Effect of cracklings hydrolysates on oxidative stability of pork meatballs fat
-
Upload
independent -
Category
Documents
-
view
0 -
download
0
Transcript of Effect of cracklings hydrolysates on oxidative stability of pork meatballs fat
www.elsevier.com/locate/foodres
Food Research International 39 (2006) 924–931
Effect of cracklings hydrolysates on oxidative stability ofpork meatballs fat
Ewa Flaczyk a,*, Magdalena Rudzinska b, Erwin Wasowicz b, Jozef Korczak a,Ryszard Amarowicz c
a Department of Human Nutrition and Food Technology, August Cieszkowski Agricultural University of Poznan, Faculty of Food Science and Nutrition,
Al. Wojska Polskiego 31, 60-624 Poznan, Polandb Institute of Food Technology of Plant Origin, August Cieszkowski Agricultural University of Poznan, Faculty of Food Science and Nutrition,
Al. Wojska Polskiego 31, 60-624 Poznan, Polandc Division of Food Sciences, Institute of Animal Reproduction and Food Research Polish Academy of Sciences, ul Tuwima 10, 10-718 Olsztyn, Poland
Received 20 January 2006; accepted 22 May 2006
Abstract
The aim of this work was to determine the effect of cracklings hydrolysates addition on the oxidative stability of fat and cholesterol inmeatballs during 7 days of refrigerated storage.
Changes in peroxide value (PV), anisidine value (AV), thiobarbituric acid reactive substances (TBARS) and cholesterol oxidationproducts (COPs) were determined. Cholesterol and oxysterols were analyzed as trimethylsilyl derivatives (TMS) by gas chromatography.Stability properties of antioxidants so employed were characterized by their inhibition activity.
Analyzed enzymatic and acid hydrolysates of cracklings (EHC and AHC) obtained from meat industry by-product showed stabilityproperties against fat and cholesterol. AHC exhibited a superior inhibition activity against fat oxidation than that of EHC because ofsmaller PV, AV and TBARS values. EHC showed a better inhibition activity against the formation of COPs in stored meatballs. After 7days of storage, inhibition of formation of the oxysterols in samples with added hydrolysates was 29–54%. The antioxidative propertiesof EHC and AHC based on PV, AV and TBARS values were weaker than that of BHT. On the other hand, BHT was a poorer anti-oxidant than the hydrolysates against cholesterol oxidation in stored meatballs.� 2006 Elsevier Ltd. All rights reserved.
Keywords: Oxidative stability; Fat; Meat; Protein hydrolysates; Cholesterol oxidation products
1. Introduction
Oxidation has been gaining a great importance becauseof its degradative influence on the quality and nutritivevalue of fat and fat-containing foods such as meat prod-ucts. A means to control lipid oxidation in meats hasbecome increasingly important with the rise in popularityof pre-cooked and convenience foods.
Lipid oxidation in muscle tissue is likely to begin imme-diately after slaughter, with biochemical changes involved
0963-9969/$ - see front matter � 2006 Elsevier Ltd. All rights reserved.doi:10.1016/j.foodres.2006.05.004
* Corresponding author. Tel.: +48 61 848 7328; fax: +48 61 848 7431.E-mail address: [email protected] (E. Flaczyk).
in the conversion of muscle to meat leading to a reductionin the antioxidative capacity. Processing techniques such asmincing and cooking disrupt muscle cell membranes andfacilitate the interaction of unsaturated fatty acids withcystolic prooxidants, thereby further accelerating the lipidand cholesterol oxidation. Meats products are often storedrefrigerated or frozen and this can lead to further oxidativechanges (Lluch, Hernando, & Perez-Munuera, 2003; Mar-aschiello, Sarraga, Esteve-Garcia, & Requeiro, 2000).
Cholesterol is a ubiquitous sterol for food products ofanimal origin, such as meat, milk, eggs and their products.Cholesterol can oxidize in foods by the same mechanism asthat of fatty acids, and yield various products such as
E. Flaczyk et al. / Food Research International 39 (2006) 924–931 925
oxysterols. Cholesterol oxidation products (COPs) exhibitmany undesirable biological activities: cytotoxity, mutage-nicity, and cancirogenicity. They play also a significant rolein initiation and development of atherosclerosis. Choles-terol oxidation products are found to inhibit HMG-CoAreductase resulting in an insufficient synthesis of cholesteroland general malfunction of the cell (Guardiola, Codony,Addis, Rafecas, & Boatella, 1996; Nielsen, Olsen, & Skib-sted, 1996; Szedlacsek et al., 1995).
Employment of optimal technology parameters and spe-cial packaging for food products limit oxidation processesof lipids and cholesterol. A more efficient means of inhibit-ing oxidation, however, is an addition of endogenous anti-oxidants. Generally, antioxidants are used as foodadditives to prevent rancidity in fats and to protect suscep-tible food constituents against oxidative damage. Becauseof concerns in recent years the safety of synthetic antioxi-dants, there has been a pronounced interest in natural ones.Protein hydrolysates of animal and plant origin have beenfound to possess antioxidant activity against lipids inmodel and food systems (Amarowicz, Karamac, & Shahidi,1999; Amarowicz & Shahidi, 1997; Shahidi & Amarowicz,1996; Wu, Chen, & Shiau, 2003). Many investigations withthe animal origin dipeptide carnosine (b-alanylo-histidine),whose antioxidant activity against lipids is well docu-mented (Decker & Crum, 1991, 1993; Decker, Crum, &Calvert, 1992; Decker & Faraji, 1990). A few years agothe influence of carnosine against lipid and cholesterol oxi-dation in chicken meat was researched. In these experi-ments, carnosine showed activity in delaing of lipid andcholesterol oxidation (O’Neill, Galvin, Morrissey, & Buck-ley, 1999). The hydrolysates from cracklings comprise pep-tides, amino acids and Maillard reactions products (PRM)which act as antioxidants (Flaczyk, Amarowicz, & Korc-zak, 2003); their activity against cholesterol oxidation inpork meatballs has not been examined.
The aim of the present investigation was to study theeffects of added enzymatic (ECH) and acid (ACH) hydrol-ysates of cracklings against oxidation of lipid and choles-terol constituents of steamed pork meatballs duringrefrigerated (2 �C) storage.
2. Materials and methods
2.1. Materials
Hydrolysates are prepared from pig cracklings, whichare obtained as by-product at a production of lard frompig fat. Dried cracklings were defated before hydrolysiswith diethyl ether (1:3 m/v) by threefold extraction. Thehydrolysates are produced with enzyme Alcalase L 2.4
and with 4.75 M HCl according to as Flaczyk et al.(2003).
The chemical characteristics of enzymatic (EHC) andacid (AHC) hydrolysates were as follows: Ntot: 11.77%,6.99%; NNH2
: 1:71%; 3:64%. The degree of hydrolysis(DH) as a ratio of N tot=NNH2
ð�100%Þ was calculated.
For EHC it was 14.51%, and for AHC 52.17%. Composi-tion of hydrolysates was characterized by HPLC filtration(Flaczyk, 2005). EHC was separated for five fraction: I was�1046 Da, main fractions (II and III) (>556 Da, and�369 Da), and small fractions (IV and V) <369 Da. Molec-ular weight of peptides of AHC were as following: the firstfraction >556 Da, main fraction �369 Da, and small frac-tion (III) <368 Da.
Pork from the shoulder region and other componentswere purchased in local stores in Poznan. Meatballs com-prised 60% ground (twice through a 9-mm plate) shoulderpork, 15% ground (the same maner) backfat, 9% cookedrice, 15% eggs and 1% salt. One portion of the batter wasthe control, to remaining portion either 2% of EHC or2% of AHC or 0.02% of BHT were added. Meatballs(50 g portion) 17 min were cooked by steam using aRational Combi-Dampfer, 105 �C to an internal tempera-ture of 72 �C. After cooling, the meatballs were vacuumpacked and stored for 7 days at +2 �C. Samples were exam-ined after cooling (0) and after 3, 5 and 7 days of storage.
All chemicals used in this study, pose standards of oxys-terols, were purchased from Sigma–Aldrich (Schnelldorf,Germany) or Merck (Darmstadt, Germany) with HPLCor GC-grade purity.
2.2. Antiradical activity
The capacity of EHC and AHC to scavenge DPPH (1,1-diphenyl-2-picrylhydrazyl) radicals after 30 min of incuba-tion at room temperature in the dark was determined asdescribed by Tang, Kerry, Sheehan, and Buckley (2002).Hydrolysates (0.8–32 mg) dissolved in 4 mL of distilledwater were added to a methanolic solution of DPPH(0.2 mM, 1 mL) (as the specific sample). The hydrolysatescapacity to scavenge free radicals (AA %) was calculatedas follows:
AA ð%Þ
¼ 1�Absorbancespecific sample�Absorbancereference sample
Absorbancecontrol sample
�100
The reference sample used consisted of was: 1 mL meth-anol without DPPH and 4 mL water with antioxidants,and the control sample composed: 1 mL 0.2 mM DPPHand 4 mL water without antioxidants. The absorbancewas measured at k = 517 nm by Metertek SP-830 spectro-photometer (Metertech Inc., Taiwan).
2.3. Chelating activity
The chelating activity of hydrolysates on Fe2+ was mea-sured according to the method of Decker and Welch (1990)by colorimetric measurement the amount of Fe2+ whichdid not chelate with hydrolysate and 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acid)-1,2,4-triazine (ferrozine). Theabsorbance was read at k = 562 nm using the SP-830 spec-trophotometer (Metertech Inc., Taiwan). Distilled water
926 E. Flaczyk et al. / Food Research International 39 (2006) 924–931
instead of hydrolysate was used as a control. Chelatingactivity (ChA %) was calculated as follows:
ChA ð%Þ
¼ Absorbancecontrol sample �Absorbancespecific sample
Absorbancecontrol sample
� 100
2.4. Lipids extraction and moisture and fat determination
The lipids of meatballs were extracted according toFolch, Less, and Stanley (1957); the moisture and the fatcontent were determined according to AOAC (1990).
2.5. a-Tocopherol
The content of a-tocopherol in extracted fat was deter-mined by HPLC (Gogolewski, Nogala-Kałucka, & Szeliga,2000). The HPLC system consisted of the Waters Model600 gradient pump, fluorimetric detector and Waters Mille-niumdata acquisition system. Samples dissolved in n-hex-ane were injected on the LiChrosorb Si 60 column(200 mm, 5 lm Merck) and the mixture of n-hexane anddioxane (97.0:3.0 v/v) was used as a mobile phase. The flowrate was 1.5 mL/min. The concentration was calculatedfrom calibration curves made for a-tocopherol.
2.6. Cholesterol analysis
The content of cholesterol in meatballs was determined bythe procedure of Przygonski, Jelen, and Wasowicz (2000). Inbrief, lipids were extracted by the Folch mixture, the samplewas saponified with 1 M KOH in methanol and the unsapon-ifiable fraction was extracted with diethyl ether. Theremaining samples were silylated by bis(trimethylsilyl)triflu-oroacetamide (BSTFA) with 1% trimethylchlorosilane(TMCS) and then were analyzed by gas chromatography(GC). An Hewlett–Packard 6890 gas chromatograph in split(1:25) mode, with a FID detector and capillary column DB-5(30 m · 0.32 mm · 0.25 lm, J&W Scientific Inc., Folsom,CA, USA) was used for the analyses. Analysis parameterswere as follows: oven temperature, 290 �C; injector,310 �C; and carrier gas, helium at 1.6 mL/min. 5a-Choles-tane was used as an internal standard.
2.7. Fatty acid composition
The fatty acid composition of extracted lipid was deter-mined by GC (IUPAC, 1987). Gas chromatography sepa-ration was performed with Hewlett–Packard 5890instrument, on Supelcowax-10 capillary column (30 m ·0.25 mm · 0.25 lm) in programmed temperature condi-tions: from 60 to 210 �C with rate 12 �C/min and in210 �C hold 25 min.
Identification of separated fatty acid methyl esters(FAME) was performed by comparison the retention dataof separated compounds with standards solutions.
2.8. Peroxide value
The peroxide value (PV) was determined by titrationwith 0.02 M sodium thiosulfate and was expressed in meqO2/kg (ISO 3960, 1996).
2.9. Anisidine value
The anisidine value (AV) was determined colorimetri-cally according to the ISO 6885 (2001).
2.10. TBARS
The TBARS values were evalued by monitoring malon-aldehyde (MDA) formation during storage of meatballs.The distillation method described by Pikul, Leszczynski,and Kummerow (1989) was used in the study. Distillateswere reacted with thiobarbituric acid (TBA) and absor-bance at 532 nm, according to the procedure of Tarladgis,Watts, Younathan, and Dugan (1960) was measured.Results were reported as MDA–TBA values (TBARS)and expressed as mg malonaldehyde kg�1 meatballs.
2.11. Cholesterol oxidation products
Cholesterol oxides were determined according to Przy-gonski et al. (2000). The following standards used for theidentification of oxysterols were purchased from Steraloids(Newport, RI, USA): 5-cholestene-3b,7a-diol (7a-OH-C),5-cholestene-3b,7b-diol (7b-OH-C), 5-cholestene-3b-ol-7-one (7-keto-C), 5a,6a-epoxy-cholestane-3b-ol (5a,6a-epoxy-C), 5b,6b-epoxy-cholestane-3b-ol and (5b,6b-epoxy-C)and 5-cholestene-3b,19-diol (19-hydroxy-C), which wasemployed as an internal standard. 5-Cholestene-3b,20-diol(20-hydroxy-C), 5-cholestene-3b,25-diol (25-hydroxy-C)and cholestane-3b,5a,6b-triol (cholestanetriol) were fromSigma (St. Louis, MO, USA). A selection of eight oxysterolswas identified using GC/MS equipment. This procedureincludes following steps:
2.11.1. Sample preparation
The internal standard (19-hydroxy-C) and butylatedhydroxytoluene (BHT, 0.006%) dissolved in chloroform–methanol (2:1; v/v) were added to the sample of meatballs.After 1 min of homogenization, the lipid fraction wasseperated and stored in a frozen (�80 �C) until the nextstep of the analysis.
2.11.2. Transesterification
To the dried sample, 2 mL of sodium methylate (10%)mixed with methyl tert-butyl ether (MTBE) (4:6; v/v) wasadded and left for 1 h. The oxysterols fraction wasextracted with chloroform and rinsed by water and 1%citric acid. Afterwards, the water layer was discardedand the chloroform extract was evaporated undernitrogen.
0
10
20
30
40
50
60
70
80
0.8 2 4 10 20 30
Concentration [mg/sample]
% D
PP
H r
ad
ical
sc
ave
ng
ing
ac
tivi
ty
EHC AHC
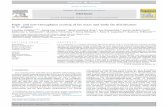
Fig. 1. The scavenging effect on 1,1-diphenyl-2-picrylhydrazyl (DPPH)radical by the crackling hydrolysates at various concentration. Eachvalues is expressed as the means ± SD (n = 5).
Table 1Regression equation of antiradical and chelating activities of hydrolysateswith their concentration
Hydrolysate Regression equation R2
Scavenging of DPPH radical
EHC y = 52.18 log10 x + 0.84 0.840 (P 6 0.001)AHC y = 67.80 log10 x + 0.18 0.980 (P 6 0.001)
Chelating activity
EHC y = 1.00x + 11.34 0.998 (P 6 0.001)AHC y = 1.42x + 18.84 0.989 (P 6 0.001)
Explanations: y, independent variable (scavenging of DPPH radical orchelating activity); x, dependent variable (concentration of hydrolysates);R2, determination coefficient.
E. Flaczyk et al. / Food Research International 39 (2006) 924–931 927
2.11.3. SPE fractionation
The SEP-PAK NH2 cartridge (Waters, MA, USA) wasconditioned with 10 mL of hexane. The sample was dis-solved in 250 lL of chloroform and transferred onto theSEP-PAK NH2 column. It was washed with 10 mL of hex-ane, 5 mL hexane–MTBE (5:1; v/v) and 5 mL hexane–MTBE (3:1; v/v). The polar fraction of oxysterols waseluted from the column with 7 mL of acetone. Solventwas evaporated in a stream of nitrogen.
2.11.4. Derivatization of oxysterols
To the dried sample, 100 lL of anhydrous pyridine and100 lL of BSTFA with 1% TMCS were added. After 4 h atroom temperature the sample was ready for GC analysis.
2.11.5. Capillary gas chromatography
Cholesterol oxidation products were determined using aHewlett–Packard 6890 capillary gas chromatograph. Sam-ple (1 lL) was introduced in a splitless mode onto a DB-5column (30 m · 0.32 mm · 0.25 lm, J&W Scientific Inc.,Folsom, CA, USA). The initial oven temperature was110 �C for 2 min, and then increased 40 �C/min to 235 �Cand then 3 �C/min to 300 �C which was hold for 10 min.The flow rate of the helium carrier gas was 1 mL/min.Identity of compounds was tentatively identified by com-parison to retention times of known standards.
2.12. Inhibition activity
Inhibition activity of antioxidants was calculated sepa-rately for all analyzed oxidation index as follows:
Inhibition activity ð%Þ
¼ OIsample without antioxidant �OIsample with antioxidant
OIsample without antioxidant
� 100
where OI is the oxidation index (PV, AV, TBARS, COPs).
2.13. Statistical analysis
Results are presented as means ± SD from five repli-cates of each experiment in two series. A P-value 6 0.05was used to denote significance differences among meanvalues determined by analysis of variance (ANOVA). ThePearson correlation was determined among differentparameters.
3. Results and discussion
The effects of protein hydrolysates on scavenging thefree radical DPPH are presented in Fig. 1. The free radi-cal-scavenging activity of EHC and AHC were examinedat concentrations ranging from 0.8 to 30 mg in analyzedsamples. According to Fig. 1, EHC showed better abilityof scavenging free radicals (30–41%) at a smaller dose(0.8–4 mg), whereas at higher dose (20–30 mg) AHC exhib-ited a better scavenging activity (54–72%). Butylated
hydroxytoluene (BHT) in such concentrations revealed99.0–99.4% ability of scavenging DPPH radical. In fact,the regression coefficients presented in Table 1 show thatthe scavenging activity of analyzed protein hydrolysatesimproved with increasing concentrations, but in logarith-mic dependence.
DPPH is a free radical compound, and has been widelyused to test the free-radical scavenging ability of varioussamples (Amarowicz, Pegg, Rahimi-Moghaddam, Barl, &Weil, 2004; Yen, Chang, & Chen, 2002). Data indicatedthat protein hydrolysates from cracklings are free-radicalscavengers, particularly of the peroxyl radical, which is amajor propagator of the oxidation chain of fat, thereby ter-minating the chain reaction (Flaczyk, 2005). Sakanaka andTachibana (2006) showed a similar, concentration-depen-dance of DPPH activity of egg-yolk protein hydrolysates,while Rajapakse, Mendis, Jung, Je, and Kim (2005) dem-onstrated such dependence for the peptides from fermentedmussels.
Chelating activity was measured by the method whereferrozine forms a complex with Fe2+ resulting in a redcolor. In the presence of a Fe2+ chelating agent, the inten-sity of the red color decreases. In our experiments (Fig. 2),AHC showed a significantly (P 6 0.01) stronger chelatingactivity (26–63%) than EHC (19–52%). The chelating activ-ity of hydrolysates depends on their concentration what aspresented by the regression coefficients (Table 1). The
0
10
20
30
40
50
60
70
5 10 20 30Concentration [mg/sample]
Che
latin
g ac
tivity
[%]
EHC AHC
Fig. 2. Fe2+ chelating activity of the crackling hydrolysates at variousconcentrations. Each values is expressed as the means ± SD (n = 5).
Table 2Chemical composition of meatballsa
Compound Content
Moisture (g/100 g) 55.45 ± 0.02Fat (g/100 g) 22.50 ± 0.03Protein (g/100 g) 22.60 ± 0.02Cholesterol (mg/100 g) 86.50 ± 0.03a-Tocopherol (mg/100 g) 0.55 ± 0.01
Fatty acids composition (%)
C 8:0 0.08 ± 0.01C 10:0 0.07 ± 0.00C 14:0 1.30 ± 0.01C 16:0 24.32 ± 0.02C 16:1 2.68 ± 0.02C 17:0 0.37 ± 0.01C 17:1 0.42 ± 0.01C 18:0 12.52 ± 0.02C 18:1 47.45 ± 0.02C 18:2 8.30 ± 0.02C 18:3 0.57 ± 0.01C 20:0 0.17 ± 0.00C 20:1 0.94 ± 0.01
a Expressed as means ± SD for 3–5 replicates.
928 E. Flaczyk et al. / Food Research International 39 (2006) 924–931
hydrolysates had a significantly (P 6 0.01) poorer chelatingcapability on Fe2+ ions at concentrations of 5–30 mg insamples, than that of ethylenediaminetetraacetic acid(EDTA) at concentrations of 200–1000 ppm, which arevery strong metal chelators. Many authors suggested thatthe presence of histidine residues could be one of the fac-tors responsible for the chelating activity. More specifi-cally, presence of histidine at the N-terminal of peptidesequence has been reported to be effective in metal ion che-lation (Chen, Muramoto, Yamauchi, & Fujimoto, 1998;Hall, 2001; Wade & Tucker, 1998). In edition, it is thoughtthat acidic and/or basic amino acids play an important rolein chelation of metal ions by the carboxyl and amino moi-eties in their side chains (Suetsuna, Ukeda, & Ochi, 2000).
As mentioned above, crackling hydrolysates showedantioxidant activity. In addition, their activity was testedusing a food model system. The proximate compositionof meatballs is presented in Table 2. They showed moistureabout 55.45%, fat and protein about 22.5%, and biologi-cally active substances such as cholesterol and a-tocoph-erol at 86.5 mg and 0.55 mg in 100 g of meatballs,respectively. Lipids of meatballs contained about 4% moreoleic acid than typical pork fat products. In our model ofexperiments, however, fatty acids of lipid meatballs wereanalyzed two times: after cooking and during 7 days ofstorage at 2 �C. We did not find any significant changesin fatty acid composition during this period of storage(P P 0.05).
Meatballs were subsequently stored in 2 �C and oxida-tive changes in lipid constituents were determined as perox-ide value, anisidine value and TBARS. The cracklingshydrolysates limited the formation of hydroperoxides,aldehydic compounds and TBARS substances in meatballsduring refrigerated storage (Table 3). EHC delayed the for-mation of lipid peroxides at the level of 23–33% and AHCat 31–41%, respectively. The best antioxidant efficacy ofhydrolysates against lipid peroxides was observed up to 5days of storage. However, synthetic antioxidant BHT pos-sessed better antioxidant activity than hydrolysates (62–70% inhibition). Both types of cracklings hydrolysatesinhibited the formation of secondary oxidation products.
Anisidine and TBARS values were a little lower in meat-balls with AHC than EHC. In particular, EHC inhibitedTBARS formation by about 20%, AHC by 30% andBHT by 47%.
Recently Sakanaka and Tachibana (2006) showed thatinhibition of the formation of TBARS in meat beef homog-enate with egg-yolk protein hydrolysates was dose depen-dent and achieved 35–91% of inhibition, and in tunahomogenate at the level 44–66%. Studied hydrolysates pos-sessed lower antioxidant activity in the meatballs systemthan evaluated by Sakanaka and Tachibana (2006). Butdirect comparison of the activity of these hydrolysates isdifficult because beef or tuna homogenates are not so com-plex system like ready-to-eat meatballs. In general, hydrol-ysates at dose ranging from 1% to 2% retarded lipidoxidation in pork patties but their activity were weakerthan synthetic antioxidants (Cuppett, 2001; Pena-Ramos& Xiong, 2003).
Lipid oxidation is controlled in meat products by anumber of different systems and components. Among thosecomponents, the biggest influence on lipid oxidation inmeat is the profile of unsaturated fatty acids and phospho-lipids and the presence of transition metal ions (Du, Nam,& Ahn, 2001; Lluch et al., 2003; Maraschiello et al., 2000).Lipid oxidations in meat products are catalyzed by hemeiron and non-heme iron. Heme iron can initiate lipidoxidation in raw meat and in meat after cooking, whereasnon-heme iron is more important in meat after cooking.Mincing meat causes damage of cellular membranes andexposes phospholipids to oxygen and light, but thermaldenaturation during cooking increases the amount ofnon-heme iron (Cuppett, 2001; Sista, Erickson, & Shewfelt,2000). Hydrolysates of cracklings demonstrated iron-chelating capability and delayed the initiation of oxidation.A better antioxidant activity of AHC against fatty acid
Table 3Peroxide, anisidine, and TBARS values* in meatballs treated with different antioxidants during storage at 2 �C
Days of storage Control EHC ACH BHT
Peroxide value (meq O2/kg fat)
0 0.4 ± 0.01a,1 0.4 ± 0.01a,1 0.4 ± 0.01a,1 0.4 ± 0.01a,1
3 2.4 ± 0.01b,4 1.8 ± 0.01b,3 1.6 ± 0.01b,2 0.9 ± 0.01b,1
5 4.5 ± 0.00c,4 3.0 ± 0.01c,3 2.7 ± 0.01c,2 1.5 ± 0.01c,1
7 6.4 ± 0.01d,4 4.9 ± 0.00d,3 4.5 ± 0.01d,2 1.9 ± 0.01d,1
Anisidine value
0 0.2 ± 0.00a,1 0.2 ± 0.00a,1 0.2 ± 0.00a,1 0.2 ± 0.00a,1
3 0.7 ± 0.01b,4 0.7 ± 0.01b,2 0.4 ± 0.01b,2 0.2 ± 0.01b,1
5 1.5 ± 0.01c,4 1.1 ± 0.01c,3 0.9 ± 0.00c,2 0.5 ± 0.00c,1
7 2.2 ± 0.01d,4 1.4 ± 0.01d,3 1.2 ± 0.01d,2 0.6 ± 0.01d,1
TBARS (mg MDA/kg of meatballs)
0 0.15 ± 0.00a,1 0.15 ± 0.01a,1 0.14 ± 0.01a,1 0.15 ± 0.01a,1
3 0.72 ± 0.00b,4 0.53 ± 0.02b,3 0.50 ± 0.00b,2 0.42 ± 0.01b,1
5 0.89 ± 0.01c,4 0.74 ± 0.02c,3 0.63 ± 0.02c,2 0.50 ± 0.02c,1
7 1.27 ± 0.01d,4 1.02 ± 0.00d,3 0.95 ± 0.01d,2 0.57 ± 0.02d,1
*Expressed as means ± SD for 3–5 replicates.a,b,c,d Separately for peroxide value, anisidine value and TBARS values averages followed by different letter correspond to time of storage and aresignificantly different at P 6 0.05.1,2,3,4 Averages followed by different numerals within the same line correspond to the type of antioxidant and are significantly different at P 6 0.05.
E. Flaczyk et al. / Food Research International 39 (2006) 924–931 929
oxidation of meatballs could be the result of a higher che-lating Fe (II) ability, which at 2% addition in model systemwas 45% (Fig. 2).
Cholesterol oxidation products were detected in themeatballs before and directly after cooking and during 3,5 and 7 days of storage at 2 �C. Thermal processingincreased the amount of oxysterols such as 7a-OH-C, 7b-OH-C and 7-keto-C, and considerably higher changes wereevident during storage. Despite the rather short time ofstorage, an increased quantity of COPs was detected, par-ticularly in the control sample. Total amounts of theseoxysterols, determined directly after cooking, ranged from4.95 to 7.06 lg/g of meatballs; the oxysterols 7a-OH-C, 7b-OH-C and 7-keto-C were dominant. Sum of these threeoxysterols in the control sample was on average 4.87 lg/gof meatballs. Meatballs with antioxidants (EHC andBHT) possessed lower quantities of these oxysterols, orhigher (AHC) in comparison with control sample(P 6 0.05).
In the first phase of research on samples with antioxi-dants, an increased quantity of 7a-OH-C, in comparisonwith the control was observed. Moreover in samples towhich AHC and BHT were added, higher amounts of5a,6a-epoxy-C were determined. It is difficult to explainsuch results: meatballs comprised a dynamic system of pro-teins, lipids, carbohydrate and minerals, which can influ-ence the transformation of COPs in meatballs. In latterstorage periods, the contents of 5a,6a-epoxy-C in meatballswith hydrolysates decreased, while that with BHTincreased, in comparison with the control. It suggests thatanother means of inhibition of cholesterol by hydrolysatesand by BHT has occurred. Probably the hydrolysates inter-act with primary COPs by a nonradical mechanism. Thequantity of 5b,6b-epoxy-C in each sample was similar overthe storage period. Apparently, the kinetics for the creation
and decomposition of this oxysterol were similar. In sam-ples with antioxidants an increase in the amount of 20-and 25-hydroxy-C, at levels of 0.09–0.56 lg/g of samplewere observed. In the cholesterol side chain, the most reac-tive group is the tertiary carbon, C25. Thus, formation of20-, 24-, 25- and 26-OH-C can occur via a direct free-rad-ical reaction, without any intermediate forms being gener-ated (Nielsen et al., 1996; Paniangvait, King, Jones, &German, 1995). 25-Hydroxy-C is recognized as a very toxicderivative of cholesterol. Only EHC retard formation ofoxysterols at the beginning of experiment. The applicationof antioxidants (AHC and BHT) in meatballs was noteffective in decreasing the rate of formation of cholesteroloxides in the initial stages of storage. Such a relationshipwith another synthetic antioxidant – butylated hydroxyani-sole (BHA) in anchovy was observed by Shozen, Ohshima,Ushio, Takiguchi, and Koizumi (1997).
From among the COPs, the most toxic derivative – cho-lestanetriol, did not form during the production of meat-balls. However, it appeared after 5 days of storage incontrol meatballs, and after 7 days in treated samples withsuch antioxidants as AHC and BHT (Fig. 3).
The lower amount of COPs in samples treated with anti-oxidants indicated inhibiting properties of the employedantioxidants against cholesterol oxidation (Fig. 3). Thehighest antioxidative efficiency was shown by EHC, whichinhibited the formation of oxysterols on average by 54%,for AHC, 29% and 37% for BHT. At the present stage ofresearch, higher protective properties of EHC against cho-lesterol oxidation in meatballs, relative to AHC, can onlybe translated by a lower degree of cracklings peptides frag-mentation and higher the peroxyl radical activity (Flaczyk,2005).
It is reasonably assumed that in analyzed meatballs, thedetermination of changes in hydroperoxides, malonaldehyde
Fig. 3. The inhibition of cholesterol oxidation products (COPs) formationby cracklings hydrolysates – enzymatic (EHC) and acidic (AHC) of andbutylated hydroxytoluene (BHT) in meatballs during 7 days of storage at2 �C. Each values is expressed as the means ± SD (n = 5).
930 E. Flaczyk et al. / Food Research International 39 (2006) 924–931
and anisidine value reflects oxidative changes in the lipidconstituent of meatballs. Oxidation of cholesterol was ana-lyzed by quantitative determination of their oxidationproducts. In Table 4 are presented some correlation coeffi-cients calculated between oxysterols content and hydroper-oxides and secondary oxidation products as TBARS oranisidine value. The correlation was positive which meansthat together with the increasing of hydroperoxides (oraldehydes or TBARS) the content of oxysterols alsoincrease. So, the content of hydroperoxides (or secondaryoxidation products estimated as TBARS) and 7-keto-Ccan be used as indicators of lipid changes, as has beenreported in the literature (Du et al., 2001; Maraschielloet al., 2000).
It is recognized that oxidation of cholesterol is corre-lated with unsaturated degree of adjacent fatty acids. Cho-lesterol is exposed to a direct attack of hydroxyl radicalsformed from fatty acids. The presence of the double unsat-urated linoleate had an important effect on the formation
Table 4Correlation coefficients* of cholesterol oxidation products with lipidoxidation indexes, in meatballs during storage at 2 �C
COPs PV Anizidin value TBARS
7a-OH-C 0.739 0.751 0.7257b-OH-C 0.636 0.604 0.6437-keto-C 0.655 0.644 0.6297a-OH-C + 7b-OH-C + 7-keto-C 0.700 0.692 0.686Total COPs 0.738 0.737 0.710
* P 6 0.001.
of oxysterols, except of prooxidants (Echarte, Conchillo,Ansorena, & Astrisaran, 2004).
7-Keto-C and 7b-OH-C are recognized as the main COPsgenerated during storage of meats products (Baggio, Miguel,& Bragagnolo, 2005; Shozen et al., 1997). In this work, sig-nificant amount of 7a-OH-C was also found. This oxysterolwas not tested by the afore-mentioned authors. According tothe accepted mechanism of cholesterol oxidation in radicalformation systems, cholesterol is oxidized to yield 7-hydro-peroxy cholesterol. The corresponding epimeric 7a- and7b-hydroxycholesterols, as well as 7-ketocholesterol, arethen derived from 7-hydroperoxy cholesterols. 7-Ketocho-lesterol is oxidized further to cholesta-3,5-diene-7-one. Itwas expected, therefore, that 7-ketocholesterol in the controlsample without any added antioxidant would be further oxi-dized and degraded to form cholesta-3,5-diene-7-one at ahigher rate than in the sample treated with antioxidants.
In the present study, the protective properties of natu-ral antioxidants against the formation of oxidation prod-ucts from fats and cholesterol in meatballs weredetected. A decrease of undesirable lipid oxidation prod-ucts in foodstuffs can be achieved by the addition of anti-oxidants from cracklings hydrolysates. Proteinhydrolysates are usually used in food technology as a tasteand flavor improver at the concentration between 0.5%and 2.0%. As the natural substances their amounts in foodare not limited. They can be used at such level, which notnegatively influences on the palatability of foods. Syn-thetic antioxidants are permitted for addition only toselected products but usually at the concentration nothigher then 0.02%. Therefore using cracklings hydroly-sates is possible and safe although at concentration 100times higher then BHT. The hydrolysates obtained fromcracklings added to meatballs in 2% amount were nearlyas effective as BHT added at allowed level in the inhibitingof lipid oxidation and were even better in retarding choles-terol oxidation products formation.
Presented results show a necessity for the continuationof such research, in the systems, where oxidation of lipidsis higher, e.g. in meatballs during frozen storage.
Acknowledgement
The authors acknowledge the State Committee for Sci-entific Research for financing this research under projectnumber 2 PO6T 039 26.
References
Amarowicz, R., Karamac, M., & Shahidi, F. (1999). Synergistic activity ofcapelin protein hydrolysates with synthetic antioxidants in a modelsystem. Journal of Food Lipids, 6, 271–275.
Amarowicz, R., Pegg, R. B., Rahimi-Moghaddam, P., Barl, B., & Weil, J.A. (2004). Free-radical scavenging capacity and antioxidant activity ofselected plant species from the Canadian prairies. Food Chemistry, 84,551–562.
Amarowicz, R., & Shahidi, F. (1997). Antioxidant activity of peptidefractions of capelin protein hydrolysates. Food Chemistry, 58, 355–359.
E. Flaczyk et al. / Food Research International 39 (2006) 924–931 931
AOAC (1990). Official methods of analysis. Washington, DC: Associationof Official Analytical Chemists.
Baggio, S. R., Miguel, A. M. R., & Bragagnolo, N. (2005). Simultaneousdetermination of cholesterol oxides, cholesterol and fatty acids inprocessed turkey meat products. Food Chemistry, 89, 475–584.
Chen, H.-M., Muramoto, K., Yamauchi, F., & Fujimoto, K. (1998).Antioxidative properties of histidine-containing peptides designedfrom peptide fragments found in the digest of a soybean protein.Journal of Agricultural and Food Chemistry, 46, 49–53.
Cuppett, S. L. (2001). The use of natural antioxidants in food products ofanimal origin. In J. Pokorny, N. Yahishlivera, & M. Gordon (Eds.),Antioxidants in food: Practical applications (pp. 285–310). Cambridge,England: Woodhead Publishing.
Decker, E. A., & Crum, A. D. (1991). Inhibition of oxidation rancidity insalted ground pork by carnosine. Journal of Food Science, 56,1179–1181.
Decker, E. A., & Crum, A. D. (1993). Antioxidant activity of carnosine incooked ground pork. Meat Science, 34, 245–253.
Decker, E. A., Crum, A. D., & Calvert, J. T. (1992). Differences in theantioxidant mechanism of carnosine in the presence of cooper andiron. Journal of Agricultural and Food Chemistry, 40, 756–759.
Decker, E. A., & Faraji, H. (1990). Inhibition of lipid oxidation bycarnosine. Journal of the American Oil Chemists’ Society, 67, 650–652.
Decker, E. A., & Welch, B. (1990). The role of ferritin as a lipid oxidationcatalyst in muscle foods. Journal of Agricultural and Food Chemistry,
38, 674–677.Du, M., Nam, K. C., & Ahn, D. U. (2001). Cholesterol and lipid oxidation
products in cooked meat as affected by raw-meat packaging andirradiation and by cooked-meat packaging and storage time. Journal of
Food Science, 66, 1396–1401.Echarte, M., Conchillo, A., Ansorena, D., & Astrisaran, I. (2004).
Evaluation of the nutritional aspects and cholesterol oxidationproducts of pork liver and fish pates. Food Chemistry, 86, 47–53.
Flaczyk, E. (2005). Antioxidant activity of enzymic and acid proteinhydrolysates with special attention to their activity against cholesterol.Monography of Poznan Agricultural University, Vol. 361.
Flaczyk, E., Amarowicz, R., & Korczak, J. (2003). Antioxidant activity ofprotein hydrolysates from by-products of the food industry. Journal of
Food Lipids, 10, 129–140.Folch, J., Less, M., & Stanley, S. (1957). A simple method for the isolation
and purification of total lipids from animal tissues. Journal of
Biological Chemistry, 227, 497–509.Gogolewski, M., Nogala-Kałucka, M., & Szeliga, M. (2000). Changes of
the tocopherol and fatty acid contents in rapeseed oil during refining.European Journal of Lipid Science and Technology, 102, 618–623.
Guardiola, F., Codony, R., Addis, P. B., Rafecas, M., & Boatella, J.(1996). Biological effects of oxysterols: current status. Food and
Chemical Toxicology, 34, 193–211.Hall, C. (2001). Sources of natural antioxidants: oilseeds, nuts, cereals,
legumes, animal products and microbial sources. In J. Pokorny, N.Yahishlivera, & M. Gordon (Eds.), Antioxidants in food: Practical
applications (pp. 159–209). Cambridge, England: WoodheadPublishing.
ISO 3960 (1996). Animal and vegetable oils and fats – determination ofperoxide value. International Organization for Standardization,Geneva, Switzerland.
ISO 6885 (2001). Animal and vegetable oils and fats – determination ofanisidine value. International Organization for Standardization,Geneva, Switzerland.
IUPAC (1987). Standard methods for analysis of oils, fats and derivatives.IUPAC Method 2.301 Report of IUPAC Working Group WG 2/87,(7th ed.). Blackwell Scientific Publications, Oxford.
Lluch, M. A., Hernando, I., & Perez-Munuera, I. (2003). Lipids in foodstructure. In Z. Sikorski & A. Kołakowska (Eds.), Chemical and
functional properties of food lipids (pp. 9–28). New York: CRC PressLLC.
Maraschiello, C., Sarraga, C., Esteve-Garcia, E., & Requeiro, G. J. A.(2000). Dietary iron and copper removal does not improve cholesteroland lipid oxidative stability of raw and cooked broiler meat. Journal of
Food Science, 65, 211–214.Nielsen, J. H., Olsen, C. E., & Skibsted, L. H. (1996). Cholesterol
oxidation in heterogeneous system initiated by water-soluble radicals.Food Chemistry, 56, 33–37.
O’Neill, L. M., Galvin, K., Morrissey, P. A., & Buckley, D. J. (1999).Effect of carnosine, salt and dietary vitamin E on the oxidative stabilityof chicken meat. Meat Science, 50, 479–488.
Paniangvait, P., King, A. J., Jones, A. D., & German, B. G. (1995).Cholesterol oxides in foods of animal origin. Journal of Food Science,
60, 1159–1174.Pena-Ramos, E. A., & Xiong, Y. L. (2003). Whey and soy protein
hydrolysates inhibit lipid oxidation in cooked pork patties. Meat
Science, 64, 259–263.Pikul, J., Leszczynski, D. E., & Kummerow, F. A. (1989). Evaluation of
three modified TBA methods for measuring lipid oxidation inchicken meat. Journal of Agricultural and Food Chemistry, 40, 756–759.
Przygonski, K., Jelen, H., & Wasowicz, E. (2000). Determination ofcholesterol oxidation products in milk powder and infant formulas bygas chromatography and mass spectrometry. Nahrung, 44, 122–125.
Rajapakse, N., Mendis, E., Jung, W.-K., Je, J.-Y., & Kim, S.-K. (2005).Purification of a radical scavenging peptide from fermented musselsauce and its antioxidant properties. Food Research International, 38,175–182.
Sakanaka, S., & Tachibana, Y. (2006). Active oxygen scavenging activityof egg-yolk protein hydrolysates and their effects on lipid oxidation inbeef and tuna homogenates. Food Chemistry, 95, 243–249.
Shahidi, F., & Amarowicz, R. (1996). Antioxidant activity of proteinhydrolysates from aquatic species. Journal of the American Oil
Chemists’ Society, 73, 1197–1199.Shozen, K., Ohshima, T., Ushio, H., Takiguchi, A., & Koizumi, C. (1997).
Effects of anti-oxidants and packing on cholesterol oxidation inprocessed anchovy during storage. Lebensmittel – Wissenschaft und
Technologie, 30, 2–8.Sista, R. V., Erickson, M. C., & Shewfelt, R. L. (2000). Lipid oxidation in
chicken muscle model system: oxidative response of lipid classes toiron ascorbate or methemoglobin catalysis. Journal of Agricultural and
Food Chemistry, 48, 1421–1425.Suetsuna, K., Ukeda, H., & Ochi, H. (2000). Isolation and characteriza-
tion of free radical scavenging activities peptides derived from casein.The Journal of Nutritional Biochemistry, 11, 128–131.
Szedlacsek, S. E., Wasowicz, E., Hulea, S. A., Nishida, H. I., Kummerow,F. A., & Nishida, T. (1995). Estrification of oxysterols by humanplasma lecithin-cholesterol acyltransferase. Journal of Biological
Chemistry, 270, 11812–11819.Tang, S. Z., Kerry, J. P., Sheehan, D., & Buckley, D. J. (2002).
Antioxidative mechanisms of tea catechins in chicken meat systems.Food Chemistry, 76, 45–51.
Tarladgis, B. G., Watts, B. M., Younathan, M. T., & Dugan, L. R. Jr.,(1960). A distillation method for the quantitative determination ofmalonaldehyde in rancid foods. Journal of the American Oil Chemists
Society, 37, 44–48.Wade, A., & Tucker, H. (1998). Antioxidant characteristics of L-histidine.
The Journal of Nutritional Biochemistry, 9, 308–315.Wu, H.-C., Chen, H.-M., & Shiau, C.-Y. (2003). Free amino acids and
peptides as related to antioxidant properties in protein hydrolysates ofmackrel (Scomber austriasicus). Food Research International, 36,949–957.
Yen, G. C., Chang, Y. C., & Chen, J. P. (2002). Antioxidant activity ofmycelia from Aspergillus candidus. Journal of Food Science, 67,567–572.