L Hondeghem and B G Katzung myocardial conduction. Test ...
-
Upload
khangminh22 -
Category
Documents
-
view
0 -
download
0
Transcript of L Hondeghem and B G Katzung myocardial conduction. Test ...
L Hondeghem and B G Katzungmyocardial conduction.
Test of a model of antiarrhythmic drug action. Effects of quinidine and lidocaine on
Print ISSN: 0009-7322. Online ISSN: 1524-4539 Copyright © 1980 American Heart Association, Inc. All rights reserved.
is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231Circulation doi: 10.1161/01.CIR.61.6.1217
1980;61:1217-1224Circulation.
http://circ.ahajournals.org/content/61/6/1217the World Wide Web at:
The online version of this article, along with updated information and services, is located on
http://circ.ahajournals.org//subscriptions/
is online at: Circulation Information about subscribing to Subscriptions:
http://www.lww.com/reprints Information about reprints can be found online at: Reprints:
document. Permissions and Rights Question and Answer information about this process is available in the
located, click Request Permissions in the middle column of the Web page under Services. FurtherEditorial Office. Once the online version of the published article for which permission is being requested is
can be obtained via RightsLink, a service of the Copyright Clearance Center, not theCirculationpublished in Requests for permissions to reproduce figures, tables, or portions of articles originallyPermissions:
by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from
Test of a Modelof Antiarrhythmic Drug Action
Effects of Quinidine and Lidocaineon Myocardial Conduction
LUC HONDEGHEM, M.D., PH.D., AND BERTRAM G. KATZUNG, M.D., PH.D.
SUMMARY The effects of quinidine and lidocaine on the maximum upstroke velocity (Vmax) of the ven-tricular myocardial action potential were compared with the effects predicted by a model over a wide range ofdriving rates, rhythm disturbances and holding potentials. These rate-, rhythm- and voltage-dependent effectswere accurately predicted by the proposed model. The model was also able to predict several previously un-documented properties of the drugs: 1) If lidocaine decreases Vmax of a pulse train, the steady state is reachedwithin a few action potentials. 2) The poststimulation recovery of Vmax in the presence of lidocaine or quinidinecan occur in a multiexponential fashion, if the membrane potential is kept at the potential where both the fast(operating mainly at more negative membrane potentials) and the slow (operating at more positive potentials)recovery processes are operative. 3) Hyperpolarization markedly attenuates the rate-dependent drug effects. 4)Combinations of lidocaine and quinidine have a superadditive effect on the Vmax of early extrasystoles.
THE EFFECT of antiarrhythmic drugs on the max-imum upstroke velocity (Vmax) of the cardiac actionpotential (AP) is generally considered an importantfactor in the therapeutic actions of such drugs.1 Ac-cording to present concepts of excitable membranefunction, antiarrhythmic drugs diminish Vmax byblocking sodium channels.2 In fact, as first proposedby Weidmann,3 Vmax is the best available experimen-tal measure of sodium conductance in cardiac cells.4The reported effects of antiarrhythmic agents on Vmaxare, however, quite different for different drugs." 2Moreover, for any individual agent the drug effect onVmax is a complicated function of the experimentalconditions,5 e.g., rate,6-8 number of action potentialsat any particular rate,9 extrasystoles and their timerelation to the steady driving rate,'0 11 extracellularpotassium concentration and resting membranepotential.'0 Such rate-, voltage- and time-dependentdrug interactions with transmembrane channels havealso been described for local anesthetics in other ex-citable tissues.'2-15
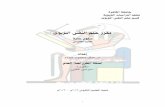
Recently, we proposed a model,2 the parameters forwhich were derived from previously published reports.This model (fig. 1) proposes that antiarrhythmic drugsinteract with membrane sodium channels in the rested(R), activated (A) and inactivated (I) states, and thateach of these interactions is characterized by anassociation (k) and dissociation (1) rate constant. Thetransitions between the rested, activated and inac-tivated states are governed by standard Hodgkin- and
From the Department of Pharmacology, University of CaliforniaSchool of Medicine, San Francisco, California.
Supported by grants from the University of California, San Fran-cisco Academic Senate, P.M.A.F., the American Heart Associa-tion, and the National Institutes of Health.
Address for correspondence: Luc M. Hondeghem, M.D., Ph.D.,Department of Pharmacology, University of California, San Fran-cisco, California 94143.
Received February 21, 1979; revision accepted November 29,1979.
Circulation 61, No. 6, 1980.1217
Huxley-type equations, but inactivation parameters ofthe drug-associated channels are shifted to morenegative potentials by an amount AV (voltage shift).Finally, we postulate that drug-associated channels donot conduct, even in the activated state; i.e., they areblocked.The published data used for the original derivation
of the model did not extend over the full range of rateand rhythm, and in some cases were available only forthe normal resting potential. However, an-tiarrhythmic drugs are frequently used in tissues ex-hibiting tachycardias, bradycardias or extrasystoles,and in tissues depolarized by ischemia. We thereforeinvestigated the effects of quinidine and lidocaine onVmax over a full range of membrane potentials, extra-systolic recovery intervals and driving rates. Apreliminary report has been presented.16
Materials and MethodsThe experimental results were obtained from right
ventricular papillary muscles of 14 guinea pigs. Theanimals were stunned by cervical dislocation and theirhearts quickly excised and transferred to a cold saltsolution. The preparations were mounted in a three-compartment, single-sucrose-gap chamber'7 in such away that less than 1 mm of myocardium protrudedinto the test chamber. The test chamber was perfusedat about 10 ml/min with a solution having the follow-ing composition (mM): NaCl 145, KCI 4, CaCl, 1.8,MgCl, 1.1 and glucose 5. The solution was bufferedwith 5 mM Tris and titrated to a pH of 7.35. The mid-dle compartment was perfused with an isotonicsucrose solution enriched with 0.025 mM CaCl2, whilethe current-injection chamber was perfused with anisotonic KCI solution. All solutions were bubbled with100% oxygen and the temperature was kept at36-37°C. The preparations were allowed toequilibrate for at least 1 hour before starting the ex-periment.Transmembrane potentials were recorded using
by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from
VOL 61, No 6, JUNE 1980
HH
UNBLOCKED: HR-- HH- C
+ DRUG - DRUG kRl 1 R
BLOCKED:
kA [Ak1T
HH' 3 HH' (
a HH'
Constants:Lidocaine:
Quinidine kR= °
kR = 04 kA = 5 104 kI = 50 (msecM-')
R = 1.0 1A = 1.5 1I = 2 10-3 (msec')kA = 2.6.104 kl = 0 (msec' M')
R = 0.05 1A = 2.7 1I = 6.10 5 (msec')
FIGURE 1. Diagram of mechanism of action of an-
tiarrhythmic drugs. R = rested; A = activated; I = inac-tivated drug-free fractions of the population of sodiumchannels; R', A' and I' = the respective states for the drug-associated fractions; HH = standard Hodgkin-Huxley rateconstants, HH' = same rate constant but with the inactiva-tion parameters shifted by an amount A V due to drugassociation, kRJ kA and k, represent the association rate con-
stants, while 1R, IA and 1, represent the dissociation rate con-
stants for the respective channel fractions.
standard 3M KCI-filled glass microelectrodes con-nected to high-input impedance and variable capacityneutralization amplifiers. The upstroke of the actionpotential was electronically differentiated using a cir-cuit that is linear from 10-1000 V/sec. After ap-propriate filtration it was fed into a sample-and-holdpeak detector."8 The output of the latter wasdelivered to a pen recorder and a digital voltmeter.The outputs of the differentiator and the peak detectorwere both continuously monitored on oscilloscopes,together with the transmembrane potential. Polaroidfilm was used for photographing the oscilloscopetraces. In the present experiments we measured theVmax and transmembrane potential of a single cell sothat nonuniformities in the preparation wouldminimally affect the results.To study rate-dependent effects, preparations were
driven by 20-second pulse trains of different ratesalternating with 20-second rest periods. Since depres-sion of Va,,x is maximal at higher driving rates, initialtrains of 5-Hz pulses were used in each experiment.Effects on Vmax were then recorded at progressivelyslower rates until a rate was found at which furtherslowing produced no increase in Vmax.To determine kinetics of poststimulation recovery
Of Vmax the preparation was subjected to 20-secondpulse trains of a fixed rate (1 Hz) and long enough (20action potentials) so that a quasi-steady-state Vmaxwas attained. These trains were separated by 20-second rest periods. After the last action potential in atrain, a single stimulus was applied at variable inter-vals. The latter interval ranged from the shortestpossible to one long enough to allow full recovery of
Vmax or 20 seconds, whichever was less. Incrementswere adjusted so that the changes in Vmax forsucceeding intervals were less than 10%. All stimuliwere obtained from an automatic increment/decre-ment programmable digital stimulator (MDC, P.O.Box 115, Novato, California 94947). The pulses con-sisted of 1-3-msec rectangular pulses of 1.2-1.5 timesdiastolic threshold strength.The membrane potential was depolarized by adding
aliquots of KCI to the perfusate of the anteriorchamber until the desired membrane potential was ob-tained . Alternatively, hyperpolarization anddepolarization were obtained by current injectionthrough the sucrose gap.19
Determinations were made before addition of drug,after 30 minutes of equilibration with the agent, and 1hour after washing out the drug. Lidocaine HCI andquinidine gluconate were used. All data were obtainedfrom single-cell impalements for the duration of theexperiments. Only preparations in which the latency(time between the onset of the stimulus and the up-stroke of the action potential) was less than 5 msecwere accepted in the present study. In addition, allpreparations in which VY,,,,, varied more than 5% as afunction of stimulus strength (1.2-1.5 times threshold)were rejected from the study; 16 of 30 preparationswere rejected.A wide range of drug concentrations was studied,
from therapeutic to toxic levels, to obtain the mostrigorous possible test of the model. Cumulative dosesfor both drugs included 2, 4, 8, 16 and 32 ,ug/ml. In sixexperiments quinidine was the first and only drug usedbecause it did not readily wash out. In the eight otherexperiments lidocaine was the first drug. Since all thelidocaine effects could readily be reversed by a wash-out period, we then superfused the preparations withquinidine.
For the prediction of the drug effects on Vnax weused the model equations and parameters describedpreviously2 and summarized in figure 1. This modelcomputes the predicted sodium conductance (GNa) asbeing directly proportional to the fraction of channelsin the activated (A) state (fig. 1). The model similarlycomputes the fraction of channels in the other poolsusing a straightforward set of differential equations.For each simulation in the present study the programwas given the membrane potential, drug-rate con-stants and concentration, and the stimulus pattern.Graphic displays of the predicted GN,, values werecompared with the normalized experimentallyobserved V,,, by plotting both sets of data on thesame time scale.
ResultsThe decrease of Vm,,,x by quinidine or lidocaine is a
function of the drug and its concentration, drivingrate, time elapsed since the previous action potential(i.e., poststimulation recovery) and the transmem-brane potential. Because these factors operatesimultaneously, we designed our experiments toisolate the effects of individual variables as shownbelow.
1218 CIRCULATION
by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from
ANTIARRHYTHMIC DRUG ACTION/Hondeghem and Katzung
B .25 Hz A .5 Hz
GNa
Vmax
a E
8 I Hz c 2Hz D
I 11 jE111141J1:171J111 ~~~~~~1111011|1111111E1111111 1 1 1 § Ei ]]1 M1!I MI1F 1111l11J!
F
GNa
Vmax
G IEj II: ;:11 : '1.| '''1-- lllllillllll
IEH11j!itltiESl:E: il11(((1711111111111111111111111111111~~
:'O V I h II
B I J K L
L LLLAAk Jk L{K -T'\
GNa ]
Vmax lflFIGURE 2. Comparison of the model-predicted (GNa) and experimentally observed (Vmax) rate-dependentdrug effects. C = control; Q =quinidine gluconate 1.5 X 10-5 M (8 .g/ml); L = lidocaine hydrochloride 3 X10-5 M (8 gg/ml); B = fraction ofsodium channels blocked; GNa = model-predicted sodium conductance;v%max = experimentally observed maximum upstroke velocity of the action potential. Vertical calibrationmarks: B = 0-{.S; GNR = 040.5; $max = 0-150 V/sec. Horizontal calibration is 10 seconds.
Driving rate markedly influences the decrease ofVmax by both quinidine and lidocaine. However, onlysteady-state data were available for lidocaine6 andonly one study of the rate of onset of quinidine effectshas been published.9 Because computer simulationsusing the model predicted a surprising differencebetween the two drugs in the rate of development ofblock during a train of action potentials, it was es-
pecially important to obtain beat-by-beat Vmax datafor the two drugs under identical conditions. Thepredicted G,, and observed Vmax effects oftherapeutic concentrations of lidocaine and quinidineduring pulse trains at the normal resting potential forseveral different driving rates are shown in figure 2. Atdriving rates slower than 0.1 Hz (not shown), and nor-mal transmembrane potential, therapeutic concen-
trations of the drugs elicit little or no activation-dependent decrease of Vmax. At fast rates (2 Hz andfaster) both drugs decrease steady-state Vmax substan-tially. However, whereas this rate-dependent effectbecomes significant at driving rates faster than 0.2 Hzfor quinidine, for lidocaine the driving rate must ex-
ceed 1 Hz in order for Vm,ax to decrease. The predic-tions of the rate-dependent drug effects on steady-stateGNa are in good agreement with the experimental
Vmax results shown. Similar results were obtained inall experiments and these results are comparable tothe rate-dependent drug effects described by otherlaboratories.6 7As noted above, the model unexpectedly predicted
that in the presence of lidocaine, if GN,, declines at all,it should quickly decrease to a quasi-steady level,whereas this steady state should develop rather slowlyin the presence of quinidine (compare figures 2H and2L). Experimentally, this slow, progressive decline forquinidine has been documented previously for a singledriving rate;9 no such data were available for lidocaineat any rate in any cardiac tissue. As shown in figure 2,under all experimental conditions where lidocainedecreased Vmax, a quasi-steady-state "max wasreached in three or fewer action potentials. This was
much faster than for quinidine, in which 20-50 actionpotentials were required to reach a steady state.
Figures 2L and 3L show that Vmma,x after an initialrapid (one or two action potentials) decline, continuedto decline at a much slower rate for the remainder ofthe train. Similar apparent deviations were frequentlyobserved at fast driving rates in preparations in whichthe diastolic membrane potential was not controlled.This slow decline of Vmax is caused by the slow
C
Q
1219
Illrli,
by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from
VOL 61, No 6, JUNE 1980
B 120mV A 1lOOmV B -85mV c -75mV D]CJ
GNo, I 1
Vmax]
B E * F -~.1l f1 1 T1
G'N ||\\i|)|] ilLlllll1 illlllllll llllltllll
Vmox~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
II]
GN1
GNa 1 t11il 11
J jt ' K
Vmox
]i l
FIGURE 3. Comparison of the model-predicted (GNa) and experimentally observed ( v'ma)voltagedependence ofdrug effects. Hyperpolarization and depolarization were elicited by current injection. Abbre-viations and calibrations the same as for figure 4. In panel E the asterisk indicates that this panel was ob-tained at -108 mV instead of -100 mV, because at the former potential the difference between GNa andV,m,X was maximal (see text).
depolarization of the resting potential that accom-
panies fast driving rates in guinea pig papillarymuscle.6 Indeed, when the depolarization was includedin our computer simulation, it also showed a slightprogressive decline of GNa of a magnitude similar tothat observed in the experiments. Conversely, if dur-ing the experiment the diastolic potential was clampedat its original value, no such additional decline of Vmaxwas observed. We therefore conclude that this slowdecline of Vmax is solely due to depolarization of thepreparation.The above rate-dependent effects of quinidine and
lidocaine are accentuated by depolarization andattenuated by hyperpolarization.
Poststimulation recovery of Vmax is another impor-tant parameter in characterizing antiarrhythmic drugaction.10 Figure 4 is a comparison of the model predic-tions of GNa and the experimentally observedpoststimulation recovery of Vmax in the presence oflidocaine or quinidine at a normal resting potential.Whereas under control conditions recovery of GN, or
Vmax was complete in a few milliseconds, this process
was greatly slowed in the presence of lidocaine or
quinidine. Both the model and the experimental
results show that poststimulation time to 50%recovery was a few hundred milliseconds for lidocaineand was prolonged to several seconds for quinidine(fig. 4). The experimental results were very similar inall preparations and resembled closely those alreadypublished for quinidine9 and lidocaine.10
However, the data of the last two studies citedsuggest that poststimulation recovery is a mono-exponential process. In contrast, the model predictedthat, at least at membrane potentials where part of theblocked channels are in the IF pool and others are inthe R' pool, dissociation of the drug from the sodiumchannels should occur along two pathways, R' to Rand I' to I (fig. 1). Since the rate constants of these twodissociation routes are quite different (R' to R is muchfaster than I' to I), the model predicted that this post-stimulation recovery should not necessarily occur witha single time constant, as previously proposed.10 In thepresent experiments there appeared to be twoprocesses in the poststimulation recovery of Vmax: afast process that was dominant at more negative mem-brane potentials and a slow one that was dominant atdepolarized (less negative than -80 mV) membranepotentials. At intermediate potentials we could some-
L
L
I]
1220 CIRCULATION
MI 11 11M11111111111111
by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from
ANTIARRHYTHMIC DRUG ACTION/Hondeghem and Katzung
B 300ms A 700ms B 3000nms
B E
GNVa
Vmax |
B I g_ J L K MMtL]
I
FIGURE 4. Comparison of the model-predicted (GNa) and experimentally observed (P'max) drug effects onpoststimulation recovery. Column headings refer to the interval between the last regular train stimulus andthe extrasystole-evoking stimulus. Abbreviations and calibrations are the same as for figure 2, except Q =
quinidine gluconate 3 X 10-5 M (16 .g/ml); L = lidocaine hydrochloride 6 X 10M (16 gg/ml); max = 0-137V/sec.
times observe both simultaneously. We have pre-viously published an example of this for quinidine,20and we observed multiple time constants in thepoststimulation recovery of Vmax in four of our pres-ent lidocaine experiments. The fast (hyperpolarized)and slow (depolarized) time constants are not alwaysresolved simultaneously in the -80- to -100-mVrange probably because the fast recovery process is somuch faster than the slow one that it tends todominate whenever it is present. However, careful ad-justment of the membrane potential allows the dem-onstration of both processes in any single preparation.The clear poststimulation recovery of Vmax in the
presence of quinidine contrasts with a previousreport,'0 and the reason for this discrepancy is an-ticipated by the proposed model. Two experimentalfactors in the protocol of Chen et al., according to themodel, would minimize such recovery: 1) post-stimulation recovery was measured for only a fewhundred to 1000 msec, whereas the time constant ofthis recovery process is several seconds; and 2) sinceonly a small fraction of the channels associates withquinidine per action potential and since the drivingrate was slow, i.e., 0.2 Hz (allowing most of the drugto dissociate from the channels), there was little rate-
dependent block, and therefore little poststimulationrecovery.Transmembrane potential. The effects of
depolarization on antiarrhythmic drug action are veryimportant.6' 81, 10, 21 However, we could find only tworeports of the effects of hyperpolarization on the ac-tion of these drugs. Weidmann,3 in a classic study ofquinidine in Purkinje fibers, and Weld and Bigger,22 ina voltage-clamp study of lidocaine on the same tissue,showed that hyperpolarization resulted in significantreversal of drug-induced block. The previous studiesrelated to steady-state conditions only and no datawere available for ventricular myocardium at hyper-polarized potentials.
Figure 3 is a comparison of the model predictionsand the experimentally observed effects of quinidineand lidocaine at a fixed rate and dose, but at variousresting potentials. For this comparison we chose a fastdriving rate (3.3 Hz), because at slower driving ratesthe drugs have little or no activation-dependent effectsat potentials more negative than -90 mV.
Lidocaine simulation at 3.3 Hz indicated that theactivation-dependent decrease of GNa should beminimal at potentials more negative than -107 mV,i.e., increase as the membrane potential becomes more
GNa flilililillSlillil ttt!181111111111111Vmax ||||| |1|| Iltjlll]11
c 7000ms_D
F
IMM..
I]
G
L
Ht
I
GNOH
Vmax
1221
111111111
by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from
VOL 61, No 6, JUNE 1980
positive and reach 50% at -80 mV. The experimentaldata of figure 3 (panels I to L) show that at -120 mVlidocaine elicited no activation-dependent decrease ofVmax. However, at -100 mV a substantial decreasewas already present, and it reached 48% at -85 mV.At more positive potentials, Vmax declined steeply andthe preparation was unable to follow this stimulationrate at -70 mV. These experimental results are thusin good agreement with the model predictions (fig. 3, Ito L). Similar results were obtained in all experiments.
For quinidine, the model predicted that at 3.3 Hz,hyperpolarization must exceed -140 mV to preventany activation-dependent decline of GNa. At -120 mVit was predicted that the activation-dependentdecrease of GNa should be less than 2%, while a declineof 50% was predicted at -98 mV. The experimentallyobserved effects of quinidine were qualitatively verysimilar to these predictions, but they were sometimesshifted by a few millivolts toward more negativepotentials, especially when hyperpolarizing currentwas passed. Such a shift is expected, especially inpreparations that require relatively large clampingcurrents. Indeed, when current is passed, the measuredpotential at the microelectrode tip is the sum of thetransmembrane potential and the voltage drop acrossthe extracellular series resistance.23 Because ofinward-going rectification, cardiac tissue requiresmore current to hyperpolarize than to depolarize.Therefore, the measured change in transmembranepotential may be somewhat erroneous, especially dur-ing large hyperpolarizing clamps. The largest suchdeviation we observed occurred in the experimentshown in figure 3. In this experiment the decline ofmax at - 120 mV was 8% larger than predicted by the
model. The deviation between the predicted GNa andthe observed Vmax values was noticeable from - 125 to-90 mV and reached a maximum of 23% at -108 mV(see figure 3F). However, this deviation between themodel predictions and the experimental results couldbe accounted for by a 6-mV potential voltage dropacross the extracellular series resistance. At - 100 mV(not shown) the predicted decline of GNa was 43%,while the observed decrease of Vmax was 54%; thedifference between the two was negligible at morepositive potentials (fig. 3, G and H). At -73 mV thepreparation could no longer follow the stimulationrate. In all experiments the results were similar to thecomputer predictions: Hyperpolarization attenuatedwhile depolarization accentuated the drug effects onVmax-
Discussion
Antiarrhythmic drugs act by their effects uponautomaticity, refractoriness and conduction.' One im-portant parameter of refractoriness and conduction isthe sodium current, which in cardiac tissue can mosteasily be measured as Vmax.2 (Refractoriness and con-duction are, however, also a function of membranepotential, membrane resistance and threshold poten-tial.)To compare the predictions of the computer model
with the present experimental results, we used the
model constants derived from previously published ex-perimental literature.2 The close concordance of thesimulation results and the present experimental resultssuggests that there is considerable homogeneityamong experimental preparations of the type used andthat the model as formulated readily tolerates minordifferences between preparations. Most important, themodel can predict drug effects under previously un-tested conditions.
According to the present results the actions oflidocaine and quinidine on V,max can be described asfollows:
Upstroke of the action potential. Both drugs havefast interaction rate constants for the activated (open)state (time constants of a fraction of a millisecond), sothat during each activation the channels reach ap-proximate equilibrium between the open (A and A',fig. 1) states. Since the affinity of lidocaine for theopen channel is much larger than that of quinidine(fig. 1), the fraction of channels that becomes blockedper activation is much larger for lidocaine than forquinidine (figs. 2-4).
Plateau of the action potential. The transitionsbetween the inactivated states (I and I', fig. 1) arerelatively slow (time constants of several hundredmilliseconds for lidocaine and several seconds forquinidine), so that during the short time of the plateaurelatively few channels block/unblock under mostconditions (figs. 2-4).
Repolarized action potential. The drug-free inacti-vated channels recover (I to R) according to standardHodgkin-Huxley type kinetics. The drug-associatedinactivated channels behave as if the transmembranepotential is decreased by 30-40 mV; similar shiftshave been observed for local anesthetics in nerve.'2Therefore, unless hyperpolarized, their recovery (I' toR') is slowed and reduced, while at slightlydepolarized membrane potentials recovery is com-pletely prevented. Blocked recovered channels (R')quickly unblock (the R-R' rate constants are fast andthe R states have low affinity for the drugs). In con-trast, blocked inactivated (I') channels only slowly un-block (see above) or not at all (see below). In this waychannels may accumulate in the I' pool, especially atfast heart rates (fig. 2), close extrasystolic coupling in-tervals (see figure 4) and in depolarized cells (fig. 3).Also, since unblocking from the inactivated state oc-curs more slowly for quinidine than for lidocaine,quinidine will trap channels at slower driving rates(fig. 2) and for longer coupling intervals (fig. 4) thanlidocaine.
Selective depression of depolarized cells has beenproposed as an important action of antiarrhythmicdrugs.21 The present model provides for at least twoimportant mechanisms for selective depression: 1)since depolarized cells unblock slowly through the IFto I route, whereas more polarized cells can in addi-tion unblock through a much faster route (1' to R' toR), it is clear that at physiologic rates and coupling in-tervals, the fraction of channels trapped in the I' statewill increase with depolarization. 2) In addition to theprevious activation-dependent selective depression,lidocaine also possesses a high affinity for the inac-
1222 CIRCULATION
by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from
ANTIARRHYTHMIC DRUG ACTION/Hondeghem and Katzung
tivated state. Our discussion will relate to how theeffects of antiarrhythmic drugs on the fast inwardcurrent may be relevant to conduction in specificarrhythmias. (Clearly these drugs also affect thedifferent outward current channels and autonomic in-fluences, all of which in turn affect automaticity,refractoriness, and conduction.) We assume that anti-arrhythmic drugs should degrade conduction in"healthy" tissues as little as possible but preferentiallymodify the "sick" conduction that causes reenttantarrhythmias in such a way as to abolish arrhythmias.Conduction can be either improved or depressed. Un-fortunately, the available antiarrhythmic drugs onlyreliably depress conduction. It was initially proposedthat type II antiarrhythmic drugs could improve con-duction,24 but this has not been confirmed by many in-vestigators.69 10, 25, 26Premature beats. During diastole the effect of anti-
arrhythmic drugs on the sodium channelsprogressively declines. For lidocaine this recoveryprocess is nearly complete in 700 msec, whereas forquinidine little or no recovery occurs during the nor-mal diastolic interval (fig. 3). Thus, while quinidine(2.5 gg/ml) can effectively depress conduction of im-pulses arising at any time during diastole, lidocaine(5 ,g/ml) is mainly effective early in diastole.The model suggests (fig. 5) that a therapeutic com-
bination of quinidine and lidocaine provides a moreeffective depression of premature conduction than theequivalent therapeutic concentration of quinidinealone. For this simulation we graphically combinedthe effects of 5 ,ug/ml of lidocaine with those of 2.1,ug/ml of quinidine. This combination provides thesame depression of the regular beat (1000 msec) as 2.5,g/ml of quinidine alone. However, it provides con-siderable extra depression of early extrasystoles. Forexample, an extrasystole occurring 50 msec afterrepolarization (dashed line in fig. 5) is depressed 27%by lidocaine (5 ,g/ml) alone, 33% by quinidine (2.5,ug/ml) alone, but 52% by the quinidine and lidocaine(2.1 gg/ml plus 5 ,ug/ml, respectively) combination.
75 -
50 -
Vmax
25 -
0 -
CONTROL
LIDOCAINE 5jpg/miQUINIDINE 2.1pg/miQ. 25,ug/miL 5pg/mI + Q. 2 lpg/mlExtra Depression by Q pl
_-I_
0 100 200 300 400
DIASTOLIC INTERVAL (msec.)
Thus, this combination allows depression of earlydiastolic conduction to a greater extent than eitherdrug can achieve alone, while not depressing conduc-tion of the regular action potential beyond the depres-sant action of quinidine alone. This is especially im-portant because therapeutic concentrations oflidocaine are well tolerated even in the presence ofhigh therapeutic concentrations of quinidine.27 29Results of a preliminary experiment testing thisprediction are shown in figure 6. In this experiment,Vmax of the early extrasystole was depressed 13% bylidocaine alone, 25% by quinidine alone, but 79% bythe combination of drugs. In contrast, the steady-state(1 Hz) Vmax was depressed by only 35% by the com-bination of drugs. Because the conditions of thispreliminary experiment were not identical to those ofthe computer simulation we cannot make firm con-clusions from the data; however, the prediction of themodel appears to be supported. For clinical applica-tion, the optimal dose combination of quinidine andlidocaine must be derived from clinical studies, andeach patient will probably have to be individuallytitrated. However, due to its high affinity for de-polarized tissue, lidocaine would be expected to bemore effective if such tissue is crucial to anarrhythmia. In arrhythmias that do not involvedepolarized tissue, quinidine might be expected to bemore effective because its action is less voltage-dependent. Finally, the early diastolic extra-depression should not be limited to quinidine andlidocaine, but should be obtainable by combining any"slow" agent with any "fast" agent.Our results indicate that a model of antiarrhythmic
drug action based on a single mechanism of action canpredict the effects of both quinidine and lidocaine onVmax over the full range of driving rates andpoststimulation recovery intervals in both normallypolarized and depolarized myocardial cells. Manyeffects predicted by the model were extrapolations intoranges previously not covered experimnentally.Moreover, although some of the predictions were
FIGURE 5. Computer-predicted effects oftherapeutic concentrations of quinidine pluslidocaine on ,max of extrasystoles. The
- t-- shaded area indicates the extra depression ofextrasystolic Vmax that can be attained by acombination of quinidine and lidocaine.This combination does not cause depressionOf $Vmax of the regular beat beyond thatobserved in the presence of quinidine alone.This extra depression provides the anti-arrhythmic activity necessary to abolish cer-tain reentrant arrhythmias (see Discussionsection).
lus L1 Jf00
500 1000
1223
Clq
by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from
VOL 61, No 6, JUNE 1980
CONTROL . LID 6
.
t
somewhat unexpected (e.g., two-beat equilibration forlidocaine; multiexponential poststimulation recoveryof Vmax), all were supported by the experimental data.Finally, application of the model can result inclinically testable hypotheses.
AcknowledgmentWe thank L. Trapp and C. Dunnebacke for typing the manuscript
and C. Cotner for making the illustrations.
References
1. Sasyniuk B, Ogilvie R: Antiarrhythmic drugs: electro-physiological and pharmacokinetic considerations. Ann RevPharmacol 15: 131, 1975
2. Hondeghem LM, Katzung BG: Time and voltage dependent in-teractions of antiarrhythmic drugs with cardiac sodiumchannels. Biochim Biophys Acta 472: 373, 1977
3. Weidmann S: The effects of the cardiac mnembrane potential onthe rapid availability of the sodium-carrying system. J Physiol127: 213, 1955
4. Hondeghem LM: Validity of Vmax as a measure of the sodiumcurrent in cardiac and nervous tissues. Blophys J 23: 147, 1978
5. Dreifus LS, de Azevado IM, Watanabe Y: Electrolyte and anti-arrhythmic drug interaction. Am Heart J 88: 95, 1974
6. Chen C, Gettes L: Combined effects of rate, membrane poten-tial and drugs on maximum rate of rise (Vmax) of action poten-tial upstroke of guinea pig papillary muscle. Circ Res 38: 464,1976
7. West TC, Amory DW: Single fiber recordings of the effects ofquinidine at atrial and pacemaker sites in the isolated rightatrium of the rabbit. J Pharmacol Exp Ther 130: 183, 1960
8. Jensen R, Katzung B: Electrophysiological actions ofdiphenylhydantoin on rabbit atria. Circ Res 26: 17, 1970
9. Heistracher P: Mechanism of action of antifibrillatory drugs.Naunyn Schmiedebergs Arch Pharmacol 269: 199, 1971
10. Chen C, Gettes LS, Katzung BG: Effects of lidocaine andquinidine on steady-state characteristics and recovery kineticsof (dV/dt) max in guinea pig ventricular myocardium. CircRes 37: 20, 1975
11. Ducouret P: The effect of quinidine on membrane electrical ac-tivity in frog auricular fibers studied by current and voltageclamp. Br J Pharmacol 57: 163, 1976
12. Courtney KR: Mechanism of frequency-dependent inhibition ofsodium currents in frog myelinated nerve by the lidocainederivative GEA 968. J Pharmacol Exp Ther 195: 225, 1975
13. Hille B: Local anesthetics: hydrophilic and hydrophobic
FIGURE 6. Preliminary experimental testof a quinidine-lidocaine combination inguinea pig papillary muscle. Each of thefour panels shows an oscilloscope display ofa steady-state (l = H,) action potential withan early extrasystole (nominally 15-msecdiastolic interval). A t the bottom ofeach ac-tion potential is its upstroke velocity inV/sec (captured in the experiment by arsample-and-hold amplifier and read out indigitalform). Upper left. responses in drug-free perfusate. Upper right. responses dur-ing superfusion with 6 gg/ml lidocaine.Lower left. responses after washout of lido-caine and exposure to quinidine, 4 gg/ml.Lower right. after adding lidocaine, 6gg/ml, to the superfusate containing 4gg/ml quinidine.
pathways for drug-receptor reaction. J Gen Physiol 69: 497,1977
14. Adams PR: Voltage jump analysis of procaine action at frogend plate. J Physiol 268: 291, 1977
15. Cahalan MD: Local anesthetic block of sodium channels innormal and pronase treated squid giant axons. Biophys J 23:285, 1978
16. Hondeghem LM, Katzung BG: Prediction of the rate, rhythmand voltage dependent effects of quinidine and lidocaine on theupstroke (Vmax) of the cardiac action potential. (abstr) 59th An-nual Meeting of the Am H Assoc, 1977
17. Reuter H, Scholtz H: Uber der Einfluss der extracellularenCa-konzentration auf Membranpotential und Kontraktionisolierter Herzpraparate bei graduierter Depolarisation.Pfluegers Arch 300: 87, 1968
18. Hondeghem LM, Cotner CL: Measurement of Vmax of thecardiac action potential with a sample/hold peak detector. AmJ Physiol 234: H312, 1978
19. Katzung B: Effects of extracellular calcium and sodium ondepolarization-induced automaticity in guinea pig papillarymuscle. Circ Res 37: 118, 1975
20. Hondeghem LM, Katzung BG: A unifying molecular model onthe interaction of antiarrhythmic drugs with cardiac sodiumchannels: application to quinidine and lidocaine. Proc WestPharmacol Soc 20: 253, 1977
21. Hondeghem LM, Grant AO, Jensen RA: Antiarrhythmic drugaction: selective depression of hypoxic cardiac cells. Am HeartJ 87: 602, 1974
22. Weld FM, Bigger JT Jr: Effects of lidocaine on the early inwardcurrent in sheep cardiac Purkinje fibers. Circ Res 37: 630, 1975
23. Goldman Y, Morad M: Measurement of transmembranepotential and current in cardiac muscle: A new voltage clampmethod. J Physiol 268: 613, 1977
24. Bigger JT, Mandel WJ: Effect of lidocaine on conduction incanine Purkinje fibers and at the ventricular muscle-Purkinjefiber junction. J Pharmacol Exp Ther 172: 239, 1970
25. Singh BN, Vaughan-Williams EM: Effect of altering potassiumconcentration on the action of lidocaine and diphenyl-hydantoin on rabbit atrial and ventricular muscle. Circ Res 24:286, 1971
26. Allen JD, Brennan FJ, Wit AL: Actions of lidocaine on trans-membrane potentials of subendocardial Purkinje fibers surviv-ing in infarcted canine hearts. Circ Res 43: 470, 1978
27. Kaplinsky E, Yahini JH, Barzilai T, Neufeld HN: Quinidinesyncope. Report of a case successfully treated with lidocaine.Chest 62: 764, 1972
28. Harrison DC: Should lidocaine be administered routinely to allpatients after acute myocardial infarction. Circulation 58: 581,1978
1224 CIRCULATION
by guest on April 29, 2014http://circ.ahajournals.org/Downloaded from