Stenting of the arterial duct: a new approach to palliation for pulmonary atresia
Transcript of Stenting of the arterial duct: a new approach to palliation for pulmonary atresia
240 Br Heart J 1992;67:240-5
Stenting of the arterial duct: a new approach to
palliation for pulmonary atresia
John L Gibbs, Martin T Rothman, Michael R Rees, Jonathan M Parsons,
Mike E Blackburn, Carlos E Ruiz
AbstractObjective-To assess the possibility of
maintaining ductal patency in neonateswith complex pulmonary atresia by per-cutaneous implantation of balloon ex-pandable stents.Patients-Two duct-dependent neo-
nates with long segment pulmonaryatresia, right sided aortic arch, and leftsided arterial duct.Results-Stents with final diameter of
3 5 or 4 mm and initial length of 7 or15 mm were successfully positioned inthe arterial duct. Two stents wererequired in one child and four in theother in order to stent the entire lengthof the duct. After the procedures theducts remained widely patent andarterial oxygen saturations remainedabove 80%. Complications of theprocedures included perforation of aperipheral pulmonary artery and car-diac perforation, both caused by guidewire manipulation. Both babies diedsuddenly, one at five weeks, and the otherat nine days after successful stenting ofthe duct. Both ducts were patent atnecropsy; the exact cause of one deathwas not clearly defined, but the secondseemed to be caused by pneumococcalsepticaemia.Conclusions-Stenting of the arterial
duct is technically feasible. It providesadequate palliation for neonates withpulmonary atresia at least in the shortterm and it seems to result in balanced,central perfusion of both pulmonaryarteries. This preliminary report sug-gests that this previously untried tech-nique may prove to be a promising andattractive alternative to neonatalaortopulmonary shunt operation.
Conventional palliative treatment of ductdependent long segment pulmonary atresiaconsists of treatment with prostaglandin E inthe short term and of surgical fashioning of anaortopulmonary shunt in the medium term. Alogical alternative to surgical aortopulmonaryanastomosis would be to prevent the arterialduct from closing. Expandable metal stentshave proved effective in the treatment ofintravascular stenoses at various sites includ-ing the pulmonary arteries and the inferiorcaval vein' and, more recently, in coronaryartery stenosis or intimal dissection after
balloon angioplasty.2 Stenting of the arterialduct in duct dependent congenital heart dis-ease has not, however, been attempted. Wereport the successful maintenance of ductalpatency by implantation of expandable stain-less steel stents. The technical procedure (per-formed in each case under general anaes-thesia), its potential complications, and itsvery considerable future promise in the pallia-tion of duct dependent congenital heart dis-ease are discussed.
Patients and methodsCASE 1A 2-6 kg boy presented with cyanosis and asoft continuous murmur when he was fourhours old. He had an extensive cleft lip andpalate and a bifid right thumb. Echocardio-graphy and later cardiac catheterisation andangiography showed severe hypoplasia of theright ventricle and tricuspid valve with anintact ventricular septum, right ventricularsinusoids, long segment pulmonary atresia,small but confluent pulmonary arteries, a rightsided aortic arch, and a left sided arterial ductarising at the origin of the left subclavianartery. He was maintained on an infusion ofprostaglandin E and at the age of five daysunderwent a Waterston aortopulmonary anas-tomosis. The right pulmonary artery wasfound to be approximately 2-5 mm indiameter behind the ascending aorta. Post-operatively the child remained duct depen-dent. Angiography showed that the shunt wascompletely occluded. In view of the failedattempt at surgical palliation and with infor-med consent from the child's parents, webelieved the alternative approach of stentingthe arterial duct was justified.
Initially we chose a titanium wire stent(William Cook, USA) that was premountedon a balloon catheter with 4 mm inflateddiameter and a 5F shaft. The 6F sheath neces-sary for delivery of the device, however,proved too large to pass retrogradely up thefemoral artery or anterogradely to the duct viathe inferior caval vein, the atrial septum, theleft ventricle and the ascending aorta. Clearly,it was essential to use a device that could bedelivered through a smaller sheath and wethen chose a Palmaz-Schatz stainless steelstent (Ethicon Ltd, Johnson & Johnson) de-signed for implantation in adult coronaryarteries (fig 1).' 2 This stent is 15 mm long,consisting of two 7 mm lengths joined by asingle 1 mm bridge, the two halves being
Killingbeck Hospital,LeedsJ L GibbsM R ReesJ M ParsonsM E BlackburnThe Royal LondonHospital, Whitechapel,LondonM T RothmanUniversity ofLomaLinda, California,USAC E RuizCorrespondence toDr J L Gibbs, Departmentof Paediatric Cardiology,Killingbeck Hospital, YorkRoad, Leeds LS14 6UQ.
Stenting of the arterial duct: a new approach to palliation for pulmonary atresia
Figure 1 A 7 mm longPalmaz-Schatz coronarystent (A) unexpanded,mounted upon a 3 7F,4 mm coronaryangioplasty ballooncatheter and (B) afterinflation to a diameter of4 mm.
easily separated into equal 7 mm lengths byvery careful cutting of the bridge (althoughthis is not recommended by the manufac-turer). After inflation to 4 mm diameter the7 mm length shortens to approximately 6 mmand the 15 mm long complete stent shortensto approximately 13 mm. The stent isoperator mounted on a balloon catheter of theoperator's choice, in this case an ACS coron-ary angioplasty balloon catheter with aninflated diameter of 4 mm and shaft of 3'7 F.At right axillary artery cutdown a 5F Van
Andel sheath (William Cook) was passed tothe aortic arch. A 4F multipurpose catheterwas passed through the sheath, bleeding beingprevented by an ACS Tuohy-Borst typerotating haemostatic valve. A superfloppy0-016 inch guide wire was manipulated downthe duct and into the peripheral left lowerlobe pulmonary artery. The catheter was thenadvanced over the guide wire into the distalpulmonary artery, the guide wire was ex-changed for a stiffer wire (intermediate 0-016inch, USCI Bard) and the multipurpose cath-eter was replaced by the balloon catheter withthe stent mounted upon it. The balloon cath-eter was positioned (dye injected through thesheath and the central balloon marker wereused to guide accurate placement) in the ductand the balloon was inflated to 4 mm at apressure of 8 atm for 20 seconds. There wastransient hypotension and a fall in oxygensaturation during balloon inflation, withprompt recovery after deflation. Repeatangiography showed the duct to be widelypatent and pulse oximetry showed an increasein peripheral oxygen saturation from 75% to90%. The stent was not visible on radio-graphic screening, but this was not consideredremarkable because the stent is rarely visible
in adult coronary arteries. Treatment withprostaglandin E was stopped and the arter-iotomy repaired.
After three hours the oxygen saturation fellto 40%. This did not improve after throm-bolytic treatment but rose again to 85% whenintravenous prostaglandin was restarted.Doppler colour flow mapping showed evi-dence of distal ductal stenosis which sugges-ted that the implanted stent was too short. Anattempt at changing to oral prostaglandin wasunsuccessful.Ten days later a second right axillary arter-
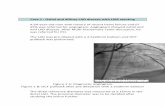
iotomy was performed and a 7 mm Johnson &Johnson stent was positioned in a similarmanner in the distal duct uneventfully using asimilar 4 mm balloon catheter (fig 2). Angio-graphy confirmed a very satisfactory stentposition in the distal part of the duct, with thestent being just visible on screening andplainly visible on the plain chest radiographon this occasion (fig 2). The duct was widelypatent and the arterial oxygen saturation hadagain risen to 90%. The child was returned tothe intensive care unit, where 15 minutes laterthe oxygen saturation again fell to 40%, therewas a fresh haemorrhage from the endo-tracheal tube and radiographic evidence ofright upper lobe consolidation. The bleedingstopped without specific treatment but thenext day oxygen saturation had not improved.Thrombolytic treatment did not cause anyfurther haemorrhage but failed to produce anyclinical improvement and prostaglandin E hadlittle effect in the dose previously used success-fully. Ultrasound examination proved unhelp-ful on this occasion. A repeat chest radio-graph, however, showed the distal stentclearly, but the longer stent was still notvisible in the proximal duct or in any otherposition in the vascular system. Retrospec-tively it became clear that the first stent hadnot, in fact, been deployed in the duct and hadbeen pushed backwards off the balloon duringthe introduction of the balloon catheter, laterto be unwittingly discarded inside the hae-mostatic valve.The child improved with high dose intra-
venous prostaglandin E. His parents en-couraged us to reattempt stenting of theproximal duct, and three days later the childwas returned to the catheter laboratory where,through a left axillary artery cutdown, a15 mm stent was easily positioned and expan-ded to 4 mm in the proximal duct overlappingthe distal stent by 1-5-2 mm. Repeat angio-graphy showed the entire duct to be widelypatent and both stents were visible on screen-ing and on the plain chest radiograph (fig 2).The oxygen saturations remained above 85%without prostaglandin E. The child wastreated with dipyridamole, aspirin, and aheparin infusion pending stabilisation of theprothrombin time on warfarin.His recovery was complicated by a further
pulmonary haemorrhage five days later,requiring reversal of anticoagulation withfresh frozen plasma and discontinuation ofwarfarin. He continued to be treated withaspirin 15 mg/kg once daily and dipyridamole
241
Gibbs, Rothman, Rees, Parsons, Blackburn, Ruiz
Figure 2 (A) A left ventriculogram in theanteroposterior projection showing the right sidedaortic arch and the left sided arterial duct(arrowed) that supplied the pulmonary arteries incase 1. (B) Delivery of the first ( 7 mm) stent to thedistal part of the duct. The balloon is seen inflatedwithin the duct, having been passed over a guidewzire inserted via the right axillary artery andpositioned in a distal pulmonary artery in the rightupper lobe. (C) After deflation of the balloon,angiography showed the duct to be widely patent.(D) A plain radiograph after the delivery of thefirst stent showing the expanded stent to be justvisible (arrowed) in the distal part of the duct.There is right upper lobe shadowing caused bypulmonary haemorrhage induced by the guide wire.(E) A plain radiograph taken after completion ofthe procedures, showiing the 7 mm and 15 mm stentsexpanded within the duct. The two stents overlap by1 5-2 mm to produce an overall stent length ofapproximately 17 mm.
5 mg/kg in three divided doses daily. He was
extubated 11 days later, when pulse oximetryshowed stable oxygen saturations between80% and 85%. Four weeks after the proce-dure he remained well saturated but mildlytachypnoeic, requiring treatment withdiuretics. He was found dead in his cot when
he was five weeks old. At necropsy the ductand both branch pulmonary arteries were
widely patent and the immediate cause ofdeath was uncertain.
CASE 2A 2-6 kg boy presented with cyanosis when he
242
Stenting of the arterial duct: a new approach to palliation for pulmonary atresia
was two days old. He was the second of twins(his twin brother had coarctation of the aorta).Investigation showed him to have atrialisomerism, a complete atrioventricular septaldefect, and long segment pulmonary atresiawith confluent but small pulmonary arteriesand with a right sided aortic arch and a leftsided arterial duct supplying the pulmonaryarteries. His parents wished to avoid surgicaltreatment and gave their informed consent toan attempt at stenting the arterial duct.Angiography via a right axillary artery cut-down and a 5F Van Andel sheath confirmed aright sided aortic arch with a long, curved,distally stenosed duct arising from the left sideof the proximal aortic arch. The left subclavianartery arose anomalously from.the descendingaorta. The arterial duct was cannulated with a4F Cobra catheter, a 0-014 inch guide wire waspositioned in the right lower lobe pulmonaryartery, and a 7 mm long Johnson & Johnsonstent was deployed in the distal duct through a4 mm (3-3F) ACX coronary angioplastyballoon catheter. A brief attempt was made toinsert a second stent more proximally in theduct, but the procedure was abandoned be-cause of increasing metabolic acidosis andtransient extreme bradycardia. The child madea prompt recovery. Treatment was continuedwith prostaglandin E, dipyridamole, andheparin.
Six days later the right femoral vein wascatheterised and a 4F Cobra catheter waspassed to the right atrium, the right ventricle,across the ventricular septal defect to theascending aorta, and thence to the arterial duct.A guide wire (0.018 inch Terumo) was posi-tioned in the right lower lobe pulmonary arteryand the catheter was exchanged for a 3-5 mm(3-6F) ACS Pinkerton balloon angioplastycatheter. During catheter manipulationthrough the right ventricle the stent becamedislodged distally from the balloon, coming tolie on the guide wire alone high in the inferiorcaval vein. The stent was retrieved by snaringthe distal end of the guide wire in the rightatrium via a second catheter in the femoral vein.Retrieval of the stent was complicated by acutetamponade caused by cardiac perforation bythe tip of the snare, but this was treatedsuccessfully by prompt pericardiocentesis andautotransfusion. The procedure was aban-doned. The child made an uncomplicatedrecovery and eight days later the duct was againcatheterised with a 6F long curved sheath viathe left femoral vein. The sheath was advancedto the ascending aorta and a guide wire waspositioned in the right lower lobe pulmonaryartery. A 15 mm stent was deployed in the ductwith its tip just inside the existing 7 mm stentin the distal duct. Repeat angiography showedsatisfactory stent positions with excellent flowto both pulmonary arteries, but the combinedlength of the stents still seemed to be too short.A further 7 mm stent was deployed in the mostproximal part of the duct. Transient markedhypotension and bradycardia occurred at thisstage, apparently owing to splinting ofthe rightventricle, the atrioventricular valve, and theaortic valve by the sheath. Final angiographyshowed a very satisfactory result.
Treatment was continued with dipyrida-mole, aspirin (15 mg/kg/day), and a heparininfusion to keep the activated partial thrombo-plastin time at 2-2-5 times the control value.The child remained well with arterial oxygensaturations above 80%. He was extubated thefollowing day and anticoagulated with war-farirt, our initial plan being to continue this foreight weeks, when we expected that endothe-lialisation of the stent would be complete.Ten days later he again became cyanosed.
Repeat aortography via the right femoral arteryshowed very localised stenosis of the ductimmediately at its junction with the pulmonaryartery (fig 3). A further 7 mm stent wasdelivered to this site and dilated to a diameter of3-5 mm without difficulty, and with promptimprovement in oxygen saturation to 90%. Atreview eight days later the child was well andgaining weight. Pulse oximetry showed read-ings between 80% and 90% throughout theday. He was discharged home, where thefollowing day he suffered two apnoeic episodesdespite remaining pink and well. He suffered afurther apnoeic episode on the way to hospitaland resuscitation was unsuccessful. Necropsyshowed that the duct was patent; there wasasplenia and pneumococci were isolated frompostmortem blood cultures. Three days laterhis twin brother was admitted to hospital after anear miss cot death at home.
DiscussionAlthough aortopulmonary shunt operationsprovide life-saving palliative treatment at lowrisk for neonates with pulmonary atresia, suchsurgical treatment has potential drawbacks.Both Blalock-Taussig (classical or modified)and Waterston shunts usually produce prefer-ential blood flow to the pulmonary artery on theside of the shunt that can result in differentialgrowth of the right and left pulmonary arteries.The Waterston anastomosis may be associatedwith distortion ofthe right pulmonary artery orpulmonary hypertension, and any kind of sur-gical intervention will inevitably result in scar-ring which may increase the risk of later, moredefinitive, surgical treatment. Non-surgicalmaintenance of ductal patency is therefore ahighly desirable goal.The recent development of stents for im-
plantation into coronary arteries had led to themanufacture of stents of suitable size for use inthe neonatal arterial duct. There are variousforms of stent-from elaborate balloon ex-pandable titanium coils to expandable stainlesssteel tubes and self expanding sprung steelcages. Self expanding stents have the potentialdisadvantage of relative lack ofoperator controlof the final stent diameter. Balloon expandablestents have the theoretical advantage that itmay prove possible to increase stent diameter ata later date simply by repeat balloon dilatation.We therefore thought that a balloon expand-able stent was best suited to our purpose. Ourinitial choice of the Cook titanium coil designwas prompted by the fact that the stent isclearly visible on radiographic screening.However, our experience suggests that adelivery system of 6F is too large to allow safe
243
Gibbs, Rothman, Rees, Parsons, Blackburn, Ruiz
Figure 3 (A) Angiography afterimplantation of three stents in case 2showing localised stenosis (arrowed) of theduct just at its junction with the pulmonaryartery. (B) After delivery of a fourthstent at this distal stenosis repeatangiography showed the duct to be widelypatent throughout its length with excellent,well balancedflow to both branchpulmonary arteries. (C) A plainradiograph showing the four ductal stentsin position. The overlap between each stenthas allowed the stents to follow the naturalcurved shape of the duct without distortingthe ductal anatomy.
percutaneous arterial access in babies of thissize. The Palmaz-Schatz stent has the distinctadvantage of being operator mounted on theballoon catheter rather than being pre-mounted by the manufacturer. This allows theoperator to choose a balloon catheter ofappropriate length, shaft size, and inflateddiameter for individual ductal anatomy. Thecatheters we chose had shaft sizes of only 3-3,3-6, and 3-7F and even with the stent mountedupon the balloon the catheter passed easilythrough a 5F sheath which, in turn, was smallenough to be introduced into the axillary arterywith ease.
Despite the repeated cardiac catheterisationsrequired in these cases before successful stent-ing of the duct, we encountered few majorcomplications. Arterial damage should beavoidable if the stent delivery system can bekept as small as 5F. A peripheral right pulmon-ary artery was perforated by a superfloppy wireand the tip of a snare fashioned from a 0014inch guide wire perforated the heart. Height-ened awareness of the possibility that evenatraumatic wires can perforate the heart orblood vessels should minimise this risk.
It seems likely that the pulmonary haemor-rhage in our first case was exacerbated byantiplatelet and anticoagulan therapy and in
our case the bleeding required warfarin treat-ment to be stopped. Despite this there has beenno suggestion of clot formation within the stentand it may be that the high flow through a 4 mmdiameter duct makes thrombosis unlikely andanticoagulant therapy unneccessary. Treat-ment with aspirin and dipyridamole does,however, seem advisable in the light ofexperience with stent implantation at othersites,' 2 at least until the stent surfaces are likelyto be completely endothelialised. We pro-visionally planned to continue these drugs forapproximately two months from the stentimplant. No complications were caused byductal damage, although dissection of the ductby a guide wire or tears induced by stentdeployment remain theoretical risks.Repeated procedures were required in both
our cases because short sections of the arterialduct remained unstented. This was partlybecause we decided to stent the ducts insections, sometimes using half length stents tomaximise flexibility of the balloon catheter/stent combination at tortuous sites. The mostdistal part ofthe duct seems to have remarkablepowers to constrict even when only a fewmillimetres is left unstented. We now ap-preciate that great care has to be taken to ensurevery accurate stent positioning at this end ofthe
244
Stenting of the arterial duct: a new approach to palliation for pulmonary atresia
duct at an initial procedure if repeated visits tothe catheter laboratory are to be avoided.There was no clear cause of death in our first
case, but as far as is possible to ascertain neitherchild died with inadequate pulmonary bloodflow. The first child had apnoeic attacks in theimmediate neonatal period which had beenattributed to airway obstruction related to hisextensive cleft palate and it is possible that thismay have contributed to his death. His pul-monary blood flow seemed to be relatively highand he had required treatment with diuretics;left ventricular volume overload may also havecontributed to his death. The second child hadextremely complex congenital heart diseaseand asplenia. His death was almost certainlydue to septicaemia, because pneumococci weregrown from the postmortem blood culture. Histwin brother's near miss cot death is also ofnotebut of uncertain relevance. We are planningfurther detailed investigation of the ducts,
including histological assessment of endothe-lialisation.
Further follow up and further experience inother patients with a wider variety of ductalanatomy is clearly required before mediumterm results of ductal stenting can be assessed.At least in the short term, however, thisprocedure seems to promise a non-surgicalalternative to aortopulmonary shunt surgery,with the very attractive benefit of providingbalanced perfusion of both lungs and thereforethe potential of balanced growth of the pul-monary arteries.
1 Mullins CE, O'Laughlin MP, Vick GW, et al. Implantationof balloon expandable intravascular grafts by catheterisa-tion in pulmonary arteries and systemic veins. Circulation1988;77:188-99.
2 Schatz RA, Baim DS, Leon M, et al. Clinical experiencewith the Palmaz-Schatz coronary stent. Initial results of amulticenter study. Circulation 1991;83:148-61.
245