Kinetic, Equilibrium and Thermodynamic Studies on Removal of Cr(VI) by Activated Carbon Prepared...
-
Upload
independent -
Category
Documents
-
view
4 -
download
0
Transcript of Kinetic, Equilibrium and Thermodynamic Studies on Removal of Cr(VI) by Activated Carbon Prepared...
Author Proof
AKinetic, Equilibrium and ThermodynamicStudies on Removal of Cr(VI) by ActivatedCarbon Prepared from Ricinus communisSeed ShellP. Thamilarasu1 and K. Karunakaran2*
1. Department of Chemistry, AMS Engineering College, Namakkal 637 013, Tamil Nadu, India
2. Department of Chemistry, Sona College of Technology, Salem 636 005, Tamil Nadu, India
A study on the removal of hexavalent chromium ions from aqueous solution by using activated carbon prepared from Ricinus communis hasbeen done. In this process, it was carbonised and activated by treating with concentrated sulphuric acid followed by heating for 5 h at 500◦C.Batch adsorption experiments are also carried out as a function of pH, contact time, initial concentration of the adsorbate, adsorbent dosage andtemperature. The experimental data are fitted well to the Freundlich adsorption isotherm. Thermodynamic parameters such as �H◦, �S◦ and �G◦are calculated, which indicated that the adsorption is spontaneous and endothermic in nature. Adsorbent used in this study is characterised byFT-IR and SEM before and after the adsorption of Cr(VI).
Keywords: adsorption, isotherm, activated carbon, chromium and seed shell
INTRODUCTION
The pollution caused because of heavy metals has receivedwidespread attention in the recent years (Bishnoi et al.,2004) due to the toxicological importance in the ecosys-
tem, agriculture and human health. It leads to the developmentof alternative technologies for the removal of these pollutants fromaqueous effluents. The use of low-cost and waste biomaterials asadsorbents of dissolved metal ions has been shown to provideeconomic solutions to this global problem (Park et al., 2005). Inthis context, our adsorbent (RCC: Ricinus communis seed shell)could be used as an effective and environment-friendly adsorbentfor the removal of Cr(VI) containing water and wastewater.
Chromium is a highly toxic pollutant generated from manyindustrial processes such as leather tanning processes, electro-plating and manufacturing of dye, paint and paper. Chromiumexists in the aquatic environment mainly in two states; trivalentchromium and hexavalent chromium. Hexavalent chromium isprimarily present in the form of chromate and dichromate ions(Khezami and Capart, 2005). The USEPA has set the permissi-ble level for chromium in drinking water at 0.05 mg/L. Thesestandards are based on the total concentration of the trivalentand hexavalent forms of dissolved chromium. Chromium has the
potential to cause the following health effects from long-termexposure to above the minimum cleanliness level (MCL); damageto liver, kidney circulatoryQ1 []and nerve tissues (USEPA, 1995).
Conventional methods for removal of dissolved heavy metalions included the chemical precipitation, chemical oxidationand reduction, ion exchange, filtration, electrochemical treatmentand evaporative recovery. However, these high-technology pro-cesses have significant disadvantages, including incomplete metalremoval, requirements for expensive equipment and monitoringsystems, high-cost reagents, energy requirements, generation oftoxic sludge and other waste products that require disposal (Aksuet al., 2002).
Adsorption on activated carbon has been found to be an effec-tive process for Cr(VI) removal, but it is too expensive. Naturalmaterials are available in large quantities; certain waste products
∗Author to whom correspondence may be addressed.E-mail address: [email protected]. J. Chem. Eng. 9999:1–10, 2012© 2011 Canadian Society for Chemical EngineeringDOI 10.1002/cjce.20675Published online in Wiley Online Library(wileyonlinelibrary.com).
| VOLUME 9999, 2012 | | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | 1 |
Author Proof
Afrom industrial and agricultural operations may have potentialas inexpensive adsorbents. Due to their low cost, after thesematerials have been expended, they could be disposed withoutexpensive regeneration. Most of the low-cost adsorbents have thelimitation of low sorptive capacity and thereby same degree oftreatment, which leads to disposal problems. Therefore, thereis need to explore ‘low-cost biosorbent’ having high adsorptioncapacity (Agarwal et al., 2006). Consequently, numerous low-cost alternatives have been studied including sawdust (Acar andMalkoc, 2004), eucalyptus bark (Sarin and Pant, 2006), greenalgae (Malkoc and Nuhoglu, 2003), seaweeds (Vijayaraghavan etal., 2005), coir pitch (Kadirvelu et al., 2001), peanut husks carbon(Ricordel et al., 2001), Zeolite tuff (Al-Haj and El-Bishtawi, 1999),activated carbon fabric cloth (Mohan et al., 2005), bagasse fly ash(Gupta et al., 1999), activated slag (Srivastava et al., 1997), greenalgae Spirogyra species (Gupta et al., 2001)Q2, nonviable cyanobacterium Nostoc muscorum biomass (Gupta and Rastogi, 2008),fertiliser industry waste material (Gupta et al., 2010), fertiliserwaste material (Srivastava et al., 1996) and bagasse fly ash—asugar industry waste material (Sharma and Park, 1999). New eco-nomical easily available and highly effective adsorbents are to bestill needed. Generally, biosorptive processes could reduce capitalcosts by 20%, operational costs by 36% and total treatment costsby 28%, compared with the conventional systems (Loukidou etal., 2004).
The literature survey indicated that R. communis has not beenused as adsorbent for the removal of chromium so far. The com-mon name of R. communis carbon is castor and it belongs to thefamily ‘Euphorbiaceae’. It is commonly cultivated in dry landsalong with groundnuts in India. The Pericarb is removed from theseed before going for the castor oil extraction. It is one of the largequantities of agricultural waste and it is used as a fuel to smallextent. RCC-activated carbon as an adsorbent is a low cost mate-rial and was used for the removal of Cr(VI) in wastewater. In thepresent work, values of well-known thermodynamic functions,kinetics and isotherms studies have been performed to elucidatethe equilibrium adsorption behaviour of Cr(VI) at different tem-peratures. Also the effect of agitation time, pH and temperatureon the adsorption has been investigated.
MATERIALS AND METHODS
Preparation of AdsorbentActivated carbon was prepared from RCC. The raw material wasprocured from local vendor. The material was washed in hot dis-tilled water to remove earthy matter and cut into small piecesand dried. The activated carbon was prepared from the abovematerial impregnated with concentrated sulphuric acid at 24 h.The impregnation of the ratio of acid volume to weight was 1:1employed. After that, the charred material was washed severaltimes in distilled water until the pH of the solution becomes neu-tral (pH 7.0). Then the material was dried and carbonised at 500◦Cusing muffle furnace. Finally, the activated material was groundedand sieved into 180–300 � using standard sieves. All the reagentsused for this study were commercially available as Analar grade(Merck, India and SD-Fine, India)Q3.
AdsorbateA stock solution of the adsorbate was prepared by dissolving2.828 g of potassium dichromate (Merck) in 1000 mL of doublydistilled water. Double distilled water was used throughout theexperiments.
Experimental Methods—Batch Mode StudiesFifty millilitres of Cr(VI) known concentration of solution and50 mg of adsorbent were taken in a 100 mL iodine flask andwere agitated at 120 rpm in a thermostatic shaker water batchat 30◦C for a 60 min of contact time. Then, the solution was cen-trifuged. The remaining concentration of Cr(VI) was measuredby using UV–Vis spectrophotometer at an absorption wavelengthof 540 nm (Systronics 169, India)Q4 with 1,5-diphenyl carbazidesolution (Merck) in acid medium. The amount of Cr(VI) adsorbedin mg/g at time (t) was computed by using the following equation(Gilcreas et al., 1965):
qt = (C0 − Ct)VM
(1)
where qt is the amount of Cr(VI) adsorbed in mg/g at time t min;C0 is the initial concentration of Cr(VI), mg/L; Ct is the concentra-tion in mg/L at a given time t,; V is the volume of Cr(VI) solutionin litre and M is the weight of activated carbon in gram. The per-centage of removal Cr(VI) solution was calculated by using thefollowing equation:
Removal (%) = C0 − Ct
C0× 100 (2)
RESULTS AND DISCUSSION
Adsorbent CharacterisationAll the parameters are analysed using standard testing meth-ods (APHA et al., 1985). Activated carbons are widely used asadsorbents due to its high adsorption capacity, high surface area,microporous structure and high degree of surface. Some impor-tant physico-chemical characteristics of RCC are given in Table 1.The pH and zero point charge (pHzpc) values, found to be 7.70and 5.86 of the adsorbent, indicate that the carbon may con-tain basic functional groups and acidic oxygen functional groups.MoistureQ5 content of the carbon has no effect on its adsorptivepower; it dilutes the carbon which is necessary to use of additionalweight of carbon during treatment process. The lower bulk den-sity value indicates the highly branched and porous carbon withmore void space. Acid soluble matter content was found slightlyhigher than the solubility in water in the carbon because of incor-porated carbonate groups in the pores of the adsorbent. RCC hashigher porosity and surface area; it shows that the RCC is use-ful for the removal of volatile, organic and inorganic contents.The lower ash content and volatile matter are attributed to lowerinorganic content and higher fixed carbon. Higher value of fixedcarbon shows that the adsorbent is having more efficiency andstability. Sodium and potassium content may be due to the pres-ence of mineral sodium and potassium in RCC. Conductivity ofthe carbon is higher due to the development of exchangeable siteson the surface of the activated carbon. High specific gravity valuesshow that the adsorbents use more number of recycling processesand have more abrasive resistance. A lower phenol adsorptioncapacity value indicates the less adsorption of organic molecules.A higher percentage removal for smaller particle size adsorbent(180–300 �) is due to the availability of more surface area. Thehigh surface area is considered to be most suitable adsorbent foradsorption (Karthikeyan et al., 2008).
| 2 | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | | VOLUME 9999, 2012 |
Author Proof
ATable 1. Characteristics of the RCC activated carbon
Parameter Value
pH 7.70pHzpc 5.86Moisture content (%) 3Bulk density (g/mL) 0.4384Solubility in water (%) 0.9273Solubility in 0.25 M HCl (%) 4.2334Porosity (%) 70.935Specific gravity (g/mL) 1.6367Volatile matter (%) 4.54Ash content (%) 7.52Fixed carbon (%) 84.94Sodium (mg/L) 62Potassium (mg/L) 2.8Phenol adsorption capacity (%) 19.14Conductivity (mS) 1.95Surface area (m2/g) 558
Effect of Adsorbent DosageThe removal of chromium by RCC at different adsorbent doses(25–300 mg) and at chromium concentration of 10 mg/L is inves-tigated. In Figure 1, the results show that the percent removal ofCr(VI) increases quickly with increase in the dose of RCC due tothe greater availability of adsorption sites of adsorbent (Mohan etal., 2006). The adsorbent dosage increase from 25 to 300 mg, theremoval percent increases from 65.9 to 80.1%. However, uptakeof Cr(VI) shows an increasing trend to the removal percentage ofadsorption. In view of this observation, it was decided to conve-niently fix the dose of activated carbon as 50 mg for the remainingexperiments for minimum usage of activated carbon and also thequantity of Cr(VI) uptake was more at 50 mg of activated car-bon, hence 50 mg was taken as the optimum dose for furtherexperimental studies.
Effect of pHThe pH is one of the most important parameters for controllingthe uptake of metal ions from their aqueous solution by adsor-bent. The effect of pH on the removal of Cr(VI) is investigatedby testing different values of pH at a temperature of 30◦C and
Figure 1. Effect of adsorbent dosage.
Figure 2. Effect of pH.
10 mg/L concentration of Cr(VI) aqueous solution. The contacttime has been fixed as 60 min for all the experiments. The exper-imental results are presented in Figure 2 for RCC. It can be seenin Figure 2 that the maximum of Cr(VI) removal (77.6%) occursat the lowest pH value. Furthermore, the adsorption efficiencyincreases with the decrease in pH. This finding has been reportedby several investigators (Selvi et al., 2001; Garg et al., 2004), whohave found that Cr(VI) removal by activated carbon is enhancedin the acidic range of pH. The favourable effect of low pH canbe attributed to the neutralisation of negative charges on surfaceof the adsorption by excess hydrogen ions, thereby facilitatingthe diffusion of hydrogenchromate ions (HCrO−
4 ) and their sub-sequent adsorption. According to the diagram by Benefield et al.(1982), HCrO−
4 is the dominant and ionic form of Cr(VI) betweenpH 2.0 and 4.0. This ionic form was found to be preferentiallyadsorbed on the surface of carbon. The negative charges couldresult from oxygenated functional groups of basic character suchas lactone or hydroxyl groups, physically adsorbed at the surfaceof the pores of activated carbon. In view of this observation, pH 2[77.6% removal of Cr(VI) by RCC] was taken as the optimum pHfor further experimental studies.
Effect of Contact TimeContact time required for the maximum removal of Cr(VI) byactivated carbon was done by 2–10 mg/L of Cr(VI) and 50 mg ofadsorbent at pH 2. There is an increase of removal in initial stagesof reaction as seen in curve. The results show that chromiumuptake is fast for the first 10–60 min and thereafter, it proceeds ata slower rate and finally attains saturation. The initial fast reac-tion may be due to increased number of vacant sites availableat the initial stage; as a result there exist increased concentra-tion gradient between adsorbate in solution and adsorbate in theadsorbent. Generally, by the time adsorption involves a surfacereaction process, the initial adsorption is fast. Then,Q6 a sloweradsorption would follow as the available adsorption site whichis gradually decreased. Maximum percentage of Cr(VI) removaloccurs at 60 min after that the percentage removal remains uni-form. Removal of Cr(VI) at this point is maximum [77.6% forRCC in 10 mg/L of Cr(VI) solution]. In this view of observation, itwas decided to conveniently fix optimum contact time of activatedcarbon as 60 min for the remaining experiments.
| VOLUME 9999, 2012 | | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | 3 |
Author Proof
ATable 2. Effect of initial concentration on removal of Cr(VI) by RCC
Equilibrium concentration (mg/L) % of Cr(VI) removal
Co (mg/L) 30◦C 40◦C 50◦C 30◦C 40◦C 50◦C
2 0.3982 0.3524 0.3136 80.09 82.38 84.324 0.8215 0.7263 0.6381 79.46 81.84 84.056 1.2871 1.1284 0.9732 78.55 81.19 83.788 1.7845 1.5376 1.3012 77.69 80.78 83.74
10 2.2395 1.9397 1.654 77.61 80.60 83.46
Effect of Initial Concentration and TemperatureIn this investigation, 50 mg of adsorbent (RCC) was treated with50 mL of Cr(VI) solution containing different concentration. Sorp-tion experiments were carried out at the most suitable pH 2 ofadsorbent. In Table 2, at 30◦C, when the initial Cr(VI) ion con-centration is increased from 2 to 10 mg/L, Cr(VI) removal percentdecreased from 80.09% to 77.61%. But the actual amount ofCr(VI) adsorbed per unit mass of the adsorbent increased withincrease in chromium concentration. As the Cr(VI) concentrationwas increased from 2 to 10 mg/L, the unit adsorption of Cr(VI) onRCC increased from 1.602 to 7.761 mg/g. The adsorption capacityof an adsorbent which is obtained from the mass balance equa-tion on the adsorbate in a system with solution volume is oftenused to acquire the experimental adsorption isotherms (Goel etal., 2005).
The rise of the adsorption capacity increases with temperaturebecause of the rise of the kinetic energy of sorbent particles.Thus the collision frequency between adsorbent and adsorbateincreases, which results in the enhanced sorption onto the sur-face of the adsorbent. Secondly, at high temperature due to bondrupture of functional groups on adsorbent surfaceQ7 there more,be an increase in number of active adsorption sites, which mayalso lead to enhanced adsorption with the rise in temperature(Tewari et al., 2005).
Adsorption IsothermsIsotherms are mathematical relationships used to describe theadsorption behaviour of a particular adsorbent–adsorbate com-bination. They help in modelling adsorption behaviour andcalculating the adsorption capacity of materials. The three adsorp-tion isotherms studied are the Langmuir, Freundlich and Tempkinisotherms.
The linear form of the rearranged Langmuir model (Langmuir,1918) is
Ce
qe= 1
Q0 · bL+ Ce
Q0(3)
The linear form of Freundlich equation (Freundlich, 1906) is
log qe = log kf + 1n
log Ce (4)
where qe is the adsorption capacity at equilibrium time (mg/g);Ce is the final concentration of Cr(VI), mg/L; Q0 and bL are Lang-muir constants measuring the monolayer adsorption capacity andenergy of adsorption, The constants Q0 and bL can be calculatedfrom the slope and intercept of the plot of Ce/qe versus Ce. Thekf and n are the empirical constants of the Freundlich isothermmeasuring the adsorption capacity and intensity of adsorptionrespectively. According to Treybal, the values of n for this sys-
tem were calculated from the slope of the curve found to bebetween 1 and 2. Our findings are in good agreement with thefindings of Treybal who mathematically evaluated values of n fora number of mass transfer operations of systems and reported thatvalues of n between 1 and 10 would represent beneficial adsorp-tion (Treybal, 1980). Our experimental data of values kf and n areconsidered qualitatively consistent with those found in adsorptionof Chromium(VI) on activated carbon (Dakiky et al., 2002). Thevalues of Q0 increased when the solution temperature increasedfrom 30 to 50◦C. The increase in the value of Q0 with increase intemperature indicates that the Cr(VI) ions are favourably adsorbedby RCC at higher temperatures which shows that the adsorptionprocess is endothermic.
The Tempkin isotherm assumes that the fall in the heat ofadsorption is linear rather than logarithmic as stated in Freundlichexpression.
The heat of sorption of all the molecules in the layer decreaseslinearly with coverage due to adsorbate and adsorbent interac-tions. The Tempkin isotherm is applied in the following form:The linear form of Tempkin equation is
qe = RT
bTln aT + RT
bTln Ce (5)
where aT is the equilibrium binding constant (L/mg); bT is theheat of adsorption; R is the gas constant; T is the temperatureand Ce is the concentration of the metal ion at equilibrium. TheTempkin constants aT and bT are calculated from the slopes andintercepts of the plot qe versus ln Ce and are given in Table 3.Tempkin equilibrium binding constant and the Tempkin constantrelated to heat of adsorption decreases with increase in tem-perature up to 50◦C. However, the model failed to explain theadsorption isotherm Cr(VI) onto RCC when compared to Fre-undlich adsorption isotherm due to its poor correlation co-efficient(r2 = 0.9765).
The essential characteristics of the Langmuir equation can bedescribed by dimensionless equilibrium parameter
RL = 1(1 + bLC0)
(6)
where bL is the Langmuir constant (energy of adsorption) and C0
is the initial concentration of Cr(VI) in mg/L. The value of RL indi-cates the shape of the isotherms is either unfavourable (RL > 1),linear (RL = 1), favourable (0 < RL < 1) or irreversible (RL = 0).Based on RL values, adsorption is favourable.
The Langmuir (figure not shown) and Freundlich isotherm forthe removal of Cr(VI) from aqueous solution by activated car-bon prepared from RCC are shown in Figure 3. The correlationcoefficients, constants of Langmuir and Freundlich isotherms arepresented in Table 3. From Table 3, it was observed that correlation
| 4 | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | | VOLUME 9999, 2012 |
Author Proof
ATable 3. Results of isotherm models for the removal of Cr(VI) by RCC
Langmuir isotherm Freundlich isotherm Tempkin isotherm
Correlation Correlation Correlationcoefficient Constants coefficient Constants coefficient Constants
Temp. (◦C) r2 Q0 (mg/g) bL (L/mg) r2 kf (mg1 − 1/n L1/n g−1) n r2 bT (kJ/mol) aT (L/mg)
30 0.98 42.55 0.098 0.999 3.728 1.104 0.972 0.557 3.04940 0.981 57.14 0.084 0.999 4.358 1.079 0.963 0.609 3.41150 0.971 119.05 0.046 0.999 10.942 1.039 0.958 0.656 4.214
Figure 3. Freundlich plot for the removal of Cr(VI) by RCC.
coefficients of Freundlich isotherm are higher than the correla-tion coefficients of Langmuir isotherm and Tempkin isotherm.This indicates that Freundlich isotherm is fitted well with theexperimental data of the present system. The good agreement ofFreundlich isotherm indicates that the surface of the activatedcarbon prepared from RCC is highly heterogeneous (Gupta et al.,1998).
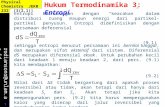
Thermodynamics StudiesThe standard free energy change (�G◦), enthalpy change (�H◦)and entropy (�S◦) were calculated from the variation of thethermodynamic equilibrium constant (K0). The values of equilib-rium constant for the adsorption process were determined by thereported method. The thermodynamic parameters were calculatedusing the following equations:
ln K0 = �S0
R− �H0
RT(7)
�G◦ = −RT ln K0 (8)
where �H◦ is the standard enthalpy of adsorption (kJ/mol), �G◦
is the standard free energy change (kJ/mol) and �S◦ is the stan-dard entropy change (J/K/mol). The values �H◦ and �S◦ can beobtained from the slope and intercept of the plot of ln K0 versus(1/T).
The computed standard free energy change, enthalpy andentropy changes along with equilibrium constants are given inTable 4. The results in Table 4 indicate that standard free energyvalues are negative which means that the reaction is spontaneous.The values of enthalpy of an adsorption process may be used todistinguish between physical and chemical adsorption. Enthalpychange values range from 11.799 to 15.896 kJ/mol; based onthese values, adsorption of Cr(VI) by RCC could be a physicaladsorption process. Positive values of standard enthalpy changesuggest that the process is endothermic in nature. Positive val-ues of standard entropy change suggest the increased randomnessat solid–solution interface. Yavuz et al. (2003) have studied theremoval of various heavy metals by kaolinite and concluded thatthe positive values of the entropy may be due to some structuralchanges in both the adsorbent and adsorbate during the adsorp-tion process (Yavuz et al., 2003).
Kinetics of Adsorption StudiesThe kinetic results obtained from the batch experiments wereanalysed using different kinetics models such as Lagergrenpseudo-first-order (Gupta et al., 2001)Q8 and pseudo-second-order(Ho and Mckay, 1998) models.
The integrated form of the pseudo-first-order kinetic model is
log (qe − qt) = log qe − k1
2.303t (9)
where qe and qt are the amounts of Cr(VI) adsorbed on adsorbent(mg/g) at equilibrium and time t, respectively and k1 is the rateconstant of first-order adsorption (min). Hence, a linear trace isexpected between the two parameters log(qe − qt) and t, provided
Table 4. Thermodynamic parameters for the removal of Cr(VI) by RCC
K0 �G◦ (kJ/mol)
C0 (mg/L) 30◦C 40◦C 50◦C 30◦C 40◦C 50◦C �H◦ (kJ/mol) �S◦ (J/K/mol)
2 4.023 4.675 5.378 −3.506 −4.014 −4.518 11.799 50.5134 3.869 4.507 5.269 −3.408 −3.918 −4.463 12.566 52.7026 3.662 4.317 5.165 −3.27 −3.806 −4.409 13.984 56.9128 3.483 4.203 5.148 −3.144 −3.736 −4.400 15.896 62.800
10 3.465 4.155 5.046 −3.131 −3.707 −4.347 15.286 60.748
| VOLUME 9999, 2012 | | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | 5 |
Author Proof
AFigure 4. Pseudo-second-order kinetics plot for the removal of Cr(VI) byRCC.
the adsorption follows first-order kinetics. The values of k1 and qe
can be determined from the slope and intercept.The adsorption may also be described by pseudo-second-order
kinetic model if the adsorption does not follow the first-orderkinetics.
The linearised form of the pseudo-second-order model is
t
qt
= 1k2qe
2+ 1
qet (10)
where k2 is the rate constant of pseudo-second-order adsorption,g/mg/min and t is time in minutes. A plot of t/qt and t shouldgive a linear relationship if the adsorption follows second order,qe and k2 could be calculated from the slope and intercept of theplot. Figure 4 is the pseudo-second-order model for removal ofCr(VI) from aqueous solution. The pseudo-first (k1) and pseudo-second-order rate constants (k2) and correlation coefficients areshown in Table 5.
A good agreement between calculated and experimental resultswas found in pseudo-second-order model. The experimental datafor the adsorption of Cr(VI) with high correlation co-efficient(r2 = 0.9999) in pseudo-second-order model. It is also to be notedthat the experimental adsorption capacities (qe) are very close tothe adsorption capacities calculated by the pseudo-second-orderkinetic model (Hamadi et al., 2001); these values are also fur-nished in Table 5.
Intraparticle DiffusionWeber and Morris (1963) suggested the following kinetic modelto investigate whether the adsorption is intraparticle diffusion or
Figure 5. Intraparticle diffusion plot for the removal of Cr(VI) by RCC.
not. According to this theory
qt = kidt1/2 (11)
The possibility of intraparticle diffusion was tested by plottingthe graph between amount of Cr(VI) adsorbed (qt) and square rootof time (t1/2) as shown in Figure 5. The intraparticle diffusionrate constant (kid) was calculated from the slope of the linearportion of curves. A gradual decrease in kid values from 0.0131 to0.0082 mg/g/min1/2 is observed when temperature is increasedfrom 30 to 50◦C. The initial curve portions show the boundarylayer diffusion and the final linear portions show the intraparticlediffusion effect. In Figure 5, the linear portions of the traces do notpass from the origin. This indicates that the mechanism of removalof Cr(VI) by RCC is complex and both the surface adsorption aswell as intraparticle diffusion contributes to the rate-determiningstep.
Fourier Transform-Infrared Spectroscopy (FT-IR)InvestigationsThe RCC spectrum at 3914–3383 cm−1 indicated the presenceof –OH groups of activated carbons. The aromatic –CH stretch-ing was observed at wave number from 3100 to 3000 cm−1. Apeak at 2857–2686 cm−1 indicated the presence of −CH and –COstretching group of aldehydes. The region from 1624 to 1585 cm−1
indicated the N–H bending of primary amines and –C–C stretchingof aromatic groups. The region from 1370 to 1350 cm−1 indi-cated –CH stretching of alkanes group. The regions from 1250to 1020 cm−1 and 910 to 665 cm−1 indicated the presence of –CNstretching and –NH stretching of aliphatic amines. The region
Table 5. Pseudo-first and pseudo-second-order constants for the removal of Cr(VI) by RCC
Pseudo-first-order kinetic model Pseudo-second-order kinetic model
C0 (mg/L) qe(exp) (mg/g) qe(cal) (mg/g) k1 (min−1) r2 P qe(cal) (mg/g) k2 (g/mg/min) r2 P
2 1.602 0.159 0.055 0.956 90.029 1.620 0.717 0.999 1.1484 3.179 0.504 0.063 0.927 84.156 3.235 0.245 0.999 1.7876 4.713 0.107 0.05 0.979 97.733 4.726 1.015 0.999 0.2738 6.216 0.119 0.056 0.991 98.076 6.231 0.976 0.999 0.241
10 7.761 0.128 0.051 0.993 98.349 7.776 0.861 0.999 0.199
| 6 | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | | VOLUME 9999, 2012 |
Author Proof
AFigure 6. (a) SEM photograph of adsorbent before adsorption of Cr(VI).(b) SEM photograph of adsorbent after adsorption of Cr(VI).
from 850 to 550 cm−1 indicated the presence of –C–Cl stretchingof alkyl halides.
After the adsorption of Cr(VI) on all activated carbons, therewas a small shift in wave number and some of the wave num-ber regions were not observed. This observation indicated theparticipation of adsorption of Cr(VI) on RCC activated carbons.Numerous chemical functional groups like carboxyl, hydroxyl,amide and halogens have been identified as potential adsorptionsites to be responsible for binding metallic ions to activated carbon(Sheng et al., 2005).
Scanning Electron Microscopic (SEM) StudiesThe SEM micrograph clearly states the porous structure of theactive carbon (Figure 6a,b). There are holes and cave-type open-ings on the surface of the specimen which would definitelyincrease the surface area available for adsorption. It is clearly seenon the surface of active carbon after adsorption in Figure 6b thatthe caves, pores and surfaces of adsorbent is covered by adsor-bate. It was evident that the adsorbent structure changed uponadsorbing the Cr(VI) ions studied.
Possible Mechanism of Adsorption of Cr(VI)For activated carbons, maximum adsorption was noticed at lowerpH range. The pH dependence of metal adsorption can largelyrelate to the type of ionic state of the functional group present inthe adsorbent and also to the metal chemistry in the solution. Highadsorption of Cr(VI) at low pH can be explained by the species ofthe chromium and adsorbent surface. At acidic pH, the predomi-nant species of chromium are Cr2O2−
7 , HCrO−4 , Cr3O2−
10 and Cr4O2−13
while at pH above 8, the stable species of chromium is CrO2−4 .
As the pH decreases in the region 2–6, the equilibrium shifts todichromate according to the overall equilibrium
2CrO42− + 2H+ → Cr2O7
2− + H2O
At still lower pH values Cr3O2−10 and Cr4O2−
13 species are formed.Thus, decreasing pH results in the formation of more polymerisedchromium oxide species. On the other hand, under acidic condi-tions, the surface of the adsorbent becomes highly protonated andfavours the uptake of Cr(VI) in the anionic form. With increase inpH, the degree of protonation of the surface decreases graduallyand hence adsorption is reduced. Furthermore, as pH increases,there is competition between the OH− and chromate ions, theformer being the dominant species at higher pH values. Thenet positive surface potential of adsorbent decreases, resultingin the weakening of electrostatic forces between adsorbent andadsorbate, which ultimately leads to reduced adsorption capac-ity. Increase in pH can be explained by solute adsorbent bindingreactions. When the oxo groups present on the surface of carbona-ceous materials come into contact with water, they react with thewater molecule as follows:
CxO + 2H2O → CxOH22+ + 2OH−
Thus hydroxyl ions released into solutions raise the equilibriumpH. When Cr(VI) ions are introduced into the system, they areabsorbed onto the positively charged surface
CxOH22+ + HCrO4
− → CxO2H3CrO3+
Combining the above reactions
CxO + HCrO4− + 2H2O → CxO2H3CrO3
+ + 2OH−
Hence, every mole of HCrO−4 adsorbed results in the release of
two moles of hydroxyl ions into solution, which eventually raisesthe solution pH. In addition, the carbonaceous material consumesprotons when it comes into contact with water and this eventuallyraises the solution pH. The high pH value causes a reduction inthe electrostatic attraction between negatively charged chromiumions and positively charged adsorbent surfaces, thereby loweringthe metal adsorption.
Test With Field SamplesTen water samples were collected from in and around NamakkalDistrict. The places of water sample collection were: 1.Salem road—Anbu nagar (Northern region), 2. Paramathimain road—Thillaipuram (Western region), 3. Thuraiyur mainroad—Ganesapuram (Eastern region), 4. Tiruchengode mainroad—Nallipalayam (Western region), 5. Trichy main road—S.K.Nagar (Southern region), 6. Velagoundampatty, 7. Pudhanchan-thai, 8. Valayapatty, 9. Aniyapauram and 10. Erumapatty. From
| VOLUME 9999, 2012 | | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | 7 |
Author Proof
ATable 6. Chemical parameters of water (a) before adsorption and (b) after adsorption
Parameter 1 2 3 4 5 6 7 8 9 10
(a)pH 8.33 7.33 7.4 7.92 7.62 8.17 6.9 7.08 7.64 7.61Total hardness 650 1050 830 860 810 400 805 880 380 475Total alkalinity 475 935 415 440 700 300 325 500 415 325Chloride 692 888 266 479 284 178 437 316 149 142Sulphate 240 220 150 230 150 110 92 87 85 70Iron 0.5 0.4 0.3 0.4 0.1 0.2 0.1 0.2 0.1 0.1Chromium 0.1 0.09 0.06 0.09 0.05 0.06 0.05 0.04 0.05 0.04Nickel 0.04 0.03 0.01 0.04 0.01 0.02 0.01 0.01 0.02 0.01Lead 0.1 0.08 0.06 0.1 0.05 0.06 0.05 0.04 0.03 0.05Fluoride 1.3 1.2 0.4 1.4 0.5 0.1 0.3 0.5 0.6 0.2
(b)pH 7.33 7.03 7.2 7.42 7.2 7.15 6.8 7 7.24 7.34Total hardness 534 750 430 560 510 350 605 660 350 355Total alkalinity 325 550 315 350 500 250 225 400 305 285Chloride 452 568 246 376 254 158 327 216 119 112Sulphate 210 170 130 180 120 100 82 76 75 60Iron Nil Nil Nil Nil Nil Nil Nil Nil Nil NilChromium Nil Nil Nil Nil Nil Nil Nil Nil Nil NilNickel Nil Nil Nil Nil Nil Nil Nil Nil Nil NilLead Nil Nil Nil Nil Nil Nil Nil Nil Nil NilFluoride Nil Nil Nil Nil Nil Nil Nil Nil Nil Nil
Units of all the parameters in mg/L except pH.
these few samples contain moderately heavy metals concentra-tion mentioned in Table 6a, which causes the harmful diseasesto human beings and these samples were treated with RCC. Aftertreatment, there is no heavy metals concentration found in thesewater samples (0.000 mg/L) as shown in Table 6b. The water qual-ity parameters of the treated water are good and in agreementwith the World Health Organisation (WHO) and Bureau of IndianStandards (BIS).
Comparison of Cr(VI) Removal With DifferentAdsorbentsThe adsorption capacity of the adsorbents for the removal ofCr(VI) has been compared with those of others reported in theliterature and the values of adsorption capacity as presented in
Table 7. Comparison of adsorption capacities of different adsorbentsfor the removal of Cr(VI)
Adsorptioncapacity
Adsorbents (mg/g) Refs.
Raw rice bran 0.07 Oliveria et al. (2005)Riverbed sand 0.15 Sharma and Weng (2007)Activated rice husk
carbon0.8 Bishnoi et al. (2004)
Wollastonite 0.83 Sharma (2001)Coconut shell
powder1.23 Pino et al. (2006)
Flyash 1.4 Banarjee et al. (2004)Activated alumina 1.6 Bishnoi et al. (2004)Nanoiron 3.56 Sharma et al. (2009)Saw dust 3.6 Baral et al. (2008)Distillary sludge 5.7 Selvaraj et al. (2003)Ricinus communis
seed shell activecarbon
7.7 Present study
Table 7. The experimental data of the present investigation arecompared with reported values. Results of our investigationrevealed that RCC has highest percent removal and adsorptioncapacity.
CONCLUSIONSR. communis seedQ9 shell activated carbon is an agriculturalwaste, abundance, cheapness and environmental friendly naturecould be used as potential adsorbent for the removal of Cr(VI)from aqueous solution contains heavy metals and polluted water.The Freundlich adsorption isotherm model describes the adsorp-tion behaviour with good correlation co-efficient. The adsorptionof Cr(VI) was depended on the pH of the solution. Based on theresults, the optimum contact time is 60 min and adsorbent dosageis 50 mg. The metal ion adsorption obeyed the pseudo-second-order kinetic model. The removal of Cr(VI) is simultaneouslyincreased with increase in the temperature from 30 to 50◦C.
ACKNOWLEDGEMENTSThe authors are thankful to the Chairman and the Management ofSona College of Technology, Salem and Annai Mathammal SheelaEngineering College, Namakkal, Tamil Nadu, India for providingnecessary research facilities to carry out this study.
REFERENCESAcar, F. N. and E. Malkoc, “The Removal of Chromium(VI) From
Aqueous Solutions by Fagus orientalis L.,” Bioresour.Technol. 94(1), 13–15 (2004).
Agarwal, G. S., H. K. Bhuptawat and S. Chaudhari, “Biosorptionof Aqueous Chromium(VI) by Tamarindus indica Seeds,”Bioresour. Technol. 97, 949–956 (2006).
AjmalQ10, M., R. A. K. Rao, R. Ahmad and M. A. Khan,“Adsorption Studies on Parthenium hysterophorous Weed:
| 8 | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | | VOLUME 9999, 2012 |
Author Proof
ARemoval and Recovery of Cd(II) From Wastewater,” J.Hazard. Mater. B135, 242–248 (2006).
Aksu, Z., F. Gonen and Z. Demircan, “Biosorption ofChromium(VI) Ions by Mowital B30H Resin ImmobilisedActivated Sludge in a Packed Bed; Comparison With GranularActivated Carbon,” Process Biochem. 38, 175–186 (2002).
Al-Haj, A. A. and R. El-Bishtawi, “Removal of Lead and NickelIons Using Zeolite Tuff,” J. Chem. Technol. Biotechnol. 69,27–34 (1999).
APHA, AWWA, WPCF, “Standard Methods for the Examinationof Water and Wastewater,” 16th ed., American Public HealthAssociation, Washington, DC (1985), pp. 445–456.
Baral, S. S., S. N. Das, G. R. Chaudhury, Y. V. Swamy and P.Rath, “Adsorption of Cr(VI) Using Thermally Activated WeedSalvinia Cucullata,” Chem. Eng. J. 139, 245–255 (2008).
Banarjee, S. S., M. V. Joshi and R. V. Jayaram, “Removal ofCr(VI) and Hg(II) From Aqueous Solutions Using Fly Ash andImpregnated Fly Ash,” Sep. Sci. Technol. 39, 1611–1629(2004).
Benefield, L. D., J. F. Judkins and B. L. Weand, “ProcessChemistry for Water and Wastewater Treatment,” EnglewoodCiffs, NJ (1982), pp. 433–435.
Bishnoi, N., M. Bajaj, N. Sharma and A. Gupta, “Adsorption ofCr(VI) on Activated Rice Husk Carbon and ActivatedAlumina,” Bioresour. Technol. 91, 305–307 (2004).
Dakiky, M., M. Khamis, A. Manassra and M. Mereb, “SelectiveAdsorption of Chromium(VI) in Industrial Wastewater UsingLow Cost Abundantly Available Adsorbents,” Adv. Environ.Res. 6, 533–540 (2002).
Freundlich, H., “Adsorption in Solutions,” Phys. Chem. 57,385–410 (1906).
Garg, V. K., R. Gupta, R. Kumar and R. K. Gupta, “Adsorption ofChromium From Aqueous Solution on Treated Sawdust,”Bioresour. Technol. 92, 79–81 (2004).
Gilcreas, F. W., M. J. Tarars and R. S. Ingols, “Standard Methodsfor the Examination of Water and Wastewater,” 12th ed.,American Public Health Association (APHA), Inc., New York(1965), pp. 213–220.
Goel, J., K. Kadirvelu, C. Rajagopal and V. K. Garg, “Removal ofLead(II) by Adsorption Using Treated Granular ActivatedCarbon: Batch and Column Studies,” J. Hazard. Mater. B125,211–220 (2005).
Gupta, V. K., M. Gupta and S. Sharma, “Process Development forthe Removal of Lead and Chromium From Aqueous SolutionsUsing Red Mudan Aluminum Industry Waste,” Water Res. 35,1124–1134 (2001a).
Gupta, V. K., K. T. Park, S. Sharma and D. Mohan, “Removal ofChromium(VI) From Electroplating Industry WastewaterUsing Bagasse Fly Ash—A Sugar Industry Waste Material,”Environmentalist 19, 129–136 (1999).
Gupta, V. K. and A. Rastogi, “Sorption and Desorption Studies ofChromium (VI) From Nonviable Cyano Bacterium Nostocmuscorum Biomass,” J. Hazard. Mater. 154(1), 347–354(2008).
Gupta, V. K., A. Rastogi and A. Nayak, “Adsorption Studies onthe Removal of Hexavalent Chromium From AqueousSolution Using a Low Cost Fertiliser Industry WasteMaterial,” J. Colloid Interface Sci. 342, 135–141 (2010).
Gupta, V. K., S. Sharma, I. S. Yadau and M. Dinesh, “Utilisationof Bagasses Fly Ash Generated in the Sugar Industry for theRemoval of Phenol and p-Nitrophenol From Waste Water,” J.Chem. Technol. Bio-Technol. 71, 180–186 (1998).
Gupta, V. K., A. K. Shrivastava and N. Jain, “Biosorption ofChromium(VI) From Aqueous Solutions by Green AlgaeSpirogyra Species,” Water Res. 35, 4079–4085(2001b).
Hamadi, N. K., D. X. Chen, M. M. Farid and M. G. Q. Lu,“Adsorption Kinetics for the Removal Chromium(VI) FromAqueous Solution by Adsorbents Derived From Used TyresSawdust,” Chem. Eng. J. 84, 95–105 (2001).
Ho, Y. S. and G. Mckay, “Kinetic Models for the Sorption of DyeFrom Aqueous Solution by Wood,” Trans. IChemE 76(B),183–191 (1998).
Karthikeyan, S., P. Sivakumar and P. N. Palanisamy, “NovelActivated Carbons From Agricultural Wastes and TheirCharacterisation,” Eur. J. Chem. 5, 409–426 (2008).
Kadirvelu, K., K. Thamaraiselvi and C. Namasivayam,“Adsorption of Nickel(II) From Aqueous Solution OntoActivated Carbon Prepared From Coirpitch,” Sep. Purif.Technol. 24, 497–505 (2001).
Khezami, L. and R. Capart, “Removal of Chromium(VI) FromAqueous Solution by Activated Carbons: Kinetic andEquilibrium Studies,” J. Hazard. Mater. 123(1), 223–231(2005).
Langmuir, I., “Adsorption of Gases on Plane Surfaces of Glass,Mica and Platinum,” J. Am. Chem. Soc. 40, 1361–1403(1918).
Loukidou, M. X., A. I. Zouboulis, T. D. Karapantsios and K. A.Matis, “Equilibrium and Kinetic Modelling of Chromium(VI)Biosorption by Aeromonas caviae,” Colloids Surf. A 242,93–104 (2004).
Malkoc, E. and Y. Nuhoglu, “The Removal of Chromium (VI)From Synthetic Wastewater by Ulothrix zonata,” Fresen.Environ. Bull. 12(4), 376–381 (2003).
Mohan, D., K. P. Singh and V. K. Singh, “Removal of HexavalentChromium From Aqueous Solution Using Low Cost ActivatedCarbons Derived From Agricultural Waste Materials andActivated Carbon Fabric Cloth,” Ind. Eng. Chem. Res. 44,1027–1042 (2005).
Mohan, D., K. P. Singh and V. K. Singh, “Trivalent ChromiumRemoval From Wastewater Using Low Cost Activated CarbonDerived From Agricultural Waste Material and ActivatedCarbon Fabric Cloth,” J. Hazard. Mater. B135, 280–295(2006).
Oliveria, E. A., S. F. Montanher, A. D. Andrade, J. A. Nobregaand M. C. Rollemberg, “Equilibrium Studies for the Sorptionof Chromium and Nickel From Aqueous Solutions Using RawRice Bran,” Process Biochem. 40, 3485–3490 (2005).
Park, D., S. Y. Yun and J. M. Park, “Studies on HexavalentChromium Biosorption by Chemically Treated Biomass ofEcklonia sp.,” Chemosphere 60, 1356–1364 (2005).
Pino, G. H., L. M. S. Mesquita De, M. L. Torem and G. A. S.Pinto, “Biosorption of Heavy Metals by Powder of GreenCoconut Shell,” Sep. Sci. Technol. 41, 3141–3153 (2006).
Ricordel, S., S. Taha, I. Cisse and G. Dorange, “Heavy MetalsRemoval by Adsorption Onto Peanut Husks Carbon:Characterisation, Kinetic Study and Modelling,” Sep. Purif.Technol. 24, 389–401 (2001).
Sarin, V. and K. K. Pant, “Removal of Chromium From IndustrialWaste by Using Eucalyptus Bark,” Bioresour. Technol. 97(1),15–20 (2006).
Selvi, K., S. Pattabhi and K. Kadirvelu, “Removal of Cr(VI) FromAqueous Solution by Adsorption Onto Activated Carbon,”Bioresour. Technol. 80, 87–89 (2001).
| VOLUME 9999, 2012 | | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | 9 |
Author Proof
ASelvaraj, K., S. Manonmani and S. Pattabhi, “Removal of
Hexavalent Chromium Using Distillery Sludge,” Bioresour.Technol. 89, 207–211 (2003).
Sheng, P. X., Y. P. Ting, J. P. Chen and L. Hong, “Sorption ofLead, Copper, Cadmium, Zinc and Nickel by Marine AlgalBiomass: Characterisation of Biosorptive Capacity andInvestigation of Mechanisms,” J. Colloid Interf. Sci. 275,131–141 (2005).
Sharma, S. and K. T. Park, “Removal of Chromium (VI) FromElectroplating Industry Wastewater Using Bagasse FlyAsh—A Sugar Industry Waste Material,” Environmentalist19(2), 129–136 (1999).
Sharma, Y. C., “Effect of Temperature on Interfacial Adsorptionof Cr(VI) on Wollastonite,” J. Colloid Interface Sci. 233,265–270 (2001).
Sharma, Y. C., V. Srivastava, C. H. Weng and S. N. Upadhyyay,“Removal of Cr(VI) From Wastewater by Adsorption on IronNanoparticles,” Can. J. Chem. Eng. 87, 921–929 (2009).
Sharma, Y. C. and C. H. Weng, “Removal of Chromium(VI) FromWater and Wastewater by Using Riverbed Sand: Kinetic andEquilibrium Studies,” J. Hazard. Mater. 142, 449–454 (2007).
Srivastava, S. K., V. K. Gupta and D. Mohan, “Kinetic Parametersfor the Removal of Lead and Chromium From WastewaterUsing Activated Carbon Developed From Fertiliser WasteMaterial,” Environ. Model. Asses. 1, 281–290 (1996).
Srivastava, S. K., V. K. Gupta and D. Mohan, “Removal of Leadand Chromium by Activated Slag—A Blast Furnace Waste,” J.Environ. Eng. 123(5), 461–468 (1997).
Tewari, N., P. Vasudevan and B. K. Guha, “Study on Biosorptionof Cr(VI) by Mucorhiemalis,” Biochem. Eng. J. 23, 185–192(2005).
Treybal, R. E., “Mass Transfer Operations,” 3rd ed., McGraw Hill,New York (1980).
USEPA, “National Primary Drinking Water Regulations, GroundWater and Drinking Water,” Consumer Factsheet onChromium (1995).
Vijayaraghavan, K., J. Jegan, K. Palanivelu and M. Velan,“Biosorption of Cobalt(II) and Nickel(II) by Seaweeds Batchand Column Studies,” Sep. Purif. Technol. 44, 53–59 (2005).
Weber, W. J. and J. C. Morris, “Kinetics of Adsorption on CarbonFrom Solution,” J. Sanit. Eng. Div. Am. Soc. Civil Eng. 89,31–60 (1963).
Yavuz, O., Y. Altunkaynak and F. Guzel, “Removal of Copper,Nickel, Cobalt and Manganese From Aqueous Solution byKaolinite,” Water Res. 37, 948–952 (2003).
Manuscript received January 7, 2011; revised manuscriptreceived August 22, 2011; accepted for publication August 23,2011.
Q1: Please check the usage of ‘circulatory’.Q2: Please specify whether it is Gupta et al. 2001a or 2001b.Q3: Please give city information for these manufacturers.Q4: Please give city information for this manufacturer.Q5: Please verify/modify the sentence for clarity.Q6: Please verify/modify the sentence for clarity.Q7: Please check the usage of ‘there more’.Q8: Please specify whether it is Gupta et al. 2001a or 2001b.Q9: Please verify/modify the sentence for clarity.Q10: Ajmal et al. 2006 has not been cited in the text. Pleaseindicate where it should be cited; or delete from the ReferenceList.
| 10 | THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING | | VOLUME 9999, 2012 |
USING E-ANNOTATION TOOLS FOR ELECTRONIC PROOF CORRECTION
Required Software
Adobe Acrobat Professional or Acrobat Reader (version 7.0 or above) is required to e-annotate PDFs. Acrobat 8 Reader is a free download: http://www.adobe.com/products/acrobat/readstep2.html.For help with system requirements, go to: http://www.adobe.com/support/.
Once you have Acrobat Reader on your PC and open the proof, you will see the Commenting Toolbar (if it does not appear automatically go to Tools>Commenting>Commenting Toolbar). If these options are not available in your Adobe Reader menus then it is possible that your Adobe version is lower than 7 or the PDF has not been prepared properly.
PDF Annotations (Adobe Reader version 7 or 8) – Commenting Toolbars look like this:
(PC, Adobe version 7)
(PC, Adobe version 8, right click on title bar (Comment & Markup) to show additional icons)
(Mac)
PDF Annotations (Adobe Reader version 9)
If you experience problems annotating files in Adobe Acrobat Reader 9 then you may need to change a preference setting in order to edit.
The default for the Commenting toolbar is set to ‘off’ in version 9. To change this setting select ‘Edit | Preferences’, then ‘Documents’ (at left under ‘Categories’), then select the option ‘Never’ for ‘PDF/A View Mode’. (the Commenting toolbar is the same as in version 8).
PLEASE DO NOT ATTEMPT TO EDIT THE ARTICLE TEXT ITSELF TO INDICATE INSERT, REPLACE, OR REMOVE TEXT
• Insert text
Click the ‘Text Edits’ button on the Commenting toolbar. Click to set the cursor location in the text and simply start typing. The text will appear in a commenting box. You may also cut-and-paste text from another file into the commenting box. Close the box by clicking on ‘x’ in the top right-hand corner. It can be deleted by right clicking (for the PC, ctrl-click on the Mac) on it and selecting ‘Delete’.
• Replace text Click the ‘Text Edits’ button on the Commenting toolbar. To highlight the text to be replaced, click and drag the cursor over the text. Then simply type in the replacement text. The replacement text will appear in a commenting box. You may also cut-and-paste text from another file into this box. To replace formatted text (an equation for example) please Attach a file (see below).
• Remove text Click the ‘Text Edits’ button on the Commenting toolbar. Click and drag over the text to be deleted. Then press the delete button on your keyboard. The text to be deleted will then be struck through. HIGHLIGHT TEXT/MAKE A COMMENT
Click on the ‘Highlight’ button on the commenting toolbar. Click and drag over the text. To make a comment, double click on the highlighted text and simply start typing. ATTACH A FILE
Click on the ‘Attach a file’ button on the commenting toolbar. Click on the figure, table or formatted text to be replaced. A window will automatically open allowing you to attach a file. To make a comment, go to ‘General’ and then ‘Description’ in the ‘Properties’ window. A graphic will appear indicating the insertion of a file. LEAVE A NOTE/COMMENT
Click on the ‘Note Tool’ button on the commenting toolbar. Click to set the location of the note on the document and simply start typing. Do not use this feature to make text edits. REVIEW
To review your changes, click on the ‘Show’ button on the commenting toolbar. Choose ‘Show Comments List’. Navigate by clicking on a correction in the list. Alternatively, double click on any mark-up to open the commenting box. UNDO/DELETE CHANGE To undo any changes made, use the right click button on your mouse (for PCs, Ctrl-Click for Mac). Alternatively click on the ‘Edit’ in the main Adobe menu and then ‘Undo’. You can also delete edits using the right click (Ctrl-Click on the Mac) and selecting ‘Delete’. SEND YOUR ANNOTATED PDF FILE BACK TO WILEY VIA [email protected] Save the annotations to your file and return as an e-mail. Before returning, please ensure you have answered any questions raised on the Query form that you have inserted all the corrections: later inclusion of any subsequent corrections cannot be guaranteed. Note: Comprehensive instructions are provided within your PDF file: to access these instructions please click on the Comments and Markup menu in the main tool bar, or click on Help.
Additional reprint and journal issue purchases
Should you wish to purchase additional copies of your article, please click on the link and follow the instructions provided: https://caesar.sheridan.com/reprints/redir.php?pub=10089&acro=CJCE 9&acro=JCB Corresponding authors are invited to inform their co‐authors of the reprint options available.
Please note that regardless of the form in which they are acquired, reprints should not be resold, nor further disseminated in electronic form, nor deployed in part or in whole in any marketing, promotional or educational contexts without authorization from Wiley. Permissions requests should be directed to mailto: [email protected]
For information about ‘Pay‐Per‐View and Article Select’ click on the following link: http://wileyonlinelibrary.com/ppv
COPYRIGHT TRANSFER AGREEMENT
111 River Street Hoboken, NJ 07030 201-748-6873
Date: To:
Production/Contribution ID # ____________ Publisher/Editorial office use only
Re: Manuscript entitled ___________________________________________________ _______________________________________________________(the "Contribution") for publication in the The Canadian Journal of Chemical Engineering (the "Journal") published by Wiley Periodicals, Inc. ("Wiley Periodicals") for the Canadian Society for Chemical Engineering (“CSChE”) Dear Contributor(s): Thank you for submitting your Contribution for publication. In order to expedite the editing and publishing process and enable Wiley Periodicals to disseminate your work to the fullest extent, we need to have this Copyright Transfer Agreement signed and returned to us as soon as possible. If the Contribution is not accepted for publication this Agreement shall be null and void. A. COPYRIGHT
1. The Contributor assigns to the CSChE, during the full term of copyright and any extensions or renewals of that term, all copyright in and to the Contribution, including but not limited to the right to publish, republish, transmit, sell, distribute and otherwise use the Contribution and the material contained therein in electronic and print editions of the Journal and in derivative works throughout the world, in all languages and in all media of expression now known or later developed, and to license or permit others to do so.
2. Reproduction, posting, transmission or other distribution or use of the Contribution or any material
contained therein, in any medium as permitted hereunder, requires a citation to the Journal and an appropriate credit to Wiley Periodicals as publisher, suitable in form and content as follows: (Title of Article, Author, Journal Title and Volume/Issue Copyright © [year] the Canadian Society for Chemical Engineering or copyright owner as specified in the Journal.)
B. RETAINED RIGHTS
Notwithstanding the above, the Contributor or, if applicable, the Contributor's Employer, retains all proprietary rights other than copyright, such as patent rights, in any process, procedure or article of manufacture described in the Contribution, and the right to make oral presentations of material from the Contribution.
C. OTHER RIGHTS OF CONTRIBUTOR The CSChE grants back to the Contributor the following: 1. The right to share with colleagues print or electronic "preprints" of the unpublished Contribution, in
form and content as accepted by Wiley Periodicals for publication in the Journal. Such preprints may be posted as electronic files on the Contributor's own website for personal or professional use, or on the Contributor's internal university or corporate networks/intranet, or secure external website at the Contributor’s institution, but not for commercial sale or for any systematic external distribution by a third party (e.g., a listserve or database connected to a public access server). Prior to publication, the Contributor must include the following notice on the preprint: "This is a preprint of an article accepted for publication in [Journal title] © copyright (year) (copyright owner as specified in the Journal)". After publication of the Contribution by Wiley Periodicals, the preprint notice should be amended to read as follows: "This is a preprint of an article published in [include the complete citation information for the final version of the Contribution as published in the print edition of the Journal]", and should provide an electronic link to the Journal's WWW site, located at the following
URL: http://www.interscience.wiley.com/. The Contributor agrees not to update the preprint or replace
it with the published version of the Contribution.
2. The right, without charge, to photocopy or to transmit online or to download, print out and distribute to a colleague a copy of the published Contribution in whole or in part, for the Colleague’s personal or professional use, for the advancement of scholarly or scientific research or study, or for corporate informational purposes in accordance with Paragraph D.2 below.
3. The right to republish, without charge, in print format, all or part of the material from the published Contribution in a book written or edited by the Contributor.
4. The right to use selected figures and tables, and selected text (up to 250 words, exclusive of the abstract) from the Contribution, for the Contributor's own teaching purposes, or for incorporation within another work by the Contributor that is made part of an edited work published (in print or electronic format) by a third party, or for presentation in electronic format on an internal computer network or external website of the Contributor or the Contributor's employer.
5. The right to include the Contribution in a compilation for classroom use (course packs) to be distributed to students at the Contributor’s institution free of charge or to be stored in electronic format in datarooms for access by students at the Contributor’s institution as part of their course work (sometimes called “electronic reserve rooms”) and for in-house training programs at the Contributor’s employer.
D. CONTRIBUTIONS OWNED BY EMPLOYER 1. If the Contribution was written by the Contributor in the course of the Contributor's employment (as a
"work-made-for-hire" in the course of employment), the Contribution is owned by the company/employer which must sign this Agreement (in addition to the Contributor’s signature), in the space provided below. In such case, the company/employer hereby assigns to the CSChE, during the full term of copyright, all copyright in and to the Contribution for the full term of copyright throughout the world as specified in paragraph A above.
2. In addition to the rights specified as retained in paragraph B above and the rights granted back to the
Contributor pursuant to paragraph C above, the CSChE hereby grants back, without charge, to such company/employer, its subsidiaries and divisions, the right to make copies of and distribute the published Contribution internally in print format or electronically on the Company's internal network. Upon payment of the Wiley Periodicals reprint fee, the institution may distribute (but not resell) print copies of the published Contribution externally. Although copies so made shall not be available for individual re-sale, they may be included by the company/employer as part of an information package
included with software or other products offered for sale or license. Posting of the published Contribution by the institution on a public access website may only be done with Wiley Periodicals’ written permission, and payment of any applicable fee(s).
E. GOVERNMENT CONTRACTS In the case of a Contribution prepared under U.S. Government contract or grant, the U.S. Government may reproduce, without charge, all or portions of the Contribution and may authorize others to do so, for official U.S. Government purposes only, if the U.S. Government contract or grant so requires. (U.S. Government Employees: see note at end). F. COPYRIGHT NOTICE The Contributor and the company/employer agree that any and all copies of the Contribution or any part thereof distributed or posted by them in print or electronic format as permitted herein will include the notice of copyright as stipulated in the Journal and a full citation to the Journal as published by Wiley Periodicals. G. CONTRIBUTOR'S REPRESENTATIONS The Contributor represents that the Contribution is the Contributor's original work. If the Contribution was prepared jointly, the Contributor agrees to inform the co-Contributors of the terms of this Agreement and to obtain their signature to this Agreement or their written permission to sign on their behalf. The Contribution is submitted only to the Journal and has not been published before, except for "preprints" as permitted above. (If excerpts from copyrighted works owned by third parties are included, the Contributor will obtain written permission from the copyright owners for all uses as set forth in Wiley Periodicals permissions form or in the Journal’s Instructions for Contributors, and show credit to the sources in the Contribution.) The Contributor also warrants that the Contribution contains no libelous or unlawful statements, does not infringe upon the rights (including without limitation the copyrights, patent or trademark rights) or the privacy of others, or contain material or instructions that might cause harm or injury. CHECK ONE:
[____]Contributor-owned work Contributor's signature
Date
Type or print name and title Contributor's signature
Date
Type or print name and title
ATTACH ADDITIONAL SIGNATURE PAGE AS NECESSARY
[____]Company/Institution-owned work(made-for-hire in the course of employment)
Company or Institution (Employer-for-Hire)
Date
Authorized signature of Employer
Date
[____]U.S. Government work
[____] Canadian Government Work
Note to U.S. Government Employees A Contribution prepared by a U.S. federal government employee as part of the employee's official duties, or which is an official U.S. Government publication is called a "U.S. Government work," and is in the public domain in the United States. In such case, the employee may cross out Paragraph A.1 but must sign and return this Agreement. If the Contribution was not prepared as part of the employee's duties or is not an official U.S. Government publication, it is not a U.S. Government work.
Note to Canadian Government Employees A Contribution prepared by a Canadian Government employee as part of his/her official duties is owned by the Canadian Government. In such case, the employee warrants that he/she has been authorized by the Canadian Government to grant exclusive worldwide publication rights to the Contribution, and hereby grants such a license. Please check the following: ____Canadian Government Work The copyright on the print and online article will read as follows: ©2008 Government of Canada. Exclusive worldwide publication rights in the article have been transferred to Wiley Periodicals, Inc.