Jawaban Tugas III
-
Upload
amira-natasya -
Category
Documents
-
view
232 -
download
0
Transcript of Jawaban Tugas III
-
7/30/2019 Jawaban Tugas III
1/6
TUGAS III
1. An ideal gas initially at 600 K and 10 bar undergoes a four-step mechanically reversible
cycle in a closed system. In step 12, pressure decreases isothermally to 3 bar; in step 23,
pressure decreases at constant volume to 2 bar; in step 34, volume decreases at constant
pressure; and in step 41, the gas returns adiabatically to its initial state.
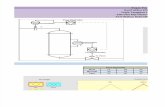
a. Sketch the cycle on a PVdiagram.
b. Determine (where unknown) both Tand Pfor states 1, 2, 3, and 4.
c. Calculate Q, W, AU, and AH for each step of the cycle.
Data: Cp = (7/2)R and Cv = (5/2)R.
Penyelesaian:
a. Diagram PV:
b. 4,1R5,2R5,3
CC
V
P
R = 8,1345 J mol-1
K-1
Hubungan antara titik2 dan 3:
3
3
2
2
T
P
T
P
V
P
(bar)
10
3
2
1
2
3
4
600 K
600 K
-
7/30/2019 Jawaban Tugas III
2/6
K4006003
2T
P
PT
2
2
3
3
Hubungan antara titik 1 dan 4:
144
1
11PTPT
K8,3782
10K600
P
PTT
4,14,111
4
1
14
c. Langkah 1-2 (proses isotermal)
U = H = 0
molJ3,587610
3lnK600
Kmol
J1345,8
P
PlnRTQ
1
2
0WQU W =Q =5876,3 J/mol
Langkah 2-3 (proses isokoris)
molJ4,2440K600400KmolJ1345,85,1TTCdTCU 1123VT
T
V
3
2
molJ3,4067K600400KmolJ1345,85,2TTCdTCH 1123PT
T
P
3
2
W = 0
QWQU
Q =2440,4 J/mol
Langkah 3-4 (proses isobaris)
molJ7,258K4008,378KmolJ1345,85,1TTCdTCU 1134VT
T
V
4
3
molJ1,431K4008,378KmolJ1345,85,2TTCdTCH 1134PT
T
P
4
3
Q = H =
431,1 J/mol
WQU
molJ6,172molJ1,4317,258QUW
Langkah 4-1 (proses adiabatis)
Q = 0
-
7/30/2019 Jawaban Tugas III
3/6
molJ2699K8,378600KmolJ1345,85,1TTCdTCU 1141VT
T
V
1
4
molJ4,4498K8,378600KmolJ1345,85,2TTCdTCH 1141PT
T
P
1
4
WQU = W
W = U =258,7 J/mol
-
7/30/2019 Jawaban Tugas III
4/6
2. An ideal gas, initially at 303.15 K (30C) and 100 kPa, undergoes the following cyclic processes in a closed
system:
(a) In mechanically reversible processes, it is first compressed adiabatically to 500 kPa, then cooled at a
constant pressure of 500 kPa to 303.15 K (30C), and finally expanded isothermally to its original state.
(b)
The cycle traverses exactly the same changes of state, but each step is irreversible with an efficiency of80% compared with the corresponding mechanically reversible process.
Calculate Q, W, U, and H for each step of the process and for the cycle. Take CP= (7/2)R and CV= (5/2)R.
Penyelesaian:
R = 8,1345 J mol-1
K-1
4,1R5,2
R5,3
C
C
V
P
a.
Diagram PV:
Langkah 1-2 (proses adiabatis)
Hubungan antara titik 1 dan 2:
122
1
11PTPT
K496500
100K15,313
P
PTT
4,14,111
2
112
Q = 0
V
P
(kPa)
500
100 1
23
313,15 K
313,15 K
-
7/30/2019 Jawaban Tugas III
5/6
molJ1,2231K15,313496KmolJ1345,85,1TTCdTCU 1112VT
T
V
2
1
molJ5,3718K15,313496KmolJ1345,85,2TTCdTCH11
12P
T
T
P
2
1
WQU = W
W = U = 2231,1 J/mol
Langkah 2-3 (proses isobaris)
molJ1,2231K49615,313KmolJ1345,85,1TTCdTCU 1123VT
T
V
3
2
molJ5,3718K49615,313KmolJ1345,85,2TTCdTCH 1123PT
T
P
3
2
Q = H =3715,5 J/mol
WQU
molJ4,1487molJ5,37181,2231QUW
Langkah 3-1 (proses isotermal)
U = H = 0
molJ8,4099
500
100lnK15,313
Kmol
J1345,8
P
PlnRTQ
3
1
0WQU
W =Q =4099,8 J/mol
Keseluruhan siklus
U = 2231,12231,1 + 0 = 0 J/mol
H = 3718,53718,5 + 0 = 0 J/mol
Q = 03718,5 + 4099,8 = 381,3 J/mol
W = 2231,1 + 1487,4
4099,8 =
381,3 J/mol
-
7/30/2019 Jawaban Tugas III
6/6