Genetic and environmental influences underlying the relationship between autistic traits and...
-
Upload
independent -
Category
Documents
-
view
1 -
download
0
Transcript of Genetic and environmental influences underlying the relationship between autistic traits and...
Available online at www.sciencedirect.com
ScienceDirect
Comprehensive Psychiatry xx (2015) xxx–xxxwww.elsevier.com/locate/comppsych
Genetic and environmental influences underlying the relationship betweenautistic traits and temperament and character dimensions in adulthood
Angelo Picardia,⁎, 1, Corrado Fagnanib,1, Emanuela Meddab, Virgilia Toccacelib,Paolo Brambillac,d, Maria Antonietta Stazib
aMental Health Unit, National Centre of Epidemiology, Surveillance and Health Promotion, Italian National Institute of Health, Rome, ItalybGenetic Epidemiology Unit, National Centre of Epidemiology, Surveillance and Health Promotion, Italian National Institute of Health, Rome, Italy
cDISM, InterUniversity Center for Behavioural Neurosciences (ICBN), University of Udine, Udine, ItalydIRCCS "E. Medea” Scientific Institute, UDGEE, Udine, Italy
Abstract
Background: In recent years, several twin studies adopted a dimensional approach to Autism Spectrum Disorders (ASD) and estimated thecontribution of genetic and environmental influences to variation in autistic traits. However, no study was performed on adults over 18 yearsof age and all but two studies were based on parent or teacher ratings. Also, the genetic and environmental contributions to the interplaybetween autistic traits and adult personality dimensions have not been investigated.Methods: A sample of 266 complete twin pairs (30% males, mean age 40 ± 12 years) drawn from the population-based Italian TwinRegister was administered the Autism-Spectrum Quotient, Temperament and Character Inventory (TCI-125), and General HealthQuestionnaire (GHQ-12). Genetic structural equation modelling was performed with the Mx program. Estimates were adjusted for gender,age, and GHQ-12 score.Results: Genetic factors accounted for 44% and 20%–49% of individual differences in autistic traits and TCI dimensions, respectively.Unshared environmental factors explained the remaining proportion of variance. Consistently with the notion of a personality profile in ASDcharacterised by obsessive temperament, autistic traits showed significant phenotypic correlations with several TCI dimensions (positive:HA; negative: NS, RD, SD, C). Genetic and unshared environmental correlations between AQ and these TCI dimensions were significant.The degree of genetic overlap was generally greater than the degree of environmental overlap.Conclusions: Despite some limitations, this study suggests that genetic factors contribute substantially to individual differences in autistictraits in adults, with unshared environmental influences also playing an important role. It also suggests that autistic traits and the majority oftemperament and character dimensions share common genetic and environmental aetiological factors.© 2014 Elsevier Inc. All rights reserved.
1. Introduction
Autism Spectrum Disorders (ASD) are a set of pheno-typically heterogeneous neurodevelopmental syndromescharacterised by difficulties in social communication andsocial interaction, and unusually restricted, repetitivebehaviours and interests [1]. These disorders are consideredto be highly heritable. In twin studies, the concordance in
⁎ Corresponding author at: Italian National Institute of Health, Centre ofEpidemiology, Surveillance and Health Promotion, Mental Health Unit,Viale Regina Elena, 299-00161 Rome, Italy. Tel.: +39 06 49904200;fax: +39 06 49904182.
E-mail address: [email protected] (A. Picardi).1 These authors contributed equally to the paper.
http://dx.doi.org/10.1016/j.comppsych.2014.12.0180010-440X/© 2014 Elsevier Inc. All rights reserved.
monozygotic twins is much higher than that in dizygotictwins [2]; also, the individual risk of ASD was found toincrease with increasing genetic relatedness [3].
Rather than treating ASD as discrete entities with acategorical approach based on a distinct boundary betweennormality and pathology, several authors [4,5] havesuggested a dimensional approach to ASD, which concep-tualises these disorders as the upper extreme of aconstellation of deficits in social adaptation and communi-cation that may be continuously distributed in the popula-tion. There are several lines of evidence for this notion:autistic traits measured in the general population show asmooth distribution throughout the normal range to theclinical extreme [6,7]; relatives of patients with ASD displayhigh levels of autistic traits [8,9]; factor analytic approaches
2 A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
did not detect discontinuities between ASD and autistic traits[10]; known risk factors for ASD (e.g., paternal age at birth)have been shown to influence autistic traits [11]; commongenetic variants that are, by their very nature, present in asignificant proportion of the general population, are believedto play a role in the aetiology of ASD [12–14]. Indeed, theDSM-5 itself has highlighted the dimensional nature of thecardinal behavioural domains of ASD, by incorporating aseverity scale to capture the ‘spectrum’ nature of ASD [1].
The dimensional approach to ASD has several potentialadvantages, such as enhancing the statistical power ofgenetic studies and averting the problem of misclassification,and it has also been incorporated in twin studies. The largemajority of these studies were performed on children andyoung adolescents, and were based on parent or teacherratings; this is an important methodological aspect becauseexternal and self-rated reports may yield diverse results, asdifferent raters can provide dissimilar perspectives onbehaviour [15,16]. The studies performed in early childhoodyielded moderate heritability estimates (40% and 44%) forparent-rated autistic traits [17,18]. In older children andyoung adolescents, most studies reported high heritability(60%–90%) for parent- and teacher-rated autistic traits[6,19–25], while heritability was found to be moderate(36%–47%) for self-reported autistic traits in 9-year-oldchildren [23]. These studies consistently reported moderateinfluences of the unshared environment, whereas they didnot yield a consistent picture regarding the influence of theshared environment; most studies found no significanteffects, while some studies in early [17,18] and middle-to-late childhood [20,23,24,26] reported modest (4%–32%)shared environmental influences. Of importance, genetic andenvironmental influences were found to be stable in thepopulation with increasing levels of autistic traits [24]; also,a strong correlation, predominantly affected by geneticfactors, was observed between narrowly defined extreme-end conditions and the full variation of autistic traits [25]. Astudy on late adolescents aged 18 years corroborated thepattern of results observed at earlier ages, as it reportedsubstantial heritability (57%) and moderate unsharedenvironmental influences (43%) on self-reported autistictraits [27].
To our knowledge, only two twin studies [23,27] werebased on self-report ratings of autistic traits, and no study wasperformed on adults over 18 years of age. Such a study wouldhave the potential to increase the understanding of develop-mental change and continuity in genetic and environmentalinfluences on ASD and autistic traits. Also, the genetic andenvironmental influences on the interplay between autistictraits and higher-order adult personality dimensions have notbeen explored. Moreover, no twin study of autistic traits tookaccount of the possible influence of state variables such asemotional distress, which has been found to be correlatedwith scores on autistic trait measures [28,29].
The present study aimed at filling these research gaps byproviding a reliable estimate of the genetic and environmen-
tal components of autistic traits in adulthood, and byexploring if and to what extent these components are relatedto those of higher-order personality dimensions. We used amultivariate, genetically informed twin design in a generalpopulation sample in order to (1) explore the genetic andenvironmental architecture of autistic traits in adults whileadjusting for emotional distress; (2) investigate the relation-ship between autistic traits and higher-order personalitytraits; (3) unravel the genetic and environmental bases ofthis relationship.
2. Methods
2.1. Subjects
The study subjects were drawn from the population-basedItalian Twin Register (ITR). The procedures that led to theestablishment of the ITR are described in detail elsewhere[30]. Currently, the ITR includes more than 25,000 twins, andis involved in both general population and clinical studies ona large variety of complex phenotypes, with behavioural andpsychiatric genetics as major research areas [31].
This study is embedded within a broad mail survey onhealth and psychological well-being in adulthood, targetingthree metropolitan areas of Northern (Milan), Central(Rome) and Southern (Palermo) Italy. From February toNovember 2010, twins aged 18–65 years, previouslyenrolled in the ITR, were invited to participate in the surveyand were asked to donate a saliva sample to be stored forfuture genetic and epigenetic studies. In the same contact, thequestionnaires on the traits of interest were sent to the twins.After excluding unmatched twins, a total of 532 subjectsfrom 266 complete twin pairs were left for the analysis. Theirmean age was 39.9 years (SD = 11.9; range 18–65).About one third (N = 158, 30%) were males, while twothirds (N = 374, 70%) were females. Most participants hadhigh school (54%) or university (34%) education. Aboutthree quarters of them (76%) worked part- or full-time, while7% were housewives, 4% were still studying, 3% wereunemployed, and 10% did not provide information abouttheir employment status. About half (49%) of participantswere married, while 6% were divorced, 2% were widowed,and 43% were unmarried.
Of the 266 pairs, 160 were monozygotic (MZ; 45 male–male, 115 female–female pairs) and 106 were dizygotic (DZ;11 male–male, 49 female–female, 46 unlike-gender pairs).
Zygosity was assigned by a standard questionnaireregarding physical similarity of the twins during infancy;this is a well-established procedure in twin studies, which isknown to be over 90% accurate. The reliability of thismethod in the ITR population was recently estimated in anindependent sample of 158 same-gender adult twin pairs byusing nine microsatellite markers; 149 pairs (94.3%) werecorrectly classified by the questionnaire.
Written informed consent was obtained from the twins,after complete description of the study.
3A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
2.2. Assessment
The Autism-Spectrum Quotient (AQ) is a self-reportquestionnaire consisting of 50 items, rated on a 4-pointagreement scale (definitely agree, slightly agree, slightlydisagree, and definitely disagree). As individuals high inautistic traits may underestimate their social impairment, theitems ask about personal preferences and habits rather thanbehavioural judgements. For 26 items, an agree response isnot characteristic for autism, whereas for 24 items theopposite is true and the scoring is reversed. Total AQ scoreswere calculated with the binary method, i.e., collapsingadjacent responses to obtain a dichotomous scoring (0-0-1-1,or 1-1-0-0 for reverse-keyed items). Total AQ score rangesfrom 0 to 50. High scores on the AQ indicate high attentionto details and poor social, attention switching, communica-tion, and imagination skills. The instrument has satisfactoryinternal consistency reliability, test–retest reliability, andconstruct validity, and it has been validated in severaldifferent countries [7,32–34]. In our study, coefficient Alphafor the total AQ was 0.70.
A complete AQ was available for 436 participants. For 21participants with no more than 3 missing items, missingvalues were replaced by the mean of the respondent’s scoreson the other items. The remaining participants either leftmore than 3 items blank (N = 5) or returned the question-naire uncompleted (N = 70).
The 12-item version of the General Health Questionnaire(GHQ-12) is a self-administered questionnaire designed tomeasure psychological distress and to detect non-psychoticpsychiatric disorders [35]. It consists of 12 items, rated on a4-point scale, relating to anxiety, depression, social dys-function, and loss of confidence. Higher scores indicategreater levels of psychological distress. The instrument iswidely used, and many studies supported its validity andreliability [36,37]. In our study, coefficient Alpha forthe GHQ-12 was 0.86. The large majority of participants(N = 518) returned a complete GHQ-12.
The TCI [38] is a true/false questionnaire to measurepersonality dimensions within the psychobiological model,which postulates that personality involves four temperamentdimensions and three character dimensions. The fourtemperament dimensions reflect individual differences inbasic emotional drives, and are called Novelty Seeking (NS),Harm Avoidance (HA), Reward Dependence (RD), andPersistence (P). Individuals scoring high in NS are quicktempered, curious, easily bored, impulsive, extravagant, anddisorderly; those high in HA tend to lack confidence and tobe cautious, fearful, easily worried, pessimist, shy, and easilyfatigued; those high in RD are tender-hearted, warm,sensitive, sociable, and dependent; while those high in Pare ambitious, industrious, perseverant, and stable, despitefrustration. The character dimensions reflect individualdifferences in higher cognitive processes, and are calledSelf-Directedness (SD), Cooperativeness (C), and Self-Transcendence (ST). Character is believed to reflect
conceptual maturity of personality in relation to self (SD),others (C) and a universal wholeness (ST). SD refers towillpower, determination, maturity, responsibility, and to theability to control, regulate and adapt behaviour in accordancewith chosen goals and values. C refers to the ability to adhereto social principles, to be tolerant and helpful, to accept otherpeople, identify with others, and cooperate with them. STrefers to the ability to tolerate ambiguity and uncertainty inlife, to spiritually accept them, and to identify oneself as partof nature and of the wider world. We used the 125-itemversion of the TCI, which has been used by Cloningerhimself and colleagues [39]. Complete data for the TCI-125were available for most participants (N from 504 to 518,depending on the subscale).
2.3. Statistical analysis
2.3.1. Sample statisticsThe AQ, TCI, and GHQ-12 scores were calculated for
each twin and were used as continuous variables insubsequent analyses. Descriptive statistics of the AQ, TCI,and GHQ-12 scores were compared across genders andzygosity groups using robust t-tests (as implemented in Stata,version 13.0) to take account of the dependence of twin data.
2.3.2. CorrelationsThe correlations (i) between the different dimensions
within a twin individual (named ‘phenotypic’), (ii) betweentwin and co-twin for the same dimension (named ‘cross-twin/within-trait’) in MZ and DZ twin pairs separately, and(iii) between one dimension in a twin and another dimensionin the co-twin (named ‘cross-twin/cross-trait’) in MZ and DZtwin pairs separately were estimated and interpreted underthe assumptions of the twin design [40]. A higher cross-twin/within-trait correlation in MZ than in DZ pairs suggestsgenetic effects on the trait. A significant phenotypiccorrelation between a pair of traits is consistent withetiological influences common to the traits. A highercross-twin/cross-trait correlation in MZ compared to DZpairs provides support for genetic factors shared by the traits.
The correlations were estimated via the maximum-likelihood method as implemented in the software Mx[41], by fitting a saturated model to MZ and DZ raw data,and by adjusting for gender, age and GHQ-12 score.
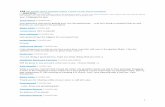
2.3.3. Genetic modellingA Cholesky decomposition incorporating additive genetic
(A), shared (familial) environmental (C) and unshared (indivi-dual-specific) environmental (E) components of variances andcovariances, and adjusted by gender, age and GHQ-12 score,was fitted to total AQ and TCI-125 dimensions scores (Fig. 1)with the software Mx [41], usingMZ and DZ raw data as input.The additive genetic component captures the average effect ofalleles without allelic or gene–gene interaction. The sharedenvironmental component reflects exposures that are commonto all members of a family, thus contributing to within-familyaggregation. The unshared environmental component subsumes
NS HA RD P SD CO
A2 A3 A4 A5 A6 A7
E2 E3 E4 E5 E6 E7
STAQ
A8A1
E8E1
Fig. 1. Path diagram of the Cholesky decomposition for total AQ and TCI-125 dimensions in one twin. Squares denote observed scores. Circles indicate latentsources of variance and covariance. Ai and Ei represent additive genetic and unshared environmental influences, respectively. Latent genetic factors correlate 1between monozygotic twins and 0.5 between dizygotic twins. Although model fitting also included shared environmental influences (Ci), the correspondinglatent sources were not shown in the diagram for reasons of clarity. AQ, autism quotient; NS, novelty seeking; HA, harm avoidance; RD, reward dependence; P,persistence; SD, self-directedness; CO, cooperativeness; ST, self-transcendence.
4 A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
factors that are specific to an individual (includingmeasurementerror) and therefore are responsible for differences betweenfamily members. This model provides: (i) ‘heritability’ of eachtrait (i.e. the proportion of total variance in the trait explained bygenetic effects); (ii) ‘genetic correlation’ between traits (i.e. ameasure of the extent to which the traits share the same geneticfactors); (iii) ‘bivariate heritability’ of traits (i.e. the proportion ofphenotypic correlation between the traits due to shared geneticeffects) [40]. A full ACE decomposition was initially fitted, andthen sub-models were tested using likelihood-ratio χ2 tests.Parameter estimates were reported under the most parsimonioussolution (best-fitting model).
3. Results
3.1. Sample statistics
Sample descriptive statistics of participants’ scores on theAQ, GHQ-12, and TCI dimensions are reported in Table 1.
Table 1Descriptive statistics of total AQ, TCI-125 dimensions and GHQ-12 scores.
Gender
Males Females
N mean ± sd N mean ± sd
Total AQ 134 17.45 ± 5.79 323 16.43 ± 6.NS 154 8.47 ± 3.76 356 8.42 ± 3.HA 154 8.60 ± 3.73 353 ⁎10.44 ± 4.RD 155 7.99 ± 2.57 362 ⁎9.45 ± 2.P 155 3.01 ± 1.54 363 3.10 ± 1.SD 149 18.13 ± 4.75 355 17.58 ± 5.C 150 18.48 ± 4.04 357 †19.50 ± 3.ST 151 4.72 ± 3.26 353 †5.58 ± 3.GHQ-12 151 10.66 ± 5.06 367 ^11.72 ± 5.
AQ, autism quotient; NS, novelty seeking; HA, harm avoidance; RD, reward dself-transcendence; GHQ, general health questionnaire.⁎ p b 0.01, † 0.01 b p b 0.05, ^ 0.05 b p b 0.10 for gender difference in mean.
No significant differences emerged between zygosity groupsin the mean scores on TCI dimensions and AQ; thissupported the assumption that MZ and DZ twins, asindividuals, come from the same reference population.
As regards gender comparisons, females scored signifi-cantly higher than males on HA, RD, CO, and ST.Furthermore, a higher – though not significantly so –mean GHQ-12 score in females was suggestive of greateremotional distress as compared to males.
3.2. Correlations
There was a significant phenotypic correlation betweenAQ and GHQ-12 scores, even after adjusting for age andgender (partial correlation = 0.26, p b 0.001), which cor-roborated our decision to adjust not only for gender and age,but also for GHQ-12 score in all genetic models. There werealso several significant phenotypic correlations between AQand TCI scores, as the AQ score was negatively correlated
Zygosity
Monozygotic Dizygotic
N mean ± sd N mean ± sd
14 275 16.64 ± 6.07 182 16.86 ± 6.0345 305 8.21 ± 3.51 205 8.76 ± 3.5810 302 10.03 ± 4.08 205 9.66 ± 4.0861 310 9.19 ± 2.62 207 8.74 ± 2.7452 308 3.11 ± 1.50 210 3.01 ± 1.5711 302 17.91 ± 5.00 202 17.49 ± 5.0359 302 19.36 ± 3.62 205 18.95 ± 3.9444 301 5.49 ± 3.47 203 5.08 ± 3.3286 315 11.31 ± 5.71 203 11.57 ± 5.57
ependence; P, persistence; SD, self-directedness; C, cooperativeness; ST,
Table 2Correlations for total AQ and TCI-125 dimensions.
Total AQ TCI-125 dimensions
NS HA RD P SD C ST
Cross-twin/within-trait correlationsMZ 0.47 0.35 0.38 0.34 0.23 0.47 0.51 0.40
(0.32, 0.58) (0.21, 0.48) (0.23, 0.51) (0.18, 0.47) (0.06, 0.38) (0.34, 0.59) (0.38, 0.62) (0.26, 0.52)DZ 0.09 0.15 0.25 −0.02 0.03 0.18 0.15 0.21
(−0.13, 0.30) (−0.07, 0.34) (0.06, 0.43) (−0.20, 0.17) (−0.16, 0.21) (−0.02, 0.35) (−0.03, 0.33) (0.00, 0.39)
Phenotypic correlationsTotal AQ −0.22 0.42 −0.28 0.07 −0.40 −0.35 0.04
(−0.31, −0.12) (0.33, 0.50) (−0.37, −0.19) (−0.03, 0.16) (−0.48, −0.32) (−0.44, −0.26) (−0.05, 0.14)
Cross-twin/cross-trait correlationsTotal AQ MZ −0.11 0.20 −0.20 0.06 −0.27 −0.25 0.05
(−0.22, 0.00) (0.08, 0.31) (−0.31, −0.08) (−0.07, 0.17) (−0.38, −0.16) (−0.35, −0.13) (−0.07, 0.16)DZ −0.07 0.01 0.06 −0.09 0.02 −0.13 0.00
(−0.23, 0.10) (−0.15, 0.16) (−0.09, 0.22) (−0.23, 0.06) (−0.13, 0.17) (−0.27, 0.02) (−0.15, 0.16)
AQ, autism quotient; NS, novelty seeking; HA, harm avoidance; RD, reward dependence; P, persistence; SD, self-directedness; C, cooperativeness; ST,self-transcendence; MZ, monozygotic; DZ, dizygotic.Values given in parentheses indicate 95% confidence intervals.
5A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
with NS, RD, SD and CO scores, and positively correlatedwith HA scores.
Table 2 shows the correlation pattern between AQ scoresand TCI dimensions. For the AQ score, cross-twin/with-in-trait correlation was much higher in MZ than in DZ pairs,with a similar pattern also generally observed for each of theTCI dimensions (MZ range: 0.23–0.51; DZ range: −0.02–0.25). This indicated that additive genetic factors contributeto individual differences in AQ and TCI scores. Moreover,no evidence of shared environmental effects emerged fromMZ vs. DZ correlations either for the AQ score or for TCIdimensions, except HA; in fact, according to quantitative
Table 3Genetic and unshared environmental effects for total AQ and TCI-125 dimension
Total AQ
NS HA RD
Genetic (A) and unshared environmental (E) proportions of varianceA 0.44 0.36 0.40 0.31
(0.30, 0.57) (0.22, 0.49) (0.26, 0.52) (0.16, 0.44)E 0.56 0.64 0.60 0.69
(0.43, 0.70) (0.51, 0.78) (0.48, 0.74) (0.56, 0.84)
Genetic (rA) and unshared environmental (rE) correlationsTotal AQ rA −0.31 0.44 −0.46
(−0.57, −0.03) (0.19, 0.64) (−0.74, −0.16)rE −0.16 0.42 −0.19
(−0.31, −0.01) (0.28, 0.55) (−0.33, −0.03)
Genetic (A) and unshared environmental (E) proportions of covarianceTotal AQ A 0.56 0.43 0.60
(0.05, 0.96) (0.17, 0.65) (0.24, 0.92)E 0.44 0.57 0.40
(0.04, 0.95) (0.35, 0.83) (0.08, 0.76)
AQ, autism quotient; NS, novelty seeking; HA, harm avoidance; RD, reward dself-transcendence.Genetic proportions of covariance are ‘bivariate heritabilities’. Values given in pa
genetic theory, the effects of shared environment are evokedwhen twice DZ correlation exceeds MZ correlation [40].
Cross-twin/cross-trait correlations of the AQ score withthe aforementioned TCI dimensions were higher in MZcompared to DZ pairs, revealing that the phenotypiccorrelations may partly originate from genetic effectscommon to autistic-like and personality traits.
3.3. Genetic modelling
Likelihood-ratio χ2 tests between nested models wereperformed. First, dropping the shared environmental
s, as estimated from the best-fitting AE Cholesky model.
TCI-125 dimensions
P SD C ST
0.20 0.46 0.49 0.41(0.06, 0.35) (0.33, 0.58) (0.36, 0.60) (0.27, 0.52)0.80 0.54 0.51 0.59(0.65, 0.94) (0.42, 0.67) (0.40, 0.64) (0.48, 0.73)
0.03 −0.53 −0.54 0.12(−0.40, 0.43) (−0.72, −0.31) (−0.75, −0.32) (−0.16, 0.41)0.09 −0.30 −0.18 −0.02
(−0.07, 0.24) (−0.43, −0.14) (−0.33, −0.02) (−0.19, 0.15)
0.14 0.60 0.73 1.38(−0.16, 0.39) (0.34, 0.82) (0.45, 0.98) (1.11, 1.65)0.86 0.40 0.27 −0.38(0.61, 1.16) (0.18, 0.66) (0.02, 0.55) (−0.65, −0.11)
ependence; P, persistence; SD, self-directedness; C, cooperativeness; ST,
rentheses indicate 95% confidence intervals.
6 A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
component from the full ACE Cholesky model provided anon-significant fit deterioration (χ2
36 = 7.15, p = 1.00).Second, genetic effects common to the dimensions weretested in the AE model by fixing the genetic correlations tozero; this led to a highly significant change in the AE modelfit (χ2
28 = 94.26, p = 4.2 × 10−9). Similarly, unsharedenvironmental correlations could not be ruled out in theAE model (χ2
28 = 133, p = 1.3 × 10−15). Therefore, themodel including additive genetic and individual-specific envi-ronmental effects on AQ score and TCI dimensions, possiblyshared between them, resulted in the best-fitting solution.
Estimates of genetic and unshared environmental effectsfor scores on the AQ and the TCI, derived from the best-fitting AE Cholesky model, are reported in Table 3. For theAQ, heritability was 44% and the remaining 56% of variancewas due to idiosyncratic environmental experiences. For TCIdimensions, genetic influences accounted for one-fifth (P) toone-half (CO) of individual differences.
The genetic correlations between the AQ and all TCIdimensions except P and ST were significant, which suggestthat the sets of genes underlying the expression of autistic-like traits and the aforementioned personality traits maysubstantially overlap. Shared genetic effects explained from43% (AQ and HA) to 73% (AQ and CO) of phenotypiccorrelation between AQ and TCI scores. Unshared environ-mental correlations between scores on the AQ and TCI werealso significant, though generally lower than geneticcorrelations. Overlapping individual-specific environmentalfactors were still responsible for important portions – from27% (AQ and CO) to 57% (AQ and HA) – of phenotypiccorrelations between AQ and TCI scores (Table 3).
The model also predicted a significant positive effectof GHQ-12 score on AQ score; the regression weight of theGHQ-12 in the marginal model for AQ score was 0.24(p b 10−6); this effect mirrored the significant positivecorrelation of 0.26 (p b 0.001), adjusted for gender andage, that was observed between the two traits at theindividual level.
4. Discussion
To our knowledge, this is the first twin study of autistictraits performed on adults who passed the late adolescencestage, as well as the first to adjust for the possible confoundingeffect of emotional distress and to investigate the geneticand environmental bases of the interplay between autistic traitsand personality as conceptualised in Cloninger’s biopsycho-social model.
Consistently with a previous study on a generalpopulation sample that reported a significant associationbetween AQ and GHQ-12 scores [29], we found a significantpositive phenotypic correlation, independent of age andgender, between AQ and GHQ-12 scores. This findingcorroborates our decision to take into account the overlapbetween scores on measures of autistic traits and emotional
distress, as well as the overlap between GHQ-12 scores andscores on some TCI subscales [42], by adjusting not only forgender and age, but also for GHQ-12 score in allgenetic models.
Our finding of a significant phenotypic correlationbetween AQ and GHQ-12 scores is consistent with theliterature. Another study on adult students found a significantpositive correlation between AQ scores and scores onmeasures of anxiety and depression, independent oftemperament [28]. Another study on young adults [27]found that higher levels of social problems related to anxietyand depression were correlated with higher AQ scores. Thesefindings suggest that the AQ may measure not only autistictraits, but also emotional distress and symptoms of anxietyand depression. This overlap may not be specific for the AQ,as it has been found in studies using other measures ofautistic traits. A study on undergraduate students found thatthe participants reporting a greater degree of autistic traits asmeasured with a short, self-report version of the SocialResponsiveness Scale reported also a higher level ofpsychiatric symptoms, especially depression and anxiety[43]. Studies on children also revealed a significantcorrelation between autistic-like and internalising traits[44], and suggested that autistic-like traits in later childhoodare partly driven by earlier internalising difficulties, and thatearly autistic-like difficulties may also have an impact onsubsequent anxiety and depression [45]. The finding of asignificant correlation between autistic traits and emotionaldistress is also consistent with clinical reports that children,adolescents, and adults diagnosed with an ASD frequentlyhave comorbid symptoms of anxiety and depression [46,47].
In our study, the AQ score showed significant phenotypiccorrelations with HA (0.42), NS (−0.22), RD (−0.28), SD(−0.40), and C (−0.35). This pattern of correlations betweenthe AQ and TCI scores is consistent with the notion of apersonality profile in autism characterised by obsessivetemperament (low NS, high HA, and low RD) and characterimmaturity (low SD and C). It also corroborates the findingsof previous studies using the same assessment instruments insamples of adult students [28,48], as well as the findings ofstudies performed on adult patients with Asperger Disorder[49] and with ASD [50–52]. The studies on non-clinicalsamples reported positive correlation between AQ and HAscores, and negative correlation between AQ and both NSand RD scores [28,48], while the studies on clinical samplesrevealed increased scores on HA and decreased scores onNS, RD, SD, and C in patients with ASD as compared withnormative groups [49–52].
The negative correlation between AQ and C appears to bemainly driven by genetic effects common to thosedimensions, as there are a considerable genetic correlation(−0.53) and a large bivariate heritability (0.73). The negativecorrelations between AQ and NS, AQ and RD, AQ and SDare mainly genetic in origin (genetic correlation −0.31,−0.46, and −0.53, respectively; bivariate heritability 0.56,0.60, and 0.60, respectively), although environmental
7A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
influences also play a role (environmental correlation −0.16,−0.19, and −0.30, respectively; environmental proportion ofcovariance 0.44, 0.40, and 0.40, respectively). The positivecorrelation between AQ and HA seems to be driven to asimilar extent from genetic and environmental effects(genetic correlation 0.44, bivariate heritability 0.43, envi-ronmental correlation 0.42, environmental proportion ofcovariance 0.57).
Currently, there is no consensus on the boundariesbetween autistic traits in the strict sense of autism-relatedphenomena and personality traits that may resemble autistictraits. The concepts of ‘character immaturity’ and ‘obsessivetemperament’ derived from the TCI literature may bear someresemblance with autistic traits. Indeed, the links betweenand the partial overlap of ASD and obsessive–compulsivedisorder (OCD) have recently received increasing attentionin the literature [53]. In both disorders a preoccupation withroutine, ritualised patterns of verbal and nonverbal behav-iour, and fixed, highly restrictive interests may appear,sometimes making it difficult to differentiate between therituals, stereotypes, and adherence to routines inherent toASD and the obsessions and compulsions typical of OCD[54]. Also, autistic traits are common in patients with OCD[55,56], and OCD is likewise frequent in individuals withASD [57]. However, the phenotypic overlap between autismand obsessionality is limited, as patients with ASD have beenshown to score much higher on the AQ than patients withOCD who, in turn, scored higher than healthy controls[7,58]. Consistently, our findings suggest that there is onlylimited, though significant, overlap between autistic traitsand temperament and character dimensions. They alsosuggest that autistic traits and the majority of temperamentand character dimensions share common genetic andenvironmental aetiological factors, and that the degree ofgenetic overlap appears to predominate on the environmentaloverlap, especially for C, NS, RD, and SD.
Genetic influences estimated in twin studies include bothinherited and heritable genetic risk factors, such as commonsingle nucleotide polymorphisms or copy number variations,and other factors that are not inherited despite contributing tothe heritability estimate, such as de novo mutations andcytogenetic abnormalities that occur in the germline and arenot inherited from parent to offspring. Consistently withprevious research, we found a significant contribution ofgenetic factors to individual differences in autistic traits. Ourheritability estimate is independent of the possible con-founding effect of emotional distress on the measurement ofautistic traits, and is similar in magnitude to those reportedby the other two studies that used self-report ratings in9-year-olds [23] and 18-year olds [27]. While studies inmiddle-to-late childhood and early adolescence reportedhigher heritability estimates [6,19–25], this might beascribed to the reliance of these studies on parent andteacher ratings, as it has been reported that the magnitude ofheritability estimates may vary depending on the informant,with teacher and parent ratings yielding high heritability
estimates (69% and 82%–87%, respectively), and self-reports giving moderate estimates (36%–47%) [23].
Given the lines of evidence supporting the notion thatASD are the upper extreme of a constellation of continuouslydistributed traits, and the finding of a genetic relationshipbetween the extremes of the continuum and trait variation inthe normal range [24], the genetic information from thisstudy may have direct relevance to understandingASD themselves.
With regard to temperament and character, heritabilityestimates for TCI dimensions ranged from 0.20 (P) to 0.49(C), suggesting an overall slightly less strong geneticinfluence as compared with the AQ. This finding isconsistent with previous twin studies, which reported thatadditive genetic effects alone best explained the sources offamilial aggregation, accounting for 27%–45% [59] and22%–49% [60] of the variance in TCI dimensions. Althoughresearch findings suggest that temperament and characterdimensions, contrary to theoretical expectations, may notdiffer greatly in heritability, their distinction remainsnevertheless valuable to relate personality measures to keydistinctions in learning and brain systems [59].
Our finding that unshared environmental effects ex-plained 56% of variance in AQ scores is consistent with theliterature. A moderate contribution of unshared environmen-tal factors to individual differences in autistic traits has beena common finding in previous twin studies [2]. In particular,the only adult study performed so far reported that unsharedenvironmental influences accounted for 43% of variance inself-reported autistic traits [27].
Unshared environmental influences make children grow-ing up in the same family different, and include anyexperience that is unique to the individual, such asdifferential prenatal effects, birth order, gender differences,de novo mutations, epigenetic processes, gene expression,life events not involving the cotwin, differences in familyinteractions or in perceptions of these interactions, differ-ences in social experiences, and differences in experienceswith activities and interests, education, and occupation [61].Therefore, these influences include, though are not limitedto, the putative environmental risk factors for ASD, such aspostnatal complications [62], advanced parental age, gesta-tional factors that may affect neurodevelopment, and folatedeficiency [63]. Unfortunately, no information was collectedabout possible environmental risk factors, so that alternativeexplanations involving factors other than those mentionedabove cannot be ruled out.
Concerning temperament and character, we found that thelargest portion of variance for TCI dimensions was explainedby unshared environmental effects, with estimates rangingfrom 0.51 (CO) to 0.80 (P). This finding is in line withprevious twin studies [59,60].
The shared environmental component of variance differsfrom one family to another and influences all children withina family to the same degree (e.g., socioeconomic status).Whereas unshared environmental effects produce differences
8 A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
among family members, shared environmental factors resultin similarities among family members. We found nosignificant contribution of shared environmental influencesto individual differences in autistic traits or temperament andcharacter dimensions. Although the power to detect theseinfluences was limited with our sample size, lack of power isunlikely to account for this finding, as the large difference incorrelations between MZ and DZ twins is highly consistentwith a model including only additive genetic and uniqueenvironmental effects. Therefore, our findings suggest thatshared environmental effects are not of major importance inexplaining the variance of autistic traits.
Previous twin studies of autistic traits did not yield aconsistent picture regarding the influence of shared environ-ment. While some studies [17,18,20,23,24,26] reportedsignificant shared environmental effects, most investigationsdid not. A study on 9-year olds reported shared environ-mental effects for self-report data, but only in males [23]. Allthe other studies that detected shared environmentalinfluences were based on parental reports [17,18,20,24,26].Given that the parent rates the behaviour of both members ofthe twin pair, rater bias may have inflated the sharedenvironmental effects in these studies. Differences betweenthe measures of autistic traits used in the various twin studiesmay also account for the inconsistency between studiesregarding the role of shared environmental influences onautistic traits. Furthermore, as suggested by the findings ofmany studies [64–66], the role of shared environmentalinfluences may decrease during development. Indeed, theonly other study performed on adults did not report asignificant influence of shared environment [27].
Several issues should be borne in mind when interpretingour findings. Some methodological challenges are inherentto twin-only designs. First, some studies have suggested thatthe process of twinning may be a risk factor for thedevelopment of autism [67,68], which would call intoquestion the generalisability of twin studies of autistic traits.However, large population-based studies do not supportthese findings [69,70].
Another limitation is that we did not include the twins’relatives in the study. Studies analysing only twins are verylimited in their ability to detect non-additive geneticvariation. For instance, a twin plus sibling design suggesteda substantial role for non-additive genetic variation inCloninger’s temperament dimensions [71]. Also, the classi-cal twin design postulates random partner selection, aspositive assortative mating (a positive correlation betweenpartners’ phenotypes) would lead to a greater resemblance inDZ twins in the face of unaltered MZ resemblance, resultingin attenuated heritability estimates. The studies that exam-ined assortative mating for autistic traits in the generalpopulation or clinical samples did not yield a consistentpicture [2]. Extended twin kinship designs requiring thecollection of data from parents, siblings, spouses, andchildren of twins would be needed to test for the possibleeffects of assortative mating, and to estimate genetic and
environmental influences in the presence of assortativemating [72]. Such designs would enable also to test directlyfor possible gene–environment correlations, which may leadto an overestimation of genetic effects (in case of acorrelation between genes and unshared environment) orshared environmental effects (in case of a correlationbetween genes and shared environment) under a classicaltwin study design.
Also, estimates of genetic and environmental influencesare quite sensitive to the specific method of measurement ofthe dependent variable. Although the AQ has satisfactorypsychometric properties and previous studies have shownthat autistic traits and behavioural problems can reliably beassessed using this instrument, further studies grounded inother measures of autistic traits in adults would be importantto complement our study.
Moreover, we relied only on self-report assessment ofautistic traits. Data from multiple raters may give importantadditional information and are likely to provide a richeraccount of autistic-like behaviours. Different raters providedifferent perspectives on behaviour, which may lead todifferent heritability estimates [23]. The availability ofmeasures based on additional informants may also reducerater bias and correlated measurement error. Infuture studies, it would be valuable to collect multipleinformant data.
Another limitation may lie in the use of a unidimensionalmeasure of autistic traits. While some researchers maintainthat autistic symptoms together represent a single underlyingdimension [10], other researchers argue that there are severaldimensions underlying autistic symptoms [73–75]. Twinstudies on children that used multidimensional measures ofautistic symptoms have reported that different dimensionsare all highly heritable but are caused by largely different setsof genetic influences, as there is only a modest degree ofgenetic overlap between dimensions [22]. Our use of aunidimensional measure prevented us to investigatethis issue.
Furthermore, the sample size was relatively small.Therefore, the power to detect shared environmental influ-ences and possible gender differences in the relativeimportance of the variance components was reduced.Although our heritability estimates are adjusted for gender,lack of statistical power did not allow us to reliably testwhether the contribution of genetic factors differs betweenmales and females, and whether gender-specific genes comeinto play. With regard to the latter issue, twin correlation insame-gender dizygotic twins (0.10) was almost identical inmagnitude to the correlation in unlike-gender twins (0.09),which provides no evidence of gender-specific genes forautistic traits. Further studies are required to elucidate whethershared environmental influences may play a role, and if thecontribution of genetic factors differs between genders.
In conclusion, our findings of moderate to substantialgenetic influences on autistic traits in an adult communitysample corroborates previous twin studies performed in
9A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
childhood, adolescence and early adulthood. The finding of asubstantial contribution of unshared environmental factors toindividual differences in autistic traits is also consistent withthe literature. Our findings also suggest that distinct profilesof temperament and character are genetically related toautistic traits in adults.
Given the limitations mentioned earlier, our findings arebest viewed as preliminary and need to be replicated bylarger, prospective studies, including the twins’ relatives, andincorporating the assessment of individual genetic back-ground and the measurement of putative environmental riskfactors. Such studies have the potential to increase theunderstanding of the role of genetic and environmentalfactors and of the interplay between genes and theenvironment in the development of autistic traits and,possibly, of ASD. The knowledge generated by such studiesmay be applied in significant ways to prevention and clinicalpractice in the field of ASD.
Acknowledgment
This study was supported by the Italian Ministry of Healthwithin the framework of the ‘Ricerca Finalizzata 2007’program. Also, Dr. Brambilla was partly supported by grantsfrom the Italian Ministry of Health (GR-2010-2317873) andby the Bial Foundation (Fellowship #262/12). We areindebted to the participants from the Italian Twin Registryfor making the study possible.
References
[1] American Psychiatric Association. Diagnostic and statistical manual ofmental disorders. 5th ed. Arlington, VA: American PsychiatricPublishing; 2013.
[2] Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits:a decade of new twin studies. Am J Med Genet B 2011;156:255-74.
[3] Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM,Reichenberg A. The familial risk of autism. JAMA 2014;311:1770-7.
[4] Gillberg CL. The Emanuel Miller Memorial Lecture 1991: autism andautistic-like conditions: subclasses among disorders of empathy.J Child Psychol Psychiatry 1992;33:813-42.
[5] Baron-Cohen S. Mindblindness: an essay on autism and theory ofmind. Cambridge, MA: MIT Press/Bradford Books; 1995.
[6] Skuse DH, Mandy WP, Scourfield J. Measuring autistic traits:heritability, reliability and validity of the Social and CommunicationDisorders Checklist. Br J Psychiatry 2005;187:568-72.
[7] Hoekstra RA, Bartels M, Cath DC, Boomsma DI. Factor structure,reliability and criterion validity of the Autism-Spectrum Quotient(AQ): a study in Dutch population and patient groups. J Autism DevDisord 2008;38:1555-66.
[8] Bishop DVM, Maybery M, Wong D, Maley A, Hallmayer J.Characteristics of the broader phenotype in autism: a study of siblingsusing the children’s communication checklist-2. Am J Med Genet B2006;141:117-22.
[9] Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A,McKenna K, et al. Autistic social impairment in the siblings ofchildren with pervasive developmental disorders. Am J Psychiatry2006;163:294-6.
[10] Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, PrzybeckT. The factor structure of autistic traits. J Child Psychol Psychiatry2004;45:719-26.
[11] Lundstrom S, Haworth CM, Carlstrom E, Gillberg C, Mill J, RastamM, et al. Trajectories leading to autism spectrum disorders are affectedby paternal age: findings from two nationally representative twinstudies. J Child Psychol Psychiatry 2010;51:850-6.
[12] Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, TrilloS, et al. A genetic variant that disrupts MET transcription is associatedwith autism. Proc Natl Acad Sci U S A 2006;103:16834-9.
[13] Chakrabarti B, Dudbridge F, Kent L, Wheelwright S, Hill-CawthorneG, Allison C, et al. Genes related to sex steroids, neural growth, andsocial-emotional behavior are associated with autistic traits, empathy,and Asperger syndrome. Autism Res 2009;2:157-77.
[14] Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, et al.Common genetic variants on 5p14.1 associate with autism spectrumdisorders. Nature 2009;459:528-33.
[15] Achenbach TM, McConaughy SH, Howell CT. Child/adolescentbehavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychol Bull1987;101:213-32.
[16] Verhulst FC, van der Ende J. Agreement between parents’ reports andadolescents’ self-reports of problem behavior. J Child PsycholPsychiatry 1992;33:1011-23.
[17] Edelson LR, Saudino KJ. Genetic and environmental influences onautistic-like behaviors in 2-year-old twins. Behav Genet2009;39:255-64.
[18] Stilp RLH, Gernsbacher MA, Schweigert EK, Arneson CL, GoldsmithHH. Genetic variance for autism screening items in an unselectedsample of toddler-age twins. J Am Acad Child Adolesc Psychiatry2010;49:267-76.
[19] Constantino JN, Todd RD. Genetic structure of reciprocal socialbehavior. Am J Psychiatry 2000;157:2043-5.
[20] Constantino JN, Todd RD. Intergenerational transmission of sub-threshold autistic traits in the general population. Biol Psychiatry2005;57:655-60.
[21] Ronald A, Happe F, Plomin R. The genetic relationship betweenindividual differences in social and nonsocial behaviours characteristicof autism. Dev Sci 2005;8:444-58.
[22] Ronald A, Happe F, Bolton P, Butcher LM, Price TS, Wheelwright S,et al. Genetic heterogeneity between the three components of theautism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry2006;45:691-9.
[23] Ronald A, Happe F, Plomin R. A twin study investigating the geneticand environmental aetiologies of parent, teacher and child ratings ofautistic-like traits and their overlap. Eur Child Adolesc Psychiatry2008;17:473-83.
[24] Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V,Happé F, et al. Evidence that autistic traits show the same etiology inthe general population and at the quantitative extremes (5%, 2.5%, and1%). Arch Gen Psychiatry 2011;68:1113-21.
[25] Lundström S, Chang Z, RåstamM, Gillberg C, Larsson H, AnckarsäterH, et al. Autism spectrum disorders and autistic like traits: similaretiology in the extreme end and the normal variation. Arch GenPsychiatry 2012;69:46-52.
[26] Constantino JN, Todd RD. Autistic traits in the general population: atwin study. Arch Gen Psychiatry 2003;60:524-30.
[27] Hoekstra RA, Bartels M, Verweij CJ, Boomsma DI. Heritability ofautistic traits in the general population. Arch Pediatr Adolesc Med2007;161:372-7.
[28] Kunihira Y, Senju A, Dairoku H, Wakabayashi A, Hasegawa T.'Autistic' traits in non-autistic Japanese populations: relationships withpersonality traits and cognitive ability. J Autism Dev Disord2006;36:553-66.
[29] Kurita H, Koyama T. Autism-spectrum quotient Japanese versionmeasures mental health problems other than autistic traits. PsychiatryClin Neurosci 2006;60:373-8.
10 A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
[30] Stazi MA, Cotichini R, Patriarca V, Brescianini S, Fagnani C,D'Ippolito C, et al. The Italian Twin Project: from the personalidentification number to a national twin registry. Twin Res2002;5:382-6.
[31] Brescianini S, Fagnani C, Toccaceli V,MeddaE,Nisticò L,D'Ippolito C,et al. An update on the Italian Twin Register: advances in cohortrecruitment, project building and network development. Twin Res HumGenet 2013;16:190-6.
[32] Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. TheAutism-Spectrum Quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathema-ticians. J Autism Dev Disord 2001;31:5-17.
[33] Wakabayashi A, Baron-Cohen S, Wheelwright S, Tojo Y. The Autism-Spectrum Quotient (AQ) in Japan: a cross-cultural comparison.J Autism Dev Disord 2006;36:263-70.
[34] Ruta L, Mazzone D, Mazzone L, Wheelwright S, Baron-Cohen S. TheAutism-Spectrum Quotient—Italian version: a cross-cultural confir-mation of the broader autism phenotype. J Autism Dev Disord2012;42:625-33.
[35] Goldberg DP. The detection of psychiatric illness by question-naire. Maudsley monograph no. 21. Oxford: Oxford UniversityPress; 1972.
[36] Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O,et al. The validity of two versions of the GHQ in the WHO study ofmental illness in general health care. Psychol Med 1997;27:191-7.
[37] Picardi A, Abeni D, Pasquini P. Assessing psychological distress inpatients with skin diseases: reliability, validity and factor structure ofthe GHQ-12. J Eur Acad Dermatol Venereol 2001;15:410-7.
[38] Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. TheTemperament and Character Inventory (TCI): a guide to itsdevelopment and use. St Louis: Center for Psychobiology andPersonality, Washington University; 1994.
[39] Tome MB, Cloninger CR, Watson JP, Isaac MT. Serotonergicautoreceptor blockade in the reduction of antidepressant latency:personality variables and response to paroxetine and pindolol. J AffectDisord 1997;44:101-9.
[40] Neale MC, Cardon LR. Methodology for genetic studies of twins andfamilies. Dordrecht: Kluwer Academic; 1992.
[41] Neale MC, Boker SM, Xie G, Maes H. Mx: statistical modeling. 7th ed.Richmond: Department of Psychiatry, Virginia CommonwealthUniversity; 2006.
[42] Jiang N, Sato T, Hara T, Takedomi Y, Ozaki I, Yamada S. Correlationsbetween trait anxiety, personality and fatigue: study based on theTemperament and Character Inventory. J Psychosom Res2003;55:493-500.
[43] Kanne SM, Christ SE, Reiersen AM. Psychiatric symptoms andpsychosocial difficulties in young adults with autistic traits. J AutismDev Disord 2009;39:827-33.
[44] Hallett V, Ronald A, Happé F. Investigating the associationbetween autistic-like and internalizing traits in a community-based twin sample. J Am Acad Child Adolesc Psychiatry2009;48:618-27.
[45] Hallett V, Ronald A, Rijsdijk F, Happé F. Association of autistic-likeand internalizing traits during childhood: a longitudinal twin study. AmJ Psychiatry 2010;167:809-17.
[46] Kim JA, Szatmari P, Bryson SE, Streiner DL, Wilson FJ. Theprevalence of anxiety and mood problems among children with autismand Asperger syndrome. Autism 2000;4:117-32.
[47] Gillott A, Furniss F, Walter A. Anxiety and high-functioning childrenwith autism. Autism 2001;5:277-86.
[48] Maes JH, Vissers CT, Egger JI, Eling PA. On the relationship betweenautistic traits and executive functioning in a non-clinical Dutch studentpopulation. Autism 2013;17:379-89.
[49] Soderstrom H, Rastam M, Gillberg C. Temperament and character inadults with Asperger syndrome. Autism 2002;6:287-97.
[50] Anckarsäter H, Stahlberg O, Larson T, Hakansson C, Jutblad SB,Niklasson L, et al. The impact of ADHD and autism spectrum
disorders on temperament, character, and personality development.Am J Psychiatry 2006;163:1239-44.
[51] Sizoo B, van den Brink W, Gorissen van Eenige M, van der Gaag RJ.Personality characteristics of adults with autism spectrum disorders orattention deficit hyperactivity disorder with and without substance usedisorders. J Nerv Ment Dis 2009;197:450-4.
[52] Vuijk R, de Nijs PF, Vitale SG, Simons-Sprong M, Hengeveld MW.Persoonlijkheidsaspecten bij volwassenen met autismespectrumstoor-nissen gemeten met de Temperament and Character Inventory(Personality traits in adults with autism spectrum disorders measuredby means of the Temperament and Character Inventory). TijdschrPsychiatr 2012;54:699-707.
[53] Jacob S, Landeros-Weisenberger A, Leckman JF. Autism spectrumand obsessive–compulsive disorders: OC behaviors, phenotypes andgenetics. Autism Res 2009;2:293-311.
[54] Paula-Pérez I. Differential diagnosis between obsessive compulsivedisorder and restrictive and repetitive behavioural patterns, activitiesand interests in autism spectrum disorders. Rev Psiquiatr Salud Ment2013;6:178-86.
[55] Ivarsson T, Melin K. Autism spectrum traits in children andadolescents with obsessive–compulsive disorder (OCD). J AnxietyDisord 2008;22:969-78.
[56] Bejerot S, Nylander L, Lindström E. Autistic traits in obsessive–compulsive disorder. Nord J Psychiatry 2001;55:169-76.
[57] Van Steensel FJA, Bögels SM, Perrin S. Anxiety disorders in childrenand adolescents with autistic spectrum disorders: a meta-analysis. ClinChild Fam Psychol Rev 2011;14:302-17.
[58] Pertusa A, Bejerot S, Eriksson J, Fernández de la Cruz L, Bonde S,Russell A, et al. Do patients with hoarding disorder have autistic traits?Depress Anxiety 2012;29:210-8.
[59] Gillespie NA, Cloninger CR, Heath AC, Martin NG. Thegenetic and environmental relationship between Cloninger'sdimensions of temperament and character. Pers Indiv Differ2003;35:1931-46.
[60] Ando J, Suzuki A, Yamagata S, Kijima N, Maekawa H, Ono Y, et al.Genetic and environmental structure of Cloninger's temperament andcharacter dimensions. J Pers Disord 2004;18:379-93.
[61] Plomin R, Asbury K, Dunn J. Why are children in the same family sodifferent? Nonshared environment a decade later. Can J Psychiatry2001;46:225-33.
[62] Ronald A, Happe F, Dworzynski K, Bolton P, Plomin R. Exploring therelation between prenatal and neonatal complications and later autistic-like features in a representative community sample of twins. Child Dev2010;81:166-82.
[63] LaiMC,LombardoMV,Baron-CohenS.Autism.Lancet 2014;383:896-910.[64] Plomin R. The Emanuel Miller Memorial Lecture 1993: genetic
research and identification of environmental influences. J ChildPsychol Psychiatry 1994;35:817-34.
[65] Eaves L, Martin N, Heath A, Schieken R, Meyer J, Silberg J, et al. Agechanges in the causes of individual differences in conservatism. BehavGenet 1997;27:121-4.
[66] Koenig LB, McGue M, Krueger RF, Bouchard Jr TJ. Genetic andenvironmental influences on religiousness: findings for retrospectiveand current religiousness ratings. J Pers 2005;73:471-88.
[67] Betancur C, Leboyer M, Gillberg C. Increased rate of twins amongaffected sibling pairs with autism. Am J Hum Genet 2002;70:1381-3.
[68] Greenberg DA, Hodge SE, Sowinski J, Nicoll D. Excess of twinsamong affected sibling pairs with autism: implications for the etiologyof autism. Am J Hum Genet 2001;69:1062-7.
[69] Hallmayer J, Glasson EJ, Bower C, Petterson B, Croen LA,Grether J, et al. On the twin risk in autism. Am J Hum Genet2002;71:941-6.
[70] Curran S, Dworzynski K,Happé F,RonaldA,AllisonC, Baron-Cohen S,et al. No major effect of twinning on autistic traits. Autism Res2011;4:377-82.
[71] Keller MC, Coventry WL, Heath AC, Martin NG. Widespreadevidence for non-additive genetic variation in Cloninger's and
11A. Picardi et al. / Comprehensive Psychiatry xx (2015) xxx–xxx
Eysenck's personality dimensions using a twin plus sibling design.Behav Genet 2005;35:707-21.
[72] Maes HH, Neale MC, Medland SE, Keller MC, Martin NG,Heath AC, et al. Flexible Mx specification of various extendedtwin kinship designs. Twin Res Hum Genet 2009;12:26-34.
[73] Goodman R. Infantile autism: a syndrome of multiple primary deficits?J Autism Dev Disord 1989;19:409-24.
[74] Happe F, Ronald A. The ‘fractionable autism triad’: a review ofevidence from behavioural, genetic, cognitive and neural research.Neuropsychol Rev 2008;18:287-304.
[75] Mandy WPL, Skuse DH. Research review: what is the associationbetween the social-communication element of autism and repetitiveinterests, behaviours and activities? J Child Psychol Psychiatry2008;49:795-808.