KULIAH 4_Siklus Menstruasi
Transcript of KULIAH 4_Siklus Menstruasi
-
8/15/2019 KULIAH 4_Siklus Menstruasi
1/15
-
8/15/2019 KULIAH 4_Siklus Menstruasi
2/15
10/03/2011
(1) CONTROL BY HYPOTHALAMUS Inhibited by combinationof estrogen andprogesteroneStimulated by highlevels of estrogen
Hypothalamus
Releasinghormone
Anterior pituitary
FSH LH
(2) PITUITARY HORMONESIN BLOOD LH peak triggers
ovulation andcorpus luteumformation
LH
FSH
FSH LH
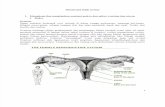
(3) OVARIAN CYCLE
Growingfollicle
Maturefollicle
OvulationCorpusluteum
Degeneratingcorpusluteum
Pre-ovulatory phase Post-ovulatory phase
Estrogen Progesteroneand estrogen
(4) OVARIAN HORMONESIN BLOOD
EstrogenProgesterone
Estrogen Progesteroneand estrogen
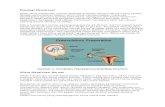
(5) MENSTRUAL CYCLE
Endometrium
Menstruation Days
ABORSI
-
8/15/2019 KULIAH 4_Siklus Menstruasi
3/15
-
8/15/2019 KULIAH 4_Siklus Menstruasi
4/15
10/03/2011
-
8/15/2019 KULIAH 4_Siklus Menstruasi
5/15
10/03/2011
Medical Abortion
• Mifepristone (RU486) – analogue of progestin norethindrone – strong affinity for the progesterone receptor,
acting as an antagonist – a single oral dose given to women 5 weeks or
less produces abortion in 85% of cases
Medical Abortion - politics
• RU486 - Mifepristone – developed in 1980’s – approved for use by French government 1988
• one day later manufacturer withdrew it from the marketsuccumbing to international boycott
• French government ordered redistribution – Prohibited in US during Reagan and Bush – Ban lifted by Clinton, clinical trials, preliminary FDA
approval 9/96 – Final approval stalled secondary to inability to
manufacture and distribute until 9/2000
-
8/15/2019 KULIAH 4_Siklus Menstruasi
6/15
10/03/2011
Medical Abortion - politics
• RU486 - Mifepristone – developed in 1980’s – approved for use by French government 1988
• one day later manufacturer withdrew it from the marketsuccumbing to international boycott
• French government ordered redistribution – Prohibited in US during Reagan and Bush –
Ban lifted by Clinton, clinical trials, preliminary FDAapproval 9/96 – Final approval stalled secondary to inability to
manufacture and distribute until 9/2000
Surgical vs. Medical: pro vs. conProvider perspective:
• Less skill needed toprovide
• Methotrexate alsotreats ectopicpregnancy
• Increased anxiety re:off site management
• More unscheduledcare: calls, ER visits
• Need to guard againstunnecessaryintervention
• Limited to 49 daysLMP
-
8/15/2019 KULIAH 4_Siklus Menstruasi
7/15
10/03/2011
Complications - rates
• Varies as a function of the gestational agethey are performed
– Major complications:• 0.25% < 7 weeks• 1% < 12 weeks• 2% over 12 weeks
Complications - immediate
• Complications of local anesthetic• Cervical shock• Cervical lacerations•
Uterine perforation• Hemorrhage• Post abortal syndrome
-
8/15/2019 KULIAH 4_Siklus Menstruasi
8/15
-
8/15/2019 KULIAH 4_Siklus Menstruasi
9/15
-
8/15/2019 KULIAH 4_Siklus Menstruasi
10/15
-
8/15/2019 KULIAH 4_Siklus Menstruasi
11/15
10/03/2011
MATERNAL RISKS WITHTWINS
• Increased minor complaints ofpregnancy
•
Increased risk of miscarriage• Increased anaemia, pre-term delivery• Hypertension• Antepartum Haemorrhage
-
8/15/2019 KULIAH 4_Siklus Menstruasi
12/15
10/03/2011
MATERNAL RISKS WITH
TWINS (contd.)• Hydramnios• Need for hospitalisation• Single fetal death in twins• Operative Delivery• Caesarean Section• Postpartum Haemorrhage
-
8/15/2019 KULIAH 4_Siklus Menstruasi
13/15
10/03/2011
-
8/15/2019 KULIAH 4_Siklus Menstruasi
14/15
10/03/2011
-
8/15/2019 KULIAH 4_Siklus Menstruasi
15/15
10/03/2011