Investigation of Nicotine Levels and Some Trace Elements in ...
Adenosine (A)2A receptor modulation of nicotine-induced locomotor sensitization. A pharmacological...
Transcript of Adenosine (A)2A receptor modulation of nicotine-induced locomotor sensitization. A pharmacological...
lable at ScienceDirect
Neuropharmacology 81 (2014) 318e326
Contents lists avai
Neuropharmacology
journal homepage: www.elsevier .com/locate/neuropharm
Adenosine (A)2A receptor modulation of nicotine-induced locomotorsensitization. A pharmacological and transgenic approach
Joanna Jastrzebska a, Ewa Nowak a, Irena Smaga b, Beata Bystrowska b,Ma1gorzata Frankowska a, Michael Bader c, Ma1gorzata Filip a,b,*, Kjell Fuxe d
a Laboratory of Drug Addiction Pharmacology, Institute of Pharmacology, Polish Academy of Sciences, Kraków, PolandbDepartment of Toxicology, Faculty of Pharmacy, Jagiellonian University, Kraków, PolandcMax-Delbrück-Center for Molecular Medicine, Berlin, GermanydDepartment of Neuroscience, Karolinska Institutet, Stockholm, Sweden
a r t i c l e i n f o
Article history:Received 18 October 2013Received in revised form28 February 2014Accepted 1 March 2014Available online 14 March 2014
Keywords:A2A receptorsNicotine locomotor sensitizationConditioned locomotionTransgenic ratsNeurochemical analyses
* Corresponding author. Laboratory of Drug AddictiPharmacology, Polish Academy of Sciences, PL 31-343Tel.: þ48 12 6623293.
E-mail addresses: [email protected], filip@i
http://dx.doi.org/10.1016/j.neuropharm.2014.03.0020028-3908/� 2014 Elsevier Ltd. All rights reserved.
a b s t r a c t
Preclinical evidence indicates an important role of adenosine (A)2A receptors in drug addiction whiletheir therapeutic relevance is still a matter of debate. We examined the influence of the A2A receptoragonist CGS 21680 and the antagonist KW 6002 on nicotine sensitization and conditioned locomotoractivity in adult (8-week old) male SpragueeDawley rats (WT). Moreover, behavioral responses tonicotine were studied in rats overexpressing A2A receptors under the control of the neuronal specificenolase (NSE) promotor. Changes in the levels of dopamine, glutamate and g-aminobutyric acid in wildtype (WT) and NSEA2A rats were determined with using LCeMS. KW 6002 significantly enhancedexpression of nicotine sensitization and conditioned locomotion, while CGS 21680 reduced all theseeffects in WT rats. A reduction of the expression of nicotine-evoked conditioned locomotor activity wasalso observed in the NSEA2A animals. The transgenic rats displayed a reduced basal tissue level ofglutamate in the prefrontal cortex and hippocampus while dopamine basal levels in the nucleusaccumbens were raised. Chronic nicotine treatment caused a significant reduction in the glutamatetissue level in the dorsal and ventral striatum, prefrontal cortex and cerebellum in wild type rats. InNSEA2A animals the same drug treatment instead produced a rise of glutamate levels in the hippocampusand dorsal striatum. Taken together, A2A receptor signaling in the rat brain can counteract locomotorsensitization and conditioned locomotion to nicotine which are related to nicotine reward-learning. It issuggested that treatment with A2A receptor agonists can help counteract the abuse actions of nicotine.
� 2014 Elsevier Ltd. All rights reserved.
1. Introduction
Nicotine addiction is the second-leading cause of death world-wide. On average, adults who smoke die 14 years earlier thannonsmokers. Nonsmokers exposed to secondhand smoke at homeor work are more vulnerable to risk of heart disease by 25e30percent and lung cancer by 20e30 percent. Now there are about 1.3billion smokers world-wide, mainly (84%) in developing countries.If current smoking trends continue, tobacco will kill 10 millionpeople each year by 2020 (WHO, 2006; EMCDDA, 2011).
on Pharmacology, Institute ofKraków, Smetna 12, Poland.
f-pan.krakow.pl (M. Filip).
Knowledge of dopamine (DA) mechanisms does not explain allfacets of nicotine addiction. The neuronal basis of this very elabo-rate area of behavior involves direct DA signaling, DA-cooperationwith other neuromodulators and DA-independent mechanisms.One modulator implicated to play an important role is adenosine(ADO). ADO binds to the G-protein-coupled ADO receptors, A1, A2A,A2B and A3, of which A1 and A2A are most highly expressed in thebrain. Consistent findings show that A2A receptors are mainlyexpressed in the striatum, while lower binding has been detected incortex, hippocampus, thalamus and cerebellum (Brown and Short,2008). Due to basal ganglia localization, A2A receptors are wellplaced to play a fundamental role in altering DA signaling and havebeen said to influence the latter via several mechanisms: directreceptorereceptor interactions, interactions at the secondmessenger level, trans-synaptically via striatal collaterals or in-terneurons, or at a post-synaptic or network level. Thus, A2A re-ceptor antagonists increased DA transmission and A2A agonists
J. Jastrzebska et al. / Neuropharmacology 81 (2014) 318e326 319
conversely decreased it, which may be partly related to theantagonistic A2A receptor-DA D2 receptor interactions in striato-pallidal g-aminobutyric acid (GABA) neurons (Knapp et al., 2001;Filip et al., 2006; Bachtell and Self, 2009). The pharmacologicalanalyses are matched by the genetic approaches in which basallevels of accumbal DA levels were unchanged (Castañé et al., 2006)or reduced (Shen et al., 2013) in A2A knockout mice that alsoshowed a suppression of the rewarding properties of nicotine(Castañé et al., 2006) and a fall in the state of phosphorylation ofdopamine- and cAMP-regulated phosphoprotein 32 kDa(DARPP32) which show important changes of relevance forneuronal plasticity linked to drug addiction.
Nicotine’s pharmacological mechanism of action depends onstimulation of several neuronal nicotinic acetylcholine receptors inthe brain. Among the receptor subtypes, a4b2, a2b4, a5, a6 and a7ones are located in the mesolimbic DA pathways and influencenicotine reward, motivation, discriminative stimulus properties aswell as nicotine withdrawal. Nicotine as the other psychostimulantoperates through neurochemical mechanism, i.e., it increases DAneurotransmission from the ventral tegmental area to structureswithin the mesocorticolimbic circuitry of the brain, particularly thenucleus accumbens and prefrontal cortex (Di Chiara and Imperato,1988; Filip et al., 2012). As recently demonstrated with geneticapproaches, deletion of A2A receptors prevents the nicotine-induced upregulation of the a-7 nicotinic receptor, built upentirely of a-7 subunits, in mice (Metaxas et al., 2013).
Role of A2A receptor in nicotine sensitization has not beeninvestigated. The aim of the present study was to investigate therole of A2A receptors in the behavioral and neurochemical re-sponses to nicotine associated with several aspects of drug addic-tion. We used pharmacological tools (the selective A2A receptorantagonist KW 6002 and the A2A receptor agonist CGS 21680) andtransgenic rats with overexpression of A2A receptor under thecontrol of the neural specific enolase promoter (NSEA2A) or theirwild-type littermates to evaluate responses induced by acute (lo-comotor activity) or repeated (locomotor sensitization, conditionedlocomotion) treatment with nicotine. The NSEA2A rats are a validmodel to study ADO/DA interactions in view of the existence ofantagonistic A2A/D2 receptor interactions in the brain (Fuxe et al.,2005). In NSEA2A rats expression of A2A receptors is enhancedmainly in the prefrontal cortex, striatum, hippocampal formationand the cerebellum (Giménez-Llort et al., 2007). In behavioralparadigms, these transgenic animals exhibited major deficits ofworking memory assessed in 6-arms radial tunnel maze, objectrecognition test and ‘repeated acquisition paradigm’ in the Morriswater maze. However, no genotype differences were found in theemotional/anxious-like behavioral test or during spontaneousmotor activity recordings (Giménez-Llort et al., 2007). In the pre-sent study behavioral analyses were matched with neurochemicalfindings in which basal tissue levels of neurotransmitters (gluta-mate (GLU), DA and GABA) as well as changes in the above neu-rotransmitters following repeated (5 days) administration ofnicotine to wild-type (WT) and NSEA2A rats were determined Thisextensive investigation on the role of A2A receptors in preclinicalnicotine addiction models gives an opportunity to construct po-tential effective pharmacotherapies. The present data including anewgenetic model provide direct experimental support for a link ofA2A receptors to the actions of nicotine.
2. Materials and methods
2.1. Behavioral experiments
2.1.1. AnimalsMale transgenic rats (NSEA2A, Giménez-Llort et al., 2007) and wild type controls
(SpragueeDawley, WT) weighing between 250 and 320 g and being 8-week oldwere used for the experiments. Animals were housed 6e8 per cage
(58 cm � 37 cm � 20 cm) in a colony room maintained at 21 � 1 �C and 40e50%humidity under a 12 h lightedark cycle (lights on at 6:00 h). The animals werehabituated to the experimental environment and were handled once a day threedays before the start of the behavioral studies. Each treatment group consisted of 6e8 rats. All the experiments were conducted during the light phase of the lightedarkcycle (between 8:00 and 14:00 h), in accordance with the National Institutes ofHealth Guide for the Care and Use of Laboratory Animals and with the approval ofthe Bioethics Commission and compliant with the Polish Law, August 21, 1997.
2.1.2. Generation of transgenic rats (NSEA2A)Generation of NSEA2A rats was described in details by Giménez-Llort et al.
(2007). Briefly, microinjection of a DNA construct was injected into the male pro-nucleus of SpragueeDawley rat zygotes with established methods (Popova et al.,2002). The construct contained a full-length human A2A cDNA cloned into anexpression vector 30 of the 1.8 kb rat neuron-specific enolase (NSE) promoter and 50
of an intron/polyadenylation cassette of SV40 virus.
2.1.3. DrugsThe following drugs were used: (�)-nicotine bitartrate (SigmaeAldrich Chem-
icals; s.c.), (E)-1,3-diethyl-8-(3,4-dimethoxystyryl)-7-methyl-3,7-dihydro-1H-pu-rine-2,6-dione (KW 6002; Tocris; UK, i.p.), (4-[2-[[6-Amino-9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]) benzenepropanoic acid hy-drochloride (CGS 21680; Tocris, UK, i.p.). Nicotine bitartrate (the doses expressed asa free base) and CGS 21680 were dissolved in 0.9% NaCl. KW 6002 was dissolved in amixture of dimethyl sulfoxide (DMSO, SigmaeAldrich, USA), Tween 80 (SigmaeAldrich, USA) and a 0.9% NaCl (1:1:8). In the case of nicotine the pH was adjusted to7.0 using 20% NaOH. All drugs were injected in a volume of 1 ml/kg. CGS 21680, KW6002 and nicotine were injected 5 min, 20 min and 0 min, respectively, before thestart of behavioral recording. The doses and pretreatment times of the drugs are inagreement with the previously published behavioral studies in which A2A receptorligands were effective in counteracting the effects of drugs of abuse and the nicotinedose used in the current study effectively induced sensitization or conditionedlocomotion (Filip et al., 2006; Zaniewska et al., 2010; Wydra et al., 2012).
2.1.4. Locomotor activity measurementThe locomotor activity was recorded individually for each animal in commer-
cially available Opto-Varimex cages (Columbus Instruments, Columbus, OH) linkedto a compatible IBM-PC. Each chamber (43 cm � 43 cm � 21 cm), equipped with a220-lx house light, was made of transparent acrylic plastic (all six sides) and washoused in a light- and soundproof wooden cubicle. The corner brackets were madeof stainless steel. Each cage was surrounded by a 15 � 15 array of photocell beamslocated 3 cm from the floor surface as reported previously (Frankowska et al., 2007).Interruptions of the photobeams demonstrated horizontal locomotor activity,defined as a distance traveled and expressed in centimeter. Measurements of lo-comotor activity began immediately after vehicle or nicotine injection and wererecorded for a total of 60 min. Animals were non-habituated to experimentalchambers before experiments.
2.1.5. Nicotine-repeated treatmentRats (WT and NSEA2A) received repeated nicotine (0.4 mg/kg) or vehicle in-
jections for 5 days in the test environment (experimental chamber). During days 6e9 of the experiment, the animals remained drug-free in their home cages. Locomotoractivity was recorded on experimental days 1e5 and 10 following an injection of achallenge dose of nicotine (0.4mg/kg; nicotine sensitization) or vehicle (conditionedlocomotor activity).
2.1.6. Basal and nicotine induced locomotor activationLocomotor activity was recorded in WT rats which received either KW 6002
(0.25e0.5 mg/kg), CGS 21680 (0.1e0.4 mg/kg) or vehicle combined with vehicle ornicotine (0.4 mg/kg). Measurements of locomotor activity in Opto-Varimex cages(see above) began immediately after second injection (vehicle or nicotine) andlasted 60 min.
2.1.7. Development of nicotine sensitizationDuring the first 5 days of experiment the WT animals received either vehicle,
KW 6002 (0.25e0.5 mg/kg), or CGS 21680 (0.2e0.4 mg/kg), in home cages beforenicotine was given in experimental chambers. On days 6e9, they remained drug-free in their home cages. On day 10, the animals received a challenge dose ofnicotine (0.4 mg/kg) and locomotor activitywas recorded immediately after nicotineinjection.
2.1.8. Expression of nicotine sensitizationDuring the first 5 days of the experiment, the WT animals received vehicle or
nicotine (0.4 mg/kg). On days 6e9, the animals remained drug-free in their homecages. Animals were then challenged on day 10, with nicotine (0.4 mg/kg), inexperimental chambers. KW 6002 (0.25e0.5 mg/kg), CGS 21680 (0.01e0.2 mg/kg)was given on day 10 of the experimentation before injection of the nicotine. Mea-surements of locomotor activity began immediately after nicotine injection andlasted 60 min.
Table 1Effects of CGS 212680 and KW 6002 on the basal locomotor activity in rats.
Drug (mg/kg, ip) Horizontal distancetraveled (cm)/60 min
ANOVA
Vehicle 1930 � 89CGS 21680 (0.2) 1523 � 241CGS 21680 (0.4) 776 � 83*** F(2,19) ¼ 17.01 p < 0.001Vehicle 2064 � 95KW 6002 (0.25) 2748 � 501KW 6002 (0.5) 1998 � 120 F(2,19) ¼ 1.59
***p < 0.001 vs. vehicle (Dunnett’s test).
J. Jastrzebska et al. / Neuropharmacology 81 (2014) 318e326320
2.1.9. Expression of nicotine-evoked conditioned locomotor activityRats were given repeated pairings of a distinct test environment (an experi-
mental chamber) with either nicotine (0.4 mg/kg) or vehicle for 5 successive days.Animals remained in their home cages during next 6e9 days of the experiment. Onthe day 10, they were challenged with vehicle in experimental chambers. KW 6002(0.125e0.25 mg/kg), CGS 21680 (0.05e0.2 mg/kg) or vehicle was given on day 10 ofthe experimentation before injection of vehicle. Measurements of locomotor activitybegan immediately after vehicle injection and lasted 60 min.
2.1.10. CGS 21680-repeated treatmentRats (WT) received repeated CGS 21680 (0.4 mg/kg) or vehicle injections for 5
days in the test environment (experimental chamber). During days 6e9 of theexperiment, the animals remained drug-free in their home cages. Locomotor activitywas recorded on experimental days 1e5 and on day 10 following an injection ofvehicle or nicotine (0.4 mg/kg).
2.2. Biochemical experiments
2.2.1. Sample preparationThe drug-naïve and drug-treated (with 5-daily nicotine/vehicle injections in
experimental chambers) WT and NSEA2A rats were decapitated and the brains werequickly removed and chilled in ice-cold saline. The prefrontal cortex, dorsal stria-tum, nucleus accumbens, hippocampus and cerebellumwere dissected out (Paxinosand Watson, 1998). Samples were immediately frozen on dry ice and storedat �80 �C. For lysate preparation, selected brain tissues were homogenized in ice-cold 0.5 M formic acid with the concentration of 5 ml/g tissue. Lysates werecentrifuged 18,000 � g for 30 min at 4 �C. The supernatant was separated and storedat �20 �C until the LCeMS analysis for DA, GABA and GLU. The sample of nucleusaccumbens and prefrontal cortex were diluted twice with 0.1 M formic acid beforethe analysis.
2.2.2. Chemicals and reagentsLC/MS grade water, acetonitrile were obtained from Merck (Germany). LC/MS
grade formic acid was obtained from POCH (Katowice, Poland). DA hydrochloride,GABA and GLU were purchased from SigmaeAldrich (St. Louis, USA). Internalstandard (IS) with isotope labeling were: 2-(3,4-dihydroxyphenyl)ethyl-1,1,2,2-d4-amine hydrochloride (DA-d4, 98 at.% D); d, l -2-aminobutyric-d6 acid (GABA-d6,98 at.% D); L-glutamic-2,3,3,4,4-d5 acid (GLU-d5, 98 at.% D) were provided bySigmaeAldrich (St. Louis, USA). A working internal standard solution of DA-d4,GABA-d6 and GLU-d5 were prepared at a concentration 25 mg/ml in LCeMS gradeacetonitrile for DA-d4, 125 mg/ml in LCeMS grade acetonitrile for GABA-d6 and GLU-d5. All standards were stored at �20 �C in the dark.
2.2.3. Liquid chromatographyemass spectrometryThe chromatographic separation was performed on an Agilent HPLC 1100 series
system (Agilent, Waldbronn, Germany), which was equipped with a degasser, abinary pump, an auto-sampler and a thermo-stated column compartment, asdescribed previously (Wydra et al., 2013). The brain sample was separated on aLiChrospher 60 RP-select B column (125 mm � 4.6 mm ID, 5 mm particle size) incombination with an appropriate guard column (4 mm � 4 mm; 5 mm particle size)(Merck, Germany). The column was thermostated at 40 �C. The mobile phase wascomposed of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B)using the following gradient program: 0e0.5 min, isocratic gradient 5.0% (B); 0.5e1.5 min, linear gradient 5.0e30.0% (B); 1.5e2.50 min, isocratic gradient 30.0% (B);2.5e4.5 min, linear gradient 30.0e100.0% (B); 4.5e5.0 min, isocratic gradient 100.0%(B); 5.0e6.0 min, linear gradient 100.0e5.0% (B); 6.0e7.0 min, isocratic gradient5.0%. The flow rate was 0.5 ml/min; the injection volume was 10 ml.
Mass spectrometric analyses were accomplished on an Applied BiosystemsMDSSciex (Concord, Ontario, Canada) API 2000 triple quadrupole mass spectrometerequipped with an electrospray ionization (ESI) interface. ESI ionization was per-formed in the positive ionization mode. High purity nitrogen used as a sheath gas,was generated with a nitrogen generator. All experiments were carried out in thepositive ionmode. The ion source parameters were as follows: ion spray voltage (IS):4500 V; nebulizer gas (gas 1): 35 psi; turbo gas (gas 2): 30 psi; temperature of theheated nebulizer (TEM): 400 �C; curtain gas (CUR): 25 psi. Nitrogen (99.9%) fromPeak NM20ZAwas used as the curtain and collision gas. The ion path parameters forDA, GABA and GLU were as follows: declustering potential (DP): 20 V; focusingpotential (FP): 300 V; entrance potential (EP): 10 V; collision cell entrance potential(CEP): 0 V; collision cell exit potential (CXP): 2 V, respectively. The quantizationanalysis was performed using the MRM mode and tandem LC/MS. The followingpairs of ions were monitored with the following values of m/z: 104.3/87.1 for GABA;110.0/93.0 for GABA-d6, 148.0/84.1 for GLU, 153.22/89.1 for GLU-d5, 154.20/137.1 forDA and 158.19/141.0 for DA-d4. Data were analyzed by using the Analyst software 1.4(Perlan Technologies).
2.3. Statistical analyses
The behavioral data are expressed as mean total activity counts (�S.E.M.) for the1 h observation period. The one-way analysis of variance (ANOVA), followed by posthoc Dunnett’s test, was applied to evaluate the treatment group on day 1 (acute
treatments) or on day 10 (repeated treatments) as well as to evaluate behavioralsensitization (on day 10, the response to nicotine in nicotine treated animals wascompared with the response to acute nicotine injection in animals treated repeat-edly with vehicle). The two-way ANOVA, followed by post hoc NewmaneKeuls’ testonly when statistically significant effects in the ANOVA, was used to analyze therepeated CGS 21680 treatment in WT rats (day of treatment) and the NSEA2A andWT rats (genotype, treatment and genotype� treatment interaction). Additionally, apriori comparisons between means representing changes from control values forWT and for NSEA2A rats were made using Student’s t-test.
Results for biochemical experiments are expressed as means (�S.E.M.). For basallevels of neurotransmitters in WT and NSEA2A rats, data were analyzed using thetwo-way ANOVA for factors genotype, region and genotype � region interaction.After chronic treatment of nicotine/vehicle to WT and NSEA2A rats, data wereanalyzed with using two-way ANOVA with factors genotype, treatment andgenotype � treatment. Post hoc comparisons were made with NewmaneKeuls’ testafter statistically significant effects in the ANOVAs. Additionally, a priori compari-sons between means representing changes from control values inside the groups(WT and NSEA2A) and between WT and NSEA2A animals after drug repeated treat-ment were made by Student’s t-test. The criterion for statistically significant dif-ferences was at p < 0.05.
3. Results
3.1. Behavioral experiments
3.1.1. Modulation of basal locomotor activity by the A2A receptorantagonist KW 6002 and the A2A receptor agonist CGS 21680
KW 6002 (0.25 and 0.5 mg/kg) did not alter the basal locomotoractivity in rats, while following injection of CGS 21680 in a dose of0.4 mg/kg (but not in dose of 0.2 mg/kg), a significant decrease inbasal locomotor activity was observed (Table 1).
3.1.2. Modulation of acute nicotine-evoked locomotion by the A2A
receptor antagonist KW 6002 and the A2A receptor agonist CGS21680
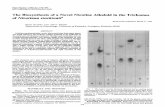
Nicotine (0.4 mg/kg) significantly (at least two-fold) enhancedthe locomotor activity of rats as compared to the effect of vehicle-treated animals (Fig. 1).
A significant group effect was detected by ANOVA for pretreat-ment with KW 6002 (F(3,20)¼ 23.13, p< 0.001). Pretreatment withKW 6002 (0.25 and 0.5 mg/kg) in a dose-dependent mannerincreased the acute locomotor effect of nicotine and a significantlystronger effect was observed after the highest dose of KW 6002(Fig. 1A).
A significant group effect was detected by ANOVA for pretreat-ment with CGS 21680 (F(4,24) ¼ 9.54, p < 0.001). Pretreatmentwith CGS 21680 in doses 0.2 and 0.4 mg/kg, but not in a dose of0.1 mg/kg, significantly attenuated the hyperactivation induced bynicotine (Fig. 1B).
3.1.3. Modulation of the development of nicotine induced locomotorsensitization by the A2A receptor agonist CGS 21680 but not the A2A
receptor antagonist KW 6002On day 10, administration of a challenge dose of nicotine
(0.4 mg/kg) to animals that received nicotine (0.4 mg/kg) repeat-edly (days: 1e5) resulted in a significant (ca. 77e98%) increase in
Dis
tanc
e tra
vele
d[cm
]/60
min
0
2000
4000
6000
8000
10000
VEHVEH NIC
VEH KW 0.25 KW 0.5
**
*****^^^
Dis
tanc
e tra
vele
d[cm
]/60
min
0
2000
4000
6000
8000
10000
****
VEHVEH NIC
^^^
VEH CGS 0.2 CGS 0.4CGS 0.1
^
A B
Fig. 1. Effects of KW 6002 and CGS 21680 on the nicotine (NIC; 0.4 mg/kg)-stimulated locomotor activity. *p < 0.05, **p < 0.01, ***p < 0.001 vs. corresponding vehicle þ vehicle(VEH þ VEH) group; p < 0.05, ^p < 0.001 vs. corresponding VEH þ NIC (Dunnett’s test).
J. Jastrzebska et al. / Neuropharmacology 81 (2014) 318e326 321
the locomotor activity compared to the effect of acute nicotine in-jection to vehicle-treated (days: 1e5) rats (Fig. 2).
A significant group effect was detected by ANOVA for pretreat-ment with KW 6002 (F(4,26) ¼ 37.29, p < 0.001). Repeated treat-ment with KW 6002 (0.25 and 0.5 mg/kg) in combination withnicotine did not alter the locomotor response of the nicotinechallenge dose, as compared with the locomotor effect of nicotinechallenge in vehicle and nicotine-treated animals (Fig. 2A).
A significant group effect was detected by ANOVA for pretreat-ment with CGS 21680 (F(4,23) ¼ 35.38, p < 0.05). A substantialdecrease in the locomotor response to the nicotine challenge wasobserved in rats treated repeatedlywith CGS 21680 only at a dose of0.4 mg/kg in combination with nicotine (Fig. 2B).
3.1.4. Modulation of the expression of nicotine locomotorsensitization by the A2A receptor antagonist KW 6002 and the A2A
receptor agonist CGS 21680On day 10 of the experiment, nicotine challenge of rats treated
repeatedly with nicotine (days 1e5) produced an increase in lo-comotor hyperactivity compared to the effect of acute nicotine invehicle-treated (days 1e5) animals (Fig. 3).
A significant group effect was detected by ANOVA for pretreat-ment with KW 6002 (F(3,20) ¼ 25.73, p < 0.001). A significant in-crease in the locomotor response to a nicotine challenge was foundin rats treated repeatedlywith nicotine after pretreatment with KW6002 in doses of 0.5 mg/kg, while the increases were non signifi-cant at 0.25 mg/kg (Fig. 3A).
A significant group effect was detected by ANOVA for pretreat-ment with CGS 21680 (F(5,35)¼ 7.92, p< 0.001). Pretreatment withCGS 21680 (0.05, 0.1 or 0.2 mg/kg) in a dose-dependent mannerdecreased considerably the locomotor effects to a nicotine chal-lenge in rats repeatedly treated with nicotine, but not in a dose0.01 mg/kg. A significant reduction almost to the control level wasseen following CGS 21680 in doses of 0.1 and 0.2 mg/kg (Fig. 3B).
3.1.5. Modulation by the A2A receptor antagonist KW 6002 and theA2A receptor agonist CGS 21680 of the expression of nicotine-evokedconditioned locomotor activity
Intermittent nicotine treatment paired with the environment(experimental chambers) for 5 days significantly enhanced (at leastby 112%) locomotor activity on day 10 compared with vehicle-treated (days: 1e5) animals exposed to the same conditions (Fig. 4).
On day 10, when KW 6002 (0.25 mg/kg, but not 0.125 mg/kg)was given to nicotine-treated rats, a significantly enhanced
locomotor activity was observed in comparison to nicotine-treatedand vehicle-challenged rats (F(3,22) ¼ 13.43; p < 0.001) (Fig. 4A).Conversely, CGS 21680 (0.1e0.2 mg/kg) but not in a dose 0.05 mg/kg, administered on day 10, decreased the conditioned locomotoractivity in rats (F(4,23) ¼ 6.31, p < 0.01) (Fig. 4B).
3.1.6. Modulation by the repeated treatment with A2A receptoragonist CGS 21680 on locomotor activity in rats
Repeated treatment with CGS 21680 (0.4 mg/kg) for 5 daysinduced significant alterations in rat locomotor activity as shown by2-way ANOVA for day � treatment interaction (F(1,14) ¼ 7.21;p < 0.001). A reduction in locomotor activity following CGS 21680(0.4 mg/kg) on day 1 (vehicle: 2658 � 158, CGS 21680: 1157 � 158;p < 0.0001) lasted till day 5 (vehicle: 1173 � 135, CGS 21680:550 � 137; p < 0.02). However, on day 10, when vehicle was givento both vehicle or CGS 21680-treated rats, there were no significantchanges in rats’ locomotor activity (vehicle: 2391�351, CGS 21680:2412 � 243; t ¼�0.06, df ¼ 14). Similarly, a nicotine challenge dose(0.4 mg/kg) resulted in similar records of locomotor activity on day10 (vehicle: 3285 � 333, CGS 21680: 3738 � 376; t ¼ �0.88,df ¼ 14).
3.1.7. Reductions of the development of nicotine induced locomotorsensitization in NSEA2A rats
Amain effect of treatment (F(1,22)¼ 42.39, p< 0.001), genotype(F(1,22) ¼ 11.99, p < 0.01), but not of treatment � genotype inter-action (F(1,22) ¼ 2.26) was observed for locomotor activity ingroups after administration of a challenge dose of nicotine (0.4 mg/kg) on day 10. However, a priori comparisons with Student’s t-testindicated significant (p< 0.001) enhancement of locomotor activityfollowing nicotine challenge inWTand NSEA2A groups on day 10. Itmeans that nicotine challenge induced the same behavioral effect(sensitization) in WT and NSEA2A rats. Additionally, Student’s t-testshowed on day 10 that repeated administration of nicotine (1e5day) and the challenge dose of nicotine (0.4 mg/kg) evokedconsiderable differences in locomotor activity in NSEA2A vs. WTgroups (p < 0.05) (Fig. 5).
3.1.8. Reduction of the expression of nicotine evoked conditionedlocomotor activity in NSEA2A rats
A main effect of treatment (F(1,19) ¼ 8.75, p < 0.05), but not ofgenotype (F(1,19) ¼ 2.36) or treatment � genotype interaction(F(1,19) ¼ 1.61) was observed for locomotor activity in groups afteradministration of vehicle on day 10. However, a priori comparisons
Dis
tanc
e tra
vele
d [c
m]/6
0 m
in
0
2000
4000
6000
8000
10000
VEH+VEH VEH+VEHVEH+NIC KW 0.25+NIC KW 0.5+NIC
NIC
REPEATED TREATMENT (1-5 DAY):
******
***
**
^^^^^^
^^^
VEH
Dis
tanc
e tra
vele
d[cm
]/60
min
0
2000
4000
6000
8000
10000
VEH+VEH VEH+VEH VEH+NIC CGS 0.2+NIC CGS 0.4+NIC
NIC
REPEATED TREATMENT:(1-5 DAY):
***
******
***^ ^^^
^^^
#
VEH
A B
Fig. 2. Effects of KW 6002 and CGS 21680 on the development of nicotine (NIC) sensitization. Rats were treated repeatedly (days 1e5) with either vehicle þ vehicle (VEH þ VEH),VEH þ NIC (0.4 mg/kg), KW 6002 (0.25e0.5 mg/kg)þNIC (0.4 mg/kg), CGS 21680 (0.2e0.4 mg/kg)þNIC (0.4 mg/kg). On day 10, the animals were given a challenge dose of NIC(0.4 mg/kg). **p < 0.01, ***p < 0.001 vs. corresponding VEH þ VEH-treated and VEH-challenged group; p < 0.05, ^p < 0.001 vs. corresponding VEH þ VEH-treated and nicotine-challenged group; #p < 0.05 vs. corresponding VEH þ NIC-treated and NIC-challenged group (Dunnett’s test).
A B
Dis
tanc
e tra
vele
d [c
m]/6
0 m
in
0
2000
4000
6000
8000
10000
12000
VEH NIC
NIC
***^^^
REPEATED TREATMENT (1-5 DAY):
**
VEH VEH KW 0.25 KW 0.5
Dis
tanc
e tra
vele
d [c
m]/6
0 m
in
0
2000
4000
6000
8000
10000
12000VEHNIC
^^^
***
^^^
NIC
REPEATED TREATMENT (1-5 DAY):
^^^
VEH VEH CGS 0.05 CGS 0.1 CGS 0.2CGS 0.01
*
Fig. 3. Effects KW 6002 and CGS 21680 on the expression of nicotine (NIC) sensitization. Rats were treated repeatedly (days 1e5) with either vehicle (VEH) or NIC (0.4 mg/kg). Onday 10, the animals were challenged with either VEH þ NIC (0.4 mg/kg), KW 6002 (0.25e0.5 mg/kg)þNIC (0.4 mg/kg), CGS 21680 (0.01e0.2 mg/kg)þNIC (0.4 mg/kg). *p < 0.05,***p < 0.001 vs. corresponding VEH-treated and VEH þ NIC-challenged group; ^p < 0.001 vs. corresponding NIC-treated and VEH þ NIC-challenged group (Dunnett’s test).
A B
Dis
tanc
e tra
vele
d[cm
]/60
min
0
1000
2000
3000
4000
5000VEHNIC
VEH
REPEATED TREATMENT:(1-5 DAY):
VEH VEH KW 0.125 KW 0.25
**
***^
Dis
tanc
e tra
vele
d[cm
]/60
min
0
1000
2000
3000
4000
5000VEHNIC
VEH
REPEATED TREATMENT:(1-5 DAY):
VEH VEH CGS 0.1 CGS 0.2
**
^^^ ^^^
CGS 0.05
Fig. 4. Effects of KW 6002 and CGS 21680 on the expression of nicotine (NIC)-evoked conditioned locomotor activity. Rats were treated repeatedly (days 1e5) with vehicle (VEH) orNIC (0.4 mg/kg). On day 10, the animals were given a challenge dose of VEH þ VEH or KW 6002 (0.125 and 0.25 mg/kg)þVEH or CGS 21680 (0.05e0.2 mg/kg) þ VEH. **p < 0.01,***p < 0.001 vs. corresponding VEH-treated and VEH þ VEH-challenged group; p < 0.05,^p < 0.001 vs. corresponding NIC-treated and VEH þ VEH-challenged group (Dunnett’s test).
J. Jastrzebska et al. / Neuropharmacology 81 (2014) 318e326322
Dis
tanc
e tra
vele
d [c
m]/6
0 m
in
0
1000
2000
3000
4000
WT VEHWT NICNSEA2A VEHNSEA2A NIC
VEH
REPEATED TREATMENT (1-5 DAY):
*
Fig. 6. Effects of nicotine (NIC) conditioned locomotor activity on WT and NSEA2A rats.Rats (WT and NSEA2A) were treated repeatedly (days 1e5) with vehicle (VEH) or NIC(0.4 mg/kg). On day 10, the animals were given a VEH. ***p < 0.001 vs. correspondingWT VEH-treated and VEH-challenged group (a priori Student’s t-test).
0
1
2
3
4
WTNSEA2A
DA (u
g/g)
*
PFCX HIP DSTR NAC CER
140
J. Jastrzebska et al. / Neuropharmacology 81 (2014) 318e326 323
with Student’s t-test indicated significant (p < 0.05) environment-triggered locomotion activity only inWTgroup on day 10 (p< 0.05)(Fig. 6.).
3.2. Biochemical experiments
3.2.1. Neurotransmitter basal levels in different brain regions of WTand NSEA2A rats
For basal tissue DA level 2-way ANOVA showed a significanteffect for region (F(4,40) ¼ 304.7; p < 0.0000001), but not for ge-notype (F(1,40) ¼ 2.96) or region � genotype interaction(F(4,40)¼ 1.47). The basal tissue concentration of DA inWTanimalsranged from 0.14 to 3.23 mg/g, with the highest levels in the dorsalstriatum and the lowest levels in the cerebellum. A priori com-parisons with Student’s t-test showed that in the NSEA2A group thebasal level of DA was increased in the nucleus accumbens(t ¼ �2.367, df ¼ 8, p < 0.05).
For basal tissue GABA level 2-way ANOVA showed a significanteffect for region (F(4,40) ¼ 81.2; p < 0.0000001), but not for ge-notype (F(1,40) ¼ 0.012) or region � genotype interaction(F(4,40) ¼ 0.14). In the WT group basal GABA levels were rangedfrom 31.33 to 102.61 mg/g; the highest concentration was observedin the nucleus accumbens and the lowest in the cerebellum. Asshown on Fig. 7, a priori comparisons with Student’s t-test did notreveal changes in the basal levels of GABA in the NSEA2A animals.
For basal tissue GLU level 2-way ANOVA showed a significanteffect for region (F(4,40) ¼ 22.2; p < 0.0000001) and genotype(F(1,40) ¼ 16.75; p < 0.0002), but not for region � genotypeinteraction (F(4,40)¼ 0.78). The basal level of GLU inWT rats was inthe range from 206.46 to 569.03 mg/g, with the highest concen-tration in the prefrontal cortex and the lowest in the dorsal stria-tum. A decrease of GLU levels was observed in the prefrontal cortex(t ¼ 4.066, df ¼ 6, p < 0.01) and hippocampus in NSEA2A rats asassessed by a priori comparisons with Student’s t-test (t ¼ 2.889,df ¼ 6, p < 0.05) (Fig. 7.)
60
80
100
120
GAB
A (u
g/g)
3.2.2. Neurotransmitter levels in different brain regions of WT andNSEA2A rats after chronic (5 days) administration of nicotine
The tissue concentration of DA in WT and NSEA2A animals afterchronic administration of nicotine did not change significantlycompared to the control groups which received the vehicle for 5days (Table 2). Repeated (5-daily) administration of vehicle
Dis
tanc
e tra
vele
d [c
m]/6
0 m
in
0
2000
4000
6000
8000
10000
12000
WT VEH WT NIC NSEA2A VEH NSEA2A NIC
REPEATED TREATMENT (1-5 DAY) :
NIC
***
^^^#
Fig. 5. Effects of nicotine (NIC) sensitization on WT and NSEA2A rats. Rats (WT andNSEA2A) were treated repeatedly (days 1e5) with vehicle (VEH) or nicotine (NIC)(0.4 mg/kg). On day 10, the animals were given a challenge dose of NIC (0.4 mg/kg).***p < 0.001 vs. corresponding WT VEH-treated and NIC-challenged group;^p < 0.001vs. corresponding NSEA2A NIC-treated and NIC-challenged group; #p < 0.05 vs. cor-responding WT NIC-treated and NIC-challenged group (a priori Student’s t-test).
0
20
40
0
100
200
300
400
500
600
GLU
(ug/
g)
**
*
Fig. 7. Basal level of neurotransmitters (WT and NSEA2A rats): DA, GLU, GABA inselected brain structures WT and NSEA2A rats. prefrontal cortex (PFCX), dorsal striatum(DSTR), nucleus accumbens (NAC), hippocampus (HIP), cerebellum (CER). *p < 0.05,**p < 0.01 vs. WT (a priori Student’s t-test).
Table 2Statistical analyses of neurotransmitter levels (DA, GLU, GABA) after chronic (5 days) administration of vehicle or nicotine in selected brain structures of WT and NSEA2A rats.
2-way ANOVA factor Brain structure
PFCX HIP DSTR NAC CER
DAGenotype F(1,16) ¼ 1.44, p ¼ 0.25 F(1,16) ¼ 0.19, p ¼ 0.67 F(1,16) ¼ 0.35, p ¼ 0.56 F(1,16) ¼ 0.27, p ¼ 0.61 F(1,16) ¼ 3.83, p ¼ 0.07Treatment F(1,16) ¼ 1.12, p ¼ 0.31 F(1,16) ¼ 2.14, p ¼ 0.16 F(1,16) ¼ 0.12, p ¼ 0.73 F(1,16) ¼ 0.01, p ¼ 0.99 F(1,16) ¼ 2.11, p ¼ 0.16Genotype � treatment F(1,16) ¼ 6.05, p < 0.05 F(1,16) ¼ 2.25, p ¼ 0.16 F(1,16) ¼ 0.01, p ¼ 0.94 F(1,16) ¼ 6.48, p < 0.05 F(1,16) ¼ 0.11, p ¼ 0.74GABAGenotype F(1,16) ¼ 0.17, p ¼ 0.69 F(1,16) ¼ 0.23, p ¼ 0.64 F(1,16) ¼ 0.24, p ¼ 0.63 F(1,16) ¼ 0.02, p ¼ 0.88 F(1,16) ¼ 2.61, p ¼ 0.12Treatment F(1,16) ¼ 0.17, p ¼ 0.69 F(1,16) ¼ 0.00001, p ¼ 1.00 F(1,16) ¼ 1.21, p ¼ 0.29 F(1,16) ¼ 0.06, p ¼ 0.81 F(1,16) ¼ 0.13, p ¼ 0.73Genotype � treatment F(1,16) ¼ 0.06, p ¼ 0.81 F(1,16) ¼ 0.09, p ¼ 0.77 F(1,16) ¼ 0.01, p ¼ 0.94 F(1,16) ¼ 0.29, p ¼ 0.60 F(1,16) ¼ 0.05, p ¼ 0.83GLUGenotype F(1,16) ¼ 22.00, p < 0.001 F(1,16) ¼ 77.47, p < 0.001 F(1,16) ¼ 0.96, p ¼ 0.34 F(1,16) ¼ 3.78, p ¼ 0.07 F(1,16) ¼ 0.33, p ¼ 0.57Treatment F(1,16) ¼ 3.97, p ¼ 0.06 F(1,16) ¼ 65,54, p < 0.001 F(1,16) ¼ 0.42, p ¼ 0.52 F(1,16) ¼ 1.01, p ¼ 0.33 F(1,16) ¼ 1.50, p ¼ 0.24Genotype � treatment F(1,16) ¼ 3.42, p ¼ 0.08 F(1,16) ¼ 37.77, p < 0.001 F(1,16) ¼ 22.96, p < 0.001 F(1,16) ¼ 3.75, p ¼ 0.07 F(1,16) ¼ 1.28, p ¼ 0.95
Dopamine (DA), g-aminobutyric acid (GABA), glutamate (GLU), prefrontal cortex (PFCX), dorsal striatum (DSTR), nucleus accumbens (NAC), hippocampus (HIP), cerebellum(CER).
J. Jastrzebska et al. / Neuropharmacology 81 (2014) 318e326324
increased accumbal DA levels in NSEA2A compared to WT rats(t ¼ �2.367, df ¼ 8, p < 0.05) (Table 3). The same increase in DAlevels was seen for basal conditions in drug-naïve animals (seeFig. 7).
GABA levels were unaffected by repeated administration ofvehicle or nicotine to both genotypes (Tables 2 and 3).
Repeated administration (5 days) of nicotine to WT and NSEA2Arats triggered a significant effect on GLU levels (p < 0.001) forgenotype � treatment interaction in the hippocampus (Table 2).Moreover, a priori comparisons with Student’s t-test between WTand NSEA2A rats indicated lower GLU levels in vehicle-treatedNESA2A rats in the prefrontal cortex (t ¼ 5.249, df ¼ 8, p < 0.001)and nucleus accumbens (t ¼ 2.336, df ¼ 8, p < 0.05) (Table 3).Instead repeated nicotine treatments resulted in a significantreduction in GLU tissue concentrations in the prefrontal cortex,dorsal striatum, nucleus accumbens and cerebellum inWT rats andin significant increases in GLU levels in the hippocampus and dorsalstriatum in NSEA2A rats.
4. Discussion
The pharmacological behavioral studies with the A2A receptorantagonist KW 6002 and the A2A receptor agonist CGS 21680 give
Table 3Neurotransmitter levels in selected brain structures of WT and NSEA2A rats after chronic
Genotype Treatment Brain structure
PFCX HIP
DA (mg/g)WT VEH 0.28 � 0.02 0.15 � 0.04
NIC 0.33 � 0.03 0.27 � 0.06NSEA2A VEH 0.32 � 0.05 0.19 � 0.03
NIC 0.21 � 0.03 0.19 � 0.05GABA (mg/g)WT VEH 45.01 � 3.13 48.24 � 2.14
NIC 46.86 � 2.60 47.29 � 1.57NSEA2A VEH 44.54 � 3.68 45.80 � 7.18
NIC 45.00 � 1.34 46.75 � 1.69GLU (mg/g)WT VEH 569.03 � 8.41 321.04 � 20.47
NIC 473.64 � 25.46## 351.45 � 13.90NSEA2A VEH 406.57 � 29.78### 87.97 � 14.75**
NIC 403.21 � 29.38 310.04 � 11.47^
Wild type rats (WT), rats with overexpression of A2A receptor (NSEA2A), vehicle (VEH), niccortex (PFCX), dorsal striatum (DSTR), nucleus accumbens (NAC), hippocampus (HIP), ce***p < 0.001 vs. WT VEH (NewmaneKeuls test).^p < 0.001 vs. NSEA2A VEH (NewmaneKeuls test).#p < 0.05.##p < 0.01.###p < 0.001 vs. WT VEH (a priori Student t-test).þþp < 0.01 vs. NSEA2A VEH (a priori Student t-test).
evidence that A2A receptor mechanisms counteract the develop-ment and/or expression of nicotine locomotor sensitization. Thus,KW 6002 significantly enhances expression of nicotine sensitiza-tion while CGS 21680 attenuates both phases of this phenomenon.The inhibitory A2A receptor mechanism is also involved in thecounteraction of the expression of nicotine evoked conditionedlocomotor activity. The transgenic approach using A2A receptoroverexpressing rats under the NSE promoter (Giménez-Llort et al.,2007) supports the pharmacological results since reductions of theexpression of nicotine evoked conditioned locomotor activity wereobserved in the NSEA2A rats vs. control rats.
Interestingly, CGS 21680 effectively counteracted both devel-opment and expression of nicotine sensitization while the A2A re-ceptor blockade with KW 6002 only affected the expression phase.The interpretation of the inhibitory actions of CGS 21680 e seenonly for the highest drug dose (0.4 mg/kg) e towards developmentof nicotine sensitizationmay derive from the sedative actions of thedrug that also reduced the daily nicotine locomotor stimulationobserved upon combined CGS 21680 þ nicotine treatment.
In line with these results central reward processes and effects ofdrugs of abuse are modulated by A2A receptor function (Filip et al.,2012). Thus, rats treated with A2A receptor agonists show a reducedinitiation of cocaine self-administration (Baldo et al., 1999).
pretreatment (5 days) of vehicle or nicotine.
DSTR NAC CER
3.23 � 0.13 1.29 � 0.11 0.14 � 0.023.15 � 0.20 1.83 � 0.24 0.20 � 0.053.32 � 0.25 1.74 � 0.15# 0.12 � 0.023.27 � 0.16 1.39 � 0.17 0.16 � 0.02
56.64 � 2.31 102.62 � 2.82 31.33 � 0.9358.07 � 3.40 108.29 � 10.37 36.73 � 3.0654.01 � 1.27 104.96 � 2.82 33.02 � 1.7555.07 � 2.54 101.85 � 6.36 37.14 � 4.10
222.90 � 23.86 259.85 � 20.89 275.47 � 28.90124.71 � 9.66# 182.45 � 25.08# 173.82 � 17.91#
* 157.67 � 38.43# 157.67 � 38.43# 251.32 � 68.11261.55 � 34.61þþ 182.26 � 25.08 247.40 � 37.01
otine (NIC), dopamine (DA), g-aminobutyric acid (GABA), glutamate (GLU), prefrontalrebellum (CER).
J. Jastrzebska et al. / Neuropharmacology 81 (2014) 318e326 325
Furthermore, an increase in A2A receptors was observed in thenucleus accumbens after extended cocaine self-administration(Marcellino et al., 2007) and this effect was strictly dependent onactive self-administration, as yoked controls passively infused withcocaine failed to show these alterations (Frankowska et al., 2013). Anumber of studies show that A2A receptor activation can reducecocaine-seeking behavior (Weerts and Griffiths, 2003; Sanchis-Segura and Spanagel, 2006; Bachtell and Self, 2009; Filip et al.,2012).
Nicotine and exposure to cigarette smoke increase DA turnoverand release in the mesolimbic DA neurons which may mediate thereinforcing and locomotor activities of nicotine (Andersson et al.,1981; Fuxe et al., 1986; Imperato et al., 1986; Stolerman et al.,1995; Pontieri et al., 1996). The major location of A2A receptors ison the dorsal and ventral striato-pallidal GABA neurons where theyinter alia form heteroreceptor complexes with D2 receptors (Fuxeet al., 2007; Trifilieff et al., 2011; Borroto-Escuela et al., 2013). Ittherefore seems likely that one mechanism for the ability of A2Areceptors to counteract nicotine sensitization is through activationof the A2A protomer in the A2A-D2 heteroreceptor complex whichreduces D2 protomer signaling via an antagonistic allosteric re-ceptorereceptor interaction and diminishes the D2 receptormediated inhibition of the dorsal striato-pallidal GABA neurons andfavors motor inhibition (Fuxe et al., 2007). Thus, with increased A2Areceptor activation the nicotine induced DA release may no longereffectively activate the D2 receptor protomer. Another mechanismcan be antagonistic crosstalk at the level of the adenylate cyclasewith A2A receptor increasing and D2 receptors reducing the activity(Fuxe et al., 2007; Ciruela et al., 2011). A2A receptor agonists werealso found to increase striatal glutamate release (Popoli et al., 1995),especially in a hemiparkinson rat model of Parkinson’s disease(Tanganelli et al., 2004), which increases motor inhibition byincreasing the drive on the striato-pallidal GABA neurons. SimilarA2A and D2 receptor mechanisms are probably involved in theaccumbaleventral pallidal GABA neurons participating in thereward network including exploration (locomotion) and potentiallyin the motivational aspects of nicotine sensitization (Filip et al.,2012). The nicotine evoked conditioned locomotor activity wasalso counteracted by A2A receptor activation indicating an associ-ation of the environmental cues with the effects of nicotine and theinvolvement of memory processes during the conditioningresponse. Our results suggest that NSEA2A can modulate theexpression of memory related to the association of environmentalcues with the effects of nicotine. These behavioral changes wereassociated to a reduction in the basal tissue levels of GLU in theprefrontal cortex and hippocampus while other neurotransmitter(DA, GABA) levels were similar in both genotypes. To further sup-port the significance of the findings on hippocampal GLU levels,repeated vehicle treatment of NESA2A rats resulted in lower GLUlevels in this region while nicotine administration evoked a sig-nificant genotype � treatment interaction on GLU levels in thesame region. The findings suggest that NSEA2A rats may trigger“OFF” signals for a normal associative contextual learning associ-ated to nicotine, probably through the modulation of prefrontalcortical-hippocampal glutamatergic circuits.
It is of substantial interest that the studies on NSEA2A rats withincreases of especially cortical A2A receptors inter alia in the pre-frontal cortex and hippocampus but also of striatal A2A receptors(Giménez-Llort et al., 2007) support the pharmacological results.The increase of brain A2A receptors may represent increases in A2A
receptor mono-homomers and/or A2A-D2 heteroreceptor com-plexes. In view of the neurochemical demonstration of significantincreases of DA levels in the nucleus accumbens and significantreductions of GLU levels in the prefrontal cortex and hippocampuswith no changes in the GABA levels in any region studied of the
NSEA2A rats we may propose the following interpretation. Thereduced basal GLU levels in the prefrontal cortex and the hippo-campus e both regions known to project to the nucleus accumbens(Mora et al., 2008) e may reflect an increased activity in their GLUefferent projections to the latter region due to overexpression ofA2A receptors in the GLU projection neurons from these regions tothe nucleus accumbens. A2A receptor activity can increase GLUrelease (see above) and as a result of an increased GLU drive on theaccumbalepallidal GABA neurons reinforcement mechanisms arereduced. In agreement, exploration (locomotion) was in factobserved. The increase of accumbens DA levels may in part reflectincreases in extrasynaptic GABA transmission from dendrites andcollaterals of the activated accumbaleventral pallidal GABA neu-rons diffusing to inhibit release of DA from the accumbal DA ter-minals. It may also in part reflect a reduced activity in themesolimbic DA neurons due to an inhibitory feedback on theventral tegmental area DA cells due to increased inhibitory feed-back from the accumbens shell-ventral tegmental area-accumbenscore pathways (Haber et al., 2000; Everitt and Robbins, 2005).
Taken together the combined results obtained with a pharma-cological and transgenic approach suggest that increasing A2A re-ceptor signaling in the brain have important inhibitory actions onnicotine induced sensitization of locomotor responses and/ornicotine evoked conditioned locomotor activity. The mechanismmay involve a direct or indirect reduction of inhibitory D2-like re-ceptor signaling in the dorsal striato-pallidal GABA neurons and theaccumbenseventral pallidal GABA neurons. It is suggested thatsuch actions by A2A receptor agonists may help counteract theabuse actions of nicotine. It should be noted that the upregulationof a-7 nicotinic receptors seen upon continuous treatment withnicotine is selectively counteracted in A2A receptor knockout mice(Metaxas et al., 2013). It remains to be determined if a-7 nicotine-A2A receptor interactions is involved in the current experimentsusing intermittent nicotine treatment.
Acknowledgments
Supported by the statutory funds of the Institute of Pharma-cology, Polish Academy of Sciences, Krakow (Poland), Faculty ofPharmacy, Jagiellonian University College of Medicine, Kraków(Poland), and Swedish Research Council (K2014-62X-00715-50-3).
References
Andersson, K., Fuxe, K., Agnati, L.F., 1981. Effects of single injections of nicotine onthe ascending dopamine pathways in the rat. Evidence for increases of dopa-mine turnover in the mesostriatal and mesolimbic dopamine neurons. ActaPhysiol. Scand. 112, 345e347.
Bachtell, R.K., Self, D.W., 2009. Effects of adenosine A2A receptor stimulation oncocaine-seeking behavior in rats. Psychopharmacology 206, 469e478.
Baldo, B.A., Koob, G.F., Markou, A., 1999. Role of adenosine A2 receptors in brainstimulation reward under baseline conditions and during cocaine withdrawalin rats. J. Neurosci. 19, 11017e11026.
Borroto-Escuela, D.O., Romero-Fernandez, W., Garriga, P., Ciruela, F., Narvaez, M.,Tarakanov, A.O., Palkovits, M., Agnati, L.F., Fuxe, K., 2013. G protein-coupledreceptor heterodimerization in the brain. Meth. Enzymol. 521, 281e294.
Brown, R.M., Short, J.L., 2008. Adenosine A(2A) receptors and their role in drugaddiction. J. Pharm. Pharmacol. 60, 1409e1430.
Castañé, A., Soria, G., Ledent, C., Maldonado, R., Valverde, O., 2006. Attenuation ofnicotine-induced rewarding effects in A2A knockout mice. Neuropharmacology51, 631e640.
Ciruela, F., Gómez-Soler, M., Guidolin, D., Borroto-Escuela, D.O., Agnati, L.F., Fuxe, K.,Fernández-Dueñas, V., 2011. Adenosine receptor containing oligomers: theirrole in the control of dopamine and glutamate neurotransmission in the brain.Biochim. Biophys. Acta 1808, 1245e1255.
Di Chiara, G., Imperato, A., 1988. Drugs abused by humans preferentially increasesynaptic DA concentrations in the mesolimbic system of freely moving rats.Proc. Natl. Acad. Sci. U.S.A. 85, 5274e5278.
EMCDDA, 2011. Annual Report: the State of the Drugs Problem in Europe.Everitt, B.J., Robbins, T.W., 2005. Neural systems of reinforcement for drug addic-
tion: from actions to habits to compulsion. Nat. Neurosci. 8, 1481e1489.
J. Jastrzebska et al. / Neuropharmacology 81 (2014) 318e326326
Filip, M., Frankowska, M., Zaniewska, M., Przegali�nski, E., M}uller, E., Agnati, L.,Franco, R., Roberts, D.C., Fuxe, K., 2006. Involvement of adenosine A2A anddopamine receptors in the locomotor and sensitizing effects of cocaine. BrainRes. 1077, 67e80.
Filip, M., Zaniewska, M., Frankowska, M., Wydra, K., Fuxe, K., 2012. The importanceof the adenosine A(2A) receptor-dopamine D(2) receptor interaction in drugaddiction. Curr. Med. Chem. 19, 317e355.
Frankowska, M., Filip, M., Przegali�nski, E., 2007. Effects of GABA(B) receptor ligandsin animal tests of depression and anxiety. Pharmacol. Rep. 59, 645e655.
Frankowska, M., Marcellino, D., Adamczyk, P., Filip, M., Fuxe, K., 2013. Effects ofcocaine self-administration and extinction on D2 -like and A2A receptorrecognition and D2 -like/Gi protein coupling in rat striatum. Addict. Biol. 18,455e466.
Fuxe, K., Andersson, K., Härfstrand, A., Agnati, L.F., 1986. Increases in dopamineutilization in certain limbic dopamine terminal populations after a short periodof intermittent exposure of male rats to cigarette smoke. J. Neural Transm. 67,15e29.
Fuxe, K., Ferre, S., Canals, M., Torvinen, M., Terasmaa, A., Marcellino, D.,Goldberg, S.R., Staines, W., Jacobsen, K.X., Lluis, C., Woods, A.S., Agnati, L.F.,Franco, R., 2005. Adenosine A2A and dopamine D2 heteromeric receptorcomplexes and their function. J. Mol. Neurosci. 26, 209e219.
Fuxe, K., Marcellino, D., Genedani, S., Agnati, L., 2007. Adenosine A(2A) receptors,dopamine D(2) receptors and their interactions in Parkinson’s disease. Mov.Disord. 22, 1990e2017.
Giménez-Llort, L., Schiffmann, S.N., Shmidt, T., Canela, L., Camón, L., Wassholm, M.,Canals, M., Terasmaa, A., Fernández-Teruel, A., Tobeña, A., Popova, E., Ferré, S.,Agnati, L., Ciruela, F., Martínez, E., Scheel-Kruger, J., Lluis, C., Franco, R., Fuxe, K.,Bader, M., 2007. Working memory deficits in transgenic rats overexpressinghuman adenosine A2A receptors in the brain. Neurobiol. Learn. Mem. 87, 42e56.
Haber, S.N., Fudge, J.L., McFarland, N.R., 2000. Striatonigrostriatal pathways in pri-mates form an ascending spiral from the shell to the dorsolateral striatum.J. Neurosci. 20, 2369e2382.
Imperato, A., Mulas, A., Di Chiara, G., 1986. Nicotine preferentially stimulatesdopamine release in the limbic system of freely moving rats. Eur. J. Pharmacol.132, 337e338.
Knapp, C.M., Foye, M.M., Cottam, N., Ciraulo, D.A., Kornetsky, C., 2001. Adenosineagonists CGS 21680 and NECA inhibit the initiation of cocaine self-adminis-tration. Pharmacol. Biochem. Behav. 68, 797e803.
Marcellino, D., Roberts, D.C., Navarro, G., Filip, M., Agnati, L., Lluís, C., Franco, R.,Fuxe, K., 2007. Increase in A2A receptors in the nucleus accumbens afterextended cocaine self-administration and its disappearance after cocainewithdrawal. Brain Res. 1143, 208e220.
Metaxas, A., Al-Hasani, R., Farshim, P., Tubby, K., Berwick, A., Ledent, C., Hourani, S.,Kitchen, I., Bailey, A., 2013. Genetic deletion of the adenosine A2A receptor
prevents nicotine-induced upregulation of a7, but not a4b2 nicotinic acetyl-choline receptor binding in the brain. Neuropharmacology 71, 228e236.
Mora, F., Segovia, G., Del Arco, A., 2008. Glutamate-dopamine-GABA interactions inthe aging basal ganglia. Brain Res. Rev. 58, 340e353.
Paxinos, G., Watson, C., 1998. The Rat Brain in Stereotaxic Coordinates, fourth ed.Academic Press, San Diego.
Pontieri, F.E., Tanda, G., Orzi, F., Di Chiara, G., 1996. Effects of nicotine on the nucleusaccumbens and similarity to those of addictive drugs. Nature 382, 255e257.
Popoli, P., Betto, P., Reggio, R., Ricciarello, G., 1995. Adenosine A2A receptor stim-ulation enhances striatal extracellular glutamate levels in rats. Eur. J. Pharmacol.287, 215e217.
Popova, E., Krivokharchenko, A., Ganten, D., Bader, M., 2002. Comparison betweenPMSG- and FSH-induced superovulation for the generation of transgenic rats.Mol. Reprod. Dev. 63, 177e182.
Sanchis-Segura, C., Spanagel, R., 2006. Behavioural assessment of drug reinforce-ment and addictive features in rodents: an overview. Addict. Biol. 11, 2e38.
Shen, H.Y., Canas, P.M., Garcia-Sanz, P., Lan, J.Q., Boison, D., Moratalla, R., Cunha, R.A.,Chen, J.F., 2013. Adenosine A2A receptors in striatal glutamatergic terminals andGABAergic neurons oppositely modulate psychostimulant action and DARPP-32phosphorylation. PLoS One 8, e80902.
Stolerman, I.P., Mirza, N.R., Shoaib, M., 1995. Nicotine psychopharmacology:addiction, cognition and neuroadaptation. Med. Res. Rev. 15, 47e72.
Tanganelli, S., Sandager Nielsen, K., Ferraro, L., Antonelli, T., Kehr, J., Franco, R.,Ferré, S., Agnati, L.F., Fuxe, K., Scheel-Krüger, J., 2004. Striatal plasticity at thenetwork level. Focus on adenosine A2A and D2 interactions in models of Par-kinson’s Disease. Park. Relat. Disord. 10, 273e280.
Trifilieff, P., Rives, M.L., Urizar, E., Piskorowski, R.A., Vishwasrao, H.D., Castrillon, J.,Schmauss, C., Slättman, M., Gullberg, M., Javitch, J.A., 2011. Detection of antigeninteractions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 51, 111e118.
Weerts, E.M., Griffiths, R.R., 2003. The adenosine receptor antagonist CGS15943reinstates cocaine-seeking behavior and maintains self-administration in ba-boons. Psychopharmacology (Berl.) 168, 155e163.
World Health Organization, 2006. Tobacco: Deadly in any Form or Disguise.Wydra, K., Golembiowska, K., Zaniewska, M., Kami�nska, K., Ferraro, L., Fuxe, K.,
Filip, M., 2013. Accumbal and pallidal dopamine, glutamate and GABA overflowduring cocaine self-administration and its extinction in rats. Addict. Biol. 18,307e324.
Wydra, K., Suder, A., Filip, M., 2012. Effects of adenosine A2A receptor ligands oncocaine self-administration and relapse in rats. Pharmacol. Rep. 64, 480e481.
Zaniewska, M., McCreary, A.C., Wydra, K., Filip, M., 2010. Differential effects of se-rotonin (5-HT)2 receptor-targeting ligands on locomotor responses to nicotine-repeated treatment. Synapse 64, 511e519.