Advances in bipolar disorder: selected sessions from the 2011 International Conference on Bipolar...
Transcript of Advances in bipolar disorder: selected sessions from the 2011 International Conference on Bipolar...
Ann. N.Y. Acad. Sci. ISSN 0077-8923
ANNALS OF THE NEW YORK ACADEMY OF SCIENCESIssue: Annals Meeting Reports
Advances in bipolar disorder: selected sessions from the2011 International Conference on Bipolar Disorder
David J. Kupfer,1 Jules Angst,2 Michael Berk,3 Faith Dickerson,4 Sophia Frangou,5 EllenFrank,1 Benjamin I. Goldstein,6 Allison Harvey,7 Fouzia Laghrissi-Thode,8 Marion Leboyer,9
Michael J. Ostacher,10 Etienne Sibille,1 Stephen M. Strakowski,11 Trisha Suppes,10 MauricioTohen,12 Robert H. Yolken,13 L. Trevor Young,6 and Carlos A. Zarate14
1University of Pittsburgh, Pittsburgh, Pennsylvania. 2Zurich University Psychiatric Hospital, Zurich, Switzerland. 3University ofMelbourne, Melbourne, Australia. 4Sheppard Pratt Health System, Baltimore, Maryland. 5Institute of Psychiatry, Kings CollegeLondon, London, United Kingdom. 6University of Toronto Faculty of Medicine, Toronto, Ontario, Canada. 7University ofCalifornia at Berkeley, Berkeley, California. 8F. Hoffmann-La Roche Ltd., Basel, Switzerland. 9Hopital Chenevier – Mondor,Universite Paris XII, Creteil, France. 10Stanford University School of Medicine, Palo Alto, California. 11University of CincinnatiAcademic Health Center, Cincinnati, Ohio. 12University of Texas Health Science Center, San Antonio, Texas. 13The JohnsHopkins University School of Medicine, Baltimore, Maryland. 14National Institute of Mental Health, Bethesda, Maryland
Address for correspondence: David J. Kupfer, MD, University of Pittsburgh, Western Psychiatric Institute and Clinic, 3811O’Hara Street, Pittsburgh, PA 15213, [email protected]
Recently, the 9th International Conference on Bipolar Disorder (ICBD) took place in Pittsburgh, PA, June 9–11,2011. The conference focused on a number of important issues concerning the diagnosis of bipolar disorders acrossthe life span, advances in neuroscience, treatment strategies for bipolar disorders, early intervention, and medicalcomorbidity. Several of these topics were discussed in four plenary sessions. This meeting report describes the majorpoints of each of these sessions and included (1) strategies for moving biology forward; (2) bipolar disorder and theforthcoming new DSM-5 nomenclature; (3) management of bipolar disorders—both theory and intervention, withan emphasis on the medical comorbidities; and, (4) a review of several key task force reports commissioned by theInternational Society for Bipolar Disorder (ISBD).
Keywords: bipolar disorders; medical comorbidity; neuroscience; diagnosis
Session on strategies for moving biologyforward
OverviewThe aim of the session “Strategies for Moving Biol-ogy Forward,” chaired by Marion Leboyer (Univer-site Paris XII), was to investigate new disease mecha-nisms such as immune–inflammatory markers andoxidative stress, while describing the utility of toolssuch as transcriptomics and multiomic profiling.Genomics has already pointed to diverse molecu-lar pathways that confer risk to bipolar disorder,though not to the anticipated extent. Etienne Sibilledescribed how he is developing methods to inte-grate results and potentially synergize the analysis ofthe Genome-wide Association Study (GWA) studies
and transcriptomic studies. Coregulated RNA tran-scripts have been shown to identify coherent genemodules with shared functions, consequently, it hasbeen hypothesized that the coregulated gene mod-ules that are enriched in genes associated with GWAresults may identify relevant biological pathwaysto explore the underlying mechanisms of bipolardisorder. Robert Yolken and Faith Dickerson haveshown that acute mania was associated with evi-dence of immune activation by: (1) elevated levels ofcytokines, such as TNF-�; (2) inflammatory macro-molecules, such as C-reactive protein (CRP); (3)antibodies to infectious agents, such as retroviruses;(4) antibodies to food antigens, such as gliadin; and(5) antibodies to brain proteins, such as the NR-2peptide of the NMDA receptor. In many cases, the
doi: 10.1111/j.1749-6632.2011.06336.xAnn. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 1
Advances in bipolar disorder Kupfer et al.
levels of these biomarkers are elevated during acutemania and are decreased six months later. Ongoingresearch should lead to the identification of a bio-logical “signature” of mania, offering potential toolsboth for diagnosis of acute mania and for predictionof illness course, and hope for new therapies. Med-ications directed at the modulation of the immuneresponse may form the basis of a new therapeuticarmamentarium for the prevention and treatmentof this disorder. Trevor Young presented work inthe field of mitochondrial dysfunction and energymetabolism in bipolar disorder. Data clearly showthat oxidative damage is present in the postsynapticmembranes and also found in the periphery. Indeed,several studies report increased oxidative stress inserum and plasma of bipolar patients. From a ther-apeutic perspective, this pathway may be of greatimportance as several mood stabilizers have antiox-idant properties. The conclusion of this session isthat studying genetic, immunoinflammation, andmitochondrial dysfunction will be very helpful tobetter understand the pathophysiology of bipolardisorder.
Genome-wide association studiesEtienne Sibille (University of Pittsburgh) openedthis session and described a series of fascinat-ing studies. Ranscriptome (the set of all expressedgenes in a tissue sample) and genome-wide associ-ation (GWA) studies have separately provided cluesto mechanisms of neuropsychiatric disorders, al-though not to the anticipated extent. Transcriptomestudies mostly focus on changes in gene expres-sion in disease states (altered expression), but alsoprovide unique opportunities for assessing the less-investigated changes in the coordinated function ofmultiple genes (altered coexpression). Moreover, re-sults from these large-scale investigations of changesin gene function are poised to interact with GWAstudies of structural changes in genes and regula-tory regions (DNA variant). Methods for integrat-ing these approaches are now being developed.
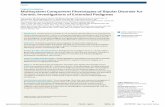
Gene arrays allow for the unbiased quantificationof expression (mRNA transcript levels) for 10,000to 20,000 genes simultaneously (see Fig. 1A). Sincegene transcript levels represent the integrated out-put of many regulatory pathways, the study of allexpressed genes provides an indirect snapshot ofcellular function under diverse conditions. For in-stance, using postmortem brain samples, this “re-
verse engineering” approach has implicated mito-chondrial dysfunction and immune/inflammation-related changes in bipolar subjects.1–3 However,current studies are still very few, were performed inheterogeneous cohorts, and utilized early and rudi-mentary versions of gene arrays. Moreover, gene ar-ray studies are subjects to similar limitations as GWAstudies, in that large number of genes are tested(n = 20–40,000) in few subjects (n = 10–100). Typ-ically, results identify 1–10% of genes affected in theillness, are characterized by very high rates of falsediscovery, and often do not properly account fornumerous clinical (e.g., drug exposure, subtypes,duration), demographic (age, sex, race), technicalparameters (RNA integrity, brain pH, postmorteminterval for brain collection), or other potentialcosegregating unknown cohort specificities. Condi-tions of postmortem brain collection also precludethe reliable identification of acute state-dependentgene changes, but are appropriate for investigat-ing stable long-term disease-related homeostaticadaptations.4
TranscriptomicsA notable derivative and less investigated aspect oftranscriptome studies is the development of genecoexpression studies. Here, two genes are definedas coexpressed in a dataset if their patterns of ex-pression are correlated across samples (see Fig. 1B).Coexpression has been shown to reflect shared func-tion between these genes, and may arise throughmultiple biological pathways, including cellular co-expression and common regulatory pathways (e.g.,hormone signaling, transcription factors).5,6 Hence,coexpression links have been used to build gene net-works, and to identify communities, or modules, ofgenes with shared functions (see Fig. 1B).7,8 Notably,by incorporating multiple interactions among largenumber of genes, the study of gene coexpressionnetworks provides one solution to tackle the com-plexity of biological changes occurring in complexpolygenic disorders.5 Indeed, the information con-tent of a large-scale gene network could be comparedto the operation of a symphonic orchestra of manyhundreds of instruments, where the intrinsic bal-ance (coexpression) between instruments (genes)provide harmonious (homeostatic) function.9
Efforts from our research team has demonstratedthat brain gene coexpression networks assemble intosmall-world and scale-free networks,10 an efficient
2 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
Figure 1. (A) Altered gene expression. Large-scale gene arrays simultaneously investigate expression levels of thousands of genes(top) and seek changes in gene transcript levels between conditions (bottom). (B) Gene coexpression. (top) Genes 3 and 4 displaycorrelated patterns of expression across samples (X axis) between two conditions (gray and black lines). This relationship is quantifiedby Pearson correlation and provides a link in the gene coexpression network (bottom). Genes 3 and 5 are not coexpressed and donot contribute to the network. Nodes (genes) of different colors and shapes are associated with different cellular functions.5,119 (C)Synergizing GWA and transcriptome studies. Genes associated with candidate DNA variants, as identified by GWA Manhattan plots(top), are overlaid onto gene coexpression networks (bottom). Gene coexpression networks that are enriched in GWA-associatedgenes are hypothesized to represent candidate biological systems for disease mechanisms.
network topology that is often observed in biolog-ical systems, but also in communication and so-cial networks.9 Interestingly, our initial study sug-gests that the structure of brain gene coexpressionnetworks is largely resilient to changes occurringin neuropsychiatric disorders (major depression,bipolar depression, and schizophrenia), and in-stead points to a peripheral network localizationof differentially-expressed genes.10 This is in strik-ing contrast to cancer- and other systemic illness-related networks, where affected genes tend todisplay a “disease/lethality–network centrality” re-lationship.11 This contrast may relate to the diffi-culty in identifying silver-bullet types of drugs inneuropsychiatry, since affected genes may attend tocontrol biological gene networks from the periph-ery, rather than through central gene hubs.10
Based on the assumptions that (1) hits on differ-ent components of a biological pathway may lead tothe same disease phenotype, even in the absence ofcommon changes across subjects, and (2) that DNAgenetic polymorphisms and gene mRNA transcriptlevels represent independent and complementarymeasures of gene structure and function, we have
hypothesized that modules of coexpressed genes,which are enriched in genes associated with GWA-identified polymorphisms, may represent candidatebiological pathways for recruitment in mechanismsof disease (see Fig. 1C). To address this question,our approach has been to identify conserved androbust gene modules based on multiple and exten-sive transcriptome studies in the human brain andto overlay onto those modules, genes that are locatednearby DNA polymorphisms associated with neu-ropsychiatric disorders, as identified by GWA stud-ies. Current efforts are addressing the difficulties incombining heterogeneous studies and the statisticaland analytical challenges that emerge from integrat-ing multiple large-scale approaches (transcriptome,coexpression network, and GWA).
In summary, the potential of transcriptome stud-ies for unbiased novel discovery and for investigat-ing basic pathological changes beyond the usualsuspects is vast, but not yet realized in neuropsy-chiatry. Early results from transcriptome studiesof altered gene expression in bipolar disordersand other major mental illnesses are promising,but results need to be replicated across multiple
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 3
Advances in bipolar disorder Kupfer et al.
cohorts and research groups. More studies areneeded using updated genetic information and tech-nological platforms. Shared access to the raw datais also necessary, as the next generation of tran-scriptome studies will need to apply novel statis-tical methods for within-study parameter integra-tion and for across-study meta-analyses, includingpermutation-based methods and accurate controlof false discovery. Currently, the study of genecoexpression networks in neuropsychiatric disor-ders is still in its infancy, but the field will benefitfrom applying network methodologies developedfor investigating other complex biological systems,including development, cancer, and brain func-tional activity. Finally, concepts and methods forintegrating functional (transcriptome) and struc-tural (DNA polymorphism GWA) studies of themolecular bases of complex neuropsychiatric dis-orders need to be developed to harness the potentialof systematic large-scale molecular and genetic in-vestigations of the brain.
Immune–inflammatory markersRobert Yolken (Johns Hopkins University) and FaithDickerson (Sheppard Pratt Health System) then de-scribed some of their new work. Mania is an abnor-mal mood state, and the defining characteristic ofbipolar disorder in which the etiology is unknown.Immunological abnormalities have been identifiedwhich may contribute to the pathophysiology of ma-nia as well as to bipolar disorder more broadly.12–16
Such factors may help explain the marked fluctua-tions in mood symptoms, which are the hallmark ofthe disorder.
In a previous study, they examined the level ofCRP, a nonspecific marker of inflammation in indi-viduals with bipolar disorder.17 CRP is a pentamericprotein that is generated in the liver and secreted inthe blood. The measurement of CRP in the bloodprovides a reliable marker of chronic inflammationcaused by infectious and other inflammatory agents.We measured the level of CRP in N = 122 outpa-tients with bipolar disorder and N = 165 controlindividuals and evaluated the symptom severity ofthe bipolar disorder patients. Within the bipolar dis-order sample, CRP was significantly associated withthe Young Mania Rating Scale (YMRS)18 score andin a multivariate analysis, CRP was the only inde-pendent predictor of YMRS score. The CRP levelsof the n = 41 individuals with YMRS > 6 were
significantly greater than the levels of the n = 81individuals with YMRS ≤, 6. The CRP levels of thegroup with YMRS > 6 were also significantly greaterthan the levels of the control group while the CRPlevels of the group with YMRS ≤ 6 did not differfrom that of controls.
Based on the results of this cross sectional study,they undertook a longitudinal study of individu-als hospitalized for symptoms of acute mania. Ouraim was to measure inflammatory markers in acutemania and to determine changes over time in thesemarkers and also to determine the correlation ofmarkers with clinical outcome, whether or not per-sons were rehospitalized for a new illness episode inthe six-month follow-up period. From blood sam-ples we measured inflammatory markers includingantibodies to intestinal antigens, antibodies to neu-roreceptors, antibodies to endogenous retroviruses,and cytokines. The sample of N = 60 participantshad a mean age of 35.4 (SD = 12.9) years andwas 30% male and 67% Caucasian. The diagnosesof study participants were divided among bipolardisorder, most recent episode manic (55%), mostrecent episode mixed (34%), and schizoaffectivedisorder, current manic episode (11%). The meanYMRS score at the time of evaluation during thehospital stay was 18.7 (SD = 8.6) and the Positiveand Negative Syndrome Scale total score19 was 75.2(SD = 11.7). For most study participants, bloodsamples were measured at three time points: the dayof hospital admission from archived samples fromthe medical admission work-up (n = 44); the dayof evaluation for the current study, on average 3–5days following hospital admission (n = 60); and ata planned six month follow-up (n = 39).
Results of the longitudinal study show that pa-tients hospitalized with mania had increased levelsof immune markers that were lower six months later.Some of the markers appear to be mania specific.Some of the markers were associated with clinicaloutcome, whether or not patients were rehospital-ized for a new illness episode during the follow-upperiod.
The results of these studies are consistent witha body of literature suggesting that acute episodesof mania are associated with evidence of immuneactivation. The pathways, which are suggested tobe involved, are shown in Figure 2. The literatureindicates an association between mania and ele-vated levels of cytokines including interferon � ,
4 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
Figure 2. Molecular processes are affected by stress and depression. From Ref. 120, with permission from John Wiley and Sons.
interleukin 2 (IL-2), IL-6, TNF-�;20 cell-mediatedimmunity, including the composition of lympho-cytes and T cell receptors;21 complement such asimmune complexes and C1q,16 and lipid levels suchas cholesterol.22 Studies indicate that many of theseabnormalities normalize following resolution ofacute mania and differ from patterns found in bipo-lar depression. Our data support the study of inter-ventions addressed at lowering levels of markers ofimmune activation as a possible treatment for ma-nia. This research may lead to improvements in thediagnosis and treatment of bipolar disorder.
Trevor Young (University of Toronto) presentedthe final report in this session in which he reviewedthe evidence for mitochondrial dysfunction and ox-idative stress in this illness. Earlier findings of ma-ternal inheritance in bipolar disorder suggested theimportance of mitochondria23 and the field movedforward quickly with the finding of increased mi-tochondrial DNA deletions in postmortem brain.24
It was then quite intriguing that several microar-ray studies on independent samples of brain tissuefound remarkable decreases in mRNAs for com-ponents of the electron transport change,25 whichis one of the core functions of the electron trans-port chain. Their lab has followed up on these dataand found compelling evidence of mitochondrialdysfunction in bipolar disorder. They reported de-creased levels of one of the components of complexI of the electron transport chain and also the ac-tivity of this complex in prefrontal cortex obtained
postmortem from patients with bipolar disorder.26
There was also increased protein carbonylation and3-nitrotyrosine levels in the same samples. Therewas a significant inverse correlation between com-plex I activity and the oxidative metabolites in thesame brain region. They next measured the brain’santioxidant system in the same samples and foundthat in bipolar disorder, as in schizophrenia and ma-jor depression, that the mean levels of glutathionewere depleted in all diagnostic groups suggestingan impact of chronic psychiatric illness on thissystem.27 Interestingly, the same findings appearto be present in another postmortem brain sam-ple from subjects with bipolar disorder and thedifferences are particularly evident in the synap-tosomal fractions (Andreazza et al., unpublisheddata), which suggests that oxidative damage mightbe present in postsynaptic membranes and couldaffect function at this level which would be con-sistent with traditional sites of pathophysiology inmood disorders.
What is particular fascinating and might be rel-evant to move the field forward is that many ofthese findings are also found in blood samples frompatients with bipolar disorder. Indeed, Konradiet al.2 found that the expression of a number ofelectron transport chain mRNAs was altered in lym-phocytes from patients with bipolar disorder. Morerecently, mitochondrial clustering has been demon-strated in lymphocytes and fibroblasts from patientswith bipolar disorder.28 There have been a number
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 5
Advances in bipolar disorder Kupfer et al.
of findings of increased oxidative stress in serumand plasma markers and a meta-analysis has shownthat several of these findings are evident acrossstudies.29 These data have been particularly impor-tant in the work of one of the International Soci-ety of Bipolar Disorders (ISBD) committees on thestudy of biomarkers (see below). Finally, this path-way might also be relevant for treatment of bipo-lar disorder since several mood stabilizers, lithium,and valproate, have antioxidant properties,30 andat least several studies suggest that the antioxidant,N–acetylcysteine, may have some mood stabilizingproperties.31 The conclusion of this presentation isthat, indeed, continued study of mitochondrial dys-function may be very helpful to gain a more fulsomeunderstanding of the pathophysiology of bipolardisorder.
Session on diagnosis
OverviewThe session on diagnosis was chaired by CarlosZarate (National Institute of Mental Health). Theconcept of bipolar disorder, initially known asmanic-depressive insanity, has gone through con-siderable revision since it was first proposed.32 Bipo-lar disorder consists of two major components, de-pression and hypo/mania, which vary greatly inseverity, duration, and course. In addition, thesecomponents may present simultaneously or sepa-rately in time. Over the years, the boundaries ofdepression, mania, and bipolar disorder continueto shift. In recent years, attention has been givento the concept of bipolar spectrum disorders.33 TheDSM was introduced in part to clarify the ambigui-ties in psychiatric diagnosis. However, even with theadvent of the DSM, there remains considerable un-certainty on diagnostic concepts of bipolar disorder.
The DSM is now going into its 5th edition, andthe bipolar disorder subworkgroup in collaborationwith the full Mood Disorder Workgroup for theDSM-5 has come up with proposed revisions ad-dressing some of these diagnostic ambiguities. It isimportant to note that when there is a proposedrevision that this is done by following a series ofrevision principles and utilizing generally acceptedvalidators of a diagnostic entity. The principles ofrevision used in the workgroup were to optimizeclinical utility, to make recommendations guidedby research evidence, to maintain continuity withprevious editions, and to set the stage for future de-
velopments in our understanding of the brain. Forthere to be a change in the criteria it is also neces-sary to elucidate the reason for change and presentevidence in support of change. Jules Angst summa-rized work and research on evidence-based effortsto redefine bipolar disorders, Ellen Frank discussedrevisions to the concept of mixed episodes in DSM-5, and Trisha Suppes covered criteria of hypomanicepisodes.
The report by Angst uses a new specifier (in-creased energy/activity levels) to understand the as-sociations among major depressive disorder, bipolarI, and bipolar II disorders. When using this specifierin the BRIDGE study (bipolar disorders: improv-ing diagnosis, guidance, and education), what wasfound was that a significant number of individu-als in a major depressive episode diagnosed withmajor depressive disorder in reality had a bipolardisorder diagnosis. When using the specifier crite-ria, the authors found that the distinction betweenBP-I versus BP-II disorders was much sharper. BP-Idisorders had higher suicide attempt rates, whereasindividuals with BP-II disorders had a greater asso-ciation with anxiety disorders. Also, both bipolar Iand II disorders, when compared with MDD, hadhigher rates of association with social phobia, sub-stance use disorders, obsessive-compulsive disorder,suicide attempts, and attention disorder hyperactiv-ity disorder. BP-II disorder compared to MDD hadsignificantly more generalized anxiety disorder andpanic.
Mixed episode, initially described as mixed statesof manic-depressive insanity, has received many re-visions since originally conceptualized.32 Early on, itwas evident that some patients with acute mania orhypomania simultaneously experience prominentdepressive symptoms. Clinicians tried to capturethis ambiguity that surrounds this condition by de-veloping diagnostic criteria. A number of diagnosticcriteria have been proposed over the years.33 DSM-IV criteria specified that in order to meet criteriafor a mixed episode that an individual was requiredto simultaneously fulfill criteria for both a manicand major depressive episode nearly every day fora period of at least one week. This was viewed astoo broad and is often not seen. Instead clinicianswere more likely to encounter individuals with asimultaneous admixture of depressive and manicsymptoms that did not meet DSM-IV criteria for amixed episode. Such clinical presentations were also
6 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
associated with many of features of this more strin-gent form (i.e., more likely to be associated with sui-cidality, longer duration of illness, concomitant al-cohol or sedative-hypnotic abuse, poorer outcome,and less adequately responsive to lithium). Thus,identifying the mixed state presentations across thespectrum would likely have major clinical impli-cations. The paper by Frank et al.59 proposes thatmixed states be identified by specifiers that can beapplied to episodes of either depression or ma-nia/hypomania and that can be applied to individu-als with a lifetime diagnosis of either major depres-sive disorder or bipolar disorder. It is believed thatthe use of specifiers would lead to earlier diagnosisand treatment.
Another challenge in the classification of bipo-lar disorder is how to define a hypomanic episode.The paper by Trisha Suppes and colleagues34 ad-dresses this important issue. Currently in DSM-IV-TR, the prototypical symptom of hypomania is el-evated mood (and/or irritable mood). The DSM-5bipolar subgroup proposes to take out one of thecriterion B symptoms, “increase in goal-directedactivity,” and to place it with “elevated mood” incriterion A. Thus, the change in criterion A wouldbe a distinct period of abnormally and persistentlyelevated, expansive, or irritable mood AND changein activity/energy levels, which should also be ab-normally and persistently increased. This suggestedchange would lead to increase specificity withoutloss of sensitivity. The other issue addressed in thepaper by Suppes is the number and duration ofhypomanic/manic symptoms necessary to meet cri-teria for a hypomanic episode. Clinicians are oftenunsure how to proceed when diagnosing a hypo-manic episode. This is not surprising as diagnosisof hypomanic episode is the most unreliable usingDSM-IV criteria. There has been an abundance ofstudies looking at whether hypomanic symptomsshould be present for two, three, four, or more daysin order for the diagnosis to be made. The work-group proposes to keep the four-day requirement,which would increase specificity with reasonablesensitivity.
The proposed DSM-IV changes are an importantstep to resolve some of the ongoing diagnostic am-biguities of bipolar disorder. Clearly, the proposedchanges are in need of testing, which will occur un-der the field trials. The DSM-5 committee is openfor comment and considering changes in diagnostic
criteria for bipolar disorder in light of these propos-als (www.dsm5.org).
Mood disorder workgroup reportsJules Angst presented first. Conventionally, mooddisorders are classified into depression, mania, andbipolar disorders. While these distinctions are veryuseful for international communication and treat-ment decisions, nature is more complex. The twocomponents, depression and mania, vary greatly inseverity and course, as do the subgroups of bipo-lar disorders (BP-I, BP-II, Md (mania with minordepressive disorders)).35 As Cassano et al.36 demon-strated in regard to patients with recurrent majordepression, there is a linear increase in the num-ber of manic symptoms as a function of depressivesymptoms over the patient’s lifetime. Bipolar dis-orders often manifest first as depression; we knowfor a fact that their diagnosis may often be delayedby up to ten years37 and that depression in patientswith bipolar or subthreshold bipolar disorder is lessresponsive to antidepressants.38,39
Depression has long dominated the field in termsof prevalence, treatment and, especially, in the esti-mates of global burden of disease. But if we assumea spectrum from depression via bipolar disordersto mania, it is important to investigate how manysubjects suffering from major depressive disordersalso manifest sub-threshold hypomania. In recentyears, reanalysis of two large epidemiological stud-ies (Early Developmental Stages of Psychopathology(EDSP) study in Munich, Germany and the Na-tional Comorbidity Study-R in the United States)found that as many as 40% of patients with DSM-IV major depressive disorder (MDD) met criteriafor sub-threshold bipolarity; the latter was stronglypredictive for BP-I disorder ten years later.40,41
The structured interviews (DIS) applied in thosestudies did not assess sub-threshold hypomania inany detail. This gap has been filled by the BRIDGEstudy, which made detailed assessments allowing anoperational definition. The study comprised 5,635patients with DSM-IV major depressive episodes(MDE) recruited in North Africa, Europe, and theNear and the Far East.42 The study validated a new“specifier” (S) definition for hypomania/mania.43
This adds increased activity/energy to DSM-IV cri-terion A (elated or irritable mood) and eliminatesall the DSM exclusion criteria (e.g., hypomania un-der antidepressants) because they rule out patients
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 7
Advances in bipolar disorder Kupfer et al.
who have a clinical profile of bipolarity. The othercriteria for BP-I-S are identical to those of DSM-IV.BP-II-S, on the other hand, differs from DSM-IVBP-II by requiring a hypomanic episode duration ofonly one day or longer (instead of the 4+ days inDSM-IV) and the lack of any exclusion criteria. Thevalidity of short hypomanic episodes of one and 2–3days was demonstrated by the BRIDGE study.42
The consequences of redefinition by the specifiercriterion are very considerable: not only does it iden-tify many more patients with affective disorders ashaving bipolar disorders, it also distinguishes moreclearly between the new bipolar subgroups and theremaining MDD in terms of validators and otherclinical characteristics (comorbidity, nonresponseto antidepressants). The current DSM-IV criteriadiagnosed 4,732 (84%) of the 5,635 patients witha MDE as having MDD, but the specifier criteriadiagnosed only 2,988 (53%) (MDD-S). Forty-sevenpercent were diagnosed as suffering from bipolardisorders. The specifier concept turned out to bemore valid than the DSM-IV classification, as illus-trated by the frequent presence of a family history ofmania, a progressively more recurrent course, morefull remission between episodes, and other clinicalcharacteristics for bipolar disorders.42
DSM-IV diagnosed 12.1% of the 5,635 MDE pa-tients as having BP-I and 2.3% as having BP-II;specifier criteria diagnosed 23.9% as having BP-I-S and 23.1% as having BP-II-S. The distinction be-tween BP-I versus BP-II disorders was much sharperwhen specifier diagnoses were applied. BP-I-S dis-orders were associated with higher suicide attemptrates, and BP-II-S disorders to a greater extentwith anxiety disorders (generalized anxiety disor-der (GAD), panic disorder (PD), and obsessive-compulsive disorder (OCD)). In comparison withMDD-S both BP-I-S and BP-II-S disorders differedin their higher rates of association with social pho-bia, OCD, binge eating, suicide attempts, substanceuse disorders, ADHD, and borderline personalitydisorder. Whereas, compared to MDD-S, BP-II-S disorder was significantly more associated withGAD and PD, there was no difference at all be-tween BP-I-S disorder and MDD-S in this respect.The strong comorbidity of BP-II-S disorders withanxiety states is clearly of great clinical interest.Figure 3 illustrates the enormous differences be-tween the varying concepts in the diagnosis of bipo-lar disorders. The DSM-IV definition gave the low-
Figure 3. Diagnosis of bipolar versus depressive disorder byvarying definitions in the Bridge Study of 5,635 patients withMDE.
est rates; these doubled with the omission of theexclusion criteria and tripled with application ofthe specifier criteria. Self-assessment using the Hy-pomania Checklist 32-R2 as a screening instrumentyielded the highest rates. It is of particular interestthat the clinicians’ initial diagnosis identified almosttwice as many patients as bipolar compared to theDSM-IV criteria.
In conclusion, the specifier definition of hypo-mania identifies with good validity many more pa-tients with MDE as having bipolar I and bipolarII disorders and thus allows a much earlier diag-nosis and hopefully a better treatment. The find-ings need replication by other studies. The DSM-5committee is considering changes in diagnostic cri-teria for bipolar disorder in light of these findings(www.dsm5.org). Changes may allow a better char-acterization of major depression as either unipolaror bipolar and give consideration to dimensionalissues.44 This is particularly relevant to the defini-tion of mixed states. While the results of the Bridgestudy and the application of the bipolarity specifiershould not necessarily change the overall proportionof subjects from the general population who sufferfrom affective disorders, it may have an impact onthe proportion of these affective patients who arerecognized as belonging to the bipolar spectrum, asopposed to the unipolar spectrum. The impact ofsuch change on treatment and outcomes should bemonitored over the next decade.
Ellen Frank (University of Pittsburgh) was thesecond presenter in this session. In order to meetcriteria for a mixed episode in the DSM-IV, an in-dividual was required to simultaneously fulfill thecriteria for both a manic episode and for a ma-jor depressive episode (except for the duration cri-terion of two weeks) nearly every day during a
8 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
period of at least one week. In reviewing these cri-teria, the Mood Disorders Workgroup of the DSM-5 Task Force concluded that while this definitionof Mixed Episode had a satisfying symmetry, itwas rather like a unicorn: beautiful to imagine, butrarely, if ever, seen in reality. Yet, the mixed episodediagnosis was actually recorded with substantial fre-quency and patients were very often referred to asbeing “mixed” in discussions among clinicians. Fur-thermore, we identified a number of negative con-sequences of the DSM-IV definition. These include(1) general confusion and lack of clarity about a pa-tients actual clinical state; (2) underestimation ofsuicide risk, inasmuch as even softer definitionsof mixed states are associated with increased riskof suicide; (3) potentially inappropriate treatmentselection, given the poor response to lithium amongpatients in a mixed state; and, finally, (4) a failureto identify those with a lifetime diagnosis of unipo-lar disorder who are at increased risk of progres-sion to bipolar disorder. We, therefore, set as ourgoal a redefinition of Mixed Episode that wouldbetter conform to clinical reality and that would al-low for the recognition of mixed states even amongindividuals with a lifetime diagnosis of unipolardisorder.
The workgroup is currently proposing mixedstates be identified by specifiers that can be applied toepisodes of either depression or mania/hypomaniaand that can be applied to individuals with a lifetimediagnosis of either unipolar or bipolar disorder.
The proposal for the with depressive featuresspecifier requires that full criteria are met for ei-ther a manic episode or a hypomanic episode andthat at least three of the following nine depressivesymptoms are present nearly every day during theepisode: subjective depression, worry, self-reproach,or guilt, negative self-evaluation, hopelessness, sui-cidal ideation or behavior, anhedonia, fatigue, andpsychomotor retardation.
The proposal for the with hypomanic featuresspecifier requires that full criteria are met for aMDE and that at least three of the following sevenmanic/hypomanic symptoms are present nearly ev-ery day during the episode: elevated mood, de-creased need for sleep (i.e., sleeps little, but reportsfeeling rested), increased goal-directed activity, in-creased energy/visible hyperactivity (as distinctfrom agitation), grandiosity, accelerated speech, andracing thoughts.
The literature on mixed states suggested that anumber of the generally accepted validators of adiagnostic entity apply to the softer definitions pro-posed by the workgroup. These validators includefamilial aggregation of both predominantly manicand predominantly depressive mixed states, priorpsychiatric history variables, concurrent psychiatricsymptoms not included in the definition, diagnosticstability over time, increased likelihood of progres-sion from a unipolar to a bipolar diagnosis amongthose with mixed depressive episodes and treatmentoutcome.
Features of psychiatric history that are consis-tently observed among those experiencing mixedstates, such as those defined by the proposed mixedspecifiers, include early onset of illness, a history ofmultiple previous episodes, suicidal behavior, co-morbid diagnoses of anxiety and alcohol or sub-stance abuse, and brain trauma. Interestingly, all ofthese are also features that tend to distinguish in-dividuals with a lifetime history of bipolar disorderfrom those with a lifetime history of unipolar de-pression.
Two major concerns of the workgroup in devel-oping the mixed specifier criteria were to select themost appropriate symptoms and to decide on thenumber of symptoms that should be required. Inselecting the specific symptoms to be included, theworkgroup focused on selecting those symptomsthat were clearly distinct from those of the pre-dominant episode polarity. This was done entirelybased on face validity, as no literature was availableto guide us. In deciding on the number of symp-toms that should be required, we looked to previousstudies of validators and to information about thelikelihood of change in diagnosis. In a longitudinalfollow-up study lasting almost 30 years, Fiedorow-icz et al.45 found a highly significant difference inthe risk of conversion from unipolar to bipolar dis-order among depressed individuals who presentedwith two vesus three or more manic/hypomanicsymptoms.
The concept of Bipolar Disorder, Not OtherwiseSpecified (NOS) as it appeared in DSM-IV, and likelyto be referred to as Bipolar Disorder, Not ElsewhereClassified (NEC) in DSM-5, will be reserved for pre-sentations of bipolar disorder that are subsyndromalby virtue of an insufficient number of symptoms tomeet the criteria for episodes of depression and/ormania or hypomania, or an insufficient episode
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 9
Advances in bipolar disorder Kupfer et al.
duration to meet those criteria and there is no re-quirement that these subsyndromal presentationsbe concurrent. By contrast, to meet the criteria forone of the proposed mixed specifiers, an individualwould be required to present in a fully syndromalepisode of depression or mania or hypomania thatis simultaneous for at least one week with at least 3of the designated symptoms of the opposite pole.
Recognizing the considerable prognostic impor-tance of mixed states, the DSM-5 workgroup onmood disorders has proposed a new definition ofsuch states that we believe is more consistent withclinical reality, is likely to be used more appropriatelyand correctly than the mixed episode diagnosis inDSM-IV, should lead to earlier recognition of thosedepressed individuals likely to develop bipolar dis-order and, perhaps most importantly, may help inthe recognition of those at risk for suicidal behavior.
Trisha Suppes (Stanford University) finished thissession with a third report. A debate often facedby psychiatrists working in mood disorders is whatcombination of symptoms is required to make adiagnosis of bipolar disorder, particularly whichsymptoms and for what duration. DSM-IV-TR of-fers guidance by distinguishing the criteria for num-ber of hypomanic symptoms and concurrent daysof hypomania and mania in bipolar disorder; how-ever, there has been increasing recognition of boththe presence and importance of subsyndromal hy-pomanic symptoms for patients experiencing dis-tress.
The Mood Disorders Workgroup for the DSM-5is making the recommendation for the addition of“activity or energy” to criterion A for hypomanicand manic episodes. The proposal for the updatedcriterion is as follows: a distinct period of abnor-mally and persistently elevated, expansive, or irrita-ble mood and abnormally and persistently increasedactivity or energy. Symptom lists (criterion B) wouldbe essentially not changed. The addition of requiringincreased activity/energy to criterion A will make ex-plicit the requirement that this hallmark symptomof bipolar I disorder be present in order to makethe diagnosis. Addition of this feature is supportedby a number of studies suggesting that activity orenergy is at least as important as mood; moreover,some studies argue that activity and energy are moreimportant than mood.46–55
The Mood Disorders Workgroup for DSM-5 isalso making the recommendation to maintain the
criterion A for an episode of hypomania at fourdays. The proposal is as follows: a distinct periodof abnormally and persistently elevated, expansive,or irritable mood and abnormally and persistentlyincreased activity or energy, lasting at least four daysand present most of the day, nearly every day, that isclearly different from the usual nondepressed mood.
The evidence is fairly compelling that maintain-ing at least a four day requirement retains the speci-ficity of the diagnosis with reasonable sensitivity.The review of the evidence suggests that decreas-ing the duration requirement would significantlyincrease the prevalence of bipolar II disorder. Thiscould, in turn, decrease specificity of the diagno-sis and add to the periodic skepticism regarding theintegrity and validity of bipolar II as a separate diag-nostic category. This recommendation is based ona review of available empirical data potentially sup-porting a change in the criteria, discussed in detailbelow. Under consideration was whether there wasjustification to change the criterion for a hypomanicepisode to two days, shortening the required periodof symptoms from four days. Some researchers havesuggested that the duration requirement be elimi-nated. For example, Angst and colleagues,47 as wellas others, have suggested that the duration require-ment should be discarded and the focus moved tochanges in activity plus clinically significant symp-toms. In one single-site observational study, char-acteristics of patients reporting two versus four dayhypomania were similar.56
The recent BRIDGE study, is one of the few stud-ies in which duration of hypomania is a specificfocus. In this large globally ascertained sample ofmore than 5,500 patients currently experiencing aMDE, an evaluation was made of various cut pointsfor duration between 1, 2–3, and 4–6 days of hypo-mania relative to a number of external validators.42
The external validators considered included: firstdegree relatives with a mood disorder; early adultonset; recurrent mood episodes; mood lability; sea-sonality; and history of suicide attempts. There wasa clear linear relationship between each of the ex-ternal validators evaluated and the duration of thehypomanic symptoms. For example, the percent ofthe population reporting family history of mooddisorders increased as number of days of hypoma-nia increased. The difference between requiring 2–3versus 4–6 in these validators was notable, in manycases improving by 20% or more.
10 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
In further support of maintaining duration crite-ria at four days, Bauer and colleagues57 found that,in a sample of 203 bipolar patients, if the hypoma-nia duration criterion was reduced from four daysto two days, the percent of hypomanic days wouldincrease twofold from 4% to 8% for each patient;the number of patients with a hypomanic episodewould double (in this sample from 44 to 96); andthe number of hypomanic episodes for all patientswould increase about threefold (from 129 to 404 inthis sample).
In sum, these data, particularly the increase instrength and number of external validators when ahypomania episode was defined by a minimum offour days,42 and the likely marked increase in preva-lence for bipolar II disorder that would occur if theentry criteria for hypomania were changed,57 weretaken by the Mood Disorder Workgroup commit-tee to support maintaining the hypomania episoderequirement at four days.
Finally, the Mood Disorders Workgroup for theDSM-5 proposes to revise Bipolar Disorder NOSto allow subcategories to be coded and to be spe-cific in definition. This is an important change fromDSM-IV-RS where NOS was defined by “examplesinclude.” This proposed change will allow the cap-ture of the well-recognized phenomena of patientsexperiencing hypomanic symptoms with notablechange from usual behavior that are fewer than fourdays in duration or of a lower symptom count thanis required to meet criteria for a full hypomanicepisode. It is important to note that in contrast to theMixed Features Specifier proposed,58 where symp-toms occur simultaneously, symptoms for BipolarNOS, by definition, occur at separate times. Specificproposed definitions include:
• Codable subcategories within Bipolar NOSthat would classify characteristic symptoms ofhypo/mania or depression that are present dur-ing separate time periods and cause distress ordysfunction, but are not of sufficient durationand/or intensity to meet criteria for a specificbipolar diagnosis.
• Subsyndromal hypomania, short duration: pa-tients who experience lifetime episodes of de-pression that meet full criteria for MDE ANDexperience hypomanic periods of sufficientnumber of criterion symptoms, but of insuffi-cient duration (≥ 2 and < 4 consecutive days).
• Subsyndromal hypomania, insufficient symp-toms: patients who experience lifetime episodesof depression that meet full criteria for MDEAND experience hypomanic periods of suffi-cient duration, but of insufficient number ofcriterion symptoms (≥2, 3 consecutive days ifmood is only irritable).
• Other Bipolar NOS: this includes atypical pre-sentations of bipolar symptoms not consideredabove that cause significant distress or psy-chosocial dysfunction.
• In summary, in this session, DSM-5 Mood Dis-order Workgroup proposals were reviewed, in-cluding the addition of activity or energy tothe mood item in criterion A; that the du-ration criterion for hypomanic episodes re-main at four days; and that specific, codableBipolar NOS categories for subthreshold moodsymptoms be added. More information aboutproposed changes in DSM-5 can be found atwww.dsm5.org.
Session on medical comorbidity
OverviewIn the comorbidity session chaired by FouziaLaghrissi-Thode (F. Hoffmann-La Roche Ltd.), anemphasis was placed on the considerable medicalcomorbidity present in bipolar disorder. The burdenof cardiometabolic conditions in bipolar disorder isvast and has an impact on levels of morbidity andmortality, and a reduced life expectancy of 10 to 25years. In this session, there was the following: a re-view of treatment disparities; recommendations forintegrative care; the examination of risk factors andhow to address them; and, finally, the current stateof intervention in an integrated care model. Severalpresentations addressed these important issues.
Cardiometabolic conditionsBenjamin Goldstein (University of Toronto) dis-cussed the burden of cardiometabolic conditions inbipolar disorder. Cardiometabolic conditions, suchas obesity, diabetes, and cardiovascular disease, area common source of morbidity and mortality inbipolar disorder. Excessive cardiovascular disease(CVD) is the leading cause of death in bipolar dis-order, contributing to 10- to 25-year shorter lifeexpectancy. Despite the fact that CVD is the leadingcause of death in the general population, standard-ized mortality ratios are fully doubled in bipolar
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 11
Advances in bipolar disorder Kupfer et al.
Figure 4. Reasons for disparities in medical care for patients with mood disorders, at the patient level, provider level, and systemlevel. Adapted from Frayne et al.121
disorder, in large part because CVD in these patientsoccurs exceedingly early in life. Recent findings fromthe United States general population suggest thatCVD patients with bipolar disorder are 14 yearsyounger than CVD patients without mood disor-ders. Indeed, evidence of increased cardiovascularrisk among youth with bipolar disorder is accu-mulating. The question of whether cardiometabolicconditions in bipolar disorder are due to the strainof having the illness (e.g., mood symptoms and theirfunctional sequelae), suboptimal lifestyle (e.g., sleepdisruption, smoking, nutrition, physical activity), orshared biological causes (e.g., inflammatory media-tors, hypothalamic–pituitary–adrenal disturbance,CACNA1C genotype) remains insufficiently an-swered. Nonetheless, in addition to concerns aboutmedical outcomes, cardiometabolic conditions areassociated with a more pernicious course of bipolardisorder.59
Disparities exist in the medical treatment of pa-tients with severe mental illness, and this has beenobserved in rigorous studies from a number of coun-tries including Canada, Denmark, Sweden, and theUnited States. For example, despite being nearlythree times as likely to die of CVD, patients withbipolar disorder or schizophrenia are no more likely
to access related hospital resources. Those who doaccess treatment are at greatly increased risk of car-diovascular death within five years, and yet are al-most half as likely to receive invasive cardiovasculartreatment as patients without these psychiatric ill-nesses. Other findings demonstrate extensive delaysin treatment from the first point of contact throughcardiac catheterization, and disparities in prescrip-tions for preventive cardiometabolic medicationspostdischarge have been documented. Potential rea-sons for these disparities have been highlighted byothers (see Fig. 4). Cardiometabolic monitoringof second-generation antipsychotics is problematic.Despite publication of joint guidelines by the Amer-ican Diabetes Association and American PsychiatricAssociation, adherence with suggested monitoringat baseline and after 12 weeks of treatment remainsdismal, with the vast majority of patients not re-ceiving monitoring. Among youth, adherence withmonitoring guidelines occurs in only 5% to 15% ofpatients.
Strategies that may prevent or delay the accu-mulation of medical burden in bipolar disorder in-clude regular medical monitoring, behavioral inter-ventions focusing on obesity prevention, and useof medications that minimize the propensity for
12 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
weight gain and metabolic disturbance. Treatmentmodels for integrating greater emphasis on medicalcomorbidity in the treatment of patients with severemental illness have recently been elaborated andpublished in landmark studies. Integrating nursecare managers into mental health clinics was shownto increase compliance with preventive services andevidence-based cardiometabolic services and to im-prove Framingham scores. Similarly, integrating su-pervised nurses into the provision of primary carefor patients with depression and diabetes or car-diovascular disease led to more fine-tuned adjust-ments of insulin, antihypertensives, and antide-pressants. Importantly, reductions in glycosylatedhemoglobin and systolic blood pressure, and im-provements in quality of life and treatment satis-faction, were also observed. Preliminary findingssuggest that similar modifications can benefit theoverall health of patients with bipolar disorderspecifically. Although these manualized interven-tions are not widely available, they share severalcore elements that can be readily integrated intotreatment. These include: psychoeducation aboutthe link between psychiatric and cardiometabolicillness, liaison with other healthcare providersabout specific patients and about monitoring andrisk factor management in general, encourag-ing self-management, and supporting patient self-efficacy and effective engagement of healthcareproviders.
In summary, medical comorbidity is salient to thecare of all patients with bipolar disorder, irrespec-tive of age or type of treatment. Familiarity withthe magnitude of this risk and with preventive andintervention strategies may improve outcomes forpatients with bipolar disorder.
Sleep and circadian rhythmsIn the second talk in the session, Allison Harvey(University of California at Berkeley) then presentedan important overview of the role of sleep and circa-dian rhythms in bipolar disorder. Bipolar disorderis a severe and chronic psychiatric illness associ-ated with significant interepisode impairment, highrates of medical morbidity, and premature mortal-ity. Three lines of evidence highlight the strong cou-pling between sleep and circadian disturbances withmood episodes, inadequate recovery, and relapserisk in bipolar disorder: (1) sleep and circadian dis-turbance is a core symptom of bipolar disorder; (2)
experimental studies suggest that sleep deprivationcan trigger manic relapse; and (3) there is evidencethat sleep deprivation can adversely affect emotionregulation the following day.60 Relatively recently, ithas become clear that bipolar disorder is also associ-ated with greater medical morbidity and prematuremortality.61 Multiple complex factors are likely tocontribute to the association between bipolar dis-order and health problems, medication side effectsperhaps being the most prominent. The questionwe ask and seek to begin to answer here is: Are thesleep and circadian problems that are core featuresof bipolar disorder one important but understud-ied contributor to the known association betweenhealth problems and bipolar disorder? Below weseek to show the plausibility of this hypothesis byhighlighting that problems relating to a selection ofhealth conditions and health behaviors have beenlinked to both sleep problems and bipolar disor-der. If sleep problems are contributors to the healthproblems experienced by bipolar patients, the publichealth implications are potentially startling becausesleep and circadian problems are modifiable. Harveysummarized the following areas:
Several longitudinal studies have identified shortsleep duration, long sleep duration, and insomniaas predictors of CVD morbidity and mortality.62
Sleep disturbance has also been associated with theincreased prevalence of traditional CVD risk fac-tors, including obesity, hypertension, and diabetesmellitus3.
There is a 1.5–2.5 fold increase in risk for CVD-related mortality for individuals with bipolar dis-order compared to the general population, makingCVD the leading cause of death in bipolar disor-der.63 Moreover, traditional CVD risk factors, suchas hypertension, obesity, and diabetes mellitus, oc-cur with greater frequency in bipolar patients thanthe general population.64
The results of a meta-analysis involving 30 stud-ies (12 in children, 18 in adults) and 634,511 par-ticipants are compelling. There was a 60–80% in-crease in the odds of being a short sleeper amongboth adults and children who were obese. Moreover,adults sleeping five hours or fewer versus more thanfive hours had significantly greater odds of beingobese and an increase of one hour per night of sleepwas associated with a decrease of 0.35 body massindex (BMI).65 Fagiolini et al.66 reported that 68%of patients with bipolar disorder were overweight,
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 13
Advances in bipolar disorder Kupfer et al.
with 32% meeting criteria for obesity (less than 20%of controls met criteria for obesity). These individ-uals suffered a range of poorer outcomes includingshorter time to recurrence of an episode, particu-larly of depression, and had a greater number ofprevious episodes of depression and mania.66 Sim-ilarly, McElroy et al.67 reported that 58% of bipolarpatients were overweight and 21% were obese. Theassociated adverse outcomes included arthritis, hy-pertension, and diabetes mellitus.
Sleep deprivation is associated with hormonal re-sponses that increase appetite, caloric intake, andinfluence the selection of foods.68 When sleep de-prived, individuals focus food selection on sugar,fat, and carbohydrates. In a comparison of 2,032 pa-tients with bipolar disorder and controls, Kilbourneet al.69 reported that patients with bipolar disorderwere more likely to report poorer eating behaviorsrelative to individuals without a serious mental ill-ness, including having fewer than two daily meals(OR = 1.32) and difficulty obtaining or cookingfood (OR = 1.48).
Sleep quality is improved by exercise70 and is aneffective treatment for chronic insomnia71 and re-duces presleep anxiety.72 Moreover, healthy partici-pants have less tolerance for exercise after sleep de-privation.73 Kilbourne et al.69 reported that patientswith bipolar disorder were more likely to reportpoor exercise habits relative to individuals withouta serious mental illness, including infrequent walk-ing (OR = 1.33) and infrequent strength exercises(OR=1.28). Another study reported predominatelysedentary routine daily activities in a sample of bipo-lar patients.74
We have reviewed evidence that sleep disturbanceis associated with an increased CVD risk, more obe-sity, poorer diet, and less exercise. We have alsoreviewed evidence that sleep disturbance is per-vasive in bipolar disorder and that bipolar disor-der is associated with higher CVD risk, more obe-sity, poorer diet, and less exercise. Taken together,it is tempting to speculate that sleep disturbancemay be an important contributor to the associa-tion between health problems and bipolar disorder.However, there is surprisingly little research thatincludes a measure of sleep and a measure of ahealth outcome in individuals with bipolar disor-der. Accordingly, we recently probed the NationalComorbidity Survey-Replication (NCS-R) to exam-
ine the prevalence of three self-reported cardiovas-cular risk factors (obesity, hypertension, and dia-betes) across bipolar respondents with chronic in-somnia symptoms, acute insomnia symptoms, andgood sleep (n = 176). Insomnia symptoms includeddifficulty falling asleep, difficulty maintaining sleep,and early morning awakening. Rates of obesity andhypertension were greater in bipolar patients withchronic (41.8%; 28.8%) and acute (43.7%; 24.1%)insomnia symptoms compared to bipolar patientswith good sleep (19.7%; 5.9%). Longer insomniasymptom duration increased odds of hypertension(OR = 2.2, 95% CI = 1.2–3.9) and a higher num-ber of insomnia symptoms was associated with el-evated rates of obesity (OR = 1.5, 95% CI = 1.0–2.1), hypertension (OR = 1.9, 95% CI = 1.1–3.6)and diabetes (OR = 4.7, 95% CI = 2.0–11.1). Al-though causal inferences are not possible given thecross-sectional design, these results add to the evi-dence that sleep disturbance may contribute to theassociation between health problems and bipolardisorder.75
With funding from NIMH, we have developedan eight session intervention for sleep and circadianproblems in bipolar disorder, combining principlesfrom motivational interviewing,76 cognitive behav-ior therapy for insomnia,77 interpersonal and socialrhythms therapy (IPSRT),78 and chronotherapy.79
The hypothesis tested is that improving sleep andcircadian problems will improve sleep, reduce moodsymptom and risk of relapse, improve quality of lifeas well as health among individuals with bipolardisorder.
Unlocking the contributors to the increased riskfor premature health-related morbidity and mor-bidity associated with bipolar disorder will be com-plex as there will be multiple factors. Herein, wepresent initial evidence, albeit all based on cross-sectional evidence, that the sleep and circadianproblems that are prominent features of bipolardisorder may contribute to several adverse healthoutcomes and may contribute to an unhealthylifestyle. Sleep and circadian problems are likelyto be modifiable with relatively simple behavioralchanges. Hence, if sleep/circadian problems do turnout to be important contributors to the healthproblems experienced by bipolar patients, therewill be an important opportunity for major healthimprovement.
14 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
Adverse health-related behaviorsIn the final report of this session, Michael Ostacher(Stanford University) reported on several other ad-verse risk factors and behaviors that increase themorbidity and mortality in bipolar disorders. Ad-verse health-related behaviors increase the risk ofmorbidity in bipolar disorder. High rates of obe-sity, smoking, drug and alcohol use, sleep cycle ab-normalities, and inadequate treatment adherenceconspire to worsen the course of bipolar disorderfor many people. Perhaps more importantly, thesemay be responsible for elevated mortality rates com-pared to the general population, mediated throughdiabetes, lipid disorders, hypertension, and heartdisease. Clinicians should be aware of the presenceof these risks, and address and promote behavioralchange and optimization of care. Smoking, alco-hol, diet, and exercise, and barriers to such changesshould be addressed from the perspective of ongo-ing treatment. Strategies for assessing these risks,monitoring motivation to change behavior, and in-tervening to promote change must be integratedinto patient care.
It has become evident that a singular focus onmood symptoms in the study and treatment of bipo-lar disorder neglects what may be the most impor-tant outcome we could measure: health. People withsevere mental illness in the United States may die25 years earlier than their counterparts in the gen-eral population. While suicide certainly accountsfor a disproportionate number of deaths in the firstdecade or two after the development of bipolar dis-order, standardized mortality ratios for people withbipolar disorder continue to be double that of thegeneral population. Nearly all of this increased mor-tality is due to medical causes. While there maybe something inherent in bipolar disorder that in-creases risk for premature mortality (abnormalitiesin inflammatory factors, for instance), it is likelythat the greatest proportion of increased risk is dueto modifiable health behaviors, primarily smokingand obesity. If patients are to be well treated in theclinic, these behaviors must be addressed.
Patients with bipolar disorder are at increased riskfor multiple problems—elevated BMI, smoking, di-abetes, lipid disorders—that individually increaserisk for cardiovascular events, but having multiplerisk factors, according to the Framingham HeartStudy, more than additively increases such risk. Inpublic sector patients in Massachusetts, marked in-
creased risk for death from cardiovascular disorderwas apparent in severely mentally ill patients, in-cluding those with bipolar disorder, as early as age25.
The association between obesity and poor moodand functional outcomes, including suicide at-tempts, in bipolar disorder is well known, as is itsprevalence. In the Pittsburgh sample, upwards of35% of subjects with bipolar disorder had BMIs inthe obese range (>30). With obesity directly relatedto rates of diabetes and lipid disturbances, its highprevalence in bipolar disorder is especially concern-ing. Because patients with mental illnesses, includ-ing bipolar disorder, often receive less comprehen-sive medical care than those without mental illness,the consequences of these risks may be greater.
Smoking, too, is highly prevalent in bipolar dis-order, with odds ratios for smoking in bipolar I andII disorders 3.5 and 3.2, respectively. Quit rates arelower in bipolar disorder and, aside from its asso-ciation with suicidal behavior, has to be consideredin understanding why mortality rates in bipolar dis-order are so high. Smoking cessation is rarely stud-ied in bipolar disorder, and patients, clinicians, andfamilies may be fatalistic about the utility of in-terventions to help patients quit. Many psychiatricclinicians are unaware of or do not help patients uti-lize established smoking cessation resources, such aspharmacological interventions, smoking cessationprograms, and quit lines.
It is time for psychiatric care to go beyond a focuson symptoms and functioning to include a focus onoverall health outcomes and decreased mortality forour patients. Systematic identification processes forrisk factors for mortality need to be part of every-day care, just as systematic diagnosis of psychiatricillnesses are. Educational efforts for providers thatemphasize the detrimental effects of smoking, obe-sity, diabetes, lipid abnormalities, and other healthrisk behaviors on both mental and physical health,and strategies (such as motivational interviewing)need to become part of practice for all psychiatriccaregivers, and needs to include careful weighing ofthe potentially detrimental health effects of manyof our medications. The integration of interven-tions for smoking cessation, dietary change, andweight management, overall physical health behav-ior change into ongoing care needs to be a primarygoal of our field. Our technologies for improvingmood and functioning are limited and improving
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 15
Advances in bipolar disorder Kupfer et al.
only incrementally; now is the time to expect theview of psychiatric care to go beyond psychiatricsymptoms and to expand to include overall health.
Session on reports from the InternationalSociety for Bipolar Disorder
OverviewThe final session, coordinated by Michael Berk (Uni-versity of Melbourne), discussed a series of reportsfrom the International Society for Bipolar Disor-ders (ISBD). Bipolar disorder is a complex, com-mon, and capricious disorder. Relative to its burden,which is between 1% and 5% of the global popula-tion, it is poorly researched and understood. Akinto what has been achieved in other major medi-cal disorders, strategic international collaborations,networks, and partnerships are essential to lever-age the resources and intellectual capital required tohave an impact on these unmet needs. In this con-text, the series of special workgroups established bythe ISBD attempts to address some of the core issuesfacing the field. The ISBD is the principal interna-tionally recognized forum to foster ongoing inter-national collaboration on education and research,with an objective to advance the treatment of allaspects of bipolar disorders, resulting in improve-ments in outcomes and quality of life for those withbipolar disorder and their significant others. In thiscontext, the ISBD has chosen a number of areasfor academic investment, including improving thequality and standardization of clinical trials, and fos-tering a better understanding and integration of thechanges in cognition, neuroimaging, and biomark-ers that are seen in the disorder.
The first issue pertains to the complexities andcontroversies regarding the design of clinical tri-als in bipolar disorders. The clinical trial evidencebase is the cornerstone of clinical decision mak-ing, and the area is bedeviled by methodologicalinconsistency that bedevils quality care. Many tri-als fail because of poor design, and others containcritical biases. This workgroup, led by Tohen andcolleagues will critically review the commonly uti-lized designs in clinical trials of bipolar disorder, aswell as suggesting novel designs, including adaptivedesigns and mixed methods designs. A review of sta-tistical techniques and cultural issues and challengesof implementing studies in emerging countries ispart of the remit of this group. A major potentialcontribution to outcomes could derive from stan-
dardisation and optimisation of clinical trial design,and the adoption of innovative methodologies in-cluding statistical methods.
It is now appreciated that rather than simply be-ing a disorder with highs and lows, with returnto one’s old self between episodes, bipolar disor-der is associated with a cascade of progressive clin-ical features. Bipolar disorder follows a progressivetrajectory; with an increased numbers of episodesand persistence of illness, there is an incrementallygreater probability of recurrence, recurrence be-comes triggered more easily, and there is a reducedlikelihood of response to treatment. An active bio-logical process of neuroprogression underpins thisstaged process, evidenced by novel evidence of bothprogressive neuroanatomical changes and cognitivedecline. The biochemical foundations of this pro-cess appear to be mediated by changes in inflam-matory cytokines, corticosteroids, neurotrophinsand oxidative stress. The consequences of this nox-ious cascade include lipid peroxidation, protein car-bonylation, DNA fragmentation and an increasedvulnerability to apoptosis. This neural toxicity hasovert functional consequences.
The Neurocognition Task Force, led by Frangouand Yatham, aims to examine cognitive deficits inbipolar disorder as part of the process of neuropro-gression in the disorder and to develop potentialtherapeutic strategies. Key goals are to standardizecognitive tests that are commonly used, ensure thatthey are validated in patients with bipolar disorder,and confirm that they target those domains thatare most relevant to the disorder. In order to estab-lish a common cognitive battery for bipolar disor-der, the ISBD put together a working group taskedwith reviewing the cognition literature to propose apreliminary neurocognitive battery for use in bipo-lar disorder. The ISBD-Battery for NeurocognitiveAssessment (ISDB-BANC) was selected as an as-semblage of recognized individual tests consideredappropriate for bipolar disorder. It initiates the pro-cedure for standardizing cognitive testing in bipolardisorder, which is a critical step to the use of compa-rable metrics across large-scale clinical studies. Thisprovides the foundation for the next generation ofcognitive remediation therapies. It is planned thatthe findings from the various task force will be inte-grated with the expectation that new strategies forassessment and treatment can be developed in thenear future.
16 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
The ISBD Neuroimaging Task Force similarly hadtwo primary goals, to assimilate neuroimaging re-search from leading bipolar disorder groups and todevelop a conceptual agreement regarding the func-tional neuroanatomy of bipolar disorder. The taskforce aimed to examine the neural systems that mod-ulate mood, as they are the probable neuroanatom-ical foundation of bipolar disorder. Affected brainareas include the amygdala, pituitary, hippocampus,and disruption of regional white matter connectiv-ity. Neuroimaging data indicate changes in key com-ponents of both structural and functional networksin bipolar disorder, both structural and functional.An important clue to the pathophysiology of the dis-order rests in follow-up studies of the onset phase ofthe disorder, adolescence. There are normal devel-opmental changes in neuroanatomy in adolescence,which overlap with the pathological changes thatoccur in bipolar disorder as part of the process ofneuroprogression. These data dovetail with the workof the neurocognition taks force, providing a neu-roanatomic substrate for the changes in functionaloutcome and cognition.
These structural and functional changes are theoutcome of a primary biochemical perturbation.The root of these changes remain opaque, howeverbiomarkers are increasingly illuminating the possi-bilities. To this end, the Biomarkers working groupof the ISBD was set up, and the report by Young andKapczinski highlights the most promising avenues.Oxidative stress and systemic inflammation appearto be key pathways underpinning neuroprogression,and they operate in concert with reductions in neu-rotrophins such as BDNF. The task group aims todevelop large scale collaborative research linkagesto examine the role of these biomarkers as modera-tors of outcome and of risk. Collaborative linkagessuch as these have a habit of growing organically,providing a critical mass of expertise to assist indeveloping solutions to complex issues of substan-tial public health significance.
Clinical trials task force reportIn the first presentation in this session, MauricioTohen (University of Texas) summarized the reportfrom the Clinical Trials Task Force. This group hasfocused on the review of commonly utilized de-signs in the treatment of bipolar disorder. The mostcommon clinical trial design for acute mania andbipolar depression is a short, two arm study, which
in general, can yield differences between efficaciousinterventions and placebo. As investigators, clini-cians and regulatory authorities may recognize thatsymptomatic treatment of manic and depressiveepisodes is a relatively small component of man-agement of bipolar disorder and, therefore, otherdesigns need to be considered.
In general, monotherapy, single point random-ization, and blinding achieve major aims. One im-portant advantage of these designs is that they arerelatively inexpensive and not subject to many ambi-guities of interpretation. Additionally, it is possibleto include an established efficacious treatment toprovide “assay sensitivity” and allow for secondaryanalyses of comparative efficacy and tolerability be-tween a new and an established drug/regimen. De-signs that employ current DSM criteria and a totalscore on a manic rating scale have inherent weak-nesses and are thus limited. DSM criteria treat allsymptoms as equal, when evidence does supportthat conclusion. Similarly, total scores are subject torater inflation to qualify individuals, thereby reduc-ing study power to detect differences; as well as over-weighting of nonspecific symptoms that may advan-tage one drug over another. For example, sedationalone can reduce many manic symptom scores, butthe aim of treatment is rarely principally sedation.
A major unmet need is that little recognition hasbeen given to the reality that most bipolar I patientshave few full manic or depressive episodes while intreatment. Rather, less severe, and generally shorterperiods of manic or depressive recrudescence occurmore often. Yet, almost no systematic experimentalstudies have been conducted on this group.
In terms of maintenance or prophylactic studies,adaptive designs could include alternative random-ized actions when manic symptoms worsen. Otherinvestigative actions would proceed without change.The analyses of results would include additional hy-potheses around the exacerbations in manic or de-pressive symptomatology
Special populations for bipolar studies fall intotwo categories. First are characteristics that are un-likely to have major impact on interventions andtheir effectiveness. These include ethnic and racialidentity, sex, and socioeconomic status. However,this does not dismiss as without benefit some stud-ies; for example, several studies have provided someevidence for greater prevalence/severity of psychoticsymptoms among African American patients.
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 17
Advances in bipolar disorder Kupfer et al.
Quality of life is of limited relevance to bipolarstudies in the short run but of major importancein maintenance stuies. Studies have consistently re-ported that functional and quality of life benefitslag behind recovery from syndromal clinical statesin bipolar disorder. However, adequate and psycho-metrically sound measures of QOL exist, and haveyielded differences among interventions. A generalweakness in these and other ancillary outcome mea-sures is that lack of adequate rater training, and oftenuse of raters who are uninvolved in the clinical careof subjects can result in loss of sensitivity of suchmeasures. Many of the comments about QOL applysimilarly to measures of functioning.
Only recently have studies persuasively shownthe plasticity of clinically significant cognitive func-tion in bipolar disorder cognitive assessment tests.Generally, these assessments required time and ef-fort that was off-putting to patients and expensiveas a component cost of trials. Cognitive tests ex-hibiting sensitivity to changes with acute treatmentsare now available; therefore, the practical barrier totheir more focused use is no longer paramount (seebelow).
Perhaps the major difference in emerging coun-tries is that costs of certain regimens and drugs areprohibitive for most patients. Therefore, studies thatcompare drugs that are available generically—andtheir cost per year ranges—warrant more attention.Emerging economies are also less likely to have fund-ing from federal revenues, therefore most potentialstudies in such countries will be ones funded by thepharmaceutical industry.
Neurocognition task force reportIn the second presentation, Sophie Frangou (KingsCollege London) summarized the report from theNeurocognition Task Force. Cognitive impairmentis part of the extended phenotype of bipolar disorderand is associated with genetic risk,80 clinical sever-ity,81–86 and psychosocial outcome.87 Examinationof cognitive deficits in bipolar disorder is thereforeintegral to our efforts to define the pathophysiologyof the disorder and to develop appropriate thera-peutic strategies. Ideally, this should involve cogni-tive tests, which are standardized and validated inpatients with bipolar disorder, targeting those do-mains that are most relevant to the disorder. A suc-cessful example of this approach is the ConsensusCognitive Battery (MCCB) developed by the Mea-
surement and Treatment Research to Improve Cog-nition in Schizophrenia (MATRICS) initiative.88,89
As a first step in establishing a common cognitivebattery for bipolar disorder, the ISBD established acommittee of experts who: (1) reviewed the litera-ture to identify cognitive measures with high sen-sitivity for bipolar disorder and to identify gaps incurrent evidence; (2) evaluated the overlap with theMCCB and the usefulness of specific MCCB sub-tests for bipolar disorder; and (3) proposed a pre-liminary battery for use in bipolar disorder that alsohighlights areas where further research is required.
The literature review considered findings frommeta-analyses of case-control studies of cognitionin bipolar disorder81–86 and from individual studiesfor specific cognitive domains with high relevancefor bipolar disorder but with a limited evidence base.The evidence available is reasonably robust for somebut not all cognitive domains of interest. Estimatesof effect size are presented for those tests with suffi-cient information to allow meta-analytic treatment.Moderate to large impairments in bipolar disorderare present in tests of attention, processing speed,memory and learning, and in response inhibitionand set-shifting. Remarkably, given the nature ofthe disorder, very few studies examined patients’performance in tests of emotional processing, socialcognition, or decision making. This is, therefore, anarea where further research is urgently required.
There is substantial empirical evidence suggestingthat the MCCB subtests focused on the domains ofattention and processing speed are suitable for bipo-lar disorder as these tests (or nearly identical ver-sions) have already been demonstrated to show highsensitivity to the disorder. The MCCB subtests forthe domains of working memory and visual learn-ing/memory have not been as extensively studied inbipolar disorder, but available evidence suggests thatthey achieve a reasonable separation between bipo-lar disorder patients and controls, with effect sizesof 0.8 and 0.6–1, respectively.90,91 Thus, it would ap-pear reasonable at the present time to include thesetests in a cognitive battery for bipolar disorder pend-ing further confirmation by future studies. In theMCCB, verbal memory/learning is assessed with theHopkins Verbal Learning Test (HVLT). This has notbeen a popular instrument in bipolar disorder be-cause of concerns about its sensitivity. Indeed, case-control effect sizes for the HVLT in bipolar disorderrange from 0.4–0.6;90,91 these values are lower than
18 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
Figure 5. Schematic of abnormalities identified from neuroimaging within the ventromedial (OFC) and ventrolateral (VLPFC)emotional networks in bipolar disorder. Although the OFC and VLPFC are actually distinct circuits, they are combined here forsimplicity. These networks modulate amygdala, which, in bipolar disorder, has functional and structural abnormalities, indicated bythe uneven amygdala figure. Connections between amygdala and prefrontal cortex are abnormal as well, illustrated by the curvingline. Prefrontal areas (OFC/VLPFC) are colored to indicate abnormalities in structure and function in these regions. Reciprocalconnection between the emotional networks and cognitive (dorsal) brain systems, through the anterior cingulate, are indicated bythe (−) on the arrow. Abbreviations: OFC, orbitofrontal cortex; VLPFC, ventrolateral prefrontal cortex; STG, superior temporalgyrus; DLPFC, dorsolateral prefrontal cortex.
the range typically reported in the meta-analyticstudies summarizing data from more complex ver-bal list-learning measures, particularly the Califor-nia Verbal Learning Test (CVLT). The CVLT maytherefore be more appropriate for bipolar disorderpatients, while the HVLT could be considered an ac-ceptable substitute particularly in study designs thatinvolve comparison with schizophrenia or repeatedtesting. The MCCB also targets reasoning and so-cial cognition. Although both domains are relevantto bipolar disorder, the specific subtests have notbeen sufficiently studied or specifically implicatedin bipolar disorder and are likely to lack assay sensi-tivity.90 The greatest knowledge gap was identified inthe assessment of the interaction between affectiveand nonaffective processes, where tasks of decision-making could prove particularly useful in bipolardisorder. Finally, after considering the validity andsensitivity of all the available tests discussed above,the committee also evaluated logistical aspects re-lating to brevity, ease of administration, reliabil-ity, repeatability, versatility and likely internationalutility.
The ISBD-Battery for Neurocognitive Assessment(ISDB-BANC) represents a collection of establishedindividual tests deemed appropriate for use in bipo-lar disorder. It comprises eight core and two flexiblesubtests (choice between HVLT or CVLT) and, ad-ditionally, inclusion of the Wisconsin Card SortingTest. The battery would be appropriate for appli-cations where a broad screening of cognitive abili-ties is required, or where repeated assessments arenecessary (e.g., treatment effects or clinical trials).Prospective studies in clinical populations will berequired to support the psychometric properties ofthe ISBD-BANC and refine its constituent compo-nents. We consider the ISBD-BANC as the initialstep in the process of standardizing cognitive test-ing in bipolar disorder.
Neuroimaging task force reportSteven Strakowski (University of Cincinnati) sum-marized the work of the Neuroimaging Task Force.The ISBD Neuroimaging Task Force met in Mi-ami in December 2010 with two primary goals: (1)to review research from leading bipolar disorder
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 19
Advances in bipolar disorder Kupfer et al.
Figure 6. Potential candidates for biomarkers in bipolar disorder. Genetics studies have identified several potential candidate genesassociated with increased risk for developing bipolar disorder, involving circadian rhythm, neuronal development, and calciummetabolism. Neuroimaging-based studies have consistently demonstrated loss of gray matter, and enlargement of striatum andamydgdala. In terms of peripheral biomarkers, repeated studies have found decreased levels of neurotrophic factors and increasedproinflammatory cytokines and oxidative stress markers.
neuroimaging groups represented at the meeting;and (2) to develop consensus around a functionalneuroanatomy for bipolar I disorder. The task forceapproached this subject working from the clini-cal assumption that bipolar disorder is a primarymood disorder; that is, that the systems most likelyto underlie the condition involve those that modu-late mood.
Although the specific control of emotional func-tion in humans is not completely defined, twoventral prefrontal networks appear to modulateemotional behavior.92–95 Both of these networks aresimilarly organized in that specific ventral prefrontalregions map to specific striatal then pallidal thenthalamic brain areas to form iterative feedback loopsthat process information and modulate amygdalaand other limbic brain areas. One network origi-nates in the ventrolateral prefrontal cortex and isthought to modulate external emotional cues; theother originates in the ventromedial (orbitofrontal)cortex and is thought to modulate internal emo-tional stimuli.92–95These networks serve as a likelysubstrates for the functional neuroanatomy of bipo-lar disorder.
Neuroimaging studies suggest that abnormalitiesin key components of these networks occur in bipo-
lar disorder. For example, Altshuler et al.96 observedexcessive amygdala activation in bipolar individu-als during mania compared with healthy subjectswhile performing a facial affect matching task. Sim-ilar findings have been reported by others.97–101 Ab-normal amygdala activation has also been observedin bipolar disorder during other mood states.102,103
Structural amygdala abnormalities are commonlyreported in adults and adolescent bipolar subjectsas well.104,105
A second common neuroimaging finding in bipo-lar disorder is abnormal ventral prefrontal activa-tion. Several studies observed decreased activationin prefrontal cortex during mania,90,106–108 whichoften occurs concurrently with amygdala overac-tivation.109 Moreover, disrupted functional con-nectivity between amygdala and ventral prefrontalcortex has been observed during mania.109 Thesefindings suggest that loss of prefrontal modulationof amygdala activation may underlie the develop-ment of mood symptoms in bipolar disorder. More-over, Strakowski et al. observed increased amygdalaactivation during euthymia that was associated withincreased ventral prefrontal activation; the latterwas interpreted to represent a compensatory mech-anism in the ”well” state to manage limbic brain
20 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
overactivity. Several investigators have observed dis-ruptions in the white matter connectivity betweenventral prefrontal cortex and amygdala suggesting astructural basis for these functional observations.110
Bipolar disorder typically begins in adolescence.Recently, in a study of new-onset adolescent bipo-lar patients, during the year after the first manicepisode, Bitter et al.111 found that bipolar subjectsdid not exhibit amygdala growth that was seen inhealthy adolescents (and adolescents with ADHD).However, at baseline, amygdala volumes amonggroups were the same, suggesting that amygdala de-velopmental abnormalities occur as a result of illnessprogression, rather than as a cause of onset. In con-trast, white matter abnormalities appear to precedethe onset of bipolar illness as observed in studiesof children at-risk for bipolar illness by virtue ofhaving bipolar parents.112
Together, these data suggest a model of bipo-lar disorder (see Fig. 5) in which abnormalitieswithin ventromedial and ventrolateral prefrontalnetworks lead to the expression of bipolar disor-der. Disruptions in regional white matter connec-tivity may occur prior to illness onset, representinga potential vulnerability for developing bipolar ill-ness. This model is illustrated in Figure 5 and pro-vides a template to guide future investigations intothe neurobiology of this important and commonillness.
Biomarker committee reportTrevor Young (University of Toronto) summa-rized the report of the Biomarker group. TheISBD Biomarker Committee began meeting at theICBD meeting in Pittsburgh in 2008, followed bygatherings at the ISBD meeting in San Paulo and sev-eral teleconferences. The Committee sought to havea comprehensive discussion of the area of biomark-ers, which might help in the diagnosis and treatmentof bipolar disorder. The group also took on a taskto draft a positional paper which is under review atthe journal Bipolar Disorders. The Committee metmost recently at the ICBD meeting in Pittsburgh inJune 2011.
It is clear that the etiology of bipolar disor-der remains uncertain, however, there have beenmany recent advances in the area of genetics, patho-physiologic mechanisms, and brain imaging. TheCommittee reviewed the data and suggested thatthere were at least several biomarkers that could be
identified from these three areas. The main poten-tial candidates for biomarkers are shown in Figure 6.Neuroimaging studies have consistently shown lossof gray matter in cortical cognitive brain network113
as well as alterations in the activation of relevant sub-frontal, anterior temporal, and ventral prefrontalregions in response to an emotional stimulus inbipolar disorder.114 Genetics has indicated severalinteresting potential candidate gene involvementranging from circadian rhythms (ARNTL, RORB),to calcium metabolism (CACNA1C), cell survival,and cortical development (BDNF, DISC1).97,115,116
With respect to peripheral biomarkers, threeareas of particular interest include inflamma-tion, mitochondrial dysfunction, and oxidativestress, and then finally changes in neurotrophicfactors.117,118
Conclusions
Research in bipolar disorder has generated remark-able insights into the mechanisms associated withthe disease risk, disease expression and treatmentresponse. The insights highlight the complexity ofbipolar disorder but also suggest that we may beclose to successfully applying basic science findingsin translational approaches to help us in our man-agement of bipolar disorder. It is too early to knowwhether some of the most interesting targets iden-tified will survive rigorous ongoing testing with ad-equate size samples and replication. To date. someof the biomarkers have already facilitated the de-velopment of new treatments, such as the work onusing antioxidants in the treatment of symptoms ofbipolar disorder. Techniques that strive to enhancebrain health and to reduce the chance of cell losshave been a mainstay of bipolar disorder and arenow supported by empirical work highlighting po-tential candidates that we may be able to measure inan ongoing fashion in patients.
Acknowledgments
The Ninth International Conference on Bipolar Dis-orders was supported in part by educational grantsfrom AstraZeneca LP, Merck Sharp & Dohme Cor-poration, Bristol-Myers Squibb, and Cyberonics,Inc. Funding for this conference was also made pos-sible by contributions from the Community CareBehavioral Health Organization (an affiliate of theUPMC Health Plan), the Fine Foundation, and theStaunton Farm Foundation.
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 21
Advances in bipolar disorder Kupfer et al.
Conflicts of interest
The authors declare no conflicts of interest.
References
1. Kato, T., C. Kakiuchi & K. Iwamoto. 2007. Comprehen-sive gene expression analysis in bipolar disorder. Can JPsychiatry 52(12): 763–771.
2. Konradi, C., M. Eaton, M.L. MacDonald, et al. 2004.Molecular evidence for mitochondrial dysfunction inbipolar disorder. Arch Gen Psychiatry 61(3): 300–308.
3. Sun, X., J.F. Wang, M. Tseng & L.T. Young. 2006. Down-regulation in components of the mitochondrial electrontransport chain in the postmortem frontal cortex of sub-jects with bipolar disorder. J Psychiatry Neurosci 31(3):189–196.
4. Beneyto, M., E. Sibille & D.A. Lewis. 2008. Human post-mortem brain research in mental illness syndromes. In:D.S. Charney & E.J. nestler (eds). Neurobiology of MentalIllness. Oxford University Press.
5. Gaiteri, C., J.P. Guilloux, D.A. Lewis & E. Sibille. 2010.Altered gene synchrony suggests a combined hormone-mediated dysregulated state in major depression. PLoS One5(4): e9970.
6. Lee, H.K., A.K. Hsu, J. Sajdak, et al. 2003. Coexpressionanalysis of human genes across many microarray data sets.Genome Research 14(6): 1085–1094.
7. Dobrin, R., J. Zhu, C. Molony, et al. 2009. Multi-tissuecoexpression networks reveal unexpected subnetworks as-sociated with disease. Genome Biol. 10(5): R55.
8. Elo, L.L., H. Jarvenpaa, M. Oresic, et al. 2007. System-atic construction of gene coexpression networks with ap-plications to human T helper cell differentiation process.Bioinformatics 23(16): 2096–2103.
9. Bullmore, E. & O. Sporns. 2009. Complex brain networks:graph theoretical analysis of structural and functional sys-tems. Nat Rev Neurosci. 10(3): 186–198.
10. Gaiteri, C. & E. Sibille. Differentially-expressed genes inmajor depression reside on the periphery of resilient genecoexpression networks. Front Neurol Neurosci, in press.
11. Goh, K.I., M.E. Cusick, D. Valle, et al. 2007. The humandisease network. Proc Natl Acad Sci USA. 104(21): 8685–8690.
12. Huang, T.L. & F.C. Lin. 2007. High-sensitivity C-reactiveprotein levels in patients with major depressive disorderand bipolar mania. Prog Neuropsychopharmacol Biol Psy-chiatry 31(2): 370–372.
13. Knijff, E.M., C. Ruwhof, H.J. de Wit, et al. 2006. Monocyte-derived dendritic cells in bipolar disorder. Biol Psychiatry59: 317–326.
14. Liu, H.C., Y.Y. Yang, Y.M. Chou, et al. 2004. Immunologicvariables in acute mania of bipolar disorder. Neuroim-munol 150: 116–122.
15. O’Brien S.M., P. Scully, L.V. Scott & T.G. Dinan. 2006.Cytokine profiles in bipolar affective disorder: Focus onacutely ill patients. J Affect Disord 90: 263–267.
16. Wadee, A.A., R.H. Kuschke, L.A. Wood, et al. 2002. Sero-logical observations in patients suffering from acute manicepisodes. Hum Psychopharmacol 17: 175–179.
17. Dickerson, F., C. Stallings, A. Origoni, et al. 2007. Ele-vated serum levels of C-reactive protein are associatedwith mania symptoms in outpatients with bipolar disorder.Prog Neuropsychopharmacol Biol Psychiatry 31(4): 952–955.
18. Young, R.C., J.T. Biggs, V.E. Ziegler & D.A. Meyer. 1978. Arating scale for mania: reliability, validity and sensitivity.Br J Psychiatry 133: 429–435.
19. Kay, S.R., A. Fiszbein & L.A. Opler. 1987. The positiveand negative syndrome scale (PANSS) for schizophrenia.Schizophr Bull. 13: 261–276.
20. Ortiz-Domınguez, A., M.E. Hernandez, C. Berlanga,et al. 2007. Immune variations in bipolar disorder: phasicdifferences. Bipolar Disord 9(6): 596–602.
21. Abeer, A. El-Sayed & H.A. Ramy. 2006. Immunologicalchanges in patients with mania: changes in cell mediatedimmunity in a sample from Egyptian patients. Egypt J.Immunol. 13(1): 79–85.
22. Cassidy, F. & B.J. Carroll. 2002. Hypocholesterolemia dur-ing mixed manic episodes. Eur Arch Psychiatry Clin Neu-rosci 252(3): 110–114.
23. McMahon F.J., O.C. Stine, D.A. Meyers, et al. 1995. Patternsof maternal transmission in bipolar affective disorder. AmJ Hum Genet 56(6): 1277–1286.
24. Kato, T., O.C. Stine, F.J. McMahon & R.R. Crowe. 1987. In-creased levels of a mitochondrial DNA deletion in the brainof patients with bipolar disorder. Biol Psychiatry 43(10):871–875.
25. C. Konradi. 2005. Gene expression microarray studiesin polygenic psychiatric disorders: applications and dataanalysis. Brain Res Brain Res Rev. 50(1): 142–155.
26. Andreazza, A.C., L. Shao, J.F. Wang & L.T. Young. 2010.Mitochondrial complex 1 activity and oxidative damage tomitochondrial proteins in the prefrontal cortex of patientswith bipolar disorder. Arch Gen Psychiatry 67(4): 360–368.
27. Gawryluk, J.W., J.F. Wang, A.C. Andreazza, et al. 2011.Decreased levels of glutathione, the major brain antioxi-dant, in post-mortem prefrontal cortex from patients withpsychiatric disorders. Int J Neuropsychopharmacol 14(1):123–130.
28. Caltaldo, A.M., D.L. McPhie, N.T. Lange, et al. 2010. Ab-normalities in mitochondrial structure in cells from pa-tients with bipolar disorder. Am J Pathol. 177(2): 575–585.
29. Andreazza, A.C., M. Kauer-Sant’anna, B.N. Frey, et al.2008. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord 111(2–3): 135–144.
30. Wang, J.F. 2007. Defects of mitochondrial electrontransport chain in bipolar disorder: Implications formood-stabilizing treatment. Can J Psychiatry 52(12):753–762.
31. Berk, M., D.L. Copology, O. Dean, et al. 2008. N-acetylcysteine for depressive symptoms in bipolar disorder –a double-blind randomized placebo-controlled trial. BiolPsychiatry 64(6): 468–475.
32. Kraepelin, E. 1989. Manic-Depressive Insanity and Para-noia. Birmingham, Classics of Medicine Library.
33. McElroy S. 1992. Clinical and research implications of thediagnosis of dysphoric or mixed mania or hypomania. AmJ Psych. 149: 1633–1644.
22 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
34. Suppes P. Proposals to the DSM-5 for bipolar disorder:Hypomania duration and the emphasis on increased ac-tivity/energy. Ann NY Acad Sci.
35. Angst, J. 2007. The bipolar spectrum. Br J Psychiatry 190:189–191.
36. Cassano, G.B., P. Rucci, E. Frank, et al. 2004. The moodspectrum in unipolar and bipolar disorder: argumentsfor a unitary approach. Am J Psychiatry 161: 1264–1269.
37. Hirschfeld, R.M.A., A.H. Young, K.D. Wagner, et al. 2004.Screening for bipolar disorder in UK adults using the mooddisorders questionnaire. (Poster), XXIV CINP Congress.Paris.
38. Hantouche, E., J.M. Azorin, S. Lancrenon, et al. 2009.Prevalence de l’hypomanie dans les depressions majeuresrecurrentes ou resistantes: enquetes Bipolact. Ann MedPsychol. 167: 30–37.
39. Rybakowski, J.K., J. Angst, D. Dudek, et al. 2010. Polishversion of the Hypomania Checklist (HCL-32) scale: theresults in treatment-resistant depression. Eur Arch PsychiatNeurol Sci. 260: 139–144.
40. Angst, J., L. Cui, J. Swendsen, et al. 2010. Major depressivedisorder with subthreshold bipolarity in the National Co-morbidity Survey Replication. Am J Psychiatry 167: 1194–1201.
41. Zimmerman, P., T. Bruckl, A. Nocon, et al. 2009. Hetero-geneity of DSM-IV major depressive disorder as a con-sequence of subthreshold bipolarity. Arch Gen Psychiatry66(12): 1341–1352.
42. Angst, J., J.M. Azorin, C.L. Bowden, et al. 2011. Prevalenceand characteristics of undiagnosed bipolar disorders inpatients with a major depressive episode: the Bridge Study.Arch Gen Psychiatry 68: 791–799.
43. Angst, J. 2009. Diagnostic concepts of bipolar disorders: aEuropean perspective. Clin Psychol Sci Prac. 16: 161–165.
44. Vieta, E. & M.L. Phillips. 2007. Deconstructing bipolardisorder: A critical review of its diagnostic validity anda proposal for DSM-V and ICD-11. Schizophr Bull. 33,886–892.
45. Fiedorowicz, J.G., J. Endicott, A.C. Leon, et al. 2011.Subthreshold hypomanic symptoms in progression fromunipolar major depression to bipolar disorder. Am J Psy-chiatry 16: 40–48.
46. Akiskal, H.S., E.G. Hantouche, M.L. Bourgeois, et al. 2001.Toward a refined phenomenology of mania: combiningclinician-assessment and self-report in the French EPI-MAN study. J Affect Disord. 67: 89–96.
47. Angst, J., A. Gamma, F. Benazzi, et al. 2003. Toward a re-definition of subthreshold bipolarity: epidemiology andproposed criteria for bipolar-II, minor bipolar disordersand hypomania. J Affect Disord. 73: 133–146.
48. Angst, J., A. Gamma, J-M. Azorin, et al. 2011. Broad consid-erations of Bipolar I and Bipolar II Disorder. InternationalConference on Bipolar Disorder (ICBD), Symposium IIDSM-5. Pittsburgh, PA, June 9–11.
49. Bauer, M.S., P. Critis-Christoph, W.A. Ball, et al. 1991.Independent Assessment of Manic and Depressive Symp-toms by Self-Rating: Scale characteristics and implicationsfor the study of mania. Arch Gen Psychiatry 48: 807–812.
50. Benazzi F. 2007. Testing new diagnostic criteria for hypo-mania. Ann Clin Psychiatry 19(2): 1–6.
51. Benazzi, F. & H.S. Akiskal. 2003. The dual factor structureof self-rated MDQ hypomania: energized-activity versusirritable-thought racing. J Affect Disord 73: 59–64.
52. Cassano, G.B., M. Mula, P. Rucci, et al. 2009. The Structureof lifetime manic-hypomanic spectrum. J Affect Disord112: 59–70.
53. Cassidy, F., E. Murry, K. Forest & B.J. Carroll. 1998. Afactor analysis of the signs and symptoms of mania. ArchGen Psychiatry 55: 27–32.
54. Gardner, R. Jr & B. Wenegrat. 1991. What is the ‘Core’symptom of mania? Arch Gen Psychiatry 50: 71–72.
55. Goodwin, F.K. & K.R. Jamison. 2007. Manic-DepressiveIllness: Bipolar Disorder and Recurrent Depression. OxfordUniversity Press. Oxford, NY.
56. Benazzi, F. & H. Akiskal. 2006. The duration of hypomaniain bipolar-II disorder in private practice: methodology andvalidation. J Affect Disord 96: 189–196.
57. Bauer, M., P. Grof, N.L. Rasgon, et al. 2006. Self-reporteddata from patients with bipolar disorder: Impact on mini-mum episode length for hypomania. J Affect Disord 96(1–2): 101–105.
58. Frank, E. 2011. Proposed revisions to the concept of mixedepisodes in DSM-5: The path travelled. International Con-ference on Bipolar Disorder (ICBD), Symposium II DSM-5. Pittsburgh, PA, June 9–11.
59. Goldstein, B.I., A. Fagiolini, P. Houck & D.J. Kupfer. 2009.Cardiovascular disease and hypertension among adultswith bipolar I disorder in the United States. Bipolar Disord11(6): 657–662.
60. Harvey, A.G., D.A. Schmidt, A. Scarna, et al. 2005. Sleep-related functioning in euthymic patients with bipolar dis-order, patients with insomnia, and subjects without sleepproblems. Am J Psychiatry 162: 50–57.
61. Kupfer, D.J. 2005. The Increasing Medical Burden in Bipo-lar Disorder. JAMA 293: 2528–2530.
62. Knutson, K.L. 2010. Sleep duration and cardiometabolicrisk: A review of the epidemiologic evidence. Best Prac ResClin Endocrinol Metab. 24: 731–743.
63. Osby, U., K. Brandt, N. Correia, et al. 2001. Excess mortal-ity in bipolar and unipolar disorder in Sweden. Arch GenPsychiatry 58: 844–850.
64. Birkenaes, A.B., S. Opjordsmoen & C. Brunborg. 2007.The level of cardiovascular risk factors in bipolar disorderequals that of schizophrenia: a comparative study. Journalof Clinical Psychiatry 68: 917–923.
65. Cappuccio, F.P., F.M. Taggart, N.B. Kandala, A. Currie, E.Peile, S. Stranges & M.A. Miller. 2008. Meta-analysis ofshort sleep duration and obesity in children and adults.Sleep 31: 619–626.
66. Fagiolini, A., D.J. Kupfer, P.R. Houck, et al. 2003. Obesityas a correlate of outcome in patients with bipolar I disorder.Am J Psychiatry 160: 112–117.
67. McElroy S.L., M.A. Frye, T. Suppes, et al. 2002. Correlatesof overweight and obesity in 644 patients with bipolardisorder. J Clin Psychiatry 63: 207–213.
68. Spiegel, K., E. Tasali, P. Penev & E.V. Cauter. 2004. Briefcommunication: Sleep curtailment in healthy young men
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 23
Advances in bipolar disorder Kupfer et al.
is associated with decreased leptin levels, elevated ghrelinlevels, and increased hunger and appetite. Ann Intern Med.141: 846–850.
69. Kilbourne, A.M., D.L. Rofey, J.F. McCarthy, et al. 2007. Nu-trition and exercise behavior among patients with bipolardisorder. Bipolar Disord. 9: 443–452.
70. M. Dworak, A. Wiater, D. Alfer, et al. 2008. Increased slowwave sleep and reduced stage 2 sleep in children dependingon exercise intensity. Sleep Medicine 9: 266–272.
71. Reid, K.J., K.G. Baron, B. Lu, et al. 2010. Aerobic exerciseimproves self-reported sleep and quality of life in olderadults with insomnia. Sleep Med. 11: 934–940.
72. G.S. Passos, D. Poyares, M.G. Santana, et al. 2010.Effect of acute physical exercise on patients withchronic primary insomnia. J Clin Sleep Med. 6: 270–275.
73. Martin, B.J. 1981. Effect of sleep deprivation on toler-ance of prolonged exercise. Eur J Appl Physiol 47: 345–354.
74. Chuang, H.T., C. Mansell & S.B. Patten. 2008. Lifestylecharacteristics of psychiatric outpatients. Can J Psychiatry53: 260–266.
75. Soehner A. & A.G. Harvey. 2011. Insomnia symptoms andcardiovascular risk in bipolar disorder: A national comor-bidity survey-replication analysis. Manuscript submittedfor publication.
76. W.R. Miller & S. Rollnick. 2002. Motivational interviewing:Preparing people to change. Guilford Press.
77. Morin, C.M. & C.A. Espie. 2003. Insomnia: A clinical guideto assessment and treatment. Kluwer Academic/PlenumPublishers.
78. E. Frank, D.J. Kupfer, M.E. Thase, et al. 2005. Two-YearOutcomes for Interpersonal and Social Rhythm Therapyin Individuals With Bipolar I Disorder. Arch Gen Psychiatry62: 996–1004.
79. Wirz-Justice A., F. Benedetti & M. Terman. 2009.Chronotherapeutics for affective disorders: A clinician’smanual for light & wake therapy. Karger.
80. D.C. Glahn, L. Almasy, M. Barguil, et al. 2010. Neurocog-nitive endophenotypes for bipolar disorder identified inmultiplex multigenerational families. Arch Gen Psychiatry67: 168–177.
81. Arts, B., N. Jabben, L. Krabbendam & van Os J. 2008.Meta-analyses of cognitive functioning in euthymic bipo-lar patients and their first-degree relatives. Psychol Med.38: 771–785.
82. Bora, E., M. Yucel & C. Pantelis. 2009. Cognitive endophe-notypes of bipolar disorder: A meta-analysis of neuropsy-chological deficits in euthymic patients and their first-degree relatives. J Affect Disord 113: 11–20.
83. Quraishi, S. & S. Frangou. 2002. Neuropsychology of bipo-lar disorder: a review. J Affect Disord 72: 209–226.
84. Robinson, L.J., J.M. Thompson, P. Gallagher, et al. 2006.A meta-analysis of cognitive deficits in euthymic patientswith bipolar disorder. J Affect Disord 93: 105.
85. Stefanopoulou, E., A. Manoharan, S. Landau, et al. 2009.Cognitive functioning in patients with affective disordersand schizophrenia: A meta-analysis. Int Rev Psychiatry 21:336–56.
86. Torres, I.J., V.G. Boudreau & L.N. Yatham. 2007. Neu-ropsychological functioning in euthymic bipolar disorder:a meta-analysis. Acta Psychiatr Scand Suppl. 434: 17–26.
87. Malhi, G.S., B. Ivanovski, Hadzi-Pavlovic D., et al. 2007.Neuropsychological deficits and functional impairment inbipolar depression, hypomania and euthymia. Bipolar Dis-ord 9: 114–125.
88. Green, M.F. & K.H. Nuechterlein. 2004. The MATRICS ini-tiative: developing a consensus cognitive battery for clinicaltrials. Schizophr Res. 72: 1–3.
89. Kern, R.S., J.M. Gold, D. Dickinson, et al. 2011. The MCCBimpairment profile for schizophrenia outpatients: resultsfrom the MATRICS psychometric and standardizationstudy. Schizophr Res. 126: 124–131.
90. Burdick, K.E., T.E. Goldberg, B.A. Cornblatt, et al. 2011.The MATRICS consensus cognitive battery in patients withbipolar I disorder. Neuropsychopharmacology 36: 1587–1592.
91. Schretlen, D.J., N.G. Cascella, S.M. Meyer, et al. 2007.Neuropsychological functioning in bipolar disorder andschizophrenia. Biol Psychiatry 62: 179–186.
92. Chen, Y.C., D. Thaler, P.D. Nixon, et al. 1995. The func-tions of the medial premotor cortex. II. The timing andselection of learned movements. Exp Brain Res. 102: 461–473.
93. Lane, R.D., E.M. Reiman, B. Axelrod, et al. 1998. Neuralcorrelates of levels of emotional awareness. Evidence of aninteraction between emotion and attention in the anteriorcingulate cortex. J Cogn Neurosci. 10: 525–535.
94. Phan, K.L., T. Wager, S.F. Taylor & I. Liberzon. 2002. Func-tional neuroanatomy of emotion: a meta-analysis of emo-tion activation studies in PET and fMRI. Neuroimage 16:331–348.
95. Yamasaki, H., K.S. LaBar & G. McCarthy. 2002. Dissociableprefrontal brain systems for attention and emotion. PNAS99: 11447–11451.
96. Altshuler L., S. Bookheimer, M.A. Proenza, et al. 2005.Increased amygdala activation during mania: a functionalmagnetic resonance imaging study. Am J Psychiatry 162:1211–1213.
97. Almeida, J.R., A. Versace, S. Hassel, et al. 2010. Elevatedamygdala activity to sad facial expressions: a state marker ofbipolar but not unipolar depression. Biol Psychiatry 67(5):414–421.
98. Blumberg, H.P., N.H. Donegan, C.A. Sanislow, et al. 2005.Preliminary evidence for medication effects on functionalabnormalities in the amygdala and anterior cingulate inbipolar disorder. Psychopharmacology 183(3): 308–313.
99. Kalmar, J.H., F. Wang, L.G. Chepenik, et al. 2009. Relationbetween amygdala structure and function in adolescentswith bipolar disorder. J Am Acad Child Adolesc Psychiatry48: 636–642.
100. Lawrence, N.S., A.M. Williams, S. Surguladze, et al.2004. Subcortical and ventral prefrontal cortical neuralresponses to facial expressions distinguish patients withbipolar disorder and major depression. Biol Psychiatry55(6): 578–587.
101. Rich, B.A. & D.T. Vinton, R. Roberson-Nay, et al. 2006.Limbic hyperactivation during processing of neutral facial
24 Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences.
Kupfer et al. Advances in bipolar disorder
expressions in children with bipolar disorder. Proc NatlAcad Sci USA 103(23): 8900–8905.
102. Altshuler, L., S. Bookheimer, J. Townsend, et al. 2008.Regional brain changes in bipolar I depression: a functionalmagnetic resonance imaging study. Bipolar Disord 10: 708–717.
103. Strakowski, S.M., C.M. Adler, S.K. Holland, et al. 2004.A preliminary FMRI study of sustained attention in eu-thymic, unmedicated bipolar disorder. Neuropsychophar-macol 29(9): 1734–1740.
104. Pfeifer, J.C., J. Welge, S.M. Strakowski, et al. 2008. Meta-analysis of amygdala volumes in children and adolescentswith bipolar disorder. J Am Acad Child Adolesc Psychiatry47(11): 1289–1298.
105. Strakowski, S.M., M.P. Delbello & C.M. Adler. 2005. Thefunctional neuroanatomy of bipolar disorder: A reviewof neuroimaging findings. Mol Psychiatry 10(1): 105–116.
106. Blumberg, H.P., E. Stern, S. Ricketts, et al. 1999. Rostraland orbital prefrontal cortex dysfunction in the manic stateof bipolar disorder. Am J Psychiatry 156(12): 1986–1988.
107. Blumberg, H.P., H.C. Leung, P. Skudlarski, et al. 2003. Afunctional magnetic resonance imaging study of bipolardisorder: state- and trait-related dysfunction in ventralprefrontal cortices. Arch Gen Psychiatry 60: 601–609.
108. Strakowski, S.M., J.C. Eliassen, M. Lamy, M.A. Cerullo,J.B. Allendorfer, M. Madore, J.H. Lee, J.A. Welge, M.P.DelBello, D.E. Fleck & C.M. Adler. 2011. Functional mag-netic resonance imaging brain activation in bipolar mania:evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry 69(4): 381–388.
109. Foland, L.C., L.L. Altshuler, S.Y. Bookheimer, et al. 2008.Evidence for deficient modulation of amygdala response byprefrontal cortex in bipolar mania. Psychiatry Res 162(1):27–37.
110. Versace, A., J.R.C. Almeida, S. Hassel, et al. 2008. Ele-vated left and reduced right orbitomedial prefrontal frac-tional anisotropy in adults with bipolar disorder revealedby tract-based spatial statistics. Arch Gen Psychiatry 65(9):1041–1052.
111. Bitter, S.M., N.P. Mills, C.M. Adler, S.M. Strakowski & M.P.DelBello. Progression of Amygdala Volumetric Abnormal-ities in Adolescents After Their First Manic Episode. J AmAcad Child Adolesc Psychiatry, in press.
112. Versace, A., C.D. Ladouceur, S. Romero, et al. 2010. Al-tered development of white matter in youth at high fa-milial risk for bipolar disorder: a diffusion tensor imagingstudy. J Am Acad Child Adolesc Psychiatry 49(12): 1249–1259.
113. Adler, C.M., J. Adams, M.P. DelBello, et al. 2006. Evidenceof white matter pathology in bipolar disorder adolescentsexperiencing their first episode of mania: a diffusion tensorimaging study. Am J Psychiatry 163(2): 322–324.
114. Adler, C.M., S.K. Holland, V. Schmithorst, et al. 2004.Abnormal frontal white matter tracts in bipolar disorder:a diffusion tensor imaging study. Bipolar Disord 6(3): 197–203.
115. Andreazza, A.C., C. Cassini, A.R. Rosa, et al. 2007. SerumS100B and antioxidant enzymes in bipolar patients. J Psy-chiatr Res. 41(6): 523–529.
116. Andreazza, A.C., M. Kauer-Sant’anna, B.N. Frey, et al.2008. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord 111(2–3): 135–144.
117. Athanasiu, L., M. Mattingsdal, A.K. Kahler, et al. 2010.Gene variants associated with schizophrenia in a Norwe-gian genome-wide study are replicated in a large Europeancohort. J Psychiatr Res. 44(12): 748–753.
118. Baumer, F.M., M. Howe, K. Gallelli, et al. 2006. A pilotstudy of antidepressant-induced mania in pediatric bipolardisorder: Characteristics, risk factors, and the serotonintransporter gene. Biol Psychiatry 60(9): 1005–1012.
119. Sibille, E., Y. Wang, J. Joeyen-Waldorf, et al. 2009. A molec-ular signature of depression in the amygdala. Am. J. Psy-chiatry 166: 1011–1024.
120. Maletic, V., M. Robinson, T. Oakes, S. Iyengar, S.G. Ball& J. Russell. 2007. Neurobiology of depression: an inte-grated view of key findings. Int. J. Clin. Pract. 61: 2030–2040.
121. Frayne, S.M., J.H. Halanych & D.R. Miller. 2005. Dispari-ties in diabetes care: impact of mental illness. Arch. Intern.Med. 165: 2631–2638.
Ann. N.Y. Acad. Sci. 1242 (2011) 1–25 c© 2011 New York Academy of Sciences. 25