tuberculous menin-
gitis. J Infect Dis 192: 2134–2141.
Venkataramana A, Pardo C, McArthur J et al. (2006). Immune
reconstitution inflammatory syndrome in the CNS of HIV-
infected patients. Neurology 67: 383–388.
1344 M. BILGRAMI AND P. O’KEEFE
Verma S, Micsa E, Estanislao L et al. (2004). Neuromuscular
complications in HIV. Curr Neurol Neurosci Rep 4: 62–67.Wallace M, Rossetti R, Olson P (1993). Cats and toxoplasmo-
sis risk in HIV-infected adults. JAMA 269: 76–77.
Wechsler A, HoD DD (1989). BilateralBell’s palsy at the time
of HIV seroconversion. Neurology 39: 747–748.Vibhagool A, Sungkanuparph S, Mootsikapun P et al. (2003).
Discontinuation of secondary prophylaxis for cryptococcal
meningitis in human immunodeficiency virus–infected
patients treated with highly active antiretroviral therapy:
a prospective, multicenter, randomized study. Clin Infect
Dis 36: 1329–1331.
Workowski K, Berman S (2010). Sexually transmitted diseases
treatment guidelines. MMWR Recomm Rep 59: 33–34.