Homocysteine and its determinants in relation to ... - DiVA-Portal
-
Upload
khangminh22 -
Category
Documents
-
view
1 -
download
0
Transcript of Homocysteine and its determinants in relation to ... - DiVA-Portal
Homocysteine and its determinants in relation to cardiovascular risk factors
and myocardial infarction
Elisabet Söderström
Department of Medical Biosciences, Clinical Chemistry Umeå, 2022
This work is protected by the Swedish Copyright Legislation (Act 1960:729) Dissertation for PhD Elisabet Söderström ISBN: 978-91-7855-730-1 (print) ISBN: 978-91-7855-731-8 (pdf) ISSN: 0346-6612, New Series No: 2171 Cover design: Inhousebyrån, Umeå University. Rubus arcticus and pedicularis sceptrum carolinum. Electronic version available at: http://umu.diva-portal.org/ Printed by: Cityprint i Norr AB Umeå, Sweden 2022
i
Table of Contents
Abstract ............................................................................................ iii Background ..................................................................................................................... iii Aims ................................................................................................................................ iii Material and methods .................................................................................................... iii Results ............................................................................................................................. iii Conclusions ...................................................................................................................... iv
Abbreviations .................................................................................... v List of original papers ..................................................................... vii Enkel sammanfattning på svenska ................................................. viii
Bakgrund ....................................................................................................................... viii Hypoteser och mål ........................................................................................................... ix Material och metoder ....................................................................................................... x Resultat ............................................................................................................................ xi Slutsatser .......................................................................................................................... xi
Background ........................................................................................ 1 Homocysteine ................................................................................................................... 1 Determinants of homocysteine ........................................................................................3 Cardiovascular disease and myocardial infarction.......................................................... 5 Atherosclerosis and atherothrombosis ............................................................................ 5 Risk marker, risk determinant and risk factor ................................................................ 7
Causality ..................................................................................................................... 7 Risk factors of cardiovascular disease ............................................................................ 8 Homocysteine, its determinants, and cardiovascular disease ...................................... 14
Aims ................................................................................................. 19 Materials and Methods ................................................................... 20
Study population............................................................................................................ 20 The Västerbotten Intervention Programme, VIP ......................................................... 20 The Northern Sweden MONICA project ....................................................................... 20 The mammography screening project, MSP ................................................................. 21 Questionnaires ................................................................................................................ 21 Blood collection .............................................................................................................. 21 Study cohort and design I, III, and IV .......................................................................... 22 Study variables I, III, and IV ......................................................................................... 23 Study cohort and design II ............................................................................................ 24 Study variables II ........................................................................................................... 24 Preanalytical factors ...................................................................................................... 26 Biochemical methods ..................................................................................................... 27 Polymorphisms analysis ................................................................................................ 28 Statistics ......................................................................................................................... 30 Ethical considerations ................................................................................................... 32
Results ............................................................................................ 33
ii
Paper I ............................................................................................................................ 33 Paper II........................................................................................................................... 34 Paper III ......................................................................................................................... 34 Paper IV ......................................................................................................................... 38
Discussion ........................................................................................ 41 Main findings .................................................................................................................. 41
General considerations ................................................................... 46 Methodological aspects ................................................................................................. 46 Sex differences ............................................................................................................... 49 Homocysteine as a risk determinant of MI ................................................................... 51
Conclusions and future perspectives .............................................. 53 Acknowledgments ........................................................................... 56
Financial support ........................................................................................................... 58 References ...................................................................................... 59
iii
Abstract
Background Globally, cardiovascular diseases (CVD), including myocardial infarction (MI) and stroke, are the leading cause of illness and death and constitute a significant part of the disease burden in Sweden and Western Europe. Age, hypertension, smoking, obesity, dyslipoproteinemia, diabetes, and impaired renal function are considered established risk factors for CVD. However, these factors do not explain all MI cases, and much is still unknown. Homocysteine is a sulfur-containing amino acid used in clinical practice as a biomarker of functional vitamin B12 and folate status. Earlier observational studies have shown associations with higher plasma homocysteine concentrations (tHcy) and CVD. The causal relationship between tHcy and MI has been debated as homocysteine-lowering prevention studies have not shown a protective effect on MI, although there may be a protective effect on ischemic stroke. Still, tHcy is a prognostic biomarker or risk determinant of MI. There is a need for more knowledge on the pathophysiologic mechanisms of how homocysteine interacts with its determinants, and other risk factors, on the risk of MI.
Aims The overall aim of the thesis was to expand knowledge about how homocysteine, as a risk determinant, can have an impact on cardiovascular disease. Specifically, the purposes were to explore the associations between tHcy, determinants of homocysteine and risk factors of CVD, and the associated risk of prospectively developing a first-ever MI.
Material and methods In papers I, III, and IV, a prospective incident nested case-referent study design was used with 545 cases of MI and 1054 matched referents. In paper II the design was cross-sectional, comparing strictly defined smokers and snus users. All study subjects emanated from the Northern Sweden Health and Disease Study (NSHDS). Blood samples stored frozen at -80ºC were later thawed, and analyses of biomarkers for renal function, lipids, B-vitamins, tHcy, cotinine, and genetic polymorphisms related to homocysteine metabolism were performed.
Results In a prospective setting, folate, but not tHcy, was positively associated with apolipoprotein A1 (Apo A1). The association was seen among referents and not among those later developing an MI.
iv
Among strictly defined smokers and snus users, cotinine was positively associated with tHcy among smokers but not among snus users, despite higher cotinine concentrations in snus users. No association was observed between tHcy and the number of cigarettes/day. The CTH G1208T and MTHFR A1298C polymorphisms were, among women, associated with a higher risk of developing a first-ever MI with a fatal outcome. No such associations were seen among men or all MI patients. Further, no associations were seen between the MTHFR C677T polymorphism and the risk of having an MI, fatal or non-fatal. Mild impairment of renal glomerular function defined by eGFRcystatin C
/eGFRcreatinine ratio and the associated risk of MI is previously not studied prospectively. In the present study, a lower eGFRcystatin C/eGFRcreatinine ratio was associated with a higher risk of later developing a first-ever MI among women, both when analyzed as a continuous variable and across the quartiles of the ratio. These associations did not appear among men.
Conclusions The independent association of folate but not tHcy with Apo A1 emphasizes the need to adjust for possible confounding effects in studies on homocysteine and endpoints or biomarkers. The results suggest a possible link between one-carbon metabolism and lipid metabolism. The independent association between cotinine and tHcy in smokers and not among snus users indicate that nicotine per se may not mediate higher tHcy concentrations. Cotinine concentrations in plasma appeared as a better predictor of tHcy than self-reported smoking data. Thus, whenever possible, self-reported smoking should be supplemented by biomarkers, such as cotinine, in epidemiological studies. After outcome stratification, fatal or non-fatal MI, the associated higher risk among women of a fatal MI and CTH G1208T and MTHFR A1298C polymorphisms, respectively, may indicate that women with the minor alleles risk having a more serious MI leading to death than women with the wild-type alleles. In a prospective setting, the eGFRcystatin C/eGFR creatinine ratio was associated with an increased risk of later developing a first-ever MI among women. The eGFRcystatin C/eGFRcreatinine ratio may be a tool, easily implemented at clinical laboratories, for evaluating the risk of having a future first-ever MI.
v
Abbreviations
AMI acute myocardial infarction
Apo A1 apolipoprotein A1
Apo B apolipoprotein B
BMI body mass index
CAD coronary artery disease
CBS cystathionine β-synthase
CI confidence interval
CHD coronary heart disease
CKD chronic kidney disease
CRP C-reactive protein
CSE cystathionine-gamma-lyase
CVD cardiovascular disease
DBP diastolic blood pressure
ECM extra cellular matrix
eGFR estimated glomerular filtration rate
H2S hydrogen sulfide
HDL-C high density lipoprotein cholesterol
HPLC high-pressure liquid chromatography
IDMS isotope depletion-mass spectrometry
LDL-C low density lipoprotein cholesterol
vi
LC-MS/MS liquid chromatography/tandem mass spectrometry
Lp(a) lipoprotein(a)
MI myocardial infarction
MMA methylmalonic acid
MONICA Monitoring of Trends and Determinants in Cardiovascular Disease
MR Mendelian randomization
MTHFR methylenetetrahydrofolate reductase
NSHDS Northern Sweden Health and Disease Study
NO nitric-oxide
OR odds ratio
RR risk ratio
SAH S-adenosyl-homocysteine
SAM S-adenosyl-methionine
SBP systolic blood pressure
SD standard deviation
SNPs single nucleotide polymorphisms
tHcy total plasma homocysteine
THF tetrahydrofolate
VIP Västerbotten Intervention Project
WHO World Health Organization
vii
List of original papers
I. Söderström E, Eliasson M, Johnson O, Hallmans G, Weinehall L, Jansson JH, Hultdin J. Plasma folate, but not homocysteine, is associated with Apolipoprotein A1 levels in a non-fortified population. Lipids Health Dis. 2013 May 22;12:74.
II. Söderström E, Nilsson TK, Schneede J, Ueland PM, Midttun Ø, Gylling B, Johansson I, Hultdin J. Plasma Cotinine Is Positively Associated with Homocysteine in Smokers but Not in Users of Smokeless Tobacco. Int. J. Environ. Res. Public Health 2021, 18, 11365.
III. Söderström E, Andersson J, Söderberg S, Van Guelpen B, Nilsson TK, Hultdin J. CTH G1208T, and MTHFR A1298C polymorphisms are associated with a higher risk of a first myocardial infarction with fatal outcome among women. (Submitted)
IV. Söderström E, Blind R, Wennberg P, Andersson J, Söderberg S, Nilsson TK, Hultdin J. Mild impairment of renal function (shrunken pore syndrome) is associated with increased risk of a future first-ever myocardial infarction in women. Scand J Clin Lab Invest. 2021 Oct;81(6):438-445
The published articles are available as open access and reprinting are permitted.
viii
Enkel sammanfattning på svenska
Bakgrund Hjärtinfarkt och stroke står för en stor del av sjukdomsbördan i Sverige. I vår västerländska befolkning har hjärt- och kärlsjukligheten minskat under 2000-talet men hör fortfarande till de sjukdomar som orsakar mest sjukdom och dödlighet globalt. Välkända riskfaktorer som ålder, högt blodtryck, övervikt, rökning, diabetes och blodfettsrubbningar anses kunna förklara en stor andel av insjuknandet, men det finns familjer som drabbas hårdare än andra och det är fortfarande mycket som inte är förklarat eller känt.
Homocystein är en aminosyra som bildas i kroppen och används i klinisk rutin i sjukvården som biomarkör för B-vitamin brist. I tidigare observationsstudier har man dessutom sett en ökad risk för hjärtkärl sjukdom vid förhöjda homocysteinnivåer. Det är inte klarlagt att homocystein i sig själv är en orsakande faktor för insjuknande i hjärtinfarkt men det är en klar prognostisk biomarkör och det är möjligt att den påverkar insjuknandet i stroke. Det är därmed intressant att undersöka hur homocystein kan bidra till den ökade risken, via andra riskfaktorer eller möjliga mekanismer. De viktigaste faktorerna som påverkar nivån av homocystein är folat och vitamin B12 men även till exempel njurfunktionen kan påverka koncentrationen i blodet.
Hjärtkärlsjukdom föregås i blodkärlen av en process som kallas åderförkalkning eller åderförfettning. Det innebär att den innersta kärlväggen i artären byggs upp av fett och fibrös vävnad och bildar till slut ett plack. Åderförkalkning har som begrepp använts länge men det är en komplex process där mycket fortfarande är okänt. Den sker över lång tid innan det leder till sjukdom och det är därför fördelaktigt att kunna studera material som är insamlat innan individer har fått symtom och blivit sjuka, vilket möjliggörs med biobanksmaterial.
Blodfettpartiklar finns i olika former i blodet och mäts till exempel som total kolesterol, low-density lipoprotein (LDL) kolesterol och high-density lipoprotein (HDL) kolesterol. I dessa lipoproteiner finns också apolipoproteiner. Apolipoprotein A1 (Apo A1) hittar man framför allt i HDL fettmolekyler och cellstudier har visat att genuttrycket av Apo A1 påverkas av homocystein. Ett möjligt samband mellan högre nivåer av HDL/Apo A1 och lägre risk för hjärtkärlsjukdom har setts. Tidigare har HDL kallats det goda kolesterolet och gör det fortfarande ibland. Enligt senare kunskap är det inte entydigt sant längre. Apolipoprotein B (Apo B) finns bland annat i LDL fettmolekyler och högre nivåer är direkt relaterade till en ökad risk för att insjukna i hjärt-kärlsjukdom.
ix
Rökning är en välkänd riskfaktor för hjärt-kärlsjukdom och i cigaretter finns nikotin tillsammans med många andra komponenter. Studier har visat att rökare har högre nivåer av homocystein än icke-rökare men orsaken är inte känd. Det är heller inte välstuderat hos snusare. Genom att mäta kotinin som är en nedbrytningsprodukt av nikotin i blod kan man jämföra sambanden hos rökare och snusare och studera om nikotinet är det som möjligen leder till högre homocystein nivåer.
Det finns några vanliga genvarianter i enzymer som påverkar homocysteinomsättningen men också bildandet av divätesulfid. Divätesulfid är ett ämne i kroppen som på liknande sätt som kväveoxid påverkar hjärtkärlfunktionen. Kväveoxid används i klinisk rutin bland annat som akut behandling i samband med kärlkramp och hjärtinfarkt medan kliniska behandlingsstudier med divätesulfid pågår i dagsläget.
Nedsatt njurfunktion är en känd riskfaktor för hjärt-kärlsjukdom. Njurfunktionen har länge bedömts med hjälp av biomarkören kreatinin i blodet, och de senaste 15 åren har även cystatin C införts som njurfunktionsmarkör i klinisk rutin inom sjukvården. Formler har utarbetats och förfinats för att beräkna (estimera) njurfunktionen i form av glomerulär filtrations hastighet (eGFR). De senaste åren har man beskrivit nedsatt njurfunktion med ett nytt begrepp; ”krympt por syndrom” och det kan liknas vid att porerna i njuren skrumpnar. Kreatinin och cystatin C påverkas olika av de skrumpna porerna, och genom att mäta dessa ämnen i blodet och jämföra dem i form av en beräknad kvot (eGFRcystatin C/eGFR kreatinin), kan de användas som ett tidigt tecken på nedsatt njurfunktion och diagnostik av krympt por syndrom. Inga prospektiva studier med avseende på eGFRcystatin C/eGFR kreatinin kvoten och hjärtinfarkt är gjorda tidigare.
Hypoteser och mål Det övergripande målet med avhandlingen var att öka kunskapen om hur homocystein som riskdeterminant kan vara relaterat till hjärt-kärlsjukdom hos kvinnor och män. Syftet var att utforska sambanden mellan homocystein, dess determinanter och kända riskfaktorer för hjärt-kärlsjukdom samt undersöka sambanden med risken att insjukna i en första hjärtinfarkt.
De specifika målen i arbetena var:
1. Att undersöka om homocystein och dess viktigaste determinanter folat, vitamin B12 och genvariationer är associerade med Apo A1 och om sambanden skiljer mellan personer som senare insjuknar i hjärtinfarkt jämfört med de personer som inte drabbas av hjärtinfarkt.
x
2. Att studera om rökning påverkar homocysteinkoncentrationer i blodplasma mer än vad snus gör. Samt att undersöka om kotinin koncentrationer är starkare kopplat till homocystein än själv-rapporterad mängd rökning.
3. Att studera om vanliga genvariationer i enzymer som påverkar homocysteinomsättningen även påverkar risken att senare i livet insjukna i en första hjärtinfarkt och om det är någon skillnad i sambanden för dem som avlidit av sin hjärtinfarkt inom 28 dagar.
4. Att undersöka om ett nytt mått för njurfunktion ”krympt-por syndrom” kvot är associerat med risken att senare i livet insjukna i en första hjärtinfarkt.
Material och metoder Studierna utgår från Northern Sweden Health and Disease Study (NSHDS), en insamlad biobank med tillhörande enkätdata och blodprover tänkta att kunna sparas och användas framöver. Materialet kommer från individer i Norrbotten och Västerbotten och är insamlat via befolkningsstudierna Västerbotten Intervention Programme (VIP), MONItoring of trend and Determinants in CArdiovascular Disease survey (MONICA) och Mammography Screening Project (MSP). Vid deltagande i befolkningsstudierna besvaras enkäter om sjukdomshistoria, aktuella mediciner och livsstils frågor. Blodtryck, längd och vikt mäts, samt kolesterol och blodsocker analyseras. Med detta upplägg kan man gå tillbaka för att undersöka vad som kan finnas i blodet innan sjukdom inträffar.
I tre av delarbetena (I, III och IV) studerade vi 545 individer som drabbats av hjärtinfarkt (fall). Alla var undersökta och hade lämnat blodprov innan de insjuknat. Dessa jämfördes mot 1054 personer (kontroller) som inte drabbats av hjärtinfarkt och med motsvarande ålder, kön, ort och tidpunkt för deltagande i NSHDS. Hjärtinfarkt diagnoserna genomgicks och klassades enligt gemensamma WHO kriterier. Information om förekomst av riskfaktorer, sjukdomshistoria och aktuell medicinering hämtades in.
Delarbete II utformades som en tvärsnittsstudie utgående från 1375 personer. Enkätdata för rökning och snusning samt information om förekomst av riskfaktorer analyserades. Vi grupperade strikt in de som endast rökte, respektive snusade eller aldrig hade använt tobak.
I delarbete III studerade vi även gruppen bland hjärtinfarkter som inneburit att patienterna avlidit inom 28 dagar och undersökte sambandet med vanliga variationer i några gener som påverkar homocystein men också tillgängligheten av divätesulfid.
xi
Material från biobanken användes till båda studiepopulationerna. Efter förvaring i lågtempererade frysar (-80º C) tinades blodproverna och njurfunktionsprover, blodfetter, B-vitaminer, homocystein, kotinin och genvariationer analyserades.
Resultat Artikel I: Homocystein var inte associerat med mängden blodfettpartiklar med Apo A1 utan istället var Apo A1 associerat med högre nivåer av folat. Detta samband sågs hos personer i kontrollgruppen som inte drabbats av hjärtinfarkt. Artikel II: Högre nivåer av nikotins nedbrytningsprodukt kotinin var associerat med högre nivåer av homocystein hos rökare men inte hos snusare. Kotinin i plasma hade ett tydligare samband med homocystein jämfört med självrapporterad mängd rökning.
Artikel III: För kvinnor sågs ett samband mellan vanliga genvarianter för enzymer som påverkar homocysteinomsättningen och ökad risk att drabbas av en hjärtinfarkt med dödlig utgång. Motsvarande samband sågs inte bland män.
Artikel IV: En lätt nedsatt njurfunktion uttryckt som ”krympt-por syndrom” kvot var för kvinnor associerat med en ökad risk att senare i livet insjukna i hjärtinfarkt. Ett tydligare samband sågs mellan den beräknade kvoten och risken jämfört med om kreatinin eller cystatin C beräkningar användes separat. Bland män sågs inte samma samband.
Slutsatser De slutsatser som avhandlingen visar, är att en av homocysteins viktigaste determinanter, folat, är relaterad till mängden av blodfettpartiklar med Apo A1 hos de som inte insjuknar i hjärtinfarkt. Detta indikerar att studier om homocystein vid sjukdom behöver ta hänsyn till dess determinanter. Resultaten stödjer också en möjlig påverkan av folat på genuttrycket av Apo A1.
Kotinin har ett samband med homocystein bland rökare men inte snusare, vilket talar för att det inte är nikotinet i tobak som orsakar högre nivåer av homocystein hos rökare utan sannolikt andra substanser som bildas när man röker tobak. Att homocystein hade ett samband med kotinin i plasma men inte till självrapporterad mängd rökning visar på behovet av att objektivt mäta biomarkörer som komplement till enkätdata.
Sambandet mellan vanliga genvariationer i enzymer som påverkar homocysteinomsättningen och ökad risk att drabbas av en hjärtinfarkt med
xii
dödlig utgång sågs bland kvinnor men inte män. Fynden talar för att de biokemiska processerna som leder till hjärtinfarkt kan skilja sig något åt hos män och kvinnor.
Lätt nedsatt njurfunktion uttryckt som sänkt kvot för krympt-por syndrom var associerat med ökad risk att insjukna i en första hjärtinfarkt för kvinnor men inte män. Kvoten kan vara en möjlig markör för hjärtkärlsjukdom i framtiden.
1
Background
Homocysteine Homocysteine is a sulfur-containing amino acid derived from methionine, an essential amino acid in the diet. It was first identified in mentally disabled children with an inborn error of metabolism; homocystinuria, in 1962 by Carson and Neill (1). The reduced function in the enzyme cystathionine β-synthase (CBS), causing very high homocysteine levels detected as homocystin in urine, was later described (2). This patient group suffered from premature atherosclerosis, stroke, and other forms of thromboembolism (3). In 1969 McCully presented the idea that moderately high homocysteine concentrations might be related to CVD, based on a comparison of a patient with an abnormality in vitamin B12 metabolism and a patient with CBS deficiency. The vascular pathology observations were very similar, and both patients had elevated homocysteine, though abnormality in separate metabolic pathways led to this conclusion.
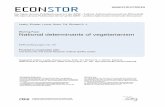
Homocysteine is formed in the methionine and folate cycles as part of the one-carbon metabolism providing methyl groups (one-carbon units) (see Figure 1). The methyl groups are essential for cellular function and are involved in synthesizing DNA, polyamines, amino acids, creatine, and phospholipids (4). The methionine and folate cycle occurs in all tissues in mammals (5).
Figure 1. Homocysteine metabolism; the folate and methionine metabolic cycle, and the transsulfuration pathway. Enzymes:1=Methionine adenosyltransferase, 2=S-Adenosylmethinonine-dependent methyltransferase, 3=S-Adenosylhomocysteine hydrolase, 4=5-Methyltetrahydrofolate:homocysteine methyltransferase, 5=Betaine:homocysteine methyltransferase, 6= cystathionine β-synthase (CBS), 7= Cystathionine gamma-lyase, 8=Serine hydroxyl-methyltransferase, 9=5,10-methylenetetrahydrofolate reductase (MTHFR), 10=Tetrahydrofolate dehydrogenase
Homocysteine
Methionine
S-Adenosyl-methionine (SAM)
S-Adenosylhomocysteine (SAH)
Methylcobalamin
5-Methyl tetrahydrofolate
Cystathionine
Cysteine
Tetrahydrofolate
9
5, 10-Methylene tetrahydrofolate
Used for DNA synthesis, reparations
CreatinineDNAProteinsPhospholipidsNeurotransmittorsMyelin sheaths
methylated products
Transsulfuration pathway
1
2
3
4
5
Betain
6
7
Dimethylglycine
PolyaminesDihydrofolate
10
8
Serine
Glycine
2
The transportation of methyl groups from folate passes through remethylation of homocysteine and conversion to S-adenosyl-methionine (SAM), the donor of methyl groups in most methylation reactions.
There are many genetic polymorphisms in methionine and folate metabolism, and rare mutations resulting in inborn errors of metabolism can also be found. They affect enzyme activity or receptor functions. The most studied gene in this metabolism is the methylenetetrahydrofolate gene. Two polymorphic variants, methylenetetrahydrofolate reductase (MTHFR) C677T rs1801133 and MTHFR A1298C rs1801131, were identified about 30 years ago and are shown to lead to a mild reduction in the enzyme function, which in turn is linked to mild and moderate hyperhomocysteinemia (6). Further on, as the MTHFR C677T polymorphism has a more pronounced effect on function, this polymorphism has most often been included in studies.
The transsulfuration pathway An alternative pathway for homocysteine is catabolism in the irreversible transsulfuration pathway. First, it is converted to cystathionine by CBS, dependent on vitamin B6 as a cofactor. In the next step, cystathionine is converted to cysteine by the B6-dependent enzyme cystathionine gamma-lyase (CSE), coded by the CTH gene. CSE can generate hydrogen sulfide (H2S) either from cysteine or homocysteine. These enzymes involved in the transsulfuration pathway are present in the liver, kidney, heart, vasculature, brain, and small intestine (7, 8), and they have been found in the circulation, secreted by endothelial cells and hepatocytes.
As H2S and cysteine are involved in a wide variety of physiological processes, the production is under strict control and critical for the maintenance of optimal cellular function. CSE can use homocysteine alone to generate H2S, and under conditions of hyperhomocysteinemia, it is responsible for the clearance of homocysteine, unlike CBS, which is unresponsive under these conditions (9). The transsulfuration pathway and the production of the peptide glutathione are linked as cysteine is one of the contained amino acids. Excesses of either SAM or homocysteine may also be excreted from the cell to the liver and kidney for extraction and metabolizing.
Homocysteine in plasma Most of the plasma homocysteine is bound to plasma proteins, mainly to albumin but exists physiologically also in reduced and oxidized (homocystin) forms (10). A small amount of homocysteine exists in free form. Current biochemical methods include a reduction process to determine the total amount of homocysteine (tHcy) in plasma (11).
3
In the literature, upper reference interval limits for fasting tHcy vary between 10-18 µmol/L for serum and plasma (12), and populations with folate enrichment often have a lower reference interval. In Sweden in the counties Norrbotten and Västerbotten, the origin of the study base used in this thesis, the reference interval at the Clinical Chemistry Departments is <15 µmol/L in serum and plasma for individuals from the ages of 12 years and above, and children 0-12 years, and pregnant women, <10 µmol/L. Albeit hyperhomocysteinemia is most often defined by tHcy values >15 µmol/L in scientific studies (13), an increase in risk can be observed at lower concentrations (14).
Determinants of homocysteine Vitamin B12 (cobalamin), and folate (vitamin B9), are the most critical determinants of tHcy. Homocysteine accumulates if there is a functional deficiency of vitamin B12, folate, or both. In most subjects, vitamin B6 is not a determinant of fasting tHcy. Elevated tHcy concentrations can be a consequence of all diseases interfering with the uptake of folate or B12, for example, atrophic gastritis, after gastric bypass, malabsorption in the small intestine, celiac disease, pancreatic disease, and inflammatory intestinal disease. Reduced hepato-biliary recirculation may also lead to B12 deficiency. Drugs that can interfere with B12 are gastric acid-blocking drugs such as proton pump inhibitors.
Vitamin B12 consists of a group of physiologically active substances with complex molecular structures, all involving a cobalt atom in the center. Vitamin B12 is transported in plasma by haptocorrin (previously transcobalamin I) and transcobalamin (previously transcobalamin II). Only the transcobalamin bound fraction can use receptor-mediated uptake in the cells and therefore is the bioactive fraction. Transcobalamin accounts for 16-20% of endogenous plasma vitamin B12, and the remaining B12 is bound to haptocorrin (15). Haptocorrin is released by granulocytes, but the function is unknown. Vitamin B12, bound to transcobalamin, is taken up by hepatocytes, stored in the liver, and released in blood to meet physiologic demands. The liver storage usually is sufficient for daily metabolic requirements for more than five years.
Reference intervals of serum or plasma vitamin B12 vary widely, depending on the laboratory and the method used. Deficiency has been defined as a concentration less than 150 pmol/L (16). It is to be noted that concentrations within reference intervals may not reflect adequate vitamin B12 status because tissue stores can be draining, while plasma concentrations may be maintained.
Folates are a family of compounds that function as coenzymes and methyl donors in the processing of one-carbon units (17). Folate in the diet is enzymatically hydrolyzed from polyglutamated forms to monoglutamates in the enterocyte
4
brush border. The monoglutamated forms are then absorbed in cells in the colon. After cellular uptake in the intestine, most folate enters the circulation as tetrahydrofolates (THF). Folate usually is present in various forms in human serum, but the dominant form is 5-methyl tetrahydrofolate (5-MTHF). If cobalamin deficiency is present, folate is trapped as 5-MTHF. Folic acid is commonly used in supplements.
Lifestyle factors have an impact on tHcy concentrations. Smoking is a known determinant, with reported higher tHcy concentrations among smokers (18-22) and lower concentrations of B-vitamins, including folate (22) and vitamin B12 (23). In smokers, the lower concentrations of folate may reflect a lower dietary intake of vegetables than in non-smokers (24).
Overconsumption of ethanol may influence tHcy. Dietary habits may be affected. A well-known fact is that high ethanol intake will reduce intestinal uptake of B-vitamins, including folate. There are also many effects on the liver (17), one resulting in reduced uptake of haptocorrin-bound B12 from plasma. Thus, recirculation of B12 via the bile fluid to the duodenum, followed by binding to intrinsic factor and receptor-mediated B12-uptake in the intestine, will be reduced. Thus, tHcy will increase despite a high B12 in plasma, which mainly consists of inactive haptocorrin-bound B12. The intestinal microbiota may also be affected which can have an effect on folate uptake (25).
The main genetic determinant of tHcy is the common variant in the MTHFR-gene, C677T (26). In Sweden, homozygotes for the minor T allele comprise about 8% of the population, see Table 1 in papers I and II, respectively. These subjects have higher tHcy, which is more pronounced in cases with low folate status (<7 nmol/L) or when P-Folate is in the lower range of the reference interval (<15 nmol/L). There are many more SNP:s influencing tHcy (27). Different variants of genes coding for enzymes in the metabolism of B12, folate, and B6 are also examples with known or possible influences on tHcy (26-28). Also, variations in receptors of cellular uptake of B12 and folate, for example, the soluble transcobalamin receptor, sCD320 (29), may affect tHcy concentrations.
The renal glomerular function influences tHcy (30). The association between tHcy and creatinine is complex. Methyl groups are needed for the synthesis of creatine. Thus, homocysteine may accumulate because of creatinine synthesis. Homocysteine may also increase due to a lower GFR, reflected by an increase in creatinine.
5
Cardiovascular disease and myocardial infarction Cardiovascular disease (CVD) includes diseases of the heart, vascular diseases of the brain, and diseases of blood vessels. CVD is the leading cause of all deaths in the world (31). The subgroup coronary heart disease (CHD) is the major cause of death and ischemic heart disease (IHD) the leading cause of all health loss globally, and in 2015 there was an estimation of 7.29 million acute MI (32). In 2020 in Sweden, CVD caused 27 973 deaths (33). A myocardial infarction (MI) occurs when the blood supply is reduced, and the affected myocardium becomes ischemic and necrotic. This is most often caused by thrombus formation in the lumen of coronary arteries.
Atherosclerosis and atherothrombosis Atherosclerosis manifests in all major vascular structures, and when it affects the hearth's own circulation, it can cause acute coronary syndromes, including MI. Stable angina, i.e., chest pain or discomfort caused by insufficient perfusion of the heart muscle, can be a consequence. Other manifestations are ischemic strokes and transient cerebral ischemic attacks. In the abdominal aorta, an aneurysm can be formed. The atherosclerotic process is a gradual, complex disorder of the arteries, evolving over time, and the exact pathophysiological process is still in many aspects unclear. Figure 2 shows an overview picture of atherosclerosis. It can be described in three phases; initiation, progression, and complications (34). The normal artery has a subendothelial trilaminar structure consisting of the intima, media, and adventitia. The healthy endothelium maintains balance in the vascular homeostasis via different functions, including constriction and dilatation of the blood vessels, anticoagulation, fibrinolysis, proliferation, and inhibition of smooth muscle cells, and leukocyte interactions (35). Endothelial cell dysfunction can impair the crucial regulation within the arterial wall. Nitric oxide (NO) is an important regulator, and its production process is subject to both transcriptional and post-translational regulation. H2S also has a prominent role in regulating endothelial and cardiovascular function (36). The atherosclerotic plaque is formed in the innermost layer of the intima. Initially, Low-density lipoprotein (LDL) particles accumulate and undergo oxidation and other modifications (34). Constituents of oxidized LDL particles may induce inflammation, and pro-inflammatory monocytes then enter the intima. Monocytes circulating in the bloodstream can bind to adhesion molecules expressed by activated endothelial cells. Chemoattractant cytokines, chemokines can promote the monocytes into the artery wall. Monocytes then mature into macrophages expressing scavenger receptors that can bind
6
lipoprotein particles and become foam cells. From the media, smooth muscle cells migrate into the intima and contribute to the accumulation of smooth muscle cells in the growing atherosclerotic plaque. During the progression of plaques, over the years, these cells can proliferate and produce extracellular matrix molecules such as collagen and elastin, as well as proteoglycans and glycosaminoglycans. This process leads to a thickening of the intimal layer and later the formation of an established plaque.
Figure 2. Atherosclerosis. By courtesy of Encyclopædia Britannica, Inc., copyright 2007; used with permission.
The leading cause of acute MI and sudden death is the rupture of vulnerable plaques in the coronary artery. Activated macrophages increase the production of the enzymes metalloproteinases that degrade the interstitial collagen. This destabilizes the fibrous cap, which increases the susceptibility of plaque rupture (37). Significant thrombus formation can alternatively result from superficial intima erosions without evidence of plaque rupture, which has been suggested to be a consequence of endothelial cell apoptosis with localized endothelial denudation.
7
Risk marker, risk determinant and risk factor Historically biomarkers have been used for many decades in etiologic and clinical research, beginning with seminal studies of infectious diseases followed by research on chronic diseases such as CVD (38). A biomarker is a biological observation, objectively measured and evaluated, that substitutes for a clinically relevant endpoint or intermediate outcome (39). Often a biomarker has the advantage of being more accessible and less expensive than direct measurement of the final clinical endpoint. Risk stratification of CVD could be improved when new variables are identified and used in conjunction with established risk factors in models (40). The term "risk" can be explained as a danger or hazard or the probability of suffering harm (41). The Swedish agency for health technology assessment and assessment of social services defines the term as "a characteristic or condition that indicates an increased risk of a person getting one or more diseases." A more distinct differentiation between related terms is (42):
• Risk marker = an attribute or an exposure that is linked to an increased risk of disease without a causal link.
• Risk determinant = an attribute or an exposure that is linked to an increased risk. A causal relationship may exist but has not been shown.
• Risk factor = risk determinant that is probably causally linked to increased disease risk and can modify the disease risk during an intervention.
Causality In epidemiological research, causality means that the exposure must always precede an effect in time. It is important to emphasize that correlation/association does not equal causation. To qualify as a causal risk factor, ideally, removal or modification of the factor will prevent or ameliorate the disease.
The classical essay by Sir Austin Bradford Hill describes nine perspectives that one should consider before deciding if an association is causally linked; strength, consistency, specificity, temporality, biologic gradient, plausibility, coherence, experimental evidence, and analogy (43). These criteria form the basis of the evaluation of causality. Still, the author Hill was ambivalent about their use and did not advocate the strict use of them, and common sense should also be applied, assessing cause and effect. Observational studies cannot prove causality. Randomized controlled trials (RCT) are considered the gold standard for establishing a causal relationship. However, to study the effect of biomarkers, an RCT is often not an alternative. One methodological approach to obtain a more reliable indication of a potential causal role of biomarkers is the use of Mendelian
8
randomization (MR) (44). The basis of MR studies is the principle of random allocation for genes, and if a gene variant is responsible for a higher concentration of a biomarker, the random allocation of the gene creates a randomized trial of low versus high concentrations of a biomarker. Since the conditions are set from birth and onward, long-term effects can be assessed. For tHcy, the MTHFR C677T polymorphism is an example (45), though there are limitations to consider regarding MR and homocysteine, compared to assumptions and the ideal model of MR depicted in Figure 3.
Figure 3. The ideal model of Mendelian randomization. No arrows are present between the gene and possible confounding factors, and the gene and the disease, respectively.
The term “reverse causality” describes the dilemma of “the chicken or the egg.” This is considered one of the most important biases in cardiovascular research and needs attention when conclusions about possible causal relationships are made (46). For the debated causal relationship between tHcy and CVD, a discrepancy has been noted between prospective and retrospective case-control studies. This may be due to reversed causation. Prospective studies minimize the risk of this bias.
Risk factors of cardiovascular disease The ongoing Framingham Heart study started in 1948 in the United States of America (USA) as a health research project in Framingham, Massachusetts. Together with other epidemiologic cohorts, they contributed in the second half of the 20th century to shift focus from treatment of established cardiovascular disease to the prevention of the disease in those at risk (47, 48). Later on, many prospective population-based and clinical studies have identified independent cardiovascular risk factors; increasing age, male sex, smoking, hypertension, dyslipidemia, obesity, and diabetes. Several of these are integrated into risk scores (49).
At the beginning of the 1980s in the USA, the Coronary Primary Prevention Trial, including men, demonstrated that cholesterol-lowering treatment reduces the
MTHFR(gene)
homocysteine (risk factor)
myocardial infarction(disease)
confounders
9
incidence of CHD (50). The cholesterol metabolism and particularly the role of the low-density lipoprotein (LDL) receptor were described by Joseph L. Goldstein and Michael S. Brown, rewarded with Nobel Prize in medicine in 1985 (51).
Between 1990 and 2017, the leading risk factors of overall early death and disability changed considerably (52), from being child wasting, short gestation for birth weight, and low birth weight for gestation, to the five leading risks in 2017; high systolic blood pressure (SBP), smoking, high fasting plasma glucose, high body-mass index (BMI), and short gestation for birthweight.
Lipids Dyslipidemia plays a central role in the development of atherosclerotic CVD, and lipid modification is a cornerstone to reducing cardiovascular risk (53). Lipids, whether synthesized or absorbed from the diet, must be transported to various tissues for lipid deposition, energy utilization, steroid hormone production, and bile acid formation. Due to their relatively aqueous insolubility, they are transported in plasma in macromolecular complexed lipoproteins. These complexes also contain apolipoproteins. Low-density lipoprotein cholesterol (LDL-C) in plasma is a measure of the cholesterol mass transported by LDL particles, the most frequent lipoprotein that carries apolipoprotein B (Apo B). Apo B contains a binding site that causes LDL-C to be deposited in tissues' extracellular matrix (ECM). In the ECM, LDL-C is prone to oxidation (54) and is involved in the atherosclerotic process. Apo B exists in two forms, apo B-100 and apo B-48, where apo B-100 is the most common in plasma in the fasting state. Every LDL particle has a single apo B polypeptide on it. The clinical benefit of LDL-C lowering therapies is determined by the reduction of the number of circulating LDL particles, and this is estimated by apo B. However, LDL particle mass is often proportional to the absolute reduction in the molarity of LDL particles as they will be concordant (55, 56). Evidence for the causal association of LDL-C and atherosclerotic CVD is considered compelling (57, 58). A log-linear relationship between the absolute change in LDL-C and the risk of atherosclerotic CVD has been demonstrated in epidemiological studies as well as in RCTs and MR studies (55, 59). Apolipoprotein A-II (Apo A-II), and A1 (Apo A1), in a ratio of 1:3, are the major proteins in high-density lipoprotein (HDL). HDL is previously described to be the most important part of reverse cholesterol transport, maintaining cholesterol homeostasis by removing excess cholesterol from peripheral cells to the liver. This process is more recently referred to as HDL-mediated trafficking
10
of cholesterol, and the pathway has been identified as more complex involving other lipoproteins as well. In contrast to the LDL-particle, which is associated with some other proteins (60), the HDL-particle is much more complex in function as it transports many different proteins. Recently, around 1000 HDL-associated proteins have been described (61). Besides proteins involved in lipid metabolism and transport, many other proteins are found engaged in hemostasis/protease inhibition, immune response, inflammation, metal binding, transport of fat-soluble vitamins, and binding to cells and heparin. The variable non-HDL is recently implemented in SCORE2 (49). This is an estimate of the total concentration of all apo B-containing lipoproteins. It is suggested that the reduced risk of atherosclerotic CVD seen with triglyceride-lowering treatment by fibrates is mediated by the changes in triglyceride-rich lipoproteins estimated by non-HDL (53, 59). It is a calculated parameter (total cholesterol - HDL cholesterol), which can be presented from clinical laboratories without further measurements. Lipoprotein(a), [Lp(a)] is recognized as one of the most proatherogenic lipoproteins. It is structurally related to LDL, and to each particle, one apo B-100 particle is linked (62). To this apo B-100, a carbohydrate-rich protein named apolipoprotein (a) [apo a] is bound with a single disulfide bond. The apo (a) can consist of different-sized isoforms, described as large, high molecular weight, or small, low molecular weight forms, depending on the number of kringle units. It is believed that LMW isoforms are more atherogenic than HMW, but probably due to their greater particle concentration than to the particle characteristics. The composition of Lp(a) in an individual is genetically determined (63). Lp(a) in mg/dL refers to the amount of cholesterol transported in Lp(a) particles. These methods do not discriminate between a few big particles vs. a high number of small particles (64). The method measuring nmol/L, i.e., number of particles, is less influenced by isoform size. Therefore, there is a discordance between Lp(a) mass and Lp(a) particles, a situation similar to that of LDL vs. apo B. There are no established lipid-lowering treatments to reduce Lp(a) specifically, but there are ongoing trials. The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors increase the numbers of LDL-cholesterol receptors on the surface of liver cells by blocking the receptor degradation and thereby increasing the turn-over of LDL-C in plasma. They have been shown to reduce Lp(a) levels (65), and this might independently reduce the CHD risk (66).
Hypertension Hypertension is an established risk factor of CVD, and high blood pressure treatment will decrease the risk of CVD, heart failure, atrial fibrillation, chronic kidney disease, and peripheral arterial disease (67). In recent years, the definition of hypertension has remained as SBP ≥140 mmHg and or diastolic blood pressure
11
(DBP) ≥ 90 mmHg (68). More intensive blood pressure lowering, including SBP below 140 mmHg, has shown additional benefits in high-risk patients (69).
Tobacco use Smoking is one of the major preventable lifestyle-related risk factors for CVD. Globally it is responsible for 10% of all CVD cases (70). Smoking delivers nicotine increases inflammation, thrombosis, and oxidation of low-density lipoprotein cholesterol (71). Other key processes in the smoking-induced atherogenesis initiation and endothelial dysfunction include a decrease of high-density lipoprotein (70).
The associated risk between CVD and moist smokeless tobacco, snus is less studied than smoking. It is mainly a Swedish phenomenon but is used in Nordic countries, and is becoming popular in the USA as well, e.g., in sports circles. A pooled analysis of observational studies on snus use and MI risk showed no associated risk (72), but an increased risk of ischemic heart disease (IHD) deaths may exist. Meta-analyses on the risk of IHD and acute MI specifically have found no excess risk among snus users (73, 74). In recent years, new forms of tobacco “heat-not-burn tobacco products” have been introduced on the market, sometimes launched as less harmful than combusted nicotine products. Current evidence suggests they are not without risk for cardiovascular health (75).
Diabetes Mellitus The two primary forms of diabetes are type 1 (T1DM) and type2 (T2DM), of which the latter is much more common. Worldwide the prevalence of diabetes continues to increase, and in 2017, 60 million adult Europeans were estimated to have T2DM, half of them undiagnosed (76). Predictions are made that more than 600 million individuals will develop T2DM By 2045, and the effects of this condition on cardiovascular health create further public health challenges. T1DM usually occurs at earlier ages and is caused by the destruction of pancreatic islet β-cells, leading to insulinopenia. T2DM differs from T1DM, and a sedentary lifestyle and obesity often accompany the former. Insulin concentrations may be normal, decreased, or increased, and the major part of this patient group has impaired insulin action.
The importance of strict lifestyle management, risk factor control, and good glucose levels is emphasized in guidelines (77), as individuals with diabetes mellitus have a doubled risk of CVD (78).
12
Obesity In the papers of this thesis, obesity was assessed by BMI, calculated after measurements of height and weight. As a marker for obesity, increased BMI is a cardiovascular risk factor traditionally used in ischemic heart disease, and it is also a risk factor for all-cause mortality (79). Obesity can be assessed with different measurements such as hip and waist circumferences. The magnitude of the relationship of hip and waist circumferences compared to BMI with CVD mortality has been broadly the same (80). BMI has been reported to be inversely associated with tHcy (81), but the contrary has been seen in adjusted models (82).
Renal function and hyperfiltration Chronic kidney disease is a well-known risk factor for CVD. This is partly due to the high prevalence of traditional risk factors such as diabetes and hypertension (83). However, the associations of renal function and albuminuria with cardiovascular risk are independent of these established risk factors. Even mild impairment of renal glomerular function is associated with an increased risk of CVD and death (84).
In general, the glomerular filtration rate (GFR), together with assessment of the integrity of the filtration barrier by investigation of present albuminuria/ proteinuria, are the most valuable indices to assess severity and progress of kidney damage.
The “gold standard” method for determining GFR is considered to be the urinary clearance of inulin, an exogenous marker, although this is not used in clinical routine. A simpler and more common exogenous alternative is the non-radioactive contrast agent iohexol, which can be administrated as a single-bolus. Though more straightforward, it still needs high-pressure liquid chromatography (HPLC) or liquid chromatography/tandem mass spectrometry (LC-MS/MS) technique to be measured. Instead, the endogenous biomarkers creatinine and cystatin C, with validated GFR estimations (eGFR), are used in high-volume throughput clinical use. Cystatin C-based GFR-estimations (eGFRcystatin C) are superior to GFR-estimations based upon creatinine (eGFRcreatinine) to predict CVD, end-stage renal disease, death, and hospitalization (85-88). GFR can be estimated with different formulas, though in Sweden, the Caucasian, Asian, Pediatric, Adult cohorts (CAPA) for eGFRcystatin C (89) and Lund-Malmö-Revised formula (LMrev) (90, 91) for eGFRcreatinine are the most commonly used. From an international perspective, CKD-EPI equations for cystatin C and creatinine-based eGFR are recommended in the USA (92).
A GFR reduction is not seen in the early stage of impaired renal function. Creatinine (0.118 kDa), a small molecule, and cystatin C, with a larger molecular mass (13.3 kDa), have different renal sieving coefficients (93). Depending on the type of kidney affection, this difference quantitated by the ratio
13
eGFRcystatin C/eGFRcreatinine is suggested as a more refined estimate of glomerular function, and this ratio can add clinical prognostic value (93-95). The term “Shrunken pore Syndrome” (SPS) was introduced in the later years, initially defined as eGFRcystatin C, being less than 60% of eGFRcreatinine (96). However, the eGFRcystatin C/eGFRcreatinine ratio is also used as a continuous variable.
Glomerular hyperfiltration implying increased GFR is often a part but not always present in the initiation of chronic kidney disease (CKD). The definition of hyperfiltration differs among studies (97), and different GFR formulas are used, which entails comparison somewhat tricky. Cardiovascular disease (98) and all-cause mortality (99) have been found to be associated with glomerular hyperfiltration based on creatinine, and only a few have used cystatin C (100). The association was independent of other cardiovascular risk factors in the cross-sectional Tromsø study on subclinical coronary atherosclerosis and hyperfiltration based on iohexol clearance (101, 102). We have shown that smoking is independently associated with a higher eGFRcreatinine among men and women, but not eGFRcystatin C (103).
C-reactive protein The sensitive, non-specific inflammation marker C-reactive protein (CRP) is well established as a biomarker of systemic inflammation. In CVD, CRP concentrations below those seen in infections, but above values considering healthy, can be a marker of the atherosclerotic process. Even lower values < 2 mg/L can predict an increased risk of CVD (104, 105). CRP itself can enhance the inflammatory and prothrombotic response, although it has not been proven that it actually participates in atherogenesis and CHD pathogenesis. The meta-analyses for the U.S. Preventive Service Task Force (106) show strong evidence that CRP and CHD events are associated. Adding CRP to risk stratification among initially intermediate-risk persons can improve risk stratification. There is not sufficient evidence that reduction of CRP levels prevents CHD events.
Genetics Evidence that a family history of CVD in a parent or sibling is associated with CVD has been developed over the years. Potentially related genes have been explored in genome-wide linkage studies and gene association studies with candidate genes (107). The genome-wide association studies (GWAS) have added the possibility of discovering new genes, and more than 320 candidate genes have been identified contributing to the risk of coronary artery disease (CAD) and MI (108). Most of the SNPs are located on DNA sequences that do not code for proteins, which makes interpretation of the biological functions and pathophysiological processes challenging. There are only a few monogenic disorders linked to CVD, for example, variants in the gene encoding LDL-receptor
14
protein, apolipoprotein B, or in the PCSK9 genes. These variants cause familiar hypercholesterolemia (109), one of the most common genetic cardiovascular diseases with a prevalence of 0.3-0.5% in Sweden, but it is considered an underdiagnosed condition. It is characterized by elevated LDL cholesterol. Though these single variations are scarce, they confer a high risk of premature CVD and MI. Still, most CAD and MI cases are polygenic disorders and, adding to this influenced by environmental factors. GWAS studies have the potential to detect associations but not causation. A given SNP that may be useful in risk prediction may not play a prominent role in the pathophysiology of the disease. The MR studies have the possibility to support causality and study life-long exposure of, for example, LDL-C compared with RCTs with LDL-lowering treatment during a shorter time (110). Several MR studies of lipids have strengthened the casual relation between CVD and LDL-C, triglycerides (58, 111), and apo B (112), respectively. The recent MR study investigated the associations of the polymorphisms in the NPC1L1 gene (target of ezetimibe) and the HMGCR gene (target of statins) with CHD (56). The results suggested that LDL's effects on the atherosclerotic CVD risk appear to be by both the absolute magnitude of exposure to LDL and the duration.
The association of HDL-C with CHD has, in contrast, not shown a similar robust association in studies (58, 112). Therefore, the causal relationship is more uncertain between CVD and HDL and apo A1, respectively.
Homocysteine, its determinants, and cardiovascular disease During the last 30 years, many studies have been published, exploring tHcy and folate concentrations with CVD, as well as prevention studies on CVD. The large secondary prevention trials with high-dose vitamin therapy of B6 (pyridoxine), B9 (folic acid), and B12 (cobalamin) were initiated in the 1990s in subjects with established vascular disease. Cardiovascular disease in the NORVIT and HOPE2 trials (113, 114) and in the VISP (115), cerebrovascular diseases were included. The treatment periods were at most five years. They showed no reduction in stroke or heart attack or improvement in mortality from these interventions. In HOPE2, a significant 24% reduction in stroke was observed from B vitamin therapy (114). A subgroup analysis of VISP participants without malabsorption of B12 or renal failure had a significant 21% reduction in adverse events (116). In subjects with advanced vascular disease and adequate dietary B vitamins, these intervention studies showed little benefit of high-dose B vitamins.
In a meta-analysis from 2010 including fourteen studies of RCT of folate supplementation and CVD (117), no overall effect of primary cardiovascular clinical end points, relative risk (RR) 1.02 and 95% confidence interval (CI) 0.93-1.13, p = 0.66) or stroke, RR 0.95 (CI 0.84-1.08), p = 0.43) were proven. Specifically, risks of primary clinical CVD events were 1.06 (CI 1.00-1.13) in strata
15
with mean baseline tHcy levels >12 µmol/L and 0.94 (CI 0.86-1.03) in strata with baseline tHcy levels <12 µmol/L(117).
In a meta-analysis using the MTHFR C677T polymorphism as a MR approach to assessing tHcy levels and CVD risk, previously unpublished data set were included to avoid publication bias (118). The study showed that lifelong moderate tHcy elevation has little or no effect on CHD. Previously published studies are suggested to reflect publication bias or methodological problems, though the tHcy concentrations were not measured in this study population.
The Cochrane meta-analyses from 2017 on tHcy-lowering interventions and MI as an outcome showed RR 1.02 (CI 0.95-1.10) (119). There is still a need for additional trials comparing tHcy-lowering interventions combined with antihypertensive medication versus antihypertensive medication and tHcy-lowering interventions at high doses versus tHcy-lowering interventions at low doses (119).
The meta-analysis of MTHFR C677T, A1298C, and MI (120), showed an increased risk of MI in African populations, but not in European, Asian, and North American populations for the MTHFR C677T polymorphism. Furthermore, in North American populations, CT genotype was associated with a decreased risk of MI. No significant associations between the A1298C polymorphism and MI were evident.
Wang et al. were first to describe the CTH G1208T rs1021737 polymorphism, previously annotated either as G1364T or C1352T, in patients with cystathioninuria (121). They also found increased tHcy concentrations among homozygotes (122). The associated risk with CVD is only studied with a retrospective design, including a small number of individuals. One recent study with coronary artery bypass grafting (CABG) patients (n=178) and controls (n=156) did not show any significant different frequency among cases and referents. Another CTH study with the insertion allele of CTH rs113044851 showed less susceptibility to sudden cardiac death (SCD) in subjects with the insertion allele in a dominant model. The possible role of the CTH gene polymorphism in cardiovascular pathology are thus unsettled.
The most recent Cochrane meta-analysis considering tHcy and renal function published 2016 showed that tHcy-lowering treatment in dialysis patients led to little or no effect on cardiovascular mortality; 4 studies, 1186 participants, RR 0.93 (CI 0.70-1.22) (123) and the secondary outcome, fatal and non-fatal MI showed RR 1.04 (CI 0.66-1.62).
16
The association between eGFRcystatin C/eGFRcreatinine ratio and tHcy has not been assessed previously.
Homocysteine and atherosclerosis The mechanistic role of tHcy in the atherosclerotic process remains largely unknown. However, some biochemical mechanisms by which high concentrations of tHcy contribute to atherosclerosis have been summarized as the following (124, 125) in Table 1. Table 1. Biochemical mechanisms of hyperhomocysteinemia suggested to contribute to the atherosclerotic process.
Process Mechanism Resulting effect Hypomethylation Increased formation of
SAH from SAM. Inhibits biological transmethylation
Metabolic conversion of Hcy to Hcy-thiolactone
Results in a chemically reactive metabolite.
Protein homocysteinylation by thiolactone may contribute to Hcy toxicity in humans, and Hcy-thiolactone and N-Hcy-protein can induce endoplasmic reticulum (ER) stress. It also leads to foam cell formation from phagocytosis of LDL aggregates
The redox status of aminothiols
Hcy represents part of the redox-thiol system, Changes in extra- or intracellular Hcy concentrations may alter redox-thiol status.
Could modulate the activity of nuclear transcription factor-kB (NF-kB) and the folding of newly synthesized glycoproteins in the ER, which depends on the proper formation of intra-chain disulfide bonds.
Oxidative stress Hcy generates reactive oxygen species through auto-oxidation.
Can cause endothelial cell injury.
Oxidative stress Decreases the expression of antioxidant enzymes or through nitrosylation by binding to nitric oxide (NO) to form Hcy-NO.
May induce cell injury
Epigenetics Epigenetics can be described as the mechanisms that regulate which gene will be transcribed and hence translated to a protein in a specific cell type. These mechanisms can be heritable states but also results from modifications in chromatin structure at histone tails site and nucleotide series, occurring without alterations in the DNA sequence (126). The epigenetic markers are propagated in mitosis and transmitted to the next generation. However, throughout life, some
17
of these markers may be changed by, for instance, starvation, toxic chemicals, radiation, hyperglycemia, cancerization, age (the “epigenetic clock”), etc. The epigenome is thus paradoxically both transgenerational and thus long-term stable, and yet to some degree dynamic throughout life. Methylation is the most studied epigenetic mechanism in mammalian cells, and 5-methyl tetrahydrofolate is the primary source of methyl groups used, for example, by DNA methyltransferases in methylation reactions. This epigenetic mechanism may either activate or silence a gene, mainly targeting promotor sites of genes. In atherosclerosis, altered DNA methylation patterns, both hypo-and hypermethylation, are suggested to be secondary to a decrease in SAM, which is highly dependent on the bioavailability of vitamin B12 and folate (127). One carbon metabolism can possibly be linked to lipid metabolism through folate-dependent epigenetic effects such as the modulation of peroxisome proliferator-activated receptor (PPAR) α in mice (128, 129). PPARs represent a family of ligand-activated nuclear hormone receptors belonging to the steroid receptor superfamily, which of three isotypes α, δ, and γ, have been described (130). Fibrates, used in CVD treatment, act as PPAR α agonists and have been shown to upregulate the human Apo A1 gene transcription, but the exact mechanism in humans is not known (131, 132). The PPAR γ acting as a transcriptional regulator of lipid metabolism appears to play an important role within the endothelium, controlling metabolic and vascular responses to high fat diet, and the pharmacologic effects of certain anti-diabetic agents (133). It is suggested that elevated concentrations of tHcy results in hypermethylation of this promoter regions resulting in reduced gene expression, contributing to cardiomyopathy. In obesity other mechanisms such as an interaction with the microbiota and mediate programmed cell death; pyroptosis in adipocytes, and interactions with transcription factors like zinc finger protein 407, are proposed (134). It has also been described that the toxic substance formaldehyde can form when THF and other folate derivates decompose by oxidation or in conjunction with other epigenetic demethylations (135). Formaldehyde can damage DNA, but the toxicity is limited by the route involving the enzyme alcohol dehydrogenase.
19
Aims
The overall aim of the thesis was to expand knowledge about how homocysteine, as a risk determinant, can be connected to cardiovascular disease. Specifically, the purposes were to explore the associations between tHcy, determinants of homocysteine and risk factors for cardiovascular disease, and the associated risk of prospectively developing a first-ever myocardial infarction.
The study-specific aims were:
1. To investigate if tHcy and its major determinants folate, vitamin B12, and MTHFR polymorphism are correlated with Apo A1 levels in a population without folate fortification. Also, to investigate if this correlation was present among those who did and did not develop a first-ever myocardial infarction.
2. To investigate if smoking is a stronger determinant for tHcy in plasma
than the smokeless tobacco product snus after adjustment for potential confounders. We also wanted to explore whether plasma cotinine concentrations were a better predictor of tHcy concentrations than self-reported smoking data.
3. To study the associations of the polymorphisms CTH G1208T, rs1021737,
MTHFR C677T, rs1801133, and MTHFR A1298C, rs1801131 with the prospective risk of developing a fatal or non-fatal myocardial infarction. Also, investigate the tHcy concentrations among MTHFR C677T, A1298G, and CTH G1208T genotypes, respectively.
4. To test the hypothesis that an impaired renal function defined by the eGFRcystatin C/eGFRcreatinine ratio independently associates with an increased risk of a first-ever myocardial infarction.
20
Materials and Methods
Study population All subjects in the studies emanate from Norrbotten and Västerbotten in Sweden and the Northern Sweden Health and Disease Study (NSHDS). It consists of three sub-cohorts; The Northern Sweden Monitoring of trends and determinants in cardiovascular disease (MONICA) project, the Västerbotten Intervention Programme (VIP), and the local Mammography screening project (MSP), merged for research purposes.
The Västerbotten Intervention Programme, VIP VIP started in 1985 in the Västerbotten municipal Norsjö as a pilot project of a community intervention program for cardiovascular and diabetes prevention. The aim was to reduce morbidity and mortality from CVD and diabetes, and the program is still ongoing (136). In the VIP, all men and women at 30, 40, 50, and 60 years of age were invited to the local primary health care center for a physical examination, answering a questionnaire, and donating blood samples for future research. The invitation of 30-year-old subjects was discontinued by the end of 1995. By December 2009, 132747 answered questionnaires, and 114791 blood samples had been collected. The mean participation has been between 57-65%.
The Northern Sweden MONICA project In the early 1980s, the World Health Organization (WHO) established the MONICA project to monitor trends and risk factors in ischemic cardiovascular morbidity and mortality (137). In the Northern Sweden MONICA project, the first participant was included in 1986. A random sample of inhabitants was invited in 1986 and 1990; 1000 men and 1000 women between the ages of 25-64 years. In the following surveys (1994, 1999, 2004, 2009, and 2014), 2500 inhabitants aged 25-74 were invited. The three latest investigations are not used in the present studies. The mean participation rate was 77%. The Northern Sweden MONICA study is continued, but the planned invitation for the 2020 survey was postponed due to the covid-19 pandemic situation.
In the Northern Sweden MONICA project, a similar approach as in the VIP was used, concerning anthropometry measurements, blood samples, OGTT, cholesterol measurements, and lifestyle and diet questionnaires completion.
21
The mammography screening project, MSP The MSP was founded in 1995. The women within the age of 40-70 years attending their local mammography screening examination every two or three years were asked to donate blood samples (138). The participants completed a short questionnaire. It was finished in 2006 and then included 28 790 women (blood samples and/or questionnaire). The participation rate was about 57% for the donation of blood samples.
Questionnaires The participants in VIP and MONICA are asked to complete a comprehensive questionnaire on several lifestyle factors, including social background, tobacco- and alcohol use, physical activity, social support, demand and control at work, migration, heredity, and history of CVD and when applicable menopause and hormonal therapy. One part includes a food frequency questionnaire. A trained nurse obtained information about medications. The questionnaires have been validated (139). MSP has a shorter questionnaire on reproductive factors, medicines, and smoking habits. In the MONICA screening of 2022, questions regarding covid-19 have been added.
Blood collection Participants are informed about fasting for at least four hours in the MONICA and VIP projects. In the MSP the time since the last meal was registered. After 15 minutes of rest in a sitting position, venous blood samples were drawn into evacuated tubes containing EDTA. Plasma was obtained by centrifugation at 1500 g for 15 minutes. Plasma, buffy coat, and packed red cells are aliquoted in smaller plastic tubes and frozen within one hour. The samples are stored frozen at -80º C until analysis or DNA preparation.
22
Study cohort and design I, III, and IV These studies were conducted with a prospective incident nested case-referent study design. The prospective design is possible since blood samples and information about exposure are available and collected before the outcome development. The term "nested" describes that the study subjects are participants in a larger study base, in this case, the NSHDS. Identification of cases and referents in the studies were performed by linking with personal numbers, the study base, and the Northern Sweden MONICA incidence registry. An incident case-referent study is less time-consuming and more cost-effective than cohort studies, where the cases are compared to all subjects in the study base. This approach is well suited, while biomarkers entail costs in this study.
Case identification All cases with fatal and non-fatal first MI and suspected fatal MI; ICD-9 410, ICD-10 I21-3 from 25 years of age, and from January 1, 1985, to December 31, 1999, were included. In the MONICA study, the recording of MI cases included the ages between 25-64 years of age. For the NSHDS, an additional recording was conducted to include individuals over 64 years of age. The case identification was made standardized, following WHO and MONICA criteria (140). Performing the case ascertainment, research nurses screened general practitioners' reports, hospital records, death certificates, and autopsy reports.
MI events that were fatal by the 28th day from the onset of symptoms were classified as fatal (paper III) (141). Exclusion criteria for MI cases were previous MI, stroke, or a cancer diagnosis within five years before and one year after MI. Cases with lipid-lowering drugs (N=5) were excluded. In paper IV, Figure 2, a flowchart describes the inclusion of study participants. Finally, 545 MI cases were included from VIP (n=431), MONICA (n=44) and MSP (n=70), respectively.
After 2009, MI cases of all ages have been included in the Northern Sweden MONICA incidence registry.
Referent selection When possible, up to six referents per case were selected from the initial study base. Matching was made for age (±2 years), sex, date of health examination, and geographical site.
For referents, exclusion criteria were MI, stroke, cancer, or death before the time of diagnosis of the index case or moving out of the region. For later biochemical analyses, the two first referents were selected. Referents with lipid-lowering drugs (n=4) were excluded. One thousand fifty-four referents were finally included (for details, see paper IV, Figure 2).
23
Study variables I, III, and IV
Blood pressure and hypertension In the MONICA survey, blood pressure was measured after 5 minutes of rest in a sitting position. In every subject, two measurements were performed, and the mean value was registered. In the VIP, blood pressure was measured after rest in a horizontal position. An adjustment was made for sitting posture based on a comparison between sitting and horizontal positions in subjects from the VIP health screening. In the MSP, blood pressure was not measured. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg and or use of antihypertensive medication during a period of 14 days before the health examination.
Body mass index (BMI) Subjects were weighed in indoor clothing and without shoes. Measurements of height were without shoes. BMI was calculated as weight (kilograms) divided by height (meters squared).
Diabetes Information on diabetes was obtained from the questionnaire, and at the health examination, fasting glucose concentrations were measured in plasma with a Reflotron® bench-top analyzer (Boehringer Mannheim GmbH, Mannheim, Germany). A two-hour glucose tolerance test was performed using 75 g glucose dissolved in 300 mL water. The definition of diabetes was a fasting plasma glucose ≥ 7.0 mmol/L and or post-load plasma glucose ≥ 11.0 mmol/L (12.2 mmol/L in VIP, in which capillary plasma was used) and or self-reported diabetes. Measurement of fasting or post-load glucose was required for classification as non-diabetic. Fasting glucose and or 2-hour oral glucose load tests were available for 444 cases and 855 referents.
Smoking Current smoking was defined as daily smoking, excluding non-, previous- and nondaily smokers.
24
Study cohort and design II The study base in paper II originates from the NSHDS, only including subjects from the VIP. Initially, subjects were selected for prospective nested case-referent studies on colorectal cancer (142, 143), case-referent ratio 1/2. The study design used was cross-sectional. The plasma analyses were collected years before the cases developed colorectal cancer (median follow-up time 8.2 years). A sensitivity analysis concerning case-referent status was performed.
Study variables II
Body mass index (BMI) BMI was calculated and classified as follows, according to WHO and the definition of normal weight < 25, overweight/pre-obesity 25–<30, and obesity ≥30 kg/m2 of adults over 20 years.
Definition of smokers and snus users Among all subjects cotinine categories were defined according to Bevital´s in laboratory cut-offs, as <5 = I (n = 831), 5–85 = II (n = 94), >85–1700 = III (n = 332), and >1700 nmol/L = IV (n = 118). Special effort was made to define smokers and snus users as strictly as possible, categorizing was made as shown in Table 2. For a subset (n=170), information about nicotine habits was missing. The frequency corresponding to the possible amount of smoking, classified by cotinine concentrations (nmol/L), is shown in Table 3.
25
Table 2 Categorizing smokers, snus users, and never tobacco users (n=1205). Missing smoking data (n=170).
N (M/W) Never tobacco users
Always only smokers
Always only snus users
Excluded
Smokers and snus users," dual users"
20 (17/3) x
Smokers and former snus users
20 (19/1) x
Smokers and non-snus users
194 (84/110) x
Former smokers and snus users
70 (66/4) x
Former smokers and former snus users
54 (51/3) x
Former smokers and non-snus users
186 (89/97) x
Non-smokers and snus users
31 (30/1) x
Non-smokers and former snus users
19 (17/2) x
Non-smoker and non-snus users
479 (214/265) x
Occasional smokers and snus users
7 (6/1) x
Occasional smokers and former snus users
2 (1/1) x
Occasional smokers and non-snus users
23 (4/19) (x)a x a
Former occasional smokers and snus users
16 (15/1) x
Former occasional smokers and former snus users
11 (10/1) x
Former occasional smokers and non-snus users
73 (26/47) x
N 552 194 54 405 Excluded -12 b -7 c Included 540 194 47
a Excluded due to high cotinine concentrations. b Cotinine concentrations >cut-off for passive smokers. c Missing analyses.
26
Table 3. 170 subjects with missing questionnaire data on nicotine habits, classified by cotinine concentrations (nmol/L).
Frequency N Percent Valid non-smoker <5 106 62.4 Passive smoker 5-<85 13 7.6 Smoker 85-<1700 20 20.0 Heavy smoker ≥1700 >25 cig per day
17 10.0
Total 170 100.0
Preanalytical factors Preanalytical factors impact the quality and usefulness of blood-derived biomarkers in a clinical or research setting. Preanalytical errors can occur at any step of the phase before the analytical phase, and it is essential to have good routines and protocols for the handling of specimens (144). Types of collection tubes, additives, time and temperature to centrifugation, degree of hemolysis, storage time, and the number of freeze-thaw cycles are all factors that may influence the obtained measurement results. Diurnal and seasonal variations can also affect population studies (38). Homocysteine is significantly affected by glycolysis, and the time and temperature prior to centrifugation are crucial (145). Unspun specimens at room temperature can double tHcy values. In obtaining correct handling, refrigerated and quickly centrifuged specimens are recommended. Of the biomarkers discussed in this thesis, triglycerides and LDL cholesterol are the ones usually recommended to be sampled after overnight fasting. For tHcy see the paper I. The other markers are not highly dependent on fasting time. In Table 4, the fasting status of the subjects in papers I, III, and IV are shown.
Table 4. Fasting status in papers I, III, and IV.
Fasting status Men Women
N % N %
>8 h 762 66.9 168 36.5
4-8 h 179 15.7 68 14.8
<4 h 41 3.6 198 43.0
Missing data 157 13.8 26 5.7
27
Biochemical methods To minimize inter-assay variability, laboratory technicians analyzed plasma specimens in batch. The positions within the triplets of one case and two referents varied randomly to avoid systematic bias. The laboratory staff was unaware of the case and referent status. In paper II Bevital AS (Bergen, Norway) performed the biochemical analyses. The coefficients of variation (C.V.s) are presented in Table 5. Table 5. Assay coefficient of variation (C.V.s) for the plasma of frozen samples.
Intra-assay CV (%)a (paper I, III, and IV)
CV (%)b according to Bevital (paper II)
Homocysteine 2.7 ; 1.9 0.9 ; 2.1 Vitamin B12 16.5 ; 10.4 4 ; 5 Folate 15.8 ; 12.1 4 ; 5 MMA n.a. 2 ; 2.6 Creatinine 1.59 ; 3.21 4 ; 4 Cotinine n.a. 2.3 ; 6 Apo A1 0.96 ; 0.61 n.a. Apo B 1.21 ; 0.81 n.a. Albumin 0.89 ; 2.25 n.a. Cystatin C 2.90 ; 1.20 n.a. CRP < 6% c n.a.
a Analyses were done in batch, and intra-assay CV for two concentrations (high and low) are presented.
b Within-and between-day CV is presented. c Inter-assay coefficient of variation.
Homocysteine, B12, folate and MMA In papers I, III, and IV, tHcy was analyzed with fluorescence polarization immunoassay on an IMx® unit (Abbott Laboratories, IL, USA). B12 and folate were analyzed using DPC® Solid Phase No Boil Dualcount radio assay (Diagnostic Products Corporation, CA, USA). In paper II, tHcy and MMA were determined by an isotope dilution gas chromatography-mass spectrometry method (G.C.–M.S.). Vitamin B12 and folate were analyzed with a microbiological assay, using Lactobacillus leichmannii for measuring vitamin B12 (146) and Lactobacillus casei to determine plasma folate (147).
Apo A1 and Apo B Apo A1 and Apo B were analyzed with immune turbidimetry on a Hitachi 911 multi analyzer with reagents from DAKO (Copenhagen, Denmark).
Albumin, cystatin C, creatinine, and CRP Albumin, cystatin C and creatinine were analyzed with a Hitachi 911 multi analyzer. Albumin was analyzed with immune turbidimetry and Cystatin C with
28
an immunological method, both with reagents from DAKO (Copenhagen, Denmark). Creatinine was analyzed by (Crea plus, enzymatic method) with kits from Roche/Boehringer (Mannheim, Germany). The enzymatic methods are not sensitive to pseudocreatinines as older color-based Jaffe methods are. CRP was analyzed with an automated high sensitive method, IMMULITE 1 (Diagnostic Products Corporation, Los Angeles, CA, USA). In paper II, creatinine concentrations were measured with LC-MS/MS.
Calculation of eGFRcystatin C/eGFRcreatinine ratio Calculation of the estimated relative glomerular filtration rate (eGFR) was made according to Caucasian, Asian, Pediatric, Adult cohorts (CAPA) for eGFRcystatin C
(89) and Lund-Malmö-Revised formula (LMrev) for eGFRcreatinine (90, 91), stated below. CAPA: eGFR = 130 × cystatin C-1.069 × age-0.117 – 7 LMrev: eGFR = ex-0.0158×age+0.438×ln(age) Female and creatinine<150: x = 2.50+0.0121×(150-creatinine) Female and creatinine≥150: x = 2.50-0.926×ln(creatinine/150) Male and creatinine<180: x = 2.56+0.00968×(180-creatinine) Male and creatinine≥180: x = 2.56-0.926×ln(creatinine/180) eGFRcystatin C/eGFRcreatinine ratio was used as a continuous variable. Creatinine results were isotope depletion mass spectrometry (IDMS) adjusted prior to the calculation of eGFR.
Cotinine Cotinine concentrations were measured with LC-MS/MS (148). The limit of detection for cotinine was 1.0 nmol/L (0.18 ng/mL).
Polymorphisms analysis
DNA-extraction and PCR DNA prepared from the buffy coat with the chloroform/phenol method was provided from the Medical Biobank, Umeå. 10 ng DNA from each sample was dispensed in 96-well plates.
MTHFR The single nucleotide polymorphisms MTHFR C677T and A1298C genotyping was performed by the TaqMan allelic discrimination method, using Minor Groove Binder (MGB) probes (paper I, III, and IV). In paper II, MTHFR 677C>T
29
was determined by MALDI-TOF mass spectrometry (149).
CTH The CTH gene polymorphism G1208T, rs1021737 was genotyped by reverse pyrosequencing (see Figure 4), as detailed in paper III, Methods section.
C/C=GG wild-type
A/A=TT homozygote variant
A/C=TG heterozygote variant
Figure 4. Pyrograms of three possible genotypes of the CTH G1208T polymorphism.
30
Statistics For most of the statistical calculations, IBM SPSS version 19.0-26.0 (IBM, Armonk, NY, USA) were used. To determine Hardy-Weinberg equilibrium on the MTHFR and CTH polymorphisms, χ2-test was used. Visual inspection and formal tests were applied to assess distributions, and when needed, data were transformed to a logarithmic scale. Missing values were treated as missing throughout the calculations. To compare baseline characteristics and study variables between groups, Chi-square test for independence was used for categorical variables (paper I, II, and IV). For comparison of continuous variables, T-test and Mann-Whitney (paper II) were used. The relationship between the tHcy concentration and the polymorphisms was investigated with the Kruskal-Wallis test. P-values < 0.05 were considered significant. Quartiles were calculated (paper I, IV) for men and women separately and based on the distribution of the referents. They were treated as categorical variables, except when testing for trend, where they were included as continuous linear variables in the regression analyses. Genotypes in papers I and III were coded in one recessive model, including wild type and heterozygote variant vs. homozygote variant, and the other, dominant model; wild type vs. heterozygote and homozygote variants. To find influencing factors of Apo A1 and B, we used linear regression analysis (paper I). In the models where we wanted to adjust for influencing factors, we used multiple regression analysis. We excluded variables that were not associated. As an exception, tHcy was included since we wanted to test the hypothesis regardless of the result in univariate analysis. In paper II, cotinine categories in all subjects were defined according to Bevital's laboratory cut-offs. Spearman's rho correlations were performed to explore associations between biochemical variables and lifestyle. In the main effects multiple regression models, we included potential confounders and significant variables from the univariate analyses. Vitamin B12 was not included since we used methylmalonic acid (MMA), mainly determined by bioavailable vitamin B12. A sensitivity analysis was performed for case-control status as cases years later developed colorectal cancer. In the nested, matched case-referent study (paper I, III, and IV), odds ratio (OR) for disease and 95% confidence intervals (CI) were calculated by conditional logistic regression analysis and the conditional maximum likelihood
31
routine designed for matched analysis, as cases and referents, had equal follow-up times. Univariable and multivariable models were used.
32
Ethical considerations The study protocols for all studies have been approved by The Research Ethics Committee of Umeå University, Umeå. The National Computer Data Inspection Board has approved the data handling procedures. The studies were conducted following the ethical principles of the Declaration of Helsinki. All subjects were informed at the time of the survey and gave written consent for future research purposes (Appendix 1). In addition, all non-fatal MI cases got written information regarding the registration in the MONICA incidence registry with the possibility to request to be removed. After selecting the cases and referents, all data handling procedures and further analyses were done using an anonymous dataset. All identifiable subject data was archived to protect research subjects' confidentiality. The identification key is stored at "Enheten för Biobanksforskning" (EBF) at Umeå University.
33
Results
Paper I The study population in papers I, III, and IV included 545 cases, 387 men, and 158 women with the outcome first-ever MI and 1054 matched referents, 752 men, and 302 women. The mean age (±SD) for the selected cases was 59 (± 7.8) years at the time of the outcome. In univariate linear regression analysis, no association was seen for tHcy and Apo A1 among referents or cases, nor after stratification for sex. In multiple regression analysis, adjusting for possible confounding factors, folate, but not tHcy, was positively associated with Apo A1 among referents, in men (β-value of 0.004, P = 0.009) and women (β-value of 0.007, P = 0.03). The association was not seen among those later developing an MI. Univariate analyses of variance of the plasma variables and alcohol intake are presented in Table 6, showing that alcohol intake defined as g/day is associated with Apo A1 and folate but not with tHcy. Data were available for a smaller subset of the material, at the most (n=642).
Table 6. Univariate analysis of variance.
Alcohol intake
Beta (S.E.) R2 P
Apo A1 0.005 (0.002) 0.013 0.002
Apo B -0.002 (0.002) <0.001 0.352
Apo B / Apo A1 ratio -0.005 (0.002) 0.008 0.014
Homocysteine -0.006 (0.029) -0.002 0.828
Vitamin B12 -1.304 (1.332) <0.001 0.328
Folate 0.093 (0.041) 0.006 0.024
34
Paper II Paper II included 1375 individuals, and after assessing strict definitions of never tobacco user (n = 540), smokers (n = 194), and snus users (n = 47), comparisons were made in a cross-sectional design. Cotinine was positively associated with tHcy among all individuals and in the defined group of smokers (Spearman’s rho correlation coefficient 0.270, p < 0.01). There was no significant association between cotinine and tHcy among snus users (Spearman’s rho correlation coefficient -0.008, p = 0.96). In multiple regression analyses, cotinine remained positively associated with tHcy (log) in all subjects and among smokers, with the effect size, partial eta2 of 0.019 (p < 0.001) and 0.029 (p = 0.023), respectively. Among the snus users, no association was observed (effect size, partial eta2 0.006, p = 0.655), though higher cotinine concentrations were present in snus users. The number of cigarettes did not show any association with tHcy concentrations (Spearman’s rho correlation coefficient 0.149, p = 0.054). This was in contrast to the correlation found between cotinine and tHcy concentrations in all subjects and among smokers. In the sensitivity analysis, adding case-referent status to the multiple regression models, the results did not change. The case-referent status was not associated with tHcy.
Paper III No associations with the risk of developing a first-ever MI were seen for all MI cases, even after stratification for sex. After stratification in fatal and non-fatal MI, a positive association for fatal MI and the CTH G1208T and MTHFR A1298C heterozygotes were observed among women, OR (95% confidence interval) 3.14 (1.16-8.54), and 3.24 (1.26-8.34), respectively, and in the dominant model 3.22 (1.22-8.51), and 2.63 (1.06-6.50) respectively. This was not found among men. The MTHFR C677T polymorphism seemed unrelated to the risk of MI, fatal or non-fatal. Another finding was that tHcy concentrations were higher in fatal cases compared to non-fatal cases, Mann-Whitney U test (P = 0.013).
Among the polymorphisms studied, a difference in tHcy concentrations remaining after stratification for sex was evident for MTHFR C677T (Table 7). For MTHFR A1298C, a difference in tHcy concentrations was seen for men. For the CTH polymorphism, no significant difference in tHcy concentrations was found among all, nor after stratification for sex. Homocysteine concentrations in the CTH polymorphism stratified for MTHFR CC-allele and CC+CT alleles (Table 8) showed no differences.
35
Table 7. Total homocysteine in relation to genotypes, in all and stratified by sex. Median values (25-75 %) are shown.
Genotypes Homocysteine N Pa
MTHFR C677T rs1801133 <0.001
CC 10.3 (8.7-12.1) 719
CT 10.6 (9.0-12.7) 566
TT 11.8 (9.9-14.9) 126
Men <0.001
CC 10.4 (8.8-12.1) 510
CT 10.7 (9.4-12.8) 402
TT 12.1 (10.1-16.3) 85
Women 0.039
CC 9.9 (8.4-12.0) 209
CT 9.8 (8.2-12.3) 164
TT 11.5 (9.3-13.0) 41
MTHFR A1298C rs1801131 0.089
AA 10.6 (9.0-12.8) 568
AC 10.4 (8.8-12.3) 659
CC 10.9 (9.0-13.3) 186
Men 0.024
AA 10.8 (9.2-12.9) 385
AC 10.4 (9.0-12.4) 483
CC 11.2 (9.2-13.3) 129
Women 0.959
AA 9.9 (8.5-12.2) 183
AC 10.0 (8.2-12.3) 176
CC 10.0 (8.4-12.4) 57
CTH G1208T rs1021737 0.148
GG 10.4 (8.9-12.5) 742
GT 10.7 (9.0-12.7) 466
TT 10.1 (9.1-11.6) 61
Men 0.533
GG 10.6 (9.1-12.7) 534
GT 10.6 (9.1-12.7) 319
TT 10.3 (9.3-11.6) 41
Women 0.095
GG 9.8 (8.3-12.0) 208
GT 10.8 (8.6-13.0) 147
TT 9.8 (7.9-11.7) 20 a P-values calculated with Kruskal-Wallis. Significant P-values <0.05 are shown in bold.
36
Table 8. Total homocysteine in relation to the CTH polymorphism, stratified for MTHFR CC-allele and CC+CT alleles. Median values (25-75%) are shown.
MTHFR 677CC n Pa MTHFR 677 CC/CT n Pa
CTH G1208T 0.81 0.168
GG 10.2 (8.6-12.0) 394 10.3 (8.8-12.3) 670
GT 10.4 (8.8-12.0) 219 10.6 (8.9-12.5) 422
TT 10.9 (9-1-11.9) 26 10.2 (8.9-11.6) 58
Men 0.822 0.53
GG 10.5 (8.8-12.4) 287 10.5 (9.1-12.4) 484
GT 10.3 (8.8-11.8) 153 10.5 (9.1-12.5) 294
TT 10.9 (9.2-11.9) 16 10.3 (9.3-11.6) 39
Women 0.185 0.237
GG 9.7 (8.3-11.8) 107 9.7 (8.2-12.0) 186
GT 10.9 (8.5-12.3) 66 10.6 (8.5-12.5) 128
TT 10.3 (8.7-11.9) 10 9.7 (7.9-11.4) 19
a P-values calculated with Kruskal-Wallis.
Table 9. Total homocysteine in relation to the CTH polymorphism, presented for folate status above and equal/below median concentrations. Median values (25th; 75th percentile) are shown.
Folate ≤ mediana n Pb Folate > mediana n Pb
CTH G1208T 0.329 0.243
GG 11.5 (9.6-13.6) 401 9.5 (8.2-11.1) 341
GT 11.7 (9.8-14.1) 265 9.7 (8.5-11.4) 200
TT 11.1 (9.7-12.0) 36 9.3 (7.7-10.5) 25
Men 0.701 0.277
GG 11.5 (9.7-13.7) 292 9.8 (8.6-11.2) 242
GT 11.3 (9.8-14.0) 181 10.0 (8.8-11.6) 138
TT 11.0 (9.8-12.0) 24 9.4 (7.7-10.9) 17
Women 0.075 0.752
GG 11.4 (9.4-12.9) 109 8.8 (7.5-10.5) 99
GT 11.9 (10.4-14.5) 84 9.1 (7.8-10.6) 63
TT 11.1 (8.4-12.1) 12 8.5 (7.5-9.8) 8 a Folate median-values were for men 9.06 and women 10.0 nmol/L. b P-values calculated with Kruskal-Wallis.
37
Homocysteine concentrations in individuals with folate status above and equal/below median concentrations show no significant difference with the CTH polymorphism (Table 9). For the MTHFR C677 polymorphism (Table 10) higher tHcy are seen among men with the TT variant and lower folate status, but no significant difference are found among women.
Table 10. Total homocysteine in relation to the MTHFR c677T polymorphism, presented for folate status above and equal/below median concentrations. Median values (25th; 75th percentile) are shown.
Folate ≤ mediana n Pb Folate > mediana n Pb MTHFR C677T <0.001 0.276 CC 11.4 (9.4-13.3) 351 9.5 (8.3-11.0) 368 CT 11.2 (9.7-13.6) 335 9.6 (8.4-11.4) 231 TT 12.9 (10.9-16.9) 88 9.8 (8.4-11.4) 38 Men <0.001 0.029 CC 11.4 (9.5-13.5) 244 9.7 (8.4-11.3) 266
CT 11.3 (9.8-13.6) 245 10.3 (9.0-11.8) 157 TT 13.1 (11.0-18.0) 60 10.4 (8.9-12.1) 25 Women 0.067 0.772 CC 11.5 (9.4-13.0) 107 9.0 (7.7-10.6) 102 CT 11.2 (9.0-13.2) 90 8.7 (7.4-10.0) 74 TT 12.1 (10.7-14.7) 28 8.4 (7.8-10.9) 13
a Folate median-values were for men 9.06 and women 10.0 nmol/L. b P-values calculated with Kruskal-Wallis.
38
Paper IV In this study, the eGFRcystatin C/eGFRcreatinine ratio did not differ between male cases and referents. However, in females, there was a difference in the eGFRcystatin
C/eGFRcreatinine ratio mean values (±SD), 0.87 (±0.17) among cases and 0.93 (±0.17) in referents, P = 0.001. A high eGFRcystatin C/eGFRcreatinine ratio was associated with a lower risk of developing a first-ever MI among women, both when analyzed as a z-scored, continuous variable and across the quartiles of the ratio. After adjustments with apo B/A1 ratio, CRP, tHcy, SBP, BMI, and diabetes, the related risk remained (OR 0.58, 95% CI 0.34–0.99). These associations did not appear among men. Sensitivity analysis excluding the MSP survey resulted in unchanged OR results. In our material, Spearman correlation coefficients were significant for tHcy vs eGFRcreatinine, eGFRcystatin C, eGFRcystatin C/eGFRcreatinine ratio for both men (-0.19, -0.22, -0.10), and women (-0.30, -0.36, -0.21). Linear regression for tHcy in our material stratified for sex showed R2 = 0.024 for eGFRcreatinine and R2 = 0.036 for eGFRcystatin C in men (Figure 5). In women, corresponding values were R2 = 0.059 for eGFRcreatinine and R2 = 0.089 for eGFRcystatin C (Figure 6). The lowest R2
was seen for the eGFRcystatin C/eGFRcreatinine ratio, in men R2 = 0.013 and women R2
= 0.022.
39
A
eGFRcreatinine B
eGFRcystatin C C
eGFRcystatin C/eGFRcreatinine
Figure 5. Linear regression tHcy and eGFR, and eGFRcystatin C/eGFRcreatinine ratio respectively, in men. One outlier tHcy = 102 µmol/L is not displayed in the figure. A) tHcy = 17.01-0.06× eGFRcreatinine; R2 = 0.024. B) tHcy = 15.03-0.04× eGFRcystatin C; R2 = 0.036. C) tHcy = 15.03-0.04× (eGFRcystatin C/eGFRcreatinine); R2 = 0.013.
40
A
eGFRcreatinine B
eGFRcystatin C C
eGFRcystatin C/eGFRcreatinine
Figure 6. Linear regression tHcy and eGFR, and eGFRcystatin C/eGFRcreatinine ratio respectively, in women. One outlier tHcy = 71.7 µmol/L is not displayed in the figure. A) tHcy = 18.18-0.09× eGFRcreatinine; R2 = 0.059. B) tHcy = 16.59-0.07× eGFRcystatin C; R2 = 0.089. C) tHcy = 14.02-3.64× (eGFRcystatin C/eGFRcreatinine); R2 = 0.022.
41
Discussion
Main findings Paper I remains the largest prospective case-referent study to date, including both men and women on the relationship between apolipoproteins, B-vitamins, tHcy, and MTHFR polymorphisms. Our findings support a positive association between Apo A1 and folate, and we have previously shown an inverse association between folate and the risk of MI (150). The association was seen among all men and women and referents but not among the individuals later developing an MI. These results are in line with animal studies (128, 129) showing a possible pathophysiological mechanism such as folate-dependent epigenetic effects in genes, as the modulation of PPAR α in mouse embryos. However, the possible mechanism in humans is unknown (131, 132). In a large study, including men (n=2775) with multiple biomarkers linked to coronary heart disease, an association with the gene cluster APOA5-A4-C3-A1 and folate after adjustment was found (151). Our findings do not support the suggested mechanism linking tHcy and Apo A1 previously shown in animal models (152-154) or the association studies between tHcy and Apo A1 in humans with CVD (152, 155, 156). These previous studies in humans include only men and fewer individuals (152, 155) compared to our study and have a cross-sectional design (156). An early small hospital-based, case-referent study (MI cases n=107) showed associations in all subjects between Apo A1 and tHcy and folate, respectively. After stratification in cases and referents, the association between tHcy and Apo A1 was seen only among cases, and no association was seen between ApoA1 and folate (157). A later minor cross-sectional study (n=258) showed a negative association between Apo A1 and tHcy (158). The study included hypothyroid, subclinical hypothyroid patients, and referents, divided into subgroups according to tHcy concentrations. This was also reported in a study on 1768 individuals defined as healthy (159). None of these studies analyzed folate, B12, or MTHFR polymorphisms. The association in our study seen between Apo A1 and folate was not evident among the cases, who later developed an MI after a median follow-up time of 4.3 (±2.5) years for men and 3.4 (±2.4) years for women. The reason could be due to lower statistical power as the beta-values for male cases and referents are similar (paper I, Table 5). In Sweden, folate fortification is not used. Instead, the Swedish Food Agency recommends supplementary folic acid intake before and during pregnancy. The absence of mandatory folate fortification in our cohort is a strength in this study
42
concerning tHcy and determinants, compared to other population-based studies, for example, in the USA. They applied voluntary folate fortification of food in 1997 and mandatory fortification in 1998. After that, serum concentrations of B-vitamins and tHcy in the population have been changed (160). In our cohort, we can thus study possible effects of folate in a lower range of the spectrum, not possible in the USA, where many subjects may have reached a threshold above which no association may be found. An example of this can be seen in Table 9, where the MTHFR C677T polymorphism was associated with tHcy in subjects with P-Folate ≤ median only.
A limitation was that we could not adjust for alcohol intake (Table 5), as alcohol data only were available in a subset of the participants. It is possible that residual confounding by alcohol use or other lifestyle factors such as a healthy diet could explain the associations. However, total alcohol intake has slowly but steadily declined over several decades in Sweden, probably switching from brandy-type of liquors to wines. The confounding by ethanol is thus likely not increase in this population.
In paper II, associations between tHcy and cotinine concentrations were observed in smokers and not among snus users. The association between tHcy and cotinine among all individuals and smokers is in agreement with prior NHANES studies (161). No previous studies have investigated the association between tHcy and cotinine concentrations among snus users. Regarding smoke-less tobacco users, only NHANES (1999-2008) had a mixed group of chewing tobacco and moist snuff users (n=69) where they reported higher tHcy concentrations and lower folate concentrations in this group compared to non-tobacco consumers (21). The association between tHcy and cotinine was not investigated in that study. Despite the fact that higher cotinine concentrations were present in snus users, no association with tHcy was evident among snus users in our study. The result could reflect possible power issues, but the different directions of the β–values in the associations in smokers compared to snus users speak against this as the only explanation. There are only a few retrospective studies investigating tHcy concentrations in previous smokers (22). One small intervention study reported no short-term changes in tHcy among smokers using conventional cigarettes, randomized to smoking abstinence (n=40), menthol cigarettes (n=42), or menthol tobacco heating systems (n=78) (162). There are no prospective studies investigating long-term changes of tHcy comparing those who continue to smoke to those who stop smoking cigarettes. Such studies would be of value. Questionnaire data to classify smokers and non-smokers are usually accurate, but when performing studies on tobacco use, analyzing cotinine is more accurate for quantifying smoking than questionnaire data of the number of smoked cigarettes/day.
43
The main findings in paper III, including and stratified for fatal MI, demonstrate that women in the dominant model for the CTH G1208T polymorphism have a potentially higher risk of developing a first-ever MI with a fatal outcome within 28 days, compared to female individuals with the wild type. These are novel findings since this study, for the first time, also includes fatal cases. One case-referent study based on a limited number of cases (163) found no association with CAD and the CTH G1208T polymorphism. The previous study had only a few female cases, notably based on another insertion allele of the CTH rs113044851. These cases were less prone to suffer from sudden cardiac death among those with the insertion allele in a dominant model (164).
The association between the MTHFR A1298C polymorphism and MI is sparsely studied. Our findings of no association with all MI agree with a recent meta-analysis (120), based on seven studies, most of them relatively small, however including paper I in this thesis as the largest. We are not aware of any previous study of MTHFR polymorphism in relation to a first MI stratified for fatal and non-fatal outcomes. Thus, the positive association of MTHFR A1298C polymorphism and fatal MI among women is novel.
Our results of no associations in the full dataset between MI and MTHFR C677T contradict an early meta-analysis (165), including six unpublished and 34 published studies before June 2001.
A meta-analysis using the MTHFR C677T polymorphism in an MR approach to assessing tHcy concentrations showed little or no effect on CHD (118). The authors concluded that previously published studies reflect publication bias or methodological problems. However, it is worth pointing out that tHcy was never measured in the dataset used; instead, an assumption of the tHcy concentrations was made, which may be affected by fortification in the population.
Our finding is supported by the results in a more recent meta-analysis, including 47 studies (120). In this meta-analysis, subgroup analysis of ethnicity showed no associations for the MTHFR C677T in European, Asian, and North American populations. At the same time, C677T polymorphism was associated with the risk of MI in African, North American, and elderly populations. Thus, there are other population factors influencing the association between MI and MTHFR C677T.
A recently published study assessing future cardiac death, including 265 patients surviving their first STEMI at a young age, suggested that homozygosity of the TT methylenetetrahydrofolate reductase C677T genotype was an independent long-term predictor of cardiac death (166). In that study, MTHFR A1298C was not assessed.
44
A possible association between the MTHFR C677T genetic polymorphism TT genotype and an increased risk for peripheral arterial disease was reported in a very recent review, including 15 studies with 1929 patients (167).
Since our study's MTHFR C677T polymorphism influenced tHcy concentrations, we explored the tHcy concentrations and the CTH polymorphism, stratified for the MTHFR C677T alleles and folate status above and equal/below median concentrations, respectively. No differences appeared for the CTH polymorphism. This is in contrast to the pattern seen for the MTHFR C677T, where individuals with folate concentrations ≤ median values show a more substantial relationship between the tHcy concentration and the polymorphic variants compared to individuals with folate concentrations above this.
In paper IV, a higher eGFRcystatin C/eGFRcreatinine ratio expressed as a z-score was associated with approximately a 40% lower risk of prospectively developing a first-ever MI among women per one SD increase in the ratio, a finding that was independent of SBP, diabetes, BMI and apo B/A1 ratio, tHcy, and CRP. This first prospective study of the eGFRcystatin C/eGFRcreatinine ratio and the risk of a first-ever MI underscores the importance of early laboratory-based vigilance of declining renal function in preventing CVD. The findings in paper IV agree with previous retrospective studies on CVD, including patients with heart failure where associated risks between eGFRcystatin
C/eGFRcreatinine ratio were described among those with poor right ventricular systolic function (168) and proteins involved in atherosclerosis (169). Among patients undergoing elective coronary bypass grafting, shrunken pore syn associated with higher mortality (170). The fact that the association only was seen among women in this study agrees with the prospective study on the risk of future surgery for aortic stenosis (171), where the associated risk was seen only among women with coronary artery disease at the time of surgery. eGFRcystatin C used as a marker of CVD has been studied prospectively in a few studies. One including only men, where the independent association with CHD was not evident after adjustments including CRP (172). In the NHANES study, eGFRcystatin C used alone was stronger associated with risk of CVD and all-cause mortality in adjusted models than adding creatinine to the eGFR (173). However, the eGFRcystatin C/eGFRcreatinine ratio was not assessed. In the prospective ULSAM study on men, cystatin C was associated with all-cause and cardiovascular mortality (174). In our prospective study, including men and women, the association with a first MI was confined to women. A meta-analysis of cross-sectional studies showed that cystatin C concentrations were associated with MI. However, that study did not address possible differences in risk for men and women (175). In contrast to other studies, no association was found in men, and
45
this is the first study reporting prospective results for women and MI. In MSP, some cardiovascular risk factors were not registered, and therefore we performed a sensitivity analysis excluding those participants, which led to unchanged OR results, supporting the overall findings. There is a well-known strong association between tHcy and renal function, with higher tHcy seen in those with low GFR (30), which can be calculated from creatinine and cystatin C in plasma. Also, as methyl groups are needed for the synthesis of creatine, tHcy is thus influenced by the production of creatinine. Despite this, we found relatively weak associations between tHcy and eGFR based on creatinine and cystatin C. A reason for this weak association is probably because most of the subjects had normal GFR: s. Very few had a reduced eGFR below 60 mL/min/1.73m2; in all individuals, only 0.8% for eGFRcreatinine, and only 6.3% for eGFRcystatin C. The eGFRcystatin C/eGFRcreatinine ratio had the lowest R2, suggesting that the eGFRcystatin C/eGFRcreatinine ratio is not associated with tHcy in the range of the ratio found in this study.
46
General considerations
Methodological aspects
Selection bias Selection bias which reflects systematic errors that arise from the way in which subjects are collected, can be a problem in case-control studies. The cases and referents (papers I, III, and IV) were identified and selected from the same defined population, minimizing selection bias that can appear if cases and referents originate from different populations. The high participation rate in NSHDS should also reduce the effect of potential selection bias due to a low participation rate. Participation in health surveys is more common among people with less risky behavior (176), which may, for example, lead to the under-representation of smokers compared to the general population. The cohort is well-defined, and this ensures that the referents represent all individuals in the study base with a risk of later becoming a case (136, 140). Exclusion of a small proportion of individuals was made due to previous diagnoses, and 0.4% of all survived cases did not consent to personal recording in the MONICA Incidence Registry (140). A minor part of the frozen plasma samples was excluded due to insufficient volume. These exclusions are random and limited and likely have no significant impact on internal validity. The genotype distributions of the referents were in Hardy-Weinberg Equilibrium which argues a selection bias of referents and genotype errors. One bias that cannot be ruled out is a selected survival bias since the inclusion criteria for collected referents are survival. The external validity has been assessed in a comparison between participants and non-participants in the northern Sweden MONICA surveys from 1986, 1990, and 1994 (177) and the VIP (178), altogether indicating that the populations are representative of the people in northern Sweden. Quality assessments of the case registry in the Northern Sweden MONICA Incidence Registry have shown good quality during the study period (139).
Information bias Information bias can be introduced if the classification of exposure or outcome is incorrect. In papers I, and IV with a prospective design, the potential misclassification of exposure would be “nondifferential” since the event was evident after data collection. One possibility would have been to use data from FFQ on intake of folate and vitamin B12. However, in an evaluation study, correlation of individual ranking between reported dietary intake and measured vitamin levels, Vitamin B12 correlated to a lesser extent than folate (179). Thus, by using biomarkers, the misclassification of exposure is minimized. In the adjusted models in papers I, and IV, self-reported smoking data is included. This might have an impact on the strength of the adjusted associations. In a previous MONICA cohort, self-reported smoking habits and biochemical markers of
47
tobacco exposure correlated well (180). The amount of smoking is not assessed in these papers, and probable underreporting should not affect differently in referents and cases. In paper II, self-reported never tobacco use, smoking, and snus use data were used in combination with cotinine assay to improve accuracy in the classification of tobacco habits. Misclassification of tobacco habits would likely result in a dilution of associations. In paper III, exposure is determined by genetic markers. Race and ethnicity can have an impact on results in genetic studies, but no differences between cases and referents are expected in this cohort.
Fluctuations in biomarkers as within-person variability could be a limitation since a single health examination, and blood sampling were performed in the studies. The sample collection done in a standardized manner after 15 minutes of rest minimizes the effects of differences in fluid distribution. Diurnal variations have probably been minimized since the majority of the samples are collected in the morning. The seasonal variation may be considered for some analytes. No variation has been found for tHcy or B12, and only a slight seasonal variation has been seen for folate (181). Also, samples from cases and the referents are matched for the time of blood sampling. All the analytes, including DNA, are stable in the freezer. Adding to this, the use of small, well-sealed tubes, which decrease evaporation and storage in low-temperature freezers (-80º C), will optimize conditions of long-time storage. There is no significant difference in storage time between cases and referents. Accredited analytical methods are used validated by external controls, which reduce potential variability related to measurement.
Multiple testing can be a problem if you have a large number of results variables in the analyses, though this is not the case in these clinical studies. Multicollinearity may exist in multiple models. To minimize the influence of possible multicollinearity in the adjusted models, we added systolic blood pressure, and diastolic blood pressure was omitted (papers I and IV). In paper II, MMA was included, while vitamin B12 was not for the same reason. If multicollinearity is present, multiple regression analysis would have difficulty separating the unique contribution of each predictor, and some of them may be reported as non-significant.
Reverse causality is one of the most important biases to consider in cardiovascular research. Inclusions of only a first-ever MI and blood samples collected at baseline of NSHDS reduce the potential problem of reverse causality from alterations in biomarkers, medical therapy, and lifestyle after an MI.
48
Confounding Confounding factors were handled by matching cases and referents and with multiple adjustments in the statistical models when possible. The matching variables are described in the materials and methods section. The matching approach has a disadvantage as the possibility of evaluating the associated risk with the matched variables is lost. However, these variables, such as age and sex, are already so well-studied that this is not a disadvantage. Stratification for sex to evaluate possible sex differences for risk markers is still possible. Potential confounders, including established CVD risk factors, were statistically adjusted for (papers I, II, and IV).
The possibility of residual confounding, such as alcohol consumption and other potential confounding factors that may be distributed differently between cases and referents and that we have not taken into account, cannot be excluded.
In paper III on genetic markers, using SNPs, one must consider that alterations in a nearby genetic locus may affect the risk if “linkage disequilibrium” is present. This is the case for MTHFR SNPs 677 and 1298, which are within the same gene on chromosome one (1p36.22), but not for CTH, situated approximately 50 million base pairs away (1p31.1), and thus not linked to the MTHFR SNPs. The possible effect of gene-environment interactions is considered partially as tHcy concentrations in the polymorphisms are presented for folate status above and equal/below median concentrations. The matching process where the geographical location of the health care unit is one criterion might complicate the possibility of finding associated risks for the specific SNPs investigated, as the effect of another unknown or not considered a genetic determinant of CVD might override the investigated associations.
Sample size and power Calculations of statistical power ahead of a study are sometimes performed. Ideally, this is done to dimension a study correctly. Estimated expected differences between cases and referents based on previous results or literature data are needed. Calculations can be done with, for example, ClinCalc (https://clincalc.com/Stats/SampleSize.aspx). As an example, eGFRcystatin
C/eGFRcreatinine ratio with a mean value of 0.90 and SD of 0.15 for referents, and a 3% difference compared to cases (enrollment ratio 2:1) show that a minimum of 726 referents and 363 cases are needed for a statistical power of 80% of finding a difference. This estimation used an α of 0.05 (probability of type I error) and a β of 0.2 (probability of a type II error). Choosing two referents instead of one for each case increases power and dilutes possible differences among referents. Thus, 545 MI cases and 1054 referents would be sufficient.
49
In many cases, a pre-study power calculation is more difficult to perform, as many study cohorts with cases and referents have already been designed before the withdrawal of blood samples from a biobank. This is the case for cohorts in this thesis, as all available MI cases according to standardized criteria during the actual time period were included. These studies have been based on power calculations for some, but not for all, variables. In situations like this, post-hoc power calculations can be performed using, for example, ClinCalc (https://clincalc.com/Stats/Power.aspx ). Using data from paper IV (Table 1) on the eGFRcystatin C/eGFRcreatinine ratio as an example showed that in women with a mean (SD) of 0.87 (0.17) for 154 cases and 0.93 (0.17) for 290 referents (6.9% difference), there was a post-hoc power of 94.3%. For men, with mean (SD) 0.91 (0.20) for 366 cases, and 0.93 (0.19) for 695 referents (3.3% difference) there was a post-hoc power of 35%. These calculations were performed using α=0.05.
Limitations Limitations in our studies are that only specific age groups are available, and in the Swedish population, the minor allele frequencies of both the CTH and the two MTHFR polymorphisms are relatively low compared to South/Central European populations. This reduces our power to detect significant effects. In paper II, the strict definition of smokers and snus users, which is a strength, entails a relatively low number of exclusively snus users since dual use of both smoking and snus use is common. Other limitations are that no other compounds in cigarette smoke, such as thiocyanate, were measured. The population was initially a case-referent cohort, which could be a possible limitation. However, adding case-referent status to the multiple regression models in a sensitivity analysis did not change the results.
Concerning renal function, one limitation is that the reference method, Iohexol-clearance, or other invasive methods, are not available in our biobank samples. We also do not have access to urine for measuring the U-albumin/creatinine ratio, another established marker for renal dysfunction.
Sex differences Male sex is considered an established risk factor of MI. The mean age (SD) of a first-ever MI among men in the Northern Sweden MONICA study is 55.4 (±6.9) years and among females 56.4 (±6.9) years (182). The age-standardized total event trends in a first-ever MI in this population have declined between 1985 and 2004, more prominent among men than women. Explanations may be different clinical manifestations, but traditional risk factors have been suggested to explain a major part. Globally smoking ranks first among men as a risk factor for all-cause all-age deaths, while among women, smoking is ranked as number eighth (52).
50
Traditional risk factors are generally lower in females compared to males in Sweden, though smoking is an exception (182). In the Northern Sweden MONICA Study, women showed better long-term survival after a first MI than men (183). Women are less susceptible to sudden cardiac death than men, but plaque erosion as the cause of AMI in 25-30% of cases of acute coronary syndrome is more common in women (184). We noted sex differences in our main findings concerning the risk of a first-ever MI in papers III and IV, where the associated risk was found among females but not among males. The sex differences in risk and pathophysiology of cardiovascular disease, in general, have been explained in various ways (185). Sex hormones, especially estrogen, have been suggested as protective, although the mechanisms are not fully understood. During menopause, estrogen levels drop 90% from pre-menopausal levels, and it may in part contribute to the rise in CVD events in post-menopausal women. LDL cholesterol levels confer similar CVD risk in women and men, while non-fasting triglycerides affect women's risk of MI to a greater extent than in men (185). In post-menopausal women, an apparent rise is seen in LDL cholesterol levels, while HDL cholesterol levels decrease. A cross-sectional study with data from the population-based Dallas Heart Study noted differences between men and women among many cardiometabolic biomarkers (186). Cystatin C and eGFRcreatinine were among those that differed, with lower concentrations of cystatin C among women than men in models adjusted for traditional risk factors. Differences in muscle mass may be another reason for creatinine (paper IV). However, in part, this is taken into account, as sex and age are included in the formula as calculating eGFRcreatinine (91). Differences based on sex or muscle mass are not seen for eGFRcystatin C (89). Also, in our studies, cases and referents were matched for age and sex. In paper IV, the different findings in men and women are in agreement with the study on the prospective risk of future surgery for aortic stenosis and the eGFRcystatin C/eGFRcreatinine ratio (171), where the associated risk in models adjusted for traditional risk factors including smoking, was found only among women with coronary artery disease at the time of surgery. Our results strengthen the indication that changes in the eGFRcystatin C/eGFRcreatinine ratio may be associated with atherosclerosis in women.
51
Homocysteine as a risk determinant of MI Homocysteine is determined by interactions between genes, diet, lifestyle, and other diseases. An association has been seen with MI and tHcy, but it has not been proven as causal. A recent MR study has not strengthened any causal relationship (187). However, there are aspects to consider when interpreting these studies, such as assumptions of MR not met (45). One example is that folate status has a known impact on the association between tHcy and MTHFR C677T (188).
If tHcy associates not only with MI but also with cardiovascular risk factors not adjusted for, the effects of these related factors might bias the association with MI. However, the association with MI persists in studies adjusted with known determinants of tHcy and established risk factors. This indicates that additional mechanisms mediate the impact of homocysteine on the risk of MI. The contribution of different genetic backgrounds may partly explain differences in findings for the genetic polymorphisms between populations. The frequencies of the MTHFR TT allele are reported lowest (<10%) in Canadian Inuit and highest (close to 60%) in Mexico (189). Also, the approach to mandatory folate fortification varies in countries, which leads to differences in gene-environment interactions, which may explain the dissimilarity in results. Multiple factors, including genes, diet, and lifestyle, influence the risk of CVD as well as tHcy. Parts of these different, multi-faceted aspects regarding homocysteine and MI have been the subjects of this thesis.
The association between Apo A1 and folate (paper I) remained after adjusting with established CVD risk factors and was evident among the referents only. Since folate is inversely associated with MI in this cohort (150), our results may suggest that it mirrors a protective effect of folate for MI, possibly mediated by epigenetic effects of methylation. However, a consequence of lower statistical power for cases cannot be excluded. The absence of an association between tHcy and Apo A1 may be explained by a low frequency among all subjects (13%) having tHcy concentrations above 15 µmol/L. An association between ApoA1 and tHcy in individuals having higher tHcy concentrations cannot be excluded. In recent years HDL-C has been challenged concerning its protective effect on CVD. The relationship between HDL-C and CVD mortality in the elderly is U-shaped (190). The many HDL-associated proteins (61) and HDL's complex functions may be why RCTs have failed to show a beneficial effect of increasing HDL or Apo A1 (191). Thus, it is no longer correct to refer to HDL-C as solely the “good cholesterol.”
Impaired renal function is a risk factor for CVD, and tHcy was associated with an increased risk of a first MI independent of cystatin C (150). In paper IV, renal
52
function estimated by eGFRcystatin C and eGFRcystatin C/eGFRcreatinine ratio were related to the risk of MI for women. This was also seen in models including tHcy. Many studies emphasize the strong correlation between glomerular filtration rate and tHcy (192). In one study, subjects older than 65 showed an increase in creatinine of 1%, which was associated with a 1% increase of tHcy (193). In our material, eGFRcreatinine and eGFRcystatin C were statistically significantly associated, but R2 values were low. Thus, it is not clinically significant, and there is no clinical value to adjust tHcy in patients with an eGFR >60 mL/min/1.73m2.
A primary strength of the studies in this thesis compared to many observational studies in this field is the design where we could study subjects that did not have previous MI, stroke, or cancer at inclusion. This minimizes the risk for reverse causation.
The cohort origins in the Northern Swedish population with a high mean participation rate and outcome, including both men and women, enable to address and identify potential sex differences. The standardized and quality assessed laboratory procedures, both preanalytical and analytical, and the strict definition of tobacco use (paper II) are other important strengths adding to good generalizability. The prospective case-referent design also has the advantage of including fatal MI cases (paper III) with a reasonably large number of female cases compared to previous studies. Albeit the findings in this paper were evident after stratification entailing the interpretations are made with caution, the findings can be regarded as hypothesis-generating.
A healthy lifestyle and optimal treatment for known causal risk factors are essential in relation to CVD. Population screening of tHcy for CVD risk is not recommended, though in young CVD patients <40 years, it is recommended to exclude homocystinuria, and in CVD patients or patients at high risk, high tHcy concentrations should be used as a prognostic factor, with >15 µmol/L belongs to a high-risk group (11). Still, there is an indication for tHcy in routine clinical diagnosis of B12- and folate deficiency in anemia and neurological diseases. A recent review concludes that the optimal concentration of tHcy where action to lower concentrations is appropriate can for major diseases such as stroke or cognitive impairment, or dementia be lower. The risk of adverse effects may be seen from 11 µmol/L, where concentrations above 13 µmol/L more certainly can lead to harmful impacts (188). The causal relationship between tHcy and CVD is not established but is still debated. There are many aspects to consider that may impact the relationship. Some of them are difficult to take into consideration or adjust for in research studies involving humans. Intervention studies are performed over a limited time
53
while the atherosclerotic process, on the other hand, is evolving over a lifetime. Adding to this, the one-carbon metabolism, including homocysteine, is a fundamental metabolic process in every tissue and the methylation processes may lead to epigenetic effects that may remain over time.
54
Conclusions and future perspectives
This thesis shows that folate, but not tHcy, was independently associated with Apo A1, which emphasizes that studies on homocysteine and endpoints or biomarkers need to adjust for the possible confounding effects. The association was evident among the referents but not among those who developed a first-ever MI. The results suggest a possible link between one-carbon metabolism and lipid metabolism via folate-dependent epigenetic effects in genes. The independent association between cotinine and tHcy among smokers but not among snus users shows that smoking is a stronger determinant for tHcy in plasma than snus use. This indicates that nicotine per se may not be the cause or mediator of higher tHcy concentrations. Instead, other substances formed from combusted tobacco may cause an increase in tHcy. This aspect is of interest for the future since newer forms of tobacco, “heat-not-burn tobacco products” containing different proportions of nicotine and generating different amounts of combustion products, are introduced on the market. However, future studies on these newer types of tobacco are needed to establish possible relationships. Plasma cotinine appears to be a better predictor of tHcy concentrations than self-reported smoking data. Whenever possible, self-reported smoking should be supplemented by biomarker assays, such as cotinine, in epidemiological studies. Our cross-sectional study design cannot establish a causal relationship. Intervention studies on smoking or snus cessation would be a possibility to evaluate the relationship further. The polymorphisms CTH G1208T rs1021737 and MTHFR A1298G rs1801131 were associated with a higher risk of prospectively developing a first-ever MI with fatal outcome among women. No associations were seen among all MI patients. For the CTH G1208T polymorphism, tHcy concentrations did not differ between subjects with GG, GT, or TT genotypes, while an expected difference was seen for MTHFR C677T. A difference in tHcy concentrations was seen for the MTHFR A1298C polymorphism among men. After outcome stratification (fatal or non-fatal MI), the results may indicate that women with the minor allele are at risk of having a more serious MI leading to death than women with the wild-type alleles. The genotype may have a differential influence on a fatal or non-fatal outcome in men and women. This may reflect differences in biochemical and pathophysiological mechanisms that need to be further studied. CVD is the leading cause of all deaths globally (31), and the possibility of additional primary prevention strategies would most likely reduce mortality. The results need to be verified in other populations in future studies. The ongoing NSHDS and the Northern Sweden MONICA incidence registry with later MI cohorts give this opportunity.
55
In a prospective setting, mild impairment of renal function defined by eGFRcystatin
C/eGFR creatinine ratio was independently associated with an increased risk of later developing a first-ever MI among women both through quartiles and continually expressed as z-scores. The risk association was stronger compared to both eGFRcystatin C and eGFRcreatinine used separately. The eGFRcystatin C/eGFRcreatinine ratio is a possible tool in accessing CVD risk. However, the potential predictive ability for the eGFRcystatin C/eGFRcreatinine ratio of MI needs to be assessed further. This requires analytical approaches that are different from those used in this thesis. If further studies confirm our findings on MI, the eGFRcystatin C/eGFRcreatinine ratio can be used in clinical practice for this indication in the future. One advantage is the easy implementation in hospital laboratory practice since no additional measurement methods are needed. The studies included in this thesis did find associations between determinants of tHcy, and Apo A1 and the risk of a future first-ever MI. Our results suggest differences in findings both in the aspect of sex and fatal vs. non-fatal MI. These findings are worth considering and evaluating further since previous studies in this field often not have approached these aspects. Also, knowledge of one-carbon metabolism is evolving in all areas, including genetics, epigenetics, proteomics, metabolomics, and microbiota. This will probably facilitate the use of personalized medicine for different diseases and conditions, including CVD, in the future.
56
Acknowledgments During the years of work that have resulted in this thesis, many people have contributed in one way or another. My warmest thank to all of you! However, I want to express my special thanks to the following people:
Johan Hultdin, my supervisor during my research training, for all the support, exciting discussions, and help with everything I have needed. Also, friendship and entertainment talk about everything and nothing in between.
Torbjörn Nilsson, my co-supervisor, for friendly and skillful guidance. Always finding creative suggestions and wise interpretations and for the great company at conferences.
Kjell Granqvist, for introducing me to the medical biosciences department when it all started, and for all the support along this journey.
All my co-authors; Mats Eliasson also for introducing me to the MONICA project and tutorial guidance, Owe Johnson, Göran Hallmans, Lars Weinehall, Jan-Håkan Jansson, Ingegerd Johansson, Ravna Blind, Bethany van Guelpen, Patrik Wennberg, Stefan Söderberg, Jørn Schneede, Per-Magne Ueland, Øivind Midttun, Björn Gylling, and Jonas Andersson for inestimable, engaging comments and discussions.
Robert Lundqvist for enthusiastic statistical guidance and boosting. Kim Ekblom, for his encouraging support and wise answers to all kinds of questions considering my research training. Lovisa Widbom and Emil Hällstig for excellent assistance with figures, formats, and pixels.
Laboratory medicine in Norrbotten County Council, with Karin Jones, Head of the Department. All former and present colleagues who have inspired and supported me at the Clinical Chemistry department in Sunderbyn and Umeå and made it possible to spend the necessary time on this thesis. Rebecca for supporting and boosting me, Mia, and Maria for "paper-celebration" and watering my only flower at my hospital office during the intense working period on my thesis.
The Biobank Research Unit, with Åsa Ågren and Ingvar Bergdahl. All participants in VIP, MONICA, and MSP and their principal investigators, maintaining these cohorts on-going and available for research.
Terry Persson and Carina Ahlgren at the department of Medical Biosciences for all administration and practical planning needed. Johanna Törmä and Ella-Karin Blomqvist at the Research Unit (FOI) in Norrbotten for help with the required practical planning.
Elinor Ljungberg, my close study mate, and friend since medical school, for sharing my research interest.
57
My very good friends in the neighborhood and Seskarö as well as in other parts of Sweden and my friends in our close faithful book group. Thanks for all the nice, fantastic dinners, excellent company, beautiful ski experiences, nice “fikas”, unforgettable boat trips, and support on distance during these last two years of restrictions.
My parents, Karin and Jan Dahl, for always believing in me and for your unconditional love. Always being there for my family and me in Norrbotten and where else in the world. My father, for inspiring me in academic research since I was young, and my mother with her creative and positive mind; "Det fixar vi, inga problem!" My brother Joel Dahl and his whole family for all your love and fantastic caring minds.
Marianne and Torsten Söderström, my parents in law for all your love and support for my family and me. We have not least beautiful memories from South Africa were we also enjoyed celebrating my first "acceptance" together! David and Johanna with families, always nice being with you!
Klara, Gustav och Olof mina älskade barn för att ni haft extra tålamod under intensiva arbetsperioder och dessutom servat med goda smoothies. Klara stort tack för hjälp med omslaget! Ni är bäst! Efter denna disputation kommer vi ta igen mysstunder som uteblivit sista tiden. Billie för troget sällskap och uppiggande promenader.
Andreas, my loving husband, and partner for life. You are inestimable, thank you for everything!
58
Financial support This work was supported by the Unit of Clinical Chemistry, Department of Medical Biosciences, Umeå University. Working Life and Welfare (former Swedish Council for Working Life and Social Research) to Ingegerd Johansson. SNF Swedish Nutrition Foundation and Swedish Research Council for Health. Visare Norr, Västerbotten County Council, and Norrbotten County Council, the latter also provided open access funding. Biobank Sweden was supported by the Swedish Research Council [V.R. 2017-00650].
59
References
1. Carson NA, Neill DW. Metabolic abnormalities detected in a survey of mentally backward individuals in Northern Ireland. Arch Dis Child. 1962;37:505-13. 2. Mudd SH, Finkelstein JD, Irreverre F, Laster L. Homocystinuria: An Enzymatic Defect. Science. 1964;143(3613):1443-5. 3. Gibson JB, Carson NA, Neill DW. Pathological Findings in Homocystinuria. J Clin Pathol. 1964;17:427-37. 4. Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu Rev Anim Biosci. 2019;7:263-87. 5. Finkelstein JD, Martin JJ. Homocysteine. Int J Biochem Cell Biol. 2000;32(4):385-9. 6. Kang SS, Wong PW, Susmano A, Sora J, Norusis M, Ruggie N. Thermolabile methylenetetrahydrofolate reductase: an inherited risk factor for coronary artery disease. Am J Hum Genet. 1991;48(3):536-45. 7. Finkelstein JD. Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost. 2000;26(3):219-25. 8. Donnarumma E, Trivedi RK, Lefer DJ. Protective Actions of H2S in Acute Myocardial Infarction and Heart Failure. Compr Physiol. 2017;7(2):583-602. 9. Sbodio JI, Snyder SH, Paul BD. Regulators of the transsulfuration pathway. Br J Pharmacol. 2019;176(4):583-93. 10. Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH. Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem. 1993;39(9):1764-79. 11. Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50(1):3-32. 12. Rifai N. Tietz textbook of Clinical Chemsitry and molecular diagnostics. 6 ed: Elsevier; 2018. 13. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39-54. 14. Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337(4):230-6. 15. Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349:g5226. 16. de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29(2 Suppl):S238-44. 17. Shulpekova Y, Nechaev V, Kardasheva S, Sedova A, Kurbatova A, Bueverova E, et al. The Concept of Folic Acid in Health and Disease. Molecules. 2021;26(12). 18. Shih CC, Shih YL, Chen JY. The association between homocysteine levels and cardiovascular disease risk among middle-aged and elderly adults in Taiwan. BMC Cardiovasc Disord. 2021;21(1):191. 19. Sobczak A, Wardas W, Zielinska-Danch W, Pawlicki K. The influence of smoking on plasma homocysteine and cysteine levels in passive and active smokers. Clin Chem Lab Med. 2004;42(4):408-14.
60
20. Iqbal MP, Yakub M. Smokeless tobacco use: a risk factor for hyperhomocysteinemia in a Pakistani population. PLoS One. 2013;8(12):e83826. 21. Marano KM, Kathman SJ, Jones BA, Nordskog BK, Brown BG, Borgerding MF. Study of cardiovascular disease biomarkers among tobacco consumers. Part 3: evaluation and comparison with the US National Health and Nutrition Examination Survey. Inhal Toxicol. 2015;27(3):167-73. 22. Ulvik A, Ebbing M, Hustad S, Midttun O, Nygard O, Vollset SE, et al. Long- and short-term effects of tobacco smoking on circulating concentrations of B vitamins. Clin Chem. 2010;56(5):755-63. 23. Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Najjar MF. Effect of cigarette smoking on plasma homocysteine concentrations. Clin Chem Lab Med. 2011;49(3):479-83. 24. Dyer AR, Elliott P, Stamler J, Chan Q, Ueshima H, Zhou BF, et al. Dietary intake in male and female smokers, ex-smokers, and never smokers: the INTERMAP study. J Hum Hypertens. 2003;17(9):641-54. 25. Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, Rasanen SM, et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018;27(3):559-71 e5. 26. Gellekink H, den Heijer M, Heil SG, Blom HJ. Genetic determinants of plasma total homocysteine. Semin Vasc Med. 2005;5(2):98-109. 27. van Meurs JB, Pare G, Schwartz SM, Hazra A, Tanaka T, Vermeulen SH, et al. Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am J Clin Nutr. 2013;98(3):668-76. 28. Kim J, Lei Y, Guo J, Kim SE, Wlodarczyk BJ, Cabrera RM, et al. Formate rescues neural tube defects caused by mutations in Slc25a32. Proc Natl Acad Sci U S A. 2018;115(18):4690-5. 29. Boachie J, Adaikalakoteswari A, Goljan I, Samavat J, Cagampang FR, Saravanan P. Intracellular and Tissue Levels of Vitamin B12 in Hepatocytes Are Modulated by CD320 Receptor and TCN2 Transporter. Int J Mol Sci. 2021;22(6). 30. Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. J Am Soc Nephrol. 2001;12(10):2181-9. 31. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-128. 32. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1-25. 33. Statistik om dödsorsaker år 2020. Socialstyrelsen; 2021. 34. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56. 35. Gimbrone MA, Jr., Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. 2016;118(4):620-36. 36. Wang R, Szabo C, Ichinose F, Ahmed A, Whiteman M, Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol Sci. 2015;36(9):568-78.
61
37. Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46(6):937-54. 38. Molecular Epidemiology: Principles and practices International Agency for Research on Cancer, France, WHO; 2011. 39. Aronson JK, Ferner RE. Biomarkers-A General Review. Curr Protoc Pharmacol. 2017;76:9 23 1-9 17. 40. Brown TM, Bittner V. Biomarkers of atherosclerosis: clinical applications. Curr Cardiol Rep. 2008;10(6):497-504. 41. 28 ed: W.B. Saunders Company; 1994. Dorlands Illustrated Medical Dictionary 42. Dahlqvist G. Fetal virusinfektion en riskfaktor för typ 1-diabetes hos barn. Lakartidningen. 2000;97(4):313-5. 43. Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295-300. 44. Bennett DA, Holmes MV. Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart. 2017;103(18):1400-7. 45. Smulders YM, Blom HJ. The homocysteine controversy. J Inherit Metab Dis. 2011;34(1):93-9. 46. Sattar N, Preiss D. Reverse Causality in Cardiovascular Epidemiological Research: More Common Than Imagined? Circulation. 2017;135(24):2369-72. 47. Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999-1008. 48. Andersson C, Johnson AD, Benjamin EJ, Levy D, Vasan RS. 70-year legacy of the Framingham Heart Study. Nat Rev Cardiol. 2019;16(11):687-98. 49. Score working group and E. S. C. Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42(25):2439-54. 50. The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251(3):351-64. 51. Raju TN. The Nobel chronicles. 1985: Joseph Leonard Goldstein (b 1940), Michael Stuart Brown (b 1941). Lancet. 2000;355(9201):416. 52. Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923-94. 53. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-88. 54. Namiri-Kalantari R, Gao F, Chattopadhyay A, Wheeler AA, Navab KD, Farias-Eisner R, et al. The dual nature of HDL: Anti-Inflammatory and pro-Inflammatory. Biofactors. 2015;41(3):153-9. 55. Cholesterol Treatment Trialists C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive
62
lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-81. 56. Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 x 2 factorial Mendelian randomization study. J Am Coll Cardiol. 2015;65(15):1552-61. 57. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-72. 58. Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539-50. 59. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA. 2016;316(12):1289-97. 60. Diffenderfer MR, Schaefer EJ. The composition and metabolism of large and small LDL. Curr Opin Lipidol. 2014;25(3):221-6. 61. Davidson WS, Shah AS, Sexmith H, Gordon SM. The HDL Proteome Watch: Compilation of studies leads to new insights on HDL function. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(2):159072. 62. Ruscica M, Sirtori CR, Corsini A, Watts GF, Sahebkar A. Lipoprotein(a): Knowns, unknowns and uncertainties. Pharmacol Res. 2021;173:105812. 63. Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. 2017;69(6):692-711. 64. McConnell JP, Guadagno PA, Dayspring TD, Hoefner DM, Thiselton DL, Warnick GR, et al. Lipoprotein(a) mass: a massively misunderstood metric. J Clin Lipidol. 2014;8(6):550-3. 65. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713-22. 66. Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, et al. Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis. JAMA Cardiol. 2018;3(7):619-27. 67. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903-13. 68. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953-2041.
63
69. Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387(10017):435-43. 70. Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509-15. 71. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731-7. 72. Hansson J, Galanti MR, Hergens MP, Fredlund P, Ahlbom A, Alfredsson L, et al. Use of snus and acute myocardial infarction: pooled analysis of eight prospective observational studies. Eur J Epidemiol. 2012;27(10):771-9. 73. Vidyasagaran AL, Siddiqi K, Kanaan M. Use of smokeless tobacco and risk of cardiovascular disease: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(18):1970-81. 74. Boffetta P, Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis. BMJ. 2009;339:b3060. 75. Fried ND, Gardner JD. Heat-not-burn tobacco products: an emerging threat to cardiovascular health. Am J Physiol Heart Circ Physiol. 2020;319(6):H1234-H9. 76. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255-323. 77. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-81. 78. Emerging Risk Factors C, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215-22. 79. Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776-86. 80. Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk--a review of the literature. Eur J Clin Nutr. 2010;64(1):16-22. 81. Ganji V, Kafai MR, Third National H, Nutrition Examination S. Demographic, health, lifestyle, and blood vitamin determinants of serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2003;77(4):826-33. 82. Elshorbagy AK, Nurk E, Gjesdal CG, Tell GS, Ueland PM, Nygard O, et al. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: does cysteine link amino acid and lipid metabolism? Am J Clin Nutr. 2008;88(3):738-46.
64
83. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339-52. 84. Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514-25. 85. Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737-45. 86. Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305(15):1545-52. 87. Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-43. 88. Nerpin E, Ingelsson E, Riserus U, Sundstrom J, Larsson A, Jobs E, et al. The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrol Dial Transplant. 2011;26(9):2820-7. 89. Grubb A, Horio M, Hansson LO, Bjork J, Nyman U, Flodin M, et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem. 2014;60(7):974-86. 90. Bjork J, Grubb A, Sterner G, Nyman U. Revised equations for estimating glomerular filtration rate based on the Lund-Malmo Study cohort. Scand J Clin Lab Invest. 2011;71(3):232-9. 91. Nyman U, Grubb A, Larsson A, Hansson LO, Flodin M, Nordin G, et al. The revised Lund-Malmo GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med. 2014;52(6):815-24. 92. Miller WG, Kaufman HW, Levey AS, Straseski JA, Wilhelms KW, Yu HE, et al. National Kidney Foundation Laboratory Engagement Working Group Recommendations for Implementing the CKD-EPI 2021 Race-Free Equations for Estimated Glomerular Filtration Rate: Practical Guidance for Clinical Laboratories. Clin Chem. 2021. 93. Grubb A. Shrunken pore syndrome - a common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology and treatment options. Clin Biochem. 2020;83:12-20. 94. Zhou H, Yang M, He X, Xu N. eGFR, cystatin C and creatinine in shrunken pore syndrome. Clin Chim Acta. 2019;498:1-5. 95. Grubb A. Glomerular filtration and shrunken pore syndrome in children and adults. Acta Paediatr [Internet]. 2021 Mar 19 Mar 19]:[1-6 pp.]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/33742469. 96. Grubb A, Lindström V, Jonsson M, Back SE, Ahlund T, Rippe B, et al. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: 'Shrunken pore syndrome'. Scand J Clin Lab Invest. 2015;75(4):333-40.
65
97. Cachat F, Combescure C, Cauderay M, Girardin E, Chehade H. A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol. 2015;10(3):382-9. 98. Dupuis ME, Nadeau-Fredette AC, Madore F, Agharazii M, Goupil R. Association of Glomerular Hyperfiltration and Cardiovascular Risk in Middle-Aged Healthy Individuals. JAMA Netw Open. 2020;3(4):e202377. 99. Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ. Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol. 2015;26(6):1426-33. 100. Palatini P, Benetti E, Zanier A, Santonastaso M, Mazzer A, Cozzio S, et al. Cystatin C as predictor of microalbuminuria in the early stage of hypertension. Nephron Clin Pract. 2009;113(4):c309-14. 101. Rognant N, Laville M. To live with normal GFR: when higher is not better. Kidney Int. 2014;86(1):10-3. 102. Eriksen BO, Lochen ML, Arntzen KA, Bertelsen G, Eilertsen BA, von Hanno T, et al. Subclinical cardiovascular disease is associated with a high glomerular filtration rate in the nondiabetic general population. Kidney Int. 2014;86(1):146-53. 103. Mickelsson M, Söderström E, Stefansson K, Andersson J, Söderberg S, Hultdin J. Smoking tobacco is associated with renal hyperfiltration. Scand J Clin Lab Invest. 2021;81(8):622-8. 104. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20-8. 105. Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132-40. 106. Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):483-95. 107. Arnett DK, Baird AE, Barkley RA, Basson CT, Boerwinkle E, Ganesh SK, et al. Relevance of genetics and genomics for prevention and treatment of cardiovascular disease: a scientific statement from the American Heart Association Council on Epidemiology and Prevention, the Stroke Council, and the Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2007;115(22):2878-901. 108. Barth AS, Tomaselli GF. Gene scanning and heart attack risk. Trends Cardiovasc Med. 2016;26(3):260-5. 109. Soutar AK, Naoumova RP. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med. 2007;4(4):214-25. 110. Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60(25):2631-9. 111. White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, et al. Association of Lipid Fractions With Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol. 2016;1(6):692-9.
66
112. Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Davey Smith G, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020;17(3):e1003062. 113. Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578-88. 114. Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567-77. 115. Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565-75. 116. Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin Intervention For Stroke Prevention trial: an efficacy analysis. Stroke. 2005;36(11):2404-9. 117. Miller ER, 3rd, Juraschek S, Pastor-Barriuso R, Bazzano LA, Appel LJ, Guallar E. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106(4):517-27. 118. Clarke R, Bennett DA, Parish S, Verhoef P, Dotsch-Klerk M, Lathrop M, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9(2):e1001177. 119. Marti-Carvajal AJ, Sola I, Lathyris D, Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:CD006612. 120. Alizadeh S, Djafarian K, Moradi S, Shab-Bidar S. C667T and A1298C polymorphisms of methylenetetrahydrofolate reductase gene and susceptibility to myocardial infarction: A systematic review and meta-analysis. Int J Cardiol. 2016;217:99-108. 121. Wang J, Hegele RA. Genomic basis of cystathioninuria (MIM 219500) revealed by multiple mutations in cystathionine gamma-lyase (CTH). Hum Genet. 2003;112(4):404-8. 122. Wang J, Huff AM, Spence JD, Hegele RA. Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration. Clin Genet. 2004;65(6):483-6. 123. Nigwekar SU, Kang A, Zoungas S, Cass A, Gallagher MP, Kulshrestha S, et al. Interventions for lowering plasma homocysteine levels in dialysis patients. Cochrane Database Syst Rev. 2016(5):CD004683. 124. Liao D, Yang X, Wang H. Hyperhomocysteinemia and high-density lipoprotein metabolism in cardiovascular disease. Clin Chem Lab Med. 2007;45(12):1652-9. 125. McCully KS. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev Clin Pharmacol. 2015;8(2):211-9. 126. Friso S, Udali S, De Santis D, Choi SW. One-carbon metabolism and epigenetics. Mol Aspects Med. 2017;54:28-36.
67
127. Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005;135(1):5-8. 128. Gueant JL, Namour F, Gueant-Rodriguez RM, Daval JL. Folate and fetal programming: a play in epigenomics? Trends Endocrinol Metab. 2013;24(6):279-89. 129. Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135(6):1382-6. 130. Janani C, Ranjitha Kumari BD. PPAR gamma gene--a review. Diabetes Metab Syndr. 2015;9(1):46-50. 131. Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116(12):3090-100. 132. Luc G, Jacob N, Bouly M, Fruchart JC, Staels B, Giral P. Fenofibrate increases homocystinemia through a PPARalpha-mediated mechanism. J Cardiovasc Pharmacol. 2004;43(3):452-3. 133. Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, et al. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest. 2009;119(1):110-24. 134. Laha A, Majumder A, Singh M, Tyagi SC. Connecting homocysteine and obesity through pyroptosis, gut microbiome, epigenetics, peroxisome proliferator-activated receptor gamma, and zinc finger protein 407. Can J Physiol Pharmacol. 2018;96(10):971-6. 135. Morellato AE, Umansky C, Pontel LB. The toxic side of one-carbon metabolism and epigenetics. Redox Biol. 2021;40:101850. 136. Norberg M, Wall S, Boman K, Weinehall L. The Vasterbotten Intervention Programme: background, design and implications. Glob Health Action. 2010;3. 137. Kuulasmaa K, Tunstall-Pedoe H, Dobson A, Fortmann S, Sans S, Tolonen H, et al. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet. 2000;355(9205):675-87. 138. Hallmans G, Agren A, Johansson G, Johansson A, Stegmayr B, Jansson JH, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort - evaluation of risk factors and their interactions. Scand J Public Health Suppl. 2003;61:18-24. 139. Eriksson M, Stegmayr B, Lundberg V. MONICA quality assessments. Scand J Public Health Suppl. 2003;61:25-30. 140. Stegmayr B, Lundberg V, Asplund K. The events registration and survey procedures in the Northern Sweden MONICA Project. Scand J Public Health Suppl. 2003;61:9-17. 141. Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90(1):583-612. 142. Myte R, Gylling B, Schneede J, Ueland PM, Haggstrom J, Hultdin J, et al. Components of One-carbon Metabolism Other than Folate and Colorectal Cancer Risk. Epidemiology. 2016;27(6):787-96.
68
143. Van Guelpen B, Hultdin J, Johansson I, Hallmans G, Stenling R, Riboli E, et al. Low folate levels may protect against colorectal cancer. Gut. 2006;55(10):1461-6. 144. Grankvist K, Gomez R, Nybo M, Lima-Oliveira G, von Meyer A. Preanalytical aspects on short- and long-term storage of serum and plasma. Diagnosis (Berl). 2019;6(1):51-6. 145. Nauck M, Bisse E, Nauck M, Wieland H. Pre-analytical conditions affecting the determination of the plasma homocysteine concentration. Clin Chem Lab Med. 2001;39(8):675-80. 146. Kelleher BP, Broin SD. Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J Clin Pathol. 1991;44(7):592-5. 147. Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43-53. 148. Midttun O, Kvalheim G, Ueland PM. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal Bioanal Chem. 2013;405(6):2009-17. 149. Meyer K, Fredriksen A, Ueland PM. MALDI-TOF MS genotyping of polymorphisms related to 1-carbon metabolism using common and mass-modified terminators. Clin Chem. 2009;55(1):139-49. 150. Van Guelpen B, Hultdin J, Johansson I, Witthoft C, Weinehall L, Eliasson M, et al. Plasma folate and total homocysteine levels are associated with the risk of myocardial infarction, independently of each other and of renal function. J Intern Med. 2009;266(2):182-95. 151. Drenos F, Talmud PJ, Casas JP, Smeeth L, Palmen J, Humphries SE, et al. Integrated associations of genotypes with multiple blood biomarkers linked to coronary heart disease risk. Hum Mol Genet. 2009;18(12):2305-16. 152. Mikael LG, Genest J, Jr., Rozen R. Elevated homocysteine reduces apolipoprotein A-I expression in hyperhomocysteinemic mice and in males with coronary artery disease. Circ Res. 2006;98(4):564-71. 153. Mikael LG, Rozen R. Homocysteine modulates the effect of simvastatin on expression of ApoA-I and NF-kappaB/iNOS. Cardiovasc Res. 2008;80(1):151-8. 154. Devlin AM, Lentz SR. ApoA-I: a missing link between homocysteine and lipid metabolism? Circ Res. 2006;98(4):431-3. 155. Liao D, Tan H, Hui R, Li Z, Jiang X, Gaubatz J, et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I Protein synthesis and enhancing HDL cholesterol clearance. Circ Res. 2006;99(6):598-606. 156. Xiao Y, Zhang Y, Lv X, Su D, Li D, Xia M, et al. Relationship between lipid profiles and plasma total homocysteine, cysteine and the risk of coronary artery disease in coronary angiographic subjects. Lipids Health Dis. 2011;10:137. 157. Christensen B, Landaas S, Stensvold I, Djurovic S, Retterstol L, Ringstad J, et al. Whole blood folate, homocysteine in serum, and risk of first acute myocardial infarction. Atherosclerosis. 1999;147(2):317-26. 158. Yang N, Yao Z, Miao L, Liu J, Gao X, Xu Y, et al. Homocysteine diminishes apolipoprotein A-I function and expression in patients with hypothyroidism: a cross-sectional study. Lipids Health Dis. 2016;15:123.
69
159. Wang Y, Liu J, Jiang Y, Zhang H, Leng S, Wang G. Hyperhomocysteinemia is associated with decreased apolipoprotein AI levels in normal healthy people. BMC Cardiovasc Disord. 2016;16:10. 160. Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999-2000. Am J Clin Nutr. 2005;82(2):442-50. 161. Beydoun MA, Beydoun HA, MacIver PH, Hossain S, Canas JA, Evans MK, et al. Biochemical and Hematological Correlates of Elevated Homocysteine in National Surveys and a Longitudinal Study of Urban Adults. Nutrients. 2020;12(4). 162. Ludicke F, Picavet P, Baker G, Haziza C, Poux V, Lama N, et al. Effects of Switching to the Menthol Tobacco Heating System 2.2, Smoking Abstinence, or Continued Cigarette Smoking on Clinically Relevant Risk Markers: A Randomized, Controlled, Open-Label, Multicenter Study in Sequential Confinement and Ambulatory Settings (Part 2). Nicotine Tob Res. 2018;20(2):173-82. 163. Giannakopoulou E, Konstantinou F, Ragia G, Gerontitis Z, Tavridou A, Papapetropoulos A, et al. Association study of the CTH 1364 G>T polymorphism with coronary artery disease in the Greek population. Drug Metab Pers Ther. 2019;34(1). 164. Zhou W, Yang Q, Yu H, Zhang Q, Zou Y, Chen X, et al. Association between an indel polymorphism within CTH and the risk of sudden cardiac death in a Chinese population. Leg Med (Tokyo). 2020;46:101736. 165. Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, et al. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288(16):2023-31. 166. Rallidis LS, Kosmas N, Stathopoulou E, Rallidi M, Gialeraki A. Homozygosity of the TT methylenetetrahydrofolate reductase C677T genotype is an independent long-term predictor of cardiac death in patients with premature myocardial infarction. Curr Med Res Opin. 2021;37(7):1079-84. 167. Liu F, Du J, Nie M, Fu J, Sun J. 5,10-methylenetetrahydrofolate reductase C677T gene polymorphism and peripheral arterial disease: A meta-analysis. Vascular. 2021;29(6):913-9. 168. Christensson A, Grubb A, Molvin J, Holm H, Gransbo K, Tasevska-Dinevska G, et al. The shrunken pore syndrome is associated with declined right ventricular systolic function in a heart failure population - the HARVEST study. Scand J Clin Lab Invest. 2016;76(7):568-74. 169. Xhakollari L, Jujic A, Molvin J, Nilsson P, Holm H, Bachus E, et al. Proteins linked to atherosclerosis and cell proliferation are associated with the shrunken pore syndrome in heart failure patients: Shrunken pore syndrome and proteomic associations. Proteomics Clin Appl [Internet]. 2021 Mar 7 Mar 7]:[1-10 pp.]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/33682349. 170. Dardashti A, Nozohoor S, Grubb A, Bjursten H. Shrunken Pore Syndrome is associated with a sharp rise in mortality in patients undergoing elective coronary artery bypass grafting. Scand J Clin Lab Invest. 2016;76(1):74-81. 171. Ljungberg J, Johansson B, Bergdahl IA, Holmgren A, Näslund U, Hultdin J, et al. Mild impairment of renal function (shrunken pore syndrome) is associated with increased risk for future surgery for aortic stenosis. Scand J Clin Lab Invest. 2019;79(7):524-30.
70
172. Luc G, Bard JM, Lesueur C, Arveiler D, Evans A, Amouyel P, et al. Plasma cystatin-C and development of coronary heart disease: The PRIME Study. Atherosclerosis. 2006;185(2):375-80. 173. Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20(10):2214-22. 174. Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358(20):2107-16. 175. Bi M, Huang Z, Li P, Cheng C, Huang Y, Chen W. The association between elevated cystatin C levels with myocardial infarction: a meta-analysis. Int J Clin Exp Med. 2015;8(11):20540-7. 176. Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643-53. 177. Peltonen M, Huhtasaari F, Stegmayr B, Lundberg V, Asplund K. Secular trends in social patterning of cardiovascular risk factor levels in Sweden. The Northern Sweden MONICA Study 1986-1994. Multinational Monitoring of Trends and Determinants in Cardiovascular Disease. J Intern Med. 1998;244(1):1-9. 178. Weinehall L, Hallgren CG, Westman G, Janlert U, Wall S. Reduction of selection bias in primary prevention of cardiovascular disease through involvement of primary health care. Scand J Prim Health Care. 1998;16(3):171-6. 179. Johansson I, Van Guelpen B, Hultdin J, Johansson M, Hallmans G, Stattin P. Validity of food frequency questionnaire estimated intakes of folate and other B vitamins in a region without folic acid fortification. Eur J Clin Nutr. 2010;64(8):905-13. 180. Eliasson M, Asplund K, Evrin PE, Lundblad D. Relationship of cigarette smoking and snuff dipping to plasma fibrinogen, fibrinolytic variables and serum insulin. The Northern Sweden MONICA Study. Atherosclerosis. 1995;113(1):41-53. 181. McKinley MC, Strain JJ, McPartlin J, Scott JM, McNulty H. Plasma homocysteine is not subject to seasonal variation. Clin Chem. 2001;47(8):1430-6. 182. Lundblad D, Holmgren L, Jansson JH, Näslund U, Eliasson M. Gender differences in trends of acute myocardial infarction events: the Northern Sweden MONICA study 1985 - 2004. BMC Cardiovasc Disord. 2008;8:17. 183. Isaksson RM, Jansson JH, Lundblad D, Näslund U, Zingmark K, Eliasson M. Better long-term survival in young and middle-aged women than in men after a first myocardial infarction between 1985 and 2006. An analysis of 8630 patients in the northern Sweden MONICA study. BMC Cardiovasc Disord. 2011;11:1. 184. Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia G, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;82(3):269-72. 185. Westerman S, Wenger NK. Women and heart disease, the underrecognized burden: sex differences, biases, and unmet clinical and research challenges. Clin Sci (Lond). 2016;130(8):551-63. 186. Lew J, Sanghavi M, Ayers CR, McGuire DK, Omland T, Atzler D, et al. Sex-Based Differences in Cardiometabolic Biomarkers. Circulation. 2017;135(6):544-55.
71
187. Miao L, Deng GX, Yin RX, Nie RJ, Yang S, Wang Y, et al. No causal effects of plasma homocysteine levels on the risk of coronary heart disease or acute myocardial infarction: A Mendelian randomization study. Eur J Prev Cardiol. 2021;28(2):227-34. 188. Smith AD, Refsum H. Homocysteine - from disease biomarker to disease prevention. J Intern Med. 2021;290(4):826-54. 189. Bauduer F, Lacombe D. Factor V Leiden, prothrombin 20210A, methylenetetrahydrofolate reductase 677T, and population genetics. Mol Genet Metab. 2005;86(1-2):91-9. 190. Li ZH, Lv YB, Zhong WF, Gao X, Byers Kraus V, Zou MC, et al. High-Density Lipoprotein Cholesterol and All-Cause and Cause-Specific Mortality Among the Elderly. J Clin Endocrinol Metab. 2019;104(8):3370-8. 191. Kjeldsen EW, Thomassen JQ, Frikke-Schmidt R. HDL cholesterol concentrations and risk of atherosclerotic cardiovascular disease - Insights from randomized clinical trials and human genetics. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(1):159063. 192. Loikas S, Koskinen P, Irjala K, Lopponen M, Isoaho R, Kivela SL, et al. Renal impairment compromises the use of total homocysteine and methylmalonic acid but not total vitamin B12 and holotranscobalamin in screening for vitamin B12 deficiency in the aged. Clin Chem Lab Med. 2007;45(2):197-201. 193. Clarke R, Lewington S, Landray M. Homocysteine, renal function, and risk of cardiovascular disease. Kidney Int Suppl. 2003(84):S131-3.