AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct Mechanisms
Mechanisms of Disease 1
-
Upload
khangminh22 -
Category
Documents
-
view
2 -
download
0
Transcript of Mechanisms of Disease 1
Mechanisms of Disease 1
Module coordinators:
Dr. S.M. Arend
LUMC, Department of Infectious Diseases
E-mail: [email protected]
Dr. I.M. Bajema
LUMC, Department of Pathology
E-mail: [email protected]
M O D U L E B O O K
Bachelor Medicine, second year
Course year 2014-2015
© 2014 Alle rechten voorbehouden
LUMC
Behoudens de in of krachtens de Auteurswet van 1912 gestelde uitzonderingen, mag niets uit deze
uitgave worden verveelvoudigd en/of openbaar gemaakt worden door middel van druk, fotokopie,
microfilm, web-publishing of op welke andere wijze dan ook en evenmin in een gegevensopzoeksysteem
worden opgeslagen zonder voorafgaande schriftelijke toestemming van de houder van de copyrights.
Voor vragen of informatie kunt u contact opnemen met:
Directoraat Onderwijs en Opleidingen, PB 9600, 2300 RC Leiden
Contents
Module committee and teachers ..............................................................................................................................................1
Preface..........................................................................................................................................................................................3
Introduction and general information .....................................................................................................................................5
1.1 Summary and relation to other modules .....................................................................................................................5
1.2 Subjects and themes........................................................................................................................................................7
1.3 Main study goals of the module....................................................................................................................................9
1.4 Prerequisites ...................................................................................................................................................................10
1.5 Teaching activ ities (study methods) ..........................................................................................................................10
1.6 English and Dutch in the module ...............................................................................................................................13
1.7 Assessment .....................................................................................................................................................................13
1.7.1 Summative tests.....................................................................................................................................................13
1.7.2 Formative tests.......................................................................................................................................................14
1.8 Exam matrix ...................................................................................................................................................................16
1.9 Study books ....................................................................................................................................................................17
1.10 Relevant websites........................................................................................................................................................17
1.11 The ‘rules of the game’ ..............................................................................................................................................17
1.12 Module schedule overview (see also Attachment 7 for complete schedule) ....................................................20
1.13 Overv iew of lectures in module Mechanisms of Disease 1.................................................................................21
1. Theme I: The immune system and its opponents ...........................................................................................................23
1.1 Theme I.A: Global overview of host defence mechanisms and ‘key players’ of the immune system ..............24
1.1.1 Introduction ............................................................................................................................................................24
1.1.2 Theme-related objectives (What you will learn) .............................................................................................24
1.1.3 Study methods and study plan ............................................................................................................................24
1.1.4 Reading list.............................................................................................................................................................25
1.1.5 SSA ..........................................................................................................................................................................27
1.2 Theme I.B. Pathology of injury and repair ...............................................................................................................29
1.2.1 Introduction ............................................................................................................................................................29
1.2.2 Theme-related objectives (What you will learn) .............................................................................................30
1.2.3 Study methods and study plan ............................................................................................................................30
1.2.4 Reading list.............................................................................................................................................................31
1.2.5 Work group 1 .........................................................................................................................................................31
1.2.6 SSA ..........................................................................................................................................................................33
2. Theme II: Microorganisms as cause of disease ..............................................................................................................35
2.1 Introduction ....................................................................................................................................................................35
2.2 Theme-related objectives (What you will learn) .....................................................................................................36
2.3 Study methods and study plan ....................................................................................................................................36
2.4 Reading list.....................................................................................................................................................................37
2.5 SSA ..................................................................................................................................................................................38
3. Theme III: Infectious Diseases ..........................................................................................................................................43
3.1 Theme III.A: Host-pathogen interactions .................................................................................................................45
3.1.1 Introduction ............................................................................................................................................................45
3.1.2 Theme-related objectives (What you will learn) .............................................................................................45
3.1.3 Study methods and study plan ............................................................................................................................46
3.1.4 Reading list.............................................................................................................................................................46
3.1.5 Virulence factors ...................................................................................................................................................47
3.1.6 SSA ..........................................................................................................................................................................48
3.1.7 Instructions Extended Matching Questions Theme III.A ...............................................................................49
3.2 Theme III.B: Clinical presentations and diagnostics..............................................................................................53
3.2.1 Introduction ............................................................................................................................................................53
3.2.2 Theme-related objectives (What you will learn) .............................................................................................54
3.2.3 Study methods and study plan ............................................................................................................................54
3.2.4 Reading list.............................................................................................................................................................55
3.2.5 Work group 2 .........................................................................................................................................................55
3.2.6 Instructions Extended Matching Questions Theme III.B ...............................................................................57
3.3 Theme III.C: Therapy of infectious diseases ............................................................................................................63
3.3.1 Introduction ............................................................................................................................................................63
3.3.2 Theme-related objectives (What you will learn) .............................................................................................64
3.3.3 Study methods and study plan ............................................................................................................................64
3.3.4 Reading list.............................................................................................................................................................65
3.3.5 Concepts of pharmacology and prescribing antimicrob ial therapy ..............................................................66
3.3.6 SSA ..........................................................................................................................................................................68
3.3.7 Tables antimicrobial therapy ...............................................................................................................................69
3.4 Essential Microorganisms ...........................................................................................................................................73
3.4.1 Tables with ‘Essential microorganis ms’ ...........................................................................................................73
3.4.2 Reading for Essential microorganisms ..............................................................................................................75
4. Theme IV: Epidemiology, prevention and control of infect ious diseases .................................................................77
4.1 General information theme IV ....................................................................................................................................77
4.1.1 Why study epidemio logy, prevention and control of infectious diseases? .................................................77
4.1.2 Theme-related objectives (What you will learn) .............................................................................................77
4.1.3 Study methods and study plan ............................................................................................................................78
4.1.4 Reading list.............................................................................................................................................................79
4.1.5 Work group 3 .........................................................................................................................................................79
4.2 Theme IV.A: Epidemiology..........................................................................................................................................81
4.2.1 Introduction ............................................................................................................................................................81
4.2.2 The infection chain ...............................................................................................................................................81
4.2.3 Reproductive rate ..................................................................................................................................................82
4.2.4 SSA ..........................................................................................................................................................................82
4.2.5 Instructions Extended Matching Questions Theme IV.A. .............................................................................83
4.3 Theme IV.B: Prevention and control .........................................................................................................................85
4.3.1 Introduction ............................................................................................................................................................85
4.3.2 Healthcare-associated infections ........................................................................................................................86
4.3.3 SSA ..........................................................................................................................................................................87
4.3.4 Instructions Extended Matching Questions Theme IV.B. ..............................................................................91
5. Theme V: A llergy ................................................................................................................................................................93
5.1 Introduction ....................................................................................................................................................................93
5.2 Theme-related objectives (What you will learn) .....................................................................................................93
5.3 Study methods and study plan ....................................................................................................................................93
5.4 Reading list.....................................................................................................................................................................94
5.5 SSA ..................................................................................................................................................................................95
6. Theme VI: Auto-immunity ................................................................................................................................................97
6.1 Introduction ....................................................................................................................................................................97
6.2 Theme-related objectives (What you will learn) .....................................................................................................97
6.3 Study methods and study plan ....................................................................................................................................98
6.4 Immunosuppressive pharmacotherapy ......................................................................................................................99
6.5 Reading list...................................................................................................................................................................100
6.6 Work group 4 ...............................................................................................................................................................100
6.7 Academic Assignment................................................................................................................................................102
6.8 SSA ................................................................................................................................................................................103
7. Theme VII: Transplantation.............................................................................................................................................107
7.1 Introduction ..................................................................................................................................................................107
7.2 Theme-related objectives (What you will learn) ...................................................................................................107
7.3 Study methods and study plan ..................................................................................................................................108
7.4 Reading list...................................................................................................................................................................109
7.5 SSA ................................................................................................................................................................................109
Attachment 1: Tab les for SSA II.1......................................................................................................................................111
Attachment 2: Tab les for SSA III.A.1 and III.A.2 ...........................................................................................................114
Attachment 3: Tab le fo r SSA IV.A.1 and IV.B.1.............................................................................................................116
Attachment 4: Tab le fo r SSA IV.B.3..................................................................................................................................117
Attachment 5: Tab le fo r SSA VI.6......................................................................................................................................121
Attachment 6: Practicals Bacteriology and Parasitology ................................................................................................122
PRACTICAL 1: Bacteriology .........................................................................................................................................122
PRACTICAL 2: Parasitology ..........................................................................................................................................127
Attachment 7: The module at a glance ...............................................................................................................................133
1
Module committee and teachers
Module coordinators
Dr. S.M. Arend
Dept. of Infectious Diseases
LUMC C5P-40
Coordinator Infectious
Diseases
LT, SM, PD, PR,
WG*
Dr. I.M. Bajema
Dept. of Pathology
LUMC P2-28
Coordinator
Immunology and
Pathology
LT, WG
* abbreviations: see next page
Secretariate
Mw. M. Veelenturf
Dept. of Pathology
P0-044
LUMC
Phone: 071 5266564
Email: [email protected]
Secretarial support practicals:
Mw. E.M. van Rijn
Dept. of Infectious Diseases
Logistical support:
D.O.O.
Module committee
Dr. S.G. van Duinen
Dept. of Pathology
WG
Dr. J. Gooskens
Dept. of Microbiology
PR
Dr. A. Lankester
Dept. of Pediatrics
SM
Dr. E.A. van Lieshout
Dept. of Parasitology
LT, PR
Dr. P.H. Nibbering
Dept. of Infectious Diseases
Dr. M.E.J. Reinders
Dept. of Nephrology
PD
Dr. R. Rissmann
Dept. of Pharmacology
LT
Prof. Dr. V.T.H.B.M. Smit
Dept. of Pathology
LT,WG
Dr. J.J.C de Vries
Dept. of Microbiology
LT,SM
2
Teachers
Immunology (various Departments)
Bekker, V. (Pediatrics) WG
Bergen, J. van (IHB§) WG
Bredius, R.G.M. (Pediatrics) SM
Claas, F.H.J. (IHB) LT
Griffioen, M. (Exp. hematology) WG
Halteren, A.G.S. van (Pediatrics) LT
Hiemstra, P.S. (Pulmonary Dis.) LT
Lankester, A.C. (Pediatrics) SM
Roelen, D. WG
Roep, B.O. (IHB) LT
Rood, J.J. van (Europdonor) LT
Schilham, M. (Pediatrics) WG
Smits, H.H. (Parasitology) WG
Tol, M.J.D. van (Pediatrics) SM
Trouw, L.A. (Rheumatology) WG
Verschuuren J.J.G.M. (Neurology) LT
§ Dept. of Immunohematology and Blood bank
Department of Infectious Diseases
Arend, S.M. LT,SM,PD,WG,PR
Bauer, M.P. SM,WG
Boer, M.G.J. de LT,SM,WG
Geluk, A. LT
Groeneveld, G.H. PR
Haverkamp, M.H. WG
Jolink, H. WG
Marbus, S.D. PR
Kroon, F.P. WG
Roestenberg, M. PR#, WG
Scheper, H. PR
Schippers, E.F. PR
Stalenhoef, J.E. WG
Visser, L.G LT,PD,PR
Vollaard, A.M. PR
Department of Microbiology
Beek, M.T. van der PR#
Bentvelsen, R.G. PR
Brienen, E.A.T. PR
Bernards, A. WG
Engel, M.F. PR
Feltkamp, M.C.W. WG
Gooskens, J. PR# (coordinator
PR1)
Klink, A.L. PR
Konstaninovski, M.M. PR
Knoester, M. WG
Kroes, A.C.M. LT,SM
Scoop, D.W.L. PR
Terveer, E.M. PR
Veldkamp, K.E. SM,PR#
Vossen, A.C.T.M. WG
Vries, J.J.C. de LT
Wong, M.C. PR
Wunderink, H.F. PR
Department of Parasitology
Brienen, E.A.T. PR
Kos, van Oosterhoud, J. PR
Kroeze, J. PR
Lieshout, E.A. van LT,PR#
(coordinator PR2)
Ramesar, J. PR
Department of Pathology
Bajema, I.M. LT, WG
Boer, H. de WG
Bosse, T. LT, WG
Bovee, J. WG
Bruijn, J.A. WG
Cleve, A. WG
Crobach, S. WG
Duine, S. van WG
Hout, M. van den WG
Kelder, T.P. WG
Koens, L. WG
Smit, V. LT, WG
Wilhelmus, S. WG
Department of Nephrology
Koning, E. de LT
Reinders, M PD
Rotmans, J PD
Pharmacology
Hessel, M.H.M. LT
Rissmann, R. LT
*Participates in:
LT = lecture
SM = seminar
PD = patient session
PR = practical (PR#=presiding practicals)
WG = work group
3
Preface
Throughout history, concepts of the causes of disease have gone hand in hand with contemporary
knowledge. Around 2500 B.C. a disease was considered to be a patient’s own fault, or the result of an
intervention by gods, demons or spirits. From 300 B.C. until about 1500 A.D., clinical observations
and autopsies (gross, morbid or macroscopic anatomy) led to new concepts about the origin of
disease. In this period, the cause of a disease was attributed to an imbalance in the bodily fluids
(humora). From 1500 until about 1800, diseases were often believed to be a consequence of
spontaneous generation of pathogens from dead material (abiogenesis). It was not until the nineteenth
century that Rudolf Virchow put forth the idea that disease was caused by alterations in cells.
Nowadays, the concept of disease can be summarized as follows: molecular changes lead to cellular
changes that result in tissue (organ) changes and subsequently cause clinical signs and symptoms.
The normal host defence mechanisms play a crucial role in the homeostasis and are a prerequisite for a
healthy life. These responses are involved in repair of injury as well as in the defence against
omnipresent viruses, bacteria, fungi and parasites. However, microorganisms may be too virulent or
the immune system may malfunction, which in both cases can result in disease.
In this module, the three disciplines immunology, pathology and infectious diseases are presented in a
fully integrated manner. These 3 disciplines were not chosen at random. Obviously, it is not difficult
to think of a number of scenarios in which they closely interact.
During this 6 weeks course, the student will not only learn different mechanisms that can lead to
disease, but will also make the first steps to become a medical expert who can recognize characteristic
clinical presentations of immune-mediated and/or infectious diseases, explain their pathogenesis and
make a targeted diagnostic plan and a rational choice from the available pharmaco-therapeutical
arsenal of immunosuppressive or antimicrobial drugs. The competence health advocate is elaborated
on during the theme ‘Prevention and control of infectious diseases’. In addition, there will be special
assignments to train and challenge the scholarly talents (‘academic assignment’). In the final theme
‘Transplantation-associated pathology’ all knowledge that was acquired throughout the module comes
together.
The coordinators of the Mechanisms of Disease 1 module wish you an enjoyable learning experience!
ME CH A N ISMS O F D ISE A SE 1
5
Introduction and general information
1.1 Summary and relation to other modules
This course elaborates on the first year modules of Medicine in which the normal anatomy, physiology
and homeostasis were taught. Disease often results from a disturbance in the structural integrity and/or
normal function of (part of) the body and/or the response to this disturbance. The second year starts with
the modules Mechanisms of Disease 1 and 2, focusing on the 7 different mechanisms of disease (see
figure).
The Pathogenesis Pie
Acute and chronic
inflammation
Disordered immunity
(immunodeficiency
and immunopathology)
Cell/tissue injury and
repair
Haemodynamic
disorders
Congenital
abnormalities (genetic,
non-genetic)
Metabolic and
degenerative
disorders
Growth disorders
(neoplastic, non-
neoplastic)
Mechanisms of disease 1Mechanisms of disease 2
In module G2MD1 the focus is mainly on inflammation, cell/tissue injury and repair and disordered
immunity but there will be some overlap with the remaining mechanisms which will be addressed in
module G2MD2. In the following course, G2MD2 , the main focus will be on neoplastic and non-
neoplastic growth disorders next to the other mechanisms of disease on the left side of the pie, for which
knowledge obtained in G2MD1 is a prerequisite.
IN TRO D U CTIO N A N D G E N E RA L IN FO RMA TIO N
6
Place of Module Mechanisms of Diseases 1 in the Curriculum Bachelor of Medicine
The concepts learned in module G2MD1 will continue to be used in all subsequent modules that address
clinical problems (’vraagstukken blokken’). In subsequent modules, additional specific diseases will be
addressed, expanding the differential diagnostic potential of the student for which a basis was laid in
module G2MD1.
ME CH A N ISMS O F D ISE A S E 1
7
1.2 Subjects and themes
A varied menu of teaching methods, including lectures, seminars, weekly patient demonstrations, work
groups, microscopy practicals and e-learning modules is offered to the students. Together, these will guide
you through the module. Moreover, we will provide you with regular test exams in order for you to assess
your state of knowledge. Your capacity to apply this knowledge to actual cases will be tested in one
component exam during the course and one final exam at the end. Collectively, these test results will
determine your final grade.
G2MD1 is organized in 7 main themes which together cover the subjects for study in coherent
components and determine the structure and contents of the module. The themes are dealt with
sequentially, continuously integrating knowledge and insight from previous themes.
THEMES of G2MD1
I. Normal host response to pathological stimuli
I. A. Brief overview of the defence mechanisms and key components of the normal immune
response
I.B. Histology of injury and response
II. Microorganisms as cause of disease
III. Infectious Diseases
III.A. Host-pathogen interactions
III.B. Clinical aspects of infectious diseases: clinical presentations and diagnostics
III.C. Clinical aspects of infectious diseases: therapy
IV. Prevention and control of infectious diseases
IV.A. Epidemiology of infectious diseases
IV.B. Prevention and control of infectious diseases
V. Allergy
Hyper-responsiveness of the immune system to non-self: allergy
VI. Auto-immunity
VI.A. Hyper-responsiveness of the immune system to self: auto-immunity
VI.B. Clinical aspects of auto-immunity: clinical presentations and diagnostics
VI.C. Clinical aspects of auto-immunity: therapy
VII. Transplantation-associated pathology
IN TRO D U CTIO N A N D G E N E RA L IN FO RMA TIO N
8
In short:
Theme I deals with the components and function of the normal immune system such as normal
anatomical barriers, specific and nonspecific cellular and humoral immunity, lymph nodes and spleen;
these components collectively represent the host defence. The histology of the response to injury or
infection is taught.
Theme II addresses the classification, structure and physiology of microorganisms, forming the link to the
next theme.
Theme III focuses on infection as the outcome of the interaction between the host and a microorganism.
Classical clinical presentations of common or interesting infections are discussed, as are the
diagnostic methods used in medical microbiology and parasitology including microscopy, culture and
susceptibility testing, serology, and molecular biological techniques. The basic principles of antimicrobial
therapy and application to simple cases are taught.
Theme IV is focused on the epidemiology, prevention and control of infectious diseases. Knowledge
about epidemiology is the basis for successful prevention and control of infectious diseases. Some
infectious diseases can spread from a carrier, patient, animal or environmental source to a new host, and
some are known for their ability to become pandemic. ‘Prevention is better than cure’ is a well-known
saying, which almost always proves to be right.
Theme V addresses the classic division of the hypersensitivity reactions into types I to IV. Basic
principles behind this subdivision together with a number of typical examples of diseases that exemplify
these principles will be dealt with. Histological changes and immunological mechanisms underlying the
different hypersensitivity reactions will be explained.
Theme VI will elaborate on the knowledge obtained in theme V, and a number of immune mediated
diseases will be presented into more detail, such as ANCA-associated vasculitis and Systemic Lupus
Erythematosus (SLE). The histopathological changes in the kidney will be the central point from which
differential diagnostic and therapeutic considerations will be discussed.
Theme VII is the last theme of this course, in which all disciplines will interact to provide a general
overview of the complex situation of solid organ and bone marrow transplantation. The clinical setting,
histopathological findings, immunological aspects, infectious threats and therapeutic challenges of the
transplanted patient will be discussed.
ME CH A N ISMS O F D ISE A S E 1
9
1.3 Main study goals of the module
Themes Relates to
competences
(**)
STUDY GOALS: The student
1. describes individual components and interactions between components of the defense mechanisms
which underlie normal response and repair mechanisms for the maintenance of tissue homeostasis
I K 1
2. recognizes and classifies histological features of acute or chronic inflammation, necrosis and repair
responses, and deduces the consequences of these processes for tissue/organ function
I T 1
3. categorizes different classes of micro-organisms, describes the structural characteristics and relates
structural components to physiology of micro-organisms
II T 1
4. explains the pathogenesis of diseases that result from an interaction between the immune system
and pathological stimuli, with focus on microorganisms, and deduces the clinical symptoms that
result from this interaction
II, III T 1,2
5. categorizes the causes of failing defense mechanisms and hyperresponsiveness of the immune
system (type I-IV allergies and auto-immune diseases) and explains the pathogenesis and clinical
symptoms of diseases resulting from these disorders
I, III, V, VI T 1,2
6. distinguishes the immunological principles of solid organ transplantation and transplantation of the
hematopoetic system, names factors which affect transplantation outcome and describes the
pathogenesis of clinical problems associated with transplantation
I, VII B 1
7. given a description of diagnostic test results in a study population, the student interprets these
results, calculates sensitivity, specificity and positive/negative predictive value and predicts the
effect of a change in pretest probability on these parameters
III, V, VI T 1,6
8. analyzes a scientific paper on immunological or infectious diseases subject III, V, VI T 1,6
For a number of infections and immune disorders that are part of the module (listed in the Module
book), the student
9. recognizes characteristic clinical presentations III, V, VI K 1,7
10. explains the pathogenesis III, V, VI T 1,7
11. selects appropriate diagnostic and therapeutic options, indicates mechanism of action of selected
immunosuppressive, immune enhancing and antimicrobial drugs
III, V, VI T 1,4,7
12. given a microscopic image, the student names the most likely microorganism, infectious syndrome,
or immune disorder
I, II, III, V,
VI, VII
B 1
13. analyzes the infection chain (reservoir, source, transmission route and host factors) and applies the
principles of prevention and control of infectious diseases to concise but realistic problems and
situations
IV T 1,3,4,5,6,7
Subjects from previous modules
recognizes normal cells, tissues and organs;
describes normal temperature and blood pressure regulation
KBT*
14. K 1
* K: kennis (knowledge); B: begrip (understanding); T: toepasssing (application)
** see next paragraph
Competences
From the CanMeds roles framework three competences are explicitly addressed in the module
G2MD1, which will be further elaborated on in the line courses:
1= Medical expert (Medisch deskundige)
2= Communicator (Communicator)
3= Collaborator (Samenwerker)
4= Manager (Organisator)
5= Health advocate (Gezondheidsbevorderaar)
6= Scholar (Academicus)
7= Professional (Beroepsbeoefenaar)
IN TRO D U CTIO N A N D G E N E RA L IN FO RMA TIO N
10
Medical Expert
The student will learn to recognize characteristic clinical presentations, make a differential
diagnosis, choose appropriate diagnostic tests, explain the pathogenesis and apply the basic
concepts of treatment of immune-based or infectious diseases.
Health Advocate
In theme IV, the rational choice of the various methods of prevention and control, such as
isolation measures, source finding and elimination, vaccination, prophylaxis, disinfection,
sterilization, antisepsis and vector control, is trained for a number of common and/or transmissible
infections including health care infections. This will help the student to become a true health advocate.
Scholar
The correct interpretation of (clinical) scientific publications is an important component of the
life-long medical learning process. In this module one or more examples will be discussed in
which specialists from various disciplines will take part. In addition it will be taught how and why
the interpretation of diagnostic test results for an individual patient depends on test sensitivity and
specificity as well as the pre-test risk of disease. Finally, important and interesting historical developments
in the field of infectious diseases are presented.
1.4 Prerequisites
Knowledge of the structure of the normal human cell and the most important physiological and
biochemical processes of the cell;
Knowledge of the normal histology of tissues and organs;
Knowledge of the concepts of molecular recognition, receptor specificity, receptor-ligand
interaction;
Understanding of normal regulation of blood pressure and temperature;
Insight into causes and mechanisms of pain sensation;
Knowledge of epidemiological terminology;
Ability to apply basic arithmetic (use of calculator allowed);
Usage of English at level B2 (Common European Framework of Reference for Languages).
1.5 Teaching activities (study methods)
This module provides a great variety of teaching activities, each of which makes a significant contribution
to the study goals. It is, however, important to realize that self-study takes up the greater part of the
scheduled time (70%).This means that the student should not rely on the contact teaching activities only.
Self-study
The assignments for self-study are described within each theme (indicated with a figure starting with the
Roman numeral of the theme, e.g. assignment II.A.4), and require basic knowledge from previous
modules. Each of the assignments specifically indicates what is expected from the student. The product of
the self-study is what has been learned from the assignments. Schemes and tables with the assignments are
provided in the appendices. After the work groups related to the themes, the answers to the self-study
assignments are placed on Blackboard. Note that effective learning requires that you first phrase your own
answers before checking them!
ME CH A N ISMS O F D ISE A S E 1
11
Lectures
The module includes in total 42 lectures.
Theme I: 10
Theme II: 4
Theme III: 8 + 1 pharmacotherapy
Theme IV: 2
Theme V: 2
Theme VI: 8 + 2 pharmacotherapy
Theme VII: 5
The titles of all lectures are provided on page 21 and the contents are summarized with the information on
study methods per theme.
Seminars
The module includes 3 different seminars during which interaction between teacher and students is an
essential part. The seminars are mostly based on cases or on specific practical situations. Each seminar is
given in 2 lecture rooms simultaneously (resp. Dutch and English version). Dutch students may attend the
English seminar (advantage: lower number of students thus higher teacher/student ratio).
-Theme I: Diagnostics of immune deficiencies (Seminar 2).
-Theme II/III: Diagnostics of infectious diseases (Seminar 1).
-Theme IV: Health advocate (‘gezondheidsbevordering’; Seminar 3).
Patient sessions
This module includes one patient session each week, together illustrating the palette of immunological,
pathological and infectious diseases problems (contents of these demos are part of the study material):
- Week 1 Febrile inflammatory illness.
- Week 2 Infection, host defence disorder.
- Week 3 Transmissible and reportable infection.
- Week 4 Allergy.
- Week 5 Auto-immune disease.
- Week 6 Transplantation-associated pathology.
Work groups
There are 4 work groups during the module. These represent an essential component of the module. Work
groups are obligatory and active participation will be assessed, see under ‘rules of the game’ in 1.11.
During each work group there will be a short formative test (open questions), followed by a discussion of
the answers.
IN TRO D U CTIO N A N D G E N E RA L IN FO RMA TIO N
12
Work group
Main themes
Subjects
General
study goals*
Disease specific
study goals* Tutor(s)
1 I
Normal host defence, acute and chronic inflammation, causes of fever, expla in
symptoms caused by inflammation 1, 2 9, 10 Immunologist or pathologist
2 II , I I I
Cases: pathogenesis of infections (host vs invader), rational selection of diagnostic
tests , therapeutic principles 3, 4, 7 9..13 Infectiologist or microbiologist
3 II , I I I , IV
Cases/subjects related to competence health advocate: prevention hospita l
infections , i solation measures , prophylaxis , active and pass ive
immunization 3 4 13
Infectiologist or
microbiologist
4 I , V, VI
Cases (or problems) with
immunodeficiency or overrespons iveness , analyze
pathogenesis , symptoms, diagnostics , therapy 2, 5 9..13
Immunologist or pathologist
*See table page 9
Practicals
The two practicals bacteriology and parasitology (these are obligatory: see ‘rules of the game’) represent
an introduction to the use of microscopy in bacteriology and parasitology, which is presented in a clinical
context. The student learns to interpret the results of the tests. At the exams, most questions related to
infectious diseases will also be patient-based. At the end of the practical there will be a short formative
test consisting of open questions, followed by a discussion of the answers.
NOTE. The practical includes assessment of Gram stains, for which colour vision is required. The
component and final exam will include assessment of Gram stains and histology. Students with problems
of colour vision should report this to the module coordinator during the module.
E-learning
G2MD1 includes e-learning modules in Theme II (1 module), Theme III (2 modules). These modules are
strongly advised as they are part of the study material for the exam. For themes II and IV additional
optional E-learning modules are available.
All e-learning modules and the TRC (Teaching Resource Center) referred to in the module book are
accessible via Blackboard → Module Mechanisms of Disease 1 → E-learning for G2MD1. The e-learning
modules are also accessible via www.medischonderwijs.nl (you may need to make a personal account)
search term ‘G2MD1’ will lead to the modules of Themes II and III, search term’ infectiepreventie’ to the
optional modules of Theme IV). More specific information about the e-learning modules is provided with
the corresponding themes.
Alternative ways of accessing the TRC are:
You can use Teaching Resource Centre Pharmacology without personalization at
http://coo.lumc.nl/TRC/default.aspx?direct=true
You can download the TRC iPad app (‘TRC pharmacology’) from the App Store or iTunes.
You can download the TRC Android app (‘TRCapp’) from Google Play.
ME CH A N ISMS O F D ISE A S E 1
13
1.6 English and Dutch in the module
The module will be attended by a number of non-Dutch speaking foreign students. All lectures will be in
English. Therefore the following arrangements have been made:
All lectures and slides will be in English.
The program also contains three seminars which will each be presented in parallel; one of these
sessions will be in English.
The patient demonstrations will be in Dutch during the interaction with the patient. In case of an
ensuing presentation or discussion without the participation of the patient , this will be done in
English on request.
One of the 7 sessions per practical and two series of workgroups will be in English.
In the module book the Dutch translation of the English for medical jargon or vice versa is
sometimes provided. It is indicated in the module book if a Dutch translation of a table or list is
available on Blackboard.
The optional e-learning modules on prevention of hospital infections are only available in Dutch.
During the lecture Prevention and control of infectious diseases, the main points of this subject will
be taught.
The exams will be in Dutch for Dutch students and in English for the others. If Dutch students
prefer an English version instead of a Dutch version, they must send a formal request by e-mail to
the secretary before the end of week 2 (September 12th
) for the component exam and before the end
of week 4 (September 26th
) for the final exam.
Please be aware that training yourself to gain access to scientific knowledge through the English language
is a vital part of your further career; you need to be able to search for medical facts and figures in books,
websites, conferences and other media. Please try to train yourself to speak up and ask your teachers
whatever you do not understand. Active participation of, and open communication between students and
teachers are vital aspects of academic training.
1.7 Assessment
The module includes summative and formative tests:
In this module, assessment consists of two summative tests, based on which the grade will be determined
(summative tests count for the grade).
Formative tests are provided for practice and self-evaluation (formative tests do not count).
1.7.1 Summative tests
Summative testing is organized in one component exam in week 4 and one large final exam, both of which
contribute to the final mark. Use of books or electronic devices is not allowed during the exams
COMPONENT EXAM (max. 20 points in total, which make up ~15% of the final grade))
NOTE. Participation in the component exam is voluntary, this means it is NOT a prerequisite for release
of the final grade. If a component exam is missed this implicates 0 points. There will be no opportunity for
IN TRO D U CTIO N A N D G E N E RA L IN FO RMA TIO N
14
a component re-exam during this module. However, the student can participate in the final exam and the
grade will be released if all obligations are fulfilled.
Date and time: Monday September 22nd
at 10:00-11:40 am
Location: USC-sportcentrum
Duration: 100 minutes
Subjects: all lectures, seminars, patient demos, e-learning, practicals and self-study assignments of themes
I, II and III.A and III.B
Competence: Medical expert
Type of exam: 15 written, mostly case-based short open questions (maximum score 7,5 points) and 25
multiple choice questions (0,5 point each, maximum score 12,5 points). The exam will include assessment
of microscopic images. For distribution of questions per theme see Exam matrix on page 16.
Assessment: MC by ICLON, open questions by module teachers based on a correct answer model. The
individual score by student number will be made known via Blackboard within one week.
FINAL EXAM (max. 95 points)
Date: Friday October 10th
at 13.00-16.00 (week 6)
Duration: 180 minutes.
Subjects: all lectures, seminars, patient demos, work group contents, practicals, e-learnings and self-study
assignments plus indicated pages from the study books.
Competences: Medical expert (±80%), Health advocate, Scholar.
Type: Multiple Choice (N=57, 1 point each), Extended Matching (N=15, 2 points each) and
Comprehensive Integrated Puzzle (one 44 puzzle consisting of 16 items worth 0.5 point per correct
answer, maximum of 8 points). For distribution of questions per theme see Exam matrix.
The final grade is determined by the sum of the points for the component exam and the final exam.
Week 1 Week 2 Week 3 Week 4 Week 5 Week 6
Component exam Final exam
Max.
95 points
Max.
20 points
Grade
~15% ~85%
1.7.2 Formative tests
Formative tests are frequently given in this module to allow the student to assess whether the level of
knowledge is sufficient or where there are gaps:
In week 1 through 5 of the module (week 36-40) a set of test questions is provided, either during lectures
with use of voting boxes (direct feedback) or via Blackboard. On one occasion a question and discussion
hour is held on Monday morning before the lectures.
ME CH A N ISMS O F D ISE A S E 1
15
On the Monday of week 6 (week 41) a larger formative test is provided via Blackboard containing
questions on all themes, of which the answers will be released the next day. On Wednesday of week 6
three response lectures are held (immunology, pathology, infectious diseases), where students can pose
questions (preferably email your questions to the coordinator of the theme that the question relates to).
This is followed by a last practice test with voting boxes.
Work groups will start with a short formative written test consisting of open questions regarding the theme
of the work group, followed by a discussion of the answers (total duration 20 minutes).
During the practical bacteriology (week 3) a short formative written test exam consisting of open
questions is given during the final 20-30 minutes, with direct discussion of the answers. The same will be
done in a short format during the practical parasitology (10 minutes). The contents of these formative tests
will be related to themes II and III, with emphasis on what was learned during the practicals (assessment
of a microscopic image in relation to a case).
Week 1 Week 2 Week 3 Week 4 Week 5
Work group
1 3 42
Practicals
B P
Week 6
Formative tests
(during PR or WG)
Formative tests
Open questions, during
WG or practical
Feedback is direct
Week 1 Week 2 Week 6Week 3 Week 4 Week 5
Formative tests
(Lecture room
or at home)
Feedback
Lecture room
with voting boxes
Feedback is directVia Blackboard
Feedback via BB or seminar
IN TRO D U CTIO N A N D G E N E RA L IN FO RMA TIO N
16
All summative and formative tests together:
Week 1 Week 2 Week 3 Week 4 Week 5
Work group
1 3 42
Practicals
B P
Week 6
Formative tests
(during PR or WG)
Formative tests
Summative tests
In addition:
All self-study assignments are de facto formative test questions.
During many lectures there will be test questions.
The practicals and work groups are mainly structured around cases with questions.
The e-learning modules contain a large number of test questions and/or learning questions through
which additional knowledge will be acquired.
1.8 Exam matrix
The subjects of the exam questions by theme are roughly as follows (minor deviation is possible):
THEME
sub-
theme TITLE MC (a 0,5 pt)
Open questions (a
0,5 pt) points per theme MC ( a 1 pt) EM (a 2 pt) CIP (a 8 pt)
Points per
theme
Subjects previous modules 4 4
I
Normal host response to
pathological stimuli 15 5 10 12 12
II
Micro-organisms as cause of
disease 5 3 4 5 5
III.A
Infectious diseases: host-pathogen
interaction 3 3
III.B
Clinical aspects of infectious
diseases: clinical presentations and
diagnostics 3 6
III.C
Clinical aspects of infectious
diseases: therapy 2 26
IV.A Epidemiology of infectious diseases 2 3
IV.B
Prevention and control of infectious
diseases 2 3 16
V.A
Hyperresponsiveness of the
immune system to non-self: allergy 3
V.B
Clinical aspects of allergy: clinical
presentations and diagnostics 3
V.C Clinical aspects of allergy : therapy 2 8
VI.A
Hyperresponsiveness of the
immune system to self: auto-
immunity 7
VI.B
Clinical aspects of auto-immune
diseases: clinical presentations and
diagnostics 2
1 (NOTE: CIP can
be about any
theme)
VI.C
Clinical aspects of auto-immune
diseases: therapy 2 19
VII VII
Transplantation associated
pathology 5 5
Subtotal 25 MC 15 Open questions 20 57 MC x 1 15 EM x 2 1 CIP x 8 95
maximum
FINAL GRADE BASED ON: COMPONENT + FINAL EXAM 115
Component exam
(~15% of final mark)
Final exam
(~85% of final mark)
III
IV
V
VI
5 7 6
The final grade is determined according to the method Cohen Schotanus (pass at 60% of the score of the
best 5% of students after adjustment for guessing).
ME CH A N ISMS O F D ISE A S E 1
17
After the final exam, students have the opportunity to comment on questions or answers during one week.
The final answers are determined taking into account these comments and the psychometric analysis in
accordance with the procedure of the national progression exam (‘voortgangstoets’). The final answer key
and an elucidation of adjusted or frequently missed questions will be published on Blackboard.
1.9 Study books
The module uses 4 obligatory study books:
Immunology: The Immune System, 3rd
edition, Peter Parham, Garland Science 2009.
Infectious Diseases: Sherris Medical Microbiology, 6th
edition. Ryan and Ray, McGraw Hill 2014.
NOTE. For students having Sherris 5th
edition published in 2010, the reading lists for the 5th
edition will be made available via Blackboard (PDF format).
Pathology: Kumar, Abbas and Fausto. Robbins and Cotran Pathologic Basis of Disease. Elsevier
Saunders, 8th
edition, 2009
Global health: Skolnik; Global Health 101, 2nd
edition 2012
With each theme or subtheme in the module book the pages or subjects to study are indicated, where the
abovementioned books are referred to briefly as ‘Parham’, ‘Sherris 6th
’, ‘Robbins’ and ‘Skolnik’.
1.10 Relevant websites
Blackboard: http://blackboard.leidenuniv.nl. Here you will find logistical information regarding
work groups and practicals, folders with PDFs of lectures and seminars, supplementary materials
regarding assignments, links to e-learning modules, translations, test exams etc.
All e-learning modules can be accessed via links on Blackboard or via www.medischonderwijs.nl.
1.11 The ‘rules of the game’
Contact
PLEASE NOTE: For questions please email the secretary Mrs. M. Veelenturf ([email protected]).
Do NOT directly email the module coordinators. The module coordinators will be around on most
teaching occasions, where you can ask questions in relation to the contents.
Lectures
Please be in the lecture room in time. If you arrive late, please take the high entrance and do not disturb
the lecture. Please refrain from talking during the lecture unless a question is asked. All lectures will be
placed on Blackboard afterwards.
Seminars
Please come in time. If you arrive late, please take the high entrance and do not disturb the seminar.
Professional behavior of students includes an active and participating attitude during seminars. Ask
your questions and raise your doubts. All seminars will be placed on Blackboard afterwards.
Patient demonstrations
IN TRO D U CTIO N A N D G E N E RA L IN FO RMA TIO N
18
NOTE: Patient demonstrations are in Dutch. Late arrivals are not allowed during a patient demonstration.
Professional behavior of students dictates that you discuss patients only with your colleague students if
this cannot be overheard by others. Patient demonstrations will not be placed on Blackboard.
Work groups
Attendance of at least 75% of the work groups, thus at least 3 work groups of this module, is obligatory
(an attendance list is signed) and thus are a prerequisite for the release of your grade. It is best to attend all
work groups. With each theme in the module book the required preparation for the corresponding work
group is indicated. Students are expected to prepare for the work group and participate actively!! The
work group teacher will make notes if students do not fulfill these requirements and the participation
assessments will be made available to the coordinator of the line professional education.
Be present on the indicated hour. The work groups start with formative test, followed by a discussion of
the answers. Students arriving late are asked to wait outside until the formative test is made, they can enter
when the discussion starts.
NOTE. Your work group number is the same as your practical group number. It is not possible to
change a work group unless there are special circumstances (in which case to can email to the module
secretary at [email protected] with a motivated request for a change). Missed work groups cannot be
caught up later.
Practicals
The two microscopy practicals (PR1 bacteriology and PR2 parasitology), are obligatory (an attendance
list is signed) and thus a prerequisite for the release of your grade. It is not possible to change practical
groups and it is NOT possible to participate in another practical group because the number of microscopes
is limited. If there is an urgent reason why you need to change practical groups you may contact the
secretary of the module ([email protected]) and ask for a motivated exception.
Missed practical
If you cannot attend a practical for legitimate reasons you should report this to the secretary with the
following requirements:
by email;
in advance and not later than 06.00 A.M. on the day of the practical;
documentation of your declared reason may be requested.
If these requirements are fulfilled you will be listed for a concise alternative teaching session on Monday
October 6th
(15.30-17.30) in the last week of the module, during which the essentials of the missed
practical(s) will be taught so that
1 you have the required knowledge at the final exam and
2 you have fulfilled your obligation for release of the grade
Your grade will only be released if all obligations (i.e. participation in at least three work groups and both
practicals or the alternative session) are fulfilled. Otherwise you can follow the work groups or practical(s)
of next year’s module.
Exam
Component exam: Enrollment for the component exam is required. However, only students who are
enrolled for the module can participate.
Final exam: Students have the opportunity to sign up for the final exam until 10 working days before the
exam (i.e. until Monday September 29th
23.59 P.M.) exactly. Students can and should check via uSis
(deelhistorie student) whether they are on the list. After the closure time, the list with registered students
ME CH A N ISMS O F D ISE A S E 1
19
will be published on Blackboard. Students who are not on the Blackboard list while the enrollment is
listed in uSis ‘deelhistorie student’ must bring a printout of (or show on smartphone) the deelhistorie to
the exam as proof of registration!! Students who are not on the list AND do not have a printed or digital
proof of registration are NOT allowed to do the exam and will be sent away.
It is possible to sign off for the exam until 10 working days before the exam (i.e. until Monday September
30th
24.00 P.M. exactly. If after that time point unforeseen circumstances arise that prohibit doing the
exam, the student must notify their study advisor at DOO and provide an explanation.
During the component exam as well as the final exam the use of books, notes, internet or mobile phone is
NOT allowed and these have to be stored and switched off. You may use a simple calculator without text
options (this will be checked).
The rules for release of blocked exam marks, (as indicated by DOO) will be followed strictly.
IN TRO D U CTIO N A N D G E N E RA L IN FO RMA TIO N
20
1.12 Module schedule overview (see also Attachment 7 for complete schedule)
Monday Tuesday Wednesday Thursday Friday
week 1
OPENING
ACADEMIC YEARLINE DAY
LT1
LT2
LT3
LT4
LT5
LT6
LT7
LT8
LT9
LT10
Pat demo 1
Test exam in
lecture room
week 2LT11
LT12
LT13
LT14LINE DAY Work group 1
LT15
LT16
LT17
LT18
LT19
LT20
LT21
Pat demo 2
(Test Questions on
Blackboard)
week 3Question hour
LT22
LT23
(afternoon)
PR1
group B,F,G
LINE DAY
PR1 group C,A,D,E
&
Work group 2
LT24
LT25
Pat demo 3
(afternoon)
PR2 (groups A,B,C)
PR2
group D,E,F,G
(Test Questions on
Blackboard)
week 4 COMPONENT EXAM
(afternoon)
SM1
SM2
SM3
LINE DAY
Voortgangstoets
(afternoon)
LT26
LT27
Pat demo 4
Work group 3
LT28
LT29
LT30
LT31
week 5 LT32
LT33
LT34
Test exam in lecture
room
LINE DAY
LT35
LT36
LT37
Pat demo 5 Work group 4
3 OCTOBER
HOLIDAY
‘LEIDENS ONTZET’
(Test Questions on
Blackboard)
week 6 LT38
LT39
LT40
LT41
(afternoon)
LT42
Pat demo 6
(Test Questions on
Blackboard)
LINE DAY
Response lectures
1. Immunology
2. Pathology
3. Infectious Diseases
No program(afternoon)
FINAL EXAM
Legend: LT: lecture; Pat demo: patient session; PR: practical, SM: seminar
ME CH A N ISMS O F D ISE A S E 1
21
1.13 Overview of lectures in module Mechanisms of Disease 1
Theme Lecture nr (abbreviated) Title Teacher
I LT1 LT2 LT3 LT4 LT5 LT6 LT7 LT8 LT9 LT10
Introduction to G2MD1 Introduction to the immune system Innate and adaptive immune responses Pathology of normal immune response Mechanisms of adaptive immunity B- and T-cell generation and diversity Pathology of inflammatory reactions Pathology of inflammatory reactions: a case Tissue injury and repair Repair mechanisms
Arend/Bajema Van Halteren Van Halteren Bajema Schilham Trouw Bajema Bajema Bosse Bosse
II LT11 LT12 LT13 LT14
Introduction to infectious diseases Bacteria Viruses Fungi and parasites
Arend Arend Kroes Van Lieshout
III LT15 LT16 LT17 LT18 LT19 LT20 LT21 LT22 LT23
Invader (virulence factors) Host versus invader Immune deficiencies and infection risk Pathology of infection Diagnostics of infectious diseases Interpretation of diagnostic test results Essential microorganisms Antimicrobial therapy Principles of antibiotic pharmacotherapy
Arend Arend Arend Smit De Vries Arend Arend Visser Hessel
IV LT24 LT25
Epidemiology of infectious diseases Prevention and control
De Boer Arend
V LT26 LT27
Mechanisms of allergy Pathology of allergy
Geluk Bajema
VI LT28 LT29 LT30 LT31 LT32 LT33 LT34 LT35 LT36 LT37
Cells in auto-immunity Pathology of auto-immunity Vasculitis Systemic Lupus Erythematodes HLA and auto-immunity Infections and auto-immunity Interactive cases Pharmacology: immune suppression 1 Pharmacology: immune suppression 2 Discussion academic assignment
Roep Bajema Bajema Bajema Verschuuren Bajema/Arend Bajema/Arend Rissmann Rissmann Bajema
VII LT38 LT39 LT40 LT41 LT42
Introduction transplantation Transplantation immunology Histopathology of Tx and rejection Islet cell transplantation Transplantation and infection risk
Van Rood Claas Bajema De Koning Arend
ME CH A N ISMS O F D ISE A S E 1
23
1. Theme I: The immune system and its opponents
Coordinator: Dr. I.M. Bajema
Already in 1796 Dr. Edward Jenner, the ‘father of immunology’ , performed the first successful
vaccination against smallpox by injecting the related cowpox virus into the son of his gardener. The
rationale for this brave experiment was his earlier observation that milkmaids were generally immune to
smallpox, while milkmaids were often infected with cowpox, a disease similar to smallpox, but much less
virulent, during their work. Jenner postulated that the pus in the blisters from cowpox protected them from
smallpox. In his time, physicians had no clue regarding the function of our immune system or the
mechanisms by which its opponents caused disease. According to Jenner’s colleagues, his unique
contribution was not that he injected cowpox virus but the fact that he proved, by subsequent challenges
with live variola (smallpox) virus, that his study subjects were protected against this infectious
microorganism by vaccination, a term derived from the Latin word vacca which means cow. Without any
knowledge of the immune system, he used its function for the purpose of preventing disease.
It took more than 150 years and substantial technical innovation before scientists collected sufficient
experimental data to understand the function of the key players of our immune system. This process was
boosted by some major breakthroughs which were rewarded by the Noble prize for Medicine. To date,
immunologists have a far more complete picture of the immune system and how we can exploit it in order
to protect our body from infections caused by known microorganisms. In Theme I, the basic knowledge of
the immune system will be presented, as summarized schematically in figure I.1.
Figure I.1: The components and key players of the immune system.
ComplementImmuno-
globulins
T cells
-Th1
-CTL
INNATE ADAPTIVE
CELLULAR
HUMORAL
Granulocytes
Macrophages
NK cells
BARRIERS
TH E ME I : TH E IMMU N E SY STE M
24
1.1 Theme I.A: Global overview of host defence
mechanisms and ‘key players’ of the immune
system
1.1.1 Introduction
Before you can appreciate how excessive immune reactions, or dysfunctional immune responses, may
cause disease, a basic understanding of the functional properties of our immune system is required.
During the first week of this course, we will provide you with some basic insights and we will discuss
some examples of immune-driven inflammatory reactions. By the end of this theme, you will know how
the human body protects itself against invading pathogens as well as some clinical consequences of
immune failure.
1.1.2 Theme-related objectives (What you will learn)
The student will be able to describe the components and function of normal human defence mechanisms
(innate and adaptive immune system) against various pathological stimuli, in particular microorganisms,
and to analyse simple clinical cases. The main topics are:
anatomical/chemical barriers and physical actions as first line of defence
normal haematopoiesis leading to the generation of various types of immune cells
key lymphoid organs and tissue sites where different steps of the immune response take place
the molecular basis of immune recognition
the mechanism of action mediated by the innate (granulocytes, macrophages, NK cells) and
acquired immune system (B and T cells) and the key cytokines involved
B- and T-cell generation and diversity including selection in the thymus
the principles of effector and memory T and B cell formation in relation to natural exposure to
pathogens and vaccination
clinical presentation and diagnosis of inborn immune deficiencies
the available therapeutic options for enhancement or restoration of dysfunctional immune
responses.
1.1.3 Study methods and study plan
Lectures Immunology
LT2: ‘Introduction to the immune system’. This first lecture focuses on the origin of immune cells,
their functional and phenotypic characteristics and their sites of action.
LT3: ‘Innate and adaptive immune responses and key cytokines’. This lecture explains how the
two distinct ‘arms’ of the immune system cooperate in clearing invading pathogens.
LT5: ‘Mechanisms of adaptive immunity’. This lecture zooms in on the mechanisms underlying T
and B cell activation and the generation of effector cells.
LT6: ‘B- and T-cell generation and diversity’. This lecture explains how B and T cells acquire
functional antigen receptors which enables them to respond to a vast number of pathogens.
ME CH A N ISMS O F D ISE A S E 1
25
LT8: ‘Pathology of inflammatory reactions: a case. This lecture will focus on the findings of an
autopsy case which shows inflammatory reactions in many organs.
Seminar 2: diagnostics of immune deficiencies
Diagnostics of classical types of inherited or acquired immune deficiencies: case-based interactive session
during which diagnostic tests must be chosen and results interpreted.
Work group 1: instructions for the work group are given under 1.2.5 of theme I.B
Self-study assignments: see 1.1.5 below
Study plan
Study the pages indicated in the reading list, preferably before attending the lectures
Follow the lectures and the seminar
Perform the self-study assignment (SSA)
Prepare for and participate actively in WG1
1.1.4 Reading list
From ‘Parham’ study:
Chapter From …up to Keywords, tables, figures
1
2
6
1-27
54-57
159-161
physical barriers fig 1.5-1.6
innate immune response fig 1.7, 1.8, 1.17,
adaptive immune response fig 1.9-1.11, 1.26, (in addition 6.20, 6.22)
neutrophil accumulation and function in inflammation fig 2.31, 2.32
immune cells and characteristic features fig 1.12, 1.14, 1.15 (+ 6.1-6.4)
primary and secondary lymphoid organs fig 1.18-1.22
2 33-40
44-47
49-53
58-59
65
the different complement systems and their function 2.3, 2.5, 2.10
pathogen lysis by complement components fig 2.12, 2.13
innate immune receptors for pathogens fig 2.19, 2.21, 2.22
inflammation induced by activated macrophages fig 2.27, 2.29, 2.36
inflammation induced by NK cells fig 2.47
3 71-75
78-85
83-85
structure of immunoglobulins and T cell receptors fig 3.1, 3.2
antigen recognition by B and T cell receptors fig 3.7, 3.8, 3.12, 3.13
antibody- and complement-mediated mechanisms for clearance of
infection fig 3.14
TH E ME I : TH E IMMU N E SY STE M
26
4 95
96-97
99-100
102-105
105-109,
(167-168)
115-119
one B cell one antibody principle fig 4.1,
antibody structure fig 4.2, 4.5
hypervariable region is the antigen-binding site (fig 4.8) and properties
of epitopes (fig 4.9 and 4.10)
mono/polyclonal antibodies and their application in clinical practice fig
4.14. 4.15
germline configuration in gene segments and RAG-induced somatic
recombination for generation of diversity fig 4.16-4.18, 4.20, 4.22,
(+6.11, 6.25)
isotype switching fig 4.22, 4.26, 4.30, 4.32
5 125-129
133-137
137, 140
143-144
146-148,
149-151
151-152
genomic organization and structure of T cell receptor complex fig 5.3,
5.6
functionally different T cells recognizing different MHC molecules fig
5.12-5.14
antigen processing into peptide fragments fig 5.20
MHC molecules are differentially expressed fig 5.23
MHC polymorphisms and T cell restriction fig 5.24, 5.25
MHC allele variation and pathogen-driven selection fig 5.33
7 187-189
199
T cell development fig 7.1, 7.3, 7.15,
positive/negative selection fig 7.16. 7.18, 7.21
8 211-213, 215,
222
224-231
237-240
241-242
T cell activation requires co-stimulation fig 8.1, 8.4, 8.10, 8.18
cytokines affecting T cell function fig 8.17, 8.19, 8.27, 8.39
CD4 T cell-driven macrophage activation fig 8.34, 8.36
CD4 T cell-driven B cell activation fig 8.37
9
254-255,
262-263
264-272
275
T helper cell -induced B cell activation, isotype switching and memory
cell formation fig 9.9, 9.19
antibodies in blood and mucosal surfaces and their function fig 9.23,
9.24, 9.25, 9.28
removal of immune complexes from the circulation fig 9.35
The key-words, figures and tables mentioned in the reading list are guidelines for the more detailed
knowledge you should have of these topics!
ME CH A N ISMS O F D ISE A S E 1
27
1.1.5 SSA
SSA I.A.1 Activation and the various effector functions of the immune system
1 Skin injury is often followed by translocation of bacteria into the deeper layer of the skin (dermis).
Describe the processes and key molecules which lead to inflammation due to, and clearance of this
primary infection.
2 Explain why it takes up to several days after initiation of inflammation before T cells specific for the
invading bacteria are detectable at the site of injury. At which sites reside the bacteria-specific B
cells?
3 Indicate the intrinsic differences in bacterial antigens which are recognized by the involved T (fig 3.7
Parham) and B cells (fig 3.12 Parham) respectively. How do these antigens arrive at the sites where
naïve T and B cells become activated (Parham page 82)?
4 Indicate the key effector molecules produced by T and B cells which assist in the clearance of
invading bacteria (fig 3.13 Parham).
5 T cells function by making contact with other immune cells (fig. 5.12 Parham). There are two types of
T cells as defined by the expression of the CD4 or CD8 co-receptor on their cell surface. Which type
of T cells are primarily involved in the induction of antibody production and subsequent clearance of
extracellular pathogens? Which MHC molecule is involved in the activation of these T cells?
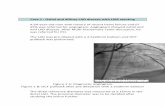
6 Study the picture of a skin biopsy of a 52 year-old male, with an itchy papule on his arm.
The description of the light microscopy is as follows:
Skin biopsy showing a hair follicle with dilated
osteum in which numerous neutrophilic granulocytes
are found. Surrounding the follicle, an inflammatory
infiltrate with predominantly lymphocytes is present.
Additional staining shows that Gram-positive cocci
are present.
Can you relate the description to what you see in the
picture? What would be your diagnosis?
SSA I.A.2 Diversity of T and B cell repertoires
During their development in the bone marrow, random joining of variable (V), diversity (D), joining (J)
and constant (C) gene segments allows the formation of a large number of unique DNA sequences
expressed by a highly diverse set of functionally different T and B cells. This process is schematically
displayed in fig 4.17 and fig. 5.3 from Parham for respectively B and T cells. The set of enzymes needed
to recombine V, D and J segments is called the V(D)J recombinase. Two of the several component
proteins facilitating recombination at the DNA level are uniquely expressed by lymphocytes; they are
specified by the recombination-activating genes (RAG) 1 and 2.
TH E ME I : TH E IMMU N E SY STE M
28
1 The number of functional gene segments available to construct both the variable and constant region
of the immunoglobulin heavy and light chains are displayed in fig 4.18 Parham. Calculate how many
different heavy (H) chains can be formed. Make similar calculations for respectively the and
chain. Now use these figures to calculate the total number of possible immunoglobulin molecules
formed by combining the available heavy and light chains.
2 Provide a rationale why, from an evolutionary standpoint, our genome does not contain a gene for
each unique immunoglobulin molecule.
3 The number of functional V, D and J gene segments available to construct a functional T cell
receptor, comprised of an and chain (fig 5.6 Parham), are displayed in Parham (fig. 5.9)
Combining the available gene segments results in at least 5.8106 different DNA sequences; each
sequence encodes a T cell receptor with a unique antigen-recognition part (=hypervariable region). As
you can see in the table, the estimated total diversity of immunoglobulins and T cell receptors
expressed by naïve lymphocytes is far greater. Explain the discrepancy.
4 Unlike the B cell receptor, the T cell receptor only serves as a cell surface receptor for activation.
Which key proteins transmit activation signals to the nucleus after the T cell receptor binds to its
cognate antigen?
5 Genetic defects which result in either the total or partial absence of one of RAG proteins are
associated with a rare syndrome called severe combined immunodeficiency disease (SCID). Describe
the composition of leukocytes in a peripheral blood sample collected from untreated SCID patients.
Are the numbers of T cells expressing the alternative T cell receptor also affected in these patients?
Note that more details on the SCID syndrome will be discussed in the seminar
ME CH A N ISMS O F D ISE A S E 1
29
1.2 Theme I.B. Pathology of injury and repair
1.2.1 Introduction
In clinical pathology, macroscopic and microscopic observations are important tools to establish a
differential diagnosis. Insight into cellular processes is essential for the understanding of the
morphological changes in organs caused by a disease. Both intracellular and extracellular changes affect
cell functioning; extracellular changes may lead to morphological changes both in cells and tissue. Our
knowledge of these changes has contributed to the identification and the classification of diseases. The
understanding of the underlying mechanisms of such changes helps to determine the cause of a disease,
explains findings at clinical examinations and provides a basis for appropriate therapy.
A disease process is characterized by its cause, development and outcome. The cause (aetiology) of
disease can be known or unknown. The mechanisms of disease leading to cellular alterations are referred
to as the pathogenesis of disease. During its development, the disease may cause tissue alterations such as
hypertrophy, atrophy, hyperplasia, metaplasia: these alterations are usually part of an adaptive process,
and can be reversible or irreversible. The functional consequences of disease lead to more or less
characteristic clinical manifestations.
Inflammation is an important and often non-specific response to tissue injury. Inflammation is not a
disease, it is rather a manifestation of the presence of a disease. The purpose of an inflammatory reaction
is to eliminate the noxious (e.g. infectious) agents and to allow repair of injured tissue. Alternatively,
inflammation may cause disease, for example a swelling caused by an inflammatory response in the throat
may compress the airway and cause suffocation. Inflammation is classified according to its time course
and cell composition. We distinguish acute inflammation (usually the initial and transient reaction) from
chronic inflammation (usually the subsequent and prolonged reaction). Chronic inflammation may (but
need not) follow an acute inflammation. Chronic inflammation may also occur as a primary reaction
(chronic inflammation "ab initio").
Inflammatory responses are usually described by the suffix ‘-itis’ (e.g. inflammation of the appendix is
called appendicitis). Although the causes of inflammatory reactions differ, the inflammatory response
invariably involves a vascular reaction, resulting in increased local blood flow and increased capillary
permeability, and a cellular reaction, resulting in the influx of leukocytes (such as granulocytes,
monocytes and lymphocytes) from blood to tissue. After an inflammatory reaction has taken place, the
inflicted damage has to be restored. This can either be accomplished by regeneration or by healing, the
latter resulting in scar formation and fibrosis.
During this subtheme, the student will become familiar with some of the basic principles of general
pathology. Via the study of morphological changes in the affected tissues and organs, the origin of a
number of fundamental cell injury mechanisms and their effects on cells and tissue will be studied. The
consequences of these changes will be related to the patient’s clinical symptoms and signs.
TH E ME I : TH E IMMU N E SY STE M
30
1.2.2 Theme-related objectives (What you will learn)
By the end of this theme, the student will be able to:
Recognize and explain the different adaptive responses of cells.
Recognize the morphological alterations (and explain the biochemical processes) that occur after
reversible and irreversible cell injury.
Explain the biochemical processes that occur after ischemia.
Explain the different mechanisms that lead to intracellular accumulations.
Describe the macroscopic and microscopic characteristics of acute and chronic inflammation.
Explain the essential differences between acute and chronic inflammatory reactions.
Describe the essential aspects of a granulomatous inflammatory reaction.
Describe the characteristics of wound healing.
Describe and understand immune-pathological processes causing the clinical signs and symptoms
of a limited number of immune mediated diseases and conditions.
Recognize, name and describe changes (morphological and structural alterations) in tissue
specimens with respect to immune mediated conditions.
1.2.3 Study methods and study plan
Lectures
LT4: Introductory lecture, "Pathology of the normal immune response". Explains the essential
features of the immune system from a pathological point of view and describes the cell types that
participate in acute or chronic inflammatory responses.
LT7: In depth lecture, "Pathology of Inflammatory Reactions": gives an overview of chapter 2
from Robbins & Cotran, focussing on stimuli and mediators of acute and chronic inflammation,
reactions of blood vessels and leukocytes in inflammation, granulomatous inflammation, and the
outcome of inflammation.
LT9: In depth lecture, "Tissue Injury and Repair": first part of the overview of chapter 1 from
Robbins & Cotran, focussing on cellular responses to injury, reversible versus irreversible injury,
adaptation in the form of hypertrophy, hyperplasia, atrophy and metaplasia.
LT10: In depth lecture, "Repair Mechanisms": second part of the overview of chapter 1 from
Robbins & Cotran, focussing on the different types of necrosis and the essential differences
between apoptosis and necrosis, intracellular accumulations, pathologic calcification and cellular
aging.
Patient demonstration: "An inflammatory disease": Illustrates the clinical and pathological signs
and symptoms caused by inflammation.
Work group: see 1.2.5
Self-study assignments: see 1.2.6
Study plan
Study the pages indicated in the reading list, preferably before the lectures;
Follow the lectures;
Perform the self-study assignment (SSA);
Prepare for and participate actively in work group 1.
ME CH A N ISMS O F D ISE A S E 1
31
1.2.4 Reading list
Robbins and Cotran:
Chapter From …up to Keywords, tables, figures
1
4-41
Etiology, pathogenesis, morphological changes,
adaptation, reversible versus irreversible cell injury,
hypertrophy, hyperplasia, atrophy, metaplasia, necrosis,
apoptosis, ischemia, hypoxia, intracellular
accumulations, steatosis, hyaline change, glycogen,
calcification
Figures: 1-1, 1-2, 1-3, 1-5, 1-6, 1-7, 1-8, 1-9, 1-10, 1-16,
1-22, 1-29, 1-30, 1-33, 1-34, 1-36.
Tables: 1-1, 1-2,
2
44-75 Acute inflammation, chronic inflammation, reactions of
blood vessels and leukocytes, leukocyte migration,
chemoaxis of leukocytes, mediators of inflammation,
cytokines, chemokines, complement system,
coagulation, serous inflammation, fibrinous
inflammation, purulent inflammation, abscess,
granulomatous inflammation
Figures: 2-1, 2-2, 2-3, 2-4, 2-5, 2-7, 2-10, 2-12, 2-13, 2-
16, 2-17, 2-18, 2-19, 2-20, 2-21, 2-22, 2-23, 2-24, 2-25,
2-26, 2-27
Tables: 2-1, 2-2, 2-3, 2-4, 2-5, 2-6, 2-7, 2-8
The key-words, figures and tables mentioned in the reading list are guidelines for the more detailed
knowledge you should have of these topics!
1.2.5 Work group 1
During this work group knowledge obtained during the lectures, seminar and self-study of themes I.A and
I.B is applied. For this work group each student should bring Parham and Robbins (book or digital)
to look up information during the work group (the students arrange that several copies of each book are
present). Students should also bring along their computers in order to look up histopathology slides on
the internet and prepare assignments.
Before the work group, the student must have studied the literature and completed the self-study
assignments of theme I. It is essential that you prepare for the work group.
During the work group
A formative test with open questions will be given to the students. Answers will be discussed directly
afterwards
students get the opportunity to ask questions that came up while doing the self-study;
the tutor will discuss with the students 2 cases which must be prepared beforehand at home. In
addition, students will work in small groups on 2 new cases, which will be distributed by the tutor
during the work group.
TH E ME I : TH E IMMU N E SY STE M
32
Cases which should be prepared before you come to Work Group 1:
Case 1: A boy with recurrent infections
A 9-months old boy is referred to the LUMC for unexplained recurrent infections in skin, gastro-intestinal
tract and the respiratory tract. An EDTA blood sample is drawn and sent to the laboratory to obtain the
basic blood cell counts. While the levels of all other leukocyte subtypes are normal, the neutrophil counts
were extremely high. Levels of immunoglobulin subtypes are normal. The pediatrician decides to collect a
skin biopsy for evaluation by the pathologist, who detects a high bacterial load, but no polymorphic or
mononuclear cells in situ. Material sent to the Microbiology Department reveals the presence of
Staphylococcus aureus in the infected skin (this bacterium will be discussed further in Theme II).
Questions:
1 As depicted in fig. 9.24 (Parham), infants display different levels of immunoglobulin subtypes
compared to adults. Describe the major subtype(s) of antibodies which will be found in the serum of
this boy and explain the major effector function of this immunoglobulin subtype(s) (see also fig 4.32
Parham).
2 Coating invading bacteria with specific antibodies, a process called opsonisation, facilitates the
uptake and degradation of bacteria by phagocytes such as macrophages and neutrophils (see also fig
3.14 Parham). Apparently, this defence mechanism is not functioning in this boy given the high
bacterial load in his skin and other mucosal tissues. Which typical feature described in the pathology
report could hint towards his diagnosis?
3 Genetic screening eventually revealed that this boy has an inherited defect in a gene encoding one of
the components of a molecule called LFA-1. At the following site
(http://www.whfreeman.com/Catalog/static/whf/kuby/content/anm/kb01an01.htm1) you can watch an
animation describing the key process of leukocyte extravasation from the circulation. Which proteins
are essential for firm binding of leukocytes to blood endothelial cells (fig 2.31 Parham)?
Case 2: A girl with fever and tonsillitis
A 13-year old girl is seen by her general practitioner (GP). She has suffered from fatigue and episodes of
fever for a substantial number of weeks. After physical examination, it becomes clear that her tonsils (fig.
1.18 Parham) are red and swollen. The GP draws an EDTA blood sample as well as a serum sample and
sends the tubes to the laboratory. From the blood cell count it becomes clear that the numbers of
mononuclear cells (fig 1.12 Parham) are substantially increased. The serum contains IgM as well as IgG
antibodies directed against Epstein-Barr virus (EBV). Hence, the diagnosis is ‘glandular fever’ or
infectious mononucleosis.
Questions:
1 Which of the above described clinical symptoms or laboratory findings indicate that B cells are the
primary target for EBV infection?
2 A primary EBV infection is usually cleared after some time. Which type of T cells are responsible for
the reduction in virus titers in the serum? What is the origin and molecular nature of the antigens
which trigger T cells involved in controlling the infection?
ME CH A N ISMS O F D ISE A S E 1
33
3 Provide a rationale for the term mononucleosis: which specific type of lymphocyte do you expect to
be increased in the blood? Which simple marker could be used to identify these cells by routine flow
cytometry analysis (see also fig. 4.14 Parham)?
4 EBV is never completely cleared from the body and persists in a latent infection stage hidden at
immune privileged sites. From here the virus may temporarily reactivate, but this usually does not
cause problems in healthy individuals. Which type of T cells are required for long-term control of
EBV reactivation?
5 Given that the virus remains present in the body, albeit at very low levels, can you predict the course
(het verloop) of the antibody response which is induced upon primary EBV infection? This question
refers to fig. 1.26 Parham.
6 Which type of patients are highly susceptible for EBV reactivation?
7 Study the 2 pictures below of a tonsil biopsy of a 15-year old female with mononucleosis infectiosa.
The description of the light microscopy depicted in the figure on the left is as follows: In some parts
of the tonsil, an intact architecture is found of the follicles, but there are also areas with necrosis and
accumulation of granulocytes.
Can you relate the description to what you see in the picture?
What do you think is the additional technique which was used to further establish the diagnosis,
represented in the figure on the right?
EBV will be further elaborated on in themes II, III and IV
1.2.6 SSA
SSA I.B.1 Microscopy assignment
Study Robbins & Cotran, Chapter 1, and answer the following questions. Figures 1-20 to which the
questions refer to, can be found on Blackboard (under Supplementary materials – Theme I).
1 Give definitions of hypertrophy, hyperplasia, atrophy and metaplasia.
TH E ME I : TH E IMMU N E SY STE M
34
2 Study Figures 1 to 4, showing you the light microscopy of 4 normal organs. Which epithelia do you
recognize?
3 Figures 5 to 8 show changes of either hypertrophy, hyperplasia, atrophy or metaplasia in the 4 organs
of question 2. Combine the figures and indicate which change took place. What could have caused
this change?
4 What are differences between apoptosis and necrosis?
5 Figures 9 to 11 show 3 different types of necrosis. Can you indicate which types?
6 Can you think of an organ which is particularly prone to fatty changes? Give a summary of the
mechanisms leading to lipid accumulation in this organ. Which of the figures 12-14 depicts fatty
changes? What are general causes of this fatty change?
7 Study Figures 15-17 of a bruise. Make a diagram which depicts mechanisms of disease reflecting the
changes in bruises from time 0 (trauma) to the end-point (bruise resolved, skin looks normal again).
8 Figures 18-20 show three stages of a myocardial infarction. Describe the mechanisms of disease
responsible for the light microscopic changes that you see.
35
2. Theme II: Microorganisms as cause of disease
Coordinator: Dr. S.M. Arend
2.1 Introduction
During evolution humans had to deal with a large number of microorganisms that inhabit the same
environment and as a result the defence systems are capable of combating most quite effectively. The
human body has even come to live in symbiosis with large numbers of microorganisms, of which bacteria
make up a large part. While human defence mechanisms are highly adequate at maintaining homeostasis
and preventing illness, a number of microorganisms can cause disease. In almost every patient with an
infectious disease inflammatory processes play a crucial role. Therefore, it is appropriate to introduce
microorganisms to the student at this place in the course.
The focus in this theme will be on the classification and structure of microorganisms, basic aspects of
the way they can cause disease (virulence factors) and on the possible types of host immune response.
In theme III on Infectious diseases the knowledge that will be acquired by studying this theme will be
extended when the interaction between host and invader is explored and the diagnosis and therapy of
infectious diseases are studied. Theme IV will focus on the epidemiology, prevention and control of
infectious diseases.
Infectious diseases can be caused by a wide variety of microorganisms: bacteria, viruses, fungi, protozoa
and worms. Protozoa and worms are together often called ‘parasites’. In this theme the student is
introduced to each of these groups of pathogens, and will learn their classification. It will gradually
become clear that infectious diseases are not always caused by one specific microorganism and that
pathogens from a different group may cause identical clinical presentations. On the other hand, one
particular microorganism may cause different clinical presentations. Although the concepts of physiology,
metabolism and genetics, i.e. DNA, RNA, protein synthesis and glucose metabolism are not completely
new to the student, it is essential to be able to distinguish between these processes in the human cell and in
a microorganism, in which specific and unique processes may take place, such as peptidoglycan synthesis,
spore formation, reproduction by fission, and the exploitation of human cells for its own ends.
War is a widely used metaphor of infectious disease. It is a struggle for power between microorganisms
and man. The weapons of the attacker are its virulence factors, and for their defence, humans have
developed defence mechanisms. The outcome of the battle depends on the balance of power (Figure II.1).
Figure II.1. Interaction between host and invader
bacterium
virus
fungus
protozoon
helminth
human
animal
Invader Host
virulence factors
defense mechanisms
bacterium
virus
fungus
protozoon
helminth
human
animal
Invader Host
virulence factors
defense mechanisms
TH E ME I I : MICRO O RG A N ISMS
36
An infectious disease is the expression of a battle between host and microorganism, but not every
encounter results in a battle. Many bacteria live in symbiosis with man and, in normal circumstances, do
not cause disease. In fact, disease is a violation of the symbiosis between man and microorganism, and
non-pathogenic organisms, i.e. commensal flora, often serve important functions in their host. Some
intestinal bacteria produce vitamin K, and as such contribute to our blood coagulation. Our commensal
flora protects us from, or hinders colonization by pathogenic microorganisms and contributes to our
defence against infectious diseases.
2.2 Theme-related objectives (What you will learn)
The student
classifies microorganisms and indicates the various cell components of and physiological processes
in bacteria, viruses, fungi, protozoa and worms, and their function;
names which body sites are normally sterile;
describes normal flora in/on humans, and explains its role in health and disease;
explains the difference between colonization, carrier state, primary pathogens and opportunistic
microorganisms and recognizes pathogenic microorganisms that can also be found as colonizing
flora;
lists possible sources of different types of pathogens (endogenous, environmental, animal or human
source);
describes the main methods for detection of microorganisms (visualization and culture);
explains the significance of structural components for the behaviour of microorganisms (e.g.
environmental requirements) and interaction with the host;
2.3 Study methods and study plan
Lectures
LT11: Introductory lecture providing an overview of the theme Infectious Diseases.
LT12: Bacteria.
LT13: Viruses.
LT14: Fungi and parasites.
These lectures focus on classification, structure, physiology and basic aspects of virulence and on the
relation between host response to components of microorganisms and symptoms/signs.
E-learning
This theme includes an e-learning module that introduce the student to diagnostics of bacterial infections
(diagnostics of other classes of pathogens are addressed in theme III):
Diagnostics of bacterial infection: introduction to laboratory methods for detection of bacteria
(indications, sample selection, staining and culture methods, determination, interpretation,
antibiotic susceptibility).
(Optional: Safe Microbiological Techniques: short module with focus on disinfection,
sterilization etc. This knowledge has more general application in medical practice.)
It is advised to study the module before the lectures. The module can be found on Blackboard (E-
learning) or at www.medischonderwijs.nl with search term G2MD1.
ME CH A N ISMS O F D ISE A S E 1
37
Work groups
Theme II-associated knowledge about microorganisms is required in the following work groups:
Work group 2: Infectious diseases (see 3.2.5 under theme III)
Work group 3: Epidemiology, prevention and control of infectious diseases (see 4.1.5 under theme IV)
Self-study assignments: see 2.5
Study plan
Study the pages indicated in the reading list
Follow the lectures
Accomplish the e-learning module
Perform the self-study assignment (SSA)
Prepare for and participate actively in the work groups
2.4 Reading list
Robbins:
Pages From …up to Keywords, tables, figures
332-336 Categories of infectious…..
Special techniques for
diagnosing infectious agents
Prions, viruses, bacteria, chlamydiae, rickettsiae,
mycoplasmas, fungi, protozoa, helminths, ectoparasites, table
8-1, fig. 8-2, fig. 8-3.
342-348 How microorganisms….… Viral
infections
Mechanisms of viral injury. Mechanisms of bacterial injury,
injurious effects of host immunity, immune evasion.
Suppurative inflammation, mononuclear and granulomatous
inflammation, cytopathic-cytoproliferative reaction, necrosis,
chronic inflammation. Fig. 8-5, fig. 8-6
The key-words, figures and tables mentioned in the reading list are guidelines for the more detailed
knowledge you should have of these topics!
Sherris 6th
edition:
Chapter Pages From .....up to… Keywords*, tables, figures
1 4-8 Infectious Agents…..The human
microbiota
*
Fig. 1-2, 1-3, Table 1-1, 1-2.
4 61-63 Culture………Culture media Fig. 4-7
6 98-105 Virus structure……….Classification of viruses
* Fig. 6-1, 6-2, 6-7
21 353-363 Bacterial structure………Bacterial growth and metabolism
* Fig. 21-1 up to and including 21-7, 21-9, 21-10,21-11, Table 21-1
21 382-388 Genetic exchange…… Bacterial
classification
*
Fig. 21-33, 21-34, 21-35, 21-36.
TH E ME I I : MICRO O RG A N ISMS
38
42 697-699 The nature of fungi……..Fungal morphology and growth
* Fig. 42-1, 42-2
48 763-771 Whole chapter *
Table 48-2, 48-3
* For keywords: see blue text in margins of Sherris.
2.5 SSA
SSA II.1 Cases
Below, 5 cases (patients A-E) are presented. A different microorganism infects each patient: a bacterium,
virus, fungus, protozoon or parasite. From these examples you will learn general concepts about the
structure, physiology and pathogenicity of microorganisms.
First study the pages indicated in the reading list.
Read the cases and compare the characteristics (structure, physiology) of the infectious agents. Use
self-study Tables II.1.1 (structure) and II.1.2 (physiology).
Name the virulence factors of the infectious agents that caused disease in patients A-E. Indicate for
each infection the pathogenesis (failing of or interaction with component of the immune system)
and type of inflammatory reaction. Use self-study Table II.1.3 (pathogenesis). The tables are
provided in the Attachments.
II.1 Patient A
Patient A is a 28-year-old woman. She consults her general practitioner (GP = huisarts) because she has
had painful micturition since one day and frequently passes small quantities of urine. She had the same
complaints two months earlier, and was then treated for cystitis. This time she feels ill and reports pain in
the lower right lumbar area. Her temperature is 38.9°C. Upon examination she is found to have right-sided
pain in the loin (slagpijn in de flank). The urine nitrite test is negative, but the white cell count in the
sediment (see Figure) is high. She is diagnosed with pyelonephritis, and a urine culture is made. Her GP
prescribes amoxicillin/clavulanic acid, 500/125 mg three times daily for 14 days. The culture yields
Escherichia coli, susceptible to nitrofurantoin, trimethoprim, amoxicillin/clavulanic acid and
cotrimoxazole and resistant to amoxicillin and sulfamethizole.
Figure Patient A: microscopy of urine with leukocytes
II.1 Patient B
Patient B is a 23-year-old man who visits the outpatient clinic because his friend observed that his skin
and eyes had become yellow. Since a week he feels unwell with complaints of nausea and fatigue. His
ME CH A N ISMS O F D ISE A S E 1
39
body temperature is 37.9 °C. He is known at the outpatient clinic with HIV infection for which he takes
antiviral therapy since two years. CD4+ counts are about 500 106/L (normal: 560-1490) and HIV (viral
load) is undetectable in the blood. Two months ago he has visited Greece. During this holiday he had
unprotected sexual contacts. The blood examination reveals increased levels of bilirubin, alkaline
phosphatase, transaminases and gamma-glutamyltransferase indicating hepatitis. IgM antibodies against
hepatitis A virus are negative. Tests for hepatitis B: HBsAg (hepatitis B surface antigen) and antibodies
against HB (hepatitis B) core antigen are both positive. The diagnosis is acute hepatitis B.
Figure Patient B: yellow sclerae in jaundice (best seen in natural light)
II.1 Patient C
Patient C is a 42-year-old man. He is seen by his GP with recurrent splits between his toes. Upon
examination he is found to have scales between the toes of both feet, especially near the left big toe. The
GP diagnoses him with foot eczema, which is a wrong term, because it is a fungal infection (tinea pedis),
which is confirmed in a skin scale preparation (see figure patient C on Blackboard). Tinea pedis is caused
by dermatophytes, such as Trichophyton mentagrophytes. The GP prescribes miconazole cream to cure
the infection.
Figure Patient C: Microscopy of KOH preparation of skin sample showing hyphae
II.1 Patient D
Patient D, a 57-year-old man returns from a business trip to South Africa. He visited various African
countries in 10 days. Four days after his return, he becomes ill and shivery, and his temperature rises to
39.4°C. He has a headache and muscular pains. Before his trip he was vaccinated against yellow fever and
hepatitis A, and to ward off malaria, he took mefloquine (Lariam®) tablets according to prescription,
which includes taking the tablets up to 4 weeks after his return. As he had been told he might get malaria
despite the mefloquine prophylaxis, patient D consults his GP. The GP thinks he might have a viral
infection (his temperature is 37.8°C), but decides to rule out malaria by testing the blood of patient D (see
figure patient D on Blackboard). The blood film shows Plasmodium falciparum, parasitemia 0.2%. No
schizonts are found. The patient is put on artemeter/lumefantrin and recovers within a few days.
More information about malaria can be found in chapter 50 of Sherris and at
http://www.dpd.cdc.gov/DPDx/HTML/Malaria.htm
TH E ME I I : MICRO O RG A N ISMS
40
Figure Patient D: Microscopy of thick blood smear showing Plasmodium falciparum parasites (red blood
cells have been lysed for optimal sensitivity; the large structure is a phagocyte). For calculation of the
parasitemia level, a thin blood smear is needed in which the red blood cells are left intact (you will
observe this yourself during the Practical Parasitology).
II.1 Patient E
Patient E, a 28-year-old woman has been referred to a specialist on account of blood in her urine. She does
not feel ill and has no further complaints. Upon inquiry she appears to have traveled through Africa for 3
months about 7 months ago. She swam in lakes and rivers regularly, and after her return home she had a
short spell of fever, skin rash and swollen lymph glands, for which she consulted her GP, who tested her
negative for malaria. After some time her complaints subsided. Upon further inquiry, she remembers to
have been itching all over shortly after a swim in Africa. The specialist requests a blood test. The blood
test shows a high count of eosinophil granulocytes. The specialist suspects schistosomiasis to explain her
hematuria, and requests serology, which confirms this diagnosis. In her urine live Schistosoma
hematobium eggs are found (see figure). Patient E is treated with praziquantel, and soon recovers.
More information about schistosomiasis is given at chapter 56 of Sherris and
http://www.dpd.cdc.gov/DPDx/HTML/Schistosomiasis.htm
Figure Patient E: Microscopy of urine showing Schistosoma egg. Note the caudal spike, characteristic of
Schistosoma hematobium (lateral spike in Schistosoma mansoni)
ME CH A N ISMS O F D ISE A S E 1
41
SSA II.2
Study how the following characteristics of microorganisms can be explained by structure or function
(information from lectures, Sherris, e-learning modules):
1 the characteristic of bacteria to stain either pink or blue/purple in a Gram stain
2 the susceptibility of viruses for drying
3 the susceptibility of viruses for detergents (soap)
4 the susceptibility of bacteria for lysozyme
5 motility of bacteria
6 acid fast staining of mycobacteria
7 survival of some bacteria and fungi under extreme circumstances
8 survival of some protozoa under extreme circumstances
9 exchange or transmission of DNA (plasmidal or chromosomal) between bacteria
10 persistence of some viruses in humans
11 the capacity of some viruses to become pandemic
TH E ME I I : MICRO O RG A N ISMS
42
Significant events in the history of infectious diseases*
1546 Girolamo Fracastoro ascribes the origin of an infectious disease to germs, and thereby introduces the ‘germ
theory’ to explain infectious diseases.
1677 Antonie van Leeuwenhoek builds microscopes and discovered “animalculi” in water and dental plaque. It is
generally believed that he saw bacteria.
1798 Edward Jenner publishes his results on cow pox (vaccinia) to inoculate against small pox. His method is
considered safer than inoculation with the small pox virus.
1850 Ignaz Semmelweis proves that puerperal fever can be prevented if physicians disinfect their hands before
they examine pregnant women
1864 Louis Pasteur demonstrates that bacteria do not arise spontaneously, giving strong evidence for the germ
theory
1876 Joseph Lister introduces antisepsis in surgery and wound care. By using carbolic acid to disinfect, he
prevented wound infection and speeded up the cure.
1876 Robert Koch publishes his findings on anthrax and validates the germ theory.
1880 Louis Pasteur develops a method to weaken a pathogen in order to use it as a vaccine.
1880 Alphonse Laveran identifies the malaria parasite in erythrocytes. It was not until 1897-99 that Sir Ronald
Ross and Batista Grassi came independently to the conclusion that the mosquito was both the vector and
host of the malaria parasite.
1881 Robert Koch develops the technique to culture bacteria in a solidified medium.
1882 Ilya Ilich Metchnikoff discovers that various cells in the body consume bacteria. He calls this phagocytosis
and postulates cellular immunity.
1882 Robert Koch isolates the tuberculosis pathogen: Mycobacterium tuberculosis.
1884 Edward Gram develops a dye system for identifying bacteria: the Gram-stain, which is still used in bacterial
diagnostics.
1885 Supervised by Louis Pasteur, the first patient is vaccinated against rabies.
1890 Emil von Behring and Shibasaburo Kitasato develop a diphtheria antitoxin serum.
1891 Paul Ehrlich proposes that antibodies are responsible for immunity to infectious diseases.
1892 Dimitri Ivanoski identifies pathogens smaller than bacteria. Filters that retain bacteria do not retain viruses.
1899 Martinus Beijerinck identifies the tobacco mosaic virus. A filtrate free of bacteria retains ability to caus e a
viral disease in plants after repeated dilutions. He calls the organism contagium vivum fluidum.
1900 Based on work of Walter Reed, it is demonstrated that Yellow Fever is caused by a virus. This is the first
report of a viral agent known to cause human disease.
1912 Paul Ehrlich announces the discovery of Salvarsan to cure syphilis.
1915-17Frederick Twort and Felix d’Herelle describe viruses that infect bacteria, and coin the name ‘bacteriophage’.
1929 Alexander Fleming publishes the first paper describing penicillin, the first antibiotic.
1935 Gerhard J. Domagk introduces Prontosil to treat streptococcal infections.
1949 John Franklin Enders, Thomas H. Weller and Frederick Chapman Robbins develop a method to grow the
polio virus, and thereby introduce the possibility to isolate a virus and study it.
1979 The WHO declares smallpox officially eliminated. The last case was seen in Somalia in 1977.
1982 Stanley Prusiner finds a new class of pathogens, prions, which cause disease both in man and animal.
*the specific names and dates will not be asked at the exam
43
3. Theme III: Infectious Diseases
Coordinator: Dr. S.M. Arend
Every student will know a number of infections and most or all will have suffered from an
infection at some time in the past. Most infections are mild and self-limited but some can be
serious or even life-threatening. Novel emerging infections are frequently in the news, there is
always a threat of a new flu pandemic and the increasing resistance of bacteria to antibiotics is a
threat to the future opportunities for treatment of infections. Whether GP or specialist, every
physician will encounter infectious diseases problems and the knowledge acquired in this theme
is therefore highly relevant for your future career.
The Theme is based on two approaches:
First the general principles are addressed in the three subthemes. In summary the subjects and
questions that will be addressed are
Theme III.A: How do microorganisms cause illness, what types of virulence mechanisms
exist and what is the relation between host defence disorders and specific classes of
microorganisms?
Theme III.B: When are diagnostic tests indicated, which diagnostic tests are suitable for
infections with which type of microorganism and how should test results be interpreted?
Theme III.C: What are the principles of antimicrobial therapy? This includes getting
acquainted with different classes of antimicrobial agents and choosing a therapy for a
simple case.
For a more focused study of infectious diseases a number of Essential Microorganisms is
chosen. These include pathogens from different classes that together represent the possible
different aspects with regard to pathogenesis, clinical syndromes or epidemiology.
The list of Essential microorganisms and the corresponding pages of Sherris to study can be
found after the subthemes on page 73. NOTE. Many but possibly not all of these Essential
Microorganisms will be discussed during lectures, seminars, patient demos, e-learning, work
groups or practicals.
ME CH A N ISMS O F D ISE A S E 1
45
3.1 Theme III.A: Host-pathogen interactions
3.1.1 Introduction
Essential to the understanding of infectious diseases is the concept that an infection is the result
of the interaction between an invader and a host (Figure III.A.1).
The invader acts through its virulence mechanisms and the host through his or her defence
mechanisms. Virulence mechanisms and defence mechanisms may both contribute to the
clinical signs and symptoms of the disease. In this theme your knowledge about virulence
mechanisms is extended. The main objective is to combine your microbiological and
immunological knowledge so that from now on you will be able to understand what is going on
in a patient with an infectious disease.
Figure III.A.1. Interaction between host and invader
3.1.2 Theme-related objectives (What you will learn)
The goals as indicated for this theme will be focused on the list of Essential Microorganisms
(see 3.4) that includes pathogens that together represent different aspects with regard to
pathogenesis, clinical syndromes or epidemiology.
For simple cases with an infection with one of these microorganisms the student can apply this
knowledge in order to explain the host-pathogen interaction, explain or choose diagnostic tests,
and recognize relevant characteristics to determine type of antibiotic treatment.
Study goals Theme III.A
The student
indicates the consequences of the interaction between host and pathogen and can apply
this knowledge to simple cases;
indicates the roles of various structural components, products and/or other characteristics
(together representing the virulence mechanisms) of bacteria, viruses, fungi, protozoa and
worms from the list of Essential Microorganisms in the pathogenesis and clinical signs
and symptoms of infectious diseases;
explains the role of the immune response in pathogenesis and signs and symptoms of
infectious diseases (immuno-pathology);
assesses a patient’s host defence and recognizes and names disorders of host defence that
lead to increased susceptibility to infectious diseases;
TH E ME II I : IN FE CTIO U S D ISE A SE S
46
indicates which specific pathogens cause infections in patients with a particular host
defence disorder, and explain the link between pathogen and disorder;
describes the spectrum of inflammatory responses to infection. From several infections
presented in the module (e.g. pneumonia, tuberculosis): the student recognizes, names
and describes changes (morphological and structural alterations) in tissue specimens with
respect to the conditions.
3.1.3 Study methods and study plan
Lectures
LT15: Invader: virulence factors of microorganisms are discussed and two clinically
relevant examples are elaborated.
LT16: Host versus invader: this lecture addresses the balance between the interaction
between host and invader and this determines the outcome as colonization, carrier,
asymptomatic (subclinical) infection, moderate illness, progressive severe illness, chronic
infection, endogenous or exogenous recurrent infection.
LT17: Immune deficiencies and infection risk: addresses how a disorder in one of the
components of the host defence system is associated with an increased risk of infection
with certain classes of microorganisms.
Patient session
A patient with an infection as a result of an immune disorder will be presented.
Illustration of the importance of the balance between host defence and virulence of
microorganisms, pathogenesis of symptoms and signs and diagnostic aspects.
Work group 2
See under 3.2.5 with theme III.B for work group preparation and contents.
Self-study assignments: see 3.1.6
Study plan
Study the pages indicated in the reading list
Follow the lectures and patient session
Perform the self-study assignments (SSA)
Prepare for and participate actively in the work group
3.1.4 Reading list
From Sherris 6th
edition study:
Chapter Start page
From… (empty indicates from start pg)
End page
up to…. (empty indicates up to end pg)
1 8 the human microbiota 12 infectious disease
2 39 adverse effects of.. 42
6 107 figure 6-8 only
6 112 only figure 6-11
ME CH A N ISMS O F D ISE A S E 1
47
6 113 only figure 6-12
6 122 human viruses 124 quantitation of viruses
7 132 140 viral transformation
7 147 148 virus induced immunosuppression
22 393 entry: beating innate host defences 403 genetics of bacterial…
43 705 708 (CORRECTION: l ine 11 from bottom: Th2 must be Th1!)
49 773 777
From ‘Parham’ study:
Chapter From …up to Keywords, tables, figures
11
329-334
333-334
337
338-348
351-356
359-361
immune invasion by genetic variati on (fig 11.1), antigenic drift (fig
11.2) or antigenic shift (fig 11.3)
latent viral infections fig 11.5
immune reaction-driven pathology
inherited immune deficiencies fig 11.9, 1.10, 11.12, 11.14)
acquired immune deficiencies such as AIDS fig 11.22-11.24
opportunistic infections in immune compromised individuals fig 11.30
all diseases that are in the list of Extended Matching Options under
Invader-host interaction: assessment of host defence (e.g. ‘Chronic
granulomatous disease’ etc.)
3.1.5 Virulence factors
During the lecture Invader virulence factors will be classified but only a few will be elaborated
on in detail. The three classes of, and most common virulence factors are:
Virulence factors aiding entry into the host (cells):
Adhesion proteins or -glycoproteins
Endocytosis induced by microorganism
Fimbriae (= pili)
Hyphae
(Lipo)teichoic acid
Virulence factors causing (direct or indirect) damage to the host
Proteins that bind iron
Enzymes inhibiting DNA- and mRNA-synthesis
Enzymes destructing tissue
Glycoproteins inducing syncytium formation
Hyphae
TH E ME II I : IN FE CTIO U S D ISE A SE S
48
Lipopolysaccharide (endotoxin)
(Lipo)teichoic acid
Peptidoglycan
Toxin that activates adenylate cyclase
Toxin acting as superantigen
Toxin inhibiting release of neurotransmitter
Toxin with cytotoxic activity
Ability to lyse infected host cells
Virulence factors that help evade or downregulate host immune responses
Antigenic shift
Antigenic variation
Biofilm
Protein inhibiting opsonisation
Intracellular survival
Capsule
Molecular mimicry
Ability to remain latently in host
During your self-study you should focus on the mechanism by which these factors contribute to
the pathogenesis and on indicating the main virulence factor(s) of each of the Essential
Microorganisms (in Sherris, the essentials are indicated in blue text in the margin).
3.1.6 SSA
SSA III.A.1
Our defence system protects us from being continuously invaded by microbes.
a. Describe the roles of natural barriers, complement, antibodies, phagocytes and cell-
mediated immunity in the defence against bacteria, viruses, fungi, protozoa and worms
respectively. Use self-study table III.A.1 in the Attachments.
b. Explain how the lower airways and urinary bladder, which are in contact with the
outside world, remain sterile.
c. Explain why antibodies play an important role in the protection against encapsulated
bacteria.
SSA III.A.2
For patients A-E (see SSA theme II under 2.5) now answer the questions: which host defence
mechanism failed, and how originated the clinical signs and symptoms (pathophysiological
explanation of signs and symptoms)? Use self-study table III.A.2 in the Attachments.
SSA III.A.3
Answer the questions to the patient cases below.
1. A 30 year-old male is taken to a hospital intensive care department after a car accident. As
fractured ribs interfere with his breathing, he is put on artificial respiration. An intravenous
catheter is brought into the right subclavian vein, and a urinary catheter into his bladder. To
facilitate his breathing, he is put on a sedative (which makes him sleep continuously), and to
prevent haemorrhage of the stomach he is given an antacid. After 3 days his blood pressure
ME CH A N ISMS O F D ISE A S E 1
49
drops and his temperature rises considerably. On suspicion of sepsis he is empirically (thus
without proof of the diagnosis) treated with antibiotics. His blood culture grows Escherichia
coli.
a. Give your opinion of the patient’s host defence
b. Explain how and why Escherichia coli causes high temperature and low blood pressure.
2. A 49 year-old female is admitted into the hospital with pneumonia, sinusitis maxillaris and
ethmoidalis, loss of weight and diarrhoea. She had pneumonia 3 and 4 years ago, and has had
sinusitis continuously thereafter. Her blood is found to contain pneumococci, and in the faeces
Giardia lamblia as well as Campylobacter jejuni are found.
a. Which immune disorder is most likely in this patient?
b. Which tests would you do to confirm this disorder?
c. What is the likely pathogenesis of the diarrhoea syndrome?
d. If the disorder you suspect is confirmed, which are the (non)-possibilities to treat the
patient?
3.1.7 Instructions Extended Matching Questions Theme III.A
During the module a formative test is provided via Blackboard including some Extended-
matching questions for this theme, as will also be the case at the final exam. During your self-
study you should therefore use this list in order to get acquainted with the options. In Dutch the
list is alphabetical (all EM options lists in Dutch for G2DM1 can be found on Blackboard-
Module G2MD1-Course documents-Translations).
Extended-matching question
Theme III.A: Invader-host interaction: assessment of host defence
Options:
a. Presence of corpus alienum (foreign body)
b. Break in skin integrity
c. Break in mucous membrane integrity
d. ‘Chronic granulomatous disease’
e. ‘Common variable immunodeficiency’
f. Complement deficiency
g. Granulocyte function disorder
h. Granulocytopenia
i. Leukocyte adhesion deficiency
j. Spleen dysfunction or asplenia
k. Incomplete emptying of urinary bladder
l. ‘Severe combined immunodeficiency’ (SCID)
m. Lack of gastric acid
n. Impaired coughing
o. Impaired ‘cell-mediated immunity’
p. Impaired intestinal peristalsis
q. Impaired colonisation resistance
r. Impaired ciliary function
s. ‘X-linked agammaglobulinaemia’
t. –
TH E ME II I : IN FE CTIO U S D ISE A SE S
50
u. –
v. –
w. –
x. No host resistance defect has played a role
Task: Indicate which disorder of host defence has likely played a major role in
the pathogenesis of the described case. If no host defence disorder
played a role you must mark compartment x on the computer form. The
exact number of requested options is indicated. When you mark
more than the indicated number of options, you will get negative points.
However, even when you do not have all options correct you will get
points.
Cases:
1. A 73-year-old man is consulting his general practitioner with dysuria (painful micturition)
since one day. Since a year he sometimes feels the urge to urinate but the urine does not come at
that moment. Often he has to go the toilet again shortly after micturition. In that case he again
produces only a small amount of urine. Examination of the urine shows many leukocytes.
Rectal examination reveals an enlarged prostate. Give one option.
2. A 30-year-old woman is treated for acute leukaemia with cytostatic drugs. She has a high
fever and from the blood Pseudomonas aeruginosa (a Gram-negative bacterium) is cultured. She
has a subclavian catheter for the administration of her medication. Since two days she has
bloody diarrhoea. Blood parameters: granulocytes 0,02109/L (normal 4,5 –10,0109/L),
thrombocytes 11109/L (normal 150-450 109/L). Give three options.
Answer 1: <k>
Answer 2: <b,c,h(a also correct)>
TH E ME II I : IN FE CTIO U S D ISE A SE S
52
Robert Koch
Robert Koch (1843-1910) was a district medical officer in
Wollstein, in what is now Poland, when he started his
research into anthrax in 1874. Without adequate quarters or
conditions to support him, he studied the blood of animals
that had recently died of anthrax, and found a high
concentration of bacteria. A rabbit inoculated with the blood
became ill and died. The tissue and blood of the rabbit
counted many bacteria. Koch managed to grow the anthrax
bacilli in vitro on the aqueous humour of a rabbit’s eye, and
found the anthrax bacilli to produce spores (see picture
below). Thus he not only found that infected animals could infect other animals, but also that healthy
animals could be infected through spores in dust and soil.
Koch published the results of his study in 1876 and
thereby validated the germ theory, which said that a
contagium, i.e. a living microorganism, caused an
infectious disease. Up till then, the role of
microorganisms in infectious disease had been denied by
famous pathologists, such as Virchow and Billroth, while
Henle had hypothesised the germ theory as early as 1840
without being able to prove it. After the discovery of
Bacillus antracis, Koch discovered two more pathogens:
Mycobacterium tuberculosis (1882) and Vibrio cholerae
(1884). Koch invented a new method to grow bacilli in a pure culture on solid media. In a publication
dated 1881 he wrote: A simple observation which anyone can repeat has led me to this approach. If a
boiled potato is cut in half and the cut surface is exposed to the air for several hours and then placed in a
moist chamber such as a moistened bell jar in order to prevent it from drying, then, depending on the
temperature of the chamber, one will find in the following day or two, on the surfac e of the potato, a
large number of very small droplets, all of which seem to be different from each other.
Microscopic investigation of the droplets showed that each droplet contained different microorganisms.
On the basis of this observation, Koch mixed the medium fluid
with gelatine, poured the mixture onto a slide, on which it
solidified (see picture on the right). Koch hereby introduced the
solid medium, which is still widely used in a bacteriology
laboratory. Koch’s research into the causative agent of tuberculosis
did not only lead to the discovery of Mycobacterium tuberculosis,
but also to the laying down of the conditions, known as Koch’s
postulates , which must be satisfied before it can be accepted that
specific microorganims cause a particular infectious disease.
53
3.2 Theme III.B: Clinical presentations and
diagnostics
3.2.1 Introduction
In the medical process diagnostics are an essential source of information and a vehicle to arrive at a
definitive diagnosis (Fig. III.B.1). In infectious diseases, laboratory diagnosis aims at the identification
of the pathogen or the host response, and when the pathogen is a bacterium, it’s susceptibility to
antibiotics is tested. Indirect diagnostic methods include serology, which is often used for viral
infections and some parasitic diseases, but also a number of specific bacterial infections.
Diagnostics serve a specific aim. A question is formulated and the tests are chosen in such a way that
an answer to this question can be obtained. Examples of indications for diagnostics in infectious
diseases are: working out a differential diagnosis, confirmation of the initially suspected diagnosis,
identification of a pathogen, antibiotic susceptibility testing, collecting basic information necessary for
choosing antibiotic therapy, for instance on a patient’s renal function. Diagnostic investigations can
also be done to obtain information about the prevalence of pathogens. In that case diagnostics serve
the community rather than an individual.
Figure III.B.1. The medical process
Diagnostics
Collecting information
Symptoms
Medical problem
Differential diagnosis
(Working)diagnosis
(empirical) Therapy
Infection
Differential diagnosis
pathogens
Empirical therapy
(if indicated)
Microbiological diagnostics
Definitive therapy
Evaluation of therapy
TH E ME II I : IN FE CTIO U S D ISE A SE S
54
3.2.2 Theme-related objectives (What you will learn)
Study goals Theme III.B:
The student
explains the diagnostic principles of infectious diseases, and applies these to simple cases;
explains the available microbiological diagnostic tests;
knows the immunological principles in clinical diagnostics (humoral immune response:
mechanism of ELISA for detection of antigen or antibodies, IgG vs IgM, IgG affinity, IgE
response to allergens or parasitic infections), cellular immune response: leukocytosis, left shift,
lymphocytosis, lymphopenia, type IV allergic response in skin test;
describes indications for diagnostic tests;
draws up a diagnostic plan for simple infection problems;
recognizes the clinical syndrome or causative microorganism from a short case description (the
‘Clinical Capsules’ in Sherris);
names the most likely pathogen(s) from an image (microscopy or macroscopy) of a diagnostic
specimen given a simple case;
interprets the results of diagnostic tests in relation to the prior probability of disease (Bayes
theorem), and calculates sensitivity, specificity and positive or negative predictive value.
3.2.3 Study methods and study plan
Lectures
LT18: Pathology of infection: in this lecture the histopathological changes will be shown of
the most important infections discussed in this theme.
LT19: Diagnostics of infectious diseases: general principles of diagnostic tests
for infections giving a framework for the self-study of this topic.
LT20: Interpretation of diagnostic test results: this depends not only on test
characteristics but also on the pre-test (prior) risk of a particular disease (focus on
sensitivity/specificity as opposed to positive and negative predictive value).
LT21: Essential microorganisms: in this lecture microorganisms are related to clinical
presentations and vice versa.
Seminar 1: diagnostics of infectious diseases
During the seminar a number cases will be presented for which you will be asked to answer the
questions essential to diagnostics of infectious diseases:
How do you define the medical problem?
What is your differential diagnosis?
Which reason(s) do you have for microbiological diagnostics?
Which patient materials do you send to the laboratory?
Which tests do you request?
What is your interpretation of the results?
Work group 2: see 3.2.5
E-learning
Two modules that continue the e-learning module in theme II:
Diagnostics of infectious diseases: this lesson guides the student through the self-study.
Diagnosis of pathogens – 25 cases: for self-assessment.
ME CH A N ISMS O F D ISE A S E 1
55
Practicals
During both practicals, standard bacteriological and parasitological diagnostic methods will be
demonstrated in the setting of clinical patient problems. Both practicals are obligatory (see
Rules of the game) and include diagnostic microscopy based on clinical cases.
Practical 1: Bacteriology. NOTE. Practical bacteriology includes a 20 minutes test exam at the
end (for details: see Assessment on pg. 13-16).
Practical 2: Parasitology. (including a 10 minutes test exam at the end)
Preparation for the practicals: at least all 3 e-learning modules regarding diagnostics of Theme II and
III.B must be completed, for Practical 2 it is useful to have studied the life cycles of the protozoa
(especially Plasmodium) and helminths from the list of Essential Microorganisms.
Study plan
Follow the lectures and seminar
Study the pages indicated in the reading list
Accomplish the e-learning modules
Prepare for and participate actively in the practicals Bacteriology and Parasitology
Prepare for and participate actively in the work group
3.2.4 Reading list
What you need to know about diagnostics of the Essential microorganisms is mainly learned from the
lecture, seminar and e-learning modules.
From Sherris 6th
edition study:
Chapter Start page
From… (empty indicates from start pg)
End page
up to…. (empty indicates end pg)
4 55 63 …….culture media
4 72 Antibody detection 76
42 708 Laboratory diagnosis 709 .. antigen and antibody detection
3.2.5 Work group 2
During this work group knowledge obtained during the lectures, seminars and self-study of themes I
and II is applied. For this work group each student should bring Sherris (book or digital) to look
up information during the work group.
It is essential that you prepare for the work group. Before the work group, the student must
have completed the self-study assignments of themes II and III.A;
have completed all four e-learning modules on diagnostics;
be acquainted with the items in the extended matching question option lists of themes III.A and
III.B.
TH E ME II I : IN FE CTIO U S D ISE A SE S
56
Students get the opportunity to ask questions that came up while doing the self-study.
NOTE. During this work group instructions and/or materials for preparing for the component exam 3
will be provided.
During the work group the students will work in small groups to answer the following questions on
cases that will be distributed by the tutor:
1 Which microorganism caused the disease?
2 Which virulence mechanisms played a crucial role?
3 Which host defences failed?
4 What mechanisms caused the clinical signs and symptoms of the disease?
5 Which (additional) diagnostic test(s) are indicated, if any?
6 Is antimicrobial therapy indicated and, if yes, what would be a rational choice?
ME CH A N ISMS O F D ISE A S E 1
57
3.2.6 Instructions Extended Matching Questions Theme III.B
During the module a formative test is provided via Blackboard including a number of Extended-
matching questions on this theme, as will also be part of the final exam. During your self-study you
should therefore use these option lists in order to get acquainted with the options. In Dutch the list is
alphabetical (all EM options lists in Dutch for G2DMD1 can be found on Blackboard-Module
G2MD1-Course documents-Translations).
Extended-matching question
Theme III.B: Diagnostics: interpretation of microscopic preparations
Options:
a. Ancylostoma duodenale
b. Ascaris lumbricoides
c. Aspergillus fumigatus
d. Candida albicans
e. Clostridium perfringens
f. Echinococcus granulosus
g. Entamoeba histolytica
h. Escherichia coli
i. Giardia lamblia
j. Mycobacterium tuberculosis
k. Neisseria gonorrhoeae
l. Neisseria meningitidis
m. Plasmodium falciparum
n. Plasmodium vivax
o. Salmonella species
p. Staphylococcus aureus
q. Staphylococcus epidermidis
r. Streptococcus pneumoniae
s. Streptococcus pyogenes
t. Schistosoma species
u. Strongyloides stercoralis
v. Toxoplasma gondii
w. Treponema pallidum
x. Trichuris trichiura
Task: Examine the microscopic preparation and indicate which organism is the cause
of the described signs and symptoms. The precise number of requested
options is indicated. When you mark more than the indicated number of
options, you will get negative points. However, you will get points even when
you do not have all options correct.
TH E ME II I : IN FE CTIO U S D ISE A SE S
58
Cases:
1. A 54-year-old woman has had an operation of the right mamma one week earlier. Now, the wound
is red and there is purulent discharge. A Gram stain of the discharge is made. Give one option.
Figure with case 1
2. A 30-year-old woman from Morocco has a swelling in the right side of her neck. Inspection reveals
that the swelling is an enlarged lymph node. A diagnostic aspiration is performed. A Ziehl-Neelsen
preparation is made from the material. Give one option.
Figure with case 2 (Sherris, Figure 27-2).
ME CH A N ISMS O F D ISE A S E 1
59
Extended-matching question
Theme III.B: Diagnostics: choosing a diagnostic test
Options
a. Antigen assay, bacterial
b. Antigen assay, protozoa
c. Antigen assay, viral
d. DNA/RNA detection (PCR)
e. Electron microscopy
f. Culture, bacterial
g. Culture, fungal
h. Culture, viral
i. Culture, worms
j. Microscopy, auramine stain
k. Microscopy, thick and thin blood smear
l. Microscopy, dark field
m. Microscopy, cysts, eggs and larvae
n. Microscopy, Gram stain
o. Microscopy, KOH-preparation
p. Microscopy, Ziehl-Neelsen stain
q. Serology
r. Toxin assay
s. -
t. -
u. -
v. -
w. -
x. No test, diagnostics not necessary
Task: Indicate for each case the preferred diagnostic test. This concerns tests that are
routinely used in daily practice. If no diagnostic is necessary mark
compartment x on the computer form. The precise number of requested
options is indicated. When you mark more than the indicated number of
options, you will get negative points. However, you will get points even when
you do not have all options correct.
Case:
3. A 62-year-old man is admitted to the hospital with bile stones and an inflamed gall bladder
(cholecystitis). He has cold chills and a rapidly rising temperature. He is pale and feels sweaty. His
blood pressure is 80/45 mmHg, his pulse rate 146/min. The doctor in charge considers a septic shock.
Give one option.
TH E ME II I : IN FE CTIO U S D ISE A SE S
60
Extended-matching questions
Theme III.B: Differential diagnosis of (possible) pathogens
Options:
a. Aspergillus fumigatus
b. Candida albicans
c. Cytomegalovirus
d. Echinococcus granulosus
e. Entamoeba histolytica
f. Epstein-Barr-virus
g. Escherichia coli
h. Giardia lamblia
i. Herpes simplex virus
j. Human immunodeficiency virus
k. Influenzavirus
l. Mycobacterium tuberculosis
m. Neisseria meningitidis
n. Plasmodium falciparum
o. Plasmodium vivax
p. Schistosoma species
q. Staphylococcus aureus
r. Staphylococcus epidermidis
s. Streptococcus pneumoniae
t. Streptococcus pyogenes
u. Strongyloides stercoralis
v. Toxoplasma gondii
w. Treponema pallidum
x. Varicella-zoster virus
Task: Indicate for each case which microorganism most likely caused the disease.
The precise number of requested options is indicated. When you mark more
than the indicated number of options, you will get negative points. However,
you will get points even when you do not have all options correct.
Cases:
4. A 3-year-old boy, who had previously been in good health, has vesicles over his whole body. Some
vesicles are broken and on other vesicles crusts have formed. The child is not very ill with light fever
(38,1 C). Give one option.
5. A 4-year-old boy is admitted because of fever (39,1 C). He vomits and is weak. Examination of the
cerebrospinal fluid shows a strongly increased number of granulocytes. Give two options.
Answers
1: <p>
2: < j>
3: <f>
4: <x>
5: <m,s>
TH E ME II I : IN FE CTIO U S D ISE A SE S
62
Alexander Fleming
In 1929 Alexander Fleming published an article in the British
Medical Journal, entitled “On the antibacterial action of
cultures of a Penicillium, with special reference to their use in
the isolation of B. influenzae”. The article begins as follows:
While working with staphylococcus variants a number of
culture-plates were set aside on the laboratory bench and
examined from time to time. In the examinations these plates
were necessarily exposed to the air and they became
contaminated with various microorganisms. It was noticed that
around a large colony of a contaminating mould the
staphylococcus colonies became transparent and were obviously undergoing lysis (photo).
Fleming realised that the fungus, which came to be called
Penicillium notatum, produced a substance with
antibacterial properties; an antibiotic. Earlier research had
shown that fungi inhibited bacterial growth. In 1870 the
British physician Sanderson had found that bacteria did not
grow in tubes exposed to the air and filled with Pasteur’s
medium in which Penicillium colonies had grown.
Sanderson’s explanation was that bacteria did not occur in
the air, but that fungi did. Lister, a famous surgeon, did not
believe this, and on the basis of his own experiments he
concluded that Penicillium inhibited bacterial growth. He
used extracts of fungal cultures to treat abscesses. The physicist John Tyndall thought that the fungus used up the
air in the culture and thus created a shortage of air, which prevented bacteria from growing.
At the time Fleming discovered penicillin, he thought of applying
it to culture bacteria, but never thought of it as a possible remedy
for infectious diseases. In 1929 there was hardly any treatment for
infections. The first chemotherapy to treat syphilis, an arsenic
compound called salvarsan, had been introduced by Paul Ehrlich
in 1912. In 1935 Gerhard J. Domagk developed prontosil, which
proved a remedy against streptococcal infections. In 1940
penicillin was refined and became available as a remedy against
infectious diseases. After the discovery that both fungi and
bacteria produced substances with antibacterial properties, the
active search in nature for such agents began, and since that time,
many antibiotics have been
developed, some of which
are listed here.
1929 Penicillin, (unrefined)
1939 Polymyxin
1940 Penicillin G
1944 Streptomycin
1945 Cephalosporin C
1947 Chloramphenicol
1948 Chlortetracyclin
1950 Erythromycin
1955 Vancomycin
1955 Amphotericin B
1955 Lincomycin
1959 Rifamycine
1960 Methicillin
1963 Gentamicin
1981 Ceftazidime
1985 Ciprofloxacin
1988 Meropenem
1989 Clarithromycin
2002 Linezolid
ME CH A N ISMS O F D ISE A S E 1
63
3.3 Theme III.C: Therapy of infectious diseases
3.3.1 Introduction
The first choice for the treatment of an infectious disease that comes to our mind is an antibiotic,
which can indeed be a powerful remedy, but by no means the only (see Table III.C.1).
Table III.C.1 Treatment of infectious diseases
Symptomatic therapy
Pharmacotherapy, not antimicrobial
Surgery
Immunotherapy
Antimicrobial therapy
Not all infections can or should be treated with antimicrobial therapy. The symptoms of many
infectious diseases can be sufficiently treated with an analgesic and antipyretic drug. Other infectious
diseases, such as abscesses, require surgery. In some cases a drug not aimed at the infectious agent is
sufficient, such as a bronchodilator in bronchitis. Immunotherapy is another option.
This theme focuses on antimicrobial therapy. Figure III.C.1 shows the interactions between the
invader, the host and the antimicrobial drug. The target site of an antimicrobial drug is the pathogen
(pharmacodynamic activity); the counteraction of the pathogen is resistance; the antimicrobial drug
may have unintentional effects in the host (side effects and toxicity); the host ingests the antimicrobial
drug, metabolises and eliminates it (pharmacokinetics) or may be allergic.
Figure III.C.1: the interactions between the host, invader and antimicrobial therapy
Pharmacodynamics in antimicrobial therapy requires a special interpretation. Instead of a receptor on
or in a human cell, as is usual in pharmacotherapy, the target site of an antimicrobial drug is the
Antimicrobial
therapy
Host
Pharmacodynamics:
activity
Invader
Resistance
Pharmacodynamics:
Side effects
and toxicity Pharmacokinetics
Defence mechanisms
Virulence mechanisms
TH E ME II I : IN FE CTIO U S D ISE A SE S
64
causative microorganism (bacterium, virus, fungus or parasite). As the structure of pathogens differs
from that of the human cell, it has become feasible to introduce drugs that have a target that is lacking
in the human body (selective toxicity). This does not imply that antimicrobial drugs are non-toxic, or
do not have side effects. The extensive use of antimicrobials has led to the development of resistance
of many microorganisms, which considerably diminished the initial success of antibiotic drugs.
3.3.2 Theme-related objectives (What you will learn)
Study goals Theme III.C.
The student
indicates the mechanisms of action of the major (groups of) antibiotics;
describes the principles of treatment of infectious diseases, and applies these to simple
problems (e.g. recognizes the indications for bactericidal antibiotics);
applies pharmacokinetic principles to simple cases, e.g. analyses serum levels in relation to
dosing schedule;
indicates the major mechanisms and the genetic background of antibiotic resistance, and the
potential ways of transmission of resistance between bacteria;
indicates the role of antibiotic drug use in the development and spread of resistance.
3.3.3 Study methods and study plan
Lectures
LT22: Antimicrobial therapy: Classes of antimicrobial drugs and mechanism of action:
examples of the mechanism of action of commonly used antimicrobial agents.
LT23: Principles of antibiotic pharmacotherapy: antibiotics are to be used prudently to
safeguard their use for future generations and this lecture addresses the principles that should
guide antimicrobial therapy.
Work group 2:
See 3.2.5 under theme III.B for work group preparation and contents. This work group includes cases
for which therapeutic decisions must be made.
E-learning
Information about specific antimicrobial drugs can be found in the study book and/or in the
TRC database.
Optional: treatment of infectious diseases: 4 cases to practice (you do not need to know the
6STEP method but should focus on the concepts explained in 3.3.5).
Study plan
Study the general information in the module book
Study the pages indicated in the reading list
Follow the lectures
(optional: TRC pharmacology database)
(optional: e-learning modules 6-step TRC Infectious Diseases)
Perform the self-study assignment (SSA)
Prepare for and participate actively in the work group
ME CH A N ISMS O F D ISE A S E 1
65
Assessment
Regarding pharmacology/pharmacotherapy, the exam will consist of multiple-choice questions on the
principles and pharmacology of drugs used in infectious diseases. You can expect that one of these
questions will require you to evaluate renal function in order to determine an appropriate therapy.
3.3.4 Reading list
From Sherris 6th
edition study:
Chapter Start page
From… (empty indicates from start pg)
End page
up to…. (empty indicates end pg)
8 151 only: general considerations
8 154 acyclovir 155 ganciclovir
23 407 Antibacterial agents and therapy 418
23 422
Bacterial resistance (only principles: table 23-2 and figure 23-7) 424
44 713 (not table 44-1) 715 including figure 44-1
50 See *NOTE
*NOTE: you may limit your study to the antibiotic drugs as listed in the table at the end of this
chapter!
These subjects can, alternatively or additionally, be studied from other sources such as the TRC (via
Blackboard) or the pharmacology textbook:
TRC database chapter "INFECTIOUS DISEASES" to learn about the various agents used as
antibiotics, antivirals, antifungals, antihelminths, antiprotozoals and antimalarials (see also
under self-study assigment).
You can read more about drugs in infectious diseases in:
Farmacotherapeutisch Kompas (www.fk.cvz.nl). See the background information in the chapter
on “drugs with infectious diseases” and also the links from the TRC to the specific drugs.
Rang & Dale Pharmacology (6th edition) Ch. 45-50: Drugs used in the treatment of infections
[p. 647-717]
Tables antimicrobial therapy
At the end of this chapter you will find tables with facts about antibacterial, antiviral, antifungal and
antiparasitic drugs. NOTE. These tables will be provided at the final examination. So, don't learn
the information presented in the tables by heart, but learn to use the information for choosing
antimicrobial therapy for the cases you find in this chapter and which you will find in your
examination. However, you need to know the mechanisms of action and adverse effects by studying
Sherris and/or the TRC and/or Rang & Dale, regarding the drugs mentioned in the table.
TH E ME II I : IN FE CTIO U S D ISE A SE S
66
3.3.5 Concepts of pharmacology and prescribing antimicrobial therapy
Concepts of pharmacology
This module teaches a number of concepts and principles of pharmacology (Table III.C.2).
Table III.C.2: Pharmacotherapeutical concepts in the treatment of infectious diseases
Pharmacodynamics
minimal inhibition concentration (MIC )
killing curve, concentration-effect curve, synergism, antagonism
measures for activity, such as time above MIC and AUC
allergy and cross-allergy
selective toxicity
antibacterial spectrum
post-antibiotic effect
Pharmacokinetics
dose, distribution volume, clearance, half-life, concentration-time curve, diffusion, diffusion
barriers, blood-brain/cerebrospinal fluid barrier
drug interaction
protein binding
active transport in kidney, cerebrospinal fluid space, and eye
Prescribing antimicrobial therapy
Before you prescribe an antimicrobial drug to a patient, it is important to first answer the following
questions:
1. Does the patient have an infection?
When it comes to the prescription of antimicrobial therapy the essential question is whether the patient
has an infection or not. The answer to this question is not always obvious; fever does not always imply
an infection, and not every inflammatory reaction implies an infection.
2. What is/are the likely pathogens causing this infection?
Symptoms and signs of an infection may be highly characteristic, but usually different microorganisms
can cause similar clinical presentations. When you have answered the question whether there is an
infection positively, the next question is: which is the (possible) pathogen or which are the (possible)
pathogens causing this infection? It does not suffice to say that the patient has for example pneumonia,
but you have to add a differential diagnosis of pathogens. For the example of pneumonia this could be:
Streptococcus pneumoniae, Legionella pneumophila, or Mycoplasma pneumoniae. During this module
you need only to recognize characteristic clinical presentations as are described during the lectures and
in the clinical capsules in Sherris.
3. Are antimicrobial agents indicated?
Not every infection requires antimicrobial therapy. The infection may be mild and self-limited or
treatment may not have a beneficial effect. An example of the latter is acute hepatitis B. Antiviral
ME CH A N ISMS O F D ISE A S E 1
67
drugs against HBV are available but in general have no place in the treatment of acute HBV infection
for lack of effect on the outcome.
4. If antimicrobial therapy is indicated, provide the rationale for the best treatment for this
patient.
If treatment is indicated, list the options and rationalize which is best suited for your patient. Table
III.C.3 can be used as an outline of things to consider for the choice for an antimicrobial drug for your
patient. Also evaluate the patient’s specific data (Table III.C.4) to determine if adjustments in drug
choice and/or dosing need to be made. Pharmacokinetics are important for a number of antibiotics and
therefore interfere with dosing. Several examples will be discussed during lectures, seminars and work
groups.
Table III.C.3. Choosing antimicrobial therapy
Selection criterion Determinants
Effectiveness Bactericidal versus bacteriostatic action*
In vitro activity
Clinical activity
Resistance a priori
Accessibility of target
Host resistance
Safety Toxicity, side effects
The span of the spectrum
Risk of development of resistance during treatment
Contraindications due to allergy, drug interactions, hearing
loss, impairment of kidney or liver function
Suitability Preferred route of administration (oral or i.v.)
Costs Costs of drug, administration and drug monitoring
* It is not always necessary to use bactericidal antibiotics. However, this is strongly preferred in the
following circumstances:
in the presence of bacteria in the blood stream (bacteraemia or sepsis)
infection of a site with limited access of normal immunity (endocarditis, meningitis)
patient with granulocytopenia.
Table III.C.4. Patient specific data
Selection criterion Determinants
Pharmacokinetic characteristics Age
Renal function
Liver function
Interactions Other drugs
Intoxications (alcohol, drugs)
Other Co-morbidity
Presence of implants (heart valve, joint prosthesis etc)
Allergy
Compliance (acceptable frequency of dosing)
TH E ME II I : IN FE CTIO U S D ISE A SE S
68
3.3.6 SSA
SSA III.C.1
The epidemiology of resistance of pathogens to antibiotics is important for the choice of empirical
treatment and dictates the possibilities for definitive therapy. For MRSA answer the following
questions:
1 Explain the name MRSA. To which antibiotic is MRSA resistant?
2 What is the mechanism of the resistance?
3 Which are the 2 types of MRSA? (Tip: http://www.kiza.nl/content/verspreiding-epidemiologie-
44)
4 What are risk groups for MRSA infection in the Netherlands?
5 What percentage of S. aureus in the Netherlands are MRSA? (Tip: Use NETHMAP 2013 at
http://www.rivm.nl/Documenten_en_publicaties/Algemeen_Actueel/Uitgaven/Infectieziekten/NE
THMAP_MARAN_2013 where you will find a report with the information on page 65-66)
6 What is the percentage in e.g. Belgium (foreign students: look up for your country of origin)?
(Tip: http://www.kiza.nl/content/verspreiding-epidemiologie-44)
Note; the indicated internet sites are in Dutch: foreign students should team up with a Dutch student.
69
3.3.7 Tables antimicrobial therapy
Antibacterial drugs
Indication
Bactericidal or
-static
Resistance
Toxicity
Spectrum
Administration
Cost per day
Penicillin G Gram-positive cocci and rods;
Gram-negative cocci
Bactericidal 80% S. aureus Allergic reaction Narrow Intravenous € 3.40
Feniticillin Gram-positive cocci and rods;
Gram-negative cocci
Bactericidal 80% S. aureus Allergic reaction Narrow Oral € 1.15
Amoxicillin Identical to Penicillin G, plus
aerobic Gram-negative rods
Bactericidal E. coli 30-40%
H. influenzae 8-10%
Allergic reaction In between narrow
and broad
Intravenous
Oral
€ 6.35
€ 0.91
Amoxicillin +
clavulanic acid (co-amoxiclav)
Identical to Penicillin G, plus
aerobic Gram-negative rods, plus
anaerobes
Bactericidal ± 5% Allergic reaction broad Intravenous
Oral
€ 8.50
€ 2.50
Flucloxacillin Gram-positive cocci , especially
S. aureus
Bactericidal < 1% S. aureus (in the
Netherlands)
Allergic reaction Narrow Intravenous
Oral
€ 18.15
€ 4.99
Cefuroxim Gram-positive cocci, aerobic
Gram-negative rods
Bactericidal <10%
P.aeruginosa always
resistant
Allergic reaction In between narrow
and broad
Intravenous € 26.32
Gentamicin Aerobic Gram-negative rods Bactericidal < 10% Ototoxicity
Nephrotoxicity
Narrow Intravenous € 19.06
Erythromycin
Clarithromycin
Gram-positive and -negative
cocci;
Mycoplasma pneumoniae,
Legionella pneumophila
Bacteriostatic Gram-positive cocci ±
5%
Nausea Narrow Intravenous (ery-
thromycin only)
Oral
i.v. € 49.80
p.o. € 3.63
Ciprofloxacin Aerobic Gram-negative rods
Legionella pneumophila
Mycoplasma pneumoniae
Bactericidal < 10% Intestinal complaints
Complaints central nervous
system
In between narrow
and broad
Intravenous
Oral
€ 143.39
€ 36.30
Metronidazole Anaerobic bacteria
Bactericidal Seldom resistance Neuropathy
Nausea and other mild side
effects
Narrow Intravenous
Oral
€ 0.50
Doxycyclin Gram-positive and -negative,
aerobic and anaerobic bacteria,
Mycoplasma pneumoniae
Bacteriostatic Gram positive cocci
5%
E. coli 30-40%
Dental discoloration in young
children (contraindicated
<8y)
Broad Intravenous
Oral
€ 3.63
€ 0.41
Nitrofurantoin Gram-negative aerobic rods and
Gram-positive cocci
Batericidal < 3% Intestinal complaints,
headache
Broad Oral € 0.50
Vancomycin Gram-positive cocci Bactericidal < 1% Red man syndrome,
intestinal complaints, loss of
hearing
Narrow Intravenous € 48.00
TH E ME I I I : IN FE CTIO U S D ISE A SE S
70
continued
anti-parasitic drugs
Indication
Resistance
Toxicity
Spectrum
Administration
Cost per day
artesunate Plasmodium falciparum, particularly
severe cases
partial resistance limited to
Cambodja
anaphylactic reactions intravenous € 381 (may vary)
arthemeter/
lumefantrine
Plasmodium falciparum, non-severe
cases
partial resistance limited to
Cambodja
Conduction abnormalities in
patients with prolonged QTc
interval
oral € 26.51 * (see
note)
atovaquone/ proguanil Plasmdium falciparum, non-severe
cases
little resistance Commonly only mild side effects oral € 31.95 *
Quinine Plasmodium falciparum, particularly
severe cases
no resistance Headache, sometimes deafness oral, parenteral € 6.87 *
chloroquine treatment of infection with Plasmodium
vivax
very common in P. falciparum
but not in other Plasmodium species
Commonly only mild side effects oral € 1.21 *
primaquine to avoid relapses of P. vivax-infections rare partial resistance Risk of haemolytic anemia oral € 4.13
pyrimethamine folic acid antagonists; together with
sulfonamids: toxoplasmosis, malaria
frequent resistance when used for
malaria prophylaxis
Higher dosages: teratogenic oral (€ 0.10/tablet)
metronidazole intestinal protozoal infections:
Entamoeba histolytica and Giardia
lamblia
occasionally non-responding
parasite strains
Neuropathy
nausea and other mild side effects
broad Intravenous
oral
€ 0.50
mebendazole broad spectrum anthelmintic, not
resorbed. Used in many nematode
infections
common in veterinary medicine
but not yet in man
Mild or none broad oral € 15.00
albendazole as mebendazole though slightly better
resorbed
as mebendazole In high dosages: leukopenia broad oral € 6.30
ivermectine Strongyloides stercoralis and some
filarial infections
common in veterinary medicine
but not yet in man
Mostly marginal fairly broad oral € 15.70
praziquantel schistosomiasis and other trematode
infections
no resistance proven, so far Mostly mild sometimes malaise fairly broad oral € 32.00
* NOTE. For antimalarials, the price refers to a complete course, because the duration of treatment differs between the drugs.
ME CH A N ISMS O F D ISE A S E 1
71
continued
Antiviral drugs
Indication
Class
Resistance
Toxicity
Administration
Cost per day
Aciclovir Herpes simplex virus
(HSV) 1 / 2, varicella-
zoster virus (VZV)
DNA polymerase inhibitor Rare, only with prolonged
use
Intestinal complaints,
reversible neurological
disorders
Intravenous
€ 65,00 - 130,00
Valaciclovir Herpes simplex virus
(HSV) 1 / 2, varicella-
zoster virus (VZV)
DNA polymerase inhibitor Rare, only with prolonged
use
Intestinal complaints,
reversible neurological
disorders
Oral € 3,00 (HSV)
€ 18,00 (VZV)
Ganciclovir Cytomegalovirus (CMV) DNA polymerase inhibitor Rare, only with prolonged
use
Neutropenia (frequent) Intravenous
€ 80,00
Valganciclovir Cytomegalovirus (CMV) DNA polymerase inhibitor Rare, only with prolonged
use
Neutropenia (frequent) Oral € 50,00
Lamivudine Human immunodeficiency
virus (HIV), hepatitis B
virus (HBV)
Reverse transcriptase/
DNA polymerase inhibitor
Often, in particular with
monotherapy
Minimal side effects Oral € 6,50 (HIV)
€ 3,00 (HBV)
Lopinavir + ritonavir Human immunodeficiency
virus (HIV)
Protease inhibitor Often, in particular with
monotherapy
Dyslipidaemia Oral € 14.50
Nevirapine Human immunodeficiency
virus (HIV)
Non-nucleoside reverse
transcriptase inhibitor
Often, in particular with
monotherapy
Hepatotoxicity, allergic
reactions
Oral € 7,50
Oseltamivir Influenza A virus,
influenza B virus
Neuraminidase inhibitor Not very common, but
increasingly reported
None Oral € 5,50
(not reimbursed)
TH E ME I I I : IN FE CTIO U S D ISE A SE S
72
continued
Antifungal drugs
Indication
Fungicidal or
fungistatic
Resistance
Toxicity
Spectrum
Administration
Cost per day
Amphotericin B Almost all yeasts and
moulds
Fungicidal Extremely rare Rare (no systemic
resorption)
Broad Oral (suspension or
compressed lozenge)
€ 0.35
Amphotericin B Almost all yeasts and
moulds
Fungicidal Extremely rare Nephrotoxicity
(significant)
Broad Intravenous € 13.74
Amphotericin B,
liposomal
Identical to
amphotericin B
Fungicidal Extremely rare Less nephrotoxic Broad Intravenous
€ 400
Fluconazole Candida species Fungistatic C.albicans: rare
Some other Candida
sp:common
Hepatotoxicity (usually
minor)
Headache
Narrow Intravenous
Oral
€ 58.22
€ 23
Caspofungin
Anidulafungin
Candida species
(severe cases or if
pretreated with azole)
Fungicidal rare Gastro-intestinal
complaints,
convulsions
Fairly broad Intravenous € 450
Voriconazole Candida species,
Aspergillus spp.,
some other yeasts and
moulds
Fungistatic (for
Candida)
Fungicidal (for
Aspergillus)
Rare Fever, transient
visual disturbances
Broad Intravenous
Oral
€ 300
€ 60
73
3.4 Essential Microorganisms
This list of microorganisms includes the most important pathogens among the many organisms that are
mentioned in your textbook. You must focus your attention on these microorganisms. See the
reading list for specific information about these microorganisms. Much of what you should know will
be addressed in the lectures, seminar diagnostics, E-learning modules and the practicals.
For your study in Sherris, you should focus on:
the main structural characteristics;
the main virulence factors (and their mechanism of action);
relation with specific host defence disorder;
the characteristic clinical presentations (Clinical capsules!) ;
appropriate diagnostic tests as learned in the subthemes;
basic aspects of therapy.
3.4.1 Tables with ‘Essential microorganisms’
Bacteria Microorganism Examples of infections
Staphylococcus aureus (Micrococcaceae) Boil, wound infection, sepsis
Coagulase-negative staphylococci, a.o. Staphylococcus
epidermidis (Micrococcaceae)
Infections of intravascular catheters and
implanted prostheses
Streptococcus species, a.o.
Streptococcus pyogenes and
Streptococcus pneumoniae (Streptococcaceae)
Tonsillitis, scarlet fever, otitis media
Pneumonia, otitis media, meningitis
Haemophilus influenzae Bronchitis, sinusitis, otitis media
Mycobacterium tuberculosis (Mycobacteriaceae) Tuberculosis
Neisseria meningitidis or meningococcus (Neisseriaceae) Meningitis
Neisseria gonorrhoea or gonococcus
(Neisseriaceae)
Urethritis (gonorrhoea)
Escherichia coli (Enterobacteriaceae) Urinary tract infection
Salmonella species (Enterobacteriaceae) Intestinal infection. Typhoid fever
Vibrio cholerae (Vibronaceae) Intestinal infection. Cholera
Campylobacter jejuni (Campylobacteriaceae) Intestinal infection
Anaerobes (Bacteroides fragilis, Peptostreptococcus,
Clostridium perfringens,C. tetani and C. difficile)
Wound infection (C. perfringens)
Tetanus (C. tetani)
Pseudomembranous colitis (C. difficile)
Borrelia burgdorferi Lyme disease
TH E ME II I : A L L E RG Y
74
Treponema pallidum Lues (syphilis)
Microorganism Examples of infections
Mycoplasma pneumoniae Pneumonia
Chlamydia trachomatis Urethritis
Coxiella burnetii Pneumonia (‘Q fever’), endocarditis
Legionella pneumophila Pneumonia
Viruses Microorganism Examples of infections
Influenzavirus (Orthomyxoviridae) Influenza
Human immunodeficiency virus (Retroviridae) AIDS
Enteroviruses (including poliovirus) Meningo-encefalitis
Herpes-simplex virus (Herpesviridae) Fever blister (koortslip)
Varicella-zoster virus (Herpesviridae) Varicella (waterpokken) and Shingles
(gordelroos)
Cytomegalovirus (Herpesviridae) Infectious mononucleosis-like illness;
Pneumonia, retinitis in patients with
impaired cell-mediated immunity
Epstein-Barr virus (Herpesviridae) Infectious mononucleosis, Post-
transplantation lymfoproliferative
disease
Hepatitis B virus (Hepadnaviridae) Hepatitis, asymptomatic carriership
Respiratory syncytial virus Bronchiolitis
Fungi and yeasts Microorganism Examples of infections
Candida albicans Stomatitis, vaginitis
Aspergillus fumigatus Pneumonia in patients with
granulocytopenia
Protozoa Microorganism Examples of infections
Entamoeba histolytica and Entamoeba dispar Amoebiasis, resp. apathogenic
Toxoplasma gondii Toxoplasmosis
Plasmodium vivax and Plasmodium falciparum Malaria
Giardia lamblia Intestinal infection
TH E ME II I : IN FE CTIO U S D ISE A SE S
ME CH A N ISMS O F D ISE A S E 1
75
Worms (helminths) Microorganism Examples of infections
Schistosoma haematobium and Schistosoma mansoni Schistosomiasis (Bilharzia)
Strongyloides stercoralis Strongyloidiasis
Taenia saginata and Taenia solium Taeniasis (T. saginata; T. solium).
Cysticercosis (T. solium)
Echinococcus granulosus Echinococcosis
Ascaris lumbricoides Ascariasis
Trichuris trichiuria Trichuriasis
Hookworm Anemia
The lifecycles of the protozoa and worms are described in Sherris. More illustrative drawings are
depicted at the website http://www.dpd.cdc.gov/DPDx/HTML/Para_Health.htm (search the names of
the parasites in the alphabetic list)
3.4.2 Reading for Essential microorganisms
From Sherris, for each of the Essential microorganisms in the above tables you should study:
The structural and physiological characteristics (for parasites, focus on life cycle and
morphology);
Pathogenesis and main virulence factors;
Association with host defence disorders;
Epidemiology and transmission;
Common clinical presentation as explained in the ‘Clinical capsule’;
Which diagnostic tests are indicated (focus on e-learning modules);
The principles of antimicrobial therapy and choice drug for a simple case;
Rational prevention and control measures.
TIP. Sherris 6th
edition contains a part on syndromes and aetiology of infectious diseases (pg 911-
930). Paragraphs I, IV, V and VIII will be helpful to learn which micro-organisms cause which type of
infections.
NOTE. A suggestion for the pages to study is provided in a table: see Blackboard under Course
documents; you can choose the table based on your edition of Sherris and based on whether you want
a schedule for all pages to study or only for the pages for the essential microorganisms. The complete
table lists all pages from Sherris for this module, thus includes the pages as are also listed under
Themes II, IIIA, IIIB, IIIC and IV. You should focus on the essentials as indicated in blue text in the
margin, clinical capsules, figures and tables.
ME CH A N ISMS O F D ISE A S E 1
77
4. Theme IV: Epidemiology, prevention and control
of infectious diseases
Coordinator: Dr. S.M. Arend
4.1 General information theme IV
4.1.1 Why study epidemiology, prevention and control of infectious diseases?
Good medical practice includes being aware of the risks of infection for an individual patient, group of
patients, health-care workers or the community, and of the potential preventive measures that can be
taken to forestall infections. Some examples of situations in which epidemiology, prevention and
control are important are:
An outbreak with a resistant microorganism on a surgical ward (e.g. MRSA) or intensive care
unit (e.g. multi-resistant Klebsiella).
A patient is admitted to the hospital with a transmissible infection (e.g. tuberculosis).
A visitor of a patient with impaired immunity has a transmissible infection (e.g. varicella).
A medical student pricks himself with a needle contaminated by blood of a patient.
Un unusually high number of patients is diagnosed with an infection (epidemic) caused by a
microorganism for which there is an environmental source (e.g. Legionella).
In a community in which many individuals are unvaccinated for religious reasons, a child is
diagnosed with an infection for which a vaccine exists (e.g. poliomyelitis).
A new strain of influenza emerges, that has a high case fatality rate but is not included in the
available vaccine.
A person will travel abroad and asks for advice regarding infection risks.
In each of these situations doing nothing is not the right thing. The situation must be analysed and
prevention and or control measures must be taken. Each situation asks for a different approach, as will
become clear during your studies for this theme.
4.1.2 Theme-related objectives (What you will learn)
General goal: For simple cases with an infection caused by one of the Essential Microorganisms and
for situations in which transmission of infection is a threat, the student analyzes the infection chain
and drafts a plan for effective prevention and/or control of the infection.
Theme IV.A. Epidemiology of infectious diseases
The student
- describes the components of the infection chain for microorganisms from the list of Essential
Microorganisms
- explains concepts of infectious diseases epidemiology and applies these to simple infection problems
Theme IV.B. Prevention and control of infectious diseases
The student
- explains the available methods for prevention and control based on the infection chain
(environmental measures, behavioral advise, various isolation methods, active and passive
immunization, disinfection, sterilization, antibiotic prophylaxis) and applies these to simple infection
problems
TH E ME IV: P RE VE N TIO N A N D CO N TRO L
78
- indicates the pathogenesis, clinical presentation and epidemiological characteristics of infections for
which vaccines are included in the present Dutch national vaccination policy
- describes the different types of isolation, knows where to find and uses existing protocols regarding
indications for isolation and choice of type of isolation
- indicates prevention and control measures for simple infection problems (case-based, outbreak or
situation in which a health care worker, including students, has an infection)
- explains why prevention and control measures can be expected to be effective in specific situations
- indicates infections from the list of Essential Microorganisms for which reporting is mandatory by
the Public Health Law as well as those infections for which immediate reporting is required
4.1.3 Study methods and study plan
Lectures
LT24: Epidemiology of infectious diseases. Illustrates epidemiological terminology and
features that are specific for infectious diseases.
LT25: Prevention and control of infectious diseases. A selection of prevention and control
measures are discussed and illustrated, as a basis for self-study.
Seminar 3: Health Advocacy
During the seminar several cases or situations with an infection problem will be presented. The
students are asked to apply the epidemiological concepts as outlined in this chapter to the case. The
students are invited to ask questions about the topic.
Patient demonstration
A patient with a transmissible infection is presented. Questions that may arise are
How did the patient acquire the infection?
Was the infection (in theory) preventable?
What did it mean to have this infection for the patient and his or her contacts?
Which measures were taken?
Did it affect his or her later behaviour?
E-learning
The e-learning modules about hygiene teach the general principles of how to be hygienic while
working in health care to prevent transmission of microorganisms, healthcare-associated infections
and occupational infections. It consists of short 8 modules including one test (see: Blackboard- Course
document – e-learning modules or via www.medischonderwijs.nl). The assignment will take at least
one hour.
Note that these modules are voluntary. However, you will be expected to master this subject when you
will go into medical practice (including VPC) so you should study them at some time!
(These e-learning modules are in Dutch; foreign students should ask their own institution about
information on this subject).
Work group 3: see 4.1.5
Self-study assignments: See 4.2.4 (2h) and 4.3.3 (NOTE: these self-study assignments are extensive
and will take you at least 4h).
ME CH A N ISMS O F D ISE A S E 1
79
Study plan
Study the pages indicated in the reading list
Follow the lectures, seminar and patient demonstration
(optional: perform the E-learning modules)
Perform the self-study assignments (SSA)
Prepare for and participate actively in the work group
4.1.4 Reading list
Concepts considered familiar: vaccine, vaccination, active and passive immunisation, as are discussed
in Parham, The Immune system.
From Sherris 6th
edition study:
Chapter Start page
From… (empty indicates from start pg)
End page
up to…. (empty indicates up to end pg)
3 43 Definitions 44 Microbial kil ling
3 49 Infection control and nosocomial… 54
5 87 93
23 429 Antimicrobial stewardship 430
PLUS: the paragraphs addressing prevention for all microorganisms that are listed under Essential
Microorganisms (see 3.4)
From Robbins study:
Chapter Start page
From… (empty indicates from start pg)
End page
up to…. (empty indicates up to end pg)
5 338 transmission 342 How microorganisms….
From Skolnik study:
Chapter Start page
From… (empty indicates from start pg)
End page
up to…. (empty indicates up to end pg)
28 31
237
NOTE; not all, exact selection will be indicated on Blackboard. Main focus will be on epidemiology. 263
Get acquainted with the options of the Extended Matching option list (see below)
4.1.5 Work group 3
Preparation for work group 3: Contents of lectures, seminar, module book, (optional: e-learning)
and self-study assignments for theme IV.A and IV.B. During work group 2 the cases for work group 3
will be provided. These must be prepared BEFORE THE WORK GROUP (AT HOME)! The group
TH E ME IV: P RE VE N TIO N A N D CO N TRO L
80
divides the cases; two or three students collaborate to prepare one case. It is, however, useful for every
student to prepare all cases. A short paper will be placed on Blackboard, you have to read this to
prepare for the pro-and contra discussion.
Work group 3 starts with a test exam (short open questions, 15-20 minutes). Students can only
participate in the test if present on time.
Cases (70-80 minutes): answer the following questions:
1 Which infection do you think this patient has, and what is/are the most likely causative
microorganism(s)? If you have problems at this stage, ask your tutor or the module coordinator
additional questions to rule in or rule out some diagnoses.
2 Analyse and describe the infection chain
3 Which measures for prevention and control are indicated in this case? Specify the measures (you
may use the list of extended matching options).
4 Is this a reportable infection and if so:
in which group is the infection listed (A, B1, B2, C: what does that implicate)?
does it have to be reported on suspicion, after diagnosis or in case of multiple cases?
Pro- and contra discussion of a topic about a short paper (20 minutes). This paper must be read at
home by all students. During the WG3 it will be decided who has to argue for or against the
proposition and who will lead the discussion..
ME CH A N ISMS O F D ISE A S E 1
81
4.2 Theme IV.A: Epidemiology
4.2.1 Introduction
Concepts such as prevalence, incidence, odds ratio, sensitivity and specificity, and methods of
epidemiological investigations are used in infectious diseases in the same way as for other diseases.
However, microorganisms as a cause of disease are special because they can be acquired from the
environment, animals or other humans and some features are unique for the epidemiology of
infectious diseases:
a patient may be the source of a disease
an individual may be a source of disease without being ill
an environmental source can cause an outbreak
in a population, individual immunity to a disease can influence its epidemiology
sometimes epidemiological investigations have to been done urgently
4.2.2 The infection chain
The scheme below (figure IV-1) can be applied to infections caused by bacteria, viruses and fungi. It
is not commonly used for parasitic infections, for which the life cycle of the parasite is often most
informative.
Figure IV.1. The infection chain
Reservoir and source
The reservoir of an infectious disease is the habitat (ecological niche) of the pathogen, where
multiplication takes place. The thing, person or animal responsible for the transmission of the disease,
is the source of the infection.
Host
The host is a living being that harbours and nourishes a pathogen. Some protozoa and worms have
various development stages in different hosts. The host in which the parasite passes its adult or sexual
existence is called the definitive or final host; the host in which it passes its larval or non-sexual
existence is called the intermediate host.
reservoir
transmission
source hostreservoir
transmission
source host
TH E ME IV: P RE VE N TIO N A N D CO N TRO L
82
Transmission
Transmission of a microorganism can be by direct or indirect contact, via the air (skin flakes, droplets,
droplet nuclei) or via a vector. This is further explained and exemplified in the lecture.
4.2.3 Reproductive rate
The reproductive rate (R) of an infectious disease is the rate at which it spreads in a community. The
basic reproductive rate (R0) is the average number of people in a non-immune community that is
directly infected by a contagious individual. The R0 of a disease depends on the attack rate, the number
of contacts that enable transmission and the duration of the contagiousness of a contagious person. In
case a vector transmits a disease, more factors are involved: the extrinsic incubation time, vector
mortality, the balance between number of vectors and hosts, and the transmission effectiveness from
vector to host and vice versa. The R0 of a great many infectious diseases has been assessed. For
measles it is 15-17; for whooping cough 15-17; mumps 10-12; rubella 7-8; diphtheria 5-6; and for
poliomyelitis 5-6.
The actual reproductive rate in a community as a rule is lower than R0 because of the number of
immune individuals in a community (either due to past infection or following vaccination), which
accounts for R = R0 X, X being the percentage of susceptible, non-immune individuals in a
community. If R > 1, the number of cases will increase and the disease becomes epidemic. If R = 1,
the disease is persistent in a community at a constant level (= endemic). If R < 1, the disease will
disappear. On the basis of such data, the number of individuals that must be immune in order to be
able to eliminate a disease can be assessed, i.e. to get measles to disappear, less than 6 percent of the
individuals in a community must be susceptible to the disease (R0 X < 1 16 X < 1 X <1/16
X <6%), in other words 94 percent must be immune.
4.2.4 SSA
IV.A.1
For a) the patients A-E (see SSA II.1 in 2.5).
b) each of the infectious diseases given in table IV-2
indicate:
1. the reservoir
2. the route of transmission
3. the geographical distribution
NB. Use self-study table IV.A.1 (Attachment).
IV.A.2
Classify the pathogens that cause the infections listed in table IV.2, all from the list of Essential
microorganisms, according to reservoir, route of transmission and geographical distribution, and
discuss the result: what strikes you making these different classifications? For the classification of
parasitic infections, the developmental stages and life cycle are helpful.
Table IV-2
Pulmonary tuberculosis Aspergillosis
Pneumococcal pneumonia Candidiasis
Cholera Amoebiasis
Herpes zoster Toxoplasmosis
Poliomyelitis Echinococcosis
ME CH A N ISMS O F D ISE A S E 1
83
4.2.5 Instructions Extended Matching Questions Theme IV.A.
During the module a formative test is provided via Blackboard including a number of Extended-
matching questions on this theme, as will also be part of the final exam. During your self-study you
should therefore use this list in order to get acquainted with the options.
Theme IV.A: Transmission of infectious diseases
Options:
a. Animal bite
b. Blood transfusion
c. Breast feeding
d. Consumption of drinking water
e. Consumption of improperly washed vegetable
f. Consumption of insufficiently heated meat
g. Contact with soil
h. Contact with animal excreta
i. Contact with droplets of airway secretion
j. Contact with hands of health-care worker
k. Contact with secretions in the birth canal
l. Contact with seawater
m. Contact with freshwater
n. Direct skin-to-skin contact with a patient
o. Human faeco-oral contact
p. Inhalation of droplet nuclei
q. Inhalation of droplets of airway secretion
r. Inhalation of small droplets from the environment
s. Inhalation of spores in the air
t. Mosquito bite
u. Prick with a used injection needle
v. Sexual contact
w. Tick bite
x. Vertical transmission (during pregnancy)
Task: Select for each patient the most likely way of transmission, by which the
patient got the infectious disease. You are requested to select exactly the
number of options as indicated. When you mark more than the indicated
number of options, you will get negative points. You will get points even
when you do not have all options correct.
Example:
Case: A 23-year-old woman has made a 3-week trip through India. During the trip
she has had no complaints. Two days after her return to the Netherlands she
has bloody diarrhoea. Examination of fresh faeces reveals haematophagic
amoebae. Give one option.
Anwer: <o>
Note that d is also theoretically possible in a setting where there
is faecal contamination of drinking water supplies, but you were
requested to indicate the MOST LIKELY way of transmission.
Sometimes more than the requested number of options are
equally correct. In that case each of these will be considered
correct.
ME CH A N ISMS O F D ISE A S E 1
85
4.3 Theme IV.B: Prevention and control
4.3.1 Introduction
During the period 1840-1940 the incidence and mortality of infectious diseases in the Western World
have decreased dramatically, as is illustrated for tuberculosis in figure IV-2. This decrease can be
ascribed to a combination of improved nutrition and living and working conditions. After 1940,
vaccination has made a major contribution to the decrease of many other infections like smallpox
(eradicated in the 70’s), polio, tetanus, diphtheria and others. The contribution made by antimicrobial
therapy is less clear, and it may even have had adverse effects like the development of drug resistance
as happened in MDR and XDR Mycobacterium tuberculosis, methicillin-resistant Staphylococcus
aureus (MRSA), vancomycin-resistant enterococci, penicillin-resistant pneumococci and ESBL-
positive Gram-negative bacteria which are all increasing worldwide. This widespread drug resistance
has serious consequences for current medical practice.
F
Figure IV-2. Decrease in tuberculosis incidence.
From: Robert Koch. A Life in Medicine and Bacteriology. T.D. Brock. ASM Press, Washington, 1999.
Health Advocacy
The competence Health Advocacy has a key place in this module. Health care workers should always
be conscious of the possibility of infections and the risks involved for other patients, healthcare
workers and communities. Pathogenic microorganisms can be acquired from many different sources
and through various transmission routes. This creates a large number of different options for
prevention. A clear understanding of the infection chain (explained in theme IV.A) is essential to
TH E ME IV: P RE VE N TIO N A N D CO N TRO L
86
establish rational prevention and control measures. The measures can be directed at the reservoir, the
source or transmission routes of pathogens, or at protection or isolation of the host (Figure IV-3), e.g.
Improvement of social conditions not only improves living conditions leading to elimination of
sources but also fortifies the hosts’ defence system thereby reducing the risk of disease.
Hygiene aims at elimination of the source and prevention of transmission. This may require vector
control if it concerns vector-mediated transmission. (The difference between disinfection and
sterilisation was explained in the COO lesson Safe Microbiological Techniques).
Immunisation protects the host from developing an infectious disease if exposed.
Antimicrobial prophylaxis aims at preventing infection with certain pathogens for which a patient
is at higher risk.
In the self-study assignments to this theme, different aspects of infection prevention and control will
be elaborated and applied.
reservoir source host
transmission
Elimination
- Cleaning
- Disinfection
- Sterilization
- Pasteurization
- Water purification
- Sewage treatment
- Adequate heating of food
- Screening of blood products
- Social development
Source isolation Protective isolation
Protection
- Vaccination
- Passive immunisation
- Antimicrobial prophylaxis
- Social development
Interruption of transmission
- Hand hygiene
- Vector control
- Asepsis
- Use of gloves, gowns, face masks
- Protection against insect bites
- Safe handling of sharps
- Safe sex
Figure IV-3. Summary of major prevention and control measures
4.3.2 Healthcare-associated infections
In order to understand infection control in healthcare institutions, you must be familiar with the
following terms:
Healthcare-associated infection
A healthcare-associated infection is an infection acquired as consequence of receiving healthcare.
Endogenous and exogenous
ME CH A N ISMS O F D ISE A S E 1
87
Endogenous healthcare-associated infections are caused by the patient’s own microorganisms.
Exogenous infections are caused by microorganisms that have been transmitted from an outside
source. Exogenous infections originating from another patient are often called cross-infections. It is
evident that endogenous and exogenous infections require completely different prevention measures.
The concepts reservoir, source, host, epidemic and endemic are used both in relation to hospital
infections and to epidemiology of infectious diseases in general (see Theme IV.A).
Prevention of healthcare-associated infections is based on active and passive immunisation (e.g.
hepatitis B vaccination and administration of hepatitis B immunoglobulin to prevent hepatitis B
infection), antimicrobial prophylaxis (e.g. to prevent post-operative wound infection), and hygiene .
Hygiene measures can be general and/or specific. General measures are taken regardless of a patient’s
disease, and comprise personal hygiene, hand hygiene, cleaning, disinfection, sterilisation and
asepsis. Asepsis means preventing contact between microorganisms and susceptible sites. A surgeon
operates aseptically, and a urinary catheter is inserted aseptically.
Depending on the disease, specific precautions may be required, such as source isolation and
protective isolation. Source isolation is implemented when a patient is contagious, such as in
tuberculosis or haemorrhagic fevers or is infected with, or colonised by multi-resistant bacteria. The
different forms of isolation are contact isolation, droplet isolation, aerogenic isolation or
combinations (see self-study assignment IV.B.4). Protective isolation measures are taken to protect
an immune-compromised host, i.e. a patient with granulocytopenia, from being exposed to potentially
pathogenic microorganisms.
4.3.3 SSA
IV.B.1. Prevention and control measures
In self-study assignment IV.A.1 you have identified the essential components of the infection chain or
life cycle. That information is the basis for prevention and control measures. Describe prevention and
control measures for the infectious diseases listed in table IV.B.1 (Attachment).
IV.B.2. Reportable infectious diseases
Until November 2008 the Infectious diseases law regulated the reporting of transmissible diseases.
From November 2008 this is laid down in the Public health law. In figure IV-4 the current rules for
reporting infectious diseases are summarized (figure in Dutch is placed on Blackboard). For the list of
Essential microorganisms look up which have to be reported, how this should be done (which
infections have to be reported already on suspicion) and which measures should be taken in the
interest of public health. On http://www.rivm.nl/cib/themas/meldingsplicht/ you find information on
the which, how and why of reportable infectious diseases. (Foreign students should look up the rules
for their country of origin.) (Note: Answers will not be placed on Blackboard)
TH E ME IV: P RE VE N TIO N A N D CO N TRO L
88
Figure IV-4. Reportable Infectious diseases
IV.B.3. Vaccination
a. Dutch national vaccination programme
Go to the ‘Nationaal Kompas Volksgezondheid’ on the internet
(http://www.nationaalkompas.nl/preventie/van-ziekten-en-
aandoeningen/infectieziekten/rijksvaccinatieprogramma ) for information about the national
vaccination programme (‘rijksvaccinatieprogramma’) and
http://www.rivm.nl/Onderwerpen/Onderwerpen/R/Rijksvaccinatieprogramma/Het_programma/Verand
eringen_in_het_programma for the recent changes in the program and English summary.
Use self-study table IV.B.3 (Appendix) listing vaccines that are included in the
‘rijksvaccinatieprogramma’. In the table, the essentials of the diseases against which the vaccination is
aimed (class of microorganism, reservoir, endemicity in the Netherlands, source, route of infection,
clinical manifestations, outcome) are already summarized (NOTE. This is part of the study material).
Answer the following questions:
Who is vaccinated, at what age and frequency?
Is it a live or subunit/component vaccine?
ME CH A N ISMS O F D ISE A S E 1
89
Which vaccination offers the best protection and which one the least protection?
In which areas of the Netherlands are the lowest levels of vaccination found?
Who is responsible for the execution of the programme and who pays for the programme?
b. Vaccination and global health
The World Health Organization (WHO) is divided into regional offices, one of these offices is the
South East Asia Region Office (SEARO). The WHO has recently published a position paper on
immunization (http://www.who.int/immunization/policy/immunization_tables/en/index.html) as has
the SEARO Office:
(http://www.searo.who.int/entity/immunization/documents/immunization_factsheet__2012.pdf)
1. Compare the WHO recommendations with those from the SEARO Region and the
immunizations included in the Dutch ‘Rijks Vaccinatie Programma’ and try to explain the
differences
2. The Indian Minister of Health and Family Welfare wants to further reduce the child mortality
in India by adding another vaccine to the existing Indian immunisation programme. He requests
you to advise him on this issue. See the following websites for additional information and give
and motivate your advice:
http://www.who.int/gho/countries/ind.pdf
http://www.ima-india.org/RECOMMENDED_IMMUNIZATION.html
http://www.who.int/immunization/policy/immunizat ion_tables/en/index.html.
IV.B.4 Isolation measures
a. Types of isolation
The different types of isolation
aerogenic isolation
protective isolation (‘beschermende isolatie’)
contact isolation
droplet isolation
strict isolation
will be explained during the lecture prevention and control, with examples of diseases or pathogens for
which these types of isolation are used. Describe the components of each type of isolation, and give
the rationale of each component.
Optional: more information on isolation can be found via the home-page of LUMC intranet –
Protocollen – Iprova – infectiepreventie. NOTE. This website is in Dutch.
b. Indications for isolation
Look up which type of isolation is indicated for
MRSA
influenza virus
infectious diarrhoea
meningococcal infection
tuberculosis
a patient with granulocytopenia (e.g. after chemotherapy)
TH E ME IV: P RE VE N TIO N A N D CO N TRO L
90
The answers to a. and b. will not be placed on Blackboard.
c. Health care worker with an infection
What should a health-care worker (including a student doing a clinical rotation) do in case of
Common cold;
’The flu’;
Diarrhoea;
Skin infection (impetigo, boil, cellulitis);
MRSA carriership.
IV.B.5 Healthcare-associated infections
Below, three cases are presented. For each, answer the following questions:
Is this a healthcare-associated infection?
If so, is it endogenous or exogenous?
What is the reservoir or the source?
What was the route of transmission?
What has led to the host’s susceptibility?
Is the disease epidemic or endemic?
Which precautions can be taken to prevent further problems?
Patient 1
A 77-year-old woman is admitted into the department of Internal Medicine. She is confused and has
lost weight. She is known to have diabetes mellitus. On day 5 of admission her temperature rises to
38.5°C. Her urinary examination on the day of admission was normal, but now counts numerous
leukocytes, and in a culture of the urine grows Escherichia coli.
Patient 2
A 62-year-old man who underwent brain surgery a year ago is transferred from a rehabilitation centre
and admitted to the department of Neurology with neurological abnormalities. He has a urinary
catheter and decubitus. On day two in the hospital his urine culture is positive for methicillin-resistant
Staphylococcus aureus (MRSA).
Patient 3
A 7 months old baby girl is in the paediatric ward after heart surgery for a congenital heart condition.
On the fifth day after the operation she has fever. A blood and an intravenous catheter tip culture are
found to have grown Klebsiella pneumoniae, which produces extended spectrum beta-lactamase
(ESBL). Five days earlier ESBL producing K. pneumoniae had been found in another child in
Paediatrics. Before that, this microorganism had never been cultured from patients in the paediatric
ward.
IV.B.6 Global burden of infectious diseases
Worldwide there are considerable differences in the causes of disease and mortality depending on the
economic situation. For this assignment, use Skolnik (the term DALYs has been introduced in year 1,
re-read pages 26 and 27 if you need to refresh this knowledge).
ME CH A N ISMS O F D ISE A S E 1
91
1 Study Table 2-3. Compare the list of the 10 most frequent causes of death and of DALYs in low-
and middle income countries, respectively high-income countries. How many are caused by
communicable diseases? Why is it valuable to have a composite indicator like DALY to measure
the burden of disease?
2 What might explain the differences between low- and high-income countries?
3 Study Table 2-8. Which are risk factors for infections in low- vs. high-income countries? Can you
think of risk factors for infections that are not in this list?
4 Find in pages 248 – 257 the burden of disease of the three ‘big killers’ malaria, AIDS and
tuberculosis. How many deaths are yearly caused by these infections? And how many DALYs are
lost? Compare this with number of death and DALYs lost by the “Neglected Tropical Diseases”.
What can you conclude?
5 How could the amount of DALYs lost be reduced? What are the limitations in this regard?
4.3.4 Instructions Extended Matching Questions Theme IV.B.
During the module a formative test is provided via Blackboard that includes a number of Extended-
matching questions on this theme, as will also be part of the final exam. During your self-study you
should therefore use this list in order to get acquainted with the options.
Theme IV.B: Prevention and control of Infectious diseases
Options:
a. Adequate heating of food
b. Antimicrobial prophylaxis
c. Asepsis
d. Use of protective gown
e. Protection against mosquito bites
f. Disinfection of skin
g. Disinfection of instruments, surfaces, devices
h. Control of animal reservoir
i. Hand hygiene
j. Use of gloves, non-sterile
k. Use of gloves, sterile
l. Cold storage of food
m. Wearing a face mask, surgical
n. Wearing a face mask (FFP1, FFP2)
o. Passive immunisation
p. Sewage treatment
q. Cleaning with water and soap
r. Screening of blood products
s. Sterilisation
t. Vaccination
u. Vector control
v. Nursing in an isolation room
w. Water purification
x. No intervention or control measure necessary
Task: Indicate which measure(s) should be taken. Mark compartment x on the computer
form when you are of the opinion that no measure is necessary. You are requested to
TH E ME IV: P RE VE N TIO N A N D CO N TRO L
92
select exactly the number of options as indicated. When you mark more than the
indicated number of options, you will get negative points. You will get points even
when you do not have all options correct.
Example
Case: Due to war activities a large number of refugees are looking for a safe place. The
refugees are housed in refugee camps. The government is afraid for a cholera
outbreak. The first cases have already been noted in the camps. Give 3 options.
Answer:< p, t, w>
Note that transmission of cholera is mainly by contaminated water, but can also be by faeco-oral
contact. Option i(hand hygiene) may help to prevent feco-oral transmission but will be of no
use when the source is contaminated water.
ME CH A N ISMS O F D ISE A S E 1
93
5. Theme V: Allergy
Coordinator: Dr. I.M. Bajema
5.1 Introduction
Four different immunopathological reactions, so called hypersensitivity reactions, are drivers of
unwanted reactions of the immune system. It is important to keep in mind that these prototypical
immune mechanisms are part of the normal immune response against infectious microorganisms or
even to harmless substances like air- or food borne allergens. Particularly in individuals who express
certain susceptibility genes (‘genetically predisposed’ individuals), these immune responses can be
excessive and, thereby, cause unwanted immune-driven pathological responses requiring
immunosuppressive therapy. In this relatively short Theme V we introduce the basic concepts of
allergy, before moving onwards to the more broader Theme VI: Autoimmunity.
5.2 Theme-related objectives (What you will learn)
By the end of theme V, the student will be able to:
explain the basic mechanism of type I, II, III and IV allergic immune responses;
explain the pathogenesis of examples of specific allergies: asthma (type I), hemolytic anemia of
the neonate (type II), hypersensitivity pneumonitis (type III) and delayed type hypersensitivity
reactions as the basis of testing for contact allergy or infection (type IV );
recognize, name and describe histological characteristics of allergic response types in tissue
specimens in relation to type(s) of immune cells involved.
indicate the main symptoms and signs of allergic disorders discussed in this module;
describe and explain the immunopathological processes causing the clinical signs and symptoms;
chose appropriate diagnostic tests for these allergic conditions.
describe the available therapeutic options in allergic disorders (drugs, desensitization by
subcutaneous immunization);
list the different classes of anti-allergic, immunosuppressive agents, indicates the mechanism of
action and recognizes the agents commonly used for allergic disorders.
5.3 Study methods and study plan
Lectures
LT26: Mechanisms of allergy. The clinical features and the immunological effector
mechanisms behind the four different types of hypersensitivity reactions are presented in this
lecture.
LT27: Pathology of allergy. The lecture provides an overview of clinicopathological aspects
of the hypersensitivity reactions, elaborating on techniques that can be used to identify which
reaction takes place.
TH E ME V: A L L E RG Y
94
Patient demonstration
A patient with allergic asthma (a common disease which is classified as a type I
hypersensitivity reaction) is interviewed followed by a case discussion.
Work group 4: In this workgroup, themes IV, V and VI will be discussed together. See 6.6 for a
description of the work group.
Self-study assignments: see 5.5
Study plan
Study the pages indicated in the reading list
Follow the lectures
Perform the self-study assignment (SSA)
Prepare for and participate actively in the work group
5.4 Reading list
From ‘Robbins and Cotran’:
Chapter From …up to Keywords, tables, figures
6 184- 208 up to: Auto-immune
diseases
Innate immunity, adaptive immunity, cells and tissues of
the immune system, types I to IV hypersensitivity
reactions
Tables: 6-2, 6-3, 6-4, 6-5, 6-6
Figures: 6-13 to 6-24
From ‘Parham’:
Chapter Pages, from …up to Keywords, tables, figures
12 365-370
376-377
377-378
379-380
380-383
384-385
allergens in relation to the 4 types of hypersensitivity
reactions (I-IV) fig 12.1-12.3
genetic basis of allergic disease
course of IgE-mediated allergic reactions (type I) fig
12.14, 12.17
anaphylactic shock fig 12.12 and 12.19
classic forms of IgE-mediated allergic diseases fig 12.21,
12.22-12.24
prevention and treatment of IgE-mediated allergic
disease
12 386-389 antibodies against (altered) components of human cells
causing type II hypersensitivity reactions fig 12.26
12 389-392 Immune complexes and complement in type III
hypersensitivity reactions fig 12.31, 12.32, 12.33, 12.34
12 392-393 T helper cells and cytokines in type IV hypersensitivity
reactions fig 12.35 and 12.36.
ME CH A N ISMS O F D ISE A S E 1
95
The key-words, figures and tables mentioned in the reading list are guidelines for the more detailed
knowledge you should have of these topics!
5.5 SSA
SSA V.1 Asthma
Asthma usually starts as allergic asthma resulting from the interplay between genetic predisposition
and the exposure to harmless environmental allergens. Repeated exposure gradually increases hyper-
responsiveness of the airways, allowing other factors such as air pollution or infection to ignite local
inflammatory responses. The end result is a chronic inflammatory disease which requires long-term
therapy. Early diagnosis and treatment are both essential to adequately treat this immune disorder.
Several therapeutic options are available.
1 Which type of T cells are involved in asthma? Describe how the main effector molecules
produced by these cells potentiate the mechanism behind this disease.
2 Two types of medication which are frequently prescribed to allergic patients are anti-histaminic
agents and corticosteroids. Describe how these drugs block the typical clinical symptoms of
asthma but do not provide a cure for these patients.
3 Why should steroid treatment be performed with more care than anti-histaminic drugs?
4 For some types of allergic diseases a process called desensitization can lead to waning of some of
the clinical symptoms (page 385 Parham). This process involves subcutaneous injections with
gradually increasing doses of the allergen involved. Explain the immunological principle of this
treatment. Why should this procedure only be performed in an outpatient setting (op de
polikliniek onder toezicht)?
SSA V.2 Glomerulonephritis
Rapidly progressive glomerulonephritis can have various causes. It is a serious condition, in which the
diagnostic work-up has to be quick in order to prevent dialysis dependency. A renal biopsy is usually
performed as well as a series of serological tests. As a typical case, a 50-year old male who has been
smoking for more than 30 years, presented with a rapidly deteriorating renal function, an active
urinary sediment, and mild proteinuria. He also complains of dyspnoea and chest pain. The
immunofluorescent staining for IgG revealed the following:
Figure: IgG staining in a glomerulus
1 Describe the pattern that you see: where is the IgG located, how is it distributed in the
glomerulus?
TH E ME V: A L L E RG Y
96
2 The pattern you see here in the IgG staning is pathognomonic for a renal disease which is
clinically characterized by rapidly progressive renal failure: which disease?
3 Which type of hypersensitivity reaction is involved in this disease?
4 Which serological test will be positive?
5 Do you have an explanation for his dyspnoea and chest pain?
97
6. Theme VI: Auto-immunity
Coordinator: Dr. I.M. Bajema
6.1 Introduction
The immune system is in constant battle with endogenous and exogenous antigens that attack our
health status. It has a variety of strategies to prevent us from developing disease. In some instances,
minor or extensive tissue damage occurs because the immune response is too strong, or because a flaw
occurs in what would otherwise be an effective method to eliminate the antigens. The various routes
by which these processes develop are known as hypersensitivity reactions, which were discussed
previously. Here we will discuss the histopathological characteristics of and mechanisms behind a
selected number of disorders of the immune system in which the immune response is directed towards
self-antigens, such as ANCA-associated systemic vasculitis, Sjögren’s Syndrome, Systemic Sclerosis
and Systemic Lupus Erythematosus.
While studying these diseases, it might be helpful to try to use the knowledge you have acquired at
this point of the Mechanisms of Disease Module in order to understand more of the pathogenesis of
these diseases. For each disease, ask yourself: which cell, which antibody, which type of
inflammation, which histological alteration is most important here? How can it lead to the clinical
manifestations that are most characteristic of this disease? And also: do we know the aetiology of this
disease? On which subjects is knowledge lacking, and what sort of research could bring us further in
order to diagnose and treat these patients?
6.2 Theme-related objectives (What you will learn)
By the end of this theme, the student will be able to:
understand the physiological basis of self/non-self-recognition by T cells;
explain the pathogenesis of auto-immune diseases caused by autoantibodies (e.g. myasthenia
gravis and SLE);
recognize and/or deduce the aetiology of the diseases and conditions discussed in this theme;
recognize, name and describe changes (morphological and structural alterations) in tissue
specimens with respect to auto-immune diseases discussed in this theme (e.g. SLE; ANCA-
associated vasculitis; diabetes; IgA nephropathy),
explain the pathogenesis of infection-associated auto-immune responses.
indicate the main symptoms and signs of auto-immune disorders discussed in this module (see
give an indication of some of the appropriate diagnostic tests for a selected number of immune
mediated diseases discussed in this theme.
describe a selected number of the available therapeutic options in a selected number of immune
mediated diseases discussed in this theme;
list the different classes of immune-suppressive or immune-modulating agents and indicates the
mechanism of action;
TH E ME VI: A U TO -IMMU N ITY
98
6.3 Study methods and study plan
Lectures
LT28: In depth lecture, "Cells and Auto-immunity": This ‘in depth’ lecture focuses on the
disease mechanism involved in type 1 diabetes and new therapies which aim at curing this
prototypical auto-immune disease.
LT29: Introductory lecture, "Pathology of auto-immunity": makes a link to the previous
theme in order to explain the basic principle of auto-immunity. Discusses the criteria for calling
a disease an auto-immune disease, discusses why some diseases are referred to as immune
mediated diseases.
LT30: In depth lecture, "Vasculitis": histopathological characteristics of vasculitis are shown,
explaining how these characteristics link up to what today is known as ANCA-associated
vasculitis. What are similarities/differences between this group of diseases and vasculitis
encountered in numerous other conditions (sepsis, malignancies, etc)
LT31: In depth lecture, "Systemic Lupus Erythematosus": focusses on SLE as a typical
example of a type III hypersensitivity reaction. SLE is the prototype of auto-immune diseases.
LT32: HLA and auto-immunity. This lecture explains how HLA acts as a dominant genetic
factor affecting susceptibility to auto-immune diseases. Some classical examples of auto-
immune-driven neurological diseases will be discussed.
LT33: Infections and auto-immunity. Two examples of diseases in which an infection is or
might be aetiologically related to the development of an auto-immune response.
LT34: Interactive cases. In this lecture things are approached not by describing the features of
a particular illness but from a clinical point of view. Several cases will be discussed and based
on the presenting symptoms and signs you must make a differential diagnosis and choose and
interpret diagnostic tests. This is how it works in practice.
LT35 and LT36: Pharmacology: immune suppression. Two lectures on the mechanism of
action of various classes of drugs used in the setting of auto-immune diseases and
transplantation.
LT37: Chimerism: Students will be given an academic assignment of which the details will be
discussed during this lecture and in 6.7.
Patient demonstration
Auto-immune disease: Illustrates the clinical and pathological signs and symptoms occurring in
this disease with emphasis on complications and treatment.
Work group 4: see under 6.6
Academic assignment: see 6.7
Self-study assignments: see 6.8
Study plan
Study the pages indicated in the reading list
Follow the lectures, seminar and patient demonstration
Perform the self-study assignment (SSA)
Prepare for and participate actively in the work group
ME CH A N ISMS O F D ISE A S E 1
99
6.4 Immunosuppressive pharmacotherapy
In G2MD1 a number of immunosuppressive drugs or therapies are studied. These relate to themes V,
VI and VII and are provided here together:
In relation to theme VI:
In relation to theme VII:
Abbreviations: GR: glucocorticoid receptor agonist; IL: interleukin; IMP: inosine-5'-monophosphate;
IS: immune suppression; GPA: granulomatosis with polyangiitis
Disease/setting Class ExamplesMechanism
of actionIndications Important side effects
Auto-immune diseases
Glucocorticoidsprednisolone,
dexamethasoneGR-agonists
AI diseases. For example SLE, GPA
Moon face, hypertension, diabetes, osteoporosis,
muscle loss
Antimetabolites azathioprine purine antagonistAI diseases.
Maintenance treatment GPA
nausea, vomiting, skin rash, cancers (bladder cancer),
anemia, infections
mycophenolatemofetil
IMP dehydrogenase
AI diseases. For example
maintenance therapy SLE
gastrointestinal side effects, leukopenia,
anaemia
Antimalarials chloroquine IS SLE
mood changes, gastrointestinal side effects, retinopathy,
cardiac toxicity
Alkylating agents cyclophosphamide Alkylating agentAIl diseases. For
example, GPA, first 3 months
nausea, vomiting, haemorrhagic cystitis,
diarrhoea, darkening of the skin/nails, hair loss) or
thinning of hair, lethargia
Disease/setting Class ExamplesMechanism
of actionIndications Important side effects
Auto-immune diseases
Glucocorticoidsprednisolone,
dexamethasoneGR-agonists
AI diseases. For example SLE, GPA
Moon face, hypertension, diabetes, osteoporosis,
muscle loss
Antimetabolites azathioprine purine antagonistAI diseases.
Maintenance treatment GPA
nausea, vomiting, skin rash, cancers (bladder cancer),
anemia, infections
mycophenolatemofetil
IMP dehydrogenase
AI diseases. For example
maintenance therapy SLE
gastrointestinal side effects, leukopenia,
anaemia
Antimalarials chloroquine IS SLE
mood changes, gastrointestinal side effects, retinopathy,
cardiac toxicity
Alkylating agents cyclophosphamide Alkylating agentAIl diseases. For
example, GPA, first 3 months
nausea, vomiting, haemorrhagic cystitis,
diarrhoea, darkening of the skin/nails, hair loss) or
thinning of hair, lethargia
See previous table
Maintenance IS therapy after renal
transplantation. High dose rejection
treatment
GR-agonistprednisolone,
dexamethasoneGlucocorticoidsTransplantation
nephrotoxicity, hypertension,
hypercholesterolemia, diabetes mellitus (tacro>
cyclo)
Maintenance IS therapy after renal
transplantation.
Calcineurininhibitor, blocks
cytokines
cyclosporin, tacrolimus
Calcineurin inhibitors
gastrointestinal side effects, leukopenia, anemia
Maintenance IS therapy after renal
transplantation. IMP dehydrogenase
mycophenolatemofetil
Antiproliferativeimmunosuppressants
anemia, leukopenia, thrombocytopenia, gastrointestinal side
effects, metabolic effects (hyperlipidemia),
proteinuria
Maintenance IS therapy after renal
transplantation.
Modulate the activity of mTOR,
inhibits IL-2 mediated signal
transduction
EverolimusMammalian target of
rapamycin (mTOR) inhibitor
gastrointestinal side effects, metabolic effects,
headache, tremor, hypersensitivity
Induction treatment renal
transplantationIL-2 antagonismbasiliximabAntibodies
hypotension, rigors, fever, shortness of breath,
bronchospasm, chills, and/or rash
Induction treatment renal
transplantation.Also rejection
treatment.
Binds to CD52 (surface lymphocyte
marker)Alemtuzumab
Disease/Setting Class ExamplesMechanism
of actionIndications Important side effects
See previous table
Maintenance IS therapy after renal
transplantation. High dose rejection
treatment
GR-agonistprednisolone,
dexamethasoneGlucocorticoidsTransplantation
nephrotoxicity, hypertension,
hypercholesterolemia, diabetes mellitus (tacro>
cyclo)
Maintenance IS therapy after renal
transplantation.
Calcineurininhibitor, blocks
cytokines
cyclosporin, tacrolimus
Calcineurin inhibitors
gastrointestinal side effects, leukopenia, anemia
Maintenance IS therapy after renal
transplantation. IMP dehydrogenase
mycophenolatemofetil
Antiproliferativeimmunosuppressants
anemia, leukopenia, thrombocytopenia, gastrointestinal side
effects, metabolic effects (hyperlipidemia),
proteinuria
Maintenance IS therapy after renal
transplantation.
Modulate the activity of mTOR,
inhibits IL-2 mediated signal
transduction
EverolimusMammalian target of
rapamycin (mTOR) inhibitor
gastrointestinal side effects, metabolic effects,
headache, tremor, hypersensitivity
Induction treatment renal
transplantationIL-2 antagonismbasiliximabAntibodies
hypotension, rigors, fever, shortness of breath,
bronchospasm, chills, and/or rash
Induction treatment renal
transplantation.Also rejection
treatment.
Binds to CD52 (surface lymphocyte
marker)Alemtuzumab
Disease/Setting Class ExamplesMechanism
of actionIndications Important side effects
TH E ME VI: A U TO -IMMU N ITY
100
6.5 Reading list
Robbins and Cotran:
Pages From …up to Keywords, tables, figures
208 –
226
Auto-immune Diseases … up
to Rejection of Tissue
Transplants
SLE, Rheumatoid Arthritis, Sjogren’s Syndrome, systemic
sclerosis, polyarteritis nodosa
Tables: 6-8, 6-9, 6-10
Figures: 6-30 – 6-33, 6-35, 6-36, 6-37, 6-38
678-679 From: The Lung
up to: Congenital Anomalies
Figure 15-1
694-696 From: Fibrosing Diseases
up to: Pneumoconioses
Fibrosing diseases, Figure 15-13, Figure 15-14, Figure
15-15
917-923 From: Nephritic Syndrome,
up to: Minimal-Change
Disease (p.923)
Table 20-5, Figure 20-9, Figure 20-10
Parham
Chapter Pages Keywords, tables, figures
13 403-407, 410-411
411-418
420-422
427-428
loss of self tolerance forms the basis of auto-immune
disorders caused by the same effector mechanisms as
hypersensitivity reactions fig 13.1 and 13.2, 13.8
the common types and the immune components
underlying these auto-immune disorders
HLA-associated risk for auto-immune disease fig 13.24
triggering of auto-immune disease by infections fig
13.31
The key-words, figures and tables mentioned in the reading list are guidelines for the more detailed
knowledge you should have of these topics!
6.6 Work group 4
Preparation for work group 4: Contents of lectures, seminar, module book and self-study assignments
for themes I, V and VI. Prepare cases 1 and 2 presented below BEFORE THE WORK GROUP.
Please bring your laptop to the work group. Work group 4 will start with a formative test exam
consisting of open questions. Answers will be discussed immediately afterwards. The two cases which
you prepared at home will be discussed and 2 new cases will be distributed for students to work on
and discuss during the work group.
ME CH A N ISMS O F D ISE A S E 1
101
Case 1: a patient with red spots on her legs
An 82-year-old woman presents to her general physician because she has red spots on her legs. They
look like this:
You, a second-year medical student has seen a
similar picture in one of the lectures of
Mechanisms of Disease 1, and you jump to a
diagnosis, which is:
Do you have any differential diagnoses?
What would you do next: would you consider taking a skin biopsy? Or would you, for instance,
ask for a serological test, and if so: which one.
In the meantime, the patient reports to you she has seen blood in her urine. You make a urine
sediment and see this:
What is this, and what does it mean?
Based on the findings in the urine sediment you ask for a number of laboratory tests and it appears
that her serum creatinine is much too high. Would you consider taking a renal biopsy?
Case 2: A lung biopsy in a patient with rheumatoid arthritis
An open lung biopsy is performed in a 46-year-old woman with rheumatoid arthritis. Because of
recent complaints consisting of dyspnoea and sneezing, an X-ray of the lungs was performed, showing
a small, nodular lesion in the right middle lobe.
Mention the differential diagnostic considerations on the basis of the information given above.
The open lung biopsy shows a lesion depicted below:
What kind of lesion is this?
Adjust your differential diagnostic considerations.
TH E ME VI: A U TO -IMMU N ITY
102
Are there any further tests you would like to do in view of your considerations?
Does your final diagnosis sufficiently explain for the patients’ complaints?
6.7 Academic Assignment
In this academic assignment, we ask you to study an academic paper on chimerism. The aim of this
assignment is to guide you into the critical appraisal of scientific publications, to study the data given
and conclusions drawn from them, and to think of new or alternative approaches that would take the
research yet one step further.
Below, you find a short introduction on the subject ‘chimerism’. The paper you have to study and a
detailed study assignment will be posted on blackboard by the end of week 3 of the module.
Chimerism
Chimerism is a medical term which is used to indicate the presence of 'non-self' cells in an individual,
mostly as a result of pregnancy, but also in the transplantation setting. The most frequently used
method to identify chimeric cells in tissues, is to search for male Y-chromosome positive cells in
female tissues by in situ hybridization. The Immunopathology research group published on chimeric
cells in renal transplants in the Lancet, in 2001. Shortly afterwards, in response to a study by Quaini et
al (NEJM, 2002), critics questioned the pre-transplantation status of pregnancy derived chimerism in
transplanted organs, stating that this could obscure the evidence for transplantation derived chimerism
in solid organ transplantation. More precisely, they argued that the Y-chromosome positivity which
was used in all studies to identify cells in female donor organs given to male recipients, could well be
the Y-chromosome positive cells of a previous pregnancy of the female donor with a son.
In response, we studied baseline levels of chimeric cells in normal solid organs of women in 2005.
Most of the work which was subsequently performed on chimerism and auto-immune disease hinged
on the hypothesis that chimeric cells may initiate a graft-versus-host-like response, thereby
contributing to the development of auto-immune disease. In short, it was argued that chimeric T-cells
of fetal origin are able to initiate an immune response at a certain time-point after pregnancy, a
pathogenic mechanism which finds its roots in experimental research performed in the 1980s,
demonstrating that a lupus-like disease can be induced in mice through chimeric T-cells.
Subsequently, we demonstrated chimeric cells at the tissue level in humans, focusing on the presence
and phenotype of chimeric cells in a variety of organs affected by Systemic Lupus Erythematosus
(SLE). Because of the limitations of current techniques that identify chimeric cells, a new technique to
detect chimeric cells on the basis of mismatches in insertions and deletions (indels) of two individuals
was successfully established at LUMC. With this technique, it became possible to pursue a number of
important research questions hitherto unanswered, that form the core of the current research grant
proposal. In short, we will investigate to whom the pool of chimeric cells belong that are present in
virtually every individual, what their relation is to pregnancy, in what amounts and how long after
pregnancy they persist, and most importantly: whether chimeric cells in humans are immune-
competent and therefore, able to initiate auto-immune diseases such as SLE.
ME CH A N ISMS O F D ISE A S E 1
103
6.8 SSA
SSA VI.1 Case: myasthenia gravis
A 25-year old woman experiencing double vision, ptosis (she could only partially lift her eyelids),
uncoordinated eye movements and weakness in her upper arms is referred to the neurology
department. The neurologists suspect myasthenia gravis , a diagnosis that is confirmed by the
presence of high levels of auto-antibodies against the acetylcholine receptor in her blood. The patient
is prescribed cholinesterase inhibitors in combination with corticosteroids. As a result of this
treatment, her neurological symptoms subside and the levels of anti-acetylcholine antibodies in her
serum decrease (although still being detectable). Two years later, this woman delivers a child that
exhibits symptoms of neonatal myasthenia gravis . Because of the medical history of the mother, this
was expected. The baby is immediately transferred to the intensive care unit, where it receives
artificial ventilation (is attached to a breathing machine) and feeding via a nasogastric tube. Antibodies
against acetylcholine receptor are initially detected in the baby’s blood, but these fall to undetectable
levels within several days. Four days after birth, the baby can be released from intensive care, and
from that moment on its development is fully normal.
Questions:
1 In contrast to most other auto-immune diseases involving auto-antibodies, the primary mechanism
of action of those directed against the acetylcholine receptor is not the infliction of tissue damage.
Specify the mechanism of action of the auto-antibodies in myasthenia gravis.
2 Specify the mechanism of action of the two drugs prescribed to this patient. Which of the two
drugs causes a decline of the levels of auto-antibodies?
3 The auto-antibodies in myasthenia gravis are primarily of IgG type. On the basis of this
information, explain why the newborn child of the woman exhibits symptoms of myasthenia
gravis. Why were the baby’s symptoms only transient?
4 Table 13.24 in Parham shows that there is an association between HLA-DR3 and risk for
developing myasthenia gravis. Explain this association on the basis of the immune effector
mechanism involved in this disease.
SSA VI.2 Case: SLE
Systemic lupus erythematosus (SLE) is a systemic auto-immune disease that can inflict immune
pathology to a diversity of tissues throughout the body. It is characterized by the presence of auto-
antibodies against DNA that cause the formation of immune complexes. Every day, many millions of
nuclei are extruded from red blood cell precursor cells in the bone marrow when these cells differentiate
into mature red blood cells. This event, amongst others, provides a rich source of antigen for the anti-DNA
auto-antibodies. Suppression of this disease involves lifelong treatment with a combination anti-
inflammatory and immunosuppressive drugs, including corticosteroids. We refer to www.nvle.org for
additional information on SLE from the patient’s perspective.
Questions:
1 SLE is caused by the formation of small immune complexes that are not efficiently cleared from
circulation. Explain why the immune complexes are small and why this hampers clearance (see
figure 12.31 in Parham).
TH E ME VI: A U TO -IMMU N ITY
104
2 Even though SLE is associated with auto-immune pathology in multiple tissues, the kidneys are in
many patients most severely affected, causing renal function deterioration. What is the
explanation for this?
3 One of the diagnostic markers of SLE is a low serum content of complement proteins, in
particular of C3. Explain the decrease of C3 concentration in the serum.
4 What are the risks of continuous treatment with anti-inflammatory drugs?
5 Symptoms of various immune disorders, including SLE, can worsen if patients contract an
infectious disease, such as the flu or even a bad cold. Explain this. (this question refers in part to
the clinical conference on SLE).
6 Describe and explain the typical pattern found by immunofluorescent markers for IgG, IgA, IgM,
C3 and C1q in lupus nephritis.
7 Comment on the clinical relevance of the classification for lupus nephritis.
8 How would you describe so-called proliferative lesions in lupus nephritis?
SSA VI.3 Case: Rheumatoid arthritis
Rheumatoid arthritis (RA) is a common auto-immune disease that affects 1-2 % of the adult population
in the developed world. Patients with RA, like those with SLE, are generally treated with a combination of
anti-inflammatory and immunosuppressive drugs. Recently, treatment with monoclonal antibodies against
the cytokine TNFα (tumor necrosis factor alpha) is also used to alleviate the symptoms.
Questions:
1 Why is it essential that RA is diagnosed early and that treatment with anti-inflammatory drugs is
initiated rapidly after diagnosis?
2 Explain why anti-TNFα antibodies can be highly effective in alleviating RA symptoms.
3 The efficacy of treatment with a preparation of anti-TNFα monoclonal antibody often decreases
after repeated administration. Explain this and provide a possible solution to circumvent this
problem. (this question refers in part to the clinical conference on rheumatoid arthritis).
4 Give a description of the typical biopsy findings in a synovial biopsy taken from a patient with
RA.
5 What sort of inflammation is found in a rheumatoid nodule? Where can they occur? Can you
think of an important differential diagnostic consideration on the basis of the histological
findings?
SSA VI.4 Case: Diabetes
A 12-year old girl, who was previously healthy, starts to perform poorly at school since several weeks. She
loses weight and displays an exceptional thirst that causes her to drink litres of liquid a day. When taken to
the doctor, increased levels of glucose are detected in her blood, leading to the diagnosis of insulin-
dependent diabetes mellitus (IDDM) or type 1 diabetes. Further analysis of her blood reveals the
presence of auto-antibodies against pancreatic β-cell antigens, confirming the diagnosis. She is prescribed
daily injections with insulin and, as a result of that, has no further complications of diabetes.
Questions:
1 Specify the type of immune reaction involved and explain how the auto-immune reaction in type I
diabetes results in high blood glucose levels.
2 If the diabetes becomes manifest in the kidneys, what kind of lesions will be found in the
glomeruli?
ME CH A N ISMS O F D ISE A S E 1
105
3 What kind of treatment can be given to patients with end stage renal failure due to diabetes?
4 Not all patients with type I diabetes display anti-β-cell auto-antibodies. What would be the
explanation for the fact that type I diabetes can develop in the absence of such antibodies?
5 In the past, the insulin used for treatment of diabetes patients was isolated from pancreases of
cattle. More recently, all patients receive recombinant human insulin. Why is this latter treatment
less likely to result in complications? (this question refers in part to the caput lecture on auto-
immune diabetes)
SSA VI.5 Lambert-Eaton myasthenic syndrome
Lambert-Eaton myasthenic syndrome (LEMS) is an auto-immune disorder that is clinically characterized
by muscle weakness and by autonomic dysfunction (e.g. dry mouth, constipation and, in men, impotence).
Similar to myasthenia gravis, this disease is caused by an auto-immune response directed against tissue-
specific antigens (calcium channels) that are expressed at the neuromuscular junction. As a result of this
auto-immune response, the transmission of nerve impulses to muscles is impaired. Approximately 50% of
LEMS patients have an underlying tumor: small cell lung cancer (SCLC). This is a tumor of neuro-
endocrine origin that expresses many proteins of neuronal origin, including the antigens expressed at the
neuromuscular junction against which the auto-immune response in LEMS patients is directed.
Questions:
1 The symptoms of LEMS are caused by the action of auto-antibodies of the IgG class, directed
against antigens expressed at the neuromuscular junction. Explain why the presence of these IgG
antibodies is indicative of an underlying auto-reactive CD4+ T-helper response.
2 Many auto-reactive CD4+ and CD8+ T cells are deleted from the T-cell repertoire in the thymus
(negative selection). Explain why this is apparently not the case for the T-cells that recognize the
target antigens expressed at the neuromuscular junction.
3 Explain how the presence of the tumor can trigger this auto-immune disease (3 points).
4 The median survival time of SCLC patients with LEMS is significantly longer than that of SCLC
patients without LEMS. Provide an explanation for this difference. (This question refers in part to
the caput lecture on neurological auto-immune disorders.)
SSA VI.6
This SSA will help you to get an overview of similarities and differences between a number of auto-
immune diseases. This is a good exercise for the question format ‘Comprehensive Integrated Puzzle’ as
will be used in the final exam.
For each of the following diseases answer the questions listed below:
SLE
Sjögren’s syndrome
GPA (granulomatosis with polyangiitis)
Churg Strauss syndrome
Rheumatoid arthritis
Systemic Sclerosis
Henoch Schönlein Purpura
TH E ME VI: A U TO -IMMU N ITY
106
Questions (use self-study table VI.6 in Attachment):
1 Which organs are mainly involved in this disease?
2 A biopsy from which organ could be helpful in establishing the diagnosis, and how?
3 Which serological test is most likely to be positive in this disease?
4 Is it known which type of hypersensitivity reaction plays a role in the development of this
disease?
5 Can you say anything about the patient’s prognosis or the general course of this diseas
ME CH A N ISMS O F D ISE A S E 1
107
7. Theme VII: Transplantation
Coordinator: Dr. I.M. Bajema
7.1 Introduction
When a vital organ or tissue is irreparably damaged or non-functional, organ or tissue transplantation can
be a life-saving treatment. A transplant can originate from a deceased donor, a living donor (both
allogeneic setting) or from the recipient him or herself (autologous setting). Transplant procedures are
generally associated with transplantation-related complications which, in some cases, can even be lethal.
The topics which will be addressed in this last Theme are focused on common immunologic, pathologic
and infectious problems seen in the setting of allogeneic transplantation; these differ between organ and
bone-marrow or stem cell transplant recipients. Infections are a major risk in both groups of transplant
recipients, because various components of the immune system can be impaired temporarily or
permanently. Prevention, timely detection and adequate treatment are of utmost importance in this patient
group.
This theme will be illustrated by aspects involved in kidney transplantation and in combined
kidney/pancreas transplantation in patients with diabetes mellitus (DM). We will focus on rejection,
chronic allograft nephropathy, viral infections in the graft, and disease recurrence. In addition, some
histopathologic aspects of Graft-versus-Host Disease will be discussed as is seen after allogeneic
hematopoietic stem cell transplantation. World-wide, the number of patients with DM, especially DM
type 2, is increasing. In DM, vascular lesions may occur at any site in the body, but they can be
particularly harmful in the kidney, causing so-called diabetic nephropathy. Diabetic nephropathy is in the
top 3 of causes leading to end stage renal failure, and thus it is one of the most important indications for
renal transplantation. In a combined kidney/pancreas transplantation, the aim is not only to restore the
patient’s renal function, but also to make the patient insulin independent. As an alternative to ‘whole
pancreas’ transplantation, methods are being developed to transplant only the islets of Langerhans .
Infections are a major risk in transplant recipients, because various components of the immune system
can be impaired temporarily or permanently. Prevention, timely detection and adequate treatment are of
utmost importance in this patient group.
7.2 Theme-related objectives (What you will learn)
By the end of this theme, the student will be able to:
explain the difference between transplantation of a solid organ or tissue and functional repair of
the hematopoietic system by hematopoietic stem cell transplantation (HSCT);
describe and explain the pathogenesis of complications associated with hematopoetic stem cell
(HSCT) and solid organ transplantation (SOTx);
explain immunopathological processes causing the clinical signs and symptoms of rejection;
explain the principle of immune-based strategies to prevent graft rejection and graft versus host
disease (GVHD) in HSCT.
recognize histological features, describe the pathogenesis of and indicate the appropriate
diagnostic test(s) for several Tx-associated conditions (acute and chronic graft rejection; drug
toxicity; BK-virus nephropathy, acute and chronic GVHD);
TH E ME VI: TRA N SP L A N TA TIO N
TH E ME VII : TRA N SP L A N TA TIO N
108
indicate the mechanism of action of different classes of anti-rejection drugs;
classify the infection risks associated with transplantation;
list the most important infections for which pre-transplantation screening is possible and relevant;
classify (opportunistic) infections associated with transplantation and relates infections with the
specific time frame after transplantation;
recognize the clinical presentations of the most frequent opportunistic infections from the list of
Essential Microorganisms (CMV, VZV, EBV, HSV, aspergillosis, toxoplasmosis) and of
Pneumocystis jirovecii;
indicate risk indicators for infection (delayed immune recovery, prolonged immunosuppressive
medication, CMV status of transplant donor and recipient, GVHD, medical devices, de novo
exposure);
indicate general and specific measures to prevent infection after transplantation.
7.3 Study methods and study plan
Lectures
LT38: Introduction to transplantation. This lecture presents the historical ‘bumpy road’ which
led to the discovery of HLA and the need for registries which nowadays help transplant
physicians to find a suitable transplant donor for their patients.
LT39: Transplantation immunology. This lecture zooms in on the role of allo-immune T and B
cells in acute or chronic allograft rejection and Graft-versus-Host Disease.
LT40: In depth lecture, Histopathology of Transplantation and Rejection: gives an overview
of the characteristic findings in solid organ transplantation, in particular in relation to renal
transplantation, in particular in relation to rejection, medication toxicity, and disease recurrence.
LT41: In depth lecture, Islet Cell Transplantation: shows techniques used for islet
transplantation and discusses advantages and disadvantages of islet cell transplantation in contrast
to whole pancreas transplantation.
LT42: Transplantation and infection risk. Relates the time-line of transplantation (solid organ
or HSCT) to defects in the various components of the host defence system and associated
infection risks and defines opportunities for primary, secondary or tertiary prevention.
Patient demonstration
"A patient with a solid organ transplantation": will illustrate the clinical history of a patient up
to the moment of transplantation, and a variety of events following renal transplantation
(rejection, infection, disease recurrence).
Study plan
Study the pages indicated in the reading list
Follow the lectures and patient demonstration
Make the self-study assignment
ME CH A N ISMS O F D ISE A S E 1
109
7.4 Reading list
Robbins and Cotran:
Pages From …up to Keywords, tables, figures
226-230 Rejection of Tissue
Transplants…. up to
Immunodeficiency
Syndromes
Acute rejection, chronic rejection, T-cell mediated
rejection, antibody-mediated rejection, graft survival
Figures: 6-39, 6-40
Parham:
Chapter From …up to Keywords, tables, figures
15 456-467 transplantation antigens and immune-driven
complications caused by effector cells responding to
mismatched antigens fig 15.1, 15.3, 15.4, 15.7, 15.11
and 15.12
472-473 prevention of T cell -mediated complications in
transplantation
The key-words, figures and tables mentioned in the reading list are guidelines for the more detailed
knowledge you should have of these topics!
7.5 SSA
SSA VII.1: Case with a renal transplant
A 46-year-old man has been waiting 5 years for a renal transplant. Ten years ago he developed a
disease by which his renal function was destroyed.
Study the glomerulus in the figure and name the lesions which
are shown here.
The immunofluorescence pattern of his native biopsy showed a pauci-immune pattern. By which
disease was his renal function destroyed?
TH E ME VII : TRA N SP L A N TA TIO N
110
A cadaveric kidney becomes available. A time-zero biopsy is taken just before transplantation, shown
in this figure:
It showed one small focus with a few lesions. What are they
called?
And what does this tell you about the quality of the kidney
transplant?
The transplantation procedure goes without complications, but 5 days after transplantation, the graft is
not functioning yet. A renal biopsy is performed, showing the lesion on the slide.
What is your diagnosis?
Two years after transplantation, cells are found in the urine with large nuclei.
Study the slide: how are these cells called?
Why?
What does their presence in the urine mean?
Would you consider taking a renal transplant biopsy?
Three years after transplantation, the patient is admitted to hospital because of an acute deterioration
of his renal function and fever. He says he has not been well for two weeks, because of a cold. What
are your differential diagnostic considerations for his condition? What are you going to do in view of
the diagnostic work-up?
The patient recovers. During 5 years, his renal transplant
is doing quite well. Then, a slowly progressive
deterioration of renal function is noticed by the clinician.
A biopsy of the renal transplant is performed.
Study the slide. What is your diagnosis?
ME CH A N ISMS O F D ISE A S E 1
111
Attachment 1: Tables for SSA II.1
Self-study Table II.1.1 Comparison of pathogen structure
Characteristic Pt. A Pt. B Pt. C Pt. D Pt. E
Pathogen
Group
Size
Cell wall
Cytoplasmic membrane
Outer membrane
Peptidoglycan
Lipo-polysaccharide
(lipo)Teichoic acid
DNA / RNA
Porins
Periplasmic space
Capsule
Envelope
Flagellas
Fimbriae
Hyphae
Ribosomes
Nucleus
Nucleocapsid
Endoplasmic Reticulum
Lysosomes
Mitochondria
Plasmids
Gastro-intestinal tract
Testis or ovarium
A TTA CH ME N TS
112
Self-study Table II.1.2 Comparison of the physiology of pathogens
Characteristics Pt. A Pt. B Pt. C Pt. D Pt. E
Pathogen
Multiplication method
Peptidoglycan
Synthesis
Penicillin-binding
proteins
DNA synthesis
RNA synthesis
Protein synthesis
Glycolysis
Fermentation
Citric acid cycle
Anaerobic breathing
Conjugation
Transformation
Transduction
Spore formation
Sexual
Reproduction
ME CH A N ISMS O F D ISE A S E 1
113
Self-study Table II.1.3 Virulence factors of pathogens.
Pt. A Pt. B Pt. C Pt. D Pt. E
Pathogen
Virulence
factor
Pathogenesis
(including
interaction
with
components
of the
immune
system)
Type of
inflammatory
reaction
A TTA CH ME N TS
114
Attachment 2: Tables for SSA III.A.1 and III.A.2
Selfstudy Table III.A.1
The role of host defence in the protection against infectious diseases
Defence
Bacteria
Viruses
Fungi
Protozoa
Worms
Barriers
Complement
Antibodies
Phagocytes
Cell mediated
Immunity
ME CH A N ISMS O F D ISE A S E 1
115
Self-study Table III.A.2
Patient A Patient B Patient C Patient D Patient E
Pathogen
Virulence
factors
Pathogenesis
Type of
inflammatory
reaction
Host defence
failure (in
general for this
pathogen, not
for this specific
patient)
Pathophysiolo
gical
explanation of
signs and
symptoms
A TTA CH ME N TS
116
Attachment 3: Table for SSA IV.A.1 and IV.B.1
Assignment IV.A.1 Assignment IV.B.1
Pathogen Reservoir Transmission Geographical
spread
Prevention and
control measures
Patient A
Patient B
Patient C
Patient D
Patient E
Pulmonary
tuberculosis
Pneumococcal
pneumonia
Cholera
Shingles
(Herpes zoster)
Poliomyelitis
Aspergillosis
Candidiasis
Amoebiasis
Toxoplasmosis
Echinococcosis
A TTA CH ME N TS
118
Table IV.B.3 ‘Rijksvaccinatieprogramma’ (National Vaccination Program)
Vaccines in the
‘Rijksvaccinatieprogramma’
(per component; actual
products may contain several
components)
All children
or selection
Age(s) Antigen only or live
vaccine
Characteristics of disease if unvaccinated: a. microorganism b. Route of infection c. Major clinical presentation d. Person to person spread possible e. endemic in Netherlands?
Level of protection
1. Diphtheria a . Corynebacterium diphtheriae b. Droplet infection; c.
necrotizing pharyngitis, tracheitis, myocarditis d. Yes e. No
2. Pertussis (kinkhoest ) a . Bordetella pertussis b. Droplet infection; c. longlasting
bronchitis with characteristic coughing pattern d. Yes e. Yes
3. Tetanus
a . Clostridium tetani b. contact with environmental source of
spores; c. generalized muscle spasms, autonomic dysfunction,
high mortality d. No e. Yes
4. Poliomyelitis a . Pol iomyelitis vi rus a. feco-oral; droplet infection, c. flaccid
para lysis d. Yes e. No, except for occasional outbreaks in
unvaccinated communities
5. HIB a . Haemophilus influenzae b. Droplet infection; contact with
a i rways secretions c. most common is upper airways infection but
most threatening infection is meningitis d. Yes e. Yes
6. Pneumococcus a . Streptococcus pneumoniae b. Droplet infection; contact with
a i rways secretions c. upper and lower airways infections d. Yes e.
Yes
7. Mumps (bof)
a . Mumps vi rus b. Droplet infection; contact with saliva c.
parotitis, orchitis or oophoritis (risk of infertility), meningitis d.
Yes e. Previously only in unvaccinated communities. Recently
outbreak that s tarted among previously vaccinated s tudents.
ME CH A N ISMS O F D ISE A S E 1
119
Table IV.B.3 continued
Vaccines in the
‘Rijksvaccinatieprogramma’
(per component)
All children or
selection
Age(s) Antigen only or live
vaccine
Characteristics of disease if unvaccinated:
a. Microorganism b. Route of infection
c. Major clinical presentation
d. Person to person spread possible
e. Endemic in Netherlands?
Level of protection
8. Measles a . Measles virus b. Droplet infection; contact with airways
secretions c. mostly fever and rash, rarely pneumonia or severe
encephalitis d. Yes e. No, except for occasional outbreaks in
unvaccinated communities
9. Rubella (rode hond)
a . Rubella vi rus b. Droplet infection; contact with a irways
secretions c. cl inical illness mild, but if during pregnancy: high risk
of intrauterine infection and intrauterine death or congenital
abnormalities d. Yes e. No, except for occasional outbreaks in
unvaccinated communities
10. MenC a . Neisseria meningitidis b. Droplet infection; c. meningitis or
sepsis d. Yes e. Yes
11. Hepatitis B virus
a . Hepatitis B vi rus b. vertical transmission; direct contact (e.g.
sexual); indirect contact (blood, needle s ticks); c. chronic
hepatitis (risk depends on age at time of infection), cirrosis, liver
cel l carcinoma d. Yes e. Yes.
12. Human PapillomaVirus a .Human Papilloma vi rus b.sexual transmission c. precancerous
cervica l lesions (detectable by PAP smear); cervix carcinoma d.
Yes e. Yes
121
Attachment 5: Table for SSA VI.6
1. Organs 2. Biopsy site
and result
3. Serology 4. Hypersensitivity
type
5. Course and
prognosis
SLE
Sjögren’s
syndrome
Granulomatosis
with poly-angiitis
Churg Strauss
syndrome
Rheumatoid
arthritis
Systemic
Sclerosis
Henoch
Schönlein
Purpura
A TTA CH ME N TS
122
Attachment 6: Practicals Bacteriology and
Parasitology
PRACTICAL 1: Bacteriology
Preparation:
E-learning modules of themes II (1 modules) and III (2 modules);
Read the introductory texts with the cases below;
For answering the questions in this practical, use Sherris. You should have this book with you
during the practical.
Microscopy
To see bacteria in a Gram stain, magnification with a 100 oil immersion objective is absolutely
necessary. The best approach for microscopy is:
examine the slide with a 10 objective, to focus and to study the presence of leukocytes;
add one drop of mineral oil;
refocus again with the 10 objective;
turn to 100 objective, focus and study stained preparations in more detail for cells and bacteria;
after microscopy, do NOT clean the stained slide (you would wipe off the bacteria), ONLY clean
the oil immersion objective with a tissue.
Blood cultures
Blood cultures are usually drawn from patients who have developed fever and have a suspicion of a
bacterial infection. Importantly, fever is not a prerequisite for a bacterial infection. Some patients are
unable to develop fever as a representing symptom of an infection because of the use of
immunosuppressive therapy or anti-inflammatory medicines with antipyretic activity. Age is another
important factor, since elderly patients also develop fever less frequently, as is uraemia. The timing
schedule for drawing a number of blood cultures is dependent on the bacteremic pattern (transient,
intermittent, or continuous) of the infection and the need to initiate antimicrobial therapy.
For a blood culture, blood is obtained by venous puncture after thorough disinfection of the skin. Blood
should not be drawn from an indwelling venous or arterial catheter. Since the number of bacteria present
in blood is often low, samples of at least 10 ml should be collected from (adult) patients. The blood is
collected in a bottle with culture medium which favours growth of aerobic bacteria and in a second bottle
favouring growth of anaerobic bacteria. Since most pathogenic bacteria are facultative aerobes, growth
usually occurs in both bottles. It is generally advised to take two blood cultures before antibiotic therapy
is (empirically) started – if empirical therapy is deemed necessary. Blood cultures are usually incubated
during 5-7 days, so a negative result will reach you only after this period. However, if blood cultures
become positive this usually happens within 24-48 hours and almost always within 72 hours, so that if
growth has not occurred within this period, you may consider the blood culture negative for practical
purposes.
When a blood culture shows bacterial growth (nowadays usually determined automatically), a Gram-stain
is made of the cultured blood (a Gram stain of freshly taken blood is not useful). If judged clinically
relevant by the medical microbiologist, this result of the Gram-stain will directly be reported to the
physician. At the moment of the first report, only the result of this Gram-stain can be reported (although
ME CH A N ISMS O F D ISE A S E 1
123
some laboratories in tertiary care facilities use mass-spectrometry for rapid identification within a few
hours). Further determination of the bacterial species involved and of its resistance pattern to antibiotics
usually takes 24 hours, but may take longer in the case of slowly growing species, mixed infections or
difficult species determinations.
Always take into account that a blood culture can be contaminated with skin bacteria. Especially
coagulase-negative staphylococci and diphteroid Gram-positive rods can be grown as contaminants from
blood cultures. Such bacterial species with low pathogenic potential only have clinical relevance in
special situations, mainly in very severely immune-compromised patients or after introduction of
biomaterials (e.g. intravenous catheters, prosthetic valves or prosthetic joints).
Case 1
A 68-year-old woman, who suffers since years from rheumatoid arthritis and is treated with
corticosteroids for this disorder, presents with increased pain of the right knee since one day. The
rheumatologist notices a red, swollen joint. The temperature is 37.8°C. Two blood cultures and an
aspirate of the knee joint are drawn and sent to the microbiology laboratory. The Gram-stain of the fluid
from the knee shows many leukocytes but no bacteria. The next day, one of the bottles from the blood
culture shows bacterial growth.
Describe the Gram-stain which is made of the blood culture.
Give two possible reasons why this blood culture becomes positive.
Which bacterial species would be responsible in each of the cases?
Would you start antibiotic treatment? If so, which treatment would you give?
Case 2
A 34-year-old man has returned since one week from a four week lasting journey to Indonesia. During his
stay there, he was in good health, apart from a few days of diarrhoea. Now he has 40°C fever since 48
hours. His heart rate is 80 per minute, his blood pressure is 130/80 mm Hg. A slightly enlarged spleen is
noticed on physical examination and a few pink coloured spots (rose spots) are visible on his abdomen.
His white blood cell count is 4.8 x 109/l. A malaria smear is negative. A blood culture is drawn and the
patient is sent home without antibiotics. On the next day, bacteria grow from the blood culture.
Describe the Gram-stain which is made of the blood culture.
Which bacterial species would you consider responsible for the infection in this patient?
A TTA CH ME N TS
124
Which antibiotic treatment would you consider?
Urine, sputum, pleural fluid, ascites, cerebrospinal fluid, pus.
Gram staining of these materials can be done directly and takes about 20-45 minutes. Rapid Gram stains
can be very useful in situations where the choice of the antibiotic regimen is critical. In Gram stains of
these specimens, the number of erythrocytes, leukocytes and squamous epithelial cells are also reported.
These numbers of cells are extremely important to judge the relevance of the bacteria which are
subsequently reported. Representative specimens from the respiratory or urinary tract should contain no
or only a few squamous epithelial cells, and in case of infection the number of leukocytes is usually high.
The presence of squamous epithelial cells indicates contamination by the flora of the upper respiratory
tract resp. vagina or preputium. It is important to differentiate squamous epithelial cells from columnar
ciliated epithelium (bronchi), alveolar cells and transitional epithelium (renal pelvis, bladder). In case of
materials that should be normally sterile, such as cerebrospinal, pleural, ascites or joint fluid, the presence
of many leukocytes indicate inflammation, which is mostly caused by bacterial infection.
Cultures are approximately tenfold more sensitive in comparison to direct smears. A negative Gram
smear of a cerebrospinal fluid specimen does not exclude bacterial infection, as the culture may become
positive. On the other hand, administration of antibiotics prior to collection of the specimen can lead to a
positive Gram-smear with bacteria, whereas bacterial growth remains negative. In these cases, bacteria
are still present, but lack viability to grow on artificial media.
Final reports of cultures of these specimens are usually available after 48 hours: 24 hours for initial
growth of bacteria, and another 24 hours for determination of bacterial species and resistance to
antibiotics. After 24 hours, a preliminary report can be given, if necessary (newly introduced
identification methods use mass-spectrometry and molecular biology, enabling more rapid identification,
but are not yet widely available). Reports may include an approximation of the number of bacteria present
in a given specimen. This can have relevance for clinical purposes: low numbers of bacteria may
sometimes be due to contamination of the specimen or to low-grade colonization of the patient with the
reported bacteria, whereas high colony counts of clinically relevant bacteria are almost always of
importance.
Whereas non-sterile specimens (urine, sputum) are routinely incubated for 48 hours only, specimens from
normally sterile fluids are often incubated for 5-7 days to allow detection of fastidious, slowly growing
bacterial pathogens as well. However, also from these specimens clinically relevant bacteria will grow
within 48 or at latest 72 hours in the majority of cases
Case 3
A 16-year-old boy suddenly became ill, with fever and chills since three hours. The physical examination
reveals a heart rate of 135 per minute, a blood pressure of 100/60 and a few petechiae on the legs. There
is nuchal rigidity. He is admitted to the paediatric intensive care and treated with broad-spectrum
antibiotics. After correction of the prolonged bleeding time, a lumbar puncture is taken.
Describe the Gram-stain which is made of the cerebrospinal fluid
Which bacterial species would you consider responsible for the infection in this patient?
ME CH A N ISMS O F D ISE A S E 1
125
Would it have been possible to diagnose this disease earlier?
Which antibiotic treatment would you give?
Case 4
A 4-year-old girl, with a history of recurrent urinary tract infections and presently on antibiotic
prophylaxis with trimethoprim, is admitted to the paediatric ward with high fever and abdominal pain. A
urinary specimen (and a blood culture) was sent to the laboratory.
Describe the Gram-stain which is made of the urinary specimen. Report also the number of
squamous epithelial cells and leukocytes!
Do you consider urinary tract infection likely in this patient? Which species is probably involved?
Which antibiotic treatment would you give?
Is it very important to obtain an antibiotic resistance pattern of this strain? Is there a risk that the
strain is resistant to the chosen treatment?
Case 5
A 68-year-old man, known with insulin-dependent diabetes mellitus for over 20 years, is seen at the
Dermatology department because of a small ulcer on the right leg, accompanied by extensive
inflammation of the surrounding skin. He is known to be allergic to penicillin. A Gram-stain of the pus on
the ulcer is made.
Describe the Gram-stain which is made of the specimen. Report also the number of leukocytes!
Which bacterial species is probably responsible for the infection?
Which antibiotic treatment would you give?
Case 6
A 23-year-old man originating from Somalia and now living in the Netherlands since 9 months, presents
with fever (38.2°C) which he has already since four months, loss of weight and night sweats. A chest X
ray shows a pulmonary infiltrate in the right apical lobe of the lung. A sputum sample is sent to the
laboratory for Gram-stain.
Describe the Gram-stain which is made of the specimen. Report also the number of epithelial cells
and leukocytes!
A TTA CH ME N TS
126
Which bacterial species is probably responsible for the infection? Which additional staining
method would you request? Describe the results of this examination (slide 6A). What would be the
next step?
Is it very important to obtain an antibiotic resistance pattern of this strain? Is there a good chance
that the strain is resistant to the chosen treatment?
ME CH A N ISMS O F D ISE A S E 1
127
PRACTICAL 2: Parasitology
In this practical you will be introduced to the techniques most often applied in the diagnostics of parasitic
infections.
Be present at the indicated time.
A laboratory coat is not necessary.
Consumption of food (including chewing gum) or drinks is not allowed in the practical room.
Bring with you Sherris 6th
edition (if you have 5th edition: the pages to prepare can be found in the
PDF with reading list for 5th edtion on Blackboard) and your course book.
Changing practicals is not allowed without special permission.
Prepare for the assignments by
reading Sherris 6th
edition pages 777-778 (Parasitic infections-diagnosis), and by reading the text
below.
Study the lecture Parasitology of theme II, which has been placed on blackboard. Pay special
attention to the life cycles, pathogenesis and diagnosis of
1 Plasmodium falciparum and P. vivax (pages 787-797)
2 Entamoeba histolytica (pages 813-818)
3 Schistosoma mansoni and S. haematobium (pages 903-905)
4 Strongyloides stercoralis (pages 858-861)
Clear information about life cycles, pathogenesis and diagnosis can also be found at
http://www.dpd.cdc.gov/DPDx/HTML/Para_Health.htm (search the names of the parasites in the
alphabetic list)
Diagnosis of malaria – background information
In order to be able to differentiate the stages of the Plasmodium parasite in the blood preparations of
assignments P1-P3 one needs to study the figure of the life cycle below.
The sexual development of Plasmodium takes place in the final host, the female Anopheles mosquito.
There the parasite divides and forms sporozoites, which enter the human circulation through a bite of a
female mosquito. Within the human host the parasite undergoes asexual multiplication. The sporozoites
pass quickly into the liver where they multiply in the liver cells for 1-2 weeks, causing no symptoms. The
parasites burst from the liver cells and the merozoites rapidly penetrate the erythrocytes and start
multiplying again. The young parasite within the red blood cell is called trophozoite. The dividing stage,
showing multiple nuclei often in combination with parasite pigment, is called schizont. After some time
the eythrocyte with the schizont ruptures and releases 8-24 merozoites into the blood, which penetrate
within seconds other erythrocytes. This process all together is called schizogony.
A TTA CH ME N TS
128
The erythrocytic cycle takes 48-72 hours, depending on the species. Some trophozoites do not go through
schizogony, but develop within erythrocytes into male and female gametocytes. A biting mosquito ingests
the gametocytes, which leads to the completion of the parasite’s life cycle.
The clinical symptoms of malaria are mainly a result of the erythrocytic cycles: the release of
haemoglobin decomposition products (malaria pigment) after rupture of the infected erythrocytes, and (in
case of Plasmodium falciparum) sequestration of maturing trophozoites and schizonts in the capillaries of
organs.
Laboratory diagnosis is based on the erythrocytic cycles. In a blood film we may see trophozoites,
schizonts and gametocytes, depending on the Plasmodium species in combination with the severity and
duration of infection. The specific morphology of these stages helps to diagnose different malaria species
(differential diagnosis), which is very important for treatment.
In falciparum malaria (Dutch: malaria tropica) caused by Plasmodium falciparum, schizogony takes place
in the organs, and gametocytes do not develop until after a number of cycli. Non-immune persons are
mostly examined earlier, as soon as complaints start. Hence, in infections caused by P. falciparum we
mostly see immature trophozoites only (except in severe infections). In infections caused by other malaria
species, each of the erythrocytic cycles can be seen in peripheral blood. In benign malaria tertiana, caused
by Plasmodium vivax, the parasitemia is often lower than in many infections with P. falciparum, the
infected erythrocytes are enlarged, the parasites are a larger (filling the red blood cell), and the surface of
ME CH A N ISMS O F D ISE A S E 1
129
the infected erythrocytes is dotted. Besides this, we often see malaria pigment sedimentation (erythrocyte
metabolic decomposition products) in the later stages. In P.ovale and P.malariae various erythrocytic
stages can also be found in peripheral blood (see Sherris table 51-1, page 791). The characteristics of
these species are not discussed in this course.
Assignment P.1 Thick blood film and thin blood film preparation
Patient 1 is a 30 years old man from Ethiopia. He came to the Netherlands 3 months before and complains
of fever. Malaria has to be excluded. Therefore blood was taken and examined for the Plasmodium
parasite in the laboratory. On slide P1 you will find both a Giemsa stained thick blood film preparation
and a Giemsa stained thin smear. Listen carefully to the instructions given
Why is a thick blood film preparation preferred to diagnose malaria, and what is the use of
making, staining and examining a thin smear preparation?
When examining P1. start with 1010 magnification in order to adjust the position of the slide and the
accessory plate. Then examine both the thick smear and the thin smear with 10100 magnification in oil
immersion. Ask assistance with the microscopy if it is not clear.
Which stages do you see in the thick smear and which in the thin smear? Describe the morphology
of the different stages.
Which species of Plasmodium are you looking at?
What is the parasitemia level (rough estimation percentage of infected erythrocytes)?
Assignment P.2. Parasitemia level
Patient 2 is a Dutch KLM-pilot (man, 50 years) who did not take malaria prophylaxis when he went to
Togo and stayed there for 2 nights. Two weeks after his return he gets high fever. His blood is examined
for malaria.
Examine preparation P2 (thick smear and thin smear are on the same slide) in the same way as
preparation P2.
Which stages do you see?
What is the parasitemia level here? Why is this important to know?
A TTA CH ME N TS
130
Assignment P.3. Differentiation between malaria species
Six months after patient 3 (a 24-year-old Dutch woman) had visited the Indonesian islands Java and Bali,
and New Guinea, she had periods of shivers and fever, which occurred at regular intervals. Here blood is
examined for malaria (P3, thick smear and thin smear at the same slide)
Observe the morphological characteristics of the parasites in P3 and name the different stages.
Compare both the infected red blood cell and the parasite itself with P1 and P2. Describe the
differences?
Which Plasmodium species do you see?
Why is the parasitemia level low? Is it important to know the parasitemia for this Plasmodium
species?
Assignment P.4. Worm eggs in urine
Patient 4 is a 36-year-old Dutch woman who went for holidays to Lake Malawi. Several months after her
return she notices bloody urine but feels otherwise well. Preparation P4 is her urine sediment. Examine
the slide, use 1010 magnification, and as soon as you find an egg, magnify to 1040 (do not use oil
immersion!).
What can you say about the aspects of the sediment (erythrocytes, leukocytes)?
Can you name the parasite species? How do you recognize the difference with a parasite of the
same genus?
Where are the worms situated who produce these eggs?
ME CH A N ISMS O F D ISE A S E 1
131
What alternative diagnostic tests could be used?
Assignment P.5 Worm eggs in faeces
Patient 5 is a 27-year-old male refugee from Afghanistan who came to Holland a few months ago. He
consulted the refugee centre physician, complaining of belly aches and diarrhoea. His stool is examined.
Preparation P5 is an unstained preparation of his stool. Examine the preparation at 1010 magnification,
and in case you find an egg, enlarge 1040 (no oil immersion!). Study the drawings of Sherris, chapter
54 (Figs. 54-3, 54-6, 54-9, 54-11) to determine the type of worm infections.
How was this patient infected with three different worm species?
If not treated, how long will these worms stay alive? Will they multiply within the human host?
Assignment P.6. Protozoal cysts in faeces
Patient 6 is a 21-year-old Dutch student who made a low budget trip through India and Nepal. He stayed
in simple hotels, often spent the night with local families, who treated him to local dishes, which he had
been advised not to eat. During the greater part of his travelling he had recurring diarrhoea, which was no
surprise to him. But he noticed blood in his stool after his return to the Netherlands. This makes him
decide to go and see his GP. Normally, in routine diagnosis, an iodine-stained preparation of his stool
would be made. For practical reasons we now examine the unstained preparation P6.
Which parasites do you see after careful examination? (NB. magnification 1040, no oil
immersion!)
Compare the sizes of the parasites in preparation P6 with those in preparation P5.Which parasitic
stages do you see in the preparation? What is the function of this stage?
An iodine-stained stool sample will be demonstrated. What is the purpose of this staining, while in
preparation P5 the stool specimen is unstained?
For which reason may it be necessary to perform microscopy on a stool sample within an hour
after production? What is the alternative if this is not possible?
What alternative diagnostic tests could be used?
ME CH A N ISMS O F D ISE A S E 1
133
Attachment 7: The module at a glance
The following three pages provide a week by week schedule, including all study and testing
methods by week, day, hour, theme and type. Hopefully this will give you a good overview of what
to expect and how to plan your studies. Please give feedback to your coordinators on the value of
this schedule.
Legend with the schedule:
IMMUNOLOGY
PATHOLOGY
INFECTIOUS DISEASES
PHARMACOTHERAPY
PATIENT DEMO
Self-study assignments
PRACTICALS
WORK GROUPS
E-learning
TEST
A TTA CH ME N TS
134
Week 1 Time Monday 1 Sept Type Theme Tuesday 2 Sept Type Theme Wednesday 3 Sept Type Theme Thursday 4 Sept Type Theme Friday 5 Sept Type Theme
08.30 LT LT5: Mechanisms of adaptive immune response LT I.A LT9: Tissue injury and repair LT I.B
09.00 I.A I.B
09.30
LT2: Intoduction to the immune system: bone
marrow, thymus, spleen. All players of the
immune system and development LT I.A
LT6: B- and T-cell generation and diversity
LT I.A
LT10: Repair mechanisms
LT I.B
10.00 I.A I.A I.B
10.30 LT3: Innate and adaptive immune responses LT I.A
LT7: Pathology of inflammatory reactions
LT I.B
Patient demonstration 1
PD I
11.00 I.A I.B I
11.30 LT4: Pathology of the normal immune response LT I.B
LT8: Pathology of inflammatory reactions:
a case LT IFormative test (vote boxes)
RC I
12.00 I.B I
12.30
13.00
13.30 SSA I.A SSA I SSA I.B SSA I Self study SSA I
14.00
14.30
15.00
15.30 Preparing for WORK GROUP 1 SSA I
16.00
16.30
17.00
17.30
Week 2 Time Monday 8 Sept Type Theme Tuesday 9 Sept Type Theme Wednesday 10 Sept Type Theme Thursday 11 Sept Type Theme Friday 12 Sept Type Theme
08.30 LT11: Introduction to infectious diseases LT II WORK GROUP 1 (THEME I) WG I LT15: Invader (virulence factors) LT III.A LT19: Diagnostics of infectious diseases LT III.B
09.00 II WG I III.A III.B
09.30 LT12: Bacteria LT II WG I LT16: Host versus invader LT III.A LT20: Interpretation of diagnostic test results LT III.B
10.00 II WG I III.A III.B
10.30 LT13: Viruses LT II WG I LT17: Immune deficiencies and infection risk LT III.A LT21: Essential microorganisms LT III
11.00 II WG I III.A III
11.30 LT14: Fungi and parasites LT II WG I LT18: Pathology of infection LT III Patient demonstration 2 LT III
12.00 II WG I III III
12.30 WG I
13.00 WG I
13.30 WG I
14.00
E-learning module Theme II Laboratory methods
for detection of bacteria COO II WG I
E-learning modules: 2 lessons diagnostics of
infectious diseases (1 teaching, 1 testing) COO III.B
14.30
SSA II. Structure, physiology and virulence
factors of different classes of microorganisms SSA II WG ISSA III.A
SSA III.A SSA and preparing for work group 2 SSA III
15.00 WG I Formative test (BB) II/III
15.30 WG I II/III
16.00 WG I
16.30 WG I
17.00 WG I
17.30 WG I
OPENING ACADEMIC YEAR LINE DAY
LT1: Introduction to G2MD1
LINE DAY
ME CH A N ISMS O F D ISE A S E 1
135
Week 3 Time Monday 15 Sept Type Theme Tuesday 16 Sept Type Theme Wednesday 17 Sept Type Theme Thursday 18 Sept Type Theme Friday 19 Sept Type Theme
08.30Discussion formative test and time for questions
WC II/IIIPRACTICAL BACTERIOLOGY↔
PR III.BLT24: Epidemiology of infectious diseases
LT IV.A PRACTICAL PARASITOLOGY PR III.B
09.00 08.30-10.30: Group C PR III.B IV.A 08.30-10.30: Group D PR III.B
09.30LT22: Antimicrobial therapy
LT III.C WORK GROUP 2 (THEME II / III) WG II / IIILT25: Prevention and control of infectious diseases
LT IV.B PR III.B
10.00 III.C WG II / III IV.B PR III.B
10.30 LT23: Principles of antibiotic pharmacotherapy LT III.C10.30-12.30: Group A
PR III.BPatient demonstration 3
PD IV 10.30-12.30: Group E PR III.B
11.00 III.C PR III.B IV PR III.B
11.30 WG II / III SSA IV.A SSA IV.A PR III.B
12.00 PRACTICAL BACTERIOLOGY PR III.B WG II / III PRACTICAL PARASITOLOGY PR III.B PR III.B
12.30 12.00-14.00: Group B PR III.B WG 12.00-14.00: Group A PR III.B SSA IV.B SSA VI.B
13.00 PR III.B WG PR III.B
13.30 PR III.B 13.30-15.30: Group D PR III.B PR III.B 13.30-15.30: Group F PR III.B
14.00 14.00-16.00: Group F PR III.B PR III.B 14.00-16.00: Group B PR III.B PR III.B
14.30 PR III.B WG II / III PR III.B PR III.B
15.00 PR III.B WG II / III PR III.B PR III.B
15.30 PR III.B 15.30-17.30: Group E PR III.B PR III.B 15.30-17.30: Group G PR III.B
16.00 16.00-18.00: Group G PR III.B PR III.B 16.00-18.00: Group C PR III.B PR III.B
16.30 PR III.B WG II / III PR III.B PR III.B
17.00 Self study Antibiotics/pharmacotherapy SSA III.C WG II / III PR III.B PR III.B
17.30 TRC antibiotics WG PR III.B Formative test (BB) PR III.B
Week 4 Monday 22 Sept Type Theme Tuesday 23 Sept Type Theme Wednesday 24 Sept Type Theme Thursday 25 Sept Type Theme Friday 26 Sept Type Theme
08.30 WORK GROUP 3 (THEME IV) WG IV LT28: Cells in autoimmunity LT VI
09.00 VGT WG IV VI
09.30 WG IV LT29: Pathology of autoimmunity LT VI
10.00 COMPONENT EXAM G2MD1 T WG IV VI
10.30 USC sportcentrum WG IV LT30: Vasculitis LT VI
11.00 WG IV VI
11.30 WG IV LT31: Systemic lupus erythematodes LT VI
12.00 WG IV VI
12.30 WG IV
13.00 SM1: Seminar diagnostics of infection SM I WG IV
13.30 SM V WG IV
14.00
SM2: Seminar diagnostics of immune
deficiencies and immunopathology SM IIILT26: Mechanisms of allergy
V WG IVSSA VI
SSA VI
14.30 LT V WG IV
15.00 SM3: Seminar health advocate SM IV LT27: Pathology of allergy V WG IV
15.30 LT V WG IV
16.00 Patient demonstration 4 V WG IV
16.30 Preparing for work group 3 WG IV
17.00 SSA V SSA V WG IV
17.30 WG IV
LINE DAY
LINE DAY
A TTA CH ME N TS
136
Week 5 Time Monday 29 Sept Type Theme Tuesday 30 Sept Type Theme Wednesday 1 Oct Type Theme Thursday 2 Oct Type Theme Friday 3 Oct Type Theme
08.30LT32: HLA and auto-immunity
LT VILT35: Pharmacology: immune-suppression 1
LT VIWORK GROUP 4 THEME V en VI
PR II.B
09.00 VI VI PR II.B
09.30LT33: Infections and auto-immunity
LT VILT36: Pharmacology: immune-supression 2
LT VI WG V
10.00 VI VI WG V
10.30 LT34: Interactive cases LT VI LT37: Academic assignment LT VI PR II.B
11.00 VI VI PR II.B
11.30 Formative test (voting boxes) RC VI Patient demonstration 5 PD VI WG V
12.00 VI WG V
12.30
13.00 PR II.B
13.30 PR II.B
14.00 SSA VI SSA VI Selfstudy pharmacology immunosuppression VI WG V
14.30 WG V
15.00 PR II.B
15.30 PR II.B
16.00 WG V
16.30 WG V
17.00
17.30
Week 6 Time Monday 6 Oct Type Theme Tuesday 7 Oct Type Theme Wednesday 8 Oct Type Theme Thursday 9 Oct Type Theme Friday 10 Oct Type Theme
08.30 LT38: Introduction transplantation WC Question hour Immunology RC all
09.00 LT VII
09.30 LT39: Transplant immunology LT VII Question hour Pathology RC all Selfstudy Selfstudy
10.00 VI
10.30
LT40: Histopathology of transplantation and
rejection LT VI Question hour Infectious Diseases RC all
11.00 VI
11.30 LT41: Islet cell transplantation LT VI Formative test (Voting boxes)
12.00 VI
12.30
13.00
13.30 LT42: Transplantation and infection risk LT VI13.00-16.00 FINAL EXAM
14.00 VI Selfstudy USC sportcentum
14.30 Patient demonstration 6 PD VI
15.00 VI
15.30
(15.30-16.30) replacement missed practical
bacteriology
16.00
16.30
(16.30-17.30) replacement missed practical
parasitology
17.00
17.30
03-okt
LINE DAY
LINE DAY