Activation Tagging Systems in Rice
-
Upload
independent -
Category
Documents
-
view
4 -
download
0
Transcript of Activation Tagging Systems in Rice
13 Activation Tagging Systems in Rice
Alexander A.T. Johnson1, Su-May Yu2 and Mark Tester1
1Australian Centre for Plant Functional Genomics, PMB 1, Glen Osmond, South Australia 5064, Australia (Emails: [email protected]; [email protected]); 2Institute of Molecular Biology, Academia Sinica, Taipei 115, Taiwan, Republic of China (Email: [email protected])
13.1 Introduction ............................................................................................. 343 13.2 Classical Activation Tagging: Enhancer Element-Mediated Gene Activation ................................................................................................ 345
13.2.1 Classical Activation Tagging in Plants ........................................... 345 13.2.2 Structure and Function of the CaMV 35S Activation Tagging System............................................................................................ 346 13.2.3 Variations to the CaMV 35S Activation Tagging System.............. 348 13.2.4 CaMV 35S Activation Tagging Resources in Rice ........................ 349
13.3 Transactivation Tagging: Transcriptional Activator-Mediated Gene Activation in Specific Cell Types ........................................................... 351
13.3.1 Gene Expression at the Cell Type-Specific Level .......................... 351 13.3.2 Origin of the GAL4 Enhancer Trapping System ............................ 352 13.3.3 GAL4 Enhancer Trapping in Plants ................................................ 353 13.3.4 Cell Type-Specific Activation of Target Genes Using GAL4 Transactivation................................................................................ 354 13.3.5 Cell Type-Specific Activation Tagging Using GAL4 Transactivation................................................................................ 357
13.4. Future Perspectives................................................................................. 359 Acknowledgements ......................................................................................... 359 References........................................................................................................ 360
13.1 Introduction
Sequencing of the 389 Mb rice genome (Oryza sativa L.) is nearly com-plete and map-based, finished quality sequence now covers 95% of the ge-nome. Determining function of the 37,544 predicted genes in the rice ge-
344 A.A.T. Johnson, S-M. Yu and M. Tester
nome, however, remains a formidable challenge that will require multiple, complementary approaches to be achieved. As with the dicotyledonous model species, Arabidopsis thaliana, rice genetic resources have been heavily invested towards the generation of random loss-of-function mu-tants, or knockouts, involving the use of mutagens such γ-rays or, more frequently, T-DNA and transposon-based systems such as Ac/Ds, En/Spm and Tos17 (see Hirochika et al. 2004 for review of rice mutant resources). To date, roughly 300,000 mutants have been generated using these strate-gies, providing invaluable genomic tools for gene mining in the model monocotyledonous species. In addition, gene targeting techniques have re-cently emerged that allow for specific rice loci to be disrupted (Terada et al. 2002; Cotsaftis and Guiderdoni 2005), yet optimization is still required before these techniques can be used to generate knockouts on order.
Despite widespread application, the traditional knockout approach is limited in its ability to fully saturate the rice genome with mutations. Genes with lethal or deleterious knockout phenotypes (particularly at the embryonic stage of development) are not amenable to the loss-of-function approach, and the investigation of large gene families is often hampered by the redundant activity of one gene member compensating for the loss of another. This is particularly relevant to the rice genome, where 29% of predicted genes have been amplified at least once to form tandem repeats, with some tandem repeats stretching up to 134 members (International Rice Genome Sequencing Project 2005). To address this significant obsta-cle and maximize the usefulness of knockout collections, gene, promoter and enhancer traps have often been included in T-DNA and transposon-based insertion systems to enable reporter visualization of native gene ac-tivity when other phenotypes are not necessarily present (Jeon et al. 2000; Ito et al. 2004; Peng et al. 2005). Trapped patterns report on spatial and developmental activity of native rice genes, although the identification of genomic elements responsible for those patterns can be laborious and not always apparent (Peng et al. 2005). RNA silencing is a well documented phenomenon in plants (Baulcombe 2004) with the clear advantage over gene knockouts of simultaneously silencing multiple members of a particu-lar gene family. The extent to which RNA silencing can be used to sup-press gene targets in the rice genome remains to be seen, with a recent study of the OsRac gene family reporting a maximum of three gene mem-bers efficiently suppressed using inverted repeat constructs (Miki et al. 2005). Continued refinements to RNA silencing technology, such as the development of artificial microRNAs with greater targeting control than traditional hairpin constructs (Schwab et al. 2006), promise to increase the efficiency and accuracy of RNA silencing in plants.
13 Activation Tagging Systems in Rice 345
While interruption or silencing of a particular coding sequence may not lead to a detectable phenotype, for a variety of reasons, dominant mutant phenotypes are more likely to result from upregulation, or activation tag-ging, of the same coding sequence. Random activation of genes in the classical sense, utilizing the CaMV 35S enhancer element, is a growing field in rice functional genomics, with two groups reporting on the devel-opment of large activation tagged populations (Jeong et al. 2002, 2006; Hsing et al. 2006). Moreover, the concurrent development of several GAL4 enhancer trapping populations in rice (Wu et al. 2003; Yang et al. 2004; Johnson et al. 2005) means that gene activation can now be targeted to specific cell types. This review provides an introduction to classical ac-tivation tagging in plants, drawing extensively from Arabidopsis research where the tagging technique primarily originated, before describing recent progress made in applying activation tagging to rice. GAL4 enhancer trap-ping technology is then presented with examples of how the technology has been used to transactivate target genes in specific cell types of rice. Fi-nally, a novel method to carry out cell type-specific activation tagging us-ing the GAL4 system, currently a powerful tool in Drosophila melanogaster genomics, is presented as an exciting application for the ex-isting GAL4 rice resources.
13.2 Classical Activation Tagging: Enhancer Element-Mediated Gene Activation
13.2.1 Classical Activation Tagging in Plants
Activation tagging provides an alternative to knockouts and RNA silencing through upregulation, rather than abolition, of native gene expression. The classical activation tagging technique uses T-DNA or transposons such as the En-I maize transposon system (Marsch-Martinez et al. 2002) to posi-tion strong activating enhancer elements, usually a tetramer of the CaMV 35S enhancer (Odell et al. 1985), throughout the plant genome. When in-tegrated in proximity to genes, the enhancer elements can interact with promoter sequences in the genome to increase expression levels and/or al-ter expression patterns of native genes. This gain-of-function approach produces dominant mutations that dramatically affect the transcriptional control of genes while still retaining a functional gene product. As such, activation tagging represents a powerful method for deregulating vital housekeeping genes that must remain transcriptionally active to ensure vi-ability.
346 A.A.T. Johnson, S-M. Yu and M. Tester
The activation approach also lends itself well to the dissection of signal transduction pathways with multiple activator and suppressor genes such as those involved in floral induction (Kardailsky et al. 1999; Lee et al. 2000) or response to internal growth factors such as auxin (Zhao et al. 2001), cytokinin (Kakimoto 1996) and brassinosteroids (Li et al. 2001, 2002). For several Arabidopsis activation tagged signal transduction mu-tants, overexpression phenotypes have been identified that have no corre-sponding loss-of-function phenotype. Protein kinase and associated signal transducing domains are particularly abundant in the Arabidopsis genome and account for more than 10% of all genes (The Arabidopsis Genome Ini-tiative 2000). Similarly, 65% of tandemly repeated genes in the rice ge-nome with more than 27 members contain protein kinase domains (Inter-national Rice Genome Sequencing Project 2005). Activation tagging may be the best method to mutate these and other highly amplified signaling genes, such as transcription factors, and could explain why many signal transduction mutants in Arabidopsis have been identified specifically through activation tagging. Finally, genes that are normally expressed at very low levels, such as those involved in phenylpropanoid biosynthesis (Borevitz et al. 2000), may only produce a recognizable phenotype when greatly overexpressed.
13.2.2 Structure and Function of the CaMV 35S Activation Tagging System
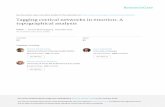
The original activation tagging T-DNA vector used for plant growth and developmental studies in tobacco (Walden et al. 1994) contained four cop-ies of the 35S transcriptional enhancer sequence (nucleotide -420 to -90 relative to transcription start of the 35S RNA promoter) cloned in tandem and placed adjacent to the right border, thus creating a 35S enhancer tetramer that could enhance, but not initiate, native gene expression upon integration into the plant genome (Fig. 13.1a). Deletion studies had previ-ously shown that the transcriptional initiation and enhancement properties of the 35S RNA promoter are physically separate, and deletions beyond -46 (such as the fragment used for activation tagging) remove transcript initiation while retaining the ability to enhance transcription (Odell et al. 1985).
Free of any insertional constraints concerning gene transcription, the 35S tetramer is a versatile enhancer element that can influence gene ex-pression both up- and down-stream of native genes, in either orientation, at genomic distances ranging from 380 bp (Wiegel et al. 2000) to 8.2 kb (Ichikawa et al. 2003) from the ATG start codon of Arabidopsis genes.
13 Activation Tagging Systems in Rice 347
RBLB
E E E E(a)
RBLB
35S(b)
RBLB
GAL4Reporter UAS(c)
RBLB
GOI UAS(d)
RBLB
UAS UAS(e)
Fig. 13.1. Schematic representations of the different activation tagging systems in plants; T-DNA constructs appear in light gray, bordered by left and right borders (LB and RB), and plant genomic elements in dark gray. (a) Classical activation tagging with a tetramer of the CaMV 35S enhancer (E) cloned next to the left bor-der of a T-DNA construct. An adjacent endogenous transcriptional unit consisting of promoter (small dashed cylinder) and coding sequence (large dashed cylinder) shows upregulated expression (indicated by arrow) due to interaction with the en-hancer element. (b) Activation tagging with the complete CaMV35S promoter (35S) cloned next to the LB of a T-DNA construct. Integration of the T-DNA di-rectly 5' of an endogenous coding sequence replaces the native promoter with the 35S promoter, resulting in constitutive overexpression of the gene. (c) Enhancer trapping with the minimal promoter-equipped gal4 gene (GAL4) cloned next to the right border of a T-DNA construct. An endogenous enhancer element (hatched arrow) drives transcription of the gal4 gene, leading to the GAL4 transcriptional activator protein binding to the UAS element (five 17 bp UAS repeats cloned in tandem, followed by a minimal promoter TATA) and activating expression of a downstream reporter gene. The resulting pattern of GAL4/reporter gene expres-sion can be highly specific, depending on the genomic enhancer, and is the defin-ing characteristic of the driver line. (d) Activation of a responder construct in spe-cific cell types using GAL4 transactivation. A gene of interest (GOI) is placed immediately downstream of the UAS element and the resulting construct is intro-duced, through sexual crosses or retransformation, into a driver line. The re-sponder construct subsequently comes under transcriptional control of the driver, forcing transcription of the GOI in the same pattern as GAL4/reporter gene ex-pression. (e) Cell type-specific activation tagging using GAL4 transactivation. UAS elements are cloned next to the LB and RB of a T-DNA construct, creating a double-sided gene transactivator that is capable of up-regulating endogenous gene expression from either border
348 A.A.T. Johnson, S-M. Yu and M. Tester
The frequency with which activation tagging produces visible, dominant activation tagged mutants has ranged from 0.07 % (Weigel et al. 2000) to 2.2% in Arabidopsis (Ichikawa et al. 2003). Differences in tagging fre-quencies may result from methylation-induced silencing of the 35S tetramer, triggered by the multimerized 35S enhancer element itself, or a prevalence of multiple T-DNA insertions in certain populations. While most studies have focused on single genes being activated by the 35S tetramer, evidence suggests that multiple genes may be activated, particu-larly in the case of genes lying closely in tandem (van der Graaff et al. 2002). The exact size of the 35S enhancer fragment varies slightly in dif-ferent activation tagging studies, but the deletion of transcriptional initia-tion and the tetramer conformation is nearly universal.
Integration of the 35S tetramer into the Arabidopsis genome usually causes massive upregulation of the adjacent gene(s), resulting in constitu-tive expression throughout most tissues of the plant and thus loss of the original expression pattern (Kakimoto 1996; Kardailsky et al. 1999; Borevitz et al. 2000; Ito and Meyerowitz 2000; Lee et al. 2000; Huang et al. 2001; Li et al. 2001, 2002; Zhao et al. 2001; Marsch-Martinez et al. 2002). However, a small number of activation tagged mutants have dis-played upregulated gene activity whilst conserving the original expression profile of the native gene (Neff et al. 1999; van der Graaff et al. 2000). In a collection of 30 activation tagged Arabidopsis phenotypic mutants, Weigel et al. (2000) identified one mutant line with upregulated expression of the FLOWERING LOCUS T gene in a similar expression pattern (predomi-nantly shoot) to that of the wild-type gene. Upregulation under the native gene expression profile could avoid deleterious effects caused by constitu-tive overexpression of a gene. An example of this is the LEAFY PETIOLE gene, originally characterised by van der Graaff (2000) in an Arabidopsis activation tagged mutant showing tissue-specific upregulation of the gene. Attempts to constitutively express the gene with the full 35S promoter re-sulted in sterile plants with severe developmental defects, indicating that tissue-specific overexpression was necessary to produce viable mutants. Clearly an increase in the frequency of tagged mutants with tissue-specific, or cell type-specific, activation of genes would be desirable, although this may require alternative promoters with tissue specific activity or the use of GAL4 enhancer trapping technology, as described later in the review.
13.2.3 Variations to the CaMV 35S Activation Tagging System
In a few instances the entire 35S promoter, rather than the enhancer tetramer, has been employed for activation tagging studies in Arabidopsis
13 Activation Tagging Systems in Rice 349
(Wilson et al. 1996; Schaffer et al. 1998; Fridborg et al. 1999). In these cases, the 35S promoter can replace a native promoter when integration of the activation tagging cassette occurs directly 5' of a coding sequence (Fig. 13.1b), resulting in constitutive overexpression of the flanking gene. This type of activation tagging requires insertions occurring in close proximity to the start codon, and the literature documents insertions ranging from 35 to 131 bp upstream of the upregulated gene (Wilson et al. 1996; Schaffer et al. 1998). Attempts have also been made to utilize enhancer fragments from promoters other than 35S, and some progress was reported with an enhancer element isolated from the cassava vein mosaic virus (Dong and Arnim 2003). The introduction of inducible promoters into activation tag-ging systems has enabled gene activation to be temporally controlled. Ma-tsuhara et al. (2000) used the Arabidopsis HSP18.2 heat-shock promoter to induce activation of several native Arabidopsis genes following heat shock at 37 °C, while Zuo et al. (2002) and Sun et al. (2003) used an estradiol-inducible activation tagging system to identify genes involved in plant phytohormone signaling. Furthermore, activation tagging has been carried out with transgenic Arabidopsis lines carrying a reporter gene such as luciferase (Luc) cloned downstream of a promoter normally activated by stress, such as the sweet potato sporamin gene (Spomin) sugar-inducible promoter (Masaki et al. 2005) or the pathogenesis-related 1 defense gene (PR-1) promoter (Grant et al. 2003). After deployment of an activation tag, such as the 35S tetramer, into the transgenic background, dominant mu-tants have been identified (through screens for reporter gene expression under non-stressed conditions) that exhibit enhanced promoter activity. Tagged genes in the dominant mutants have been shown to function as key regulators of the targeted promoters and other similar sequences.
13.2.4 CaMV 35S Activation Tagging Resources in Rice
Jeong et al. (2002) was the first group to report activation tagging in any monocot species with their description of 13,450 activation tagged lines in japonica rice. The plants were produced using the traditional 35S enhancer tetramer (nucleotide -417 to -86 relative to transcription start) located next to the left border of a T-DNA construct, and dominant mutants were de-tected at a frequency of 0.3%. However, RT-PCR analysis of ten randomly selected lines with 35S tetramer insertions within 4.5 kb of a native rice gene showed that four lines had significantly increased expression of that gene. These results suggest that integration of the 35S tetramer into the rice genome activates expression of adjacent genes (within 4.5 kb) in roughly half of the insertion events, despite the fact that dominant mutant
350 A.A.T. Johnson, S-M. Yu and M. Tester
phenotypes were detected at a much lower frequency. Detailed analysis of two activation tagged lines demonstrated that at least one of them, with in-creased expression of the Cf2/Cf5 gene, showed elevated gene expression under the same expression profile as the native gene. More recently, Jeong et al. (2006) described a much larger expression study of genes located closest to the 35S tetramer in 112 activation tagged lines, taken from the same population of plants (which currently totals 47,932 lines). Over half of the lines, 52%, showed elevated expression of the adjacent gene. Gene activation was found to extend significantly further than 4.5 kb, with one analyzed gene showing strong upregulation at a distance of 10.7 kb from the 35S tetramer. Interestingly, and unlike previous reports in Arabidopsis, 70% of the tagged lines showed elevated expression under the endogenous gene profile, with the remaining lines showing more general expression profiles and frequent ectopic expression in the leaf.
The higher frequency of gene activation in rice compared to Arabidop-sis, particularly under the native gene profile, likely results from the fact that most Arabidopsis activation tagged mutants were identified in screens for visible dominant phenotypes, while the expression analyses carried out by Jeong et al. (2006) utilized randomly selected transformants determined to have the activation tagging T-DNA construct integrated in intergenic regions. Indeed, the study identifies many activation tagged lines with upregulated gene expression that do not appear to have a phenotype. An examination of expression patterns in the 0.3% of activation tagged lines with dominant mutant phenotypes would determine if constitutive overex-pression of activated genes predominates in these lines, or if the endoge-nous expression pattern is retained here as well. Closer examination is also needed to verify that the dominant phenotypes are truly a consequence of the activation tagging element and not a result of tissue culture-induced somaclonal variation. Finally, the high frequency of gene activation de-tected by RT-PCR highlights the fact that more sensitive screens may be necessary to identify mutants in populations of rice activation tagged lines.
A large population of 45,000 activation tagged lines of rice was recently described by Hsing et al. (2006) using a modification of the classical CaMV 35S activation tagging system. The study cloned eight tandem re-peats of the 35S enhancer (nucleotide -343 to -46 relative to transcription start) adjacent to the left border of a T-DNA construct, creating a 35S oc-tamer that can activate gene expression upon integration into the rice ge-nome. The octamer configuration may serve as a more potent activator than the traditional tetramer, with genetic distances as far as 12.5 kb from the ATG start codon reportedly leading to gene activation in certain lines (S. M. Yu et al., unpublished). Tagging efficiency has not been reported
13 Activation Tagging Systems in Rice 351
for this population, nor has the predominant pattern of overexpression been documented. However, detailed characterization of a GA-deficient mutant line showing upregulated expression of the GA2ox gene suggests that the line has constitutive overexpression of the gene, as the phenotype (inhib-ited stem and reproductive organ development) does not differ from that obtained by general overexpression of the gene by the full 35S promoter.
The two populations of classical activation tagged lines reported in rice comprise a much larger population of plants (more than 90,000 independ-ent transformants) than that reported for Arabidopsis. In addition, the CaMV 35S enhancer element appears to function equally well, if not bet-ter, in the Jeong et al. (2006) population as compared to published Arabi-dopsis studies. The 35S enhancer has been shown to activate rice genes at genetic distances as far as 12.5 kb, far exceeding the average gene density of one gene per 9.9 kb in the rice genome (International Rice Genome Se-quencing Project 2005). This result suggests that most insertions of the 35S enhancer element into the rice genome (excluding insertions in coding sequences, leading to knockouts) have the potential to activate at least one native rice gene, if not several. For all of these reasons, the described clas-sical activation tagging resources are likely to yield valuable mutants that traditional loss-of-function strategies have missed. However, additional analyses of the Hsing et al. (2006) population are required to ascertain the efficiency and pattern of activation tagging in rice with a 35S octamer en-hancer element, as well as any silencing issues implicated with eight re-peats of the enhancer sequence.
13.3 Transactivation Tagging: Transcriptional Activator-Mediated Gene Activation in Specific Cell Types
13.3.1 Gene Expression at the Cell Type-Specific Level
The recent increase of gene expression studies involving specific tissues and cell types of plants represents a rapidly growing trend in plant biology to deconstruct organs into their constituent parts, and firmly heralds the ar-rival of microgenomics in plants (Brandt 2005; Moore et al. 2006). Two technological breakthroughs largely responsible for this development - la-ser capture microdissection (LCM; Kerk et al. 2003; Nakazono et al. 2003) and fluorescence-activated cell sorting (FACS; Birnbaum et al. 2003) - now enable researchers to isolate and perform transcriptome analyses on cell types as numerically small as the Arabidopsis quiescent center (Nawy
352 A.A.T. Johnson, S-M. Yu and M. Tester
et al. 2005). These studies have identified markedly diverse gene expres-sion profiles in different cell types and developmental stages of plants (Birnbaum et al. 2003), highlighting the dynamic nature of gene expres-sion across various organs, tissues and cell types of plants.
Despite the fact that native genes are frequently expressed in a cell type-specific manner, the majority of functional genomic studies in plants in-volve overexpression of target transgenes throughout the entire plant using strong constitutive promoters such as CaMV 35S or the rice Actin1 pro-moter. Constitutive overexpression of transgenes can lead to pleiotropic ef-fects not related to normal functioning of the gene and a resulting misin-terpretation of its role. A more desirable option is to target transgene expression to particular tissues and cell types where it is known or pre-dicted to be active. However, until recently, a general paucity of plant promoters with spatial and/or developmental-specific activity has limited the ability to do this. The development of GAL4 resources in Arabidopsis and more recently, rice, reduces the need for characterized cell type-specific promoters and now enables the expression and analysis of trans-gene expression in nearly all tissues and cell types of these model organ-isms.
13.3.2 Origin of the GAL4 Enhancer Trapping System
The development of GAL4 enhancer trapping technology in Drosophila melanogaster (Brand and Perrimon 1993) marked a revolution in fly de-velopmental biology and multi-cellular organisms in general. Providing re-searchers for the first time with a powerful tool to routinely manipulate or destroy (ablate) specific cell types while leaving the rest of the organism untouched, the GAL4 system has come to be known as the fly geneticist’s “swiss army knife” (Duffy et al. 2002). Today more than 5,000 Drosophila GAL4 enhancer trap lines have been developed and characterized, allow-ing nearly all cell types of this model organism to be targeted.
The GAL4 enhancer trapping system makes use of the 881 amino acid GAL4 transcriptional activator protein originally isolated from Saccharo-myces cerevisiae. The GAL4 protein acts as a potent transcriptional activa-tor in yeast by binding to a 17 bp DNA sequence known as the Upstream Activation Sequence (UAS) element, found in the promoter region of ga-lactose-inducible genes. For GAL4 enhancer trapping in other organisms, the gal4 coding sequence is cloned behind a minimal promoter and launched into the genome using the P-element transposon for flies, or T-DNA for plants. Integration of the construct into the host genome can
13 Activation Tagging Systems in Rice 353
place the minimal promoter-equipped gal4 gene under transcriptional con-trol of local promoter and enhancer elements, so that GAL4 is produced in a pattern reflective of native gene activity. GAL4 expression is conven-iently visualized through a GAL4-responsive reporter gene such as GFP or GUS (uidA), included on the same T-DNA construct for plants, cloned downstream of 5-6 tandem repeats of the UAS element (Fig. 13.1c). Bind-ing of GAL4 to the UAS sequence leads to amplification and increased expression of the reporter gene (Moore et al. 2006), resulting in far higher frequencies of enhancer trapping than that reported with traditional enhan-cer trapping constructs. Depending on the native gene driving expression of GAL4, expression patterns of the transcriptional activator can range from whole organism to individual cell types.
13.3.3 GAL4 Enhancer Trapping in Plants
Enhancer trapping in plants with a modified version of the GAL4 protein (a GAL4:VP16 fusion protein engineered for efficient expression in plants, hereafter referred to as GAL4) was first reported in Arabidopsis, where T-DNA integration of a GAL4:GFP enhancer trapping construct yielded GFP expression frequencies of 30% in T1 lines (Haseloff 1999). More than 12,000 Arabidopsis GAL4 lines have been developed, and many display highly specific GFP expression patterns throughout the plant. A catalogue of root-specific lines, with patterns specific to cell types such as the peri-cycle and lateral root cap, has been collated to facilitate study of the root meristem and is available at http://www.plantsci.cam.ac.uk/Haseloff/. The deployment of GAL4 technology in rice followed several years later with the development of three large enhancer trapping populations in japonica rice: 31,443 independent transformants in cvs. Zhonghua 11 and Zhonghua 15 (Wu et al. 2003); 100,000 independent transformants in cv. Nipponbare (Yang et al. 2004; Peng et al. 2005) and a second cv. Nipponbare popula-tion containing roughly 13,000 independent transformants (Johnson et al. 2005). Comprising nearly 145,000 transformants in total, these rice enhan-cer trap lines now represent the largest set of GAL4 resources in any single plant species, and perhaps any organism. The three rice populations were transformed with similar GAL4 enhancer trapping constructs, although two populations used six repeats of the UAS element and the GUS reporter gene in their enhancer trapping construct (Wu et al. 2003; Yang et al. 2004; Peng et al. 2005) while the population described by Johnson et al. (2005) employed five repeats of the UAS element and the GFP reporter gene. The populations show significantly different frequencies of reporter gene expression, ranging from 32% in the GFP lines to 84.3% in the GUS
354 A.A.T. Johnson, S-M. Yu and M. Tester
populations, and the noted variation in construct design may account for these differences. As with the Arabidopsis GAL4 library, many rice lines with cell type-specific reporter gene expression profiles have been identi-fied (see Fig. 13.2) and searchable databases for various patterns through-out the rice plant can be found at http://129.127.183.5 (Johnson et al. 2005) and http://rmd.ncpgr.cn/ (Zhang et al. 2006).
Fig. 13.2. Three different GAL4 driver lines from the Johnson et al. (2005) collec-tion showing markedly different patterns of GFP reporter gene expression specific to various cell types of the rice shoot: (a) collar or lamina joint; (b) leaf vascular cells; (c) collar and stomata. The spatial and/or developmental characteristics of endogenous plant enhancers determine the GAL4/GFP expression pattern of each individual driver. Green color due to fluorescence of GFP; red due to autofluores-cence of chlorophyll
13.3.4 Cell Type-Specific Activation of Target Genes Using GAL4 Transactivation
The potent activator properties of GAL4 enable the cell type-specific ex-pression patterns of interesting GAL4 lines to be harnessed to drive the expression of other transgenes in equally specific fashion. For this to oc-cur, a GAL4 enhancer trap line is first selected on the basis of reporter gene expression in a particular cell type(s) of interest. The selected enhan-cer trap line is termed the “driver” line because it drives expression of the GAL4 transcriptional activator protein in a pattern of interest. A second transformant is then generated with a transgene of interest placed immedi-ately downstream of the UAS sequence element to which GAL4 binds, creating the so-called “responder” line (Fig. 13.1d). By crossing the driver and responder lines (or re-transforming the driver with the responder con-
13 Activation Tagging Systems in Rice 355
struct), the GAL4-responsive gene of interest comes under transcriptional control of the GAL4 expression pattern present in the driver line, resulting in transactivation of the gene of interest specifically in the cell type(s) of interest.
Transactivation of genes using GAL4 enhancer trapping technology has long been routine in Drosophila (Brand and Perrimon 1993; Phelps and Brand 1998), and a similar trend in the plant literature is now emerging with five published reports in Arabidopsis (Bougourd et al. 2000; Kiegle et al. 2000; Boisnard-Lorig et al. 2001; Sabatini et al. 2003; Gallois et al. 2004) and two in rice (Johnson et al. 2005; Liang et al. 2006). The study by Johnson et al. (2005) was the first to demonstrate with the GUS reporter gene (uidA) that a target transgene could be transactivated by GAL4 driver lines in specific cell types of the rice plant representing the root, seed, leaf and floral organs. Figure 13.3a presents the confocal image of a rice en-hancer trap line identified in this collection with GFP fluorescence specific to xylem parenchyma cells of the root; a vascular cell type known to be important in controlling the composition of the xylem transpiration stream
Fig. 13.3. Transactivation of the GUS reporter gene (uidA) in xylem parenchyma cells of the rice root. (a) Confocal laser scanning microscopy image of a GAL4 driver line showing GFP expression specifically in xylem parenchyma cells of the root; beginning in the zone of cell differentiation (indicated by arrow). Green color due to fluorescence of GFP; red due to fluorescence of propidium iodide; scale bar = 50 μm. (b-c) Stereomicroscopy images of the same GAL4 line trans-formed with a UAS:uidA responder construct. Histochemical GUS staining shows that expression of the reporter gene remains specific to the xylem parenchyma, consistently appearing in the zone of cell differentiation and becoming more in-tense in mature regions of the root
356 A.A.T. Johnson, S-M. Yu and M. Tester
(Tester and Davenport 2003). An identical GUS staining pattern was ob-tained upon re-transformation of the enhancer trap line with a UAS:uidA responder construct (Fig. 13.3b-c), providing clear evidence that GAL4-mediated transactivation of genes in rice is robust and cell type-specific. The ability to target transgene expression to specific cell types of interest, such as the xylem parenchyma, represents a more biologically relevant method to express genes of interest than that provided by constitutive pro-moters. In addition, genes reported to show toxicity when expressed con-stitutively, such as certain sodium transporter genes, may be more amena-ble to the GAL4 system of expression.
More recently, six different GAL4 enhancer trap lines from the Wu et al. (2003) collection, showing GUS expression patterns primarily in repro-ductive cell types such as the stigma or lemma/palea, were used as drivers to investigate the function of 10 rice transcription factors (Liang et al. 2006). The responder lines, each carrying one of the transcription factor genes cloned downstream of the UAS element, were crossed to the differ-ent drivers, thus enabling functional analysis of transcription factor expres-sion in several different organs represented by the GAL4 drivers. The study demonstrated that certain transcription factors reveal phenotypes upon activation in specific organs and not in others (such as a leaf pheno-type, but not floral, for OsMADS15). In addition, crosses of the responder lines to one reference driver showing GUS expression in most organs of the plant (essentially a constitutive driver) frequently produced more se-vere phenotypes than with the specific driver lines, causing lethality in the case of OsMYBS3. The OsMYBS3 transcription factor produced a viable phenotype when activated specifically in the stigma (short stigma), a result that again highlights the utility of tissue-specific expression systems, such as GAL4 transactivation, when aiming to perturb specific plant processes.
Transactivation involving any of the lines described by Yang et al. (2004) has not, to our knowledge, been published. However, the sheer number of GAL4 rice lines currently available to the scientific community, as well as the fact that transactivation has been clearly demonstrated in two of the GAL4 populations, suggests that cell type-specific transactivation of target genes is a rapidly emerging technique in rice that will see increased application to functional genomic studies in the near future. In addition, al-ternative transcriptional activator systems, using proteins other than GAL4, are currently being developed for use in rice that may extend the application of transactivation work even further by providing transcrip-tional activators with greater freedom to operate (www.cambia.org). Alter-native transcriptional activators would also enable the production of “dual” driver lines, where the combination of two enhancer trapping constructs,
13 Activation Tagging Systems in Rice 357
based on different transcriptional activator proteins, would enable the si-multaneous targeting of transgene expression to different cell types of the plant.
13.3.5 Cell Type-Specific Activation Tagging Using GAL4 Transactivation
The development of large GAL4 populations in rice, and successful im-plementation of transactivator technology to drive targeted gene expres-sion, indicates that a more specialized form of activation tagging is now possible, one where tagging is carried out in specific cell types of the plant. This novel gain-of-function strategy uses random genomic integration of the GAL4-binding UAS element to dissect and analyze the function of en-dogenous genes in individual cell types. Unlike classical activation tagging with the 35S enhancer element, the UAS strategy forces random gene acti-vation specifically in predetermined cell types of interest, thereby reducing pleiotropic effects caused by constitutive gene activation and yielding valuable functional data that is relevant to only the targeted cell type.
Shortly after the GAL4 enhancer trapping system was first described in Drosophila, a UAS-based gene activator construct was developed enabling activation tagging studies to be carried out in specific cell types (Rørth et al. 1996). The gene activator consisted of multiple repeats of the UAS element, plus a minimal promoter for transcript initiation, positioned to-wards one end of a P-element transposon. In what the authors termed a “modular misexpression screen”, the gene activator was launched through-out the fly genome using P-element mutagenesis and a library of flies was generated, each containing a novel genomic insertion of the UAS element. The insertion process ensured that some UAS insertions occurred in close proximity to, or inside of, native genes, thereby creating GAL4-responsive endogenous genes and what we have termed “endogenous responder” lines. Subsequent sexual crosses of the endogenous responders to selected driver lines resulted in activation of the endogenous genes in specific cell types of interest. The sexual crosses revealed high frequencies of activa-tion tagged mutants when an eye-specific GAL4 driver was used, with 4% of endogenous responders yielding a dominant phenotype upon crossing to this line.
Toba et al. (1999) developed a significantly improved version of the original fly random gene activator construct by positioning UAS minimal promoter elements at both ends of a P-element transposon. The double-sided activator activates genes in both orientations, and 64% of the result-
358 A.A.T. Johnson, S-M. Yu and M. Tester
ing endogenous responder lines revealed a detectable dominant phenotype in combination with at least one of the four different GAL4 driver lines they were crossed to. The fact that several endogenous responders pro-duced phenotypes in combination with certain GAL4 drivers, and not with others, indicates that many genes only produce a detectable phenotype when activated in specific cell types. Correspondingly, many endogenous responders produced radically different phenotypes, ranging from rough eyes to lethality, when crossed to the four different driver lines, demon-strating that native genes can play fundamentally different roles when acti-vated in different cell types, information that conventional constitutive ex-pression would “average out” as a single phenotype or miss entirely.
Cloning of the UAS insertion site in 47 mutant endogenous responder lines showed that more than half contained insertions of the enhancer ele-ment at -50 to +100 nucleotides relative to the native transcriptional start of an endogenous gene, and most of the activated transcripts were spliced and polyadenylated correctly (Toba et al. 1999). Close proximity of the UAS element to the 5' region of activated genes, most likely due to the fact that the UAS element carries a minimal promoter that initiates transcrip-tion, greatly facilitated the cloning of activated genes and appeared to limit activation to one specific gene. The remarkably high activation frequency reported in this study could be attributed to significantly amplified gene expression achieved through binding of GAL4 to the UAS, resulting in prominent phenotypes, as well as the fact that cell type-specific activation tagging may result in larger numbers of viable phenotypes than that de-rived from classical activation tagging.
The development of large GAL4 resources in both rice and Arabidopsis has yet to lead to cell type-specific activation tagging populations, al-though the idea of using GAL4 technology in this fashion has long been promoted for plants (see CAMBIA review in Finkel 1999). Experience with Drosophila would suggest that such tagging efforts will soon follow and find broad application in both model species, especially as increasing numbers of well characterized GAL4 driver lines become available to the scientific community. A double-sided T-DNA gene activator carrying UAS minimal promoter repeats at both ends, similar in concept to the vec-tor described by Toba et al. (1999), was recently developed for use in rice transformations and is currently being integrated into several GAL4 en-hancer trap lines (Fig. 13.1e; Johnson and Tester, unpublished results). The double-sided activator has the potential to activate native genes from either border, however, the average density of one gene per 9.9 kb in the rice ge-nome would ensure that most T-DNA insertions result in single gene acti-vations.
13 Activation Tagging Systems in Rice 359
13.4. Future Perspectives
The immediate task for classical activation tagging in rice is to utilize the large resources at hand for gene discovery programs. While the majority of Arabidopsis activation tagging papers focus on the characterization of in-dividual mutant lines showing interesting phenotypes, rice activation tag-ging has so far mostly reported on population development and expression analyses of genes lying adjacent to the activation element. The expression studies have conclusively demonstrated that the CaMV 35S activation strategy works efficiently in rice and, in fact, indicate that more stringent screens are probably necessary to detect phenotypes associated with the high levels of gene activation uncovered in the tagged populations. Trans-activation tagging is also an area of rice functional genomics where large efforts have been devoted towards resource development. Further work is needed to demonstrate that transactivation is possible in all of the existing GAL4 collections - however, successful GAL4 transactivation of the GUS reporter gene (Fig. 13.3; Johnson et al. 2005) as well as 10 transcription factor genes (Liang et al. 2006) verifies that targeted expression studies, using most if not all cell types of rice, are now possible. Further charac-terization of expression patterns in the three GAL4 collections to uncover specific GAL4 drivers, particularly in the lines described by Yang et al. (2004), will accelerate the use of GAL4 enhancer trapping technology for this purpose. Finally, activation tagging in specific cell types, still unreal-ized in plant biology, is one of the most promising methods to emerge from GAL4 technology and will likely see widespread application to rice activation tagging efforts in the near future.
Acknowledgements
We thank Gynheung An for advance access to activation tagging data that greatly facilitated the writing of this manuscript. We would also like to recognize Drs. Emmanuel Guiderdoni and Julian Hibberd as co-developers of the GAL4/GFP rice enhancer trap collection. Drs. Venkatesan Sundare-san and Michael Ayliffe are thanked for critical review of the manuscript.
360 A.A.T. Johnson, S-M. Yu and M. Tester
References
Baulcombe D (2004) RNA silencing in plants. Nature 431:356–363 Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Ben-
fey PN (2003) A gene expression map of the Arabidopsis root. Science 302:1956–1960
Boisnard-Lorig C, Colon-Carmona A, Bauch M, Hodge S, Doerner P, Bancharel E, Dumas C, Haseloff J, Berger F (2001) Dynamic analyses of the expression of the HISTONE:YFP fusion protein in Arabidopsis show that syncytial en-dosperm is divided in mitotic domains. Plant Cell 13:495–509
Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging iden-tifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12:2383–2394
Bougourd S, Marrison J, Haseloff J (2000) An aniline blue staining procedure for confocal microscopy and 3D imaging of normal and perturbed cellular pheno-types in mature Arabidopsis embryos. Plant J 24:543–550
Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401–415
Brandt SP (2005) Microgenomics: gene expression analysis at the tissue-specific and single-cell levels. J Exp Bot 56:495–505
Cotsaftis O, Guiderdoni E (2005) Enhancing gene targeting efficiency in higher plants: rice is on the move. Transgenic Res 14:1–14
Dong Y, von Arnim AG (2003) Novel plant activation-tagging vectors designed to minimize 35S enhancer-mediated gene silencing. Plant Mol Biol Rep 21:349–358
Duffy JB (2002) GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis 34:1–15
Finkel E (1999) Australian center develops tools for developing world. Science 5433:1481–1483
Fridborg I, Kuusk S, Moritz T, Sundberg E (1999) The Arabidopsis dwarf mutant shi exhibits reduced gibberellin responses conferred by over-expression of a new putative zinc finger protein. Plant Cell 11:1019–1031
Gallois JL, Nora FR, Mizukami Y, Sablowski R (2004) WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev 18:375–380
Grant JJ, Chini A, Basu D, Loake GJ (2003) Targeted activation tagging of the Arabidopsis NBS-LRR gene, ADR1, conveys resistance to virulent patho-gens. Mol Plant Microbe Interact 16:669–680
Haseloff J (1999) GFP variants for multispectral imaging of living cells. Meth Cell Biol 58:139–151
Hirochika H, Guiderdoni E, An G, Hsing Y, Eun MY, Han C, Upadhyaya N, Ramachandran S, Zhang Q, Pereira A, Sundaresan V, Leung H (2004) Rice Mutant Resources for Gene Discovery. Plant Mol Biol 54:325–334
Hsing Y-I, Chern C-G, Fan M-J, Lu P-C, Chen K-T, Lo S-F, Ho S-L, Lee K-W, Wang Y-C, Sun P-K, Ko R, Huang W-L, Chen J-L, Chung C-I, Lin Y-C,
13 Activation Tagging Systems in Rice 361
Hour A-L, Wang Y-W, Chang Y-C, Tsai M-W, Lin Y-S, Chen Y-C, Chen S, Yen H-M, Li C-P, Wey C-K, Tseng C-S, Lai M-H, Chen L-J, Yu S-M (2006) A rice gene activation/knockout mutant resource for high throughput func-tional genomics. Plant Mol Biol (In Press)
Huang S, Cerny RE, Bhat DS, Brown SM (2001) Cloning of an Arabidopsis pata-tin-like gene, STURDY, by activation T-DNA tagging. Plant Physiol 125:573–584
Ichikawa T, Nakazawa M, Kawashima M, Muto S, Gohda K, Suzuki K, Ishikawa A, Kobayashi H, Yoshizumi T, Tsumoto Y, Tsuhara Y, Iizumi H, Goto Y, Matsui M (2003) Sequence database of 1172 T-DNA insertion sites in Arabi-dopsis activation-tagging lines that showed phenotypes in T1 generation. Plant J 36:421–29
International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436:793–800
Ito T, Meyerowitz EM (2000) Over-expression of a gene encoding a cytochrome P450, CYP78A9, induces large and seedless fruit in Arabidopsis. Plant Cell 12:1541–1550
Ito Y, Eiguchi M, Kurata N (2004) Establishment of an enhancer trap system with Ds and GUS for functional genomics in rice. Molecular Genetics and Genom-ics 271:639–650
Jeon J, Sichul L, Ki-Hong J, Jun S, Jeong D, Lee J, Kim C, Jang S, Lee S, Yang K, Nam J, An K, Han M, Sung R, Choi H, Yu J, Choi J, Cho S, Cha S, Kim S, An G (2000) T-DNA insertional mutagenesis for functional genomics in rice. Plant J 22:561–570
Jeong DH, An S, Kang HG, Moon S, Han JJ, Park S, Lee HS, An K, An G (2002) T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol 130:1636–1644
Jeong DH, An S, Park S, Kang HG, Park GG, Kim SR, Sim J, Kim YO, Kim MK, Kim SR, Kim J, Shin M, Jung M, An G (2006) Generation of a flanking se-quence-tag database for activation-tagging lines in japonica rice. Plant J 45:123–132
Johnson AAT, Hibberd JM, Gay C, Essah PA, Haseloff J, Tester M, Guiderdoni E (2005) Spatial control of transgene expression in rice (Oryza sativa L.) using the GAL4 enhancer trapping system. Plant J 41:779–789
Kakimoto T (1996) CKI1, a histidine kinase homolog implicated in cytokinin sig-nal transduction. Science 274:982–985
Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral in-ducer FT. Science 286:1962–1965
Kerk NM, Ceserani T, Tausta SL, Sussex IM, Nelson TM (2003) Laser capture microdissection of cells from plant tissues. Plant Physiol 132:27–35
Kiegle E, Moore C, Haseloff J, Tester M, Knight M (2000) Cell-type specific cal-cium responses to drought, NaCl, and cold in Arabidopsis root: a role for en-dodermis and pericycle in stress signal transduction. Plant J 23:267–278
362 A.A.T. Johnson, S-M. Yu and M. Tester
Lee H, Suh S, Park E, Cho E, Ahn JH, Kim S, Lee JS, Kwon, YM, Lee I (2000) The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev 14:2366–2376
Li J, Lease KA, Tax FE, Walker JC (2001) BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana. Proc Nat Acad Sci USA 98:5916–5921
Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidop-sis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110:213–222
Liang D, Wu C, Li C, Xu C, Zhang J, Kilian A, Li X, Zhang Q, Xiong L (2006) Establishment of a patterned GAL4/VP16 transactivation system for discover-ing gene function in rice. Plant J 46:1059–1072
Marsch-Martinez N, Greco R, Arkel VG, Herrera-Estrella L, Pereira A (2002) Ac-tivation tagging using the En-I maize transposon system in Arabidopsis. Plant Physiol 129:1544–1556
Masaki T, Tsukagoshi H, Mitsui N, Nishii T, Hattori T, Morikami A, Nakamura K (2005) Activation tagging of a gene for a protein with novel class of CCT-domain activates expression of a subset of sugar-inducible genes in Arabidop-sis thaliana. Plant J 43:142–152
Matsuhara S, Jingu F, Takahashi T, Komeda Y (2000) Heat shock tagging: a sim-ple method for expression and isolation of plant genome DNA flanked by T-DNA insertions. Plant J 22:79–86
Miki D, Itoh R, Shimamoto K (2005) RNA silencing of single and multiple mem-bers in a gene family of rice. Plant Physiol 138:1903–1913
Moore I, Samalova M, Kurup S (2006) Transactivated and chemically inducible gene expression in plants. Plant J 45:651–683
Nakazono M, Qiu F, Borsuk LA, Schnable PS (2003) Laser-capture microdissec-tion, a tool for the global analysis of gene expression in specific plant cell types: identification of genes expressed differentially in epidermal cells or vascular tissues of maize. Plant Cell 15:583–596
Nawy T, Lee JY, Colinas J, Wang JY, Thongrod SC, Malamy JE, Birnbaum K, Benfey PN (2005) Transcriptional profile of the Arabidopsis root quiescent center. Plant Cell 17:1908–1925
Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, Tsubuki M, Honda T, Takatsuto S, Yoshida S, Chory J (1999) BAS1: A gene regulat-ing brassinosteroid levels and light responsiveness in Arabidopsis. Proc Nat Acad Sci USA 96:15316–15323
Odell JT, Nagy F, Chua N (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313:810–812
Peng H, Huang H, Yang Y, Zhai Y, Wu J, Huang D, Lu T (2005) Functional analysis of GUS expression patterns and T-DNA integration characteristics in rice enhancer trap lines. Plant Sci 168:1571–1579
Phelps CB, Brand AH (1998) Ectopic gene expression in Drosophila using GAL4 system. Methods 14:367–79
Rørth P (1996) A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc Natl Acad Sci USA 93:12418–12422
13 Activation Tagging Systems in Rice 363
Sabatini S, Heidstra R, Wildwater M, Scheres B (2003) SCARECROW is in-volved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev 17:354–358
Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carré IA, Coupland G (1998) The late elongated hypocotyl mutation of Arabidopsis disrupts cir-cadian rhythms and the photoperiodic control of flowering. Cell 93:1219–1229
Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D (2006) Highly spe-cific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18:1121–1133
Sun J, Niu QW, Tarkowski P, Zheng B, Tarkowska D, Sandberg G, Chua NH, Zuo J (2003) The Arabidopsis AtIPT8/PGA22 gene encodes an isopentenyl transferase that is involved in de novo cytokinin biosynthesis. Plant Physiol 131:167–176
Terada R, Urawa H, Inagaki Y, Tsugane K, Iida S (2002) Efficient gene targeting by homologous recombination in rice. Nature Biotech 20:1030–1034
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815
Toba G, Ohsako T, Miyata N, Ohtsuka T, Seong KH, Aigaki T (1999) The gene search system: a method for efficient detection and rapid molecular identifica-tion of genes in Drosophila melanogaster. Genetics 151:725–737
van der Graaff E, Dulk-Ras AD, Hooykaas PJ, Keller B (2000) Activation tagging of the LEAFY PETIOLE gene affects leaf petiole development in Arabidopsis thaliana. Development 127:4971–4980
van der Graaff E, Hooykaas PJ, Keller B (2002) Activation tagging of the two closely linked genes LEP and VAS independently affects vascular cell num-ber. Plant J 32:819–830
Walden, R, Fritze K, Hayashi H, Miklashevichs E, Harling H, Schell J (1994) Ac-tivation tagging: a means of isolating genes implicated as playing a role in plant growth and development. Plant Mol Biol 26:1521–1528
Weigel D, Ahn JH, Blázquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrandiz C, Kardailsky I, Malancharuvil EJ, Neff MM, Nguyen JT, Sato S, Wang ZY, Xia Y, Dixon RA, Harrison MJ, Lamb CJ, Yanofsky MF, Chory J (2000) Activation Tagging in Arabidopsis. Plant Physiol 122:1003–1013
Wilson K, Long D, Swinburne J, Coupland G (1996) A Dissociation insertion causes a semidominant mutation that increases expression of TINY, an Arabi-dopsis gene related to APETALA2. Plant Cell 8:659–671
Wu C, Li X, Yuan W, Chen G, Kilian A, Li J, Xu C, Li X, Zhou DX, Wang S, Zhang Q (2003) Development of enhancer trap lines for functional analysis of the rice genome. Plant J 35:418–427
Yang Y, Peng H, Huang H, Wu J, Jia S, Huang D, Lu T (2004) Large-scale pro-duction of enhancer trapping lines for rice functional genomics. Plant Sci 167:281–288
364 A.A.T. Johnson, S-M. Yu and M. Tester
Zhang J, Li C, Wu C, Xiong L, Chen G, Zhang Q, Wang S (2006) RMD: a rice mutant database for functional analysis of the rice genome. Nucleic Acid Res 34:745–748
Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291:306–309
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vege-tative-to-embryonic transition in Arabidopsis. Plant J 30:349–359