Exchange-Coupled Bimagnetic Cobalt/Iron Oxide Branched Nanocrystal Heterostructures
Interfacial interactions between poly[L-lysine]-based branched polypeptides and phospholipid model...
-
Upload
independent -
Category
Documents
-
view
0 -
download
0
Transcript of Interfacial interactions between poly[L-lysine]-based branched polypeptides and phospholipid model...
ary
MA-DPH,nteractionteric Eurete theented for
n intoxcept Eor bioactive
Journal of Colloid and Interface Science 267 (2003) 18–24www.elsevier.com/locate/jcis
Interfacial interactions between poly[L-lysine]-based branchedpolypeptides and phospholipid model membranes
R. Szabó,a F. Hudecz,a and F. Reigb,∗
a Research Group Peptide Chemistry at Eötvös L. University, Hungarian Academy of Sciences, P.O. Box 32, H-1518 Budapest 112, Hungb Department of Peptide and Protein Chemistry, Institute for Chemical and Environmental Research, CSIC, Jordi Girona 18,
08034 Barcelona, Spain
Received 30 October 2002; accepted 29 May 2003
Abstract
The interaction of five poly[L-lysine]-derived branched chain polypeptides of poly[Lys(Xi )] (X iK) or poly[Lys(Xi -DL-Alam)] (XAK) withlipid bilayers (DPPC and DPPC/PG, 8:2) was studied by fluorescence polarization techniques. Two fluorescent probes, DPH and Twere utilized to monitor changes of motion in the internal and/or in the polar head regions, respectively. Results indicate that the iof polypeptides with neutral (DPPC) bilayers is mainly dependent on the polarity and electrical charge of side chains. The amphoiKshows the highest level of interaction. Polycationic polypeptides (HiK, PiK, TAK) have a relatively small effect on the transition temperatof the lipids, while the polyanionic Succ-EAK has no effect at the alkyl chain region of the bilayer. Data with TMA-DPH indicalack of pronounced interaction between the polypeptides and the outer surface of the liposome. Similar tendency was documDPPC/PG vesicles. Polypeptides, HiK, and PiK induce significant changes in the transition temperature, thus indicating their insertiothe hydrophobic core of the bilayer without marked effect on the polar head region. Results suggest that these polypeptides (eiK)have no destabilizing effect on liposomes studied. These properties are considered as beneficial for their use as safe carriers fmolecules. 2003 Elsevier Inc. All rights reserved.
Abbreviations:DPH: (1,6-diphenyl-1,3,5-hexatriene); TMA-DPH: [1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5 hexatrienep-toluensulfonate]; DPPC:dipalmitoyl phosphatidylcholine; PG: phosphatidylglycerol
andby
citytionem-ulttor-
rvedsedy-canh asr-
cleicllsal-signepi-tionandk-
vede-
uno-car-9].
-00%in
1. Introduction
Cells take up macromolecules, including proteinsbioconjugates, derived from proteins or polypeptidesendocytosis via specific receptors with broad specifi(e.g., mannose or scavenger) [1]. During the internalizaprocess macromolecules have to interact with the cell mbrane and they may influence its fluidity. This could resin altered lateral receptor mobility and thus several receprelated functions such as cell adhesion [2]. It was obsethat decrease of Ca2+ response is associated with decreamembrane fluidity in prion-infected cells [3]. Some hdrophilic molecules, even negatively charged peptides,penetrate the membrane [4]. Polycationic polymers sucpolylysine [5] or histidylated polylysine [6] are able to pe
* Corresponding author.E-mail address:[email protected] (F. Reig).
0021-9797/$ – see front matter 2003 Elsevier Inc. All rights reserved.doi:10.1016/S0021-9797(03)00604-0
forate the cell membrane and thus serve as carriers of nuacids. In the course of gene or drug delivery into living ceit is important that the carrier molecule not disturb the bance of the cell membrane [7]. The selection and/or deof macromolecules of bioconjugates of drugs, genes, ortopes could be improved by establishing structure–funcrelationship between the structure of the macromoleculeits capability to interact with phospholipid bilayers mimicing biological membranes.
Polymeric polypeptides based on poly[L-lysine] habeen widely developed to explore their potential uses aslivery systems. Various drugs such as methotrexate, damycin, amyloride, and GnRH analog coupled to theserier molecules exhibited markedly improved efficacy [8,For example, coupling of daunomycin to poly[Lys(Glui-DL-Alam)] (EAK) resulted in compensation for the immunosuppressive effect of the drug and produced 66–1long-term survivors (>60 days) against L10 leukaemia
R. Szabó et al. / Journal of Colloid and Interface Science 267 (2003) 18–24 19
were
sti-tides
-desL-
theithtion,y in-
ela-ands-thes
isof
ers
rn-ho-i-ingingre-underic
esm-n-s”
hedno-
onof
ctedTa-fol-
n-nyl;A:ul-
a.omide,sednol
eree-
a
mice [10]. Carrier-dependent anti-Leishmania donovanief-fects of methotrexate branched polypeptide conjugatesobserved in vitro and in vivo [11].
Recently a systematic study was initiated to invegate the interaction between branched chain polypepwith the general formula of poly[Lys(Xi-DL-Alam)] (XAK),wherei ∼= 1 andm ∼= 3, and phospholipid mono- or bilayers with different compositions [12–14]. These polypepticontain oligopeptide side chains composed of oligo[Dalanine] and an additional amino acid (X) coupled toα-amino group of the terminal alanine. The results wthese polypeptides indicate that the side-chain composinamely the charge and length of the branches, markedlfluence its effect on model membranes.
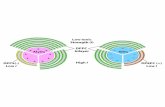
The aim of the present study was to establish a corrtion between the structural properties of the polymerstheir influence on the fluidity of phospholipid bilayers uing a new group of polylysine-based compounds withgeneral formula (poly[Lys(Xi)]) X iK. These polypeptidepossess a single amino acid (X= Pro, His, or Glu) cou-pled to theε-amino groups of the polylysine backbone. Thstudy was extended by the inclusion of two new XAK typepolypeptides with polycationic (poly[Lys-(Thri-DL-Alam)](TAK)) or anionic (poly[Lys-(Succ-Glui-DL-Alam)] (Succ-EAK)) character. The chemical structure of these polymis given in Fig. 1.
In this communication we describe our findings conceing the interaction of branched polypeptides with phosplipid bilayers with two different lipid compositions: dipalmtoyl phosphatidyl choline (DPPC) and DPPC contain20% negatively charged phosphatidyl glycerol (PG). Ustwo fluorescent probes, DPH and TMA-DPH [15], asporter molecules incorporated into the liposomes, we fothat polymeric polypeptides studied, except the amphotEiK with Glu, have no destabilizing effect on liposomes.
2. Experimental
Abbreviations for amino acids and their derivativfollow the revised recommendation of the IUPAC-IUB Comittee on Biochemical Nomenclature, entitled “Nomeclature and Symbolism for Amino Acids and Peptide(recommendations of 1983). Nomenclature of brancpolypeptides is in accordance with the recommendedmenclature of graft polymers (IUPAC-IUB CommissionBiochemical Nomenclature, (1984) [16]. For the sakebrevity, codes of branched polypeptides were construby us using the one-letter symbols of amino acids (ble 1). The abbreviations used in this paper are thelowing: XiK, poly[Lys(Xi)], X = Pro (PiK), His (HiK),or Glu (EiK); poly[Lys(Xi-DL-Alam)], X = Thr (TAK)or Succ–Glu (Succ–EAK). All amino acids are of L-cofiguration unless otherwise stated. Z: benzyloxycarboBoc: tert-butyloxycarbonyl; Pcp: pentachlorophenyl; NCN -carboxy anhydride; Succ: succinyl; DMSO: dimethylsfoxide.
2.1. Chemicals
DPH, TMA-DPH, DPPC, and PG were from SigmAmino acids, acetic acid, and diethyl ether were frReanal (Budapest, Hungary). Benzyloxicarbonyl chlor1-hydroxy benzotriazole, and diethylamine were purchafrom Fluka (Buchs, Switzerland), while pentachlorophewas from Merck (Darmstadt, Germany).
2.2. Synthesis
Experimental details of the synthetic procedures wdescribed previously [17–19]. Briefly, poly[L-Lys] was prpared by the polymerization ofN -carboxy-N -benzyloxycar-bonyllysine anhydride under conditions that allowed
Fig. 1. Schematic presentation of XK type polypeptides, where X= Pro (PK), X= Glu (EK), X = His (HK), and of XAK type polypeptides, where X=Succ–Glu (Succ–EAK), X= Thr (TAK), derived from poly[L-Lys].
20 R. Szabó et al. / Journal of Colloid and Interface Science 267 (2003) 18–24
Table 1Characteristics of branched chain polymeric polypeptides with poly[Lys(Xi )] or poly[Lys(Xi -DL-Alam)] formula
Polymer Abbreviationa Amino acid compositionb DPnc Mw
d ± 5%
Lys Ala (m) X (i)
Poly[Lys(Proi )] PiK 1.00 – 0.95 84 24800Poly[Lys(Hisi )] HiK 1.00 – 0.56 93 15400Poly[Lys(Glui )] EiK 1.00 – 1.00 94 25700Poly[Lys(Thri -DL-Alam)] TAK 1.00 3.10 1.00 60 29300Poly[Lys(Succ-Glui -DL-Alam)] Succ-EAK 1.00 4.00 1.00 80 51400
a Code and branched chain polymeric polypeptides based on one-letter symbol of amino acids.b Amino acid composition was determined by amino acid analysis as after hydrolysis in 6 M HCl at 105◦C for 24 h.c Number of average degree of polymerization determined by sedimentation equilibrium measurements.d Average molecular mass of polymers, calculated from the average degree of polymerization (DPn) of poly[Lys] and of the side chain composition.
eticter-agedein
tiveseocon-
ng
heou-9].dia-izedd-isin-9.0
ia-ried.
p-eck-sis,vac-
of
lvedndlearsofsiontedfor
llar-risd inter.
ringA-
PE-er-
ribeduo-etion-mes
andred.turehEx-for
o-tzky
number average degree of polymerization (DPn) approxi-mately 100. Protecting groups were cleaved by HBr in acacid (35%, m/V). The size of the polypeptides were demined by sedimentation equilibrium analysis, the averrelative molar masses (Mw andMz) were determined, anthe polydispersity factor (Mz/Mw) and an average degreof polymerization (DPn) were calculated. Data are givenTable 1.
2.3. Poly[Lys(Xi)](X iK) polypeptides
The amino acids were coupled to theε-NH2-groupof lysine as protected pentachlorophenyl ester deriva(Z-X-OPcp, where X= Glu or Pro; Boc-X-OPcp, wherX = His) using the in situ active ester method. Z or Bprotecting groups were cleaved with HBr or acetic acid ctaining 35% HBr (m/V) [18].
2.4. Poly[Lys(Xi-DL-Alam)](XAK) polypeptides
XAK-type polypeptides were synthesized by graftishort oligomeric DL-Ala chains to theε-NH2-group of ly-sine residues using N-carboxy-DL-Ala-anhydride [17]. Tsuitably protected and activated Glu/Thr residue was cpled to the end of branches of AK by amide bond [1After the removal of protecting groups, samples werelyzed against distilled water, freeze-dried, and characterPoly[Lys-(Succ-Glui-DL-Alam)] (Succ-EAK) was preparefrom EAK as follows: 10 mg (18.5 mmol) EAK was dissolved in 2 ml of 0.1 M carbonate buffer (pH 9.2). To thsolution 220 µl (220 mmol) succinic anhydride dissolvedDMSO (c = 100 mg/ml) was added in aliquots with continuous stirring in 30 min. The pH was adjusted betweenand 9.2 with 0.1 M NaOH. After 4 h the solution was dlyzed against distilled water for 2 days and then freeze-d
2.5. Amino acid analysis
The amino acid constitution of the polymeric polypetides was determined by amino acid analysis using a Bman 6300 analyzer (Fullerton, USA). Prior to the analythe samples were hydrolyzed in 6 M HCl in sealed and euated tubes at 105◦C for 24 h.
.
2.6. Preparation of liposomes
Liposomes were prepared using DPPC or a mixtureDPPC and PG (80/20, mol/mol) [20] as follows. Either20 mg DPPC or 16 mg DPPC and 4 mg PG were dissoin chloroform were mixed in a round-bottomed flask asolvent was eliminated by rotary evaporation from the csolution at 55◦C in vacuum for 15 min. The lipid film wadried in high vacuum for 1 h and hydrated with 4 ml0.1 M sodium acetate adjusted to pH 7.4. This suspencontaining multilamellar vesicles (MLV) was then sonicausing an ultrasounds tip, with the tube immersed in ice8 × 2 min. Between the sonication events N2-flux was ap-plied for 1 min. Finally, the suspension of small unilamevesicles was placed into a 55◦C water-bath for 1 h for annealing, followed by centrifugation to eliminate metal deband remaining MLV. The size of the liposomes, measurea Malvern Autosizer [21], was lower than 80 nm in diame
2.7. Fluorescence studies
Fluorescence studies were carried out by measuchanges in fluorescence intensity and polarization of TMDPH and DPH probes located in the bilayers, using aLS50 spectrofluorometer provided with a four cuvettes thmostated holder. Liposomes (SUVs) prepared as descabove were incubated with different concentrations of flrescent probes in the dark at 55◦C for 60 min. Fluorescencintensity was measured to determine the PL/probe relaship saturation. Once selected the optimal value, liposowere mixed either with polymer solution (0.2 mg/ml) orwith 0.1 M sodium acetate (pH 7.4), used as reference,fluorescence intensity as well as polarization were measuThis process was carried out by duplicate, and temperawas increased from 28 to 55◦C step by step leaving enougtime between lectures to allow the system to equilibrate.citation and emission wavelengths were 355–430 nmTMA-DPH or 365–425 nm for DPH. The degree of flurescence polarization was calculated according to Shiniand Barenholz [22] applying the equation
(1)P = (I‖ − I∗⊥G
)/(I‖ + I∗⊥G
),
R. Szabó et al. / Journal of Colloid and Interface Science 267 (2003) 18–24 21
ar-
ntoxci-
earibed
1).
d
inobyere
theese
e ofentows:an bers
g aain,g,t thee ofoly-
of
o 8andre-er
hos-ers
ellH),
sur-
eres in
lay-nsi-
r-r
mo-o-ionsure-noting
ts ob-
en-0%PPC
iza-d
olu-d in
re,edpo-e toion-in
s ofcro-ofges
fivet allof
ger
cat-ion
her,al-
eryture
whereI‖ andI⊥ are the intensities measured with its polization plane parallel (I‖) and perpendicular (I⊥) to that ofthe exciting beam.G is a factor used to correct polarizatioof the instrument and is given by the ratio of verticallyhorizontally polarized emission components when the etation light is polarized in the horizontal direction.
3. Results and discussion
3.1. Chemical structure of the polymeric polypeptides
Two types of branched polypeptides derived from linpolylysine were prepared. In the case of polymers descrwith the formula of poly[Lys(Xi)](X iK) a single amino acid(Glu, His, or Pro) was coupled to theε-NH2 groups of thelysine residues using in situ active ester method (Fig.The oligo (DL-Ala) side chains of poly[Lys(Xi-DL-Alam)](XAK) polymers was grafted to theε-NH2 groups of thelysine residues by polymerization of DL-Ala-N -carboxy an-hydride where theε-NH2 groups of the polylysine serveas multifunctional initiator. To theα-NH2 groups of theterminal alanine residues of the branches a further amacid (X = Glu or Thr) was coupled and derivatizedsuccinylation (Succ-Glu) (Fig. 1). The polypeptides wcharacterized by their average relative molar mass andcomposition of the side chain. The characteristics of thmacromolecules are summarized in Table 1.
Regarding the amino acid composition and the degresubstitution by amino acid X each polymer had a differoverall electrical charge that has been estimated as follEiK possessing Glu residues at almost each side chain cconsidered as a neutral, but zwitterionic molecule. PolymTAK and PiK can be considered as a polycation bearinpositive charge at the terminal of each monomer side chwhile HiK, due to the intrinsic basicity of the imidazole rinbehaves as a polycation, but with two positive charges aside terminal of the monomer unit. Due to the presencsuccinylated Glu at the end of the branches Succ-EAK pmer has two negative charges per monomer side chain.
Based on the length of the branches two groupspolypeptides can be classified. Polymers like HiK, PiK,and EiK have short and similar side chains (equivalent tcarbon atoms), but the length of the branches of TAKSucc-EAK is equivalent to 17 and 24 carbon atoms,spectively. All these parameters will condition their furthinteraction with lipid bilayers.
3.2. Interaction of polymers with phospholipid bilayers
The interaction between branched polypeptides and ppholipid membranes was investigated using lipid bilaywith DPPC and DPPC/PG (80/20, mol/mol). A fluores-cent probe with positive electrical charge TMA-DPH as was a hydrophobic one, 1,6-diphenyl-1,3,5-hexatriene (DP
e
were used to analyze the effect of polymers on the outerface and on hydrophobic core of bilayers.
For these studies small unilamellar vesicles (SUVs) wused, so that light scattering was not important. Changefluorescence intensity and/or polarization of probes in biers were determined as a function of temperature. Tration from gel to liquid crystalline state (Tc ∼ 40–41.5◦Cfor DPPC andTc ∼ 32◦C for DPPC/PG, 80/20) was chaacterized by the analysis ofTc values in the presence oabsence of polymers. The optimal probe/phospholipidlar ratios were determined by incubation of plain lipsomes with increasing volumes of concentrated solutof each label followed by fluorescence intensity measments. Up to a certain label/lipid relationship there wasincrease in the fluorescence of the samples thus indicathat the system was already saturated and stable. Resultained were as follows: TMA-DPH:phospholipid= 1:144(mol/mol), and DPH:phospholipid= 1:240 (mol/mol) [12].Under conditions applied (neutral pH, low polymer conctration, probe/phospholipid ratio) liposomes containing 2PG were negatively charged while those composed of Dcould be considered neutral.
Changes in fluorescence intensity as well as polartion of DPH or TMA-DPH after incubation of DPPC anDPPC/PG liposomes with polymer solution or acetate stion are described in the next paragraph and summarizeFigs. 2–5.
3.3. Polarization fluorescence studies with DPH
Liposomes labeled with a hydrophobic fluorophoDPH, located in the inner core of the bilayer, were mixwith polymer solutions and fluorescence intensity andlarization were measured at different temperatures. Dutheir high hydrophobicity, DPH molecules, after incubatwith liposomes at temperatures aboveTc, are able to penetrate the external part of the bilayer and localize mainlyits internal core. This location, in between the acyl chainphospholipids, makes them extremely sensitive to the miviscosity of their environment. Any change in the motionacyl chains affects their own motion and results in chanin the polarization or anisotropy of the samples.
Polarization/temperature curves recorded for thepolymers under study and DPPC liposomes indicated thaof them except Succ-EAK interact with the internal corebilayers. Figures 2a–2c show this process recorded for EiK,HiK, and PiK. One can appreciate that differences are larat temperatures under or aroundTc. Moreover, for EiK, thepolarization curve runs under that of the reference, indiing a depolarization of the probe that reflects a fluidificatof the system. In contrast, for HiK and PiK, the polariza-tion values corresponding to the samples are slightly higsuggesting a rigidifying effect. Transition temperatures cculated from the first derivatives are given in Table 2. A vstrong effect was observed for the transition temperain DPPC bilayer with DPH for EiK (�T = −8.4). This
22 R. Szabó et al. / Journal of Colloid and Interface Science 267 (2003) 18–24
icles
bi-
f po-
ionssingcule.rface-andem-or-nedAKfptideom-inoleethe) inoupswith-itive”
cedrithob-
Pe
la-riza-d of
y of
, ase re-Hher attem-
-PG
f po-
(a)
(b)
(c)
Fig. 2. Polarization/temperature curves recorded for DPH/DPPC vesin the presence or absence of branched polypeptides: (a) poly[Lys(Glu1.0)](EiK) —Q—; (b) poly[Lys(His0.56)] (HiK) —Q—; (c) poly[Lys(Pro0.95)](PiK) —Q—; control —2—.
Table 2Effect of polymeric constructs on the transition temperature of DPPClayers
Polymer Code �T (T − Tc)a
DPH TMA-DPH
Poly[Lys(His0.56)] HiK 0.6 −1.7Poly[Lys(Pro0.95)] PiK 2.0 −0.3Poly[Lys(Glu1.0)] EiK −8.4 0Poly[Lys(Thr1.0-DL-Ala3.1)] TAK 0 .6 0.2Poly[Lys(Succ-Glu1.0-DL-Ala4.0)] Succ-EAK 0 0
a Transition temperatures were calculated from the first derivative olarization versus temperature sigmoidal curves.
finding could be explained by pronounced ionic interactbetween the amphoteric polypeptide and DPPC possealso one positive and one negative charge per moleThe attraction forces generated changes in the outer suof the bilayer. Interestingly PiK, one of the three polycationic polypeptides studied, interacted with DPPCexhibited a somewhat higher effect on the transition tperature (�T = 2.0) than the other two compounds (fboth TAK and HiK �T = 0.6). This difference in the effect of the three polycationic polypeptide could be explaiperhaps by steric and electronic factors. Polypeptide Tcontains positively chargedα-amino groups at the end othe branches; however, the distance between the polypebackbone and the amino group is relatively large as cpared to that of the polypeptide backbone and the imgroup in PiK. This could result in a sterically more favorabclose interaction for PiK with the amphoteric DPPC. In casof HiK we have not only one, but two positive charges (α-amino group and the imidazole moiety in the side chainthe branches. The neighboring arrangement of these grgenerate higher charge density and upon interactionDPPC not only is the attraction between HiK and the phospholipid present, but also the repulsion between the poscharges of HiK and DPPC exists. The lack of such “built-indouble density repulsion could result in more pronouninteraction of PiK with DPPC. Differences with a similatendency in the binding of polycationic polypeptides wthe negatively charged DPPC-PG liposome were alsoserved (Table 3). In this case the most marked effect ofiKas compared to HiK or TAK could also be explained by thabove structure-based interpretation.
Plain liposomes composed of DPPC/PG (8/2, molar)beled with DPH showed, as was to be expected, polation/temperature changes, softer than those composepure DPPC, indicating a decrease in the cooperativitthe transition. After incubation with HiK and PiK, polar-ization/temperature curves adjusted better to a sigmoidcan be appreciated in Figs. 3a, 3b and Table 3. Thessults suggest that the presence of polymer molecules,iKand PiK, in the media has a rigidifying effect, increasing tcooperativity of the process. This effect is also strongelow temperatures and disappears almost completely atperatures aroundTc (Table 3). TAK the third polycationic
Table 3Effect of polymeric constructs on the transition temperature of DPPC(8:2) bilayers
Polymer Code �T (T − Tc)a
DPH TMA-DPH
Poly[Lys(His0.56)] HiK 4.9 2.8Poly[Lys(Pro0.95)] PiK 7.0 −0.7Poly[Lys(Glu1.0)] EiK 0.1 0Poly[Lys(Thr1.0-DL-Ala3.1)] TAK 1 .0 0Poly[Lys(Succ-Glu1.0-DL-Ala4.0)] Succ-EAK 0 0
a Transition temperatures were calculated from the first derivative olarization/temperature sigmoidal curves.
R. Szabó et al. / Journal of Colloid and Interface Science 267 (2003) 18–24 23
-PGtides.
ra-
itho-Ta-tureundlv-
] forpho-bethet theeencomept
es-soft
torim-
PCptide
-PGptide
sat-hetne
f neg-t thetionin
ter.ithe]truc-tabi-ryses
asss ofive,ro-
thisly-thisr-
(a)
(b)
Fig. 3. Polarization/temperature curves recorded for DPH/DPPC(8:2) vesicles in the presence or absence of branched polypep(a) poly[Lys(His0.56)] —Q—; (b) poly[Lys(Pro0.95)] —Q—; con-trol —2—.
structure has only a small rigidifying effect with a tempeture increase of 1◦C (not shown). EiH and Succ-EAK showno interaction with vesicles in this experiment.
3.4. Polarization fluorescence studies with TMA-DPH
Experiments carried out with liposomes saturated wTMA-DPH showed soft interactions between the lipid plar zone of the bilayer and the polymeric constructs (bles 2 and 3). As a common trend polarization/temperacurves show a lower slope (without sudden changes arothe Tc), than was previously described for samples invoing DPH. This behavior has already been described [23other molecules that localize at the polar heads of phoslipids, and is mainly due to the external localization of promolecules far away from the acyl chains that experiencefusion process. In most cases it was not possible to adjuscurves to a sigmoid and only qualitative differences betwsamples and references could be reported. Liposomesposed of DPPC showed no interaction with polymers excwith the histidine containing derivative (Table 2). The prence of this polymer in the incubation media promotes afluidification of the bilayers for lipids in gel state;Tc thus is1.7◦C lower than that of the reference (DPPC), Fig. 4; upthis transition polarization/temperature curves are supeposable for both samples (reference and HiK).
-
Fig. 4. Polarization/temperature curves recorded for TMA-DPH/DPvesicles in the presence or absence of branched polypepoly[Lys(His0.56)] (HiK) —Q—; control —2—.
Fig. 5. Polarization/temperature curves recorded for TMA-DPH/DPPC(8:2) vesicles in the presence or absence of branched polypepoly[Lys(His0.56)] (HiK) —Q—; control —2—.
Concerning vesicles composed of DPPC/PG (0.8/0.2)urated with TMA-DPH, the presence of polymers in tmedia, except for the HiK derivative, has almost no effecon the microviscosity of the system (Fig. 5, Table 3). Ocan suggest that the presence of a certain percentage oative charge on the surface of the liposome can attracpositively charged polymer side chains and this interacresults in a restriction in motion of the atoms involvedthis area. Other polymers have no effect on this parame
Comparing the physicochemical results obtained wthe two liposomal compositions and the five poly[L-lysinderivatives, one can conclude that these polymeric constions are by themselves safe, as far as the lack of deslization of bilayers is concerned. All of them modify vesoftly the microviscosity of bilayers, and in some ca(HiK/DPPC-PG) a beneficial effect has been reportedtheir presence results in an improvement of compactnebilayers. The only exception is the glutamic acid derivatwhich has a fluidifying effect that could eventually compmise the stability of vesicles.
Nevertheless, one can not exclude the possibility thatdrawback could be beneficial if the direct interaction of pomers (EiK) with cellular membranes was concerned, aslocal fluidification could help the penetration of polymeassociated molecules into the cells.
24 R. Szabó et al. / Journal of Colloid and Interface Science 267 (2003) 18–24
up-ern-rianiann-e
z in
an,
ar,
on,
ar-
ingca-
ate
46
in:hn
.ys.
io-
980)
93)
ly-
te
6)
7–
g-
Acknowledgments
Experimental work summarized in this paper was sported by grants from the Hungarian–Spanish Intergovmental Programme (5/1998, 2/2001), from the HungaResearch Fund (OTKA, No. T-03838), from the HungarMinistry of Welfare (ETT, No. 12/2000) and from the Hugarian Ministry of Culture (Medichem, NFKP 1/047). Wgreatly acknowledge the help of Dr. Leocadio Rodriguethe mathematical treatment of the data.
References
[1] S.K. Basu, Biochem. Pharmacol. 40 (1995) 1941–1946.[2] P.Y. Chan, M.B. Lawrence, M.L. Dustin, L.M. Ferguson, D.E. Gol
T.A. Springer, J. Cell Biol. 115 (1991) 245–255.[3] K. Wong, Y. Qiu, W. Hyun, R. Nixon, J. van Cleff, J. Sanchez-Salaz
S.B. Prusiner, S.J. DeArmond, Neurology 47 (1996) 741–750.[4] J.M. Leenhouts, P.W.J. van den Wijngaard, A.I.P.M. de Kro
B. de Krujiff, FEBS Lett. 370 (1995) 189–192.[5] Y. Wu, C.H. Wu, Biochemistry 27 (1988) 887–892.[6] R. Mahajoub, P. Midoux, Bioconjugate Chem. 12 (2001) 92–99.[7] F. Hudecz, Gy. Kóczán, J. Reményi, in: Gy. Keri, I. Toth (Eds.), H
wood Academic/Taylor & Francis, London, 2002, pp. 553–578.[8] F. Hudecz, Anti-Cancer Drugs 6 (1995) 171–193.
[9] F. Hudecz, in: A. Agelli, N. Boden, S. Zhang (Eds.), Self-AssemblPeptide Systems in Biology, Medicine and Engineering, Kluwer Ademic, Dordrecht, 2001, pp. 139–160.
[10] D. Gaal, F. Hudecz, Eur. J. Cancer 34 (1998) 155–161.[11] Gy. Koczan, A.C. Ghose, A. Mookerjee, F. Hudecz, Bioconjug
Chem. 13 (2002) 518–524.[12] I.B. Nagy, M.A. Alsina, I. Haro, F. Reig, F. Hudecz, Biopolymers
(1998) 169–179.[13] F. Hudecz, I.B. Nagy, Gy. Kóczán, M.A. Alsina, F. Reig,
E. Chiellini, J. Sunamoto, C. Migliaresi, R.M. Ottenbrite, D. Co(Eds.), Kluwer Academic/Plenum, New York, 2001, pp. 103–120.
[14] I.B. Nagy, Zs. Majer, F. Hudecz, Biopolymers 58 (2001) 152–164[15] J.-G. Kuhry, G. Duportail, C. Bronner, G. Laustriat, Biochim. Bioph
Acta 845 (1985) 60–67.[16] IUPAC-IUB Commission on Biomedical Nomenclature, Eur. J. B
chem. 138 (1984) 9–37.[17] F. Hudecz, M. Szekerke, Collect. Czech. Chem. Commun. 45 (1
933–940.[18] G. Mezõ, J. Kajtár, F. Hudecz, M. Szekerke, Biopolymers 33 (19
873–885.[19] G. Mezõ, J. Kajtár, I.B. Nagy, M. Szekerke, F. Hudecz, Biopo
mers 42 (1997) 713–730.[20] I.B. Nagy, M.A. Alsina, I. Haro, F. Reig, F. Hudecz, Bioconjuga
Chem. 11 (2000) 30–38.[21] Y. Cajal, M.A. Alsina, F. Rabanal, F. Reig, Biopolymers 38 (199
607–618.[22] N. Shinitzky, Y. Barenholz, Biochim. Biophys Acta 515 (1978) 36
394.[23] A. Fernandez, M.A. Alsina, I. Haro, R. Galantai, F. Reig, Lan
muir 14 (13) (1998) 3625–3630.
![Page 1: Interfacial interactions between poly[L-lysine]-based branched polypeptides and phospholipid model membranes](https://reader039.fdokumen.com/reader039/viewer/2023051108/633df5f7df741406dc0b4c83/html5/thumbnails/1.jpg)
![Page 2: Interfacial interactions between poly[L-lysine]-based branched polypeptides and phospholipid model membranes](https://reader039.fdokumen.com/reader039/viewer/2023051108/633df5f7df741406dc0b4c83/html5/thumbnails/2.jpg)
![Page 3: Interfacial interactions between poly[L-lysine]-based branched polypeptides and phospholipid model membranes](https://reader039.fdokumen.com/reader039/viewer/2023051108/633df5f7df741406dc0b4c83/html5/thumbnails/3.jpg)
![Page 4: Interfacial interactions between poly[L-lysine]-based branched polypeptides and phospholipid model membranes](https://reader039.fdokumen.com/reader039/viewer/2023051108/633df5f7df741406dc0b4c83/html5/thumbnails/4.jpg)
![Page 5: Interfacial interactions between poly[L-lysine]-based branched polypeptides and phospholipid model membranes](https://reader039.fdokumen.com/reader039/viewer/2023051108/633df5f7df741406dc0b4c83/html5/thumbnails/5.jpg)
![Page 6: Interfacial interactions between poly[L-lysine]-based branched polypeptides and phospholipid model membranes](https://reader039.fdokumen.com/reader039/viewer/2023051108/633df5f7df741406dc0b4c83/html5/thumbnails/6.jpg)
![Page 7: Interfacial interactions between poly[L-lysine]-based branched polypeptides and phospholipid model membranes](https://reader039.fdokumen.com/reader039/viewer/2023051108/633df5f7df741406dc0b4c83/html5/thumbnails/7.jpg)