Development of a chitosan–polyglutamate based injectable polyelectrolyte complex scaffold
Methodology for the Randomised Injecting Opioid Treatment Trial (RIOTT): evaluating injectable...
Transcript of Methodology for the Randomised Injecting Opioid Treatment Trial (RIOTT): evaluating injectable...
BioMed CentralHarm Reduction Journal
ss
Open AcceMethodologyMethodology for the Randomised Injecting Opioid Treatment Trial (RIOTT): evaluating injectable methadone and injectable heroin treatment versus optimised oral methadone treatment in the UKNicholas Lintzeris*1,3, John Strang1, Nicola Metrebian1, Sarah Byford1, Christopher Hallam2, Sally Lee1, Deborah Zador4 and RIOTT GroupAddress: 1Institute of Psychiatry, King's College London, 16 De Crespigny Park, London, SE5 8AF, UK, 2The Alliance, Room 312 Panther House, 38 Mount Pleasant, London, WC1X 0AN, UK, 3National Drug and Alcohol Research Centre, University of New South Wales, Sydney, 2052, Australia and 4South London and Maudsley NHS Trust. Denmark Hill, London, SE5 8AF, UK
Email: Nicholas Lintzeris* - [email protected]; John Strang - [email protected]; Nicola Metrebian - [email protected]; Sarah Byford - [email protected]; Christopher Hallam - [email protected]; Sally Lee - [email protected]; Deborah Zador - [email protected]; RIOTT Group - [email protected]
* Corresponding author
AbstractWhilst unsupervised injectable methadone and diamorphine treatment has been part of the Britishtreatment system for decades, the numbers receiving injectable opioid treatment (IOT) has been steadilydiminishing in recent years. In contrast, there has been a recent expansion of supervised injectablediamorphine programs under trial conditions in a number of European and North American cities,although the evidence regarding the safety, efficacy and cost effectiveness of this treatment approachremains equivocal. Recent British clinical guidance indicates that IOT should be a second-line treatmentfor those patients in high-quality oral methadone treatment who continue to regularly inject heroin, andthat treatment be initiated in newly-developed supervised injecting clinics.
The Randomised Injectable Opioid Treatment Trial (RIOTT) is a multisite, prospective open-labelrandomised controlled trial (RCT) examining the role of treatment with injected opioids (methadone andheroin) for the management of heroin dependence in patients not responding to conventional substitutiontreatment. Specifically, the study examines whether efforts should be made to optimise methadonetreatment for such patients (e.g. regular attendance, supervised dosing, high oral doses, access topsychosocial services), or whether such patients should be treated with injected methadone or heroin.
Eligible patients (in oral substitution treatment and injecting illicit heroin on a regular basis) are randomisedto one of three conditions: (1) optimized oral methadone treatment (Control group); (2) injectedmethadone treatment; or (3) injected heroin treatment (with access to oral methadone doses). Subjectsare followed up for 6-months, with between-group comparisons on an intention-to-treat basis across arange of outcome measures. The primary outcome is the proportion of patients who discontinue regularillicit heroin use (operationalised as providing >50% urine drug screens negative for markers of illicit heroinin months 4 to 6). Secondary outcomes include measures of other drug use, injecting practices, health andpsychosocial functioning, criminal activity, patient satisfaction and incremental cost effectiveness. The studyaims to recruit 150 subjects, with 50 patients per group, and is to be conducted in supervised injectingclinics across England.
Published: 27 September 2006
Harm Reduction Journal 2006, 3:28 doi:10.1186/1477-7517-3-28
Received: 28 March 2006Accepted: 27 September 2006
This article is available from: http://www.harmreductionjournal.com/content/3/1/28
© 2006 Lintzeris et al; licensee BioMed Central Ltd.This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Page 1 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
BackgroundThe British system of injectable opioid treatmentConventional approaches to maintenance substitutiontreatment using oral methadone are effective for most her-oin users entering treatment (see [1] for review). However,there are a proportion of patients who do not benefit fromsuch approaches – up to 50% of patients drop out ofmaintenance treatment within 12 months, and of thosewho remain in treatment, a substantial minority (up to15% of most programs) continue to inject heroin on a reg-ular basis (e.g. daily), and continue to experience consid-erable drug related harm [2,3].
One response for those individuals who fail to benefitfrom conventional substitution treatment has been theprescription of injectable opioids – methadone anddiamorphine (pharmaceutical heroin). This has been adistinctive, yet dwindling feature of the 'British system'over the past 40 years [4]. The majority of patients treatedwith injectable opioids have received injectable metha-done ampoules, and much smaller proportions havereceived injectable diamorphine (approximately 90% and10% of injectable prescriptions in 1995 respectively [5]).Unlike recent developments in Switzerland, Germany,Spain and the Netherlands, where treatment centres havebeen established to deliver supervised injectable opioidtreatment (IOT), the British system has had limited capac-ity for supervised dosing. The majority of IOT patientsreceive daily to weekly supplies of methadone or diamor-phine ampoules from clinics or community pharmaciesfor take home unsupervised consumption [6].
However, in the past decade, the role of IOT for heroindependence has been steadily diminishing in Britain.Injected methadone ampoules accounted for 8.7% ofNHS opioid prescriptions for heroin dependence in Eng-land and Wales in 1995, but only 1.9% in 2003 [4]. In1995, diamorphine accounted for two per cent of all opi-oid prescriptions for opiate dependence [5], and by 2000this had fallen to approximately 1% [6]. Whilst exactnumbers are unavailable, we estimate that in 2006, thereare between 2,000 to 3,000 patients prescribed injectablemethadone ampoules, and up to 500 patients prescribeddiamorphine in the NHS for opioid dependence. Thistotal number in IOT has remained relatively static over thepast decade, with little 'turn-over' and few new patientscommencing IOT. Yet during this same period there hasbeen a marked expansion of numbers in opioid substitu-tion (largely oral methadone and buprenorphine) treat-ment in Britain, from fewer than 50,000 to over 100,000,reflecting both a probable increase in the number of her-oin users and the proportion in treatment. Thus, whilstthe number of heroin dependent users and the number ofpatients entering substitution treatment in Britain appearsto be steadily increasing over the past decade, the role of
IOT has proportionally diminished, with few new patientscommencing this form of treatment. The diminishing roleof IOT in the UK may be due to a number of factors [6,7]:
- Limited evidence supporting IOT – the past decade hasseen an increasing emphasis within modern health sys-tems that clinical activity be based upon the principles ofevidence-based practice [8,9]. Further, there is generalconsensus as to the quality of evidence required to estab-lish such an evidence base [10,11]. Whilst it is possible toidentify individuals or groups of clients who have beensuccessfully treated with IOT, this does not provide suffi-cient evidence to establish the safety and efficacy of thistreatment approach. Adequately controlled trials, com-paring IOT to treatment approaches considered 'goldstandard' for this patient population are required. Whilsttrials of heroin treatment conducted in Switzerland[12,13] and the Netherlands [14] provide useful informa-tion on the potential benefits of prescribing heroin, thedifferences between the trial designs and treatment con-text of these trials make it unclear how far these results canbe applied to a UK setting. Here in the UK, only one RCTof 96 subjects conducted in the 1970's has comparedunsupervised IOT (diamorphine) to oral methadone([15]- reviewed below). In contrast to the limited evi-dence base of IOT, there has been an ever increasing bodyof evidence supporting other substitution approaches,such as oral methadone and sublingual buprenorphinetreatment.
- Concerns regarding diversion of medication – programswith low levels of supervision may be less expensive todeliver, however, widespread proliferation of treatmentprograms without the capacity for supervision (even of'unstable' patients) are likely to be associated with diver-sion of some medication onto the 'illicit market'. Twelvepercent of patients in the Hartnoll trial [15] self-reportedselling part of their take away diamorphine ampoules. Ina recent survey of 192 opioid dependent patients enteringtreatment in south London, approximately 20% reportedhaving ever used illicitly obtained injectable methadoneor diamorphine [16]. Doctors concerns over the possibil-ity of diversion of prescriptions to others has led to areluctance by some to prescribe injectable treatments [6].Concerns regarding diversion and poor adherence mayalso limit the doses prescribed within IOT, which may inturn reduce treatment effectiveness.
- Concerns that the provision of injectables may prolongthe drug use and injecting 'careers' of patients, with simi-lar concerns that it is difficult to 'move' patients ontomore conventional treatment approaches once they havebeen exposed to prescribed injectable opioids [15,17].This can also result in 'silting up' of limited treatmentplaces, restricting its availability to new patients. This is
Page 2 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
particularly relevant to the British system where the rou-tine availability of injectable take-aways for unsupervisedconsumption at home may remove incentives for clientsto move onto oral methadone programs.
- High cost – IOT is considerably more expensive than oralmethadone treatment. The main additional costs are med-ication-related (e.g. approximate medication costs alone(without VAT) for oral methadone (100 mg/day) are lessthan £500 per year, £1500 per year for injectable metha-done (100 mg/day), and £6500 per year using injectablediamorphine (400 mg/day) licensed in the UK [18]; withadditional costs involved in dispensing and supervision.In a London trial comparing supervised injectable to oralmethadone treatment, injectable treatment was found tobe 4 to 5 times more expensive to deliver [19]. Given theincreasing resource pressures placed upon health services,and without evidence of its cost-effectiveness over con-ventional treatment, IOT may be seen by many fundingbodies as an unaffordable 'luxury'.
Current evidence base for IOTThere have been several recent reviews of the evidencebase for IOT [20,21]. Three published RCTs have com-pared injectable diamorphine to oral methadone; and onehas compared injectable methadone to oral methadone.
Hartnoll and colleagues [15] reported on treatment reten-tion and self-reported heroin use in 96 heroin users enter-ing treatment, randomised to either take-awaydiamorphine ampoules (n = 44), or oral methadone (n =52). Overall, self-reported heroin use was comparablebetween the two groups. Whilst the injectable diamor-phine group had significantly better treatment retentionat 12 months, the majority continued to use illicit heroinin small amounts. In contrast, there was greater treatmentdrop out in the oral methadone group, and some patientsstopped using illicit heroin, whilst others continued to uselarger amounts of heroin. The results did not demonstratea clear superiority for either treatment, and it was almost20 years before the next controlled trial.
Perneger and colleagues ([12]) reported on the first RCTof supervised injectable diamorphine, in which 51 Swissheroin users with a history of poor performance in priormethadone programs were randomised to either injecta-ble heroin (n = 24) or oral methadone (n = 27). Treatmentretention was high in both groups, and the heroin treat-ment group self-reported significantly less heroin use thanthe methadone group. However, a considerable propor-tion of those randomised to oral methadone respondedwell (33% achieving abstinence and 19% had very lowlevels of heroin use), and 38% of patients randomised tothe oral methadone (wait list) Control group chose not toenrol in diamorphine treatment when available six
months later. Initiating IOT would have been unnecessary(and costly) in this patient group.
The experience from this RCT led to an understanding thatIOT should be seen as a 'second line' treatment approach,generally confined to those patients who are failing torespond to their current episode of methadone treatment.This was the basis for the next RCT [14] conducted by theCCBH in the Netherlands, in which 174 methadonepatients with a history of regular heroin injecting wererandomly allocated to either continue their oral metha-done treatment (n = 98), or to commence injectablediamorphine (n = 76) (with oral methadone doses alsoavailable). Treatment retention was comparable, and theinjectable diamorphine group were reported to have hada better global response in parameters such as social func-tioning, psychological health and criminality.
These findings suggest that the addition of injectablediamorphine conferred benefits to patients performingpoorly in methadone treatment over oral methadonetreatment alone. However, there were two key limitationswith the study. No data were reported for what are gener-ally considered to be primary outcomes of treatment forheroin dependence – levels of illicit heroin use and ongo-ing high-risk injecting practices. We will return to thisissue later. More importantly however, the study did notexamine whether the addition of injectable heroin con-ferred benefits over 'enhanced' or 'optimal' methadonetreatment. There were no specific attempts to optimise theconditions of methadone treatment for those patientsrandomised to the methadone only group. Hence, it maybe that some patients were performing poorly at enrol-ment due to an inadequate methadone dose, poor psy-chosocial services or infrequent attendance. Withoutspecific measures to optimize their treatment, it may notbe surprising that many continued to have poor out-comes. Indeed the different outcomes between the tworandomized groups could be due to the considerable dif-ferences in opioid doses used, and not due to the type orroute of opioid used (the mean methadone dose in theOral Methadone group was approximately 70 mg,whereas the mean methadone equivalent dose in theInjectable Diamorphine group was greater than 200 mgdaily). It should be noted that Hartnoll et al trial [15] hada similar dose disparity (in the opposite direction, withhigher equivalent oral methadone than heroin doses),thereby limiting the interpretation of two of the threepublished RCTs.
There are many reasons why patients may continue regu-lar heroin injecting during their methadone treatment.These may be patient related (e.g. strong desire to con-tinue injecting); alternatively, poor outcomes are oftenrelated to how treatment is delivered. The components of
Page 3 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
effective methadone treatment are well established [22],with more successful programs incorporating: higherdoses of methadone (in particular above 80 mg); ade-quate levels of supervision and monitoring; access to psy-chosocial services; and positive therapeutic relationshipsbetween patients and service providers. In many treat-ment systems, optimal treatment conditions are notalways available (such as access to adequate methadonedoses, counselling or welfare services), and under somecircumstances, continued heroin use by a patient in meth-adone treatment can be reduced by enhancing the condi-tions of their treatment – thereby diminishing theindication for IOT for that individual.
The need for further researchThe need for further controlled studies of IOT has beenhighlighted by a number of clinicians, researchers, usergroups and political authorities (e.g. [7,19,23,24]). Sys-tematic reviews of the evidence regarding IOT have con-cluded (a) the provision of IOT in supervised injectingclinics is feasible, (b) that IOT appears to be at least aseffective as conventional substitution treatment in achiev-ing certain outcomes such as treatment retention; how-ever (c) the existing evidence base is insufficient: "Nodefinitive conclusions about the overall effectiveness ofheroin prescription is possible because of non-compara-bility of the experimental studies available. Heroin use inclinical practice is still a matter of research in most coun-tries" [20]. We note that there are several RCTs of injecta-ble heroin treatment that have been recently conducted orin progress (in Germany, Spain and Canada), and thefindings of these trials will substantially build upon theavailable evidence base.
Given the concerns regarding IOT and its relatively lim-ited evidence base, the principles of rational therapeuticswould suggest that IOT should be seen as a 'second line'treatment modality limited to patients in methadonetreatment who meet the following criteria: (i) have a pro-tracted history of heroin dependence and injecting, (ii)have adhered to conditions of effective methadone treat-ment for an extended period of time, yet (iii) continue toregularly inject illicit heroin and experience related harms[25]. To date however, no controlled trial of this targetgroup has adequately examined whether the prescribingof injectable opioids is more effective than attempts atenhancing the conditions of oral methadone treatment inthose patients performing poorly in their current treat-ment episode.
Further, the social and historical context in which treat-ment occurs must be considered. Recent guidance [25]emphasises that all patients entering IOT should have fullsupervised dosing (as with patients commencing oral sub-stitution treatment), through the establishment of new
'European-style' clinical services. This is a new develop-ment for UK services, and it may be that the feasibility ofdelivering supervised IOT in Swiss, Dutch or German cit-ies may be difficult to replicate in Britain, where there maybe different patterns of drug use, geo-demographics, andimportantly, different expectations among service users ofIOT – given the long tradition of unsupervised IOT in thiscountry.
Of particular relevance to the British setting is the limitedevaluation of injectable methadone treatment. The major-ity of IOT in the UK is in the form of injectable metha-done, and yet there has been only one RCT of injectablemethadone, a pilot study in which 40 heroin users enter-ing treatment were randomly allocated to either equiva-lent doses of oral methadone or injectable methadone.There was no significant difference in treatment retention,illicit heroin use or other outcomes between the twogroups [19]. The use of injectable methadone rather thandiamorphine may have particular advantages (e.g. onlyone daily injection, less expensive) or disadvantages (e.g.side effects, inadequate substitute for heroin). Furtherresearch is required to establish the role of different inject-able opioids.
Another concern in the interpretation of earlier researchhas been the reliance on self-report data in evaluatingillicit heroin use. The research examining the validity andreliability of self-reported heroin use (see [26] for review)indicates that "self-report of illicit behaviours are suffi-ciently reliable and valid to provide descriptions of druguse, drug-related problems and the natural history of druguse" (p. 261–262). However, the conditions required toachieve this include that (a) there are no negatively per-ceived consequences for the client arising from any self-disclosure (such as loss of take-away privileges, loss of facewith a therapist or researcher, or even discontinuation ofthe program); and (b) self-report coincides with, and canbe corroborated by, objective measures such as urine drugscreens. Hence, the evaluation of illicit heroin use in opi-oid substitution treatment trials normally involves theconfidential collection of self-report data by independentresearchers, together with regular urine drug screens.
It is difficult to achieve these conditions when evaluatingdiamorphine treatment. The detection of morphine inurine drug screens (UDS) may be a suitable marker forheroin use in methadone patients, but is useless as amarker in diamorphine prescribed patients. Further, thepossible perception by some clients that unfavourableoutcomes for a trial-based diamorphine program couldresult in its discontinuation or scaling back, could serve asa motive for clients to under-report their illicit heroin use,particularly in the absence of UDS. Under such circum-stances, the validity and reliability of self-reported illicit
Page 4 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
heroin use may be questioned. In response, there havebeen efforts to establish objective measures of illicit her-oin use that do not rely on the detection of morphine. Apromising technique includes the identification ofpapaverine metabolites in UDS – papaverine is an opiatefound in illicit 'brown' heroin commonly used in westernEurope, but not present in pharmaceutical diamorphine[27,28]. Recent research suggests papaverine metabolites(hydroxy- and dihydroxypapaverine) are a suitablemarker for illicit heroin use, with high sensitivity, specifi-city and negative predictive values compared to the detec-tion of morphine in UDS of methadone orbuprenorphine prescribed patients [28]. This approachprovides an avenue for objective assessment of illicit her-oin use in diamorphine-prescribed patients.
The development of the Randomised Injectable OpioidTreatment Trial occurred as a response to calls from theHome Office to expand IOT in Britain [24], the need tobetter establish an evidence base for IOT, and to examinethe role of injectable methadone and diamorphine treat-ment delivered under the conditions identified in new UKnational guidance [25]. Specifically, the central questionto be addressed is whether efforts should be made to opti-mise conventional treatment for such patients (e.g.encouraging high doses, supervised dosing, psychosocialinterventions, and regular attendance) in order to reduceregular illicit heroin use, or whether such patients shouldbe treated with injected methadone or injected heroin in
newly developed supervised injecting clinics. To date, thishas not been addressed in published RCTs of IOT.
The research component of the trial was primarily fundedby a Research Grant from Action on Addiction, a nationalvoluntary sector charity and the Big Lottery. Clinical serv-ices (including the establishment of new supervisedinjecting clinics) were funded by the Home Office andNational Treatment Agency, together with local healthauthority funding. The research is being conducted byresearchers at the National Addiction Centre (Institute ofPsychiatry, Kings College London and the South Londonand Maudsley NHS Trust).
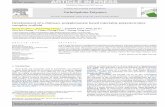
MethodologyOverviewRIOTT examines the role of IOT for the management ofheroin dependence in patients not responding to conven-tional substitution maintenance treatment. The study is aprospective, open-label three-way RCT (See figure 1). Eli-gible patients (in oral methadone treatment but stillinjecting illicit heroin on a regular basis) are randomisedto one of three conditions: (i) optimised oral methadonetreatment (Control group); (ii) injected methadone treat-ment; or (iii) injected heroin treatment. Approximately150 subjects (50 in each group) are followed up for 6-months, comparing between-group differences on anintention-to-treat analysis between the Control Groupand the Injectable Methadone Group, and between theControl Group and the Injectable Heroin Group; compar-
Overview of Research Design for RIOTTFigure 1Overview of Research Design for RIOTT.
6 months
Between group comparisons
Between group comparisons
Experimental Group
Injectable Methadone Group
Control Group
Optimised oral methadone
treatment
Experimental Group
Injectable Heroin Group
3 months
n=50
n=50
n=50
Subjects>3 years injecting
in treatment >6 monthsregular heroin injecting
no active significantmedical / psychiatric
condition
informed consent
N=150
Page 5 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
ing outcomes across a range of measures, including druguse, injecting practices, measures of global health and psy-chosocial functioning, criminality, treatment retention,incremental cost effectiveness, and measures of client sat-isfaction. It is a multi-site trial conducted at four sites inLondon (two sites), Brighton (South East England), andDarlington (North East England).
Research hypothesesThe aim of the study is to examine the safety, efficacy andcost effectiveness of treatment with optimised oral meth-adone compared to injectable methadone or injectablediamorphine, for patients in maintenance treatment whocontinue to inject illicit heroin regularly. The trial is notdesigned to directly compare injectable methadone toinjectable diamorphine treatment, which are both 'exper-imental' conditions. Hence, the research hypotheses forinjectable heroin treatment are:
i) that a selected group of patients (not responding to cur-rent oral methadone treatment) receiving injectable her-oin treatment, will make greater reductions in their illicitheroin use, other drug use and criminal activity, andgreater improvements in their health and social function-ing, than if provided with optimised oral methadonetreatment;
ii) providing injectable heroin to a selected group ofpatients (not responding to current oral methadone treat-ment) results in a greater economic benefit per extra unitof resource invested in the treatment, than only offeringoptimised oral methadone;
iii) The formal null hypothesis is that: there is no differ-ence between the two treatments
The research hypotheses for injectable methadone treat-ment are:
i) A selected group of patients (not responding to currentoral methadone treatment) receiving injectable metha-done treatment, will make greater reductions in theirillicit heroin use, other drug use and criminal activity andgreater improvements in their health and social function-ing, than if provided with optimised oral methadonetreatment;
ii) providing injectable methadone to a selected group ofpatients (not responding to current oral methadone treat-ment) results in a greater economic benefit per extra unitof resource invested in the treatment, than only offeringoptimised oral methadone;
iii) The formal null hypothesis is that: there is no differ-ence between the two treatments
The primary outcome measure is illicit heroin use asmeasured by the proportion of subjects in each group whocease regular illicit heroin use, operationalised as provid-ing at least 50% UDS negative for markers of illicit heroinduring months 4 to 6 of treatment (allowing a sufficientperiod for the study treatment to take effect). We estimateat least 50% negative UDS in once weekly random UDS tobe consistent with no more than approximately one (or atmost two) days heroin use per week on average – whichrepresents a discontinuation of regular heroin use, and isa clinically meaningful reduction for this particularpatient population (selection criteria include using illicitheroin on at least 50% of days and 100% positive UDSscreens for heroin use). The full range of measures forillicit heroin use is described in the Outcome Measuressection below.
The secondary outcome measures are:
- other illicit drug and alcohol use,
- high risk injecting practices and complications,
- indices of general health and psychosocial functioning,
- criminal activity,
- treatment retention,
- adverse events,
- cost-effectiveness in enhancing quality of life indices andreducing illicit heroin use.
- patient satisfaction with each of the three treatmentapproaches
- treatment goals and priorities of patients entering thetrial;
- likely demand for any future expansion of IOT underthese conditions.
SubjectsSelection criteriaThe trial targets patients in methadone treatment whocontinue to inject heroin on a regular basis. Specific crite-ria are:
1. Aged between 18 and 65 years at recruitment to study.
2. At least 3-year history of injecting heroin use.
3. In continuous methadone treatment for at least 6months this episode.
Page 6 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
4. Regular injecting heroin use in preceding 3 months (asevidenced by opiate-positive urine drug screens and self-report in clinical records), and heroin use on at least 50%of days (15 days) in the preceding month on self-report.
5. Evidence of regular injecting on clinical examination.
6. No significant and active medical (e.g. hepatic failure)or psychiatric condition (e.g. active psychosis, severeaffective disorder).
7. Not alcohol dependent or regularly abusing benzodi-azepines according to DSM-IVR criteria.
8. Not pregnant, breastfeeding, or planning to becomepregnant during the study period.
9. Resident of catchment area of participating agency.
10. Able and willing to participate in the study procedures(e.g. no impending prison sentence) and provideinformed consent.
Sample and power calculationPrimary statistical comparisons will be made between theControl and Injectable Methadone groups; and betweenthe Control and Injectable Heroin groups. The primaryendpoint for the sample size calculation is the proportionof subjects in each group who cease regular illicit heroinuse (operationalised as providing at least 50% UDS nega-tive for markers of illicit heroin) during months 4 to 6 oftreatment – allowing a sufficient period for the study treat-ment to take effect.
Due to the differences in selection criteria and reportedoutcome measures in previous studies of IOT, the availa-ble evidence does not allow confident predictions of treat-ment effect size for the different conditions. Nevertheless,some estimates can be made based on earlier trials[12,14,19,29]. Assuming 20% of subjects in the opti-mized oral methadone group cease regular illicit heroinuse (as operationalised above), and 50% of patients ineach of the IOT groups cease regular illicit heroin use,using a three-way randomisation schedule with equalnumbers assigned to each group, and with α = 0.05, β =0.85, then approximately 50 subjects per group should besufficient to detect significant differences between theControl group and each of the Experimental Injectablegroups – 150 subjects in total.
Recruitment and treatment allocationInformation regarding the trial is made available topatients in participating catchment areas through a varietyof means, including written information at clinics, exist-ing service providers, and through service user group
meetings. Patients receiving oral methadone treatmentwho inject illicit heroin regularly may refer themselves, orbe referred by their keyworker to the RIOTT clinical teamat each site. Those identified as potentially eligible areassessed and screened by RIOTT clinical staff to establisheligibility and informed consent. The assessment, screen-ing and consent process usually takes approximately twoweeks from initial presentation.
Randomisation for the trial is conducted independentlyby the Clinical Trials Unit of the Institute of Psychiatry,King's College London. Subjects are randomly allocatedusing minimization techniques into one of the three studyconditions in a 1:1:1 ratio within each treatment site, withstratification on two criteria: a) regular cocaine/crack use(> 50% days in previous 4 weeks on self-report); and b)receiving optimised oral methadone treatment at baseline(doses of at least 80 mg/day and supervised at least 5 days/week).
Randomisation occurs at the end of the two-week assess-ment and consent period. Patients receive pre- and post-randomization counseling by the clinical team prior tobeing informed of their treatment allocation by aresearcher. Treatment is initiated on the next working day.
Treatment conditionsThe study is an open-label trial in which clients, cliniciansand researchers are aware of treatment allocation. Thesame clinical staff deliver all three treatment conditions ateach site, thereby minimizing the potential for bias as aresult of differences in staff expertise or enthusiasm. Thetreatment conditions are:
(1) Optimised oral methadone treatmentPatients randomised to the Control group are to receive'optimised oral methadone treatment'. The key principlesfor treatment in this group entail:
- High methadone doses. Doses are individualised with theaim of reducing illicit opiate use. Methadone doses inexcess of 80 mg (and generally >100 mg) are encouraged(but not mandated) in this patient group, with a maxi-mum upper dose of 300 mg identified.
- Supervised dosing: Oral methadone is consumed undersupervision on at least 5 days per week during the first 3months. Thereafter, the level of supervised dosing mayreduce (conditional upon the patient's substance use,medical, psychiatric and social circumstances) to threedays a week, which is the minimum attendance requiredto enable random urine collection for drug screening.
- Frequent reviews and ancillary services. Patients areassigned a key worker, with weekly sessions scheduled
Page 7 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
during the initial three-month period. Thereafter, the fre-quency of scheduled reviews may reduce to two-weekly.All patients have monthly reviews with a study medicalofficer, and have access to a psychologist for individualCBT-based therapy. Patients also have access to otherancillary services (e.g. group programs) available at eachsite, as for any patient of the service. It should be empha-sized that participation in ancillary services such as coun-seling or group activities are voluntary, and not arequirement of continuation in the study.
- Treatment Care Plans addressing drug-related, physical,psychological and social issues are developed in consulta-tion with the client, key worker, study medical officer, andother relevant parties within the first month of the study,and reviewed at 3 and 6-months. Other health or socialservices are engaged accordingly.
- Urine drug screens. Random urine drug screens collectedweekly.
(2) Treatment with Injectable Methadone or (3) Injectable DiamorphineAspects of treatment other than medication issues (suchas key worker and medical reviews, ancillary services, andurine drug screens) are the same for the Injectable Metha-done and Injectable Diamorphine groups as described forthe Optimised Oral Methadone Group.
- High doses of injectable methadone or diamorphine. For theInjectable Methadone group, initial doses are convertedfrom oral to injectable methadone. Whilst there is limitedevidence regarding conversions between injected and oralmethadone in this population, the available data suggeststhat oral methadone has a mean bioavailability of approx-imately 80%, with large individual variation (rangingfrom 40 to 100% in previous research) [30-33]. Hence,the study uses a conversion formula of injected metha-done dose = 0.8 × oral methadone dose (separate researchunderway at the National Addiction Centre is examiningthe biavailaibility of injectable methadone (IM and IV) inlong term oral methadone patients). Doses are subse-quently titrated (generally upwards) and individualisedwith the aim of reducing illicit opiate use. Patients canalso choose to have oral methadone supplements. Maxi-mum doses of injectable methadone are 200 mg per day(plus up to 100 mg oral methadone), to a total dose of300 mg per day.
Injectable Diamorphine group: the dose conversionsbetween oral methadone and injected diamorphine arebased upon the work of Seidenberg and colleagues [34],developed for the Swiss, and more recently used by theGerman and Canadian heroin trials. The dose equivalencebetween oral methadone and diamorphine is not linear.
At low doses, the conversion rate from oral methadone(total daily dose) to injected heroin (total daily dose) isapproximately 1:3; whilst at higher doses, the conversionrate approximates 1:5. Other factors that impact uponmethadone metabolism (e.g. concomitant medications,medical conditions) are taken into consideration at trans-fer. Doses are subsequently titrated and individualisedwith the aim of reducing illicit opiate use. Patients areencouraged to retain a small oral methadone dose (e.g. 20to 40% of their initial dose) in order to prevent opiatewithdrawal between injecting sessions, and to facilitateany transitions between oral methadone and injecteddiamorphine (effectively having a 'loading dose' of meth-adone). It is expected that most patients will use injecteddiamorphine doses in the range of 300 to 600 mg per day,with an upper total daily dose of 900 mg (450 mg perinjection). Patients can also have up to 100 mg oral meth-adone supplementary to diamorphine, making their totaloral methadone equivalent dose approximately 300 mg.
- Supervised dosing. All doses of prescribed injectable opio-ids are supervised throughout the 6-month study period.Treatment typically involves once-a-day injection ofmethadone, or twice-a-day injection of diamorphine.Patients have a degree of autonomy in the frequency ofattendance for dosing and the mix of injected opioids/oralmethadone – patients unable or unwilling to attend forinjectable opioid treatment have access to oral methadonedoses. This flexibility of attendance for on-site injectingaims to minimize the inconvenience of IOT, and reflectsthat many patients entering RIOTT may not be injectingevery day, and hence, it may not be therapeutically neces-sary for patients to increase their frequency of injecting.An example of this dosing flexibility is provided in Table1. Patients must attend a minimum of 4-days-a-week foronsite IOT (to ensure integrity of the treatment condi-tion). The principles of IOT used in RIOTT are consistentwith recent national guidance [25].
All doses of injectable opioids are supervised onsite in theparticipating clinics. Two injecting sessions operate eachday, 7-days a week. Patients self-administer their injec-tions, with the choice of intravenous, intramuscular orsubcutaneous routes. Injecting sites and routes are
Table 1: Example of flexibility in IOT prescription
Drug & route Dose & time
Regime A Diamorphine (IV or IM)Diamorphine (IV or IM)Methadone (oral)
200 mg morning200 mg afternoon30 mg evening
Regime B Diamorphine (IV or IM)Methadone (oral)
200 mg (daily)100 mg (daily)
Regime C Methadone (oral) 160 mg
Page 8 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
recorded daily, and routinely assessed throughout thetrial.
MedicationsTrial medications include (i) oral methadone solution (1mg in 1 ml) or concentrate (10 mg in 1 ml); (ii) injectedmethadone ampoules (50 mg in 1 ml, 50 mg in 2 mls, 10mg in 1 ml ampoules licensed in the UK for IM or IV injec-tion); and (iii) diamorphine: the trial uses 10 gram freeze-dried diamorphine ampoules licensed and imported fromSwitzerland (Diaphin®), which are reconstituted by a trialpharmacist under aseptic conditions to a concentration of100 mg/1 ml. Each injection of diamorphine is dispensedas a 'loaded' syringe by the pharmacist, and self-injectedby the patient. The trial has a Clinical Trial Authorisationfrom the UK Medicine and Health Regulatory Authorityfor importation and use of Diaphin® ampoules.
Treatment post-trialAs injectable methadone and injectable diamorphine arelicensed and available in Britain, the trial does not need toconsider issues of 'compassionate grounds' for continua-tion of treatment. At the end of the 6-month study period,the nature of ongoing treatment is decided on an individ-ual basis by clinicians in consultation with each patient,in keeping with the recent NTA Guidance Report [25], andsubject to available clinical resources. The basis of thisdecision will be the extent to which each patient has dem-onstrated a positive clinical response and has obtainedsignificant benefit from their study treatment.
Outcome measures and data collectionThe study utilises a combination of data collection meth-ods for the outcome domains, including self-report data,UDS, and clinical records. Structured interviews with sub-jects are conducted by independent (IoP) researchers atbaseline (during the pre-trial treatment phase), 3 and 6months after randomisation. Semi-structured interviewsare conducted at various time points over the trial.
EfficacyThe primary outcome for the trial is illicit heroin use,measured using self-report data and UDS results, usingpapaverine metabolites as markers of illicit heroin use[28]. The secondary outcomes include changes in othertypes of substance use, high-risk injecting practices, indi-ces of general health and psychosocial functioning, crimi-nal activity, treatment retention, and indices of patientsatisfaction. Outcome measures are described in Table 2.
SafetyAdverse events to injected prescription heroin have beendescribed in Swiss heroin clinics [35], however furtherinformation comparing the adverse profile of injecteddiamorphine, oral and injected methadone in chronicaddict populations is needed to better characterise thesafety profile of these medications. Adverse events mayoccur as a result of the drug (e.g. methadone, heroin), orthe route of administration (IV, IM, oral). Indeed, anecdo-tal reports suggest that some patients experience consider-able adverse reactions (e.g. pain, 'burning') with
Table 2: Outcome measures
Outcome Outcome measures
Illicit heroin use • Self-reported data at 3 and 6 month interviews (including number of days used, routes of administration, average amount/cost, and frequency of use in past month as measured by the Opiate Treatment Index (OTI) Q score [43]; self-reported overdoses.• Random weekly UDS result, testing for papaverine metabolites.
Other drug use • Self-reported data at 3 and 6 month interviews regarding use of other opioids, alcohol, benzodiazepines, cannabis, cocaine. Measures include number days used, average cost/amount used.• Random weekly UDS result
High-risk injecting practices • Self-report data at 3 and 6 month interviews regarding participation in risk practices for blood borne virus transmission in preceding month using modified Injecting Risk Questionnaire [44]• Self-report data regarding injecting practices in past month (including sites, routes, adverse events, complications) and clinical examination of injecting sites (monthly medical reviews)
General health status and psychosocial functioning • Self-report data using SF-36 [45], EQ-5D [46, 47] and OTI Psychosocial Adjustment Section collected at 3 and 6 month interview• Hospital Anxiety Depression Scale [48] completed at monthly medical review.
Changes in criminality • Self – report data using modified Crime Section of Maudsley Addiction Profile [49] and OTI collected at 3 and 6 month interview
Measures of patient expectation and satisfaction • Treatment Perceptions Questionnaire [50]• Drug Use Expectations and User Nominated Outcome Instrument structured and semi-structured interviews examining patient perceptions of positives and negatives of using illicit heroin, and key outcomes/goals of treatment, as identified by service users (developed for the trial). Interviews conducted with service-user researcher at baseline, 3 and 6 months• Semi-structured interviews with service-user researcher examining patient perspectives of the relative advantages and disadvantages of each of the three treatment approaches.
Page 9 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
intravenous methadone, however these have not beenadequately documented in the published literature.RIOTT will examine adverse events between the threetreatment groups over time.
There are also potential concerns regarding the cardio-res-piratory effects of injectable opioids. Previous studiesexamining self-administration of injectable heroin andmethadone have identified significant hypoxia in somepatients [36,37]. However, it is unclear to what extent therisk of hypoxia is influenced by treatment conditions (e.g.,injected drug, route of administration, dose). There havealso been recent concerns regarding the use of high dose(oral and injected) methadone and prolongation of QTcintervals, a condition associated with cardiac arrythmias(such as torsade de pointes) and sudden death [38,39]. Itremains unclear whether injected methadone poses agreater risk of QTc prolongation than equivalent doses oforal methadone. The acute effects of injected diamor-phine upon ECG changes have not been previouslyreported. To address these concerns, indices of respiratoryand cardiac function are examined in relation to the drugsused (methadone versus diamorphine), route of adminis-tration (oral, IV and IM), and dose, compared to pre-ran-domisation baseline measures.
The prescription and supervised injection of diamorphine(and methadone) allows the opportunity to study thephysiological, subjective and cognitive-performanceeffects of these drugs. Despite widespread use of injectedheroin in many societies, there is to date remarkably littlepublished literature about the impact of injected heroinor methadone upon these parameters. The capacity tostudy the effects of heroin under clinical/laboratory con-ditions will enhance our understanding of the acuteeffects of heroin use in dependent users.
Another aspect of safety of this treatment approach is thatof 'community safety'. One of the major issues facingthose providing drug treatment services is the possiblecommunity backlash when such services are proposedwithin a local community. The establishment of a super-vised injecting clinic raises potential concerns for the localcommunity. Examples may include fears of local residentsand businesses regarding the congregation of drug usersregularly attending the clinic; or concerns regarding intox-icated clients being a nuisance to the local community. Asa part of the overall RIOTT, a community impact evalua-tion will be conducted to: a) investigate the impact on thelocal community of supervised injectable maintenanceclinic; b) document the expectations, fears and experienceof the local community; and c) compare and contrast themethods used by different services for addressing commu-nity interaction. The study design incorporates a blend ofepidemiological and social research methodologies in
order to gather data from a number of complimentarysources.
Cost effectivenessIOT is likely to be more expensive than optimised oralmethadone treatment, yet may be associated with betteroutcomes and/or cost-savings elsewhere. The economicevaluation will take a broad cost perspective, includingcosts borne by the health, social, voluntary and criminaljustice sectors, and costs to the economy in the form ofproductivity losses. Detailed information on the resourcesassociated with the three treatment interventions are col-lected from the relevant clinics and include staff time,equipment, study medications, dispensing services andthe treatment of adverse events. Data on the use of allother services (including health, social and voluntary sec-tor services), days off work due to illness, criminal justicesector contacts and crimes committed are collected usinga service use schedule, based on one designed at the Uni-versity of York for the economic evaluation of alcohol anddrug interventions and successfully applied to evaluationsof brief interventions [40]. Self-report data are collected atresearch interviews at baseline and at the 3 and 6 monthfollow-up points. Cost-effectiveness will be explored interms of illicit heroin use, the primary outcome measure,and in terms of quality adjusted life years, using the EQ-5D measure of health-related quality of life (see Table 2).
Patient satisfaction and experiencesA service-user researcher (CH) employed through a serviceuser-organisation, the Alliance, conducts structured andsemi-structured interviews with subjects throughout thefollow-up period to gain information on expectations of,and satisfaction with treatment (see Table 2). Semi-struc-tured interviews will also explore the broader experienceof prescribed and non-prescribed heroin use, exploringthe contextual influence of the clinical setting itself in theconstruction of individual's experiences of heroin use. It isexpected that interviews with a service user researcher maylend greater validity to subject responses when examiningissues such as treatment goals, perceptions about illicitheroin use and treatment.
Data analysisQuantitative data will be recorded by hand in Case RecordForms, coded, and entered into a database using the Sta-tistical Package for Social Science-12 software by aresearcher. Data will be analyzed on an intention-to-treatbasis. The primary outcome measure (illicit heroin use –operationalised as the proportion of subjects who provide>50% UDS negative for markers of illicit heroin use dur-ing the 4th to 6th months of study treatment) will be ana-lysed using the logistic regression model. Continuousoutcomes collected at 3 time points (baseline, 3 monthsand 6 months follow-ups), will be modelled using
Page 10 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
repeated measurement with mixed models approach toexamine the differences between treatment and controlgroups and the time course. Correlated responses will bedealt with by covariance structures. Endpoints that arecoded as categorical data will be treated as multivariatebinary responses in order to fit the repeated measure-ments. Odds ratios will be used to present differences intreatment retention between the control and IOT groups.
Semi-structured interviews collecting qualitative data aretape recorded (with subject consent) and transcribed,with all identifying information removed from the tran-scripts. Data is analysed in hard copy form, using qualita-tive thematic analysis [41].
DiscussionDespite IOT being part of the British treatment system,there have been few studies examining the safety andeffectiveness of this approach, particularly of injectablemethadone treatment, which comprises the majority ofIOT in this country. The primary aim of RIOTT is to com-pare the safety, efficacy and cost effectiveness of injectablemethadone and diamorphine treatment (delivered undersupervised conditions), to optimised oral methadonetreatment, in methadone patients who regularly injectillicit heroin. Such research should better inform clini-cians and policy makers as to how to respond to thispatient group, and to inform decision making as towhether additional resources should be directed to IOT,or to enhancing the capacity for oral methadone treat-ment to be delivered under optimised conditions.
The establishment of RIOTT also enables a variety of'nested' studies examining a range of issues regardinginjectable heroin and methadone. Some of these studieshave been briefly discussed, such as projects examiningthe cardio-respiratory effects of these drugs in chronic opi-ate dependent individuals. Other projects being examinedinclude cognitive-performance effects of injectable opio-ids compared to oral methadone, and pharmaco-geneticresearch examining responses to opioid treatment.Despite the widespread proliferation of heroin use acrossthe world, and despite reports of widespread misuse ofmethadone by injection [42], there has been remarkablylittle laboratory research examining the pharmacologicaleffects of these drugs at high doses in chronic opiate users.Such research may lead to a better understanding of themechanisms behind heroin-related harms such as cogni-tive impairment and overdose.
A key point in embarking on another RCT of injectableheroin treatment relates to the extent to which there is asufficient evidence base currently available to scientifi-cally 'prove' the efficacy of heroin prescribing – in short –do we need more heroin trials? Underlying this is the
question 'how much evidence is required in order to sci-entifically establish the efficacy and safety of a particulartreatment'? The experience of substitution opiate treat-ment (methadone, LAAM, buprenorphine) suggests thatconsiderable and unequivocal evidence is required inorder to defend such treatments against politicised critics– be they politicians, academics, service providers, localcommunity or (ex)drug user groups – much more so thanin most other areas of medicine. This applies to an evengreater extent with the highly controversial issue of inject-able opioid prescribing. A particularly strong evidencebase will be a minimum requirement in establishing IOTas an available option – the findings of two or three RCTsalone are unlikely to convince sceptics of such a conten-tious treatment paradigm. The most recent systematicCochrane review of heroin prescribing suggests that differ-ences in study design limit our capacity to draw conclu-sions on the available published trials in this area. Thecompletion and dissemination of the various interna-tional trials in Germany, Spain, Canada and Britain isrequired in order to establish this evidence base, particu-larly as we should not pre-empt the findings of such stud-ies in assuming that they will all show similar results.Should the broad safety and efficacy of IOT be establishedthrough the various trials underway internationally, it willenable researchers to address the next 'level' of clinical-research questions. This may include issues around theavailability of take-away doses of injectables, the selectionof which opioids should be prioritised, and whetherpatients can be successfully transitioned from injecting tonon-injecting (e.g. intranasal, oral) routes.
We should also recognise that, as with the expansion ofmethadone and buprenorphine treatment internationallyover the past 20 years, any expansion of IOT will often bepreceded by calls for 'local' research that addresses localconcerns and regulatory requirements. Interestingly,whilst Phase III RCTs are often conducted in order toachieve registration of a new medicine, in the British con-text however, licensing of IOT is not the issue. Ratherresearch is required to establish its role within the treat-ment system after over a decade of decline, and at a timewhere health systems are requiring a greater evidence baseof efficacy and cost-effectiveness.
Competing interestsThe author(s) declare that they have no competing inter-ests.
Authors' contributionsNL and JS are the Chief Investigators, and NM is the TrialCo-ordinator and Co-investigator. These three authorshave equally contributed to the drafting of this report. SB,SL, and CH are the health economist, statistician and serv-ice user researcher for the trial and have contributed to the
Page 11 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
relevant sections of the paper. All authors read andapproved the final manuscript.
AcknowledgementsThe RIOTT group consists of clinicians and researchers who have contrib-uted to the development or implementation of the research project, but have not contributed sufficient to be authors of this paper. They include (in alphabetical order): G Achunine (trial pharmacist, South London and Maud-sley (SLaM) NHS Trust), O Bowden Jones (Site Investigator, Central North West NHS Trust), T Carnwath (Site Investigator, County Durham and Darlington NHS Trust), Luciana Forzisi (Researcher, NAC), H Williams (Site Investigator, South Downs Health NHS Trust), S Mayet (Clinical Research Fellow, NAC), H McCrossan (Service Manager, SLaM NHS Trust); P Miller (Health Services Researcher, NAC), T Mitchell (Researcher, NAC), R van der Waal (Team Leader, SLaM NHS Trust), D Zador (Site Investigator, SLaM NHS Trust). The authors would also like to acknowledge Lesley King Lewis and staff at Action on Addiction, staff and clients at SLaM Trust, C Murphy (Clinical Trials Unit, Institute of Psychiatry, Kings College London), Big Lottery, The National Treatment Agency.
The study is funded by The Big Lottery Research Grants and Action Addic-tion (research aspects of the trial); the National Treatment Agency and local DAT and PCT authorities (clinical services). NL was funded by an Aus-tralian government NHMRC Neil Hamilton Fairley Clinical Research Fel-lowship during the development of the study.
References1. Mattick RP, Breen C, Kimber J, Davoli M: Methadone mainte-
nance therapy versus no opioid replacement therapy for opi-oid dependence. Cochrane Database Syst Rev 2003, (2):CD002209..
2. Gossop M, Marsden J, Stewart D, Kidd T: The National Treat-ment Outcome Research Study (NTORS): 4-5 year follow-up results. Addiction 2003, 98:291-303.
3. Teesson M, Ross J, Darke S, Lynskey M, Ali R, Ritter A, Cooke R:One year outcomes for heroin dependence: Findings fromthe Australian Treatment Outcome Study (ATOS). DrugAlcohol Depend 2005, Epub ahead of print:.
4. Strang J, Gossop M: Heroin Addiction and the British System:Treatment and Policy responses. Volume 2. London ,Routledge; 2005.
5. Strang J, Sheridan J, Barber N: Prescribing injectable and oralmethadone to opiate addicts: results from the 1995 nationalpostal survey of community pharmacies in England andWales. BMJ 1996, 313:270-272.
6. Metrebian N, Carnwath T, Stimson GV, Storz T: Survey of doctorsprescribing diamorphine (heroin) to opiate-dependent drugusers in the United Kingdom. Addiction 2002, 97:1155-1161.
7. Zador D: Injectable opiate maintenance in the UK: is it goodclinical practice? Addiction 2001, 96:547-553.
8. Davidoff F, Haynes B, Sackett D, Smith R: Evidence based medi-cine. BMJ 1995, 310:1085-1086.
9. Sackett DL, Rosenberg WMC, Gray JAM, Richardson WS: Evidencebased medicine: what it is and what it isn’t. BMJ 1996,312:71-72.
10. Cochrane Library: The Cochrane Manual . The Cochrane Col-laboration; 2006.
11. NHMRC: How to review the evidence: systematic identifica-tion and review of the scientific literature. Canberra , Com-monwealth of Australia; 1999.
12. Perneger TV, Giner F, del Rio M, Mino A: Randomised trial of her-oin maintenance programme for addicts who fail in conven-tional drug treatments. British Medical Journal 1998, 317:13-18.
13. Uchtenagen A, Gutzwiller F, Dobler-Mikola A: Medical Prescrip-tion of Narcotics Research Programme Final Report of thePrincipal Investigators. Zurich , Institution fur Sozial-und praven-tivmedizin der Universitat Zurich; 1998.
14. van den Brink W, Hendricks VM, Blanken P, Huijsman I, van Ree JM:Medical co-prescription of heroin: two randomised control-
led trials. Utrecht, The Netherlands , Central Committee on theTreatment of Heroin Addicts (CCBH); 2002.
15. Hartnoll R, Mitcheson M, Battersby A, Brown G, Ellis M, Fleming P,Hedley N: Evaluation of heroin maintenance in controlled tri-als. Archives General Psychiatry 1980, 37:877 -8883.
16. Ridge G, Lintzeris N, Gossop M, Witton J, Clark K, Strang J: TheSeven Boroughs study. London , National Addiction Centre, Insti-tute of Psychiatry, Kings College London and South London andMaudsley Trust ; 2004.
17. Battersby M, Farrell M, Gossop M, Robson P, Strang J: "Horse trad-ing": prescribing injectable opiates to opiate addicts - adescriptive study. Drug and Alcohol Review 1992, 11:35 -342.
18. British Medical Association, Royal Pharmaceutical Society of GreatBritain: British National Formulary. Volume 50. London , BMJPublishing Group Ltd and Royal Pharmaceutical Society of Great Brit-ain; 2005.
19. Strang J, Marsden J, Cummins M, Farrell M, Finch E, Gossop M, Stew-art D, Welch S: Randomized trial of supervised injectable ver-sus oral methadone maintenance: report of feasibility and 6-month outcomes. Addiction 2000, 95():1631 -11645.
20. Ferri M, Davoli M, Perucci CA: Heroin maintenance for chronicheroin dependents. The Cochrane Database of Systematic Reviews2005:Art. No.: CD003410. DOI: 10.1002/14651858.CD003410.pub2..
21. Stimson GV, Metrebian N: Prescribing heroin: What is the evi-dence? York , Joseph Rowntree Foundation; 2003.
22. Ward J, Mattick R, Hall W: Methadone maintenance treatmentand other opioid replacement therapies. Amsterdam , Har-wood Academic Publishers; 1998.
23. Drucker E, Vlahov D: Controlled clinical evaluation of diacetylmorphine for treatment of intractable opiate dependence.Lancet 1999, 353(9164):1543-1544.
24. House of Commons Home Affairs Committee: The government'sdrug policy: Is it working? Volume 1: Report and Proceedingsof the Committee. London , The Stationery Office Ltd; 2002.
25. National Treatment Agency for Substance Misuse: Injectable her-oin (and injectable methadone). Potential roles in drugtreatment: Full guidance report. London , National TreatmentAgency; 2003.
26. Darke S: Self-report among injecting drug users: A review.Drug Alcohol Depend 1998, 51:253-263.
27. Musshoff F, Trafkowski J, Madea B: Validated assay for the deter-mination of markers of illicit heroin in urine samples for thecontrol of patients in a heroin prescription program. J Chro-matogr B Analyt Technol Biomed Life Sci 2004, 811(1):47-52.
28. Paterson S, Lintzeris N, Mitchell TB, Cordero R, Nestor L, Strang J:Validation of techniques to detect illicit heroin use inpatients prescribed pharmaceutical heroin for the manage-ment of opioid dependence. Addiction 2005, 100(12):1832-1839.
29. Metrebian N, Shanahan W, Stimson GV, Small C: Prescribing drugof choice to opiate dependent drug users: a comparison ofclients receiving heroin with those receiving injectablemethadone at a West London drug clinic. Drug and AlcoholReview 2001, 20:267-276.
30. Dale O, Hoffer C, Sheffels P, Kharasch ED: Disposition of nasal,intravenous, and oral methadone in healthy volunteers. ClinPharmacol Ther 2002, 72:536-545.
31. Gourlay GK, Cherry DA, Cousins MJ: A comparative study of theefficacy and pharmacokinetics of oral methadone and mor-phine in the treatment of severe pain in patients with cancer.Pain 1986, 25:297-312.
32. Nilsson MI, Meresaar U, Anggard E: Clinical pharmacokinetics ofmethadone. Acta Anaesth Scand Supp 1982, 74:66-69.
33. Wolff K: Characterization of methadone overdose: clinicalconsiderations and the scientific evidence. Ther Drug Monit2002, 24:457-470.
34. Seidenberg A, Honneger U: Methadon, Heroin und andere Opi-oide. Gottingen , Hans Huber Verlag; 1998.
35. Dursteler-MacFarland KM, Stohler R, Moldovanyi A, Rey S, BasdekisR, Gschwend P, Eschmann S, Rehm J: Complaints of heroin-main-tained patients: A survey of symptoms ascribed to diacetyl-morphine. Drug Alcohol Depend 2006, 81 :231-239.
36. Dursteler-Mac Farland KM, Stormer R, Seifritz E, Hug I, Muller-SpahnF, Ladewig D, Stohler R: Opioid-associated effects on oxygensaturation. Addiction 2000, 95:285-287.
Page 12 of 13(page number not for citation purposes)
Harm Reduction Journal 2006, 3:28 http://www.harmreductionjournal.com/content/3/1/28
Publish with BioMed Central and every scientist can read your work free of charge
"BioMed Central will be the most significant development for disseminating the results of biomedical research in our lifetime."
Sir Paul Nurse, Cancer Research UK
Your research papers will be:
available free of charge to the entire biomedical community
peer reviewed and published immediately upon acceptance
cited in PubMed and archived on PubMed Central
yours — you keep the copyright
Submit your manuscript here:http://www.biomedcentral.com/info/publishing_adv.asp
BioMedcentral
37. Stoermer R, Drewe J, Dursteler-Mac Farland KM, Hock C, Mueller-Spahn F, Ladewig D, Stohler R, Mager R: Safety of injectable opi-oid maintenance treatment for heroin dependence. Biol Psy-chiatry 2003, 54:854-861.
38. Kornick CA, Kilborn MJ, Santiago-Palma J, Schulman G, Thaler HT,Keefe DL, Katchman AN, Pezzullo JC, Ebert SN, Woosley RL, PayneR, Manfredi PL: QTc interval prolongation associated withintravenous methadone. Pain 2003, 105:499-506.
39. Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD,Mehler PS: Torsade de pointes associated with very-high-dosemethadone. Ann Intern Med 2002, 137:501-504.
40. Barrett B, Byford S, Crawford MJ, Patton R, Drummond C, Henry JA,Touquet R: Cost-effectiveness of screening and referral to analcohol health worker in alcohol misusing patients attendingan accident and emergency department: a decision-makingapproach. Drug Alcohol Depend 2006, 81(1):47-54.
41. Miles MB, Huberman AM: Qualitative Data Analysis. NewburyPark , Sage; 1984.
42. Fiellin DA, Lintzeris N: Methadone syrup injection in Australia:a sentinel finding? Addiction 2003, 98:385.
43. Darke S, Hall W, Wodak A, Heather N, Ward J: Development andvalidation of a multi-dimensional instrument for assessingoutcome of treatment among opiate users: The OpiateTreatment Index. British Journal of Addiction 1992, 87:733-742.
44. Stimson GV, Jones S, Chalmers C, Sullivan D: A short question-naire (IRQ) to assess injecting risk behaviour. Addiction 1998,93(3):337-347.
45. Ware JEJ, Sherbourne CD: The MOS 36-item short-form healthsurvey (SF-36). I. Conceptual framework and item selection. Med Care 1992, 30:473-483.
46. Brooks R: EuroQol: the current state of play. Health Policy 1996,37:53-72.
47. EuroQol Group: A new facility for the measurement of health-related quality of life. Health Policy 1990, 16:199-208.
48. Zigmond AS, Snaith RP: The hospital anxiety and depressionscale. Acta Psychiatr Scand 1983, 67(6):361-370.
49. Marsden J, Gossop M, Stewart D, Best D, Farrel M, Edwards C, Leh-mann P, Strang J: The Maudsley Addiction Profile (MAP): abrief instrument for assessing treatment outcome. Addiction1998, 93:1857 -11868.
50. Marsden J, Nizzoli U, Corbelli C, Margaron H, Torres M, Prada DeCastro I, Stewart D, Gossop M: New European instruments fortreatment outcome research: reliability of the maudsleyaddiction profile and treatment perceptions questionnaire inItaly, Spain and Portugal. Eur Addict Res 2000, 6(3):115-122.
Page 13 of 13(page number not for citation purposes)