The Loss of the External Ear Opening in Scincid Lizards
Transcript of The Loss of the External Ear Opening in Scincid Lizards
Journal of Herpetology, Vol. 36, No. 4, pp. 544–555, 2002Copyright 2002 Society for the Study of Amphibians and Reptiles
The Loss of the External Ear Opening in Scincid Lizards
ALLEN E. GREER
Australian Museum, 6 College Street, Sydney, New South Wales 2010, Australia; E-mail: [email protected]
ABSTRACT.—Scincid lizards have probably lost the external ear opening at least 17 times. Based on featuresof the middle ear, especially the orientation and attachment of the extracolumella and columella, five basictypes of ear loss are recognized. These five types are strongly associated with major lineages. Ear loss seemsto be associated with both small size and fossorial habits.
Scincid lizards have undergone numerous in-dependent evolutionary modifications in a num-ber of morphological features that make theman ideal group in which to study the details ofevolutionary change. One of these recurrent evo-lutionary trends is the reduction and loss of theexternal ear opening. In this paper, I review thetaxa in which the loss of the external ear open-ing has occurred, describe the basic morpholog-ical features of this loss, and discuss the ecolog-ical features that are associated with it.
MATERIALS AND METHODS
Observations on the morphology of the for-mer position of the external ear opening weremade in most cases on intact specimens or in afew cases from the literature or illustrations. Ob-servations on the shape of the quadrate and theorientation and attachments of the columellaand extracolumella were made on intact speci-mens, cleared-and-stained specimens and driedskulls. Observations on the auditory groovewere made on intact specimens (morphologyfollows Baird, 1970). Unfortunately, many spe-cies without an external ear opening are knownfrom too few specimens to warrant dissectionof the middle ear structures and hence areknown only from their external ear morphology.
The taxa lacking an external ear opening havebeen associated here into presumed lineages (bynumber in Appendix 1) on the basis of relation-ships where known. Where knowledge of exist-ing relationships fails to provide a clear indica-tion of the groups in which the ear opening hasbeen lost, the following conservative criteriahave been applied. If the external ear openingis absent in only some members of a genus, itis assumed that the ear opening has been lostonly once in that genus. All genera in which theear opening is absent and which occur on thesame continent or major, long isolated island(e.g., Madagascar) are considered to haveshared a unique common ancestor lacking anear opening. Any genus in which the ear open-ing is interspecifically variable and which isunique in this regard on a continent is included
in a lineage with the other earless genera on thatcontinent. When there is more than one genusin which the ear opening is interspecifically var-iable on any one continent, the one genusthought to be most closely related to the earlessgenera is considered to be part of the single lin-eage embracing those genera; the residual gen-era in which the ear opening is interspecificallyvariable are considered to have lost their earopenings independently.
The convention for phylogenetic interpreta-tion outlined above is likely to be highly con-servative. For example, the external ear openingis interspecifically variable in the southwestAsian genus Ophiomorus and hence on the geo-graphical criteria outlined above would be con-sidered as having lost the external ear openingonly once. However, in a recent cladistic analy-sis of the group, the external ear opening wasinferred to have been lost twice, once in the rel-atively primitive O. persicus and once in the oth-er earless species (Greer and Wilson, 2001).
For brevity’s sake, species with an external earopen are called ‘‘eared,’’ and those without anexternal ear opening are called ‘‘earless.’’ Thenumber of digits on the manus and pes, whichis used as a morphological proxy for the degreeof fossoriality, is given as manus/pes (e.g., 5/5); clawless stylar limbs are scored as 0.5, andthe absence of limbs as 0.
Museum abbreviations follow Leviton et al.,1985, except for the Australian Museum whichis AM.
RESULTS
There are five basic patterns in the loss of theexternal ear opening in skinks. The major dif-ference between the five patterns relates pri-marily to the orientation and attachment of theextracolumella and columella.
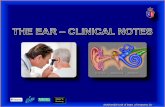
In the first pattern, the extracolumella andcolumella project laterally and the extracolu-mella attaches to the inside of the skin in theregion where the tympanic membrane would beif it were present, and there is always at least aslight auricular dimple or crease based on the
545EAR LOSS IN SKINKS
FIG. 1. The primitive (A, C) and derived (B, D) external morphologies in patterns 1 and 2 of ear loss inskinks. Pattern 1 (A–B) in which the extracolumella attaches to the medial side of the skin causing a dimple(or crease)—(A) Calyptotis ruficauda (AM R 141598) with the primitive condition consisting of a large externalear opening and only slightly recessed tympanum (extracolumella’s attachment to medial side of tympanumindicated by dashed line) and (B) Calyptotis lepidorostrus (AM R 139461) with the derived condition consistingof a dimple where the extracolumella attaches to the medial side of the skin. Pattern 2 (C–D) in which theextracolumella attaches to the medial surface of the anterior slip of the depressor mandibulae which has movedanteriorly across the position of the former tympanum and the extracolumella attaches to the medial side ofthis muscle, resulting in a smooth external surface—(C) Nannoscincus gracile (AM R 147885) with the primitivecondition consisting of a small external ear opening and (D) Nannoscincus mariae (AM R 148007) with thederived condition consisting of no external trace of the external ear opening’s former position.
attachment of the extracolumella to the skin(Fig. 1A–B). In most species, the round window(lateral aperture of the recessus scalae tympani)is large, specifically, larger than what would berequired to carry the glossopharygeal nerve;however, in some Anomalopus and in Isopachys,the round window is reduced to a small fora-men that would do little more than accommo-date this nerve. An auditory groove is present.
Pattern 1 occurs only in sphenomorphine ly-gosomines (Appendix 1: lineages 11–17), withone possible exception. In the nonlygosomineOphiomorus persicus (Appendix 1: lineage 4) theextracolumella projects laterally just beneath theposteroventral inflection of the quadrate (be-
low), but to what it attaches is unclear. That itmay not be the inside of the skin is suggestedby the fact that, in all known cases of the extra-columella attaching to this position, there is anauricular dimple or crease evident externally;however, this species lacks any indication of theformer position of the external opening (pers.obs.). In the other earless species of Ophiomorus,the extracolumella attaches directly and stronglyto the quadrate’s posteroventral inflection (pat-tern 5, below).
Pattern 1 occurs in all earless spehnomor-phines as far as is known except for the south-east Asian Isopachys anguinoides which has pat-tern 3 (below). Within lygosomines, pattern 1
546 ALLEN E. GREER
probably evolved at least seven times (Appendix1).
This pattern appears to have evolved throughtwo processes: the encroachment of the rim ofthe external ear opening toward the center ofthe tympanum, and the scleratization of the re-duced tympanum. That these two processesmay have occurred more or less synchronouslyis suggested by the small, scleratized tympanaseen as a variant within essentially earless spe-cies, for example, the east Australian Calyptotisscutirostrum (pers. obs.) and in the small-earedclose relatives of earless species, for example,the New Guinean Sphenomorphus microtympanusvis a vis S. anotus (Greer, 1973:figs. 2 and 1, re-spectively). That scleratization of the tympanummay have preceded encroachment of the rim ofthe tympanum is suggested by the scleratizationof the relatively large tympanum in the north-eastern Australian Calyptotis thortonensis (Greer,1983) and in the Phillipines Sphenomorphus lu-zonensis (pers. obs.).
In the second pattern, the extracolumella andcolumella project laterally and the extracolu-mella attaches to the medial surface of the an-terior slip of the depressor mandibulae whichappears to have shifted anteriorly into the spacepreviously occupied by the tympanum. There isno dimple or crease to indicate the former po-sition of the external ear opening (Fig. 1C–D).The round window (lateral aperture of the re-cessus scalae tympani) is large. An auditorygroove is present.
Pattern 2 occurs only in eugonglyline lygo-somine skinks (Appendix 1; lineages 9–10): theAustralian Menetia surda, some individuals ofMenetia greyii and probably M. amaura (if it is infact distinct from M. greyii; pers. obs.) and theNew Caledonian Nannoscincus mariae. The spe-cies in both groups have eared congeners; henceit is likely the pattern has evolved at least twiceif not three times (twice in Menetia).
The most parsimonious derivation of this pat-tern type is by the anterior encroachment of theanterior slip of the depressor mandibulae acrossthe medial aspect of the tympanum. This se-quence implies an intermediate condition of theextracolumella attaching partly to the tympa-num and partly to the muscle slip. Such a stagehas not yet been observed.
In the third pattern, the extracolumella andcolumella are orientated anterolaterally, andtheir distal end abuts the posterolateral side ofthe quadrate. There is no auricular dimple orcrease. The round window (lateral aperture ofthe recessus scalae tympani) is reduced to asmall foramen for the glossopharyngeal nerve.It remains to be determined whether an audi-tory groove is present or absent.
This pattern occurs only in the southeast
Asian sphenomorphine Isopachys anguinoides(Appendix 1: lineage 15 [part]).
This pattern appears to have evolved frompattern 1, which is the only other pattern seenin the sphenomorphines and is the pattern thatoccurs in both the other species of Isopachys andin relatives close to the genus (Greer, 1997; Ap-pendix 1). This pattern has evolved only once.
In the fourth pattern, the extracolumella andcolumella project anterolaterally to the quadrate,and the extracolumella connects strongly withthe fascia just lateral to the temporal muscula-ture and loosely with the inside of the skin.There is no auricular dimple or crease. Theround window (lateral aperture of the recessusscalae tympani) is reduced to a small foramenfor the glossopharyngeal nerve (Rieppel, 1982;pers. obs.). An auditory groove is absent.
This pattern occurs in all species of the southAfrican genera Acontias, Acontophiops, and Ty-phlosaurus (de Villiers, 1939; Brock, 1941; van derMerwe, 1944; Toerien, 1963; Rieppel, 1980, 1981:fig. 5a, 6a, 10a, 12a, 1982; Appendix 1: lineage8). These three genera constitute a strongly sup-ported lineage within the nonlygosomineskinks, the acontines (Greer, 1970; Rieppel, 1981,1982), suggesting that the group’s unique earpattern probably evolved only once.
The evolutionary origin of this pattern is ob-scure. There are no obvious antecedent condi-tions among other nonlygosomines, and in theonly other earless nonlygosomines, the extra-columella and columella are orientated in vir-tually the opposite direction (below).
In the fifth pattern, the extracolumella andcolumella are orientated dorsoposteriorly andabut the inside of the posteroventral inflectionof the dorsal head of the quadrate (Rieppel,1980:fig. 8a, 1981:fig. 14a, 15b–e, 1982:fig. 2a).There is no auricular dimple or crease. In mosttaxa, the round window (lateral aperture of therecessus scalae tympani) is relatively large; inBrachymeles vermis and Nessia layardi, the roundwindow is small but still larger than a mere fo-ramen for the glossopharyngeal nerve. An au-ditory groove is present in Feylinia currori but isabsent in Typhlacontias gracilis (pers. obs.).
This pattern, at least as far as the extracolu-mella-quadrate relationship is concerned, occursin all the earless nonlygosomines except theacontines, and it occurs only in nonlygosomines(Appendix 1: lineages 2–3, 5–7). It probablyevolved five times.
The earless Nessia layardi has the columellaprojecting laterally, but the extracolumella turnsdorsoposteriorly to abut the ventral inflection ofthe dorsal part of the quadrate. This may havebeen the precursor to the condition in most oth-er species in which the columella itself turns to-ward the ventral inflection of the quadrate’s dor-
547EAR LOSS IN SKINKS
sal process and articulates with it through ashort, stout cartilaginous extracolumella. In-deed, the ultimate precursor to pattern 5 mayhave been the extracolumella’s primitive (i.e., ineared forms) connection to the paroccipital pro-cess through its dorsal process (Baird, 1970:fig.4).
The only skink lineage to have lost the exter-nal ear opening but for which no details of themiddle ear morphology are available is thesoutheast North American Neoseps reynoldsi (Ap-pendix 1: lineage 1). This species is probablymost closely related to the sympatric Eumecesegregius (Telford, 1959), which has a relativesmall external ear opening compared to its con-geners. Although most specimens of Neoseps rey-noldsi have a minute external ear opening, whichis often covered by an overlapping anteriorscale, careful inspection shows that at least somespecimens lack an external ear opening (pers.obs.). What other modifications may have ac-companied the loss of the external ear openingin such individuals awaits further investigation.
The only other species besides Menetia greyiiand Neoseps reynoldsi in which the external earopening is said to be variable is Ablepharus pan-nonicus. There is speculation that this usuallyeared species may grade into the earless A. gray-anus (Mertens, 1965, 1970, but see Anderson,1999).
In all species lacking an external ear opening,the thin lateral rim or conch of the quadrate thatanchors the anterior edge of the external earopening in eared forms has been reduced, leav-ing only the thicker medial rod, which forms thebase of the suspension for the lower jaw.
DISCUSSION
Perhaps the most remarkable aspect of themiddle ear morphology outlined here is thestrong association of the patterns with major lin-eages (Appendix 2). With only one exception,pattern 1 occurs only in the sphenomorphines,where it evolved at least seven times. Pattern 2occurs only in the eugongylines where itevolved at least twice. Pattern 4 occurs only inacontines. And pattern 5 occurs only in nonly-gosomines other than acontines. The fact thatpatterns 1 and 5 have evolved repeatedly intheir respective broad groups, sphenomorphi-nes and nonlygosomines, suggests that func-tional or developmental constraints act to chan-nel the kind of ear loss that occurs in eachgroup.
Furthermore, some groups appear moreprone to ear loss than others. For example, inthe two major groups of lygosomines to haveexperienced ear loss, the sphenomorphines andthe eugonglylines, there are more earless line-
ages (above) and species (x2 5 24.1, P , 0.001)in the former than in the latter.
Ecologically, the loss of the external ear open-ing appears to be strongly associated with twodifferent features: fossoriality and small size.Fossoriality is evident in lineages 1–8 and 12,and small size is evident in lineages 9–11 (Ap-pendix 1). A combination of small size and oc-cupation of moist leaf litter (precursor to fos-soriality?) or fossoriality is evident in lineages13–17 (Appendix 1).
Statistical analysis of the frequency of pre-sumed fossorial habits and small size amongthe earless species supports these ecological as-sociations with loss of the external ear opening.Using the total number of digits on the manusand pes as an index of fossoriality in an inverserelationship (fewer digits 5 greater fossoriality),then for all skinks, earless skinks have fewerdigits in general than do eared skinks (Mann-Whitney U 5 118128, P , 0.0001, N 5 120 and1124, respectively). A similar significant rela-tionship holds for all sphenomorphine species(the group that accounts for seven of the 17 cas-es of ear loss; Mann-Whitney U 5 14286, P ,0.0001, N 5 47 and 424, respectively), and fortwo of the three largest genera in which the earopening is interspecifically present or absent:Brachymeles (1 Davewakeum; Mann-Whitney U 554, P 5 0.0011, N 5 9 and 6, respectively) andScelotes (Mann-Whitney U 5 81, P 5 0.015, N 57 and 14, respectively). However, there is a sig-nificant inverse relationship in the third genus,Ophiomorous (Mann-Whitney U 5 0.000, P 50.029, N 5 8 and 2, respectively); that is, in thislineage, earless species tend to have more digitsthan eared species. But those earless species thatdo have a relatively high number of digits (N 57) are nonetheless clearly fossorial as far as isknown (in loosely consolidated sand) while theecological relationships of the earless limblessspecies (N 5 1) are uncertain (Greer and Wilson,2001). Functionally, as skinks spend more timebelow ground, they may not only have less needfor detecting airborne vibrations but may alsobe at greater risk of having their ear openingsclogged with substrate particles.
The close association between ear loss andfossoriality probably explains why, among ly-gosomines, the sphenomorphines have lost theear opening more often than the eugongylines(above); the former group gives the overall im-pression of being more fossorial than the lattergroup. This impression is supported by a com-parison of the total number of digits in the twogroups with the former having significantlyfewer total digits than the latter (Mann-WhitneyU 5 85614, P 5 0.004, N 5 471 and 330, re-spectively).
The reduction and virtual loss of the lateral
548 ALLEN E. GREER
aperture of the recessus scali tympani and theloss of the auditory groove are both features ofburrowing lineages in skinks as they are in oth-er lizards (Baird, 1970). Both a large lateral ap-erture and an auditory tube could be argued asfunctioning to equalize pressure in the innerand middle ears, respectively, and their reduc-tion and loss implies that such pressure equal-ization functions have been reduced or lost theirsignificance. Perhaps an increasing reliance onground pressure waves in at least some burrow-ers over airborne pressure waves has caused thischange. The longer wave lengths of groundpressure waves may require both heavier bonestructure and tighter connections with sur-rounding structures than is provided by themore delicate structure and lighter connectionsassociated with airborne pressure waves.
With regard to size, comparison of the maxi-mum snout–vent length between earless andeared species in skinks consisting of less that150 mm snout–vent length (to eliminate obvious‘‘giants’’; Greer, 2001) and with at least five dig-its on each foot (to reduce, if not eliminate, thefossorial species) showed that earless speciestend to be significantly smaller than eared spe-cies (Mann-Whitney U 5 15016, P , 0.0001, N5 20 and 879, respectively). However, the rela-tionship does not hold up within individual lin-eages (e.g., Ablepharus; Mann-Whitney U 5 5, P5 0.14, N 5 1 and 5, respectively, although theearless species is by far the smallest: 36 mm vs.50–65 mm), Lipinia (Mann-Whitney U 5 16.5, P5 0.11, N 5 4 and 17) and Menetia (digits 4/5;Mann-Whitney U 5 5.0, P 5 0.74, N 5 2 and6). Functionally, as skinks become smaller, theirtympanums—if they fail to scale interspecifical-ly in isometry or in negative allometry withhead size—may become an increasing impor-tant source of water loss. Hence, closure wouldbe a countervailing option.
Acknowledgments.—I thank H. Finlay for thedrawings in Figure 1.
LITERATURE CITED
ANDERSON, S. C. 1999. The Lizards of Iran. Societyfor the Study of Amphibians and Reptiles, St. Lou-is, MO.
BAIRD, I. L. 1970. The anatomy of the reptilian ear. InC. Gans and T. S. Parsons (eds.), The Biology ofthe Reptilia. Vol. 2, pp. 193–275, Academic Press,London.
BOHME, W. 1981. A new lygosomine skink from Thai-land. Bollettino del Museo Civico di Storia Natur-ale Verona 8:375–382.
BOURRET, R. 1937. Notes herpetologiques surl’Indochine francaise. XV. Lezards et serpents re-cus au Laboratoire des Sciences Naturelles del’Universite au cours de l’annee 1937. Descriptionsde deux especes et de deux varietes nouvelles. Bul-
letin General de l’Instruction Publique (Hanoi) 4:57–80.
BROADLEY, D. G. 1990. The herpetofaunas of the is-lands off the coast of south Mocambique. Arnoldia9:469–493.
. 1994. The genus Scelotes Fitzinger (Reptilia:Scincidae) in Mozambique, Swaziland and Natal,South Africa. Ann. Natal Museum 35:237–259.
BROCK, G. T. 1941. The skull of Acontias meleagris witha study of the affinities between lizards andsnakes. Journal of the Linneaean Society of London(Zoology) 41:71–88.
BROWN, R. M., J. A. MCGUIRE, J. W. FERNER, AND A.C. ALCALA. 1999. New species of diminutive liz-ard (Squamata; Lygosominae: Sphenomorphus) fromLuzon Island, Republic of the Philippines. Copeia1999:362–370.
BROWN, W. C. 1956. A revision of the genus Brachy-meles (Scincidae) with descriptions of new speciesand sub-species. Breviora 54:1–19.
BROWN, W. C., AND A. C. ALCALA. 1961. A newsphenomorphid lizard from Palawan Island, Phil-ippines. Occasional Papers of the California Acad-emy of Sciences 32:1–4.
BRYGOO, E. R., AND R. ROUX-ESTEVE. 1981. Un genrede lezards scincines d’Afrique: Melanoseps. Bulletindu Museum National d’ Histoire Naturelle 3:1169–1191.
COPLAND, S. J. 1952. A mainland race of the scincidlizard Lygosoma truncatum (Peters). Proceedings ofthe Linnean Society of New South Wales 77:126–131.
COUPER, P. J., J. A. COVACEVICH, S. P. MASTERSON, AND
G. M. SHEA. 1996. Coggeria naufragus gen. et sp.nov., a sand-swimming skink from Fraser Island,Queensland. Memoirs of the Queensland Museum39:233–241.
DE VILLERS, C. G. S. 1939. Uber den Schadel des su-dafrikanischen Schlangenartigen Scinciden Acon-tias meleagris. Anatomischer Anzeiger 88:289–368.
GREER, A. E. 1970. A subfamilial classification of scin-cid lizards. Bulletin of the Museum of Compara-tive Zoology 139:151–183.
. 1973. Two new lygosomine skinks from NewGuinea with comments on the loss of the externalear in lygosomines and observations on previouslydescribed species. Breviora 406:1–25.
. 1983. The Australian scincid lizard genus Ca-lyptotis De Vis: resurrection of the name, descrip-tions of four new species, and discussion of rela-tionships. Records of the Australian Museum 35:29–59.
. 1997. fbDoes the limbless lygosomine skinkIsopachys borealis really lack pectoral and pelvic gir-dles? Journal of Herpetology 31:461–462.
. 2001. Distribution of maximum snout–ventlength among species of scincid lizards. Journal ofHerpetology 35:383–395.
GREER, A. E., AND H. G. COGGER. 1985. Systematicsof the reduce-limbed and limbless skinks currentlyassigned to the genus Anomalopus (Lacertilia: Scin-cidae). Records of the Australian Museum 37:11–54.
GREER, A. E., AND G. D. F. WILSON. 2001. Commentson the scincid lizard genus Ophiomorus, with a cla-
549EAR LOSS IN SKINKS
distic analysis of the species. Hamadryad 26:273-282.
HEYER, W. R. 1972. A new limbless skink (Reptilia:Scincidae) from Thailand with comments on thegeneric status of the limbless skinks of southeastAsia. Fieldiana (Zoology) 58:109–129.
HIKIDA, T. 1982. A new limbless Brachymeles (Sauria:Scincidae) from Mt. Kinabalu, north Borneo. Cop-eia 1982:840–844.
LANG, M., AND W. BOHME. 1990. Description andphylogenetic position of a new species of Isopachysfrom central Thailand and southern Burma (Squa-mata:Scincidae). Bulletin de l’Institut Royal des Sci-ences Naturelles de Belgique. Biologie. 60:231–240.
LAURENT, R. F. 1964. Reptiles et Amphibiens del’Angola. (Troisieme Contribution). Museo doDundo, No. 67, Lisboa, Portugal.
LEVITON, A. E., R. H. GIBBS JR., E. HEAL, AND C. E.DAWSON. 1985. Standards in herpetology and ich-thyology. Part I. Standard symbolic codes for in-stitutional resource collections in herpetology andichthyology. Copeia 1985:802–832.
MERTENS, R. 1965. Bemerkungen uber einige Eide-chsen aus Afghanistan. Senckenbergiana Biologica46:1–4.
. 1970. Die Amphibien und Reptilien West-Pakistans. Stuttgarter Beitrage zur Naturkunde216:1–5.
MINTON, S. A. 1966. A contribution to the herpetol-ogy of West Pakistan. Bulletin of the AmericanMuseum of Natural History 134:27–184.
RIEPPEL, O. 1980. The sound-transmitting apparatusin primitive snakes and its phylogenetic signifi-cance. Zoomorphology 96:45–62.
. 1981. The skull and the jaw adductor mus-culature in some burrowing scincomorph lizardsof the genera Acontias, Typhlosaurus and Feylinia.Journal of Zoology London 195:493–528.
. 1982. The phylogenetic relationships of thegenus Acontophiops Sternfeld (Sauria: Scincidae),with a note on mosaic evolution. Annals of theTransvaal Museum 33:241–257.
SMITH, M. A. 1935. Fauna of British India. Reptiliaand Amphibia. Vol. II. Sauria, London.
TAYLOR, E. H. 1950. Ceylonese lizards of the familyScincidae. University of Kansas Science Bulletin 33:481–518.
TELFORD JR., S. R. 1959. A study of the sand skink,Neoseps reynoldsi Stejneger. Copeia 1959:110–119.
TOERIEN, M. J. 1963. The sound-conducting systemsof lizards without tympanic membranes. Evolution17:540–547.
VAN DER MERWE, N. J. 1944. Die skedelmorfologie vanAcontias meleagris (Linn.). Tydskrifvir Wetenskapen Kuns 5:59–88.
Accepted: 5 January 2002.
550A
LL
EN
E.G
RE
ER
APPENDIX 1.Skink taxa that lack an external ear opening, with details of four morphological features that may be associated with the absence of an external ear opening. Thenumbers in parentheses after a generic name indicate the number of species lacking an external ear opening/total number of species in the genus. The numbers inparentheses after the species name are the number of digits on the manus/pes (most primitive formula observed in cases of variation), with 0.5 indicating a clawlesslimb and 0 indicating limbless. The reference is for the auricular depression, unless noted otherwise. All other observations are based on personal observation. Thenumbered groups represent the lineages—estimated conservatively (see text)—within which the loss of the external ear opening is likely to have occurred. Symbols: 1,feature present; 2, feature absent, ?, feature unknown.
TaxonAuricular
depressionQuadratal
flangeColumella
typeLateral aperture
of r.s.t.Auditorygroove Reference
Scincinae(1) Neoseps (1/1)—SE North America
reynoldsi (part) (0.5/2) 2 ? ? ? ? Pers. obs.
(2) Brachymeles (8/16; 1/16 indeterminate)—Philippines and SE Asia9apus (0/0) 2 ? ? ? ? Hikida, 1982:fig. 1E; pers. obs.bonitae (2/0.5)cebuensis (3/2)elerae (4/4)minimus (0/0)samarensis (3/3)tridactylus (3/3)vermis (0/0)
2???22?
1?????1
5?????5
1?????
1small
?????1?
Pers. obs.
Brown, 1956Pers. obs.Pers. obs.
Davewakeum (1/1)—SE Asiamiriamae (0/0) 2 1 5 1 ? Heyer, 1972; pers. obs.
(3) Nessia (2/8)—Sri Lankaderaniyagalai (0/0)layardi (0/0)
1slight
2?1
?5
?1small
??
Taylor, 1950Pers. obs.
(4) Ophiomorous (8/10)—SW Asiapersicus (3/2) 2 1 1? 1 ? Pers. obs.
(5) Ophiomorus (8/10)—SW Asiablanfordi (4/3) 2 ? ? ? ? Pers. obs.brevipes (4/3)chernovi (4/3)nuchalis (4/3)raithmai (3/3)streeti (3/3)tridactylus (3/3)
222222
??????
5??5?5
1??1??
??????
Pers. obs.Pers. obs.Pers. obs.Pers. obs.Pers. obs.Pers. obs.
551E
AR
LO
SSIN
SKIN
KS
APPENDIX 1. Continued..
TaxonAuricular
depressionQuadratal
flangeColumella
typeLateral aperture
of r.s.t.Auditorygroove Reference
(6) Scelotes (7/22)—southern Africaanguina (0/0)arenicolor (0/0)caffer (3/3)guentheri (0/0.5)inornata (0/0)insularis (0/0)vestigifer (0/0.5)
?2222??
1?1????
555????
?11????
???????
Pers. obs.Pers. obs.Pers. obs.Pers. obs.Pers. obs.Broadley, 1990Broadley, 1994
Feylinia (6/6)—central Africaboulengeri (0/0) ? ? ? ? ?currori (0/0)elegans (0/0)grandisquamis (0/0)macrolepis (0/0)polylepis (0/0)
2?2??
1????
555?5
11??1
1????
Rieppel, 1981; pers. obs.Pers. obs.Pers. obs.
Pers. obs.Melanoseps (5/5)—central Africaater (0/0)loveridgei (0/0)occidentalis (0/0)rondoensis (0/0)schebeni (0/0)
222??
1????
5????
1????
?????
Greer, 1970Brygoo and Roux-Esteve, 1981:fig. 2Brygoo and Roux-Esteve, 1981:fig. 3; pers. obs.
Scolecoseps (3/3)—central Africaacontias (0/0)boulengeri (0/0)litipoensis (0/0)
?22
???
?5?
?1?
???
Greer, 1970D. Broadley, pers. comm.
Typhlacontias (6/6)—southern Africabrevipes (0/0.5)gracilis (0/0)johnsonii (0/0)punctatissimus (0/0)rohani (0/0)rudebecki (0/0)
?2?22?
?1?1??
55?55?
1?????
?2????
Greer, 1970Greer, 1970
Laurent, 1964:fig. 25; pers. obs.Greer, 1970, as T. ngamiensis
(7) Cryptoscincus (1/1)—Madagascarminimus (0/0) 2 ? ? ? ? Pers. obs.
Paracontias (5/5)—Madagascarbrocchii (0/0)hildebrandti (0/0)
22
11
55
?1
??
Pers. obs.Pers. obs.
552A
LL
EN
E.G
RE
ER
APPENDIX 1. Continued..
TaxonAuricular
depressionQuadratal
flangeColumella
typeLateral aperture
of r.s.t.Auditorygroove Reference
holomelas (0/0)milloti (0/0)rothschildi (0/0)
222
1??
5??
1??
1??
Pers. obs.Pers. obs.Pers. obs.
(8) Acontines—southern Africa See Greer, 1970:163Acontias (8/8) 2 1 4 ? 2 Rieppel, 1981; pers. obs.breviceps (0/0)gracilicauda (0/0)lineatus (0/0)litoralis (0/0)meleagris (0/0)
????2
?????
444?4
222?2
????2
Pers. obs.Pers. obs.Pers. obs.
de Villiers, 1939; Brock, 1941; van der Merwe, 1944;Toerien, 1963; pers. obs.
percivali (0/0)plumbeus (0/0)poecilus (0/0)
???
???
44?
22?
???
Pers. obs.Pers. obs.
Acontophiops (1/1)lineatus (0/0) 2 1 4 2 ? Rieppel, 1982; pers. obs.
Typhlosaurus (9/9) 2 1 4 ? 2 Rieppel, 1980, 1981, 1982; pers. obs.aurantiacus (0/0)braini (0/0)caecus (0/0)cregoi (0/0)
????
????
??4?
??22
????
Pers. obs.Pers. obs.
gariepensis (0/0)lineatus (0/0)lomii (0/0)meyeri (0/0)vermis (0/0)
?2???
?????
?4??4
????2
?2???
Toerien, 1963
Pers. obs.
LygosominaeEugongylini
(9) Nannoscincus (1/9)—Australia and New Caledoniamariei (5/5) 2 1 2 1 1 Pers. obs.
(10) Menetia (3/9)—Australiaamaura (4/5)greyii (some) (4/5)surda (4/5)
222
???
?22
??1
??1
Pers. obs.Pers. obs.Pers. obs.
Sphenomorphini
553E
AR
LO
SSIN
SKIN
KS
APPENDIX 1. Continued..
TaxonAuricular
depressionQuadratal
flangeColumella
typeLateral aperture
of r.s.t.Auditorygroove Reference
(11) Ablepharus (1/6)—SW Asiagrayanus (5/5) 1 ? 1 1 ? Smith, 1935; Minton, 1966; Anderson, 1999
(12) Anomalopus (7/7)—AustraliaAnomalopusleuckartii (2/0.5)mackayi (2/2)verreauxii (3/0.5)
111
111
111
22
1minute
1?1
Greer and Cogger, 1985; pers. obs.Greer and Cogger, 1985; pers. obs.Greer and Cogger, 1985; pers. obs.
Vermisepsbrevicollis (0/0)gowi (0/0)pluto (0/0)swansoni (0/0)
1111
1?11
1111
1minute
21minute,slit-like
2
1111
Greer and Cogger, 1985; pers. obs.Greer and Cogger, 1985; pers. obs.Greer and Cogger, 1985; pers. obs.Greer and Cogger, 1985; pers. obs.
Coeranoscincus (2/2)frontalis (0/0)reticulatus (3/3)
11
11
11
1minute,slit-like
1?1
Greer and Cogger, 1985; pers. obs.Greer and Cogger, 1985; pers. obs.
Coggeria (1/1)naufragus (3/3) 1 ? 1 1 ? Couper et al., 1996; pers. obs.
Ophioscincus (3/3)coolooloensis (0/0)ophioscincus (0/0)truncatus (0/0)
111
111
111
?11
111
Greer and Cogger, 1985; pers. obs.Greer and Cogger, 1985; pers. obs.Greer and Cogger, 1985; Copland, 1952;
pers. obs.
(13) Calyptotis (3/5)—Australialepidorostrum (5/5)scutirostrum (5/5)thortonensis (5/5)
111
11?
11?
11?
11?
Pers. obs.Pers. obs.Pers. obs.
Saiphos (1/1)equale (3/3) 1 1 1 1 1 Pers. obs.
(14) Hemiergis (5/5)—Australiadecresiensis (3/3)initialis (5/5)millewae (5/5)peronii (4/4)quadrilineatum (2/2)
11111
111?1
11111
11111
11111
Pers. obs.Pers. obs.Pers. obs.Pers. obs.Pers. obs.
554A
LL
EN
E.G
RE
ER
APPENDIX 1. Continued..
TaxonAuricular
depressionQuadratal
flangeColumella
typeLateral aperture
of r.s.t.Auditorygroove Reference
(15) Isopachys (4/4)—SE Asiaanguinoides (0/0)borealis (0/0)gyldenstolpei (0/0)roulei (0/0)
2111
1111
3111
2??2
????
Hyer, 1972; pers. obs.Lang and Bohme, 1990; pers. obs.Heyer, 1972; pers. obs.Heyer, 1972; pers. obs.
Larutia (4/4)—SE Asialarutensis (2/2)miodactylus (2/1)sumatrensis (2/2)trifasciatum (2/2)
?11
1faint
1???
1?1?
1???
1???
Pers. obs.Pers. obs.Pers. obs.Pers. obs.
Leptoseps (2/2)—SE Asiaosellai (4/4)poilani (5/4)
1faint
1faint
??
??
??
??
Bohme, 1981; pers. obs.Pers. obs. (contra Bourret, 1937)
Parvoscincus (2/2)—Philippinespalawanensis (5/5)sisoni (5/5)
11
??
??
??
??
Brown and Alcala, 1961:fig. 2; pers. obs.Pers. obs.
‘‘Siaphos’’ (1/1)—SE Asiatridigitum (3/5) 1 ? ? ? ? Pers. obs.
Sphenomorphus (6/ca 120)—southeast Asia and Indo-Australian Archipelagocophias (5/5)luzonense (5/5)parvum (5/5)sanana (5/5)surdum (5/5)tagapayo (5/5)
111111
??????
??????
??????
??????
Pers. obs.Pers. obs.Pers. obs.Pers. obs.Pers. obs.Brown et al., 1999
(16) Lipinia (5/20) SE Asia—SW Pacificinfralineolatum (5/5)nitens (5/5)quadrivittatum (5/5)relictum (5/5)subvittatum (5/5)
11111
??2??
??1??
1?1??
?????
Pers. obs.Pers. obs.Pers. obs.Pers. obs.Pers. obs.
(17) Sphenomorphus (1/ca 120)—New Guineaanotus (5/5) 1 ? ? ? ? Greer, 1973:fig. 1b; pers. obs.
555EAR LOSS IN SKINKS
APPENDIX 2Material Examined
The features of the middle ear were examined inthe following specimens.Ablepharus grayanus: MCZ 84084; Acontias breviceps:MCZ 38559; A. g. gracilicauda: MCZ 100905; A. lineatus:MCZ 21416, 21659; A. meleagris: BMNH 63.2.21.21,MCZ 11934; A. percivali occidentalis: MCZ 67861, 67859;A. p. percivali: MCZ 40180; A. p. tasmani: MCZ 96905;A. plumbeus: AM R 76334, BMNH 94.6.29.38, MCZ14233, 21452; Anomalopus gowi: AM R 63130; A. brevi-collis: AM R 114084, QM 33853; A. leuckartii: AM R43949; A. mackayi: AM R 13138; A. pluto: AM R 94362;A. swansoni: AM Palmer 5186, R 104139; A. verreauxii:AM R 6437 114043, MCZ 10263; Brachymeles bonitae:MCZ 20129; B. tridactylus: AM 98395; B. vermis: MCZ26587; Calyptotis lepidorostrum: AM R 59246; C. scuti-rostrum: AM R 43061, 90434; Coeranoscincus frontalis:AM R 3823, QM J 45355; C. reticulatus: AM R 4795;Coggeria naufragus: QM J 59670; Davewakeum miriamae:FMNH 182546; Feylinia currori: AM R 97270, BMNH1903.12.2.18, CAS 55112, MCZ 106990; F. elegans: MCZ42886; ; F. grandisquamis: MNHN 1206.77; F. polylepis:MCZ 61215; Hemiergis decresiensis: AM R 93911, MCZ49173, SAM 3237; H. initialis: MCZ 74976, WAM13633; H. millewae: AM R 115996, SAM 3069B; H. per-
onii; AM R 115711, MCZ 24595, 24648, 24652; H. quad-rilineatum: MCZ 33210, WAM 35048; Isopachys angui-noides: AM R 112447; MCZ 74098; I. borealis: ZFMK45714; I. gyldenstoplei: FMNH 178324; I. roulei: FMNH196172, 196198, MCZ 74099; Larutia larutensis: BMNH1946.8.3.19, MCZ 39265; L. sumatrensis: NHW 10172.1;Lipinia quadrivittatum: AMNH 86665, FMNH 152400;Melanoseps ater: subspecies: misukuensis: MCZ 50955;subspecies rondoensis: MCZ 52487; Menetia greyii: AMR 102024; M. surda: WAM 27981; Nannoscincus mariei:AM H 52097, R 125851, 146485, MCZ 92393; Nessialayardi: BMNH 1964.1720, MCZ 4122, unregistered;Ophiomorus brevipes: FMNH 141550; O. persicus:FMNH 141557; O. tridactylus: AMNH 75610, CAS84679; O. raithmai: AMNH 85846; Ophioscincus coolool-ensis: QM J 27381, 27384; O. ophioscincus: AM R 47642;O. truncatus: AM R 8666, 153851, 153868; Paracontiasbrocchi: MNHN 1979.8271; P. hildebrandti: MCZ 7767;P. holomelas: MNHN 7792; Saiphos equale: AM R 7242,41197, AMNH 27266, MCZ 35344; Scelotes anguina:MCZ 131887; S. arenicolor: MCZ 14205; S. caffer: MCZ131886; Scolecoseps boulengeri: MCZ 18357 (paratype);Typhlacontias brevipes: MCZ 96702; T. gracilis: AM R76274, 76276; T. punctatissimus: TM 24471; T. rohani:FMNH 142787, 142791; Typhlosaurus caecus: AMNH50669.
Journal of Herpetology, Vol. 36, No. 4, pp. 555–561, 2002Copyright 2002 Society for the Study of Amphibians and Reptiles
Effect of Water Temperature and Oxygen Levels on the DivingBehavior of Two Freshwater Turtles: Rheodytes leukops and
Emydura macquarii
TONI E. PRIEST AND CRAIG E. FRANKLIN1
Department of Zoology and Entomology, University of Queensland, Brisbane, Queensland 4072, Australia
ABSTRACT.—Rheodytes leukops is a bimodally respiring turtle that extracts oxygen from the water chieflyvia two enlarged cloacal bursae that are lined with multi-branching papillae. The diving performance of R.leukops was compared to that of Emydura macquarii, a turtle with a limited ability to acquire aquatic oxygen.The diving performance of the turtles was compared under aquatic anoxia (0 mmHg), hypoxia (80 mmHg) andnormoxia (155 mmHg) at 15, 23, and 308C. When averaged across all temperatures the dive duration of R.leukops more than doubled from 22.4 6 7.65 min under anoxia to 49.8 6 19.29 min under normoxic conditions.In contrast, aquatic oxygen level had no effect on the dive duration of E. macquarii. Dive times for both specieswere significantly longer at the cooler temperature, and the longest dive recorded for each species was 538 minand 166 min for R. leukops and E. macquarii, respectively. Both species displayed a pattern of many short divespunctuated by occasional long dives irrespective of temperature or oxygen regime. Rheodytes leukops, onaverage, spent significantly less time (42 6 2 sec) at the surface per surfacing event than did E. macquarii (1066 20 sec); however, surface times for both species were not related to either water temperature or oxygen level.
Aquatic respiration is one of several strategiesemployed by freshwater turtles to extend dive
1 Corresponding Author. E-mail: [email protected]
duration (Belkin, 1968; Ultsch et al., 1984; Stoneet al., 1992a). The capacity for aquatic respira-tion varies greatly among taxa, ranging from 4%of total oxygen uptake in the snapping turtleChelydra serpentina (at 258C; Bagatto and Henry,
556 T. E. PRIEST AND C. E. FRANKLIN
1999a) to 37.5% in the softshell Apalone spiniferus(at 258C; Stone et al., 1992a). Although the de-gree of aquatic respiration has been quantifiedfor many species and under many conditions,the implications of aquatic respiration on divingbehavior remain unresolved.
Two abiotic factors that affect diving behaviorof bimodally breathing turtles are aquatic oxy-gen level and temperature (Ultsch, 1985). A pos-itive correlation between aquatic oxygen leveland dive duration has been consistently report-ed for the highly aquatic softshells (Ultsch et al.,1984; Ultsch, 1985; Stone et al., 1992b). Resultsfor other taxa are less clear, with the moderatelyaquatic Chelydra serpentina (Ultsch et al., 1984),Chrysemys picta (Ultsch, 1985), and Sternotherusminor (Belkin, 1968) showing a positive relation-ship, whereas the moderately aquatic K. subru-brum (Stone et al., 1992b) does not.
Temperature is an important confoundingfactor in studies of diving behavior of bimodallybreathing turtles. Decreasing temperature re-sulted in an increase in the relative contributionof aquatic respiration in C. picta (Herbert andJackson, 1985), Elseya latisternum (King and Hea-twole, 1994b), and C. serpentina (Gatten, 1980).At lower temperatures, it may therefore be ex-pected that the magnitude of the increase indiving time in response to increased partialpressures of oxygen (PO2) would be greater;that is, turtles would dive relatively longer inhigh PO2 water at low temperatures comparedto low PO2 water. In support of this, S. odoratusdisplayed a positive relationship between PO2
and dive duration at 38C and 108C (Ultsch, 1985)but not at 238C (Stone et al., 1992b). In contrast,C. picta displayed a relationship at 108C (Ultschet al., 1984; Ultsch, 1985) but not 38C (Ultsch andJackson, 1982).
In light of the contradictory results of thestudies on the effects of aquatic PO2 and tem-perature on diving behavior, this study investi-gated the effect of temperature and PO2 on thediving behavior of two Australian pleurodires,Rheodytes leukops and Emydura macquarii, whichvary in their capacity for aquatic respiration.
Unlike the crypodires that obtain aquatic ox-ygen partly via the buccopharynx, pleurodirescan obtain aquatic oxygen via enlarged cloacalbursae (King and Heatwole, 1994a). Cloacal res-piration has reached its pinnacle in R. leukops, achelid from northeast Australia. The cloacal bur-sae of this turtle are greatly enlarged and arelined with highly vascularized, multibranchedpapillae (Legler and Cann, 1980; Legler andGeorges, 1993). By rhythmic ventilation of thesestructures, R. leukops is able to extract, on aver-age, 41% of its oxygen requirements from thewater (Priest, 1997; C. E. Franklin, M. Gordos,and T. Priest, unpubl. data). Emydura macquarii
does not have extensive cloacal modificationsand has a limited capacity for aquatic respira-tion, extracting approximately 11% of its totaloxygen consumption from the water (Leglerand Georges, 1993; Priest, 1997).
We hypothesize that diving time of both R.leukops and E. macquarii will be dependent onwater temperature and that only R. leukops willrespond to changes in aquatic oxygen level andthat the degree of the response will be depen-dent on temperature.
MATERIALS AND METHODS
Six R. leukops (mean mass: 1325 g 6 193.9 SE;range: 595–1810 g) and five E. macquarii (meanmass: 1595 g 6 104.9 SE; range: 1200–1800 g)were used in this study. Emydura macquarii werecollected from the Albert River, Brisbane and R.leukops from the Fitzroy R., Rockhampton,Queensland. Turtles were housed in two 2000-liter covered outdoor holding tanks at 23 6 28Cwith water depth 250–300 mm. A full spectrumlight was positioned above each tank, and abasking platform was provided, although R. leu-kops were never observed basking. Turtles werefed twice weekly with chopped meat and a va-riety of fruit and vegetables. The water was con-tinually filtered and was changed after everyfeeding.
Experiments were conducted at 158C, 238C,and 308C and at three aquatic PO2s: anoxia (0mmHg), hypoxia (80 mmHg), and normoxia(155 mmHg), for each temperature. The temper-atures chosen approximate the water tempera-tures over a year in the turtles’ natural homerange. Turtles were placed in a rectangular tank(500 mm 3 1000 mm) that was filled to a depthof 250 mm with tap water and housed in a con-trolled temperature room. The water was con-tinually circulated and filtered and the feedingregimen maintained. Rheodytes leukops wereplaced in the tank in pairs and E. macquarii inone pair and one group of three. Because theexperimental tanks were relatively large and nointerindividual aggression was observed, anyeffect of studying the turtles in groups ratherthan individually should be minimal. Turtleswere marked with colored paint to allow indi-vidual recognition. Each group was allowed oneweek at 238C to become accustomed to the tank.Preliminary videotaping demonstrated that av-erage dive time reached a plateau after this time.The temperature was then changed to the ex-perimental temperature and the first PO2 levelset. Temperatures were randomly selected, andthen within each temperature the PO2 was againrandomly selected. To minimize possible ther-mal stress to the animals, the test temperaturewas maintained until all three PO2 trials for thattemperature had been completed. Maintaining
557DIVING BEHAVIOR OF FRESHWATER TURTLES
FIG. 1. Average dive duration (min) of (A) Rheod-ytes leukops (N 5 6) and (B) Emydura macquarii (N 55) at 15, 23, and 308C under aquatic anoxia (0 mmHg),hypoxia (80 mmHg), and normoxia (155 mmHg). Barsare the mean of each treatment and error bars are 1SE.
the test temperature until all PO2 treatmentswere completed may have allowed some ther-mal acclimation to occur; however, randomizingPO2 treatments and having multiple groups thatwere therefore presented with both temperatureand PO2 in different order should remove anybias caused by possible thermal acclimation.
To maintain the oxygen level a TPS dissolvedO2 electrode (ED500) was suspended in the tankand connected to a TPS oxygen analyzer, model2052A. The O2 analyzer was connected to aMann Industries UTC/R Universal temperaturealarm (thermocouple inputs) that was wired sothat when the input from the oxygen analyzermoved beyond a preset level the alarm wastripped and a solenoid valve opened. The sole-noid controlled the flow of either N2 or O2
through an airstone into the tank. The oxygenelectrode was suspended at the outflow of thefilter system to ensure a good flow of water overthe electrode.
The controlled temperature room was main-tained on a 12:12 h light:dark photic regime. Theduration of the trials was varied depending onthe experimental temperature, being 24, 18, and6 h for 15, 23 and 308C, respectively. The turtleshad access to room air for the entire duration ofthe experiment, and basking platforms were notsupplied.
Experiments were videotaped during day-light hours only with a National F10 video cam-era and National AGG010 timelapse videocas-sette recorder. All tapes were later viewed andthe emergence and submergence time of everydive during the experimental period was re-corded.
Statistical Analysis.—Dive time, surface time,and longest dive time were analyzed using arepeated measures three-way ANOVA. Analysisof dive and surface time was performed on themean values for each turtle at each of the ex-perimental conditions. When an effect wasshown to be significant, differences among tem-perature, PO2, and species were detected usingthe least significant difference technique. Prob-ability values less than 0.05 were taken as sig-nificant. Results are presented as means of treat-ments 6 SE, unless otherwise stated.
RESULTS
Dive Duration.—There was a significant dif-ference in the way the species responded tochanges in aquatic PO2 (F 5 6.51, P , 0.0025).Average dive time for R. leukops more than dou-bled from 22.4 6 7.65 min to 49.8 6 19.29 minwhen PO2 was increased from 0 to 155 mmHg(Fig. 1). In contrast, aquatic oxygen level had noeffect on dive duration of E. macquarii (Fig. 1).Rheodytes leukops had on average significantlylonger dives than E. macquarii in normoxic water
at all of the experimental temperatures (Figs. 1–2). In hypoxic water, dives for R. leukops weresignificantly longer at 15 and 238C only. Underaquatic anoxia average dive duration of R. leu-kops and E. macquarii was not significantly dif-ferent (Fig. 1)
Decreasing temperature resulted in signifi-cantly longer dive durations for both species,but the magnitude of the response differed (F5 10.03, P , 0.0001). Decreasing the tempera-ture from 30 to 158C resulted in a sevenfold in-crease in dive time from 10.1 6 2.7 to 70.5 615.0 min in R. leukops and a fivefold increase inaverage dive duration from 6.7 6 1.2 to 31.3 68.3 min for E. macquarii. Average dive times ofE. macquarii at 23 and 308C were not signifi-cantly different.
The response to changes in PO2 shown by R.leukops was not dependent on temperature (F 51.87, P , 0.1256). Dive times under normoxiawere approximately twice the length of divesunder anoxia irrespective of temperature.
Frequency of Dive Durations.—The longestdives recorded for R. leukops were at 158C andin normoxic water; the longest single dive re-corded was 538 min. Although R. leukops was
558 T. E. PRIEST AND C. E. FRANKLIN
FIG. 2. Average maximum dive duration (min) of(A) Rheodytes leukops (N 5 6) and (B) Emydura mac-quarii (N 5 5) at 15, 23 and 308C under conditions ofaquatic anoxia (0 mmHg), hypoxia (80 mmHg) andnormoxia (155 mmHg). Bars are the mean of eachtreatment, error bars are 1 SE.
FIG. 3. Frequency distributions of dive times forRheodytes leukops (N 5 6) at 158C under aquatic anoxia,hypoxia, and normoxia. Observations for all individ-uals within a treatment have been combined. Bars are15-min intervals.
capable of extremely long dives, 41% of divesunder the aforementioned conditions were lessthan 30 min (Fig. 3). For E. macquarii underequivalent conditions, the longest dive was 166min, and 61.5% of dives were less than 30 min(Fig. 4). The extreme right skew of the dive timehistograms was evident for both species and un-der all temperatures and aquatic oxygen levels(Fig. 3).
Surfacing.—Surface times for both specieswere not significantly related to either watertemperature or oxygen level (F 5 1.95, P . 0.15;Fig. 5). Rheodytes leukops, however, spent signif-icantly less time at the surface after each divethan did E. macquarii, spending on average 426 2 sec at the surface between dives comparedwith 107 6 20 sec for E. macquarii (F 5 29.48, P, 0.0001).
DISCUSSION
Dive Duration.—Both aquatic oxygen level andtemperature had a significant impact on the div-ing behavior of R. leukops, as would be expectedbased on previous studies of highly aquatic tur-tles. In contrast, the diving behavior of E. mac-quarii was influenced by water temperatureonly. Previous studies have demonstrated a cor-
relation between PO2 and dive duration in tur-tles with aquatic uptake capabilities similar toE. macquarii (Belkin, 1968; Ultsch et al., 1984;Ultsch, 1985); however, direct comparisons ofthe species in the different experiments are con-founded by methodological differences. Withthe exception of the work of Stone et al. (1992)with A. spinifera, all these studies were con-ducted under forced submergence and gener-ally concluded with the death of the turtles.Forced submergence leads to many physiologi-cal adjustments that are not seen in freely div-ing individuals. Such adjustments include a se-vere bradycardia and dramatically reduced met-abolic rate (Belkin, 1964; Herbert and Jackson,1985), increased anaerobic metabolism, and insome species increased aquatic respiration (Ba-gatto and Henry, 1999b). Studies with forciblysubmerged animals may overestimate the rela-tive contribution of aquatic respiration, and theturtles therefore may show a response thatwould not necessarily be present when divingfreely. For example, S. odoratus under forced sub-mergence displayed a correlation betweenaquatic PO2 and maximum dive duration but
559DIVING BEHAVIOR OF FRESHWATER TURTLES
FIG. 4. Frequency distributions of dive times for Rheodytes leukops (N 5 6) and Emydura macquarii (N 5 5)at 15, 23, and 308C in normoxic water (155 mmHg). Observations for all individuals within a treatment havebeen combined. Bars are 15-min intervals.
did not show one under freely diving conditions(Ultsch et al., 1984; Stone et al., 1992b). It istherefore not surprising that under freely divingconditions only the highly aquatic R. leukops andnot E. macquarii displayed a correlation betweenPO2 and dive duration.
A correlation between temperature and diveduration is well documented in freshwater tur-tles (Fuster et al., 1997). The few studies thathave examined the percent aquatic uptake atdifferent temperatures have found that it in-creases at low temperatures (Gatten, 1980; Her-bert and Jackson, 1985; King and Heatwole,1994b). Accordingly dive times would be ex-pected to be proportionally longer at low tem-peratures in high PO2 water compared to lowPO2 water. Rheodytes leukops and E. macquarii,however, did not show such an interaction be-tween temperature and PO2. Ultsch (1985), whostudied A. spinifera, C. serpentina, and C. concinnaand looked at survival time in relation to tem-perature and PO2, also observed no interaction,that is, the effect that increasing aquatic PO2 hadon diving duration did not depend on temper-ature.
The hypothesis that R. leukops uses its aquatic
respiratory ability to extend dive duration be-yond that of E. macquarii is supported by thisstudy. In anoxia, dive times of R. leukops and E.macquarii were approximately equal. In hypoxicwater, dives of R. leukops were approximatelytwice as long as those of E. macquarii, and innormoxia the dives were three times longer, in-dicating the more oxygen that is available themore R. leukops is able to extend its dives be-yond those of E. macquarii. This contrasts withthe results of Stone et al. (1992b), who conclud-ed that A. spinifera used their superior aquaticrespiration to reduce the time spent at the sur-face rather than to increase dive duration be-yond that of S. odoratus. These discrepanciesmay have been caused by differences in behav-ior and activity level between the species. Apa-lone spinifera in a later study had an aquatic O2
uptake of 21.7% (Bagatto and Henry, 1999b). Inaddition, S. odoratus may have become habitu-ated to the experimental chamber, as normoxictrials were always conducted first. Bagatto andHenry (1999b) also found that dive times of A.spinifera in oxygenated water were twice as longas those originally recorded by Stone et al.(1992b).
560 T. E. PRIEST AND C. E. FRANKLIN
FIG. 5. Average time spent at the surface after eachdive for both (A) Rheodytes leukops (N 5 6) and (B)Emydura macquarii (N 5 5) at 15, 23, and 308C andunder conditions of aquatic anoxia (0 mmHg), hyp-oxia (80 mmHg), and normoxia (155 mmHg). Bars arethe mean of each treatment, and error bars are 1 SEof the mean.
The relatively high proportion of short divesobserved in this study is a common feature ofstudies with freshwater turtles (Stone et al.,1992b) and is supported by studies of oxygenuse by diving turtles. These studies showed thatafter diving, turtles still had sufficient oxygenstores in their lungs and tissues to sustain con-tinued respiration (Burggren and Shelton, 1979;Gatten, 1984; Burggren et al., 1989). In additionthis pattern may be a result of different activitylevels based on appetite and foraging or endog-enous rhythms of daily activity levels.
Surface Duration.—Surface duration was notrelated to either water temperature or oxygencontent for either species, but R. leukops had sig-nificantly shorter surface times under all con-ditions. Rheodytes leukops is a highly aquatic tur-tle that spends the majority of its time on theriver bed rather than in the water column (Leg-ler and Cann, 1980). Emydura macquarii are lessaquatic and are often observed engaged in bothaerial and aquatic basking (Manning and Grigg,1997). It is likely that E. macquarii would havespent time at the surface when not actually re-plenishing oxygen stores, whereas R. leukops re-mained at the surface only long enough to re-
plenish oxygen stores, as demonstrated by thefact that E. macquarii had much more variablesurface periods than R. leukops. Stone (1992b)also demonstrated that A. spinifera, the speciesin their study that was most dependent onaquatic respiration, had the shortest and leastvariable surface period.
At a given temperature, R. leukops dived lon-ger than E. macquarii at high PO2, yet surfacetime was unrelated to PO2. The lack of correla-tion between dive and surface interval and theshort surface interval suggests that the turtleswere using aerobic metabolism.
Ecological Implications.—Rheodytes leukops iscommonly found in the riffle zones of its river-ine habitat. These riffles are areas of relativelyshallow, flowing water with a high level of sat-urated oxygen. Rheodytes leukops is a bottomdwelling turtle that forages on and among therocks and debris of the substrate for aquatic in-vertebrates and algae (Legler and Cann, 1980;Cann, 1998). Cloacal respiration would thereforeserve to greatly increase the amount of time thespecies can spend foraging and thus reduce thetime and energy spent traveling to the surface.Emydura macquarii, in contrast, is a generalistand can be found in impoundments and largewaterholes as well as riverine habitats. The ben-thic zone of deep waters are frequently anoxic,a habitat where cloacal respiration would be ofno advantage.
Acknowledgments.—This study was funded bya University of Queensland Foundation Grantand an Australian Research Council Grant toCEF. Financial assistance was also generouslyprovided by Australian Geographic. All exper-imental procedures were approved by the Uni-versity of Queensland animal ethics and exper-imentation committee (AEEC ZOO/375/96-00/URG/H). Turtles were collected under Depart-ment of Environment Scientific Purposes permitC6/000064/96/SAA.
LITERATURE CITED
BAGATTO, B. P., AND R. P. HENRY. 1999a. Aerial andaquatic respiration in the snapping turtle, Chelydraserpentina. Journal of Herpetology 33:490–492.
. 1999b. Exercise and forced submergence inthe pond slider (Trachemys scripta) and softshellturtle (Apalone ferox): Influence on bimodal gas ex-change, diving behavior and blood acid-base sta-tus. Journal of Experimental Biology 202:267–278.
BELKIN, D. A. 1964. Variations in heart rate duringvoluntary diving in the turtle Pseudemys concinna.Copeia 1964:321–330.
. 1968. Aquatic respiration and underwatersurvival of two freshwater turtle species. Respira-tion Physiology 4:1–14.
BURGGREN, W. W., AND G. SHELTON. 1979. Gas ex-change and transport during intermittent breath-
561DIVING BEHAVIOR OF FRESHWATER TURTLES
ing in chelonian reptiles. Journal of ExperimentalBiology 82:75–92.
BURGGREN, W. W., A. SMITS, AND B. EVANS. 1989. Ar-terial oxygen homeostasis during diving in theturtle Chelodina longicollis. Physiological Zoology62:668–686.
CANN, J. 1998. Australian Freshwater Turtles. Beau-mont Publishing Pte Ltd., Singapore.
FUSTER, J. F., T. PAGES, AND L. PALACIOS. 1997. Effectof temperature on oxygen stores during aerobicdiving in the freshwater turtle Mauremys caspica le-prosa. Physiological Zoology 70:7–18.
GATTEN, R. E. 1980. Aerial and aquatic oxygen uptakeby freely-diving snapping turtles (Chelydra serpen-tina). Oecologia 46:266–271.
. 1984. Aerobic and anaerobic metabolism offreely-diving loggerhead musk turtles (Sternotherusminor). Herpetologica 40:1–7.
HERBERT, C. V., AND D. C. JACKSON. 1985. Tempera-ture effects on the responses to prolonged sub-mergence in the turtle Chrysemys picta bellii. II.Metabolic rate, blood acid-base and ionic changes,and cardiovascular function in aerated and anoxicwater. Physiological Zoology 58:670–681.
KING, P., AND H. HEATWOLE. 1994a. Non-pulmonaryrespiratory surfaces of the chelid turtle Elseya latis-ternum. Herpetologica 50:262–265.
. 1994b. Partitioning of aquatic oxygen uptakeamong different respiratory surfaces in a freelydiving pleurodian turtle, Elseya latisternum. Copeia1994:802–806.
LEGLER, J. M., AND J. CANN. 1980. A new genus andspecies of chelid turtle from Queensland, Austra-lia. Contributions to the Scientific Natural HistoryMuseum of Los Angeles County 324:1–18.
LEGLER, J. M., AND A. GEORGES. 1993. Family Cheli-dae, Chapter 21. In R. E. Jones (ed.), Fauna of Aus-
tralia, Australian Government Printing Service,Canberra, Australian Capital Territory, Australia.
MANNING, B., AND G. C. GRIGG. 1997. Basking is notof thermoregulatory significance in the ‘‘basking’’freshwater turtle Emydura signata. Copeia 3:579–584.
PRIEST, T. E. 1997. Bimodal respiration and dive be-haviour of the Fitzroy River turtle, Rheodytes leu-kops. Unpubl. honors thesis, University of Queens-land, Brisbane, Queensland, Australia.
STONE, P. A., J. L. DOBIE, AND R. P. HENRY. 1992a.Cutaneous surface area and bimodal respiration insoft-shelled (Trionyx spiniferus), stinkpot (Sternoth-erus odoratus), and mud turtles (Kinosternon subru-brum). Physiological Zoology 65:311–330.
. 1992b. The effect of aquatic oxygen levels ondiving and ventilatory behavior in soft-shelled(Trionyx spiniferus), stinkpot (Sternotherus odoratus),and mud turtles (Kinosternon subrubrum). Physio-logical Zoology 65:331–345.
ULTSCH, G. R. 1985. The viability of nearctic fresh-water turtles submerged in anoxia and normoxiaat 3 and 108C. Comparative Biochemistry andPhysiology 81A:607–611.
ULTSCH, G. R., AND D. C. JACKSON. 1982. Long-termsubmergence at 38C of the turtle, Chrysemys pictabellii, in normoxic and severely hypoxic water I.Survival, gas exchange and acid-base status. Jour-nal of Experimental Biology 96:11–28.
ULTSCH, G. R., C. V. HERBERT, AND D. C. JACKSON.1984. The comparative physiology of diving inNorth American freshwater turtles. 1. Submer-gence tolerance, gas exchange and acid-base bal-ance. Physiological Zoology 57:620–631.
Accepted: 16 January 2002.
Journal of Herpetology, Vol. 36, No. 4, pp. 561–571, 2002Copyright 2002 Society for the Study of Amphibians and Reptiles
A New Phrynobatrachus from the Upper Guinean Rain Forest,West Africa, Including a Description of a New Reproductive Mode
for the Genus
MARK-OLIVER RODEL1 AND RAFFAEL ERNST
Theodor-Boveri-Institute (Biocenter of the University), Department of Animal Ecology and Tropical Biology(Zoology III), Am Hubland, D-97074 Wurzburg, Germany
ABSTRACT.—We describe a new species of Phrynobatrachus from the Western part of the Upper Guineanrain forest, West Africa. Phrynobatrachus phyllophilus sp. nov. differs from all other known West AfricanPhrynobatrachus by a combination of morphological and acoustical characters. It is most similar to Phry-nobatrachus guineensis from which P. phyllophilus is distinguished by its almost white belly, presence ofonly one dark bar on femur and tibia, shape of the thumb in reproductive males, advertisement call, repro-ductive mode, and selection of different forest types. Phrynobatrachus phyllophilus is the first known speciesof the genus that deposits small clutches of eggs rich in yolk on leaves, in close vicinity to extremely smallpuddles on the forest floor. Its preferred habitats are swampy areas of primary rain forest. We also describethe tadpole of P. phyllophilus and the advertisement call of P. guineensis.
562 M.-O. RODEL AND R. ERNST
Since 1993, we have investigated anurans inTaı National Park (TNP), Ivory Coast. Until now,we have recorded 13 Phrynobatrachus specieswithin the park’s borders (unpubl. data). In 1998we reported on the breeding behavior of Phry-nobatrachus guineensis, a minute frog originallydescribed in 1961 by Guibe and Lamotte, fromMont Tonkoui, Ivory Coast and Mont Nimba,Guinea. Phrynobatrachus guineensis and Phryno-batrachus dendrobates are unique among Phryno-batrachus with respect to their reproductivemode: eggs are deposited in tree holes, or othersmall, water-filled cavities like empty fruit cap-sules and snail-shells (Rodel, 1998; unpubl. data;R. C. Drewes, pers. comm.). Phrynobatrachus gui-neensis inhabits relatively dry parts of the rainforest, normally not providing any sources ofopen water, other than the above-mentionedbreeding sites. From the beginning of our in-vestigations, we became aware of another Phry-nobatrachus species, similar to P. guineensis, thatseemed to prefer rather swampy parts of theforest. With the descriptions of West AfricanPhrynobatrachus species given by Guibe and La-motte (1961, 1963), it was not possible to sepa-rate it from P. guineensis. Examination of voucherspecimens of P. guineensis, collected by Lamotteand coworkers, and Schiøtz, revealed that bothspecies were well represented in Paris and Co-penhagen collections, although often mixedwithin samples from even one locality. In 1999and 2000, we finally succeeded in recording theadvertisement calls of both species, and we col-lected data on the reproductive behavior of thenew species, enabling us to properly differenti-ate it from P. guineensis by means of morpholog-ical and acoustical characters.
MATERIALS AND METHODS
Study Areas.—The TNP is the largest protect-ed area of rain forest in West Africa. Yearly pre-cipitation reaches 2200 mm in the southwestand 1700 mm in the northeast of the park. Mostprecipitation occurs from April to July and fromSeptember to November. The first dry periodlasts from December to February; normally asecond one occurs in August. Temperaturesvary between 20–338C, with daily temperaturedifferences of up to 108C. The mean annual tem-perature is about 258C. Humidity fluctuatesfrom 85% (day) to 90–100% (night). During thedry season, humidity may drop below 60%,even in closed forest. Our main investigationarea was located 23 km southeast of the smalltown of Taı and comprised about 30 km2 of pri-
1 Corresponding Author. Present address: Universi-ty of Mainz, Department of Zoology, Saarstrasse 21,D-55099 Mainz, Germany; E-mail: [email protected]
mary and secondary rain forest around the Sta-tion de Recherche en Ecologie Tropicale (SRET,58509N, 78209W, formerly CRE and IET). Be-tween 1991 and 1999, mean annual precipitationat the SRET station was 1854 mm (SD 5 249;range 1424–2194 mm; R. Noe, pers. comm.). Ad-ditionally we made some short investigations inSouth-Eastern Mont Peko National Park (MPNP,68589N, 78109W), situated within the zone ofmoist semideciduous forest (Parren and deGraaf, 1995). The whole area is situated withinthe equatorial climate (Riezebos et al., 1994); flo-ristically, it belongs to the Guinea-Congo region(Guillaumet, 1967).
Field Data.—Data were collected irregularly indifferent parts of the forest and regularly along10 transects, 600 m in length. Six transects wereset up in primary and four in secondary forest.Data collection and transect conception are de-scribed in more detail in Rodel et al. (2001).
Measurements.—Frogs were sacrificed in achlorbutole solution and preserved in 4% form-aldehyde or 70% ethanol. All adults were sub-sequently transferred to ethanol. Larvae of dif-ferent stages preserved in 4% formaldehyde,were transferred to 70% ethanol after twomonths. Measurements were taken with a dialcaliper (6 0.1 mm) or a measuring ocular in adissecting microscope (6 0.1 mm, Zeiss StemiSV 6). Measurements taken on frogs weresnout–vent length (SVL), interorbital distance(IO), distance from a line between the anteriorborder of the eye to snout tip (ES), femur length(FE), tibia length (TI), and length of the foot in-cluding the longest toe (TA). The webbing for-mula is according to Rodel (2000). On tadpoles,we measured body length, body width (mea-sured at the plane of the eyes); tail length, finheight, height of tail axis, and body height. No-menclature of morphological features followsVan Dijk (1966), Altig and Johnston (1989), andAltig and McDiarmid (1999). The labial toothrow formula is according to Dubois (1995), andstaging of tadpoles is according to Gosner(1960). The tadpole description is a summary ofall examined specimens from Gosner stages 34–41 (N 5 10). The description of the coloration isbased on living frogs and tadpoles. Drawingswere done after color slides or with the aid of acamera lucida. We included only those frogswithin the type series that we had access to dur-ing writing of the paper.
Specimens Examined and Voucher Specimens.—Museum specimens originated from or are de-posited in the following collections: the Museumnational d’Histoire naturelle, Paris (MNHN); theForschungsinstitut und Naturmuseum Sencken-berg, Frankfurt/M. (SMF); the Staatliches Muse-um fur Naturkunde, Stuttgart (SMNS); the Zoo-logical Museum at the University of Copenhagen
563A NEW PHRYNOBATRACHUS FROM WEST AFRICA
FIG. 1. (A) Amplectant (A) pair and (B) male of Phrynobatrachus phyllophilus sp. nov. from Taı National Park,Ivory Coast. Drawn after color slides.
FIG. 2. Throat of a reproductive Phrynobatrachusphyllophilus sp. nov. male (SMNS 9721.1, holotype).
(ZMUC), the Zoologisches Forschungsinstitutund Museum Alexander Koenig, Bonn (ZFMK),and the Zoologische Staatssammlung des bayer-ischen Staates, Munchen (ZSM). For further in-vestigations, some specimens remain in the pri-vate collection of the senior author (CR) and willbe deposited in the collections mentioned above.
Acoustical Data.—Recordings were made inthe field using a Sony WM-D6C tape recorderand directional microphones (Sony ECM-Z157,and Sony ECU-959C) and were analyzed on aDSP Sona-Graph 5500 (Kay). Call duration,notes per call, note length, pulses per note, in-ternote interval and frequency range were de-termined.
RESULTS
Phrynobatrachus phyllophilus sp. nov.Phrynobatrachus guineensis (part): Guibe and La-
motte, 1961, 1963; Schiøtz 1964b, 1964c.Phrynobatrachus guineensis (part?): Lamotte,
1966, 1971.
Holotype.—SMNS 9721.1, male, Taı National
Park, Ivory Coast, SRET station, swampy area inprimary rain forest, 58509N, 78209W, 28 Septem-ber 1999, Ernst and Rodel leg.
Paratypes.—SMNS 9721.2, female, same dataas holotype; SMNS 9724.1–18, 4 males, 11 fe-males, 3 juveniles, Taı National Park, IvoryCoast, Guiroutou, 58259N, 78109W, 1993, Rodelleg.; ZSM 354/2001, male, same data; ZFMK74223, male; same data; SMNS 9722.1–8, 2males, 3 females, 3 juveniles, same locality asholotype, January to March 1999, Ernst and Ro-del leg.; SMNS 9723.1–10, 10 tadpoles, same lo-cality as holotype, 17–30 June 2000, Ernst andRodel leg.; CR phyllophilus 1–4, 1 male, 3 fe-males, Mont Peko National Park, Ivory Coast,68589N, 78109W, river Bihi, gallery forest, 21 June2000, Ernst and Rodel leg.; ZMUC R075278,male, R075290, female, R075292, male, R075304,female, all from Monts Loma, Sierra Leone, for-est, 98109N, 11879W, 12 June 1963, Schiøtz leg.
Diagnosis.—Very small and compact frog witha moderately pointed snout (Figs. 1–2); thumbof reproductive males not swollen (Fig. 3A); bel-ly almost white (Fig. 4A–B); femur and tibiawith one broad black bar (Fig. 4C); finger andtoe tips enlarged to discs (Fig. 3A, C); moder-ately webbed feet; males with small femoralglands (Fig. 4A); inhabits swampy areas of rainforest; small clutches with eggs rich in yolk areoviposited terrestrially on leaves near water.
Description of the Holotype.—Measurements inmillimeters. Small and compact male in repro-ductive condition, SVL 14; snout moderatelypointed; femur length 7; tibia length 7.8; footlength including longest toe 10; head width 4;interorbital-distance 2.2; distance from eye tosnout tip 3; nostrils closer to snout tip than toanterior corner of eyes; canthus rostralis dis-tinct; loreal region slightly concave; small butdistinct tympanum; with the exception of twolarger dorsal warts in shoulder region, skin of
564 M.-O. RODEL AND R. ERNST
FIG. 3. Hands and feet of Phrynobatrachus phyllo-philus sp. nov. (A; SMNS 9721.1, male holotype), Phry-nobatrachus guineensis (B; CR guineensis, male), P. phyl-lophilus (C; SMNS 9721.2, female paratype), and P. gui-neensis (D; CR guineensis, female).
FIG. 4. Ventral side of throat and belly and dorsalaspect of femur and tibia in Phrynobatrachus phyllophi-lus sp. nov. (A and C, SMNS 9721.1, male, holotype;B, SMNS 9721.2, female, paratype), and Phrynobatra-chus guineensis (D and F, CR guineensis 1, male; (E, CRguineensis 2, female).
back and ventrum smooth; throat with severalfolds that run almost parallel to lower jaw (Fig.2); relative finger length: 1 5 2 , 3 . 4; relativetoe length: 1 , 2 , 3 , 4 . 5; thumb not swol-len, with distinct subarticular tubercle (Fig. 3A);finger and toe tips enlarged to discs, clearly sur-passing width of subarticular tubercles; fingerswithout webbing; toes moderately webbed, aweb hem reaching discs, webbing formula: 1 (0),2 i/e (1-0), 3 i/e (2-1), 4 i/e (1), 5 (1) (Fig. 3C);tarsal tubercle present; inner and outer metatar-sal tubercle present; small but distinct femoralglands (Fig. 4A).
Color in Life.—Back and head dark brown;dorsal warts bordered black; black lateral bandsstretch caudoventrad from behind the tympa-num to belly and vanish before reaching thehips; above these bands, in front of hips a largeyellow spot on flanks; a large red spot layswithin this yellow area; one black spot anteriorto hips; femur and tibia show one black trans-verse bar each; anal region bordered by a blacktriangle; a white line stretching from this tri-
angle to outer parts of femur; foot and toes withblack transverse bars; toe and finger tips gray;upper and lower jaw with black and white bars;throat black; pectoral region and belly white; afew small black spots laterally to pectoral re-gion; ventral parts of femur and tibia dark gray;knee and border of tibia with black points.
Color in Preservation.—Almost as in life; throatdark brown; yellow and red spots on flanks fad-ed; ventral parts of femur and tibia almostwhite.
Variation.—SVL in adults ranges from 12 to 17mm in males, and 15–23 mm in females; femalesare significantly larger than males (Mann-Whit-ney U-test, Z 5 29.673, P 5 0.001, N 5 131);further measurements are summarized in Ta-bles 1 and 2; back with at least two larger warts;the rest of the dorsal skin may be smooth, gran-ular or warty; roughness of the skin changeswithin individuals; reproductive males normal-ly have smooth skin; throats of nonreproductivemales may be almost white with a few blackspots; females have white to gray throats withirregular dark spots and patches that occasion-ally may nearly cover the whole throat; a fewsmall black spots may be present in pectoral re-gion; especially in females two dark spots arealmost always present in this region (Fig. 4B);
565A NEW PHRYNOBATRACHUS FROM WEST AFRICA
TABLE 1. Comparison of morphological features (x 6 SD, range) in Phrynobatrachus phyllophilus sp. nov. andPhrynobatrachus guineensis; f 5 female, m 5 male, for other abbreviations see material and methods section;compare Table 2.
P. guineensisf (N 5 5)
P. guineensism (N 5 24)
P. phyllophilusf (N 5 25)
P. phyllophilusm (N 5 18)
SVLIOESFETITA
17.5 6 1.9, 15.7–19.82.3 6 0.4, 1.8–2.82.9 6 0.5, 2.3–3.69.3 6 1.3, 8.1–10.9
10.0 6 1.4, 8.5–12.012.9 6 1.5, 11.2–14.5
16.0 6 0.5, 15.1–17.11.7 6 0.2, 1.1–2.12.3 6 0.2, 2.0–2.78.4 6 0.4, 7.4–9.28.9 6 0.5, 7.5–9.8
11.8 6 0.5, 10.7–12.6
19.4 6 1.6, 16.0–22.23.2 6 0.4, 2.4–4.03.0 6 0.3, 2.4–3.99.4 6 0.8, 7.9–11.6
11.1 6 0.8, 10.0–12.814.8 6 1.1, 13.0–18.1
14.4 6 0.8, 12.7–15.72.4 6 0.3, 2.0–3.02.3 6 0.3, 2.0–3.07.4 6 0.4, 6.5–8.08.3 6 0.8, 7.0–9.9
11.0 6 0.8, 10.0–12.7
TABLE 2. SVL of Phrynobatrachus species living sympatric with Phrynobatrachus phyllophilus sp. nov.; data fromliving adult frogs, measured in Taı National Park, Ivory Coast, in 1999 and 2000; in parentheses, we give valuesof museum specimens that exceeded our field data or were not present in these; * 5 probably synonyms, butas the types from Phrynobatrachus alticola Guibe and Lamotte, 1961 seem to be lost (A. Ohler, pers. comm.) thiscould not be clarified within this paper; degree after Guibe and Lamotte (1963).
Species m Mean SD Range N f Mean SD Range N
phyllophilusguineensisalticola*tokba*gutturosusfraterculusannulatusvilliersialleniliberiensisplicatus
mmmmmmmmmmm
14.315.613.7—
18.517.9—
11.418.424.235.1
2.01.91.2—1.31.0—0.81.71.93.0
12.0–17.012.0–17.0
12.0–15.0 (18.0)(13.5–15.0)
16.0–20.0 (15.0)17.1–19.020.5–24.0
9.0–14.512.0–21.519.0–33.020.5–41.5
701064742
1743396959
fff—fffffff
19.418.616.5—
19.8——
13.923.530.832.1
1.51.41.4—2.5——1.22.33.73.9
15.0–23.016.0–20.1 (22.0)15.0–18.0 (20.5)
—18.0–23.0
(25)824.4
10.0–16.013.0–27.021.0–35.527.0–38.0
6165
5—
11112446513
dorsal color ranges from beige to dark brown;black lateral bands are always present; clearspots on flanks are normally lacking (Fig. 1A);when present, they may be white, yellow, yellowwith a red central part, or red with a fine or-ange border; these clear patches may be restrict-ed to the hip area or stretch from behind theeyes to the femur (Fig. 1B); rarely a broad or-ange to red transverse band may cross the back;almost all specimens examined have only onedark transverse bar on femur and tibia, rarely asecond or even third, but very faint black trans-versal line may be present; dark transversal barsmay be present on arms and fingers; outermetatarsal tubercle may be indistinct; femoralglands were not visible in males that have beenpreserved in formaldehyde before being trans-ferred to ethanol.
Vocalization.—The advertisement call of P.phyllophilus sp. nov. comprises a series of differ-ent brief clicks with a metallic quality. It alwaysstarts with groups of 1–3 short and one longernote (Fig. 5A). The short ones last 0.04 sec andcomprise 6–7 pulses. The longer note lasts 0.21–0.32 sec and comprises 21–31 pulses (N 5 4males). This group (0.44–0.75 sec) of notes is re-
peated for up to 8 sec. This introductory se-quence is followed by a long lasting (8–10 sec)sequence of equally built up notes with a meanduration of 0.05 sec (6 0.01, 0.04–0.06, N 5 4)each (Fig. 5B). These notes comprise 5–10 pulses(7.9 6 1.6, N 5 4). The notes are separated bypauses of 0.10–0.13 sec (0.11 6 0.01, N 5 4). Thefinal part lasted 0.17 sec, comprising about 20pulses (Fig. 5B). Frequency range of the wholecall was between 3500 and 6083 Hz. Maximumfrequency intensity was between 4500 and 5000Hz. Schiøtz (1964c) described and figured callswith a lower frequency than ours, from rain for-ests in Sierra Leone. He assigned these calls toP. guineensis. However, his recordings are struc-turally identical to our recordings of P. phyllo-philus and therewith different from the call of P.guineensis (Fig. 6C). The call of P. guineensissounds similar to the human ear but is struc-turally different, shorter and much less conspic-uous in the forest. Phrynobatrachus guineensismales always call within or near water filledcavities. Acoustic properties of P. phyllophilus andP. guineensis calls are summarized in Table 3.
Tadpole Description.—Body ovoid in dorsalview (Fig. 6A); in lateral view body oval to
566 M.-O. RODEL AND R. ERNST
FIG. 5. Waveform (upper) and sonogram (lower) ofadvertisement calls in Phrynobatrachus phyllophilus sp.nov. (A–B) and Phrynobatrachus guineensis (C) from TaıNational Park, Ivory Coast; A 5 initial phase; B 5 endof middle part and final sequence; compare text andTable 3.
TABLE 3. Acoustic properties (x 6 SD, range) of advertisement calls of Phrynobatrachus phyllophilus sp. nov.and Phrynobatrachus guineensis. Given are mean 6 standard deviation, range and sample size; * 5 in the presenceof a female; ** 5 further harmonics at 4500–5000 Hz and 5700–6200 Hz are visible in other recordings.
Parameter phyllophilus sp. nov. guineensis guineensis*
Note length (sec)
Pauses between notes (sec)
Call duration (sec)
Notes per callLower frequency (Hz)Upper frequency (Hz)
0.05 6 0.01,0.35–0.60, N 5 4
0.11 6 0.01,0.10–1.13, N 5 4
ø 20
. 203,5006,083
0.02 6 0.01,0.01–0.04, N 5 4
0.13 6 0.02,0.10–0.20, N 5 4
0.93 6 0.18,0.74–1.15, N 5 4
6–10, N 5 42,2504,500**
0.01 6 0.002,0.01–0.02, N 5 2
0.04 6 0.02,0.01–0.08, N 5 21.6–2.0, N 5 2
38–40, N 5 22,8006,200
slightly compressed (Fig. 6B); body length about1.7 times body width (measured at the plane ofthe eyes); body length 0.5 tail length; eyes dor-solaterally; nostrils laterally, hard to see fromdorsal view, closer to snout tip than to anteriorcorner of the eyes; tail straight, if extrapolated,axis of tail passes through eyes; tail moderatelywebbed, fin height 1.1 times body height; dorsalfin originates at dorsal tail-body junction; dorsalfin slightly curved, ventral fin nearly parallel totail axis; tail tip pointed; small oral disc anter-oventral, bordered by single row of papillaewith rostral gap, caudal with long filamentouspapillae; a few additional papillae are groupedin oral angles; jaw sheaths thin and evenlybroad U-shaped; labial tooth row formula 1:111/3, third tooth row in lower lip very short(Fig. 6C); spiracle sinistral, visible dorsally; ventopens medially, positioned basicaudally.
In life, tadpoles are uniform brown, the finhyaline with a very few small dots. The ven-trum is slightly lighter. In preservation, areasaround eyes and tip of the snout become lighterin color. Two tadpoles were preserved five daysafter hatching. At that time, they had reachedstage 33–34. They measured 11.0 and 11.3 mmtotal length. Body length was 3.9 and 4.0 mm,respectively. Another eight tadpoles were pre-served 18 days after hatching. They were instage 37–41, the later ones ready to metamor-phose. The largest tadpole (stage 41) measured4.7 mm body length and 13.0 mm total length.SVL of metamorphosed froglets is about 5 mm.
Natural History.—All calling males were con-cealed beneath leaves in close vicinity to minutepuddles on the forest floor. None of these breed-ing sites exceeded a surface area of 1 m2. Waterdepth rarely exceeded a few centimeters. Nor-mally one male initiated calling and other malesimmediately joined the chorus. On 25 Septem-ber 1999, we heard a chorus in a swampy area.While recording the call of the holotype, it suc-ceeded in attracting a female (SMNS 9721.2,paratype). The amplectant frogs were trans-
567A NEW PHRYNOBATRACHUS FROM WEST AFRICA
FIG. 6. Tadpoles of Phrynobatrachus phyllophilus sp. nov. (A-C; SMNS 9745; stage 34) and Phrynobatrachusguineensis (D-F; CR guineensis; stage 34) in dorsal (A, D) and lateral view (B, E); oral disc (C, F; keratodontssketched, no accurate count); scale bar 5 1 mm.
ferred to a terrarium. They deposited a clutchof 41 large eggs, rich in yolk, in the dry part ofthe terrarium on a dead leaf, the following day.This clutch did not develop. On 5 June 2000, wecollected two amplectant pairs (A and B) nextto small puddles. Both pairs were transferred toseparate terraria with both aquatic and dryparts. Both pairs oviposited the same day. PairA attached a clutch of 24 eggs (1.2 mm diameter,jelly layer very thin) on a vertical leaf in the drypart of the terrarium. Pair B deposited 26 eggs(1.2 mm diameter, with jelly 4 mm) on humidgravel close to water. On 7 June, we partly flood-ed clutch B. Clutch A was moistened. On 9 June,larval development was only visible in clutch A.On 13 June, larvae of clutch A were ready tohatch, whereas in clutch B the eggs had died. Afourth clutch, comprising 34 eggs was attachedto a leaf outside water, close to a small puddle.Embryonic development lasted 9–10 days, larvaldevelopment until metamorphosis lasted aboutthree weeks.
Between January 1999 and October 2000, werecorded 378 P. phyllophilus on our transects inTNP. During 93 transect hours, 13 specimenswere found in secondary habitats (0.1 frog/h).In primary forest, we detected 1.3 frogs perhour (365 frogs in 289 transect hours). We reg-istered most of these frogs (294) in swampy ar-eas of the forest. The preferred habitat com-
prised a sparse understorey and canopy, and anearly closed tree-storey. Leaf litter coverage atthese sites was about 80%. We recorded 164 call-ing males. Only 11 males were heard between1900 and 2200 h. All others called during day-time. About half of the calling males were re-corded in June (81). The remaining calling activ-ity was nearly equally distributed from May toNovember. During the rainy season, the major-ity of the population was made up by adults.During the end of the dry season, most speci-mens were juveniles. During that time, P. phyl-lophilus congregates around small puddles insmall, dried up river beds. At MPNP, we foundP. phyllophilus during a very dry period in a gal-lery forest close to a small river. The surround-ings mainly consisted of secondary rain forestand cacao plantations.
Distribution.—Phrynobatrachus phyllophilus hasbeen recorded from the Western part of the Up-per Guinean rain forest. Voucher specimens re-ferable to this species originated from forests inSierra Leone, Guinea, Liberia, and South-West-ern Ivory Coast (see type series and Appendix1 for exact localities). As forests in Eastern IvoryCoast and Western Ghana have not been the tar-get of intensive herpetological investigations, P.phyllophilus may occur there as well. Accordingto published descriptions, a record from La-
568 M.-O. RODEL AND R. ERNST
motte (1967) in Lamto, Central Ivory Coastprobably refers to P. guineensis.
Etymology.—The name is composed of theGreek words for leaves (phyllon) and loving(philein). It was chosen to point on the closeassociation of this species with leaf litter. Malescalled exclusively while sitting underneathleaves and clutches were most often attached toleaves.
DIFFERENTIAL DIAGNOSIS AND DISCUSSION
The genus Phrynobatrachus is one of the mostdiverse African anuran genera. Currently about66 species, occurring exclusively in Sub-SaharanAfrica, are recognized (Poynton, 1999), althoughthe genus is in urgent need of thorough revision(Rodel, 2000). Description of new species there-fore should be made only with extreme caution.In this paper, we were able to show that P. phyl-lophilus sp. nov. is unique, compared with allother West African Phrynobatrachus species, withrespect to its advertisement call, its develop-mental mode, and a specific combination ofmorphological and coloration characters.
Phrynobatrachus phyllophilus is most similar toP. guineensis. Separation from the later specieswas difficult because the descriptions of Guibeand Lamotte (1961, 1963) comprise a mixture ofcharacters of both species. In Lamotte’s collec-tion, both species are represented. Taxonomieanalysis is also complicated by the fact that thetype series of P. guineensis appears lost (A. Ohl-er, pers. comm.). Fortunately, Guibe and La-motte (1961, 1963) published excellent drawingsof the female holotype of P. guineensis and citeseveral characters that are absent in P. phyllophi-lus. This especially concerns the pattern of thebelly, the shape of the thumb in reproductivemales, and a special variation in dorsal colorpattern. The belly of male and female P. guineen-sis always shows large black spots. In P. phyllo-philus, no more than a few black points may bepresent in this region. The thumb of reproduc-tive males in P. guineensis is extremely swollen(Fig. 3B); no subarticular tubercle is visible. Inthat state, thumbs in male P. phyllophilus are notswollen, but a subarticular tubercle is clearlydiscernible (Fig. 3A). Guibe and Lamotte (1961,1963) cite a specimen with a clear vertebral linethat continues on the femur, tibia, and metatar-sus. We never observed such a color pattern inP. phyllophilus; instead this is a rather commoncolor morph in P. guineensis (see Bohme, 1994;unpubl.). Species affiliation therewith was as-sured. We refrain from designating a neotypefor P. guineensis, because we have no specimensfrom the original type locality, Mont Tonkoui,Ivory Coast, available. All P. guineensis have yel-low hips and orange toe and finger tips, char-acters that are always absent in P. phyllophilus.
Rarely individuals from other Phrynobatrachusspecies may show only one dark bar on femurand/or tibia (e.g., P. alleni, P. guineensis; pers.obs.). Yellow or red spots on the flanks may bealso present in P. guineensis (compare figures inSchiøtz, 1964b; Rodel, 1998).
Differentiation of P. phyllophilus from otherspecies is much easier. Phrynobatrachus phyllophi-lus differs from P. alleni, P. liberiensis, P. natalensis,and P. plicatus by its smaller size. Phrynobatrachusalleni and P. plicatus differ from all other WestAfrican Phrynobatrachus by their dorsal ridges,in the shape of an X on their backs. Phrynoba-trachus phyllophilus differs from P. alticola, P. tokba,P. gutturosus, P. fraterculus, and P. calcaratus by itsmore developed webbing. Phrynobatrachus phyl-lophilus differs from P. latifrons, P. accraensis, P.francisci, P. natalensis, P. alleni, and P. plicatus byits less developed webbing. P. phyllophilus differsfrom P. calcaratus, P. villiersi, P. taiensis, and P. an-nulatus by the lack of an eyelid tubercle. Phry-nobatrachus phyllophilus males differ from male P.alleni, P. accraensis, and P. latifrons by the yellowthroat of the later three species. Phrynobatrachusphyllophilus differs from P. ghanensis, P. villiersi, P.annulatus, P. fraterculus, and P. guineensis by thedifferent ventral coloration (compare figures inGuibe and Lamotte, 1963; Schiøtz, 1964a; Perret,1988).
Advertisement call descriptions for West Af-rican Phrynobatrachus have been published for P.accraensis, P. alticola, P. alleni, P. latifrons, P. fran-cisci, P. fraterculus, P. gutturosus, P. natalensis, P.liberiensis, and P. plicatus (Schiøtz, 1964c; Rodel,2000). The most obvious differences between theadvertisement calls of P. phyllophilus and P. gui-neensis are the structure of the call and thelength of single notes. The latter are alwaysmuch shorter in P. guineensis (Table 3; Kruskal-Wallis test, x2 5 30.14, df 5 1, P , 0.001). Phry-nobatrachus phyllophilus calls have a more com-plex structure, comprising different initial, mid-dle and final sequences, whereas the whole callof P. guineensis is of equal structure (Fig. 5A–C).However, the call of P. guineensis is apparentlyaltered in presence of a female, it is longer withmore, shorter notes that are separated by muchshorter pauses (Table 3).
Phrynobatrachus phyllophilus and P. guineensiswere sympatric but only rarely lived in syntopy.Although we recorded P. guineensis nearly al-ways in drier parts of the forest, P. phyllophilusclearly preferred swampy habitats. Phrynobatra-chus phyllophilus shared this habitat with P. alleni,P. plicatus, P. gutturosus, P. liberiensis, and P. vil-liersi. But it was only P. villiersi that regularly,and P. alleni that occasionally, made use of thesame breeding sites: small puddles on the forestfloor.
Despite the fact that members of the genus
569A NEW PHRYNOBATRACHUS FROM WEST AFRICA
Phrynobatrachus live in habitats ranging fromdry savannas to primary rain forests and a widerange of altitudes, their known reproductivepatterns are astonishingly uniform (Altig andMcDiarmid, 1999). With the exception of P. gui-neensis, that deposits its few large eggs abovesmall water-filled cavities (Rodel, 1998), and P.alticola, that oviposits on leaves on the forestfloor (Rodel and Ernst, 2002), all other West Af-rican Phrynobatrachus species, oviposit clutchesof several hundred to a few thousand smalleggs that float in a single layer on the surfaceof stagnant or slow running water (Stewart,1967; Wager, 1986; Rodel, 2000). All but one tad-poles described, P. alticola (Rodel and Ernst,2002), are exotrophic, lentic, and benthic (Altigand McDiarmid, 1999; Rodel, 2000). Reproduc-tive modes of West African Phrynobatrachus areknown from P. accraensis, P. alleni, P. alticola, P.calcaratus, P. francisci, P. guineensis, P. latifrons, P.natalensis, P. plicatus, and P. villiersi (Lamotte andDzieduszycka, 1958; Barbault and Trefaut Ro-drigues, 1979; Barbault and Pilorge, 1980; Rodel,1998, 2000; Rodel and Ernst, 2002; unpubl.).Phrynobatrachus phyllophilus differs from all thesespecies by terrestrially ovipositing small clutch-es, of large eggs rich in yolk, on leaves close towater. The tadpoles are exotrophic. They differfrom tadpoles of P. guineensis by the labial toothrow formula, the presence of filamentous papil-lae on the lower lip, a shorter tail, a lower dorsalfin, and a fin that originates more distad (Fig.6A–F; Rodel, 1998).
The validity of P. phyllophilus was revealed af-ter intensive fieldwork, shedding light on callvariation and reproductive behavior. In additionto this species, we have good evidence for ad-ditional, hitherto overlooked species, alreadyrepresented in museum collections. In 1983, La-motte stated that there is probably no place inWest Africa where amphibian diversity exceeds40 species. For the TNP, to date we have record-ed 56 amphibian species (unpubl. data), and ad-ditional species are likely still undiscovered. Anincreased effort of herpetological fieldwork inthe Upper Guinean forest would be timely, es-pecially given high rates of deforestation (Chat-elain et al., 1996).
Acknowledgments.—MOR was supported by apostdoctoral scholarship from the German Aca-demic Exchange Service (DAAD). Analyzing andpublication of the data was part of the BIOLOG-program of the German Ministry of Educationand Science (BMBF; Project W08 BIOTA-West, 01LC0017). The following colleagues helped insending specimens from their collections: A. Du-bois and A. Ohler (MNHN), J. B. Rasmussen(ZMUC), and A. Schluter (SMNS). M. Lamottemade possible access to his collection. A. Schluter
assisted in sound analyzing. TROPENBOS—Coted’Ivoire helped with transportation and variousadministrative services. Lodging facilities in TNPwere provided by the ‘‘Centre de Recherche enEcologie’’ and the ‘‘Projet Autonome pour laConservation du Parc National de Taı.’’ Workingin MPNP and TNP was kindly permitted by therespective park directors, the Commandants A.Dje Bi Ta and K. N’Dri. The ‘‘Projet Autonomepour la Conservation du Parc National de Taı,’’the ‘‘Tai Monkey Project,’’ and BirdLife Inter-national provided logistic support. Researchpermission was given by the ‘‘Ministere del’Enseignement Superieur et de la RechercheScientifique,’’ of the Republic of Ivory Coast.The access permit to MPNP and TNP was is-sued by the ‘‘Ministere de la Construction etde l’Environnement.’’ G. G. Gbamlin, C. Y.Ouoro, and R. Jamba were from invaluable helpduring fieldwork. These supports are grateful-ly acknowledged.
LITERATURE CITED
ALTIG, R., AND G. F. JOHNSTON. 1989. Guilds of an-uran larvae: relationships among developmentalmodes, morphologies and habitats. HerpetologicalMonographs 3:81–109.
ALTIG, R., AND R. W. MCDIARMID. 1999. Diversity:familial and generic characterizations. In R. W.McDiarmid and R. Altig (eds.), Tadpoles: The Bi-ology of Anuran Larvae, pp. 295–337. Universityof Chicago Press, Chicago.
BARBAULT, R., AND T. PILORGE. 1980. Observations surla reproduction et la dynamique des populationsde quelques anoures tropicaux V. Phrynobatrachuscalcaratus. Acta Oecologica 1980:373–382.
BARBAULT, R., AND M. TREFAUT RODRIGUES. 1979. Ob-servations sur la reproduction et la dynamique despopulations de quelques anoures tropicaux IV.Phrynobatrachus accraensis. Bulletin de l’Institut fon-damental d’Afrique noire Serie A 41:417–428.
BOHME, W. 1994. Frosche und Skinke aus dem Re-genwaldgebiet Sudost-Guineas, Westafrika. II.Ranidae, Hyperoliidae, Scincidae; faunistisch-oko-logische Bewertung. Herpetofauna 16:6–16.
CHATELAIN, C., L. GAUTIER, AND R. SPICHIGER. 1996.A recent history of forest fragmentation in south-western Ivory Coast. Biodiversity and Conserva-tion 5:37–53.
DUBOIS, A. 1995. Keratodont formula in anuran tad-poles: proposals for a standardization. Journal ofZoological Systematic and Evolutionary Research33:I–XV.
GOSNER, K. L. 1960. A simplified table for staginganuran embryos and larvae with notes on identi-fication. Herpetologica 16:183–190.
GUIBE, J., AND M. LAMOTTE. 1961. Deux especes nou-velles de batraciens de l’Ouest Africain apparten-ant au genre Phrynobatrachus: Ph. guineensis n. sp.et Ph. alticola n. sp. Bulletin du Museum Nationald’Histoire Naturelle 2e Serie 33:571–576.
. 1963. La reserve naturelle integrale du MontNimba. XXVIII. Batraciens du genre Phrynobatra-
570 M.-O. RODEL AND R. ERNST
chus. Memoires de l’Institut fondamental d’Afriquenoire 66:601–627.
GUILLAUMET, J.-L. 1967. Recherches sur la vegetationet la flore de la region du Bas-Cavally (Coted’Ivoire). Memoires Office de la Recherche Scien-tifique et Technique Outre-Mer 20:1–247.
LAMOTTE, M. 1966. Types de repartition geograp-hique de quelques batraciens dans l’Ouest Africain.Bulletin de l’Institut fondamental d’Afrique noireSerie A 28:1140–1148.
. 1967. Les batraciens de la region de Gpakobo(Cote d’Ivoire). Bulletin de l’Institut fondamentald’Afrique noire Serie A 29:218–294.
. 1971. Le Massif des Monts Loma (Sierra Le-one), Fasciule I; XIX. Amphibiens. Memoires del’Institut fondamental d’Afrique noire 86:397–407.
. 1983. Amphibians in savanna ecosystems. InF. Bourliere (ed.), Ecosystems of the World 13,Tropical Savannas, pp. 313–323. Elsevier ScientificPublishing Co., Amsterdam, The Netherlands.
LAMOTTE, M., AND S. DZIEDUSZYCKA. 1958. Contri-bution a l’etude des batraciens de l’Ouest Africain,VII. Le developpement larvaire de Phrynobatrachusfrancisci. Bulletin de l’Institut fondamentald’Afrique noire Serie A 20:1071–1086.
PARREN, M. P. E., AND N. R. DE GRAAF. 1995. Thequest for natural forest management in Ghana,Cote d’Ivoire and Liberia. The Tropenbos Foun-dation (Tropenbos series No. 13), Wageningen, TheNetherlands.
PERRET, J.-L. 1988. Les especes de Phrynobatrachus(Anura, Ranidae) a eperon palpebral. Archives dessciences 41:275–294.
POYNTON, J. C. 1999. Distribution of amphibians inSub-Saharan Africa, Madagascar, and Seychelles.In W. E. Duellman (ed.), Patterns of Distribution ofAmphibians, a Global Perspective, pp. 483–539.John Hopkins University Press, Baltimore, MD.
RIEZEBOS, E. P., A. P. VOOREN, AND J. L. GUILLAUMET.1994. Le Parc National de Taı, Cote d’Ivoire. Tro-penbos Series 8, Wageningen, The Netherlands.
RODEL, M.-O. 1998. A reproductive mode so far un-known in African ranids: Phrynobatrachus guineen-sis Guibe and Lamotte, 1961 breeds in tree holes(Anura: Ranidae). Herpetozoa 11:19–26.
. 2000. Herpetofauna of West Africa. Vol. I.Amphibians of the West African savanna. EditionChimaira, Frankfurt/M., Germany.
RODEL, M.-O., AND R. ERNST. 2002. A new reproduc-tive mode for the genus Phrynobatrachus: Phryno-batrachus alticola has nonfeeding, nonhatching tad-poles. Journal of Herpetology 36:121–125.
RODEL, M.-O., G. SCHORR, AND R. ERNST. 2001. ZurBiologie von Cardioglossa leucomystax (Boulenger,1903), im Taı-Nationalpark, Elfenbeinkuste. Sala-mandra 37:239–260.
SCHIØTZ, A. 1964a. A preliminary list of amphibianscollected in Ghana. Videnskabelige Meddelelserfra Dansk Naturhistorisk Forening 127:1–17.
. 1964b. A preliminary list of amphibians col-lected in Sierra Leone. Videnskabelige Meddelelserfra Dansk Naturhistorisk Forening 127:19–33.
. 1964c. The voices of some West African am-phibians. Videnskabelige Meddelelser fra DanskNaturhistorisk Forening 127:35–83.
STEWART, M. M. 1967. The amphibians of Malawi.State University Press, New York.
WAGER, V. A. 1986. Frogs of South Africa, their fas-cinating life stories. Delta, Craighall, South Africa.
VAN DIJK, D. E. 1966. Systematic and field key to thefamilies, genera and described species of SouthernAfrican anuran tadpoles. Annals Natal Museum18:231–286
Accepted: 16 January 2002.
APPENDIX 1
Additional Specimens Examined
Phrynobatrachus accraensis.—Liberia: MNHN 22specimens without number, Grassfield, Nimba coun-ty; MNHN 28 specimens without number, Yuelliton;Guinea: MNHN 1998.1540, 45, 46, 50, 51, Bord de Ca-vally, Mt. Nimba; Ivory Coast: SMNS 9719.1–7, Gui-routou, Taı National Park; SMNS 9720, same locality,1996; SMNS 9753, SRET, Taı National Park.
Phrynobatrachus alleni.—Liberia: MNHN 1998.1597–98, Grassfield, Nimba County; MNHN 1998.1190, Mt.Nimba, Aguesse; MNHN 1998.2501–10, 2333–2400,Mt. Nimba; MNHN 1998.2625, Mt. Nimba, Grassfield;MNHN 1998.2401–2500, Yaa River, Nimba County;Ivory Coast: MNHN 1998.1595–96, Tabou; SMNS9727.1–50, Guiroutou, Taı National Park; SMNS9725.1–2, 9726.1–2, 9728.1–2, SRET transects, Taı Na-tional Park.
Phrynobatrachus alticola (possibly synonymous withP. tokba).—Liberia: MNHN 1998.1197, 1998.1522, 24,27, 90–94, 1998.2522–2524, 27, 1998–2664, 69, 70, 1998–2676, 1993–5508, Grassfield, Nimba county; MNHN1998–2643, 45–47, Mt. Alpha, Nimba; MNHN 50 spec-imens without number, New Mine Road (Seka valleyforest), Mt. Nimba; MNHN 1998.2618–20, 24, Mt.Nimba; MNHN 1998.1533, old Bapa ouest; Guinea:MNHN 1997.0799, 1590–9, Mt. Nimba; MNHN1995.882, 1590–05, A16, Route de Tonkoui; IvoryCoast: MNHN 1997.0800, 1590–10, Tonkoui; SMNS,9732, Guiroutou, Taı National Park; SMNS 9733.1–19,SRET transects, Taı National Park; SMNS 9730.1–3,same locality; SMNS, 9729.1–4, same locality; SMNS9731.1–2, same locality.
Phrynobatrachus annulatus.—Liberia: MNHN 1998.1552–53, Yuelliton Road, Nimba county; Ivory Coast:SMNS 9735, SRET transects, Taı National Park; SMNS9734, same locality.
Phrynobatrachus calcaratus.—Ivory Coast: MNHN1998.1582–84, Lamto; MNHN 1998.1559–60, 1998.2529, Tabou; SMNS 9736.1–4, 9737, Guiroutou, Taı Na-tional Park; SMNS 8958.1–7, South-Western ComoeNational Park; SMNS 8961.1–14, Ananda.
Phrynobatrachus francisci.—Ivory Coast: MNHN1998.1585–92, Lamto; SMNS 9742, 8959.1–32, SMF78642–45, South-Western Comoe National Park.
Phrynobatrachus fraterculus.—Sierra Leone: ZMUCR074944, R075020, 3 ml. S. of Joru; ZMUC R075825–29, Gola F.R.; Liberia: MNHN 1998.1593–94, Mt. To-cadeh; MNHN 1998.1193–94, New-camp road side,South-East Nimba, Nimba County; MNHN 1998.1520,Mt. Nimba, Grassfield; Guinea: MNHN 1921.153–57(syntypes), Macenta; Ivory Coast: SMNS 9744.1–2,SRET transects, Taı National Park; SMNS 9743, samelocality; MNHN 1958.348, MNHN A 360, Nimba Zie-la.
571A NEW PHRYNOBATRACHUS FROM WEST AFRICA
Phrynobatrachus guineensis.—Sierra Leone: ZMUCR075277, 93, 306, Mts Loma, forest; ZMUC R075840,South Kambui F.R.; Guinea: ZFMK 56335, foret deZiama, Bnaemn riviere de Malweta, South of Seredou;Liberia: MNHN 1993.4259–65, 1998.1196, 99–1200,1998.1576–79, 1998.2573–80, 2656, 2662, 65–68, 73, 94,2627, Grassfield, forest, Nimba county; MNHN1998.1537–42, Mt. Yuelliton, Nimba county; MNHN1993.4244–58, 1998.2622, Mt. Nimba; MNHN1993.5505–06, New Camp, Nimba County; IvoryCoast: MNHN 1993.4266–68, l‘Orumbo Boka (Lamto);SMNS 9745.1–16, 9746.1–2, 9747.1–3, 9748, Taı Nation-al Park, SRET station; CR guineensis 1–12, Guiroutou,Taı National Park.
Phrynobatrachus gutturosus.—Liberia: MNHN 1998.1501–12, Grassfield, Nimba county; MNHN 1998.2581–2617, Mt. Nimba; MNHN 1921.280, 280 A, 280D, 280 F, 280 H, 281 C, 282, 282 E, 282 F, 282 I, (syn-types), Sanikole; Ivory Coast: MNHN 1998.1561–70,Lamto; SMNS 9738, 9751.1–6, Guiroutou, Taı NationalPark; SMNS 9749.1–2, SRET station, Taı National Park;SMNS, 9739.1–2, same locality; SMNS 9752, South-Western Comoe National Park; SMNS 9750, Westernpark, Mt. Peko National Park.
Phrynobatrachus latifrons.—Ivory Coast: SMNS8957.1–48, South-Western Comoe National Park;SMNS 8990.1–2, Ananda.
Phrynobatrachus liberiensis.—Liberia: MNHN 1998.1571–73, 1998.1599–600, 1998.1797, 99, 1998.2630,1998.2671–72 1 4 specimens without number, Grass-field, Nimba county; MNHN 1998.2517, 19–20, Mt.Nimba, Aguesse; MNHN 1998.2621, Mt. Nimba;
MNHN 1998.2543, 44, 46, New camp foret; IvoryCoast: MNHN 26 specimens without number, Taı;SMNS 9758, Guiroutou, Taı National Park; SMNS9755, Mt. Nienokoue, rocky river at base of mountain,Taı National Park; SMNS 9756, near mountain, Mt.Peko National Park; SMNS 9754, Noma, Mt. SangbeNational Park; SMNS 9718, 9757, SRET station, Taı Na-tional Park.
Phrynobatrachus natalensis.—Sierra Leone: MNHN1998.1795–96, Sokurela; Ivory Coast: MNHN, 10 spec-imens without number, Dampleu; SMNS 9759.1–10,Southern park, Comoe National Park; SMNS 9760,Western park, near village, Mt. Sangbe National Park;SMNS 8960.1–3, SMF 78634, 78636, South-WesternComoe National Park.
Phrynobatrachus phyllophilus.—Type series and Libe-ria: MNHN 1998.1554–58, Mt. Tocadeh; MNHN1989.4743–44, North Grassfield, Nimba County; SierraLeone: ZMUC R075280, 86, 95, 97, 98, 303, Mts. Loma.
Phrynobatrachus plicatus.—Liberia: MNHN 1998.2518, Mt. Nimba, Aguesse; MNHN 176.180–84, Mt.Nimba, Grassfield; MNHN 1999.2511, Nimba expe-dition, Konia, Lota county; Ivory Coast: MNHN1998.1181–92, Lamto, Bandama; MNHN 26 specimenswithout number, Taı; SMNS, 9761.1–2, 9763.1–2,9764.1–3, 9765, Guiroutou, Taı National Park; SMNS9762.1–3, SRET station, Taı National Park.
Phrynobatrachus tokba.—Guinea: MNHN 1921.144–152 (syntypes), N’Zebela and N’Zerekore.
Phrynobatrachus villiersi.–Ivory Coast: SMNS 9768.1–9, Guiroutou, Taı National Park; SMNS 9767.1–2,9769.1–2, SRET, transects, Taı National Park.
Journal of Herpetology, Vol. 36, No. 4, pp. 571–578, 2002Copyright 2002 Society for the Study of Amphibians and Reptiles
A New Species of Phyllomedusa Wagler, 1830 (Anura: Hylidae) fromCentral Brazil
REUBER ALBUQUERQUE BRANDAO
Departamento de Ecologia, Universidade de Brasılia. Brasılia-DF.70 910-900, Brazil; E-mail: [email protected]
ABSTRACT.—A new species of Phyllomedusa, related to Phyllomedusa megacephala, is described from thehigh plateaus of the state of Goias and Distrito Federal, Brazil. The new species is characterized by mediumsize, small finger pads, short and narrow head, thin body, vertical snout in profile, very granulate belly, chestwithout reticular pattern, transversal bars in the mandible, and flanks with reticular black, sepia, or purplepattern over yellow or orange background.
The genus Phyllomedusa Wagler, 1830, is dis-tributed from Costa Rica to Argentina and com-prises 30 species (De la Riva, 1999; Frost, 2000).It consists of five species groups (‘‘Phyllomedusaburmeisteri’’ group sensu B. Lutz, 1950; ‘‘Phyllo-medusa hypochondrialis’’ group sensu Boker-mann, 1965; ‘‘Phyllomedusa buckleyi’’ group sen-su Cannatella, 1980; ‘‘Phyllomedusa perinesos’’group sensu Cannatella, 1982; and ‘‘Phyllome-
dusa tarsius’’ group sensu De La Riva, 1999),even though some species are not currentlyaligned with a species group (Frost, 2000).
The ‘‘P. hypochondrialis’’ group contains spe-cies characterized by an abruptly ending snout,poorly developed finger pads, thumb equal orsmaller than the second finger and first toe larg-er than the second one (Bokermann, 1965).Based on larval characters, Cruz (1982) included
572 REUBER BRANDAO
in this group the following species: P. hypochon-drialis (Daudin, 1802); P. burmeisteri Boulenger,1882; Phyllomedusa rhodei Mertens, 1926; P. dis-tincta B. Lutz, 1950; Phyllomedusa centralis Bok-ermann, 1965; and Phyllomedusa ayeaye (B. Lutz,1966). Later, Pombal and Haddad (1992) real-located P. burmeisteri and P. distincta to the ‘‘P.burmeisteri’’ group (sensu Lutz 1950). Amongthese species, P. ayeaye, P. centralis, and P. mega-cephala (Miranda-Ribeiro, 1926; currently a syn-onymy of P. hypochondrialis, sensu Frost, 2000),are distinguished by a reticulated pattern on theflanks and hidden parts of the limbs (Boker-mann, 1965; B. Lutz, 1966; Miranda-Ribeiro,1926); a medial thickening on the upper part ofthe tadpole’s beak (Cruz, 1982); habitat use(open areas, mostly as ‘‘campo rupestre’’ androck-field cerrado on top of plateaus and moun-tain ranges; Cruz, 1982; Cardoso et al., 1989);and by their occurrence at high altitudes.
Phyllomedusa ayeaye is known from only onelocality: Morro do Ferro in the Serra da Manti-queira mountain range, near Pocos de Caldas,Minas Gerais State, Brazil (B. Lutz, 1966). It isconsidered an endangered species because of itsextremely restricted distribution and to the on-going destruction of its habitat (Nascimento,1998). The tadpole of P. ayeaye was described byCruz (1982). Phyllomedusa centralis is endemic tothe Chapada dos Guimaraes National Park re-gion, Mato Grosso State, Brazil (Bokermann,1965). Phyllomedusa megacephala was describedfrom a single individual, and the type localityremains unknown (Miranda-Ribeiro, 1926). Thisname has been attributed to populations of Phyl-lomedusa from Serra do Cipo, MG (Feio et. al., inpress) whose tadpoles were described by Cruz(1982) as P. centralis.
Herein, I describe a new species of Phyllome-dusa from the high plateus of Central Brazil, re-lated to P. megacephala. Further, I evaluate the va-lidity of the name P. megacephala for populationsfrom Serra do Cipo.
MATERIALS AND METHODS
Specimens used for description were depos-ited in the Colecao Herpetologica da Universi-dade de Brasılia (CHUNB) and in the MuseuNacional, Rio de Janeiro (MNJR), and thoseused for comparisons were at these institutionsand in the Museu de Zoologia da Universidadede Sao Paulo (MZUSP), the Werner BokermannCollection (WCAB, housed at the MZUSP), andthe Adolpho Lutz Collection (AL, housed atMNRJ; see Appendix 1). Paratypes were depos-ited in CHUNB and MNRJ.
Thirteen measurements were taken fromadults with a digital caliper (0.01mm): totallength; head, arm, hand, thigh, tibia, tarsus, andfoot length; head width and height; ocular di-
ameter; tympanum diameter and interorbitaldistance, following Heyer et. al., 1990.
Measurements taken from tadpoles and ter-minology used follow Altig (1970) and Johnsonand Altig (1986). The following measurementswere taken: total length; body and tail lengths;maximum body and tail heights; eye–nostrildistance and interorbital distance; maximumbody width and ocular diameter. Developmen-tal stages follow Gosner (1960). Tadpole illustra-tion was made with a drawing tube. Identifica-tion of tadpoles was based on a series of indi-viduals in different stages of development andin tadpoles kept in captivity until metamorpho-sis. Tadpoles are deposited in CHUNB withnumbers 17474 (one tadpole) and 24581 (six tad-poles).
Phyllomedusa oreades sp. nov.Figures 1–2
Holotype.—CHUNB 12510, adult male collect-ed in Serra da Mesa, Goias State, Brazil (138459S;488189W; 940 m), nearly 1 km after the first gateof the Serra da Mesa hydroelectric plant, on theroad that connects the plant to Minacu, beforethe Ava-Canoeiros Indian reserve, on 25 Febru-ary 1998. Collected by R. A. Brandao, G. J. Zer-bini, T. L. Abreu, M. Kanegae, and V. Braz.
Paratypes.—CHUNB 12511–12516 (six males),collected along with the holotype; CHUNB12517 (one female) collected at Vila de Sao Jorge,Chapada dos Veadeiros, Goias State, Brazil(148099S; 478529W; 1400 m) by T. L. Abreu andM. Kanegae on 1 April 1998; MNRJ 23679 (onemale), Reserva Ecologica do IBGE (RECOR),Brasılia, Distrito Federal, Brazil (158559S;478559W; 1000 m), by V. Dias, no date; MNRJ23680 (one female) collected in the ARIE doCorrego Capetinga, Fazenda Agua Limpa-Dis-trito Federal, Brazil (158579S; 478599W; 1100 m)by O. Pires-Jr in November 1997.
Diagnosis and Comparison with Other Species.—Phyllomedusa oreades is similar to P. ayeaye, P. cen-tralis, and P. megacephala and characterized bymedium size, small finger pads, short and nar-row head, vertical snout in lateral view, externaledge of the upper lip not completely visible dor-sally, lower lip bearing transverse stripes, venterhighly granular and lacking reticular marks;and body flanks bearing a black, sepia or darkpurple reticular drawing over a yellow or or-ange background.
Phyllomedusa oreades is readily distinguishedfrom P. ayeaye by less reticulated flanks and alarger tympanum; from P. megacephala by its ver-tical snout, short head, and by having the exter-nal edge of the upper lip only partially visibledorsally; from Phyllomedusa aff. megacephala ofSerra do Cipo (Minas Gerais State, Brazil) byhaving a vertical snout (obtuse in P. aff. mega-
573NEW PHYLLOMEDUSA FROM CENTRAL BRAZIL
FIG. 1. Holotype of Phyllomedusa oreades sp. n. (CHUNB 12150), Body (A), Head (B), Hand (C), and Foot (D).
574 REUBER BRANDAO
FIG. 2. Adult female (MNRJ 23680) of Phyllomedusa oreades (Photo: A. Sebben).
TABLE 1. Morphological measurements (millime-ters) of adult Phyllomedusa oreades sp. n. (N 5 10).
Mean 6 SD Range
Total lengthHead lengthHead widthHead heightEye diameter
36.48 6 2.5611.35 6 0.4912.21 6 0.86
8.59 6 0.784.22 6 0.40
33.01–42.6410.61–12.3811.00–13.85
7.48–9.613.29–4.72
Larger tympanumdiameter
Interorbital distanceArm lengthHand lengthThigh lengthTibia lengthTarsus lengthFoot length
2.67 6 0.387.50 6 0.20
27.21 6 2.9810.05 6 0.8114.32 6 1.9314.54 6 1.8310.03 6 1.3412.67 6 1.14
1.92–3.057.22–7.78
22.80–32.958.75–11.43
11.40–16.7311.36–17.59
7.87–12.5710.77–14.53
cephala) and a more reticulated pattern of thelower eyelid; from P. hypochondrialis by lacking asupramaxilar white stripe, tympanic membranelarger and more evident, and head 1.23 higher;and by having the flanks, arms, fingers, legs,and toes reticulated (banded in P. hypochondri-alis). Phyllomedusa centralis bears no reticulatedpattern on the lower eyelid, total length 1.23larger and head length 1.33 larger than P. or-eades, whereas P. rohdei is 1.33 larger and has arobust body, and colored face (P. oreades sp. n.
shows only the reticulated pattern on the exter-nal margin of eyelids).
Description of the Holotype.—General aspectslender (Fig. 1); head as long as wide; bodylength approximately three times the headwidth; snout slightly round in dorsal view (Fig.1A), ending abruptly in lateral view (Fig. 1B);loreal region slightly concave; nostrils small,placed laterally; eyes large, placed dorsolateral-ly; interorbital distance larger than eye diameter(Table 1); tympanum nearly oval; larger tym-panum diameter (1.63 the eye diameter); su-pratympanic fold beginning on top of tympa-num and ending at the base of the mandible;parotoids and vocal sacs undistinguishable; vo-cal sac under the tongue, vocal slits circular;tongue longer than wide, free on posterior endwith no groove and narrower on the base; vo-merine teeth absent; choanae rounded; fingersdeveloped; arm thin and forearm robust; fingerslacking any trace of webs; relative finger length:I,II,IV,III; finger tips poorly developed; tipsof fingers I and II approximately equal to eachother and smaller than tips of fingers III and IV,which are equal to each other (Fig. 1C). Subar-ticular tubercle present only on the base of fin-ger I; series of tubercles on fingers II, III, andIV; large trapezoidal palmar tubercle on thebase of finger I; two elliptical and parallel tu-bercles on the base of finger III; nuptial pad ev-
575NEW PHYLLOMEDUSA FROM CENTRAL BRAZIL
FIG. 3. Distribution of Phyllomedusa oreades sp. n. inthe State of Goias, GO, and in Distrito Federal, DF.Dots are the cities of Brasılia, DF; and Goiania, GO;the line delimits the area over 800 m; the shaded areasare over 1000 m.
ident, dorsally visible at the base of finger I.Snout–vent length (33.01mm) 2.93 that of thethigh length; toe tips size similar to each otherexcept for the fourth, which is slightly larger(Fig. 1D); relative length of the toes II,I,III,V,IV; series of tubercles on toes III, IV, andV; plantar callosities developed; toes webbingabsent. Dorsal skin smooth and ventral skingranulated; fine granulation on the abdomen.
Measurements of Holotype.—In millimeters: To-tal length 33.01; head length 10.61; head width11.00; head height 7.48; eye diameter 3.29; tym-panum diameter 1.92; interorbital distance 7.22;arm length 30.02; hand length 8.91; thigh length11.40; tibia length 11.36; tarsus length 7.87; footlength 11.00.
Coloration.—In life, dorsal coloration leafgreen; flanks and hidden parts of the legs or-ange with an irregular reticulated black, sepiaor purple pattern. Finer reticulated pattern onthe axilla, fingers, and toes; axilla, forearm, andhands with finely reticulated and narrowermarks, dark sepia to black over yellow back-ground. Arms without green pattern, forearmwith dorsolateral green coloration extendingfrom the elbow to the external dorsal surface ofthe hand. Tarsus dorsally green and metatarsuswith a dorsolateral green coloration in such away that only the green color is seen while rest-ing. Lower eyelid margin with a yellow semi-circle with dark reticular marks. External mar-gin of eyes surrounded by a thin yellow halo inlarger individuals. Venter pinkish white, lackingreticular marks. In preservative, the green colorof the dorsum becomes light-blue, and the yel-low and orange of the legs and body turnswhite.
Variation.—Measurements of the type seriesare shown in Table 1. Males are smaller thanfemales and have more discrete flanks patterns.Females have the external margins of eyes sur-rounded by a yellow halo and sometimes by re-ticulated marks. Although the parotoid gland isnot clearly evident, it is more evident in femalesthat, for this reason, appear to have larger heads(Fig. 2).
Etymology.—In the Greek mythology, the or-eades are mountain nymphs, besides being thephytogeographic region corresponding to cen-tral Brazil, as proposed by K. F. P. Von Martiusin 1824 (Rizzini, 1979). Therefore, the specificepithet refers to the occurrence of the species incentral Brazil, always in open physiognomies onthe top of plateaus and mountains.
Distribution.—Phyllomedusa oreades occurs onplateaus in the State of Goias (Serra da Mesa,Chapada dos Veadeiros, Serra dos Pirineus) andin the Distrito Federal (ARIE do Capetinga, Re-serva Ecologica do IBGE) always at altitudesabove 900 m (Fig. 3). All areas in Goias were
characterized by ‘‘campo limpo’’ or ‘‘campo cer-rado’’ physiognomies on quartzitic outcrops.The Brasılia site lacks rock outcrops.
Description of the Tadpole.—A tadpole at stage37 (Gosner, 1960; CHUNB 17474) shows a totalwidth of 46.80 mm. In lateral view, it has anelongated and trapezoidal shape (Fig. 4A); bodynarrow; dorsally trapezoidal in shape, maxi-mum body width at the level of the eyes; bodywidth approximately one-third of the totallength (Table 2); tail narrow with an elongatedand acute tip; dorsal fin lower than the ventralfin; dorsal fin beginning at the posterior bodymargin and extending to the tail tip; ventral finbeginning anterior to the anal tube and extend-ing from the terminal portion of the body to thetail tip; ventral fin depth approximately twicethe depth of the dorsal fin, narrowing abruptlyat the tip of the tail; tail curved toward the ven-tral surface. Musculature developed and evidentalong the tail. Eyes large, placed dorsolaterally.Nostrils small, dorsolateral; spiracle ventral,with an exposed opening, displaced to the leftand placed over the transversal midline. Analtube round and placed on the right side of theventral fin. Mouth anterior, small; two rows ofpapillae on posterior margin, one row of smallpapillae in the anterior margin, with a wide me-dian interruption; small and scattered lateralpapillae in the transversal mouth midline; twoanterior rows of denticles, the second interrupt-ed medially; three rows of posterior denticles
576 REUBER BRANDAO
FIG. 4. Tadpole (Gosner stage 37) of Phyllomedusaoreades sp. n. (CHUNB 17474) in lateral (A) and dorsal(B) views and oral disc structures (C).
TABLE 2. Morphological measurements (millime-ters) of tadpoles of Phyllomedusa oreades sp. n. (N 5 7).
Mean 6 SD Range
Total lengthBody lengthBody widthBody heightOrbital diameterInterorbital distanceEye–nostril distanceDisc lengthTail lengthTail height
46.28 6 2.3815.18 6 0.938.32 6 0.377.18 6 0.592.78 6 0.127.18 6 0.393.06 6 0.223.25 6 0.15
31.09 6 1.668.92 6 0.82
42.95–50.6813.79–16.467.70–8.77
13.79–16.462.51–2.936.42–7.752.64–3.363.11–3.53
29.16–34.227.69–9.79
with no reductions or interruptions, the firstrow wider than the second, and the second wid-er than the third; keratodont rows formula:(LTRF) 2(2)/3; beak narrow and wide, complete-ly pigmented, and finely serrated; upper jaw en-larged at medial portion and lower jaw Vshaped (Fig. 4C).
In life, tadpole coloration is greenish graywith darker dots scattered mostly on the dorsalregion and the muscular portion of the tail,forming discrete transverse bars. In formalde-hyde, tadpole color is grayish-brown and dor-sally transparent. A pear-shaped blotch is onthe dorsum and a omega (s)-shaped blotch isbetween the nostrils, both corresponding to el-ements of the cartilaginous skeleton. Dorsallyand laterally the body is bluish-gray, internal or-gans are not visible. Dorsal and ventral fins aretransparent, with transversal pigmented stripes.The caudal musculature is whitish.
Comparisons with Other Tadpoles of the Phyllo-medusa hypochondrialis Group.—The tadpoles of P.ayeaye, P. hypochondrialis, P. aff. megacephala fromSerra do Cipo, and P. rohdei were described byCruz (1982). The tadpoles of P. oreades are char-acterized by their body shape and by a pig-mented abdomen. It is readily distinguishedfrom tadpoles of P. hypochondrialis and P. rohdeiby the opaque abdomen, presence of a medialthickening of the upper beak, and by a freeopening of the spiracle. It differs from the tad-pole of P. ayeaye, by an opaque abdomen, bodyshape, and tail size. It differs from the tadpole
of P. aff. megacephala by the opaque abdomenand by lacking the interruption of the posteriorrows of papillae. Tadpoles of P. oreades, P. ayeaye,P. centralis, and P. aff. megacephala share the me-dian thickening of the upper beak, the absenceof an operculum over the opening of the spira-cle, and the occurrence in brooks and rivulets(Cruz, 1982; Feio et. al., in press.; R. A. Brandaoand G. J. Zerbini, pers. obs.). However, the P.ayeaye tadpoles occur in ponds (Cardoso et al.,1989).
Natural History Notes.—On 28 February 1998,active males of Phyllomedusa oreades were ob-served in an area of ‘‘campo sujo,’’ an open cer-rado physiognomy, with short and scatteredscrubs (Eiten, 1990), over a lithosoil with manysmall outcrops of quartzite, on Serra da Mesa,Minacu (Goias, Brazil). Common plants in thearea belong to the families Velloziaceae, Erio-caulaceae, Poaceae, and Cyperaceae. Around2000 h, some males were observed calling andmoving slowly over the grassy vegetation to-ward a brook, where other males were callingnear small waterfalls. Around 2300 h, severalmales concentrated in small ponds. The heightof the calling perches varied from 20 to 150 cm(N 5 4). When disturbed, they seized callingand remained still. After capture, all individualsdisplayed a tanatosis behavior lasting from 1.5to 5 min (N 5 7). The vocalization is similar tothat of P. hypochondrialis, although more discrete,being easily mistaken with the sound of the wa-terfalls. In this locality, no females were ob-served. Temperature and relative air humidityvaried between 23.98C and 82% (1940 h) to20.58C and 89% (0100 h).
Tadpoles were found in a clean pond, formedby a small waterfall, approximately 100 cmdeep. The substrate was covered with whitepebbles. Tadpoles showed both diurnal andnocturnal activity and stayed still in the middleof the water column in a characteristic position,nearly vertical, with the mouth toward the sur-face, with only the tip of the tail moving. When
577NEW PHYLLOMEDUSA FROM CENTRAL BRAZIL
disturbed, they fled to deeper regions, wherethey hid under roots or rock crevices. Tadpolesof Hyla pseudopseudis, Leptodactylus syphax, andEpipedobates flavopictus, and potential predatorsLethocerus sp. (Hemiptera, Belostomatidae) anda side-necked turtle, Phrynops vanderhaegei Bour,1973 (Chelonia, Chelidae) were also observed inthe same pond.
Two young that completed development inthe laboratory had the same apparence as theadult (total length 5 15.30 and 16.74 mm).
DISCUSSION
Based on tadpoles’ external morphology,Cruz (1982) proposed two subgroups within P.hypochodrialis species group. Tadpoles of P. hy-pochondrilais and P. rohdei are characterized byan arc-shaped upper beak, width of the poste-rior third row of denticles (less than half of thetwo other ones), skin projection forming anoperculum over the aperture of the spiracle,height of the lower fin three times that of theupper fin, and inhabiting lentic habitats. A sec-ond subgroup would consist of tadpoles of P.ayeaye and P. aff. megacephala, which are charac-terized by a rounded thickening on the medialportion of upper beak, the width of the poste-rior third row of denticles (larger than half ofthe other ones), lack operculum, and the heightof the lower fin is less than three times theheight of the upper fin. The tadpole of P. oreadesfits this second subgroup as does the tadpole ofP. centralis (pers. obs.). The tadpoles of this sub-group occur in lotic habitats, with exception ofP. ayeaye tadpole (Cardoso et al., 1989).
Phyllomedusa megacephala is known only fromthe holotype. Miranda-Ribeiro (1926) suggestedthat the specimen was collected in the State ofRio de Janeiro. This was supported by Boker-mann (1966). The specimen was probably col-lected in the late 19th century (U. Caramashi,pers. comm.). If the locality is correct, the spe-cies may occur in mountain fields in the state ofRio de Janeiro.
Although Phyllomedusa from the Serra doCipo has been identified as P. megacephala (as inFeio et al., in press), a comparison of these spec-imens with the holotype of P. megacephala refutesthis assumption. Phyllomedusa megacephala isreadily distinguished from the other species ofthe group by the reticulated drawing on theflanks and by having all the edge of the upperlip visible in dorsal view. Therefore, I suggestthat the Phyllomedusa from Serra do Cipo is an-other species, although further comparationsmust be done for a proper identification. Its tad-pole has already been shown in the literature(Cruz, 1982).
Acknowledgments.—G. Zerbini, T. Abreu, M.
Kanegae, and V. Braz for field assistance. A. Seb-ben for the photography. M. Carvalho (UFMT-Cuiaba), U. Caramaschi (MNRJ), and M. Rod-rigues (MZUSP), for the access to the collectionsunder their responsibility. P. Eterovick for lend-ing material. P. Eterovick, G. Colli, J. Pombal Jr.,and A. Sebben for critical reading of the prelim-inary versions of the manuscript. A. Garda, G.Colli, W. Quatman, and A. Gainsbury for En-glish improvements. A. F. B. Araujo, J. Marinho-Filho, R. Cavalcanti, and H. Garbogini (ProjetoMonitoramento das Populacoes Animais noAHE Serra da Mesa-GO, UnB/FURNAS) for thefacilities for working in Serra da Mesa.
LITERATURE CITED
ALTIG, K. L. 1970. A key to the tadpoles of the con-tinental United States and Canada. Herpetologica26:180–207.
BOKERMANN, W. C. A. 1965. Tres novos batraquios daregiao central de Mato Grosso, Brasil (Amphibia,Salientia). Revista Brasileira de Biologia 25:257–264.
. 1966. Lista Anotada das Localidades Tipo deAnfıbios Brasileiros. RUSP, Sao Paulo, Brazil.
CANNATELLA, D. C. 1980. A review of the Phyllome-dusa buckleyi group (Anura: Hylidae). OccasionalPapers of the Museum of Natural History, Univer-sity of Kansas 87:1–40.
. 1982. Leaf-frogs of the Phyllomedusa perinesosgroup (Anura: Hylidae). Copeia 1982:501–513.
CARDOSO, A. J., G. V. ANDRADE, AND C. F. B. HADDAD.1989. Distribuicao espacial em comunidades deanfıbios (Anura) no sudeste do Brasil. RevistaBrasileira de Biologia 49:241–249.
CRUZ, C. A. G. 1982. Conceituacao de grupos de es-pecies de Phyllomedusinae brasileiras com baseem caracteres larvarios (Amphibia, Anura, Hyli-dae). Arquivos da Universidade Federal Rural doRio de Janeiro 5:147–171.
DE LA RIVA, I. 1999. A new Phyllomedusa from south-western Amazonia (Amphibia, Anura, Hylidae).Revista Espanola de Herpetologia 13:123–131.
EITEN, G. 1990. Vegetacao do Cerrado. In M. N. Pinto(org.), Cerrado–Caracterizacao, Ocupacao e Per-spectivas, pp. 9–65. Editora Universidade de Bra-sılia, Brasılia, Brazil.
FEIO, R. N., W. C. A. BOKERMANN, AND I. SAZIMA. InPress. Anfıbios Anuros da Serra do Cipo, MinasGerais. In Serra do Cipo: Ecologia e Evolucao. G.W. Fernandes (ed). Atheneu Editora, Belo Horizon-te, Brazil.
FROST, D. E. 2000. Amphibian Species of the World:An Online Reference. V2.20. (http://research.amnh.org/herpetology/amphibia).
GOSNER, K. L. 1960. A simplified table for staginganuran embryos and larvae with notes in identi-fication. Herpetologica 16:183–190.
HEYER, W. R., A. S. RAND, C. A. G. CRUZ, O. L. PEIX-OTO, AND C. E. NELSON. 1990. Frogs of Boraceia.Arquivos de Zoologia, Sao Paulo 31:231–410.
JOHNSON, G. F., AND K. L. ALTIG. 1986. Identificationcharacteristics of anuran tadpoles. HerpetologicalReview 17:36–37.
LUTZ, B. 1950. Anfıbios anuros da colecao Adolpho
578 REUBER BRANDAO
Lutz V. Locomocao e estruturas das extremidades,Va Phyllomedusa (P.) burmeisteri distincta A. Lutz,Vb Aplastodiscus pervirides A. Lutz. Memorias doInstituto Oswaldo Cruz 48:599–637.
. 1966. Pithecopus ayeaye, a new Brazilian hylidwith vertical pupils and grasping feet. Copeia1996:236–240.
MIRANDA-RIBEIRO, A. 1926. Notas para servirem aoestudo dos Gymnobatrachios (Anura) Brasileiros-Tomo Primeiro. Archivos do Museu Nacional, Riode Janeiro 27:1–227.
NASCIMENTO, L. B. 1998. Phyllomedusa ayeaye (B. Lutz,1966). In A. B. M. Machado, G. A. B. Fonseca, R.B. Machado, L. M. S. Aguiar, and L. V. Lins (eds.),Livro Vermelho das Especies Ameacadas de Extin-cao da Fauna de Minas Gerais, pp. 453–455. Fun-dacao Biodiversitas, Belo Horizonte, Brazil.
POMBAL JR., J. P., AND C. F. B. HADDAD. 1992. Especiesde Phyllomedusa do grupo burmeisteri do Brasil Ori-ental, com descricao de uma especie nova (Am-phibia: Hylidae). Revista Brasileira de Biologia 52:217–229.
RIZZINI, C. T. 1979. Tratado de Fitogeografia do Bras-il–Aspectos Sociologicos e Florısticos. Hucitec/Edusp, Sao Paulo, Brazil.
Accepted: 7 February 2002.
APPENDIX 1
Specimens Examined
Phyllomedusa ayeaye.—Minas Gerais State, Brazil. Po-cos de Caldas, Morro do Ferro; AL 3722–3723; AL3726–3727; Phyllomedusa centralis.—Mato Grosso State,Brazil. Chapada dos Guimaraes, Mata Fria; CHUNB12518–12520; CHUNB 12570–12571; MNRJ 23681;Phyllomedusa hypochondrialis.—Para State, Brazil. Belem,Embrapa; MNRJ 13671–13675. Goias State. Caldas No-vas, UHE Corumba; CHUNB 14202. Serranopolis, SVSPousada das Araras; CHUNB 14218. Pirenopolis, SVSVaga Fogo; CHUNB 14211. Minacu, UHE Serra daMesa; CHUNB 04915; Phyllomedusa megacephala.—MNRJ 257. Phyllomedusa aff. megacephala.—Minas Ger-ais State, Brazil. Jaboticatubas, Serra do Cipo; MNRJ11307–11308. Conceicao do Mato Dentro, Serra doCipo; MZUSP 56889–56891; WCAB 45824–45828;Phyllomedusa moschata.—Rio de Janeiro State, Brazil. Ter-esopolis; MNRJ 0258; MNRJ 5239–5244; Phyllomedusaoreades.—Distrito Federal, Brazil. Reserva Ecologica doIBGE; MNRJ 23679. ARIE do Capetinga, Fazendaagua Limpa; MNRJ 23680. Goias State. Serra da Mesa,Minacu; CHUNB 12510–12516. Sao Jorge, Alto Paraısode Goias; CHUNB 12517. Serra dos Pirineus, Pireno-polis; CHUNB 21907–21908; Phyllomedusa rohdei.—SaoPaulo State, Brazil. Baraquecaba, Sao Sebastiao; MNRJ17046–17051; Phyllomedusa sp.—Bahia State, Brazil. Ma-racas; MNRJ 13602–13611.
Journal of Herpetology, Vol. 36, No. 4, pp. 578–589, 2002Copyright 2002 Society for the Study of Amphibians and Reptiles
Ontogenetic Scaling of the Cranial Horn Array inPhrynosoma orbiculare (Squamata: Phrynosomatidae)
G. LAWRENCE POWELL,1 ANTHONY P. RUSSELL, AND MICHAEL J. RYAN2
Vertebrate Morphology Research Group, Department of Biological Sciences, University of Calgary,2500 University Drive NW, Calgary, Alberta, Canada T2N 1N4
ABSTRACT.—Phrynosoma orbiculare bears one pair of horns on the midline parietal bone (P2), two pairson each squamosal bone (S1 and S2), and one horn on each frontal bone (F0). Reduced major axis (RMA)regression statistics for the relationship of each horn to head length, and of head length to body length,were estimated and compared for significant differences in slopes, size at smallest head length, and size atgreatest head length. F0 and P2 increase at different but low rates, but S1 and S2 grow at a significantly fasterrate and approach P2 length toward the upper end of the head length range. This suggests an adaptive rolefor the cranial horn array in adult lizards. No sexual differences were found in these relationships, but therewere significant subspecific differences in the relationships of head length to body length and of S1 and S2
to head length. This allometry of the cranial horn array is hypothesized to represent a plesiomorphic con-dition among the horned phrynosomatids.
The scaling of crania to body size, and rela-tive growth within crania, have not received a
1 Corresponding Author. E-mail address: [email protected]
2 Present address: Royal Tyrrell Museum of Pa-laeontology, P.O. Box 7500, Drumheller, Alberta, Can-ada T0J 0Y0.
great deal of attention. Most ontogenetically ori-ented studies bearing upon these phenomenaare concerned with domestic mammal species(e.g., Hughes and Tanner, 1970; Leamy andBradley, 1982; Weijs et al., 1987; Herring, 1993;Fiorello and German, 1997) and may lack gen-erality (Emerson and Bramble, 1993). The rela-tively circumscribed set of cranial functions per-
579PHRYNOSOMA HORN SCALING
mits the framing of testable hypotheses con-cerning change in these functions through on-togeny. Functions can thus be integrated withmorphology in the formulation of testable hy-potheses about morphological and phylogeneticconstraints (Emerson and Bramble, 1993) or evo-lutionary processes (e.g., Geist, 1987).
In reptiles, the dermatocranium is frequentlymodified to give rise to tubercles, spines andcrests (Rieppel, 1993). Lizards of the genusPhrynosoma are characterized by well-devel-oped, bone-cored horns (or spines) originatingon the parietal, squamosal, and other cranialand mandibular bones (Reeve, 1952; Presch,1969; Montanucci, 1987), constituting a syna-pomorphy for the genus (Etheridge and deQueiroz, 1988; Frost and Etheridge, 1989). Thegenus displays a wide variety of cranial hornconfigurations and sizes, each of which is gen-erally characteristic of one species (e.g., Reeve,1952; Baur and Montanucci, 1998). However,Montanucci (1987) also noted variation in hornnumber not only within species but asymmet-rically within individuals. It is commonlyagreed that the cranial horn array is defensivein nature (Bryant, 1911; Smith, 1946; Reeve,1952; Presch, 1969; Pianka and Parker, 1975;Munger, 1986; Sherbrooke, 1981, 1987, 1990,1991; Sherbrooke and Montanucci, 1988), and inthe more spinose Phrynosoma species, it hasbeen shown to have a defensive function againstat least some predators (Sherbrooke, 1981, 1987,1991; Sherbrooke and Montanucci, 1988; Holteand Houck, 2000). However, it is difficult to en-vision how cranial horns may be an effectivedefence when relatively short in adults (e.g.,Phrynosoma douglasi; Dumas, 1964) or in juve-niles of species that display greater expressionlater in ontogeny.
Here, we examine the ontogenetic develop-ment of the cranial horns in Phrynosoma orbicu-lare through an analysis of their scaling relation-ships with head size, as a prelude to a broader-based comparative study of this genus. A recentphylogenetic analysis of Phrynosoma (Reederand Montanucci, 2001) provides a new workinghypothesis for the exploration of evolutionarytransformations within the genus. One suchtransformational pattern is the relative devel-opment and expression of the cranial horn ar-ray.
Phrynosoma orbiculare has short horns in com-parison to most of its congeners (Reeve, 1952;Horowitz, 1955; Montanucci, 1979) and puta-tively exemplifies the simplest pattern of cranialhorn development among the extant species ofthe genus (although Montanucci [pers. comm.]is uncertain as to whether this morphology rep-resents a true plesiomorphy or a secondarily de-rived state approximating the inferred plesio-
morphic condition). Isometric growth of the cra-nial horn array relative to head size would sug-gest that its size and proportions in adulthoodare simply a result of overall increase in bodysize. Positive or negative allometry of the cranialhorns with regard to the head would suggestthat more complex hypotheses concerning theselective pressures affecting the size of the arrayare required. Although any hypothesized func-tion of the cranial horn array is beyond thescope of this analysis, our results will permitthe formulation of hypotheses concerning thesepossible functions in P. orbiculare in particularand among Phrynosoma species in general.
MATERIALS AND METHODS
Data Collection.—Mensural data were takenfrom 84 preserved specimens of P. orbiculare(Appendix 1). Snout–vent length (SVL) wasmeasured ventrally to the nearest millimeter.Head length (HL), to the nearest millimeter, wasmeasured as the dorsal straight-line distancefrom the anterior tip of the premaxilla to theposterior midline edge of the parietal (Fig. 1);this did not include the midline parietal horn(P1) when it was present. All measurements re-ported in this study are estimated by the meansof three repetitions of each. Body length (BL)was estimated for each lizard by subtracting HLfrom SVL; this measure eliminates the contri-bution of head length to total length and thusallows examination of the scaling of cranialhorns against a noncranial estimator of size.
Four horns (Fig. 1) were measured on eachlizard; the medial parietal horn (P1) and thethird squamosal horn (S3) were not consistentlypresent in this series and were thus rejected formodeling purposes. The other horns were con-sistently present, as follows: one on the poste-rior margin of the parietal (P2), two on the pos-terior margins of the squamosal (S1 and S2), andone (F0) on the posterior supraorbital margin ofthe frontal. These are also known, respectively,as occipital, temporal and supercilliary horns(e.g., Bryant, 1911; Reeve, 1952; Baur and Mon-tanucci, 1998). The perpendicular distance fromthe tip of the horn to the midpoint of the horn’sbase (horn length) was measured, to the nearest0.1 mm, for each horn on the left side of eachspecimen’s head (Fig. 1).
Horns were frequently missing their kerati-nous sheaths, exposing the deeper cutaneouscovering of the bony core. Measurement of suchstructures would constitute an additional sourceof variance in the analysis, and thus, individualhorns lacking sheaths were discarded from theanalyses pertaining to that horn. Measuring thecorresponding horn on the right side was dis-allowed, as it might have introduced variancefrom fluctuating asymmetry. Thus, sample size
580 G. L. POWELL ET AL.
FIG. 1. Dorsal view of the head of Phrynosoma orbiculare showing features measured. HL, head length; P2,length of parietal horn 2; S1, length of squamosal horn 1; S2, length of squamosal horn 2; F0, length of frontalhorn. S3 (squamosal horn 3) and P1 (parietal horn 1) are labeled here but were not measured.
for any one horn and associated mensural char-acters will differ from those of other horns, eachbeing a smaller subset of the whole sample, andindividual lizards were not consistently repre-sented throughout the analysis as a whole. Datafrom all individuals were used for analysis ofthe relationship between body length and headlength.
Analysis.—All mensural data were log10-trans-formed for analysis; all subsequent references tothe variables defined above refer to the trans-formed values unless otherwise noted. A num-ber of Phrynosoma species display strong sexualsize dimorphism (Fitch, 1981; Powell and Rus-sell, 1984, 1985; Zamudio, 1998). Thus, we ini-tially divided the data by sex for subsequentanalysis until appropriate tests indicated thatthe data could be pooled. Distributions of vari-ables for the dataset as a whole, pooled by sex
and for the various subsets of it, were examinedby means of Box plots and histograms; normal-ity of each distribution was tested by Kolmo-gorov-Smirnov One-Sample tests.
We first examined sexual dimorphism in thescaling relationships between body length andhead length. The least-squares regression modelis generally considered to be inappropriate formodeling allometric relationships, the preferredmodel being the Reduced Major Axis (RMA;Imbrie, 1956; Gould, 1966; Seim and Saether,1983; Ricker, 1984; Jones, 1988; McArdle, 1988;LaBarbara, 1989; but see Harvey and Pagel,1991; Kimura, 1992). Slope and intercept of theregression of head length on body length foreach sex were estimated according to Miller andKahn (1962) and Ricker (1984). Standard devi-ation of the slope was estimated as in Ricker(1984), and that of the intercept as in Miller and
581PHRYNOSOMA HORN SCALING
Kahn (1962). The absolute dispersion about theaxis (standard deviation of the residuals) andrelative dispersion about the axis (amount ofshape variation as a proportion of the sample’saverage shape) were calculated for each regres-sion as in Jones (1988). Difference in slope be-tween the pair of lines was tested by Clarke’s(1980) approximation of the t-statistic, and dif-ferences in intercept tested by a Z-statistic asdescribed by Miller and Kahn (1962) and Jones(1988). A lack of significant difference betweenlines in slope (t12 5 0.805, P 5 0.787, df 540.037) and in intercept (Z 5 0.699, P 5 0.242)indicated that a regression analysis usingpooled data was appropriate. The RMA sloperelating head length to body length was com-pared to that expected for an isometric relation-ship by the approximation of t defined by Ricker(1984:1903).
Because the horns are all dermatocranialstructures that are adjacent to one another and,in two cases (S1 and S2) arise from the samebone, their statistical independence could not beassumed. Thus, each of the four horns measuredon an individual lizard was considered to bepart of a series of iterative homologues (Ghise-lin, 1976), that is, similar structures produced bythe same developmental and genetic constraintsexpressed in different parts of the body (Wag-ner, 1989, 1994; Roth, 1994). The development ofthe horns from three different cranial bones(Bryant, 1911; Reeve, 1952; Presch, 1969; Mon-tanucci, 1987) argues against this assumption;alternatively, that they are all developed fromdermal cranial bones suggests the iteration of adevelopmental process common to all suchbones. As iterative homologues, lengths of cra-nial horns could be considered to be repeatedestimates of lengths of a structure, the horn. Assuch, their scaling relationship to head lengthwas first examined through a repeated-mea-sures analysis of covariance, with head lengthas a covariate. The variance-covariance matrix tobe used was first tested for equality of variancesand of covariances by Box’s x2, based uponWilks’ L (Morrison, 1976), which indicated(Box’s x2 5 317.89, P , 0.001, df 5 8) that theassumption of compound symmetry requiredfor the repeated-measures model (Morrison,1976; Potvin et al., 1990) is not supported. Thatdistributions of neither the horn lengths norhead length are normal (P , 0.001 in all cases)also militated against the use of a repeated-measures model. Accordingly, RMA regressionstatistics were estimated for the relationships ofP2, S1, S2, and F0 to head length. Initial regres-sion statistics were calculated for each horn ondata pooled by sex. Pairwise comparisons ofRMA slope and intercept between the sexeswere made as described above. The acceptance
level for each set of four simultaneous pairwisecomparisons was set by a stepwise Bonferroniadjustment (Rice, 1989), initially calculated forfour pairwise comparisons. There were no sig-nificant differences between the sexes in slope(P2: t12 5 20.425, P 5 0.338, df 5 21.453; S1: t12
5 0.116, P 5 0.546, df 5 26.778; S2: t12 5 20.973,P 5 0.170, df 5 23.244; F0: t12 5 1.192, P 5 0.877,df 5 22.980) or intercept (P2: Z 5 0.461, P 50.678; S1: Z 5 20.001, P 5 0.500; S2: Z 5 0.756,P 5 0.755; F0: Z 5 20.898, P 5 0.185), and sothe data for each horn were pooled and regres-sion statistics as described above (Miller andKahn, 1962; Ricker, 1984; Jones, 1988) estimatedfrom the pooled subsamples.
The deviation from the regression line foreach observation was calculated as the height ofthe triangle with vertices defined by the bivar-iate coordinates of the observed value, the po-sition of the predicted x-value for that observa-tion on the regression line, and the position ofthe predicted y-value for that observation on theregression line. This estimate is not that of theresiduals for an RMA line, which minimizes thesum of the areas of the triangles as calculatedabove (Gould, 1966; Miller and Kahn, 1962;Ricker, 1984; Jones, 1988); however, it incorpo-rates the differences of x and of y, in proportionto their magnitudes, from their predicted val-ues, without the complication of squared values.The lengths of the lines joining observed andpredicted x-values and observed and predictedy-values were calculated as observed minus ex-pected, and the calculated deviation determinedto be negative or positive according to its po-sition above or below the regression line. Thedistributions of deviations for each regressionwere examined for normality by means of Kol-mogorov-Smirnov One-sample tests, and devi-ations were plotted against a variable correlatedwith body size to examine for size-related pat-terns of variation. The ratio of the coefficient ofvariation of the ordinate variable to that of theabcissal variable was calculated for each RMAline as a measure of the difference in error ofthe two variables, a ratio approaching unity in-dicating that the RMA slope estimate is robust(Kimura, 1992) and preferable to the corre-sponding least squares slope estimate (McArdle1988). The RMA slopes relating each of the hornlengths to head length were compared to theslope expected for an isometric relationship bythe approximation of t defined by Ricker (1984).The a for each of these four tests was deter-mined by a stepwise Bonferroni adjustment(Rice, 1989); because none of the variables in-volved had a normal distribution, it is unlikelythat the subsample used to calculate any partic-ular slope was binormal, and thus all of the sig-nificance levels calculated for t would be ap-
582 G. L. POWELL ET AL.
FIG. 2. (A) Plot of log10 transformed values ofhead length against body length for Phrynosoma orbi-culare, with regression line superimposed. (See text forregression statistics). (B) Distribution of deviationfrom reduced major axis regression of head lengthagainst body length. (C) Bivariate plot of deviationsfrom reduced major axis regression of head lengthagainst body length, plotted against BVL. (D) Boxplots of subspecies-specific deviations from reducedmajor axis regression of head length against bodylength calculated from pooled sample.
proximate (Ricker, 1984). RMA slopes for the re-lationships of each cranial horn length to bodylength were calculated and tested for isometryin the same way.
Differences in slope between pairs of regres-sions describing the relationship of horn lengthto head length were tested by Clarke’s (1980)approximation of the t-statistic, with a set bymeans of a stepwise Bonferroni adjustment(Rice, 1989), initially calculated for four pairwisecomparisons and using the Dunn-Sidak approx-imation for a at each step. This approximationfor the acceptance level is conservative whenthere is a lack of independence between individ-ual tests (Sokal and Rohlf, 1995), which mightbe expected in pairs of regressions describingthe scaling of two cranial horns. Pairwise dif-ferences between lines at the smallest and at thegreatest head lengths were tested by a Z-statisticas estimated by Miller and Kahn (1962) andJones (1988). The rejection level for this test sta-tistic was also set by means of a stepwise Bon-ferroni adjustment using the Dunn-Sidak for-mula for a, initially calculated for 12 pairwisecomparisons.
Some variation in horn morphology and rel-ative head length in P. orbiculare is apparentlyassociated with subspecific divergence (Horo-witz, 1955; Montanucci, 1979). Accordingly, wegrouped deviations for each RMA line by sub-specific status when possible. The subspecificdesignations of Montanucci (1979) were fol-lowed if locality data (Baur and Montanucci,1998) sufficed for assignment on this basis, forspecimens which had not been cataloged bysubspecies. However, a number of specimens(24 of 84) had not been identified to subspecieswhen cataloged or had insufficient locality datafor us to make such an assignment; these wereomitted from this part of the analysis. The solepublished intraspecific phylogeny (Horowitz,1955) does not allow us to effectively partitionphylogenetic variance in accounting for thesedifferences. Differences in horn length amongthese groups were examined by Kruskal-WallisANOVAs of deviations, one for each horn. Ac-ceptance levels for the four ANOVAs were de-termined by a stepwise Bonferroni adjustment(Rice, 1989). The same procedure was followedfor head length deviations. The differencesamong subspecies in horn orientation com-mented upon by Montanucci (1979) should notaffect estimates of horn length.
RESULTS
Distributions of Morphological Variables.—His-tograms of the pooled data indicated a strongbias toward larger animals in the sample. Dis-tributions of all variables in the pooled data, andin various subsets thereof, were significantly
different from normal (P , 0.0001 in all instanc-es). However, the distribution of body sizes rep-resented by this sample spanned the range fromhatchling to mature adult for both sexes and forthe subsets of the dataset defined by sheathpresence for each horn. Coefficients of variationfor all of the horn lengths were larger than forany of the other variables.
Relationship between Head and Body Dimen-sions.—There was a high correlation betweenhead length and body length (r 5 0.961), andthe ratio of their respective CVs is 1.095, vali-dating the choice of an RMA model (McArdle,
583PHRYNOSOMA HORN SCALING
TABLE 1. Reduced major axis statistics for relationships of four cranial horn lengths to head length inPhrynosoma orbiculare, with ratios of coefficients of variation of each horn length to head length.
P2 S1 S2 F0
Nr
400.961
550.952
490.934
450.892
Intercept(SD)
21.664(0.0941)
22.177(0.1087)
22.204(0.1326)
21.557(0.1178)
Slope(SD)
1.857(0.0815)
2.223(0.0925)
2.229(0.1134)
1.504(0.1015)
Absolute dispersion about axisRelative dispersion about axisCV ratio
0.06625.3284.51
0.08276.6066.022
0.09737.9266.66
0.09347.9819.375
1988; Kimura, 1992). The distribution of the de-viations from the RMA regression was signifi-cantly nonnormal (P , 0.0001; Fig. 2B). Therewas some tendency to underestimate the headlength of large individuals (Fig. 2C), but the rel-ative dispersion (2.84) and absolute dispersion(0.057) about the line were small. The slope ofthe RMA regression (0.65; SD 5 0.020) was sig-nificantly less than the isometric slope (t 5217.743, P , 0.001, df 5 81), indicating negativeallometry; the head became relatively smallerwith greater body size (Fig. 2A).
There were significant differences among thesubspecies in central tendency of deviation (Fig.2D) from this pooled regression (Kruskal-WallisH 5 13.982; P 5 0.007). Some of the variance inthis relationship must thus be attributed to in-traspecific phylogeny.
Horn Growth.—The smallest slope was for therelationship between F0 and head length, fol-lowed by that for P2 and head length; the slopesfor the two squamosal horns were very large(Table 1). Standard deviations of slope and in-tercept for the four regressions did not differgreatly among lines (Table 1). The deviations ofthe four regressions were not normally distrib-uted (P , 0.0001 for all instances). Plots of de-viations against an index of lizard size suggestthat S1 lengths for small and large lizards tend-ed to be overestimated, and those for medium-sized lizards underestimated, but there were noobvious size-associated patterns for the othercranial horn regressions (Fig. 3A). Ratios of CVsof each of the four horns to that of head lengthranged in value between 4.5 and 9.4 (Table 1),indicating a tendency toward greater error inhorn length measurement than in head lengthmeasurement and thus that the RMA modelmay not be the most robust for these data(McArdle, 1988; Kimura, 1992). However, thecorrelation coefficients for these relationshipswere high (Table 1), confirming that we are notestimating parameters for variables betweenwhich little or no structural relationship exists(Harvey and Pagel, 1991). The large absolute
sizes of the RMA slope estimates (Table 1) willbe associated with lower error in estimationeven with a high CV ratio (Kimura, 1992). Fi-nally, all of the regressions of horn length onhead length have similar, small absolute disper-sions and relative dispersions (Table 1), indicat-ing robust estimation of slopes and intercepts.
All of the reduced major axis slopes for hornlengths were significantly greater than 1, the ex-pected slope for isometric scaling (P2: t 510.252, P , 0.001, df 5 38; S1: t 5 13.064, P ,0.001, df 5 53; S1: t 5 10.608, P , 0.001, df 547; F0: t 5 4.853, P , 0.001, df 5 43; maximumaccepted stepwise Bonferroni adjusted rejectionlevel a 5 0.0127). With regard to body length,the reduced major axis slopes for horn lengthwere mostly significantly greater than that ex-pected for isometry (P2: t 5 3.019, P 5 0.002, df5 38; S1: t 5 5.571, P , 0.001, df 5 53; S1: t 55.684, P , 0.001, df 5 47) with the exception ofthat for F0 (t 5 0.174, P 5 0.431, df 5 43; max-imum accepted stepwise Bonferroni adjustedrejection level a 5 0.0127 for four comparisons).Cranial horn growth rates thus exceed headlength growth rates and largely exceed bodylength growth rates.
The slopes of the regression lines for the twosquamosal horns did not differ significantlyfrom one another, but all other possible pair-wise comparisons of slopes indicated significantdifferences (Table 2A).
At the smallest head length, the line for P2
was significantly higher than those for the otherhorns (Table 2B; Fig. 3B). The lines for the twosquamosal horns had no significant vertical dif-ference at this head length, and neither dis-played significant vertical difference from thatfor F0 (Table 2B; Fig. 3B). At the greatest headlength, there was no significant vertical differ-ence between the line for P2 and either of thosefor the squamosal horns, although the line forP2 is significantly higher than that for F0 (Table2C; Fig. 3B). There was no significant verticaldifference between the lines for the squamosalhorns, but both of these were significantly high-
584 G. L. POWELL ET AL.
FIG. 3. (A) Plots of log10-transformed values of each cranial horn length on head length, with individualRMA regression lines superimposed, for Phrynosoma orbiculare (see Table 1 for regression statistics). (B) Plot ofall RMA regression lines of each cranial horn on head length superimposed upon the same set of axes, showingchange in proportions in the cranial horn array with increase in head size in Phrynosoma orbiculare. P2, lengthof parietal horn; S1, length of squamosal horn 1; S2, length of squamosal horn 2; F0, length of frontal horn.
er than the line for F0, at the greatest head length(Table 2C; Fig. 3B).
There were two significant differences amongthe subspecies in central tendency of deviations(Fig. 4; P2: Kruskal-Wallis H 5 4.301; P 5 0.367;S1: Kruskal-Wallis H 5 16.773; P 5 0.002; S2:Kruskal-Wallis H 5 20.611; P , 0.001; F0: Krus-kal-Wallis H 5 5.846; P 5 0.211; maximum ac-cepted stepwise Bonferroni adjusted rejectionlevel a 5 0.0127). Differences (among the sub-species examined) in scaling of S1 and of S2 tohead length thus contributed nonsize-associated
variance, possibly phylogenetic, to these analy-ses.
DISCUSSION
Growth and Proportion.—The total sample un-derrepresented small animals, but inspection ofdeviation plots (Fig. 2) indicates that large de-viations are not exclusively associated withsmall body size. Museum series are frequentlybiased toward adult, or large, individuals (Al-berch, 1985), but our sample encompasses theontogenetic size range, and in the absence of
585P
HR
YN
OSO
MA
HO
RN
SCA
LIN
G
TABLE 2. (A) T12-values for pairwise slope comparisons between RMA regressions for horn lengths against head length in Phrynosoma orbiculare. Maximum acceptedstepwise Bonferroni adjusted rejection level a 5 0.025; *, significant difference at a lower adjusted a. (B) Z-statistics for pairwise vertical difference between RMAregressions for horn lengths against head length in Phrynosoma orbiculare at minimum head length (0.947). Maximum accepted stepwise Bonferroni adjusted rejectionlevel a 5 0.00833; *, significant difference at a lower adjusted a. (C) Z-statistics for pairwise vertical difference between RMA regressions for horn lengths against headlength in Phrynosoma orbiculare at maximum head length (1.313). Maximum accepted stepwise Bonferroni adjusted rejection level a 5 0.00833; *, significant differenceat a lower adjusted a.
DependentVariable
A
P2 S1 S2
B
P2 S1 S2
C
P2 S1 S2
S1
2.5150.74
P , 0.025*
T12
degrees of freedomprobabilitysignificance
6.06P , 0.0053
*
Zprobabilitysignificance
1.85P . 0.01
NSprobabilitysignificance
S2
2.1846.73
P , 0.025*
0.022147.36
P . 0.05NS
5.44P , 0.0053
*
0.37P . 0.05
NS
1.95P . 0.0083
NS
0.39P . 0.025
NS
F0
2.4842.74
P , 0.0167*
4.66135.34
P , 0.008*
4.20118.96
P , 0.0053*
7.77P , 0.0053
*
1.63P , 0.0125
NS
1.09P . 0.0167
NS
14.81P , 0.0053
*
12.80P , 0.0053
*
11.24P , 0.0053
*
586 G. L. POWELL ET AL.
FIG. 4. Box plots of subspecies-specific deviationsfrom reduced major axis regressions of cranial hornlengths on head length, calculated from pooled sam-ples; (A) second parietal horn (P2); (B) first squamosalhorn (S1); (C) second squamosal horn (S2); (D) frontalhorn (F0).
disjunctions in scaling trajectories between ju-venile and adult lizards, the parameter esti-mates presented here should approximate thoseof the true scaling relationships.
The scaling model of head length growth asa function of body length indicates that headlength becomes a proportionately smaller partof total length as absolute size increases, a com-mon pattern among vertebrates (Emerson andBramble, 1993). This change in proportion is ac-companied by a shift in the relative sizes of thefour cranial horns because of differences in theirgrowth rates. In very small individuals, withrelatively large heads, the parietal horns are rel-atively large, whereas the squamosal horns andthe frontal horns are similarly sized and muchsmaller (Fig. 3B). At the greatest observed head
length, the squamosal and parietal horns are ofapproximately the same length, the frontalhorns being much smaller in proportion (Fig.3B). All cranial horns displayed strong positiveallometry relative to head length and to bodylength. This supports a function for the hornswhich is developed in adults but poorly devel-oped in subadults, or that changes through on-togeny.
The osteological (Bryant, 1911; Smith, 1946;Presch, 1969; Montanucci, 1987; Etheridge andde Queiroz, 1988; Frost and Etheridge, 1989)and other morphological synapomorphies(Pianka and Parker, 1975; Sherbrooke, 1981) typ-ifying Phrynosoma species appear to be part ofa suite of morphological, physiological and eco-logical characteristics associated with myrme-cophagy, which perhaps explains this suite inits entirety as a set of coadaptations imposed orpermitted by diet and its consequences (Piankaand Parker, 1975; Pianka, 1986). Phrynosoma or-biculare has short horns relative to most of thespecies of its genus (Reeve, 1952; Montanucci,1987; Baur and Montanucci, 1998; Reeder andMontanucci, 2001) and can be expected to bearconsequent costs in terms of inefficient anti-predator defence (Munger, 1986; Sherbrooke,1991), but the cranial horn array still displaysstrong positive allometry. This implies that theconnection between defense and cranial hornarray development in this genus may be morecomplex than has been hitherto realized. Dataon the nature of the principal predators encoun-tered within the range of P. orbiculare, and theintensity of predation pressures, would be use-ful in formulating and testing hypotheses rele-vant to this possibility. It has been suggested,for instance, that potential predators, particular-ly snakes, are relatively rare in habitat occupiedby P. orbiculare (R. Montanucci, pers. comm.).
It is unlikely that relatively large cranial hornarrays facilitate social signaling among adult P.orbiculare. There does appear to be a relation-ship between head bob amplitude and the spe-cies-specific degree of cranial horn developmentin the six Phrynosoma species examined by Lynn(1965). The head-bob display of P. orbiculare hasbeen described as ‘‘a fine trembling,’’ withoutthe forepart of the body being raised on theforelegs, as is seen in congeners with better-de-veloped cranial horns (B. Baur, pers. comm.),suggesting that horn development, which in anycase is not sexually dimorphic, is not significantin such signaling.
We have described the differences in scalingbetween the four horns of the cranial array inone short-horned species of Phrynosoma. A com-parative study of the relationship, through on-togeny, between clearly defined possible selec-tive pressures and cranial morphology in Phry-
587PHRYNOSOMA HORN SCALING
nosoma species is required for evaluation of thescaling of the cranial horn array in P. orbiculare.On the basis of what we have found for thisspecies, however, we predict that interspecificvariations in the magnitude of positive allome-try of the squamosal horns produces much ofthe variance in the homologous portions of thecranial horn array observed among the speciesof this genus.
Geographical Variation in Cranial Horn Allome-try.—Differences were found in the scaling ofhead length to body length, and of cranial hornlength to head length, among the subspeciesrepresented in our sample (Fig. 4). The devia-tions of the sample of Phrynosoma o. bradti in-dicate proportionately longer parietal hornsthan in the other subspecies sampled (Fig. 4A).Horowitz (1955), however, found that both P. o.orientale and P. o. orbiculare had proportionatelylonger parietal horns than P. o. bradti. Montan-ucci (1979) noted that P. o. boucardii has promi-nent frontal horns, which was not indicated bythe deviation values for this subspecies in ouranalysis (Fig. 4D). These disparities from re-ported relative horn lengths may be caused bythe limited number of P. orbiculare subspecies inthe pooled sample used to calculate the param-eters of the RMA regressions for which the de-viations were calculated (Fig. 4), but they sug-gest that the relative lengths of the cranial hornarrays considered characteristic of the subspe-cies of P. orbiculare be reexamined. Significantdifferences among the subspecies examinedhere were in the deviations of the two squa-mosal horns (Fig. 4B–C), those displaying thegreatest allometry in the sample as a whole (Fig.3B), and we suggest that the greatest variationin the cranial horn array among the subspeciesof P. orbiculare may be caused by allometric dif-ferences in the squamosal horns.
Acknowledgments.—For providing access tospecimens under their care, we thank J. Rosado,C. Cole, D. Good, J. Dixon, R. Reynolds, G.Schneider, J. Simmons, and J. Vindum. We aregrateful to L. Harder and L. Linton for muchuseful advice during the early stages of the pro-duction of this paper and P. Bergmann for refin-ing the calculation of deviations. We particularlythank W. Sherbrooke, B. Baur, and R. Montan-ucci for reading and commenting upon an ear-lier draft of the ms and for sharing theirthoughts on Phrynosoma horns; any remainingerrors are of course ours, and these individualsare not responsible for our interpretations of thedata. The comments of G. Smith and two anon-ymous reviewers resulted in an improved fin-ished product.
LITERATURE CITED
ALBERCH, P. 1985. Museum collections and the evo-lutionary study of growth and development. In E.H. Miller (ed.), Museum Collections: Their Rolesand Future in Biological Research, p. 29–41. BritishColumbia Provincial Museum Occasional PaperNo. 25. Victoria, BC, Canada.
BAUR, B., AND R. R. MONTANUCCI. 1998. Krotene-chsen. Verlag Elke Kohler, Frankfort, Germany.
BRYANT, H. C. 1911. The horned lizards of Californiaand Nevada of the genera Phrynosoma and Anota.University of California Publications in Zoology 9:1–84.
CLARKE, M. R. B. 1980. The reduced major axis of abivariate sample. Biometrika 67:441–446.
DUMAS, P. C. 1964. Species-pair allopatry in the gen-era Rana and Phrynosoma. Ecology 45:178–181.
EMERSON, S. B., AND D. M. BRAMBLE. 1993. Scaling,allometry and skull design. In J. Hanken and B. K.Hall (eds.), The Skull. Vol. 3. Functional and Evo-lutionary Mechanisms, p. 384–421. University ofChicago Press, Chicago.
ETHERIDGE, R., AND K. DE QUEIROZ. 1988. A phylog-eny of Iguanidae. In R. Estes and G. Pregill (eds.),Phylogenetic Relationships of the Lizard Families:Essays Commemorating Charles L. Camp, p. 283–367. Stanford University Press, Stanford, CA.
FIORELLO, C. V., AND R. Z. GERMAN. 1997. Heteroch-rony within species: craniofacial growth in giant,standard, and dwarf rabbits. Evolution 51:250–261.
FITCH, H. S. 1981. Sexual size differences in reptiles.University of Kansas Museum of Natural HistoryMiscellaneous Publications 70:1–72.
FROST, D. R., AND R. ETHERIDGE. 1989. A phylogeneticanalysis and taxonomy of iguanian lizards (Rep-tilia: Squamata). University of Kansas Miscella-neous Publications 81:1–65.
GEIST, V. 1987. On speciation in Ice Age mammals,with special reference to cervids and caprids. Ca-nadian Journal of Zoology 65:1067–1084.
GHISELIN, M. T. 1976. The nomenclature of corre-spondence: a new look at ‘‘homology’’ and ‘‘anal-ogy.’’ In R. B. Masterton, W. Hodos, W., and H.Jerison (eds.), Evolution, Brain and Behavior: Per-sistent Problems, p. 129–142. Lawrence ErlbaumAssociates, Publishers, Toronto, ON, Canada.
GOULD, S. J. 1966. Allometry and size in ontogenyand phylogeny. Biological Reviews 41:587–640.
HARVEY, P. H., AND M. D. PAGEL. 1991. The compar-ative method in evolutionary biology. Oxford Uni-versity Press, Oxford.
HERRING, S. W. 1993. Epigenetic and functional influ-ences on skull growth. In J. Hanken and B. K. Hall(eds.), The Skull. Vol. 1. Development, p. 129–142.University of Chicago Press, Chicago.
HOLTE, A. E., AND M. A. HOUCK. 2000. Juvenile great-er roadrunner (Cuculidae) killed by choking on aTexas horned lizard (Phrynosomatidae). South-western Naturalist 45:74–76.
HOROWITZ, S. B. 1955. An arrangement of the sub-species of the horned toad, Phrynosoma orbiculare(Iguanidae). American Midland Naturalist 54:204–218.
HUGHES, P. C. R., AND J. M. TANNER. 1970. A longi-tudinal study of the growth of the black-hoodedrat; methods of measurements and rates of growth
588 G. L. POWELL ET AL.
for the skull, limbs, nose-rump and tail length.Journal of Anatomy 106:349–370.
IMBRIE, J. 1956. Biometrical methods in the study ofinvertebrate fossils. Bulletin of American Museumof Natural History 108:211–252.
JONES, B. 1988. Biostatistics in paleontology. Geosci-ence Canada 15:3–22.
KIMURA, D. K. 1992. Symmetry and scale dependencein functional relationship regression. SystematicBiology 41:233–241.
LABARBARA, M. 1989. Analyzing body size as a factorin ecology and evolution. Annual Review of Ecol-ogy and Systematics 20:97–117.
LEAMY, L., AND D. BRADLEY. 1982. Static and growthallometry of morphometric traits in randombredhouse mice. Evolution 36:1200–1212.
LEVITON, A. E., R. H. GIBBS JR., E. HEAL, AND C. E.DAWSON. 1985. Standards in herpetology and ich-thyology. Part I. Standard symbolic codes for in-stitutional resource collections in herpetology andichthyology. Copeia 1985:802–832.
LYNN, R. T. 1965. A comparative study of display be-havior in Phrynosoma (Iguanidae). SouthwesternNaturalist 10:25–30.
MCARDLE, B. H. 1988. The structural relationship: re-gression in biology. Canadian Journal of Zoology66:2329–2339.
MILLER, R. L., AND J. S. KAHN. 1962. Statistical anal-ysis in the geological sciences. John Wiley andSons, New York.
MONTANUCCI, R. R. 1979. Notes on systematics ofhorned lizards allied to Phrynosoma orbiculare (Lac-ertilia: Iguanidae). Herpetologica 35:116–124.
. 1987. A phylogenetic study of the horned liz-ards, genus Phrynosoma, based on skeletal and ex-ternal morphology. Contributions to Science of theNatural History Museum of Los Angeles County390:1–36.
MORRISON, D. F. 1976. Multivariate Statistical Meth-ods. 2nd ed. McGraw-Hill Book Co., Toronto, ON,Canada.
MUNGER, J. C. 1986. Rate of death due to predationfor two species of horned lizard, Phrynosoma cor-nutum and P. modestum. Copeia 1986:820–824.
PIANKA, E. R. 1986. Ecology and Natural History ofDesert Lizards. Princeton University Press, Prince-ton, CT.
PIANKA, E. R, AND W. S. PARKER. 1975. Ecology ofhorned lizards: a review with special reference toPhrynosoma platyrhinos. Copeia 1975:141–162.
POTVIN, C., M. J. LECHOWICZ, AND S. TARDIF. 1990.The statistical analysis of ecophysiological re-sponse curves obtained from experiments involv-ing repeated measures. Ecology 71:1389–1400.
POWELL, G. L., AND A. P. RUSSELL. 1984. The diet ofthe eastern short-horned lizard (Phrynosoma doug-lassi brevirostre) in Alberta and its relationship tosexual size dimorphism. Canadian Journal of Zo-ology 62:428-440.
. 1985. Growth and sexual size dimorphism inAlberta populations of the eastern short-hornedlizard, Phrynosoma douglassi brevirostre. CanadianJournal of Zoology 63:139-154.
PRESCH, W. 1969. Evolutionary osteology and rela-tionships of the horned lizard genus Phrynosoma(family Iguanidae). Copeia 1969:250–275.
REEDER, T. N., AND R. R. MONTANUCCI. 2001. Phylo-genetic analysis of the horned lizards (Phrynoso-matidae: Phrynosoma): evidence from mitochondri-al DNA and morphology. Copeia 2001:309–323.
REEVE, W. L. 1952. Taxonomy and distribution of thehorned lizard genus Phrynosoma. University ofKansas Science Bulletin 34:817–916.
RICE, W. R. 1989. Analyzing tables of statistical tests.Evolution 43:223–225.
RICKER, W. E. 1984. Computation and uses of centraltrend lines. Canadian Journal of Zoology 62:1867–1905.
RIEPPEL, O. 1993. Patterns of diversity in the reptilianskull. In J. Hanken and B. K. Hall (eds.), The Skull.Vol. 2. Patterns of Structural and Systematic Diver-sity, p. 344–390. University of Chicago Press, Chi-cago.
ROTH, V. L. 1994. Within and between organisms: re-plicators, lineages and homologues. In B. K. Hall(ed.), Homology. The Hierarchical Basis of Com-parative Biology, p. 301–337. Academic Press, NewYork.
SEIM, E., AND B.-E. SAETHER. 1983. On rethinking al-lometry: which regression model to use? Journal ofTheoretical Biology 104:161–168.
SHERBROOKE, W. C. 1981. Horned Lizards: UniqueReptiles of Western North America. SouthwestParks and Monuments, Globe, AZ.
. 1987. Defensive head posture in horned liz-ards (Phrynosoma: Sauria: Iguanidae). SouthwesernNaturalist 32:512–515.
. 1990. Predatory behavior of captive greaterroadrunners feeding on horned lizards. WilsonBulletin 102:171–174.
. 1991. Behavioral (predator-prey) interactionof captive grasshopper mice (Onychomys torridus)and horned lizards (Phrynosoma cornutum and P.modestum). American Midland Naturalist 126:187–195.
SHERBROOKE, W. C., AND R. R. MONTANUCCI. 1988.Stone mimicry in the round-tailed horned lizard,Phrynosoma modestum (Sauria: Iguanidae). Journalof Arid Environments 14:275–284.
SMITH, H. L. 1946. Handbook of Lizards. ComstockPublishing Associates, Comstock, NY.
SOKAL, R. R., AND F. J. ROHLF. 1995. Biometry. ThePrinciples and Practice of Statistics in BiologicalResearch. W. H. Freeman and Co., New York.
WAGNER, G. P. 1989. The biological homology con-cept. Annual Review of Ecology and Systematics20:51–69.
. 1994. Homology and the mechanisms of de-velopment. In B. K. Hall (ed.), Homology: The Hi-erarchical Basis of Comparative Biology. AcademicPress, New York.
WEIJS, W. A., P. BRUGMAN, AND E. M. KLOK. 1987. Thegrowth of the skull and jaw muscles and its func-tional consequences in the New Zealand rabbit(Oryctolagus cuniculus). Journal of Morphology 194:143–161.
ZAMUDIO, K. R. 1998. The evolution of female-biasedsexual size dimorphism: a population level com-parative study in horned lizards (Phrynosoma).Evolution 52:1821–1833.
Accepted: 8 February 2002.
589PHRYNOSOMA HORN SCALING
APPENDIX 1Specimens Examined
List of specimens of Phrynosoma orbiculare examinedin this study. Institutional abbreviations conform withusages given in Leviton et al., 1985.
AMNH 118128, 62321, 75866; CAS 156548–49,156561, 169778; MCZ 169817, 169818, 169819, 169821,169822, 169823, 169825, 169826, 169827, 169828,169829; TCWC 19615, 22021, 38505, 38506, 38507,
38554, 38555, 38556, 38558, 40689, 51615, 54227, 54228,54229, 54428, 54429, 54430, 6414, 6415, 944; USNM194851, 293489, 293490, 293491, 293492, 293493,293494, 293495, 293496, 47157, 47158, 47159, 47874,47875, 47877, 47878, 47879, 47880, 47881, 47882; MVZ24350, 52143, 67418, 68953; KU 25861, 25866, 25882;UMMZ 102229, 102601, 102603, 102605, 111504,113640, 113641, 113642, 114153, 114154, 114155, 3855,95191, 99921.
Journal of Herpetology, Vol. 36, No. 4, pp. 589–597, 2002Copyright 2002 Society for the Study of Amphibians and Reptiles
Diet of Crotalus lepidus klauberi (Banded Rock Rattlesnake)
ANDREW T. HOLYCROSS,1,2 CHARLES W. PAINTER,3 DAVID B. PRIVAL,4 DON E. SWANN,4
MICHAEL J. SCHROFF,4 TAYLOR EDWARDS,4 AND CECIL R. SCHWALBE5
2Department of Biology, Arizona State University, Tempe, Arizona 85287-1501, USA;E-mail: [email protected]
3Endangered Species Program, New Mexico Department of Game and Fish, P.O. Box 25112,Santa Fe, New Mexico 87504, USA
4School of Renewable Natural Resources, Biosciences East, University of Arizona, Tucson, Arizona 85721, USA5United States Geological Survey, Sonoran Desert Field Station, 125 Biosciences East, University of Arizona,
Tucson, Arizona 85721, USA
ABSTRACT.—We describe the diet of Crotalus lepidus klauberi (Banded Rock Rattlesnake) using samplescollected in the field and from museum specimens, as well as several records from unpublished reports.Most records (approximately 91%) were from the northern Sierra Madrean Archipelago. Diet consisted of55.4% lizards, 28.3% scolopendromorph centipedes, 13.8% mammals, 1.9% birds, and 0.6% snakes. Sceloporusspp. comprised 92.4% of lizards. Extrapolation suggests that Sceloporus jarrovii represents 82.3% of lizardrecords. Diet was independent of geographic distribution (mountain range), sex, source of sample (stomachvs. intestine/feces), and age class. However, predator snout–vent length differed significantly among preytypes; snakes that ate birds were longest, followed in turn by those that ate mammals, lizards, and centi-pedes. Collection date also differed significantly among prey classes; the mean date for centipede recordswas later than the mean date for squamate, bird, or mammal records. We found no difference in the elevationof collection sites among prey classes.
Descriptive natural history studies are essen-tial for both the development of biological the-ory and conservation efforts (Dodd, 1987;Greene, 1994). Dietary studies are particularlyimportant for understanding snake biology, be-cause diet is a primary force in the evolution ofsnake morphology and behavior (Gans, 1961;Cock Buning, 1983; Greene, 1983; Mushinsky,1987; Greene, 1992, 1997; Rodrıguez-Robles etal., 1999; Rodrıguez-Robles and Greene, 1999).As landscapes become increasingly fragmentedand climate change alters species distributions(Meffe and Carroll, 1997), a thorough knowl-edge of diet of affected taxa will be vital forsuccessful conservation. Based in part on the
1 Corresponding Author.
difficulty of obtaining large sample sizes, infor-mation regarding the diet of most snake speciesis anecdotal (Mushinsky, 1987). Herein, we de-scribe and evaluate variability in the diet of Cro-talus lepidus klauberi (Banded Rock Rattlesnake),and discuss autecological and conservation im-plications. A collaborative approach, combiningdata from field studies with data obtained frommuseum specimens and historical records, per-mits us to progress beyond the anecdotal.
As its common name implies, Crotalus lepidus(Rock Rattlesnake) is predominantly saxicolousand found on ridges and in drainages in aridand semiarid hillsides or mountains from 300–2930 m (Stebbins, 1985). Crotalus lepidus is dis-tributed from southeastern Arizona, southernNew Mexico and west Texas south to Jalisco,
590 A. T. HOLYCROSS ET AL.
Mexico, (Stebbins, 1985). Four subspecies arerecognized, C. l. klauberi, Crotalus lepidus lepidus,Crotalus lepidus maculosus, and Crotalus lepidusmorulus (Liner, 1994; Crother, 2000). With the ex-ception of a quantitative treatment comparingthe diet of high- and low-elevation populationsof C. l. lepidus (Beaupre, 1995), published infor-mation on the diet of C. lepidus consists primar-ily of isolated observations (Appendix 1). Here-in, we provide a quantitative analysis of noveldata for C. l. klauberi at the northern end of itsdistribution in Chihuahua, northern Sonora,southeastern Arizona, and southwestern NewMexico.
MATERIALS AND METHODS
Fecal samples were obtained from field en-counters with C. l. klauberi in the Animas (NM;1994–1999), Chiricahua (AZ; 1995–1996 and1999), Huachuca (AZ; 1998), and Peloncillo (AZand NM; 1995–1998) mountains in the course ofother studies. Sampling spanned April to Oc-tober, although search effort was generally bi-ased toward late summer and early fall (July toOctober). We marked individuals using passiveintegrated transponders (see Jemison et al.,1995), recorded snout–vent length (SVL; mm)and mass (g) and determined sex by probing(Schaefer, 1934). Although most fecal sampleswere obtained directly via gentle palpation, afew feces were naturally voided and collectedfrom individual holding containers (althoughsnakes were not held for this purpose). All feceswere preserved for later identification. We didnot attempt to palpate stomach contents fromlive specimens but include data from one spon-taneously regurgitated prey. Also included inour analyses are detailed, quantitative data onprey remains identified from the feces of wild-caught C. l. klauberi in two unpublished reports(Barker, 1991; McCrystal et al., 1996). Camp-bell’s (1934) prey records were included amongthe museum specimens we examined. In thecourse of collecting specimens and pursuingother fieldwork, we observed several hundredC. l. klauberi, made repeated observations on fiveradio-tracked individuals (Smith et al., 2001),and recorded many observations relevant to for-aging behavior.
In addition, we examined 256 specimens of C.l. klauberi (Appendix 2) from the following in-stitutional collections: ASU, CAS, CM, KU,LACM, MSB, MVZ, SDSNH, UAZ, and UMMZ(symbolic codes follow Leviton et al., 1985). Weexamined the stomach and posterior 3 cm of theintestine for prey remains. Prey from specimenshoused in captivity prior to preservation orwhich appeared to have been fed in captivity(allopatric or domestic prey) were omitted.
Our combined sample includes prey remains
from C. l. klauberi collected in the HuachucaMountains (AZ; N 5 36), Animas Mountains(NM; N 5 33), Peloncillo Mountains (AZ andNM; N 5 29), Chiricahua Mountains (AZ; N 526), Sierra San Luis (Sonora and Chihuahua; N5 13), Sierra Madre Occidental (Sonora andChihuahua; N 5 7), Sierra del Nido (Chihua-hua; N 5 5), Santa Rita Mountains (AZ; N 5 2),Organ Mountains (NM; N 5 2), San AndresMountains (NM; N 5 2), Dos Cabezas Moun-tains (AZ; N 5 1), San Mateo Mountains (NM;N 5 1), and Martin Canyon (NM; N 5 1).
Lizard remains were identified to the greatesttaxonomic resolution possible based on wholeremains or diagnostic scale characters for resi-dent lizards. Mammals were identified to genususing characteristics (gross morphology, medul-la configuration, and scale pattern) of dorsalguard hairs (see Moore et al., 1974). Centipedeswere identified as Scolopendra spp. from wholeremains or exoskeletal remnants of chelicerae,leg, and/or body segments. Birds were diag-nosed as such from feathers or feather frag-ments.
For statistical analyses we (1) grouped preyinto four classes (mammals, squamates, centi-pedes, and birds) and (2) classified snakes asadults ($ 350 mm SVL) or juveniles (, 350 mmSVL) based on reproductive data presented byGoldberg (2000). We compared diet amongmountain ranges with N . 20 (Animas, Chiri-cahua, Huachuca, and Peloncillo mountains).Statistics were computed using BIOMstat ver-sion 3.3 or SPSS version 10.1. Means are report-ed 6 1 SE. Data used in parametric analyseswere normal (Shapiro-Wilk, P . 0.05 for alltests). Missing or unavailable data for some var-iables explains variation in sample size amongtests. Nominal significance level was set at a 50.05 and Dunn-Sidak adjusted (Sokal and Rohlf,1995) for multiple tests of the same dataset us-ing the same test statistic (Cabin and Mitchell,2000). Specifically, for four RxC tests of inde-pendence using all prey records a9 5 0.013 andfor three one-way ANOVA tests a9 5 0.017.
RESULTS
A total of 159 prey were identified (Table 1).In the field, 81 (20%) of approximately 404 cap-tures (including recaptures) yielded 89 prey re-cords: 88 identified from feces and one fromstomach contents. Eight fecal samples containedremains of two prey species (centipede 1 lizard,N 5 6; centipede 1 mammal, N 5 2). Four in-dividuals contained identifiable prey on discretecapture events separated by more than twomonths (centipede, centipede, N 5 2; mammal,lizard, N 5 2). Sixty-seven (26%) of 256 C. l.klauberi museum specimens contained 70 iden-tifiable prey; 43 identified from intestinal con-
591ROCK RATTLESNAKE DIET
TABLE 1. Prey consumed by Crotalus lepidus klauberiin this study (including prey records reported inCampbell, 1934; Barker, 1991; and McCrystal et al.,1996). Numbers in brackets indicate percents and to-tals by major taxonomic grouping (Arthropoda, Aves,Mammalia, Squamata).
Prey taxonPercent
total N
ArthropodaScolopendra spp.
[28.3]28.3
[45]45
AvesUnidentified bird
[1.9]1.9
[3]3
MammaliaUnidentified mammalPeromyscus spp.Chaetodipus spp.‘‘shrew’’
[13.8]5.05.02.51.3
[22]8842
SquamataUnidentified snakeUnidentified lizardCnemidophorus spp.Sceloporus spp.Sceloporus clarkiiSceloporus jarroviiSceloporus virgatusUrosaurus ornatus
[56.0]0.65.73.2
25.80.6
18.21.30.6
[89]195
411
2921
Total 100.0 159FIG. 1. Association between prey class and (A)
predator (Crotalus lepidus klauberi) snout–vent length(N 5 159), (B) collection date ( N 5 157), and (C)elevation (N 5 120).
tents and 27 from stomach contents. Three spec-imens contained more than one prey type(mammal 1 centipede, N 5 1; lizard 1 centi-pede, N 5 1; lizard 1 lizard, N 5 1). Small ar-thropod parts (, 3 mm) occurred exclusively inspecimens that contained well-digested insec-tivorous lizards, and were considered the resultof secondary ingestion.
We identified remains of 88 (55.4%) lizards,45 (28.3%) centipedes, 22 (13.8%) mammals,three (1.9%) birds and one (0.6%) snake (Table1). Sceloporus spp. accounted for 92.4% of iden-tifiable lizards. Sceloporus jarrovii comprised87.9% of Sceloporus identified to species. Extrap-olation suggests S. jarrovii comprised approxi-mately 82% of lizards consumed and 45% ofprey overall. One of two S. virgatus, the only S.clarkii, and four of five Cnemidophorus spp. wereobtained from specimens collected in the Pelon-cillo Mountains. All centipedes were identifiedas Scolopendra spp., four of which were found insamples containing other prey. Peromyscus spp.comprised 57% of identified mammals (Table 1).Three of four mammals eaten by Peloncillospecimens were Chaetodipus spp. The two small-est (282 and 284 mm SVL) C. l. klauberi that con-sumed mammals had both eaten shrews (eitherSorex arizonae or Notiosorex crawfordi). It was notpossible to identify birds beyond class, becauseof the limited amount and condition of remains.
Remains of the only snake consumed were re-moved from a specimen (MVZ 73094) collectedat approximately 2740 m in the Sierra del Nido,Chihuahua, the highest elevation collection sitein this dataset. Direction of ingestion was deter-mined for 13 lizards, four mammals, and twocentipedes. Judging by orientation in the stom-ach, all 17 tetrapods were swallowed headfirstwhereas one centipede was swallowed tail-firstand the other headfirst.
Predator SVL differed significantly amongprey classes (ANOVA, F3,155 5 6.38, P , 0.001;Fig. 1A). Snakes that ate birds were longest(mean SVL 5 484 6 24 mm, N 5 3), followedby those that ate mammals (mean SVL 5 437 619 mm, N 5 22), squamates (mean SVL 5 4136 11 mm, N 5 89), and centipedes (mean SVL5 353 6 12 mm, N 5 45). In post hoc multiplecomparisons, SVL of snakes that ate centipedesdiffered significantly from snakes that ate squa-mates or mammals (Tukey HSD, P , 0.004); allother pairwise comparisons were not signifi-cant. Date of collection differed significantlyamong prey classes (ANOVA, F3,153 5 4.66, N 5157, P , 0.004; Fig. 1B). Mean date of squamateconsumption was earliest (17 July), followed bybirds (24 July), mammals (27 July), and centi-pedes (16 August). In posthoc multiple compar-
592 A. T. HOLYCROSS ET AL.
FIG. 2. Scatter-plot of prey records by predator(Crotalus lepidus klauberi) SVL and date of collection.Solid circles indicate centipede records, and open cir-cles indicate all other prey. The dashed and solid linesrepresent the association (Kendall’s Robust Line-fit)between the variables for all snakes that ate prey (SVL5 477.4 2 0.38 [Julian date]) and those snakes that atecentipedes (SVL 5 290.8 1 0.25 [Julian date]), respec-tively.
isons, only centipede and squamate records dif-fered significantly in mean date of collection(Tukey HSD, P 5 0.001). Using Kendall’s RobustLine-fit, we found that among snakes that con-tained prey, SVL 5 477.4 2 0.38 (Julian date).The slope describes a slight decrease in SVL(approximately 4 cm) over the course of the ac-tive season that is significantly different from azero slope (t 5 20.1133, P 5 0.035; Fig. 2).Among snakes that ate centipedes, SVL 5 290.81 0.25 (Julian date), but the slope did not differsignificantly from zero (t 5 0.0919, P . 0.37;Fig. 2).
Prey class was independent of predator ageclass (RxC test of independence, GWilliams 5 7.4,df 5 3, P 5 0.06, N 5 158), mountain range(GWilliams 5 15.1, df 5 9, P . 0.08, N 5 124),source (stomach vs. intestine/feces) of sample(GWilliams 5 4.7, df 5 2, P 5 0.20 N 5 159), andsex (GWilliams 5 2.7, df 5 3, P . 0.44, N 5 158).The elevation at which specimens were collecteddid not differ significantly among prey classes(ANOVA, F3,116 5 0.94, N 5 120, P 5 0.42; Fig.1C).
DISCUSSION
Most general works suggest C. lepidus dietconsists mainly of lizards, especially Sceloporusspp., but also note that mammals, snakes, frogs,birds, and centipedes are consumed (Gloyd,1937; Armstrong and Murphy, 1979; Stebbins,1985; Tennant, 1985; Lowe et al., 1986; Ernst,1992; Degenhardt et al., 1996; Price, 1998; Bart-lett and Tennant, 2000; Werler and Dixon, 2000).However, snakes, frogs, and birds are infre-quently reported, and explicit reports of centi-pedes being eaten in the wild have not beenpublished in the primary literature (Appendix1). Tennant (1985) presumed limited geographic
variability in diet when he described C. l. klaub-eri diet as identical to that of C. l. lepidus. How-ever, comparison of our data (Table 1), Beaupre’s(1995) data, and literature records for both sub-species (Appendix 1) suggests noteworthy geo-graphic variation in diet (e.g., the absence ofcentipede records from C. l. lepidus). Data hereindemonstrate that, although lizards and mam-mals are important prey of C. l. klauberi, centi-pedes also constitute a significant portion ofdiet.
Our field observations suggest that rocky out-crops and talus are favored hunting grounds.We often found C. l. klauberi in loose S-shapedpostures against sides of rocks, or in vertical fis-sures in rocks, with the head directed towardthe lip of the rock. We assume these snakes areambush hunting for diurnal S. jarrovi that areparticularly abundant and active in these habi-tats. These behaviors are not uncommon amongsnakes that habitually prey on saxicolous lizards(e.g., Downes and Shine, 1998), and our obser-vations are similar to previous descriptions offoraging posture and ambush-site selection byC. lepidus (Beaupre, 1995; McCrystal et al., 1996).Juvenile C. l. klauberi use their orange tails tolure lizards (Kauffeld, 1943b; Starrett and Ho-lycross, 2000) and unlike many congeners, oftenretain this pigmentation into early adulthood.
The proportion of centipedes consumed issurprising given the dearth of literature recordsdocumenting centipedes as natural prey of C.lepidus (Appendix 1) and in comparison to pro-portions in the diets of other small rattlesnakes(e.g., Sistrurus miliarus, Hamilton and Pollack,1955; Crotalus enyo, Taylor, 2001; Crotalus w. ob-scurus, Holycross et al., in press; Sistrurus caten-atus edwardsii, Holycross and Mackessy, 2002).The presence of centipedes in the diet of thesesmall, generally primitive rattlesnakes and someother pit vipers (Bothrops asper, Greene, 1992)suggests that they might have been a significantpart of the diet of early rattlesnakes or perhapsmore generally, early pit vipers. Unlike sympat-ric and often syntopic populations of C. w. ob-scurus, C. l. klauberi continue to eat centipedesinto adulthood, thus contributing to their pro-portional prevalence. The pervasiveness of soli-tary centipede records in diets of several smallrattlesnakes, in addition to observations in cap-tivity (Rubio, 1998), suggests direct predationon live centipedes rather than secondary inges-tion or incidental scavenging. Crotalus lepidusmay occasionally take other arthropods (e.g., in-sects, scorpions) early in life, as suggested byseveral records from the wild and observationsin captivity (Appendix 1).
Adult C. l. klauberi prey infrequently on mam-mals when compared with adult congeners(Klauber, 1972; Mushinsky, 1987), including
593ROCK RATTLESNAKE DIET
smaller, sympatric C. w. obscurus (Holycross etal., in press). Peromyscus boylii is the most fre-quently trapped small mammal in many habi-tats occupied by C. l. klauberi (Hoffmeister andGoodpaster, 1954; Cook, 1986), and is especiallyabundant in talus (62% of captures; Holycrosset al., in press). Thus, mammals appear to bean abundant, yet relatively unexploited, re-source. These disparities hint at the possibilityof innate differences in foraging behaviors. Forexample, are C. l. klauberi more likely to establishambush postures in response to lizard chemicalcues than mammalian odors? Although suchhypotheses remain untested, it is interesting tonote that C. l. klauberi scored significantly lowerthan C. v. viridis in ability to trail rodent prey inlaboratory trials (Chiszar et al., 1986).
Smaller C. l. klauberi ate smaller, more elon-gate prey, consistent with the prediction thatprey diameter/predator head size and prey/predator body mass ratios limit smaller snakesto smaller, more ingestible prey (Reynolds andScott, 1982; Greene, 1983; Arnold, 1993). Nev-ertheless, proportion of prey classes consumeddid not significantly differ between age classes,indicating that variability in prey consumed issize-based, and not related to reproductive ma-turity.
In this study, mean elevation of collection sitesdid not differ significantly among prey classes.In contrast, C. l. lepidus from a xeric, low-eleva-tion site ate a significantly higher proportion ofmammalian prey than specimens from a rela-tively mesic, high-elevation site in south Texas(Beaupre, 1995). However, the low-elevationpopulation exhibited greater activity at nightwhen rodents are more likely to be active, andlow defecation rates suggest this populationmay be resource-limited (Beaupre, 1995). In ourarea, C. l. klauberi generally occur at higher ele-vations and appear to be primarily diurnal andcrepuscular, although individuals may becomenocturnal during warm months (Degenhardt etal., 1996; ATH, pers. obs.).
Although mean elevation of collection sitesdid not differ significantly among prey classes,our data suggest that composition of some preyclasses might vary with elevation. The species oflizards and rodents in the Peloncillo sample ap-pear to differ from the other sites in our sample,perhaps because this mountain range is lowerin elevation and less wooded. Sceloporus jarroviiand P. boylii, both of which are important preyin high-elevation habitats, appear to be lessabundant in the Peloncillo Mountains (ATH,pers. obs.). Conversely, several lizards (e.g.,Cnemidophorus exsanguis, C. sonorae, and Scelopo-rus clarkii) and certain rodents (e.g., Chaetodipusspp.) appear to be more abundant (ATH, pers.obs.).
The later mean collection date for centipederecords might be based, in part, on a confound-ing effect. On average, snakes that ate centi-pedes were smaller than those that ate otherprey (Fig. 1A), and there was a negative asso-ciation between snake SVL and date (Fig. 2).However, centipede records are evenly distrib-uted among snake lengths across all seasons(Fig. 2), suggesting that seasonal bias in the sizeof snakes that ate centipedes does not accountfor the seasonal variation. Thus, the differencebetween the mean date of collection for centi-pede and lizard prey probably reflects seasonalvariation in prey activity patterns or encounterrates. Two trends in the data appear to influencethis difference. First, centipede records are in-frequent in the spring and early summer, andsecond, the proportion of centipedes in the fallis dramatically higher. Centipedes cannot closetheir spiracles (Pechenik, 2000) and lose waterrapidly when humidity is low (Lewis, 1981) inthe spring and early summer. Increased surfaceactivity by Scolopendra spp. coincides with therainy season (J. Schmidt, pers. comm.) in Julyand August when centipedes begin to increasein frequency in the diet of C. l. klauberi. In ad-dition, the proportion of centipedes relative tolizards eaten (most records from adult snakes)shifts conspicuously in early September, per-haps because of decreasing activity of lizards inthe fall. Crotalus l. klauberi are born with the ad-vent of the rainy season, when neonate Scelopo-rus jarrovii and increased surface activity byScolopendra spp. provide an abundant food re-source for neonatal, gape-limited predators.
Although we expected a prevalence of lizardsin the diet of C. l. klauberi, the proportion of themodal prey genus, Sceloporus, was surprising.When compared to the mean proportion withwhich 51 other lizard-eating snakes consumetheir modal prey genus (Rodrıguez-Robles andGreene, 1999; combining their original data onR. lecontei with their summary of data for 50other species), it falls in the upper tail (10%) ofthe distribution (Fig. 3). Clearly, C. l. klauberi re-lies heavily on its modal prey genus. But is C. l.klauberi a ‘‘specialist?’’ The operational difficul-ties of labeling a species an ecological or evo-lutionary specialist are addressed by Rodrı-guez-Robles and Greene (1999). From an eco-logical perspective, a specialist must consume aprey species out of proportion to its availability.Although Sceloporus jarrovii is certainly the mostabundant lizard in many habitats occupied byC. l. klauberi, it would be difficult to determinewhether it is consumed in proportion to theavailability of prey overall. Indeed, defining‘‘abundance’’ for ambush predators is problem-atic, since prey encounter rates may not neces-sarily reflect their abundance in the environ-
594 A. T. HOLYCROSS ET AL.
FIG. 3. Distribution of the percentage of the modalgenus (among lizard prey) in the diet of 51 snakes thatprey primarily on lizards (data from: Rodrıguez-Ro-bles and Greene, 1999). The sample mean is indicatedby m and percentage of Sceloporus spp. in the diet ofCrotalus lepidus klauberi is indicated by Clk.
ment. Nevertheless, appropriately sized (ingest-ible) rodents appear to be an abundant resourcein C. l. klauberi habitat. Indeed, adult C. w. obscu-rus, a slightly smaller and sympatric rattlesnake,prey primarily on rodents (62.3%), but also relyon lizards (26.4%; Holycross et al., in press).
From an evolutionary perspective, dietaryspecialization implies the presence of adapta-tions that aid in the acquisition of a particularprey type. Crotalus l. klauberi consume Sceloporusjarrovi and Scolopendromorph centipedes pri-marily, and appear to have maintained preda-tory behaviors that maximize foraging successfor these prey. Ambush site selection and for-aging posture described earlier appear to be ad-aptations for preying on saxicolous lizards.Likewise, orange tail tips and caudal luring ap-pear to be adaptations for luring lizards. How-ever, C. lepidus falls in a clade of small, primitiverattlesnakes (Klauber, 1972) that, like manysmall viperids, prey primarily on lizards(Greene, 1992) and perhaps centipedes. Al-though reliance on lizard and centipede preymight represent retention of a plesiomorphicdiet and related adaptations, C. l. klauberi doesnot fully exploit rodent resources that seem tobe readily available based on trapping data anddiet of similarly sized, sympatric members ofthe same guild (Holycross et al., in press). Thus,although considering C. l. klauberi a specialist isoperationally difficult, recognition of a retainednarrow trophic niche seems warranted.
Acknowledgments.—We thank those who ded-icated one or more summers of field assistance:H. Amrhein, M. Avent, D. Bell, H. Blankenship,J. Borgmeyer, T. Britton, C. Cole, G. Cook, P.Daniel, T. Devitt, P. Dickey, D. Emmons, B. Fe-dorko, M. Feldner, O. Fourie, G. LeGalliard, D.Germaine, M. Goode, B. Hamilton, A. Holland,L. Ireland, L. Kamees, D. Kirk, E. Koeck, S. Kol-vek, J. Lokke, J. Manzer, J. Martinez, J. Parsons,
R. Phillips, D. Pires, A. Powers, R. Reed, B. Rich-ardson, J. Sifert, J. Sigala Rodrıguez, L. Slone, L.Smith, B. Starrett, T. Tully, M. Wall, and F. Ward.We also thank the innumerable unnamed vol-unteers that assisted us in the field for shorterdurations. We thank M. Douglas (ASU), J. Vin-dum (CAS), S. Rogers (CM), J. Simmons (KU),K. Beaman (LACM), J. Giermakowski (MSB), C.Cicero (MVZ), B. Hollingsworth (SDSNH), G.Bradley (UAZ), and R. Nussbaum and G.Schneider (UMMZ) for loaning specimens un-der their care. Financial support provided by theWallace Research Foundation. The following or-ganizations provided funding for related proj-ects and thus facilitated acquisition of samplesin the field: New Mexico Department of Gameand Fish (NMDGF) Endangered Species Pro-gram, Arizona Game and Fish Department(AGFD) Nongame Branch, AGFD Heritage Fund(IIPAM I95048), AGFD Nongame Check-offFund, New England Herpetological Society,Southwest Parks and Monuments Association,Malpai Borderlands Group, National Park Ser-vice, Animas Foundation, U.S. Forest ServiceRocky Mountain Research Station, and U.S. Fishand Wildlife Service. Access to private landsgranted by the Animas Foundation. Field spec-imens handled under authority of scientific col-lecting permits (ATH: AGFD SP605602,SP648632, SP711300, SP779370, SP841338 andNMDGF 2824; CRS: AGFD SP774669, SP849158and CNM 98-005). Institutional Animal Careand Use Committee protocols: ASU 93-280R,UA 97-080, and UA 94-067. A. Price, B. Graves,and an anonymous reviewer provided helpfulcomments that improved the manuscript.
LITERATURE CITED
ARMSTRONG, B. L., AND J. B. MURPHY. 1979. The Nat-ural History of Mexican Rattlesnakes. University ofKansas Museum of Natural History Special Pub-lication No. 5. Lawrence.
ARNOLD, S. 1993. Foraging theory and prey-sizepredator-size relations in snakes. In R. A. Seigeland J. T. Collins (eds.), Snakes: Ecology and Be-havior, pp. 87–115. McGraw-Hill, New York.
AXTELL, R. W. 1959. Amphibians and reptiles of theBlack Gap Wildlife Management Area, BrewsterCounty, Texas. Southwestern Naturalist 4:88–109.
BARKER, D. G. 1991. An investigation of the naturalhistory of the New Mexico ridgenose rattlesnake,Crotalus willardi obscurus. Report to EndangeredSpecies Program, New Mexico Department ofGame and Fish, Santa Fe.
BARTLETT, R. D. AND A. TENNANT. 2000. Snakes ofNorth America: Western Region. Gulf PublishingCo., Houston, TX.
BEAUPRE, S. J. 1995. Comparative ecology of the mot-tled rock rattlesnake, Crotalus lepidus, in Big BendNational Park. Herpetologica 51:45–56.
BRYSON JR., R. W., J. BANDA, AND D. LAZCANO. 2002.
595ROCK RATTLESNAKE DIET
Crotalus lepidus maculosus. Diet. Herpetological Re-view 33:139-140.
CABIN, R. J., AND R. J. MITCHELL. 2000. To Bonferronior not to Bonferroni: when and how are the ques-tions. Bulletin of the Ecological Society of America81:246–248.
CAMPBELL, B. 1934. Report on a collection of reptilesand amphibians made in Arizona during the sum-mer of 1933. Occasional Papers of the Museum ofZoology, University of Michigan (289), Ann Arbor.
CHISZAR, D., C. RADCLIFFE, AND F. FEILER. 1986. Trail-ing behavior in banded rock rattlesnakes (Crotaluslepidus klauberi) and prairie rattlesnakes (C. viridisviridis). Journal of Comparative Psychology 100:368–371.
COCK BUNING, T. DE. 1983. Thermal sensitivity as aspecialization for prey capture and feeding insnakes. American Zoologist 23:363–375.
CONANT, R. 1955. Notes on three Texas reptiles, in-cluding an addition to the fauna of the state. Amer-ican Museum Novitates 1726:1–6.
COOK, J. A. 1986. The mammals of the AnimasMountains and adjacent areas, Hidalgo County,New Mexico. Occasional Papers of the Museum ofSouthwestern Biology (4), University of New Mex-ico, Albuquerque.
CROTHER, B. I. (CHAIR) 2000. Scientific and standardEnglish names of amphibians and reptiles of NorthAmerica north of Mexico, with comments regard-ing confidence in our understanding. Society forthe Study of Amphibians and Reptiles Herpetolog-ical Circular (29), St. Louis, MO.
DEGENHARDT, W. G., C. W. PAINTER, AND A. H. PRICE.1996. The amphibians and reptiles of New Mexico.University of New Mexico Press, Albuquerque.
DICKERMAN, R. W., AND C. W. PAINTER. 2001. Crotaluslepidus lepidus. Diet. Herpetological Review 32:46.
DODD JR., C. K. 1987. Status, conservation, and man-agement. In R. A. Seigel, J. T. Collins, and S. S.Novak (eds.), Snakes: Ecology and EvolutionaryBiology, pp. 478–513. McGraw-Hill Publishing Co.,New York.
DOWNES, S., AND R. SHINE. 1998. Sedentary snakesand gullible geckos: predator-prey coevolution innocturnal rock-dwelling reptiles. Animal Behav-iour 55:1373–1385.
ERNST, C. H. 1992. Venomous Reptiles of NorthAmerica. Smithsonian Institution Press, Washing-ton, DC.
FALCK, E. G. J. 1940. Food of an eastern rock rattle-snake in captivity. Copeia 1940:135.
GANS, C. 1961. The feeding mechanism of snakes andits possible evolution. American Zoologist 1:217–227.
GLOYD, H. K. 1937. A herpetological consideration offaunal areas in southern Arizona. Bulletin of theChicago Academy of Sciences 5:79–136.
GLOYD, H. K., AND H. M. SMITH. 1942. Amphibiansand reptiles from the Carmen Mountains, Coahui-la. Bulletin of the Chicago Academy of Sciences 6:231–235.
GOLDBERG, S. R. 2000. Reproduction in the rock rat-tlesnake, Crotalus lepidus (Serpentes: Viperidae).Herpetological Natural History 7:83–86.
GREENE, H. W. 1983. Dietary correlates of the origin
and radiation of snakes. American Zoologist 23:431–441.
. 1992. The ecological and behavioral contextfor pitviper evolution. In J. A. Campbell and E. D.Brodie Jr. (eds.), Biology of the Pitvipers, pp. 107–117. Selva, Tyler, TX.
. 1994. Systematics and natural history, foun-dations for understanding and conserving biodi-versity. American Zoologist 34:48–56.
. 1997. Snakes: The Evolution of Mystery inNature. University of California Press, Berkeley.
HAMILTON JR., W. J., AND J. A. POLLACK. 1955. Thefood of some crotalid snakes from Fort Benning,Georgia Natural History Miscellanea 140:1–4.
HARRIS JR., H. S., AND R. S. SIMMONS. 1977. Addi-tional notes concerning cannibalism in pit vipers.Bulletin of the Maryland Herpetological Society 13:121–122.
HOFFMEISTER, D. F., AND W. W. GOODPASTER. 1954.The mammals of the Huachuca Mountains, south-eastern Arizona. Illinois Biological Monographs24(1), University of Illinois Press, Urbana.
HOLYCROSS, A. T., AND S. P. MACKESSY. 2002. Varia-tion in the diet of Sistrurus catenatus (Massasauga),with emphasis on Sistrurus catenatus edwardsii(Desert Massasauga). Journal of Herpetology 36:454-464.
HOLYCROSS, A. T., C. W. PAINTER, D. G. BARKER, AND
M. E. DOUGLAS. In Press. Foraging ecology of thethreatened New Mexico Ridge-nosed Rattlesnake,Crotalus willardi obscurus. In G. W. Schuett, M. Hog-gren, M. E. Douglas, and H. W. Greene (eds.), Bi-ology of the Vipers. Eagle Mountain Publishing,Eagle Mountain, UT.
JEMISON, S. C., L. A. BISHOP, P. G. MAY, AND T. M.FARRELL. 1995. The impact of PIT-tags on growthand movement of the rattlesnake, Sistrurus mili-arus. Journal of Herpetology 29:129–132.
KAUFFELD, C. F. 1943a. Fieldnotes on some Arizonareptiles and amphibians. American Midland Nat-uralist 29:342–358.
. 1943b. Growth and feeding of new-bornPrice’s and green rock rattlesnakes. American Mid-land Naturalist 29:607–614.
KLAUBER, L. M. 1972. Rattlesnakes: Their Habits, LifeHistories, and Influence on Mankind. 2nd ed. 2 vol.University of California Press, Berkeley.
LEVITON, A. E., R. H. GIBBS JR., E. HEAL, AND C. E.DAWSON. 1985. Standards in herpetology and ich-thyology. Part I. Standard symbolic codes for in-stitutional resource collections in herpetology andichthyology. Copeia 1985:802–832.
LEWIS, J. G. E. 1981. The Biology of Centipedes. Cam-bridge University Press, Cambridge.
LINER, E. A. 1994. Scientific and Common Names forthe Amphibians and Reptiles of Mexico in Englishand Spanish. Society for the Study of Amphibiansand Reptiles Herpetological Circular (23), St. Lou-is, MO.
LOWE, C. H., C. R. SCHWALBE, AND T. B. JOHNSON.1986. The Venomous Reptiles of Arizona. ArizonaGame and Fish Department, Phoenix.
MARR, J. C. 1944. Notes on amphibians and reptilesfrom the central United States. American MidlandNaturalist 32:478–490.
MCCRYSTAL, H. K., C. R. SCHWALBE, AND D. F. RETES.
596 A. T. HOLYCROSS ET AL.
APPENDIX I.Summary of Original Prey Records for Crotalus lepidus in the Literature
An asterisk (*) denotes prey consumed in captivity. A plus (1) indicates observations that may be the resultof prior ingestion by insectivorous prey. Crotalus lepidus lepidus, Crotalus lepidus klauberi, and Crotalus lepidusmorulus are denoted by Cll, Clk, and Clm, respectively.
Prey Ssp. Reference
ArthropodaScolopendra spp.‘‘arthropods’’‘‘crickets’’‘‘large grasshopper’’
ClkCllClkCll
Barker, 1991; Rubio, 1998*Beaupre, 19951
McCrystal et al., 1996*Conant, 19551
AvesBird Cll Klauber, 1972
Mammalia‘‘mouse’’ Clk Kauffeld, 1943a*; Woodin, 1953; McCrystal et al., 1996*‘‘mammal’’ Cll Klauber, 1972; Reynolds and Scott, 1982‘‘mammal’’ Clm Bryson et al., 2002Dipodomys spp.Microtus spp.Mus musculusPerognathus spp.Peromyscus spp.Sigmodon spp.
CllCllCllCllCllCll
Beaupre, 1995Falck, 1940*Falck, 1940*Beaupre, 1995Falck, 1940*; Beaupre, 1995Beaupre, 1995
1996. Selected aspects of the ecology of the Ari-zona ridge-nosed rattlesnake (Crotalus willardi wil-lardi) and the banded rock rattlesnake (Crotalus lep-idus klauberi) in Arizona. Final Report to ArizonaGame and Fish Department, Phoenix.
MEFFE, G. K., AND C. R. CARROLL. 1997. Principles ofConservation Biology. 2nd ed. Sinauer Associates,Inc., Sunderland, MA.
MILSTEAD, W. W., J. S. MECHAM, AND H. MCCLINTOCK.1950. The amphibians and reptiles of the StocktonPlateau in northern Terrell County, Texas. TexasJournal of Science 2:543–562.
MOORE, T. D., L. E. SPENCE, C. E. DUGNOLLE, AND W.G. HEPWORTH. 1974. Identification of the dorsalguard hairs of some mammals of Wyoming. Wy-oming Game and Fish Department, Cheyenne.
MUSHINSKY, H. R. 1987. Foraging ecology. In R. A.Seigel, J. T. Collins, and S. S. Novak (eds.), Snakes:Ecology and Evolutionary Biology, pp. 302–334.McGraw-Hill Publishing Co., New York.
PECHENIK, J. A. 2000. Biology of the Invertebrates. 4thed. McGraw Hill, Boston, MA.
PRICE, A. H. 1998. Poisonous Snakes of Texas. TexasParks and Wildlife Press, Austin.
REYNOLDS, R. P., AND N. J. SCOTT JR. 1982. Use of aMammalian Resource by a Chihuahuan SnakeCommunity. In N. J. Scott Jr. (ed.), HerpetologicalCommunities, pp. 99–118. U.S. Fish and WildlifeService, Washington, DC.
RODRIGUEZ-ROBLES, J. A., AND H. W. GREENE. 1999.Food habits of the long-nosed snake (Rhinocheiluslecontei), a ‘‘specialist’’ predator? Journal of Zool-ogy (London) 248:489–499.
RODRIGUEZ-ROBLES, J. A., D. G. MULCAHY, AND H. W.GREENE. 1999. Feeding ecology of the desertnightsnake, Hypsiglena torquata (Colubridae). Cop-eia 1999:93–100.
RUBIO, M. 1998. Rattlesnake: Portrait of a Predator.Smithsonian Institution Press, Washington, DC.
SCHAEFER, W. H. 1934. Diagnosis of sex in snakes.Copeia 1934:181.
SMITH, L. J., A. T. HOLYCROSS, C. W. PAINTER, AND M.E. DOUGLAS. 2001. Montane rattlesnakes and pre-scribed fire. Southwestern Naturalist 46:54-61.
SOKAL, R. R., AND F. J. ROHLF. 1995. Biometry. 3rd ed.W. H. Freeman Co., New York.
STARRETT, B. L., AND A. T. HOLYCROSS. 2000. Crotaluslepidus. Caudal luring. Herpetological Review 31:245
STEBBINS, R. C. 1985. A Field Guide to Western Rep-tiles and Amphibians. Houghton Mifflin Co., Bos-ton, MA.
SWINFORD, G. W. 1992. Status of the mottled rock rat-tlesnake, Crotalus lepidus lepidus, in southeasternNew Mexico. Report to New Mexico DepartmentGame and Fish, Santa Fe.
TAYLOR, E. N. 2001. Diet of the Baja California rattle-snake, Crotalus enyo (Viperidae). Copeia 2001:552–554.
TENNANT, A. 1985. A Field Guide to Texas Snakes.Texas Monthly Press, Austin.
WERLER, J. E., AND J. R. DIXON. 2000. Texas snakes:identification, distribution, and natural history.University of Texas Press, Austin.
WILLIAMSON, M. A. 1971. An instance of cannibalismin Crotalus lepidus (Serpentes: Crotalidae). Herpe-tological Review 3:18.
WOODIN, W. H. 1953. Notes on some reptiles fromthe Huachuca area of southeastern Arizona. Bul-letin of the Chicago Academy of Science 9:285–296.
Accepted: 8 February 2002.
597ROCK RATTLESNAKE DIET
APPENDIX I. Continued.
Prey Ssp. Reference
SquamataLacertilia‘‘lizard’’ Cll Marr, 1944; Conant, 1955; Klauber, 1972; Swinford,
1992; Beaupre, 1995‘‘lizard’’ Clk Kauffeld, 1943a; Barker, 1991; McCrystal et al., 1996‘‘lizard’’ Clm Klauber, 1972Anolis nebulosus Clm Bryson et al., 2002Cnemidophorus spp.Cnemidophorus gularisCnemidophorus tigrisCophosaurus texanus
CllCllCllCll
Beaupre, 1995Milstead et al., 1950Axtell, 1959*Beaupre, 1995
Holbrookia texanaEumeces laticepsPhrynosoma cornutumPhrynosoma hernandesiSceloporus spp.Sceloporus cf. clarkiiSceloporus jarrovii
CllClkCllCllClkClkClk
Axtell, 1959*Kauffeld, 1943a*Milstead et al., 1950Dickerman and Painter, 2001McCrystal et al., 1996Woodin, 1953Campbell, 1934; Kauffeld, 1943a*; Woodin, 1953;
Klauber, 1972; McCrystal et al., 1996*Sceloporus jarrovii Clm Bryson et al., 2002Sceloporus grammicus (reported as
Sceloporus microlepidotus disparil-is)
Cll Gloyd and Smith, 1942
Sceloporus merriamiSceloporus poinsettiSceloporus undulatusUrosaurus ornatusUrosaurus ornatus
CllCllClkClkCll
Beaupre, 1995Marr, 1944Kauffeld, 1943a*Woodin, 1953*Milstead et al., 1950; Beaupre, 1995
Serpentes‘‘snake’’Crotalus lepidus
CllClk
Beaupre, 1995Williamson, 1971*; Harris and Simmons, 1977*
Gyalopion canum (reported as Fici-mia cana)
Cll Milstead et al., 1950
Virginia striatula (reported as Hal-dea striatula)
Cll Miltead et al., 1950*
AmphibiaAmbystoma tigrinumAcris crepitansPseudacris triseriataRana pipiensRana sylvaticaSyrrhophus marnockii
CllCllCllCllCllCll
Falck, 1940*Falck, 1940*Falck, 1940*Falck, 1940*Falck, 1940*Milstead et al., 1950
APPENDIX 2Specimens Examined
ASU 1624, 1811, 2101, 3651, 15611, 16119, 31334,31398, 33115; CAS 1814, 34747, 48021–033, 66377,84131, 101364, 122764, 138859; CM 16754–56, 40182,67042; KU 5330, 5552, 5554–55, 6646–48, 6654–56,49586, 128604, 157865–66; LACM 2968–975, 64278,75329, 76442, 126001, 134035–36, 134053, 136977; MSB4113, 4115, 8374, 8399, 25231, 25356–58, 30447, 31013–14, 31191, 31359, 31643, 32050–51, 32053, 32060–61,32220–22, 32319, 38305, 44700, 48115, 49551–52,50228, 52175, 52719, 52917–18, 53314, 56044, 60065,
60068, 60694, 60917–18, 61796; MVZ 22404, 54568–69,67194, 67200, 67231, 68225, 71018–20, 73094, 84508,229798–99, 229956–57; SDSNH 1636, 2143, 2160, 3035–37, 3076, 3078, 3082, 3108, 3110–14, 3116, 3119, 3121,3123, 3125–26, 3223–26, 3458, 3475–76, 3504, 20969,21343, 21495–96, 23054–55, 23057, 40849–851, 43968,59418; UAZ 27562–69, 27571–582, 27584–87, 28124,28573, 30243, 35006, 35047, 35079, 36314, 36681, 37833,37834, 38091–92, 39794, 40067–071, 42088–2116,42431, 42907, 43679, 46124, 46271, 46555, 48774–76,49921, 50248, 51793, 51942; UMMZ 54019, 71322–25,72546, 75798–800, 129099, 174482.