Combined application of δ13C and molecular ratios in sediment cores for PAH source apportionment in...
Transcript of Combined application of δ13C and molecular ratios in sediment cores for PAH source apportionment in...
Organic Geochemistry 37 (2006) 674–687
www.elsevier.com/locate/orggeochem
OrganicGeochemistry
Combined application of d13C and molecular ratios insediment cores for PAH source apportionment in the
New York/New Jersey harbor complex
Beizhan Yan a,1, Teofilo A. Abrajano a,*, Richard F. Bopp a, Lucille A. Benedict a,Damon A. Chaky b, Eric Perry a, Jing Song a, Dennis P. Keane a
a Department of Earth and Environmental Science, Rensselaer Polytechnic Institute, 110 8th Street, Science Center, Room 1C25, Troy,
NY 12180-3590, USAb Lamont-Doherty Earth Observatory of Columbia University, Palisades, NY 10964, USA
Received 22 June 2004; received in revised form 8 August 2004; accepted 28 January 2006Available online 27 April 2006
Abstract
Sub-basins within the New York/New Jersey (NY/NJ) Harbor host variable amounts and sources of polycyclic aro-matic hydrocarbons (PAHs). Analyses of radionuclides 137Cs and 7Be were used to assign approximate dates to individualsections of sediment cores collected from four major sub-basins of the harbor complex. Sampling sites range from heavilycontaminated Newark Bay and Passaic River to less contaminated Raritan Bay and Jamaica Bay. PAHs were identifiedwith GC–MS and quantified by GC respectively. Gas chromatography–isotope ratio mass spectrometry (GC/IRMS) wasapplied to determine d13C values of pyrene (d13CPy), a principal 4 ring parent PAH. Two end-member PAH source com-ponents, petroleum-related PAHs (PRPAH) and combustion-related PAHs (CRPAH), were calculated from a carbon iso-topic mass balance equation based on assumptions that petroleum-related d13CPy is �29&, and that combustion-derivedd13CPy is �24&. Temporal trends of PRPAH in these cores are similar to those of the petroleum-derived unresolved com-plex mixture (UCM), whereas CRPAH resembled trends of ‘‘Total’’ PAH (TPAH). Three molecular ratios, fluorantheneto fluoranthene plus pyrene (Fl/(Fl + Py)), high molecular weight 4–6 ring PAH to total PAH (Ring456/TPAH) and par-ent to total (Par/(Par + Alkyl)), correlate strongly with each other, and show a moderate to strong positive correlationwith d13CPy. In contrast, the ratios of anthracene to phenanthrene plus anthracene (A/(Pa + A)), benzo[a]anthracene tobenzo[a]anthracene plus chrysene (BaA/(BaA+Chy)), and indeno[1,2,3-cd]pyrene to indeno[1,2,3-cd]pyrene plusbenzo[ghi]perylene (IP/(IP + Bghi)) show no, weak, or even negative correlation with the four well correlated ratios(d13CPy, Par/(Par + Alkyl), Fl/(Fl + Py), and Ring456/TPAH). Combined application of the four well correlated molecu-lar indicators suggests that PAH contribution from combustion became more important in most areas of the NY/NJ Har-bor during the last three decades of the 20th century.� 2006 Elsevier Ltd. All rights reserved.
0146-6380/$ - see front matter � 2006 Elsevier Ltd. All rights reserveddoi:10.1016/j.orggeochem.2006.01.013
* Corresponding author. Tel.: +1 518 276 6036; fax: +1 518 2762012.
E-mail address: [email protected] (T.A. Abrajano).1 Current address: Department of Chemistry, University of
Idaho at Idaho Falls, Idaho Falls, ID 83402, USA.
1. Introduction
Sediments in the highly urbanized New York/New Jersey (NY/NJ) Harbor complex are known
.
B. Yan et al. / Organic Geochemistry 37 (2006) 674–687 675
to be contaminated by various chemical compoundsand heavy metals (Bopp et al., 1993a; Gunster et al.,1993; Crawford et al., 1995; Wolfe et al., 1996;Mitra et al., 1999). Following estuary-wide multi-year intensive bioeffects surveys conducted by theNational Oceanic and Atmospheric Administration(NOAA), polycyclic aromatic hydrocarbons(PAHs) have emerged as a leading concern in thisarea (Wolfe et al., 1996). These surveys have shownthat among all contaminants analyzed (PAHs, pes-ticides, PCBs, and 16 trace and heavy metals),amphipod toxicity was most strongly associatedwith levels of PAHs in the NY/NJ Harbor (Wolfeet al., 1996). Although a number of pioneering stud-ies have investigated spatial and temporal distribu-tions of PAHs in the NY/NJ Harbor areas (Wolfeet al., 1996; Gigliotti et al., 2002), most were focusedon concentrations of principal parent (unsubsti-tuted) PAHs. Alkyl-substituted PAHs that varysubstantially in their distribution can provide addi-tional important information for PAH sourceapportionments (Sporstol et al., 1983; Pereiraet al., 1999; Mitra et al., 1999; Yunker et al., 2002).
Traditionally, PAH source apportionment stud-ies have relied on a number of molecular criteriato distinguish between pyrogenic (combustion-
Table 1Quantified PAHs and their abbreviations in sediment samples
Compounds Abbreviations Compou
*Naphthalene Na Benzo[gh
Biphenyl Bph Benzo[c]*Acenaphthylene Acy Tetrame*Acenaphthene Ace *Benzo[aDibenzofuran DBFu *Chrysen*Fluorene F TrimethDibenzo[b,d]thiophene DBT *Benzo[b*Phenanthrene Pa *Benzo[k*Anthracene A Benzo[a]4H-cyclopenda[def]phenanthrene 4HCP Benzo[e]2-Phenylnaphthalene 2PN *Benzo[a*Fluoranthene Fl Perylene*Pyrene Py Indeno[7Benzonaphthofurans BNFu *Indeno[Retene Ret Picene1,2-Benzofluorene 12BF *Dibenzo2,3-Benzofluorene 23BF *Benzo[gBenzo[b]naphthothiophenes BNTH Anthrar
Alkylated seriesC1–C4 Naphthalene C1–C4 Na C1–C2 DC1–C3 Phenanthrene/anthracene C1–C3 P/A C1 PAHC1–C2 Fluoranthene/pyrene C1–C2 F/PC1–C2 Benzo[a]anthracene/Chrysene C1–C2 B/C
The compounds denoted with * are EPA PAHs, the sum of all 16 EPAshown in this table.
a Due to co-elutions chrysene and triphenylene are treated as a single
derived, e.g. emissions from wood and motor vehi-cles) and petrogenic (petroleum-derived, e.g. oilspills, leakage of crankcase and lubrication oil)(Wakeham, 1996; Budzinski et al., 1997; Yunkeret al., 1999). These criteria are based on overallPAH molecular fingerprints or relative concentra-tions of isomers with the same mass. Currentlyapplied criteria include: Fl/(Fl + Py), the ratio offluoranthene (Fl) to fluoranthene plus pyrene (Py),both have a mass of 202; A/(Pa + A), the ratio ofanthracene(A) to PAHs with mass 178 (A and phen-anthrene (Pa)); BaA/(BaA + Chy), the ratio ofbenzo(a)anthracene (BaA) to the sum of mass 228PAHs ( BaA+ Chrysene(Chy) ); C0/(C0 + C1)P/A
and C0/(C0 + C1)F/P, ratios of parent PAHs withmass 178 (Pa, A) or 202 (Fl, Py) to the parent PAHsplus C1 alkyl homologues at the same mass respec-tively; and IP/(IP + Bghi), the ratio ofindeno[1,2,3-cd]pyrene (IP) to the sum of IP andbenzo[g,h,i]perylene (Bghi). In a previous study ofrecently deposited (post-1990) harbor sediments(Yan et al., 2004), two other indicators were used:Ring456/TPAH, the ratio of high molecular weight4–6 ring PAHs (including all alkylated 4–6 ringPAHs as well) to TPAH, the sum of all quantifiedparent plus alkyl PAHs (Table 1); Par/(Par +
nds Abbreviations
i]fluoranthene BghiFphenanthrene BcPthyloctahydrochrysenes C48HChy]anthracene BaAe + triphenylenea Chy
yltetrahydrochrysenes C34HChy]fluoranthene BbF]fluoranthene BkFfluoranthene BaFpyrene BeP]pyrene BaP
Per,1,2,3-cdef]chrysene + Dibenzo[aj]anthracene IC + DjA1,2,3-cd]pyrene IP
Pi[ah]anthracene DAhi]perylene Bghi
ene AA
ibenzo[b,d]thiophene C1–C2 DBTs with mass 252 C1 m252
PAHs is calculated asP
PAH. TPAH is the sum of all the PAH
compound.
0 5 10
km
HACKENSACKRIVER
PASSAICRIVER
MA
NH
ATT
AN
STATENISLAND
RARITANBAY
ARTHURKILL
GEORGEWASHINGTON
BRIDGE
NEWARKBAY JAMAICA BAYJB 6,13
NB13, 20
RB17, 29
P5
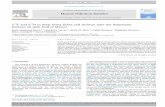
Fig. 1. Sampling sites of seven sediment cores in this study fromin the New York/New Jersey Harbor complex.
676 B. Yan et al. / Organic Geochemistry 37 (2006) 674–687
Alkyl), the sum of the parent PAHs with masses128, 178, 202, and 228 (Na, Pa, A, Fl, Py, BaA,Chy) divided by these parent PAHs plus theirrelated alkyl PAHs. Each of these ratios should the-oretically increase with enhanced contributionsfrom combustion (Yunker et al., 2002; Yan, 2004).
Compound-specific carbon isotope analysis(CSCIA) was introduced by O’Malley et al. (1994)as a method of utilizing distinct carbon isotope dif-ferences between petrogenic and pyrogenic PAHsources in sediments for source apportionment stud-ies (O’Malley et al., 1994; Mazeas and Budzinski,2001; Okuda et al., 2002a; Okuda et al., 2002b).Petrogenic sources (e.g. fresh fuel oil, crankcaseoil) typically have a low d13C value for principalparent PAHs like Fl and Py (around �29 to�27&), whereas pyrogenic compounds from petro-leum and wood combustion have relatively highervalues ranging from �22& to �26& (O’Malleyet al., 1994; Abrajano et al., 2003). McRae andcoworkers have observed significant variations ind13C value during coal conversion processes(McRae et al., 1996; McRae et al., 1998).
In our studies of dated NY/NJ Harbor sedi-ments, we have found the dominant PAH sourcesin recent sediments are from petroleum usageincluding direct oil-spill (petrogenic) and petroleumcombustion (pyrogenic) sources (Yan et al., 2004).In addition, we have shown recently, based on ourstudy of Central Park Lake (NY) sediments, thatcoal and petroleum combustion related PAHs canbe distinguished using a combination of molecularindicators (Yan, 2004). Typically, coal combustionproducts have abundant high molecular weight(HMW) PAH, limited amount of unresolved com-plex mixtures (UCM), and ratios of Fl/(Fl + Py)and 1,7/(1,7 + 2,6)-DMP around 0.55 and 0.67,respectively (Yan et al., 2005). 1,7/(1,7 + 2,6)-DMP, the ratio of 1,7-dimethylphenanthrene(DMP) to the sum of 1,7-DMP plus 2,6-DMP wasfirst used as an indicator of softwood combustionby Benner et al. (1995), but we suggested that theratio of 1,7/(1,7 + 2,6)-DMP could also be used todifferentiate various pyrogenic sources of PAHs(e.g. wood, coal, petroleum combustion) (Yanet al., 2005). Petroleum-combustion derived hydro-carbons have abundant UCM, a ratio of around0.45 for Fl/(Fl + Py), and a 1,7/(1,7 + 2,6)-DMPratio around 0.37–0.45. Therefore, by using thismolecular ratio, we can estimate relative contribu-tions from various combustion sources (petroleum,coal, and wood) (Yan, 2004; Yan et al., 2005).
In this study, dated sediment cores were selectedfrom a sediment archive of over 25 years of exten-sive coring in the NY/NJ Harbor areas. By compar-ing the pyrene d13C values with molecular ratios, weattempt to evaluate the usefulness and limitations ofthese widely used molecular indicators. The implica-tions of the isotopic and molecular variations in sed-iment cores from the NY/NJ Harbor complex willalso be explored.
2. Methods
Four sampling sites (Fig. 1) were selected fromthe NY/NJ Harbor areas including heavily contam-inated areas like Newark Bay (NB) and the PassaicRiver (PR), and less contaminated sites such asRaritan Bay (RB), and Jamaica Bay (JB). Two cores(core JB6 and JB13) were collected from JamaicaBay located west of John F. Kennedy InternationalAirport (JFK) with one core (JB6) collected in 1982and another taken 6 years later near the samelocation (Bopp et al., 1993b). The Passaic River
B. Yan et al. / Organic Geochemistry 37 (2006) 674–687 677
(PR) core was collected in 1985 from an area con-taining petroleum refineries and storage facilities(Bopp et al., 1993b). Two Newark Bay cores(NB13 and NB20) were taken in 1984 and 1985respectively (Keane, 1998) near the center of thebay (Bopp et al., 1991). For this study, we analyzed137Cs dated sediments from the upper 44 cm of coreNB13, corresponding to deposition dates of �1964to �1984 and lower part of NB20 (36 cm until bot-tom, deposition approximate from 1954 to 1964)(Bopp et al., 1991). Two Raritan Bay cores were col-lected in 1980 (RB17) and 1990 (RB29). Core RB17was used to study deposition prior to 1980, whilethe surface sample (0–2 cm) of RB29 with measur-able 7Be has been used to characterize deposition�1990 (Bopp et al., 1993b). Cores from sites ofNB, PR and JB and core RB29 were segmented into2 or 4 cm depth sections soon after collection. RB17was sliced into 2.5–3 cm sections (Keane, 1998).Shortly after collection, core sections were dried at35 �C under a flow of air filtered through Florisil�
to minimize contamination from laboratory air.Once dried, samples were homogenized to a finepowder with a mortar and pestle and stored in air-tight aluminum cans or pre-fired glass vials untilanalysis.
Detailed descriptions of radionuclide datingmethods have been discussed previously (Boppet al., 1991, 1993a, 2006). Briefly, activities of parti-cle-associated radionuclides including 137Cs and 7Bewere measured by gamma-ray spectrometry usingeither a lithium-drifted germanium or an intrinsicgermanium detector. 137Cs is derived from falloutfrom the atmospheric testing of nuclear weaponsthat yielded the first detectable levels of 137Cs inabout 1954 and peaked in 1963 (Olsen et al.,1981). 7Be is naturally created in the upper levelsof the atmosphere through spallation of N2 andO2 by cosmic rays. Due to its short half-life (53.4days), the presence of 7Be in the top few centimetersin a sediment core is evidence of a recent depositionwithin one year of core collection. On the basis ofthree time horizons noted above (1954 and 1963by 137Cs and collection year by 7Be), approximatedates of deposition were assigned to individual coresections. The gamma-spectrometry procedureemployed in our lab also allows quantification oftotal and supported 210Pb (Chillrud et al., 1999).In the cores examined here, however, low surfaceexcess Pb-210 activities, a result of the high massaccumulation rates, greatly limits the utility of thisdating tool (Bopp et al., 2006).
Sediments were Soxhlet extracted using an azeo-tropic acetone/hexane mix (60/40 (v/v)) over night,then the extract was concentrated to 2–4 ml in aKuderna-Danish device. In order to remove themore polar compounds, alumina column chroma-tography was performed on the concentratedextracts. Following alumina cleanup, activated cop-per powder was added to remove elemental sulfur.The extract was separated into two fractions on sil-ica: the first fraction coeluted with hexane contain-ing the low polarity saturated hydrocarbons, andPCBs; the second fraction with CH2Cl2 containingmore polar compounds like PAHs and pesticides(Bopp, 1979).
Hydrocarbon compounds were identified by GC–FID and GC–MS with the help of variouspurchased standards and the Standard ReferenceDatabase of the National Institute of Standardsand Technology (NIST). For the alkane fraction,the total saturated hydrocarbons (TSH) were quan-tified by integrating all the resolved alkane peaksand ‘‘hump’’ UCM as one peak. The UCM was sub-sequently calculated by subtracting the resolvedpeaks from TSH. Various high-purity PAH stan-dards, which included the 16 EPA PAHs, C1–C4
alkyl PAHs homologues, thiophene series andretene, were purchased from Accustandard, Chiron,or Supelco.
GC–FID analysis was performed on a FisonsInstruments 8000-series gas chromatographequipped with an EL980 FID electrometer. Com-pound separation was done on a 0.32 mm ID,60 m, 0.25lm film thicknesses, DB-5 capillary col-umn with a temperature-programmed run (50–150 �C at 10 �C min�1; 150–300 �C at 4 �C min�1;isothermal at 300 �C for 60 min). Flame ionizationdetection provides a linear response for a widerange of PAH concentrations along with a predict-able decrease of area responses (peak area per ngcarbon) with increased retention times. Arearesponses were used for calibration of most ofthe parent PAHs with available standards. Forthose without available standards, area responseswere interpolated from the trend of the arearesponse vs. retention time for known standards(R2 = 0.97). GC–MS analysis was done using aShimadzu GCMS-QP5050 with the GC columnoven programmed at from 50 to 280 at 5 �C/min,held at 50 �C for 3 min, and then at 280 �C for20 min. The column used for GC–MS analysiswas similar to that used for GC/FID analysis buthalf the length (30 m).
678 B. Yan et al. / Organic Geochemistry 37 (2006) 674–687
CSCIA was performed with a with a HewlettPackard 5890 gas chromatograph coupled with acombustion interface to a VG Optima isotope ratiomass spectrometer (IRMS) as previously reported(O’Malley et al., 1994; Smirnov et al., 1998). Thetemperature program was the same as the GCPAH program. For samples with lower signal:UCMratios, we have previously employed additionalHPLC separation to improve measurement preci-sion of d13C but showed the effect of this added stepto be quite insignificant (<0.3&) (O’Malley, 1994).Even worse, incomplete recovery of some lowmolecular weight PAHs led to dramatic discrepan-cies in GC/IRMS measured d13C values and theactual value (i.e., measured by conventional IRMS).More recently, we utilized another method of clean-up combining the use of size exclusion (PhenomenexPhenogel, 100 A) and a thin layer (silica gel, 60 A,500 lm thickness) chromatography on a standardsediment PAH extract from Halifax Harbor (NRCHS3B, National Research Council, Canada) (Abraj-ano, unpublished). Whereas repeat analyses of d13Cfor pyrene (d13CPy) in NRC HS3B show improve-ment in multiple injection precision (1r < 0.2&),the actual measured values are identical to thed13CPy measured without this added clean-up stepto within 0.2&. Consequently, all GC/IRMS analy-ses reported here are for PAH extracts without priorHPLC clean-up.
Stabilities of extracted samples stored in a refrig-erator have been investigated. For example, similar
1930
1950
1970
1990
100 1000 1000
JB
NB
PR
RB
App
roxi
mat
e de
posi
tion
year
ΣPAH(
Fig. 2.P
PAH (lg/g dry weight) depth profiles in the four cores from th1954 in cores NB and RB was estimated under the assumption that th
molecular distributions and ratios (e.g. Fl/(Fl + Py)) have been observed in recent GC deter-mination and those determined about two decadesago (Yan, 2004). To assure data quality in thePAH analysis, over 5% of the total number of sam-ples analyzed in our lab were extracted and analyzedmore than once using different sample weights (cf.Keane, 1998). About 10% of the sample extractswere injected twice, providing duplicate chromato-graphic runs. Analytical results from both types ofduplicates showed a relative percent difference ofless than 15% (Bopp, unpublished data). QA/QCmethodology for the GC–IRMS follows that ofO’Malley et al. (1994).
3. Results and discussion
3.1. Spatial and temporal distributions of
hydrocarbons from the NY/NJ Harbor complex
‘‘Total’’ PAH (P
PAH) is commonly defined asthe sum of 3–6 ring parent PAH compounds, butthis measure clearly underestimates petrogenicsources that tend to have abundant alkyl PAHs.To better compare our results with other studies,we use
PPAH as defined (Fig. 2), but we also pres-
ent TPAH as sum of all quantified parent and alkylPAHs (Table 1, Fig. 3). The range of
PPAH con-
centrations (dry weight) varies considerably, bothspatially and temporally. For example, around1980, the concentration sequence in four sites from
0 100000 10000001930
1950
1970
1990
ppb)
e NY/NJ Harbor complex. Approximate deposition date prior toere is the same deposition rate before 1954 as that after 1954.
1930
1940
1950
1960
1970
1980
1990
1930
1940
1950
1960
1970
1980
1990
1930
1940
1950
1960
1970
1980
1990
1930
1940
1950
1960
1970
1980
1990
0 1 1 2 2 1 0 2 5 4 0
0 1900 3800
UCM
17 34 51
TPAH
800 1600 2400
0 4 8 9 6
PRPAHs
25 200 375
2 0 2 0 0 3 8 0
C R P A H s
2500 3850 5200
5 0 6 7 8 4
150 220 290
9 5 1 5 5 2 1 5
110 300 490
Raritan Bay
0 1 0 2 0
2 16 30
2 1 0 1 8
PRPAHs C R P A H s
PRPAHs C R P A H s PRPAHs C R P A H s
Passaic River
Jamaica Bay Newark Bay
UCM TPAH
TPAH TPAHUCM UCM
App
roxi
mat
e D
epos
ition
Yea
rA
ppro
xim
ate
Depo
sitio
n Y
ear
Fig. 3. Temporal trends of UCM, TPAH, calculated petroleum-derived PAHs and combustion- derived PAHs (lg/g dry weight) in foursub-basins in New York/New Jersey Harbor complex. Trends indicated by dashed lines follow the top scale; solid lines follow the bottomscale.
B. Yan et al. / Organic Geochemistry 37 (2006) 674–687 679
highest to lowest is the Newark bay (70 lg/g), Pas-saic River (60 lg/g), Jamaica Bay (9.4 lg/g), andRaritan Bay (1.9 lg/g), which is consistent withother reports in these areas (Crawford et al., 1995;Gigliotti et al., 2002). The considerable geographicvariations in PAH concentrations suggest that localPAH sources are most important.
In core PR, concentrations of principal parentPAHs (Fl, Py, BaA, Chy, etc.) in all but the top sec-tion are higher than their effects range-median(ERM) guideline values (amphipod toxicity). Thissuggests that PAHs in the Passaic River could havesignificant effects on benthic organisms (Long et al.,1995). In all but the oldest sections of the NB cores,concentrations of these principal compounds arelower than their ERM, but greater than ERL
(effects range-low) (Long et al., 1995). In contrast,all above mentioned principal PAHs in core JBand RB are close to or below their correspondingERL values.P
PAH depth profiles from NB, RB and JB showelevated levels around 1940s–1950s, then generallydeclining to the 1980s. These decreasing temporaltrends are consistent with those recorded in NJ Har-bor sediments (Huntley et al., 1995) and manyobservations around the world (Sanders et al.,1993; Simcik et al., 1996; Fernandez et al., 2000;Okuda et al., 2002a, Lima et al., 2003), but oppositeto the increasing trend observed by Van Metre et al.(2000). This substantial decline in PAH concentra-tion since the 1950s has been attributed to a majorshift in energy use (e.g. residential heating and
Table 2The Pearson correlation matrix of various concentrations
TPAH UCM PRPAHs CRPAHs
TPAH 1.00UCM 0.49 1.00PRPAHs 0.54 0.67 1.00CRPAHs 0.98 0.39 0.37 1.00
Note: TPAH is concentration sum of all quantified PAHs insamples, UCM are unresolved complex mixture; PRPAHs is thepetroleum related PAHs, CRPAHs are the combustion relatedhydrocarbons, both are calculated from the mass balance ofisotope ratios (detailed explanations in text).
680 B. Yan et al. / Organic Geochemistry 37 (2006) 674–687
power generation) from predominantly coal-derivedenergy to a greater petroleum and gas usage (Hiteset al., 1980; Pereira et al., 1999). Evidence suggeststhat coal combustion has the potential to releasemore PAHs than petroleum or natural gas per unitof power generated (Rogge et al., 1993; Oros andSimoneit, 2000; Schauer et al., 2001).
Degraded or weathered petroleum typically hasmuch higher UCM abundance relative to resolvedpeaks. In contrast, wood and coal combustion nor-mally has lower UCM abundance than resolvedpeaks (Oros and Simoneit, 2000; Fine et al.,2001). In most of the sediments from cores RBand JB, the hump is generally broad and high com-pared with saturated hydrocarbon peaks, indicat-ing saturated hydrocarbon sources are mainlyfrom petroleum usage. Compared with a broadUCM range (i.e., large molecular weight range)in samples from other sites, elevated UCM in coreJB tends to have a lower molecular weight distri-bution (C15–C21), probably indicating biodegrada-tion of jet fuel from the JFK (Fig. 1), and it islikely that there is a direct input from the airport(Bopp et al., 1993b).
The carbon preference index (CPI), ratio of thesum of the odd carbon-numbered alkanes (fromC25 to C35) to the sum of the even carbon-number(from C24 to C34), is widely used to distinguish sat-urated hydrocarbon sources between higher plants(CPI > 5), and petroleum (CPI � 1) (Wakeham,1996). In all our cores, CPI is mainly around 1–2,indicating dominant petroleum sources for the satu-rated hydrocarbons rather than higher plantsources.
3.2. d13CPy and PAH sources over time
Previously, we proposed a method for quantify-ing PAH sources from carbon isotopic mass balanceequations (e.g., O’Malley et al., 1994; Abrajanoet al., 2003). However because similar stable isoto-pic patterns of major PAH compounds (Fl, Py,BaA, Chy) were always observed among samplesin this study and previous studies (Stark et al.,2003), in the present work, pyrene (Py) is selectedto define the two end-members of petroleum relatedPAHs (PRPAHs) and combustion related PAHs(CRPAHs). Using this approach, we assign d13Cof Py (d13CPy) from petrogenic source a value of�29&, while that from combustion sources as�24& (O’Malley et al., 1994; Abrajano et al.,2003). The mass balance equation is as follows:
d13Csample ¼ �29‰ � fPetro þ�24‰ � ð1� fPetroÞð1Þ
where fPetro is the fraction from petroleum. Eq. (1)assumes 2-source mixing, hence the fraction fromcombustion should be 1 � fPetro. If d13C of Py inthe sample is known, then PRPAHs can be calcu-lated from Eq. (1) and the following equation:
PRPAHs ¼ TPAHs � fPetro ð2Þwhere we use TPAH rather than
PPAH becauseP
PAH underestimates ‘‘total PAH’’ especiallywhen there are significant inputs from petroleum re-lated sources (Readman et al., 2002; Yan, 2004).
As reported previously, d13CPy from petrogenicor combustion sources show a range of values with2r variation of � ± 2& for combustion-producedpyrene (n = 42) and a narrower 2r variations of� ± 1& for petrogenic pyrene (n = 31) (O’Malleyet al., 1994; Abrajano et al., 2003). In the presentpaper, we selected the mean values of �29& and�24& as representative ratios for the two sources,respectively, and this inevitably leads to some uncer-tainties (2r of up to 60%) in any estimated PRPAHsand CRPAHs fraction. Regardless of the specificd13CPy values selected, however, the calculatedtrends of these two end-members in each coreshould be qualitatively similar, hence the correlationanalysis with other source indicators remainsrobust.
Depth profiles of two independent parameters,PRPAHs and UCM, from the four sites are shownin Fig. 3. Even minor fluctuations in these twoprofiles are similar, whereas the calculated combus-tion-related PAH resemble the profile of TPAH.PRPAHs show a stronger correlation with theUCM (Pearson correlation R=0.67, n = 42,p < 0.001) than CRPAHs do (R = 0.39) (Table 2).Similar historical trends and strong correlationbetween PRPAHs and UCM in these four cores
Tab
le3
Th
eP
ears
on
corr
elat
ion
mat
rix
of
iso
top
era
tio
san
dva
rio
us
mo
lecu
lar
rati
os
(all
the
sam
ple
sfr
om
NY
/NJ
Har
bo
r)
d13C
Py
Fl/
(Fl
+P
y)A
/(P
a+
A)
BaA
/(B
aA+
Ch
y)C
0/(
C0
+C
1)P
/AC
0/(
C0
+C
1)F
/Py
IP/(
IP+
Bgh
i)R
ing4
56/T
PA
HP
ar/(
Par
+A
lkyl
)
d13C
py
1.0
0
Fl/
(Fl
+P
y)0
.66
1.00
A/(
Pa
+A
)0
.09
�0.
111.
00B
aA/(
BaA
+C
hy)
�0
.20
�0.
47�
0.14
1.00
C0/(
C0
+C
1) P
/A0
.01
0.00
0.33
�0.
091.
00C
0/(
C0
+C
1) F
/P0
.49
0.60
0.06
�0.
420.
061.
00IP
/(IP
+B
ghi)
0.0
10.
160.
12�
0.43
0.20
0.11
1.00
Rin
g456
/TP
AH
0.2
90.
210.
40�
0.39
0.01
0.17
0.35
1.00
Par
/(P
ar+
Alk
yl)
0.5
70.
560.
29�
0.31
0.16
0.49
0.11
0.76
1.00
No
te:d1
3C
Py
isth
est
able
carb
on
iso
top
eco
mp
osi
tio
no
fP
y;se
ete
xtfo
rm
ole
cula
rin
dic
ato
rd
efin
itio
n.
B. Yan et al. / Organic Geochemistry 37 (2006) 674–687 681
suggest that the trend of PRPAHs calculated by thecarbon mass balance equation can reflect the rela-tive importance of petroleum input.
3.3. Correlation of pyrene d13C with molecular
indicators
Pearson correlation analysis was used to eluci-date the relationship of the aforementioned molecu-lar criteria and d13CPy. As discussed above, if initialmolecular characteristics are retained from sourcesto sediments, the molecular ratios should correlatewith each other and with d13CPy. Theoretically,d13CPy should be positively correlated with themolecular ratios as configured because d13CPy willincrease as petroleum combustion contributionincreases (O’Malley et al., 1994).
3.3.1. Fl/(Fl + Py)
Fluoranthene and pyrene are two of the mostabundant PAH compounds in sediment samples(Wakeham et al., 1979a,b; Fernandez et al., 2000).Yunker et al. (2002) reviewed the variation of Fl/(Fl + Py) ratios in petroleum/crude, single-sourcecombustion and environmental samples. They pro-posed that the petroleum/crude to combustionboundary ratio appears closer to 0.4 than 0.5, thelatter being the demarcation used by others previ-ously. Yunker et al.’s (2002) work also suggests thatpetroleum combustion products have a range of Fl/(Fl + Py) of around 0.4–0.5, while PAHs producedfrom plant biomass burning (including grass, woodor coal) have ratios typically greater than 0.50(Yunker et al., 2002). Correlation coefficientsamong various indicators in this study are shownin Table 3. Fl/(Fl + Py) correlates strongly withd13CPy (R = 0.66, n = 42, p < 0.001) when all sam-ples from the four sampling sites are considered.However, the correlation coefficients between Fl/(Fl + Py) ratio with d13CPy vary considerably fromsite to site when only the samples within individualsites are considered (Table 4). It is likewise notablethat the correlation appears to be weakest whereTPAH are highest (i.e., Passaic River, NewarkBay). In core PR and the upper portion of coreNB, very small variations in d13CPy ratios and Fl/(Fl + Py) ratios were observed, indicating a relativestable contribution from combustion and petroleumsources (Fig. 4). In contrast, cores JB and RBshowed larger temporal changes in both d13CPy
and Fl/(Fl + Py) ratios (Fig. 4), suggesting the fluc-tuations of major PAH contribution between petro-
Table 4Correlation coefficients of d13CPy with Fl/(Fl + Py) and Par/(Par + Alkyl) and Ring456/TPAH from four sampling sites
Jamaica Bay (n = 9) Newark Bay (n = 15) Passaic River (n = 8) Raritan Bay (n = 10)
Fl/(Fl + Py) 0.63 0.38 �0.48 0.83BaA/(BaA + Chy) 0.16 0.46 0.03 �0.80C0/(C0 + C1)F/P 0.04 0.55 0.15 0.60Ring456/TPAH 0.59 0.32 0.52 0.64Par/(Par + Alkyl) 0.52 0.66 0.48 0.72
1930
1940
1950
1960
1970
1980
1990
1930
1940
1950
1960
1970
1980
1990
1930
1940
1950
1960
1970
1980
1990
1930
1940
1950
1960
1970
1980
1990
Raritan Bay
δ13
Cpy Par/(Par+Alkyl)
Passaic River
Jamaica Bay Newark Bay
δ13
Cpy Par/(Par+Alkyl)
δ13
Cpy Par/(Par+Alkyl) δ13
Cpy Par/(Par+Alkyl)
Ring456/TPAHFl/(Fl+Py) Ring456/TPAHFl/(Fl+Py)
Ring456/TPAHFl/(Fl+Py) Ring456/TPAHFl/(Fl+Py)
-26.9 -25.4 -23.9 0.27 0.38 0.50
0.43 0.46 0.49 0.43 0.55 0.68
-26.2 -25.2 -24.3 0.32 0.43 0.54
0.41 0.45 0.49 0.55 0.66 0.77
-26.1 -25.7 -25.3 0.32 0.36 0.40 -27.8 -26.1 -24.3 0.31 0.51 0.71
0.44 0.48 0.53 0.53 0.61 0.70 0.34 0.46 0.59 0.56 0.72 0.87
Petroleum Combustion
App
roxi
mat
e D
epos
ition
Yea
rA
ppro
xim
ate
Dep
ositi
on Y
ear
Fig. 4. Temporal trends of correlated indicators (refer to text definitions) in samples from four cores in New York/New Jersey Harborcomplex. Trends indicated by dashed lines follow the top scale; solid lines follow the bottom scale. For all indicators, a tendency towardsthe right suggests increasing contributions from combustion, while to the left, petroleum.
682 B. Yan et al. / Organic Geochemistry 37 (2006) 674–687
genic and combustion sources. The stronger correla-tion of d13CPy and Fl/(Fl + Py) in cores JB and RBsuggest that these two ratios are indeed sensitiveenough to reflect major shifts in PAH sourcesbetween petrogenic and pyrogenic sources (cf.
Abrajano et al., 2003). The weaker correlation inthe more heavily polluted areas is presumably dueto enhanced importance of variability withinsources (e.g., amongst combustion sources suchas motor vehicle emissions, combustion from oil
B. Yan et al. / Organic Geochemistry 37 (2006) 674–687 683
refineries, residence heating, wood combustion,incinerator products, etc.) where no large shifts inpetrogenic/combustion ratio occur or where com-bustion overwhelmingly dominates PAH sources(e.g., in highly industrialized areas). The poor corre-lation between d13CPy and Fl/(Fl + Py) may alsoarise where the specific petrogenic and combustionPAH sources for an area overlap in their Fl/(Fl + Py) or d13CPy ratios. For example, the ratiosof Fl/(Fl + Py) from kerosene (petrogenic PAHsource) and gasoline combustion were reported tobe 0.46 and 0.44, respectively, in a recent study(Yunker et al., 2002, and reference therein).
3.3.2. A/(Pa + A)
The ratio of A/(Pa + A) has been considered as asensitive indicator by Yunker and other scientists(Budzinski et al., 1997; Readman et al., 2002). Gen-erally, a ratio < 0.1 is indicative of petrogenicsources, while greater than 0.1 implies combustionsources. In the present study, A/(Pa + A) has a dif-ferent trend and weak correlation with d13CPy andother molecular indicators (Table 3). For instance,in all but surface sediments from sites RB, boththe d13CPy (ranging from �27.7 to �27.0&) andFl/(Fl + Py) ratio (0.34–0.4) indicate a dominantpetrogenic source. However, A/(Pa + A) ratios aregreater than 0.25 suggesting a dominant combustionsource. The inconsistency of ratio A/(Pa + A) withthe other ratios is also observed in 1990s harbor sur-face sediment study and a Central Park Lake sedi-ment core (Yan, 2004). We attribute thisdiscrepancy to environmental behavior differencesbetween two isomers Pa and A. Previous work hasshown that anthracene (A) has a much higherpotential for photolysis than Pa, hence the applica-bility of this molecular indicator in ‘‘weathered’’PAHs may be questionable (Masclet et al., 1986;Sanders et al., 1993).
3.3.3. BaA/(BaA + Chy)
Yunker et al. (2002) proposed that a ratio ofBaA/(BaA + Chy) less than 0.2 was indicative ofpetrogenic inputs, whereas ratios greater than 0.35point to combustion sources. However, the presentstudy shows that BaA/(BaA + Chy) negatively cor-relates with d13CPy and Fl/(Fl + Py) when samplesfrom all the four sites are considered. Indeed, thecorrelation coefficients varied substantially from siteto site, but are very weak in general (Table 4). BaA/(BaA + Chy) shows a low negative correlation withd13CPy in cores from Raritan Bay (RB17 and RB29)
and Passaic River, but a low positive correlation incores JB and NB. As with the ratio of A/(Pa + A),the weaker correlation and the aberrant variationsin correlation coefficients between the ratio ofBaA/(BaA + Chy) and d13CPy may be caused bythe different environmental behaviors of these twoisomers. BaA has a shorter half-life when exposedto sunlight, atmosphere, compared with chrysene(Masclet et al., 1986; Kamens et al., 1988). Regard-less of the ultimate explanation for the observedresults, they clearly imply that caution is necessarywhen using BaA/(BaA + Chy) as tracer to sourcePAHs.
3.3.4. IP/(IP + Bghi)
Yunker et al. (2002) proposed that IP/(IP +Bghi) ratios less than 0.2 may suggest a petrogenicinput, between 0.20 and 0.50 likely imply liquid fos-sil fuel combustion, while over 0.5 may be indicativeof plant biomass combustion (including coal com-bustion). Our correlation studies show that IP/(IP + Bghi) has little to no correlation with thed13CPy (R = 0.01), and no or very weak correlationwith other molecular ratios (e.g. Fl/(Fl + Py))(Table 3).
3.3.5. Alkyl PAH series (C0/(C0 + C1)P/A; C0/
(C0 + C1)F/P)High C0/(C0 + C1)P/A and C0/(C0 + C1)F/P ratios
generally indicate combustion contribution,whereas low ratios implicate petrogenic sources(Wakeham et al., 1979a; Sporstol et al., 1983; Yun-ker et al., 2002). Our post-1990 harbor sedimentstudy showed that both these indicators have goodcorrelations with the Fl/(Fl + Py) ratio (R is 0.92and 0.94 for P/A and F/P series respectively,n = 13) (Yan et al., 2004). In the NY/NJ Harborsediments, C0/(C0 + C1)P/A showed poor correla-tion with d13CPy(R = 0.01, n = 42), whereas theC0/(C0 + C1)F/P is moderately correlated withd13CPy (R = 0.49, p < 0.01) (R = 0.60) (Table 3).F/P series appears to be poorly correlated withd13CPy in Jamaica Bay (R = 0.04, n = 9) despitethe fact that other molecular ratios are correlatedstrongly (Table 4). The stronger correlation of thesetwo ratios with d13CPy in post 1990s harbor sedi-ments and weaker historical correlation in oldersediment cores may be due to the alteration ofengine types and catalytic conversion systems inthe past several decades. It is well known that variedcombustion temperature and ratios of air to fuelratios in different engine types as well as post-engine
684 B. Yan et al. / Organic Geochemistry 37 (2006) 674–687
pollution control devises could substantially alterthe alkyl PAH composition in motor vehicle emis-sions (Baek et al., 1991).
3.3.6. Ring456/TPAH
Ratios or percentages of LMW (2–3 ring) to totalor HMW (4–6 ring) parent PAHs have been appliedin several studies to distinguish petrogenic andpyrogenic PAH sources (Bence et al., 1996; Wanget al., 1999; Mai et al., 2002). However, Benceet al. (1996) found that a few years after the ExxonValdez oil spill, most of the 2–3 ring parent PAHswere lost due to evaporation and degradation.Hence, source apportionment assignments using2–3 ring parent PAHs may become compromisedafter moderate weathering. Nevertheless, it is alsoknown that some of the alkylated Na and Pa homo-logues especially the C3–C4 homologues couldremain after moderate weathering and depositionin anaerobic conditions (Wang et al., 1999). Forpost-1990s sediments, Ring456/TPAH, the ratio oftotal 4–6 ring PAHs (including parent and alkylatedhomologues) to total PAH, was used as a proxy forthe ratios of LMW to total or HMW parent PAHs(Yan et al., 2004), and was found to strongly corre-late with Fl/(Fl + Py) and two alkyl series molecularindicators. Further analyses have found thatRing456/TPAH is sensitive to inputs from freshoil spills as long as no extensive degradation occurs(e.g. complete loss of the 2 and 3 ring parent andalkyl PAHs) (Yan et al., 2004). After moderate tostrong weathering, the ratio may become indifferentto petrogenic contributions. Concurrent researchhas found that the representative ratios of petro-genic and fossil fuel combustion sources can be setas 0.3 and 0.7, respectively (Yan, 2004).
In the NY/NJ Harbor sediments, Ring456/TPAH shows only fair correlation with d13CPy
(R = � 0.29, p < 0.1, n = 42) when all of the sedi-ment samples are included, but show quite similartrends (Fig. 4) within each specific core site. Thiscould imply either that the molecular characteristicsof petrogenic and combustion sources are distinctfrom site to site or there were distinct weatheringpathways after PAH deposition in each specific siteover the last several decades.
3.3.7. Par/(Par + Alkyl)
Dominance of alkyl substituted over parentPAHs from petrogenic sources is seen in the Pa/A,F/P Na and BaA/Chy series (Wang et al., 1999).LMW Na and P/A series degrade more easily, lead-
ing to a prominence of HMW PAHs in sediments ingeneral (e.g. Fl/(Fl + Py) and BaA/Chy series)(Wang et al., 1999). Despite possible degradation,the BaA/Chy series from oil spills could retain theiralkyl dominance even after extensive weathering(e.g., Bence et al., 1996). Consideration of all fouralkyl series (Na, P/A, F/P, and 228 series) mayallow a determination of the presence of oil spillsand the degree by which the oil has been degraded(Wang et al., 1999). Therefore a ratio like Par/(Par + Alkyl), the total C1–C4 homologues of themasses 128,178, 202, and 228 to the parent PAHswith these masses (Na, Pa, A, Fl, Py, BaA, Chy),can be used to distinguish petrogenic and pyrogenicsources even after extensive weathering in sediments(Yan et al., 2004). In the harbor sediments, a corre-lation coefficient of 0.57 (p < 0.001) between Par/(Par + Alkyl) and d13CPy was observed. Par/(Par + Alkyl) also presents a very strong correlationwith other molecular ratios (e.g. 0.56 with Fl/(Fl + Py) and 0.76 with Ring456/TPAH,p < 0.001). As with Ring456/TPAH, correlationcoefficients range from 0.48 in core PR to 0.72 incore RB (Table 3), indicating its fairly stable corre-lation with d13CPy in all four areas. The strong cor-relation in each individual area and whole harborcomplex implies that Par/(Par + Alkyl) may be areliable PAH source indicator over large geographic(or even temporal) scales.
3.4. Combined application of d13CPy, Fl/(Fl + Py),
Par/(Par + Alkyl), and Ring456/TPAH
The above discussions suggest that d13CPy andFl/(Fl + Py), Par/(Par + Alkyl), and Ring456/TPAH are promising indicators that could be usedas PAH tracers in the NY/NJ Harbor areas.Fig. 4 shows trends of those four ratios in each site.Despite the parallel trends of
PPAH levels in most
areas of the NY/NJ Harbor from the 1950s to the1980s (Fig. 2), depth profiles of d13CPy and the threemolecular ratios are completely different from site tosite (Fig. 4), suggesting varied PAH sources in dif-ferent areas. Generally, in most sediment samples,Fl/(Fl + Py) and 1,7/(1,7 + 2,6)-DMP ratios (notshown) are below 0.48 and 0.40 respectively. Thisis especially true for sediments deposited withinthe past half century, where the ratios are well belowthe corresponding ratios for coal-combustion (0.56,0.66 respectively). Interestingly, these ratios arewithin the range of 1,7/(1,7 + 2,6)-DMP ratios offossil fuel combustion products. In contrast, the
B. Yan et al. / Organic Geochemistry 37 (2006) 674–687 685
top section of core RB29, Fl/(Fl + Py) shows a highratio of 0.65 and 1,7/(1,7 + 2,6)-DMP of 0.52, sug-gesting a high possibility for PAH input from coalcombustion for these recent sediments. However,the ratio of U/R (UCM to resolved hydrocarbons)in the saturated fraction is 23, much higher thanthe coal generated ratio from coal smoke (2.9–3.3)(Oros and Simoneit, 2000), and comparable withpetroleum dominant ratios (�20) seen in CentralPark Lake, New York City (Yan et al., 2005).Therefore, it is likely that in these four sites, petro-leum-combustion related usage is the dominantPAH source over the past several decades.
However, we note that prior to 1960, higher con-centrations of TPAH are observed in the sedimentsfrom NB and RB. As mentioned above, the decreas-ing
PPAH trends since the 1940s to the present
have been attributed to a major fuel change fromcoal to petroleum combustion (Hites et al., 1980).The energy shift probably can explain observationsfrom Newark Bay sediments. From the 1930s to1940s in core NB, d13CPy is around �24& to�25.7& indicating a combustion dominant source.Fl/(Fl + Py) ratios are around 0.5, suggesting a pos-sible source from coal or wood combustion. How-ever, 1,7/(1,7 + 2,6)-DMP ratios would be higher(> 0.6) than those determined in the 1940s (�0.40)if the major source was wood burning (Benneret al., 1995; Yan, 2004; Yan et al., 2005). Therefore,the dominant PAH sources in Newark Bay aroundthe 1940s were most likely from mixture of petro-leum and coal combustion sources (power genera-tion) (Rod et al., 1989). Using mass balanceequations based on 1,7/(1,7 + 2,6)-DMP, we foundthat 30–50% of TPAHs are from coal combustion(Yan, 2004). However, the energy shift may notexplain the PAH decline in the case of RaritanBay. From the 1940s to the 1970s, all four indicatorsremain relatively constant with d13CPy (around �27to �28&) and Fl/(Fl + Py) ratios (from 0.3 to 0.4)showing dominant petroleum usage source, proba-bly resulting from substantial petroleum usage inthe nearby Arthur Kill. In most of the harbor sam-pling sites (e.g. the Passaic River, Raritan Bay andJamaica Bay), a gradual increase in combustionfrom 1970s to 1980s can be observed (Fig. 4). Mostindicators in these three sites support this finding.
4. Summary
Concentrations of PAH are observed to decreasefrom the 1940s to the 1980s in sediments from many
areas within the NY/NJ Harbor complex, Fl/(Fl + Py), Par/(Par + Alkyl), and Ring456/TPAHsare significantly correlated. Combined applicationsof these PAH source indicators have found that inRaritan Bay, petroleum dominance is observedfrom the 1950s to the 1970s with a switch to com-bustion dominance from the 1970s to the 1990s. InJamaica Bay the dominant PAH source switchesfrom combustion to petroleum before �1970 thenback to combustion until �1990. Sediment recordsin the Passaic River were dominated by petroleumcombustion from the 1960s to the 1980s. In NewarkBay, mixtures from coal and petroleum combustionare the two major PAH sources before the 1950s,then coal combustion decreases in the last half-cen-tury. Combined applications of selected indicatorssuggest that in most areas of the NY/NJ Harbor,combustion source has increased in relative impor-tance since the early 1970s.
Acknowledgement
Funding for this project was provided by the Na-tional Science Foundation (Hydrogeology Pro-gram) and the Hudson River Foundation(A90095). Three anonymous reviewers and E.A.Canuel are specially thanked for their insightful sug-gestions and efforts to improve the quality of thismanuscript.
Associate Editor—Elizabeth Canuel
References
Abrajano, T.A., Yan, B., O’Malley, V.P., 2003. High-molecularweight petrogenic and pyrogenic hydrocarbons in aquaticenvironments. In: Lollar, B.S., (Ed.), Treatise on Geochem-istry, Environmental Geochemistry, pp. 475–510.
Baek, S.O., Field, R.A., Goldstone, M.E., Kirk, P.W., Lester,J.N., Perry, R., 1991. A review of atmospheric polycyclicaromatic hydrocarbons: sources, fate and behavior. Water,Air, and Soil Pollution 60, 279–300.
Bence, A.E., Kvenvolden, K.A., Kennicutt, M.C., 1996. Organicgeochemistry applied to environmental assessments of PrinceWilliam Sound, Alaska, after the Exxon Valdez oil spill – areview. Organic Geochemistry 24 (1), 7–42.
Benner, B.A., Wise, S.A., Currie, L.A., Klouda, G.A., Kline-dinst, D.B., Zweidinger, R.B., Stevens, R.K., Lewis, C.W.,1995. Distinguishing the contributions of residential woodcombustion and mobile source emissions using relativeconcentrations of dimethylphenanthrene isomers. Environ-mental Science & Technology 29, 2382–2389.
Bopp, R.F., 1979. The Geochemistry of Polychlorinated Biphe-nyls. Ph.D. Thesis, Department of Earth Science. ColumbiaUniversity, New York.
686 B. Yan et al. / Organic Geochemistry 37 (2006) 674–687
Bopp, R.F., Chillrud, S.N., Shuster, E.L., Simpson, H.J., 2006.Contaminant chronologies from Hudson River sedimentaryrecords. In: Levinton, J., Waldman, J. (Eds.), The HudsonRiver Estuary. Cambridge University Press, pp. 383–397,chapter 26.
Bopp, R.F., Chillrud, S.N., Simpson, H.J., Virgillio, A., 1993a.Pollutant chronologies in New York Harbor, Raritan Bayand Jamaica Bay. Final Report, New Jersey Sea GrantContract NJMSC R/E-17P.
Bopp, R.F., Simpson, H.J., Chillrud, S.N., Robinson, D.W.,1993b. Sediment-derived chronologies of persistent contam-inants in Jamaica Bay, New York. Estuaries 16 (3B), 608–616.
Bopp, R.F., Gross, M.L., Tong, H., Simpson, H.J., Monson, S.J.,Deck, B.L., Moser, F.C., 1991. A major incident of dioxincontamination: sediments of New Jersey estuaries. Environ-mental Science & Technology 25, 951–956.
Budzinski, H., Garrigues, P., Bernard, G., Bellocq, J., Hinrichs,K., Rullkotter, J., 1997. Identification of polycyclic aromatichydrocarbons in sediments from the Amazon fan: occurrenceand diagenetic evolution. In: R.D. Flood, D.J.W. Piper, A.Klaus, L.C. Peterson (Eds.), Proceedings of the OceanDrilling Program, Scientific Results, 155, pp. 555–564.
Chillrud, S.N., Bopp, R.F., Simpson, H.J., Ross, J., Shuster,E.L., Chaky, D.A., Walsh, D.C., Chin Choy, C., Tolley, L.R.,Yarme, 1999. Twentieth century atmospheric metal fluxes intoCentral Park Lake, New York City. Environmental Science &Technology 33, 657–662.
Crawford, D.W., Bonnevie, N.L., Wenning, R.J., 1995. Sourcesof pollution and sediment contamination in Newark Bay,New Jersey. Ecotoxicology and Environmental Safety 30, 85–100.
Fernandez, P., Vilanova, R.M., Appleby, P., Grimalt, J.O., 2000.The historical record of atmospheric pyrolytic pollution overEurope registered in the sedimentary PAH from remotemountain lakes. Environmental Science & Technology 34,1906–1913.
Fine, P.M., Cass, G.R., Simoneit, B.R.T., 2001. Chemicalcharacterization of fine particle emissions from fireplacecombustion of woods grown in the northeastern UnitedStates. Environmental Science & Technology 35, 2665–2675.
Gigliotti, C.L., Brunciak, P.A., Dachs, J., Glenn IV, T.R.,Nelson, E.D., Totten, L.A., Eisenreich, S.J., 2002. Air-waterexchange of polycyclic aromatic hydrocarbons in the NewYork–New Jersey, USA, Harbor Estuary. EnvironmentalToxicology and Chemistry 21 (2), 235–244.
Gunster, D.G., Bonnevie, N.L., Gillis, C.A., Wenning, R.J.,1993. Assessment of chemical loadings to Newark Bay, NewJersey from petroleum and hazardous chemical accidentsoccurring from 1986–1991. Ecotoxicology and EnvironmentalSafety 25, 202–213.
Hites, R.A., LaFlamme, R.E., Windsor, J.G., Farrington, J.W.,Deuser, W.G., 1980. Polycyclic aromatic hydrocarbons in ananoxic sediment core from the Pettaquamscutt River (RhodeIsland, USA). Geochimica et Cosmochimica Acta 44, 873–878.
Huntley, S.L., Bonnevie, N.L., Wenning, R.J., 1995. Polycyclicaromatic hydrocarbon and petroleum hydrocarbon contam-ination in sediment from the Newark Bay Estuary, NewJersey. Archives of Environmental Contamination and Tox-icology 28, 93–107.
Kamens, R.M., Guo, Z., Fulcher, J.N., Bell, D.A., 1988.Influence of humidity, sunlight, and temperature on the
daytime decay of polyaromatic hydrocarbons on atmosphericsoot Particles. Environmental Science & Technology 22, 103–108.
Keane, D.P., 1998. Temporal Trends of Saturated and PolycyclicAromatic Hydrocarbons in the sediments of the Hudson andPassaic River Systems. Master thesis, E & ES. RPI, Troy,NY.
Lima, A.C., Eglinton, T.I., Reddy, C.M., 2003. High resolutionrecord of pyrogenic polycyclic aromatic hydrocarbons depo-sition during the 20th century. Environmental Science &Technology 37, 53–61.
Long, E.R., Macdonald, D.D., Smith, S.L., Calder, F.D., 1995.Incidence of adverse biological effects within ranges ofchemical concentrations in marine and estuarine sediments.Environmental Management 19 (1), 81–97.
Mai, B., Fu, J., Sheng, G., Kang, Y., Lin, Z., Zhang, G., Min,Y., Zeng, E.Y., 2002. Chlorinated and polycyclic aromatichydrocarbons in riverine and estuarine sediments fromPearl River Delta, China. Environmental Pollution 117,457–474.
Masclet, P., Mouvier, G., Nikolaou, K., 1986. Relative decayindex and sources of polycyclic aromatic hydrocarbons.Atmospheric Environment 20 (3), 439–446.
Mazeas, L., Budzinski, H., 2001. Polycyclic aromatic hydrocar-bon 13C/12C ratio measurement in petroleum and marinesediments: Application to standard reference materials and asediment suspected of contamination from Erika oil spill.Journal of Chromatography A 923, 165–176.
McRae, C., Love, D., Murray, I.P., Snape, C.E., Fallick,A.E., 1996. Potential of gas chromatography isotope ratiomass spectrometry to source polycyclic aromatic hydro-carbon emissions. Analytical Communications 33, 331–333.
McRae, C., Snape, C.E., Fallick, A.E., 1998. Variations in thestable isotope ratios of specific aromatic and aliphatichydrocarbons from coal conversion processes. Analyst 123,1519–1523.
Mitra, S.M., Dellapena, T.M., Dickhut, R., 1999. Geochemicalfactors affecting sediment/pore water distribution of PAH’s inthe Hudson River. Estuarine, Coastal and Shelf Science 49,311–326.
Okuda, T., Kumata, H., Naraoka, H., Ishiwatari, R., Takada,H., 2002a. Vertical distributions and d13C isotopic composi-tions of PAHs in Chidorigafuchi Moat sediment, Japan.Organic Geochemistry 33, 843–848.
Okuda, T., Kumata, H., Zakaria, M.P., Naraoka, H., Ishiwatari,R., Takada, H., 2002b. Source identification of Malaysianatmospheric polycyclic aromatic hydrocarbons nearby forestfires using molecular and isotopic composition. AtmosphericEnvironment 36, 611–618.
Olsen, C.R., Simpson, H.J., Peng, T.H., Bopp, R.F., Trier, R.M.,1981. Sediment mixing and accumulation rate effects onradionuclide depth profiles in Hudson Estuary sediments.Journal of Geophysical Research 86, 11020–11028.
O’Malley, V.P., 1994. Compound-Specific Carbon Isotope Geo-chemistry of Polycyclic Aromatic Hydrocarbons in EasternNewfoundland Estuaries. PhD Thesis, Memorial Universityof Newfoundland.
O’Malley, V.P., Abrajano, T.A., Hellou, J., 1994. Determinationof the 13C/12C ratios of individual PAH from environmentalsamples: can PAH sources be apportioned. Organic Geo-chemistry 21 (6/7), 809–822.
B. Yan et al. / Organic Geochemistry 37 (2006) 674–687 687
Oros, D.R., Simoneit, B.R.T., 2000. Identification and emissionrates of molecular tracers in coal smoke particulate matter.Fuel 79, 515–536.
Pereira, W.E., Hostettler, F.D., Luoma, S.N., van Geen, A.,Fuller, C.C., Anima, R.J., 1999. Sedimentary record ofanthropogenic and biogenic polycyclic aromatic hydrocar-bons in San Francisco Bay, California. Marine Chemistry 64,99–113.
Readman, J.W., Fillmann, G., Tolosa, I., Bartocci, J., Villeneuve,P., Catinni, C., Mee, L.D., 2002. Petroleum and PAHcontamination of Black Sea. Marine Pollution Bulletin 44,48–62.
Rogge, W.F., Hildemann, L.M., Mazurek, M.A., Cass, G.R.,1993. Sources of fine organic aerosol. 2. Noncatalyst andcatalyst-equipped automobiles and heavy-duty diesel trucks.Environmental Science & Technology 27, 636–651.
Rod, S.R., Ayres, R.U., Small, M., 1989. Reconstruction ofhistorical loadings of heavy metals and chlorinated hydro-carbon pesticides in the Hudson-Raritan basin, 1880–1980.Final Project Report to the Hudson River Foundation.
Sanders, G., Jones, K.C., Hamilton-Taylor, J., 1993. A simplemethod to assess the susceptibility of polynuclear aromatichydrocarbons to photolytic decomposition. AtmosphericEnvironment 27 (2), 139–144.
Schauer, J.J., Kleeman, M.J., Cass, G.R., Simoneit, B.R.T., 2001.Measurement of emissions form air pollution sources. 3. C1-C29 organic compounds from fireplace combustion of wood.Environmental Science & Technology 35, 1716–1728.
Simcik, M.F., Eisenreich, S.J., Golden, K.A., Liu, S.P.., Lipiatou,E., Swackhamer, D.L., Long, D.T., 1996. Atmosphericloading of polycyclic aromatic hydrocarbons to Lake Mich-igan as recorded in the sediments. Environmental Science &Technology 30, 3039–3046.
Smirnov, A., Abrajano, T.A., Smirnov, A., Stark, A., 1998.Distribution and sources of polycyclic aromatic hydrocarbonsin the sediments of Lake Erie, Part 1. Spatial Distribution,Transport, and Deposition. Organic Geochemistry 29 (5–7),1813–1828.
Sporstol, S., Rainer, N.G., Lichtenthaler, R.G., Gustavsen, K.O.,Urdal, K., Oreld, F., 1983. Source identification of aromatichydrocarbons in sediments using GC/MS. EnvironmentalScience & Technology 17, 282–286.
Stark, A., Abrajano, T.A., Hellou, J., Metcalf-Smith, J.L., 2003.Molecular and isotopic characterization of polycyclic aro-
matic hydrocarbon distribution and sources at the interna-tional segment of the St. Lawrence River. OrganicGeochemistry 34, 225–237.
Van Metre, P.C., Mahler, B.J., Furlong, E.T., 2000. Urbansprawl leaves its PAH signature. Environmental Science &Technology 34, 4064–4070.
Wakeham, S.G., 1996. Aliphatic and polycyclic aromatic hydro-carbons in Black Sea sediments. Marine Chemistry 53, 187–205.
Wakeham, S.G., Schaffner, C., Giger, W., 1979a. Polycyclicaromatic hydrocarbons in recent lake sediment �1. Com-pounds having anthropogenic origins. Geochimica et Cos-mochimica Acta 44, 403–413.
Wakeham, S.G., Schaffner, C., Giger, W., 1979b. Polycyclicaromatic hydrocarbons in recent lake sediment �2. Com-pounds derived from biogenic precursors during early dia-genesis. Geochimica et Cosmochimica Acta 44, 415–429.
Wang, Z., Fingas, M., Page, D.S., 1999. Oil spill identification.Journal of Chromatography A 843, 369–411.
Wolfe, D.A., Long, E.R., Thursby, G.B., 1996. Sediment toxicityin the Hudson-Raritan Estuary: Distribution and correlationswith chemical contamination. Estuaries 19 (4), 901–912.
Yan, B., 2004. PAH Sources and Depositional History inSediments from the Lower Hudson River Basin. Ph.D.Thesis, Earth & Environmental Sciences. Rensselaer Poly-technic Institute, Troy.
Yan, B., Benedict, L., Chaky, D.A., Bopp, R.F., Abrajano, T.A.,2004. Levels and patterns of PAH distribution in sedimentsfrom New York/New Jersey Harbor complex. NortheasternGeology and Environmental Sciences 26, 113–122.
Yan, B., Abrajano, T.A., Bopp, R.F., Chaky, D.A., Benedict, L.,Chillrud, S.N., 2005. Molecular tracers of saturated andpolycyclic aromatic hydrocarbon inputs into Central ParkLake, New York City. Environmental Science & Technology39 (18), 7012–7019.
Yunker, M.B., Macdonald, R.W., Brewer, R., Mitchell, R.H.,Goyette, D., Sylvestre, S., 2002. PAHs in the Fraser Riverbasin: a critical appraisal of PAH ratios as indicators of PAHsource and composting. Organic Geochemistry 33 (4), 489–515.
Yunker, M.B., Macdonald, R.W., Goyette, D., Paton, D.W.,Fowler, B.R., Sullivan, D., Boyd, J., 1999. Natural andanthropogenic inputs of hydrocarbons to the Strait ofGeorgia. The Science of the Total Environment 225, 181–209.