Serotonin transporter binding after recovery from eating disorders
-
Upload
independent -
Category
Documents
-
view
1 -
download
0
Transcript of Serotonin transporter binding after recovery from eating disorders
ORIGINAL INVESTIGATION
Serotonin transporter binding after recoveryfrom eating disorders
Ursula F. Bailer & Guido K. Frank & Shannan E. Henry &
Julie C. Price & Carolyn C. Meltzer & Carl Becker &
Scott K. Ziolko & Chester A. Mathis & Angela Wagner &
Nicole C. Barbarich-Marsteller & Karen Putnam &
Walter H. Kaye
Received: 29 March 2007 /Accepted: 5 July 2007# Springer-Verlag 2007
AbstractRationale Several lines of evidence suggest that alteredserotonin (5-HT) function persists after recovery fromanorexia nervosa (AN) and bulimia nervosa (BN).Objectives We compared 11 subjects who recovered (>1year normal weight, regular menstrual cycles, no bingeingor purging) from restricting-type AN (REC RAN), 7 whorecovered from bulimia-type AN (REC BAN), 9 who
recovered from BN (REC BN), and 10 healthy controlwomen (CW).Materials and methods Positron emission tomography(PET) imaging with [11C]McN5652 was used to assessthe 5-HT transporter (5-HTT). For [11C]McN5652, distri-bution volume (DV) values were determined using a two-compartment, three-parameter tracer kinetic model, andspecific binding was assessed using the binding potential(BP, BP=DVregion of interest/DVcerebellum−1).Results After correction for multiple comparisons, the fourgroups showed significant (p<0.05) differences for [11C]
PsychopharmacologyDOI 10.1007/s00213-007-0896-7
Parts of the manuscript were presented at the 44th American Collegeof Neuropsychopharmacology (ACNP) Annual Meeting, December11–15, 2005, Waikoloa, Hawaii.
U. F. Bailer :G. K. Frank : S. E. Henry :C. C. Meltzer :A. Wagner :W. H. Kaye (*)Western Psychiatric Institute and Clinic, University of Pittsburgh,Iroquois Building, Suite 600, 3811 O’Hara Street,Pittsburgh, PA 15213, USAe-mail: [email protected]
W. H. KayePsychiatry, University of California San Diego,8950 Villa La Jolla Drive, Suite C207,La Jolla, CA 92037, USA
U. F. BailerDepartment of Biological Psychiatry,University Hospital of Psychiatry, Medical University of Vienna,Vienna, Austria
J. C. Price : C. C. Meltzer : C. Becker : S. K. Ziolko :C. A. MathisDepartment of Radiology, Presbyterian University Hospital,School of Medicine, University of Pittsburgh,Pittsburgh, PA, USA
C. C. MeltzerDepartments of Radiology and Neurology,Emory School of Medicine,Atlanta, GA, USA
C. C. Meltzer :W. H. KayeSchool of Medicine, University of Pittsburgh,Pittsburgh, PA, USA
G. K. FrankLaboratory for Developmental Brain Research,University of Colorado at Denver and Health Sciences Center,The Children’s Hospital,1056 E. 19th Avenue,Denver, CO 80218, USA
A. WagnerDepartment of Child and Adolescent Psychiatry,J.W. Goethe University of Frankfurt/Main,Frankfurt/Main, Germany
N. C. Barbarich-MarstellerNew York State Psychiatric Institute, Department of Psychiatry,College of Physicians and Surgeons,Columbia University Medical Center,New York, NY, USA
K. PutnamDepartment of Environmental Health,Division of Epidemiology and Biostatistics,University of Cincinnati School of Medicine,Cincinnati, OH, USA
McN5652 BP values for the dorsal raphe and antero-ventralstriatum (AVS). Post-hoc analysis revealed that REC RANhad significantly increased [11C]McN5652 BP compared toREC BAN in these regions.Conclusions Divergent 5-HTT activity in subtypes ofeating disorder subjects may provide important insights asto why these groups have differences in affective regulationand impulse control.
Keywords Anorexia nervosa . Bulimia nervosa .
Serotonin transporter . Positron emission tomography .
Serotonin . 5-HTTLPR
Introduction
Anorexia nervosa (AN) and bulimia nervosa (BN) aredisorders of unknown etiology that most commonly havetheir onset during adolescence in females. These disordersare characterized by the relentless pursuit of thinness,obsessive fears of being fat, and aberrant eating behaviors,such as restrictive eating and episodes of purging and/orbinge eating (American Psychiatric Association 1994).
Physiologic and pharmacologic studies show that dis-turbances of serotonin (5-HT) activity occur in people whoare ill with eating disorders (EDs; Brewerton and Jimerson1996; Walsh and Devlin 1998; Wolfe et al. 1997). Such 5-HT disturbances may contribute to appetite dysregulation(Blundell 1984; Leibowitz and Shor-Posner 1986), anxiousand obsessional behaviors and extremes of impulse control(Cloninger 1987; Higley and Linnoila 1997; Lucki 1998;Mann 1999; Soubrie 1986). Importantly, disturbances in 5-HT measures appear to persist after individuals recoverfrom AN and BN. It has been demonstrated that individualswho recovered from AN and BN have elevated cerebrospi-nal fluid (CSF) concentrations of 5-hydroxyindoleaceticacid (5-HIAA; Kaye et al. 1991a), reduced 5-HT2A receptorlevels (Bailer et al. 2004; Frank et al. 2002; Kaye et al.2001a), and increased 5-HT1A receptor levels (Bailer et al.2005). Moreover, those recovered from AN and BN showaltered behavioral responses to 5-HT challenges (Franket al. 2001; Kaye et al. 2003; Smith et al. 1999; Ward et al.1998). Recent imaging studies, using single photonemission computed tomography (SPECT) with [123I]beta-CIT found reduced 5-HTT binding in people who are illwith bulimic-type EDs (Kuikka et al. 2001; Tauscher et al.2001). Steiger found that individuals who recovered fromBN had reduced platelet binding of paroxetine (Steigeret al. 2005b); however, recent studies have shown thatperipheral measures of 5-HT receptor levels (5-HT2A and 5-HTT) do not correlate with central measures in healthycontrols. (Cho et al. 1999; Uebelhack et al. 2006; Yathamet al. 2000).
To date, no studies have explored whether central 5-HTTabnormalities continue after individuals with EDs haverecovered. The aim of this study was to use positronemission tomography (PET) imaging with [11C]McN5652to determine if alterations of 5-HTT persist after recoveryfrom AN and BN. Persistence of these abnormalities intorecovery may indicate a “trait” phenomenon, possiblylinked to the pathogenesis of the illness. In contrast,normalization of the 5-HTT alterations upon recoverywould suggest a “state” phenomenon.
The boundaries between subtypes of eating disorders arepoorly understood and somewhat controversial. Reasons forsubdividing recovered restricting-type anorexia nervosa(REC RAN) and recovered bulimia-type anorexia nervosa(REC BAN) is that they have differences in affectivemodulation and perhaps response to selective serotoninreuptake inhibitor (SSRI) medication. In addition, otherstudies from our group show that they are different in termsof binding of the 5-HT1A receptor (Bailer et al. 2005).Finally, this is reasonably consistent with the Diagnosticand Statistical Manual of Mental Disorders (DSM IV;American Psychiatric Association 1994) practice of cate-gorizing subgroups as AN, AN-BN, and BN.
A secondary, exploratory aim of this study was toexamine the possible effects of the functional polymor-phism in the promoter region of the 5-HTT gene (5-HTTLPR) on in vivo expression of 5-HTT in healthywomen and those who recovered from EDs. Some, but notall, genetic studies find altered frequency of a short allelepolymorphism in the 5-HTTLPR in people with AN andBN (Di Bella et al. 2000; Gorwood 2004; Lauzurica et al.2003; Matsushita et al. 2004; Monteleone et al. 2005;Steiger et al. 2005a). The correlation of genotypes withimaging findings can be problematic, given the fact that thesample size is small. Thus, we report exploratory findings.
Materials and methods
Twenty-seven women who recovered from EDs [7 womenrecovered from bulimic-type anorexia nervosa (REC BAN),11 women who recovered from restricting-type anorexianervosa (REC RAN), 9 women who recovered frombulimia nervosa (REC BN)] were recruited as previouslydescribed (Bailer et al. 2005; Wagner et al. 2006) Allindividuals underwent four levels of screening: (1) a briefphone screening, (2) an intensive screening assessingpsychiatric history, lifetime weight, binge eating andmethods of weight loss/control, and menstrual cycle historyas well as eating pattern for the past 12 months, (3) acomprehensive assessment using structured and semi-structured psychiatric interviews conducted by phone or inperson, and (4) a face- to-face interview and physical
Psychopharmacology
examination with a psychiatrist. To be considered “recov-ered,” subjects had to (1) maintain a weight above 85%average body weight (Company 1959), (2) have regularmenstrual cycles, and (3) have not binged, purged, orengaged in significant restrictive eating patterns for at least1 year before the study. Restrictive eating pattern wasdefined as regularly occurring behaviors, such as restrictingfood intake, restricting high-caloric food, counting calories,and dieting. Additionally, subjects must not have usedpsychoactive medication such as antidepressants or metcriteria for alcohol or drug abuse or dependence, majordepressive disorder, or severe anxiety disorder within 3months of the study. Ten healthy control women (CW) wererecruited through local advertisements and had no historyof an ED, psychiatric or neurologic disorder, or seriousmedical illness. This study was conducted according to theinstitutional review board regulations of the University ofPittsburgh, and all subjects gave written informed consent.
Blood was drawn for assessment of β-hydroxybutyrate(BHBA), a plasma ketone body that is relatively sensitiveto reflecting the presence of starvation (Fichter et al. 1990),as well as for evaluation of gonadal hormone levels(estradiol, E2). The Structured Clinical Interview forDSM-IV Axis I Disorders (First et al. 1996) was used toassess the lifetime prevalence of Axis I psychiatricdisorders. The ED diagnosis was made by a modifiedversion of module H of the SCID I. Current psychopathol-ogy was assessed with a battery of standardized instrumentsincluding the Beck Depression Inventory (BDI; Beck et al.1961), the Spielberger–Trait Anxiety Inventory (STAI;Spielberger et al. 1970), the Frost MultidimensionalPerfectionism Scale (MPS; Frost et al. 1990), the EatingDisorders Inventory (EDI-2; Garner 1990)), the Yale–Brown Obsessive Compulsive Scale (Y-BOCS; Goodmanet al. 1989a,b), the Yale–Brown–Cornell Eating DisorderScale (YBC-EDS; Mazure et al. 1994; Sunday et al. 1995),the Barratt Impulsiveness Scale (BIS; Barratt and Patton1983), and the Temperament and Character Inventory (TCI;Cloninger et al. 1994) for assessment of harm avoidance,novelty seeking, and reward dependence.
All subjects were scanned on the ECAT HR+PETscanner (CTI PET systems, Knoxville, TN) in three-dimensional (3D) imaging mode during the first 10 daysof the follicular phase of the menstrual cycle. Details ofPET and magnetic resonance imaging (MRI) acquisitionand co-registration are described elsewhere (Lopresti et al.2001). Immediately after slow bolus intravenous injectionof 13.8±1.8 mCi [11C]McN5652, dynamic emission scan-ning with arterial blood sampling (input function) wasperformed over 90 min. A scanning time of 90 min wasused to achieve time stable measurements of distributionvolume (DV) across regions for [11C]McN5652 (Frankleet al. 2004). The following regions of interest (ROI) were
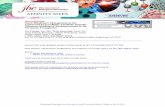
hand drawn on the coregistered MR images by techniciansblind to subject diagnosis and applied to the dynamic PETdata to generate time–activity curves: subgenual cingulate,thalamus, antero-ventral striatum, dorsal caudate, andcerebellum (as a reference region; Bailer et al. 2005;Drevets et al. 2001; Frank et al. 2005). For the dorsalraphe nucleus, due to the lack of identifiable anatomicboundaries on the MR, the ROI was placed directly on thePET image in the following manner. Based on thecoregistered MRs, the brain stem was subdivided into arostral (midbrain/upper pons) and caudal region (medulla/pons) to approximate the dorsal and median raphe nuclei,respectively. A dorsal midbrain raphe nucleus was drawnon the dynamic PET image, using circular fixed 6 mmradius ROIs placed over the area of highest radioactivity.The dorsal raphe was drawn over three contiguous planes,and the data from those three planes were sampled. Theinferior border of the dorsal raphe nucleus was identified bythe interpeduncular cistern on the MR. Previous imagingstudies of our group in EDs (Bailer et al. 2004, 2005, 2007;Frank et al. 2005) have found alterations in these ROIs andtherefore guided our choice of brain regions to investigatein our subjects in this study. Figure 1 shows examples ofMR and PET image data acquired at the level of the dorsalraphe.
For the kinetic analyses, regional [11C]McN5652 DVvalues were determined using a two-compartment, three-parameter tracer kinetic model (Lopresti et al. 2001).Specific 5-HTT binding was assessed using bindingpotential (BP), where BP was derived as the differencebetween the ROI DV value and the cerebellar DV value,normalized to the cerebellar DV [BP=(DVROI−DVCER)/DVCER] (Parsey et al. 2000).
An MR-based partial volume effect correction methodwas applied to correct the PET data for the dilutional effectof expanded CSF spaces accompanying normal aging anddisease-related cerebral atrophy (Meltzer et al. 1996, 1999).
For the genotyping data, S and L alleles were determinedusing DNA amplification (polymerase chain reaction, PCR)and established flanking primers. Amplification productswere resolved by electrophoresis and visualized withethidium bromide staining and UV transillumination,according to Edenberg and Reynolds (1998). Genotypingwas performed blind to diagnosis and imaging results.
Global BP differences were estimated by multivariateanalysis of variance (MANOVA) to explore if meandifferences among the three ED groups and controls werelikely to occur by chance. A new linear combinationconsisting of all the dependent ROI’s variables (subgenualcingulate, antero-ventral striatum, thalamus, dorsal caudate,and dorsal raphe) controlled the experiment-wise error ratebefore evaluating specific regions and allowed overallassessment of global BP patterns. Multiple comparisons
Psychopharmacology
using the Tukey–Kramer method identified the significantgroup pairs for statistically significant ANOVAs. Correla-tions were examined with Pearson correlation coefficients.A repeated measures analysis of variance (ANOVAR) wasapplied to examine potential group differences in radio-labeled metabolites of [11C]McN5652. Values are expressedas mean±standard deviation (SD). Standard statisticalsoftware packages (SAS Version 9.1) were used.
Results
Demographic data and behavioral assessment data areshown in Table 1. The repeated measures analysis of theunmetabolized fraction of [11C]McN5652 (5 time points)indicated no differences between the four groups over the
time course (data not shown). The regional [11C]McN5652BP values followed the rank order of 5-HTT binding(Parsey et al. 2000) as shown in Table 2.
MANOVA results for global BP differences weresignificant at the 5% level. Follow-up univariate ANOVAsignificantly distinguished the groups apart for [11C]McN5652 BP values in the regions of antero-ventralstriatum (F3,35=5.19, p=0.004) and dorsal raphe (F3,35=4.64, p=0.008; Table 2). Post-hoc analysis revealed that theREC RAN had significantly increased [11C]McN5652 BPcompared to REC BAN in these regions. REC BAN hadsignificantly lower [11C]McN5652 BP in the antero-ventralstriatum compared to REC BN. Illustrative scatterplots forthe dorsal raphe and antero-ventral striatum are shown inFig. 2.
[11C]McN5652 BP was not related to lifetime history ofmajor depressive disorder (n=16; 5 REC RAN, 4 RECBAN, and 7 REC BN), obsessive–compulsive disorder (n=18), alcohol abuse (n=3) or alcohol dependence (n=1), anyanxiety disorder (n=11), use of birth control pills (n=14),levels of plasma β-hydroxybuteric acid (BHBA), length ofrecovery, age, or current or low body mass index (BMI) inREC ED (all REC subgroups together) subjects. There wasno relationship between [11C]McN5652 BP and measuresof anxiety, depression, obsessionality, or impulse control atthe time of the study.
Genotyping data were available in 24 REC ED subjectsand 10 CW. ED subjects showed allele frequencies of 58%for the L and 42% for the S allele and a genotypedistribution of 29.2% LL (n=7), 58.3% SL (n=14), and12.5% SS (n=3). CW had frequencies of 60% for the L and40% for the S allele and genotype frequencies of 40% LL(n=4), 40% SL (n=4) and 20% SS (n=2). This smallnumber of subjects showed no significant differences inallele or genotype frequencies between REC ED and CW.Because of the small sample, all subjects were consideredtogether (REC ED and CW) when compared to [11C]McN5652 BP. The subjects with SS alleles had lower [11C]McN5652 BP than subjects with LL alleles for the dorsalcaudate (SS, 0.43±0.06; LL, 0.60±0.12; p=0.01), but notother brain regions (Fig. 3). Findings were similar for thedorsal caudate when only the REC ED subjects wereconsidered (SS, 0.44±0.08; LL, 0.63±0.09; p=0.03).
Discussion
This study has several major findings. First, REC RAN hadelevated [11C]McN5652 BP in the dorsal raphe and antero-ventral striatum in comparison to REC BAN. Furthermore,REC BAN had decreased [11C]McN5652 BP in the antero-ventral striatum in comparison to REC BN. These findingssuggest that subtypes of ED have differences in 5-HT
Fig. 1 This figure is representative of a recovered anorexic women,restricting type (REC RAN). The image is an SUV normalization of alate sum (30 to 90 min) [11C] McN5652 positron emissiontomography (PET) scan (left) and the subject’s associated coregisteredSPGR magnetic resonance (MR) image (right). The SUV image wascreated from the late sum by division (of each pixel) by the subject’sinjected dose in mCi and multiplying by the subject’s weight in kg.Also shown are examples of the regions-of-interest that were used togenerate the PET time-activity data. DRP Dorsal raphe nucleus; CERcerebellum
Psychopharmacology
function, which may provide important insights into whythese groups have differences in affective regulation andimpulse control.
To our knowledge, imaging studies of 5-HTT have notbeen done in individuals with RAN, whether ill orrecovered. Other imaging studies have found reduced 5-HTT levels in symptomatic BN subjects. Tauscher et al.(2001) found reduced [123I]beta-CIT binding in ten indi-viduals who were ill with BN. Two of those individuals had
a history of AN, but specific imaging data for those twoindividuals were not identified. Kuikka et al. (2001) foundreduced [123I]beta-CIT binding values in subjects ill withbinge eating disorder. In addition, Steiger et al. (2005b)showed that individuals remitted from BN have reducedplatelet [3H]paroxetine binding. We found that reduced[11C]McN5652 BP appeared to occur mainly in the remittedBAN, not the REC BN subjects. New studies, seeking toreplicate these findings with [11C]DASB, a more specific
Table 2 Regional [11C]McN5652 binding potential (BP) between groups
CW (n=10) REC RAN(n=11)
REC BAN(n=7)
REC BN(n=9)
ANOVA Uncorrectedp value
Tukey-KramerMultipleComparison
Mean SD Mean SD Mean SD Mean SD df F
Dorsal raphe 1.169 0.337 1.316 0.288 0.749 0.332 1.027 0.348 3.35 4.65 0.008 2>3Antero-ventral striatum 0.743 0.129 0.835 0.117 0.549 0.236 0.767 0.133 3.35 5.19 0.004 2>3; 3<4Thalamus 0.693 0.221 0.744 0.201 0.519 0.085 0.722 0.327 3.35 1.54 0.073Dorsal caudate 0.526 0.129 0.553 0.137 0.575 0.180 0.565 0.155 3.35 0.19 0.882Subgenual cingulate 0.239 0.052 0.237 0.105 0.236 0.066 0.224 0.086 3.35 0.06 0.752
Group comparisons by ANOVA. The cerebellar DV values were similar between groups (CW, 21.23±5.07; REC RAN, 20.56±5.62; REC BAN,20.58±4.75; REC BN, 22.30±3.54; p=.860).CW healthy comparison women; REC RAN recovered anorexic women, restricting type; REC BAN recovered anorexic women, bulimic-type; RECBN recovered bulimic women
Table 1 Demographic values between groups
CW (n=10) REC RAN(n=11)
REC BAN(n=7)
REC BN(n=9)
ANOVA
Mean SD Mean SD Mean SD Mean SD Sig. Group Diff.
Age (years) 27.93 6.89 24.74 5.48 23.66 4.17 24.44 5.27 0.396Current BMI (kg/m2) 22.66 2.02 20.74 2.44 21.75 2.27 23.24 2.76 0.119Low BMI (lifetime) (kg/m2) 20.37 1.60 13.33 (9) 2.25 15.14 1.53 18.59 1.76 <0.001 2, 3<1, 4Age of Onset (years of age) 15.91 3.21 15.83 (6) 1.94 17.38 (8) 4.69 0.622Length of Recovery (months) 43.36 49.52 12.00 (6) 13.56 17.13 (8) 10.16 0.143Estradiol (pg/mL) 43.10 42.33 19.56 (9) 26.68 25.2 (5) 25.37 90.56 99.65 0.083β-hydroxybuteric Acid (mg/dL) 0.83 0.31 0.52 (9) 0.31 0.52 (4) 0.21 1.25 (8) 1.46 0.215Cortisol (mcg/dl) 15.00 4.67 13.78 (9) 3.87 16 (5) 2.45 16.11 6.17 0.729EDI 2 –Drive for Thinness (“worst ever”)(Garner 1990)
0.30 0.67 18.60 (10) 4.09 18.57 3.36 19.78 1.39 <0.001 1<2, 3, 4
Perfectionism (MPS) (Frost et al. 1990) 53.80 12.26 111.82 17.10 113.43 20.32 99.11 22.03 <0.001 1<2, 3, 4Novelty Seeking (TCI (Cloninger 1987)) 20.90 4.98 15.27 7.42 21.71 8.50 20.32 8.28 0.210Harm Avoidance (TCI) 8.40 4.22 18.00 6.26 12.86 10.78 19.88 6.28 0.004 1<2, 4Trait Anxiety (STAI) (Spielberger et al. 1970) 28.70 5.14 45.00 12.81 38.71 12.35 47.33 9.80 0.002 1<2, 4Self Control (BIS (Barratt and Patton 1983)) 80.90 21.14 72.27 21.64 88.71 22.22 98.33 25.92 0.092Yale-Brown Obsessive-Compulsive Scale (YBOCS)(Goodman et al. 1989a,b)
0.00 0.00 4.64 6.39 8.00 (6) 8.99 11.63 (8) 10.46 0.013 1<4
Yale-Brown-Cornell Eating Disorders Scale(YBC-EDS) (Mazure et al. 1994; Sunday et al. 1995)
0.30 0.95 6.18 6.62 6.67 (6) 5.79 5.88 (8) 5.06 0.034
Depression (BDI) (Beck et al. 1961) 1.50 2.27 8.36 6.82 6.00 4.97 9.11 9.84 0.063
Group comparisons by ANOVASig. Significance; CW healthy control women; REC RAN recovered anorexic women, restricting type; REC BAN recovered anorexic women,bulimic-type; REC BN recovered bulimic women; BDI Beck Depression Inventory; TCI Temperament and Character Inventory; BIS BarrattImpulsiveness Scale; STAI State Trait Anxiety Inventory; MPS Multidimensional Perfectionism Scale
Psychopharmacology
radiotracer for a reliable quantification of 5-HTT parame-ters in regions of moderate 5-HTT density, including thelimbic regions (Frankle et al. 2004), are in progress.
Still, this finding raises the possibility that these data canbe used to understand who might respond to medication.The eating disorders are thought to share a commonvulnerability, as crossover between subtypes is common(Herzog et al. 1996) and subtypes are cross-transmitted infamilies (Kendler et al. 1995; Lilenfeld et al. 1998; Stroberet al. 2000). However, other factors appear to differentiatesubtypes of AN and BN, including degree of weight loss,affect regulation, self-control, and perhaps response toSSRI medication (Kaye et al. 2004). It should be notedthat we only studied recovered individuals with “pure”subtypes. For example, RAN individuals had never en-gaged in bulimic behaviors.
While BN individuals show a response to higher dosesof fluoxetine (Fluoxetine Bulimia Nervosa CollaborativeStudy Group 1992), the efficacy of such medication hasbeen questioned, as relatively few individuals abstain frombinge and purge behaviors, and relapse during treatment iscommon (Walsh 1991). It remains controversial whether
SSRIs are effective in RAN individuals (Kaye et al. 2001b;Walsh et al. 2006). Our clinical experience (Kaye et al.1991b) suggests that individuals with RAN respond betterto fluoxetine than those with BAN did and that someindividuals with BN can be relatively insensitive to highdoses of SSRIs. There is little data determining whetherBAN and BN individuals have differential responses toSSRIs. While highly speculative, our findings raise theprovocative possibility that decreased 5-HTT function maybe related to poor response to SSRI medication, whereasindividuals with increased 5-HTT activity may respond tohigher SSRI doses. Thus, PET and radioligand studies maybe a useful tool for investigating and managing medicationresponse in treatment resistant individuals.
In general, the REC RAN individuals had elevated 5-HTT binding, suggesting they have relatively greater 5-HTuptake and reduced extracellular 5-HT compared to RECBAN and BN. In support of this possibility, the REC BANand BN individuals tend to have higher binding of 5-HT1A
post-synaptic receptors and autoreceptors (Bailer et al.2005; Kaye et al., unpublished data), which may be acompensatory means of downregulating raphe activity
Genotype
SS LL
[11
C]M
cN
56
52
BP
do
rsa
l ca
ud
ate
0.2
0.3
0.4
0.5
0.6
0.7
0.8
p = .01
Genotype
SS LL
0.2
0.3
0.4
0.5
0.6
0.7
0.8
p = .03
Fig. 3 Scatterplot of [11C]McN5652 binding potential (BP)in the dorsal caudate in the CWand REC eating disorder sample(left graph) and REC eating dis-order only (right graph) by5-HTLPR genotype (SS andLL only)
CW
REC RAN
REC BAN
REC BN
[11C
]McN
5652 B
P
0.2
0.4
0.6
0.8
1.0
1.2
CW
REC RAN
REC BAN
REC BN
[11C
]McN
5652 B
P
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
2.0Fig. 2 Scatterplots of the [11C]McN5652 binding potential(BP) values in the dorsal raphe(left plot) and antero-ventralstriatum (right plot). CWHealthy control women; RECRAN recovered anorexicwomen, restricting type; RECBAN recovered anorexicwomen, bulimic-type; REC BNrecovered bulimic women
Psychopharmacology
(Cooper 1996; Hajos et al. 2003). Moreover, reduced 5-HTT activity, in terms of genotypes (Steiger et al. 2005a),has been associated with affect dysregulation, which tendsto be more common in the bulimic subgroups. Furthermore,in people with impulsive aggressivity, reduced 5-HTTbinding was found in the anterior cingulate cortex, a regioninvolved in affect regulation (Frankle et al. 2005). Imagingstudies (using SPECT or PET) in other psychiatric disorderswith altered affect regulation have revealed inconsistentresults. In obsessive–compulsive disorder, increased(Pogarell et al. 2003), unaltered (Simpson et al. 2003), ordecreased 5-HTT binding (Hesse et al. 2005; Stengler-Wenzke et al. 2004; Zitterl et al. 2007) was found. Resultsremain contradictory in depression as well (see Hesse et al.2004 for review).
The functional polymorphism in the promoter region ofthe human 5-HTT gene (SLC6A4) has been implicated inthe modulation of mood and antidepressant response(Murphy et al. 2004). We included data on possiblerelationships between 5-HTTLPR and [11C]McN5652 BP,despite our concerns that the sample was small, because ofthe interest in this topic. Several lines of evidence show thatthe genotype with two copies of the long (L, 528 bp) alleleleads to greater 5-HT reuptake compared to genotypeshaving either one or two copies of the short (S, 484 bp)allele (Lesch et al. 1996). As 5-HTT regulates 5-HTconcentrations in the synaptic cleft by recycling released5-HT, individuals with the S allele are likely to have higherextracellular 5-HT due to decreased 5-HT reuptake (Leschet al. 1996; Sundaramurthy et al. 2000). In other disorders,relationships between 5-HTTLPR genotypes and imagingmeasures of the transporter measures have been reported tooccur (Heinz et al. 2000; Little et al. 1998) or not (Parseyet al. 2006; Shioe et al. 2003; Willeit et al. 2001). Some, butnot all studies, have found increased S allele frequency inBN and AN (Di Bella et al. 2000; Fumeron et al. 2001;Hinney et al. 1997; Lauzurica et al. 2003; Matsushita et al.2004; Sundaramurthy et al. 2000). The data in our studysuggest that SS homozygous individuals have reduced 5-HTT binding in the dorsal caudate. In individuals with BN,the S allele has been associated with poor response to SSRItherapy (Monteleone et al. 2005) and outpatient multimodalgroup therapy (K. Bruce, personal communication), as wellas greater affective instability and impulsivity (Steiger et al.2005a). Together, these data further support the possibilitythat there is a group of individuals with ED who havedecreased 5-HTT function, and these individuals mayrespond poorly to treatment. The subjects in our studywere all white women, 18 to 45 years old, studied in theirearly follicular menstrual phase. Other studies have beenless homogenous, which may obscure potential findings.
In terms of limitations, the sample size is small, andreplication of these findings in larger samples is clearly
needed. The major limitation of the radioligand used in thisstudy is the high nonspecific binding, precluding reliablemeasurement of 5-HTT in the human neocortex; wetherefore restricted our ROI analysis to regions for whichthe derivation of [11C]McN5652 BP has been found reliable(Parsey et al. 2000) and in which we previously foundalterations. Persistent alterations in monoamine activityafter recovery raise the question of whether this is apremorbid vulnerability for developing ED symptoms.Alternatively, it is possible that chronic disturbances ofnutrition during the ill state might contribute to a persistent“scar” in recovered individuals, caused by chronic malnu-trition and emaciation. Patients were off medication (e.g.,SSRIs) for greater than 3 months, so it is unlikely thatpersistent effects of 5-HT active medication accounted forthe results. Fourteen ED subjects (3 REC RAN, 5 RECBAN, and 6 REC BN) were on SSRIs in the past. Therewas no difference in [11C]McN BP across ROIs betweensubjects who were or were not on SSRIs in the past withineach group and within the entire group of REC EDsubjects.
In summary, these data support and extend studiessuggesting that a disturbance of 5-HTT neuronal functionpersists after normalization of weight and nutritional statusin people who have had eating disorders.
Acknowledgements The authors are indebted to the participatingindividuals for their contribution of time and effort in support of thisstudy. We would like to thank W. Gordon Frankle for review of thismanuscript, Eva Gerardi and Katherine Plotnicov for editorialassistance, and the University of Pittsburgh Medical Center PETFacility staff for their invaluable contribution to this study. This studywas supported by grants from National Institute of Mental Health(NIMH) MH46001, MH42984, K05-MD01894, NIMH TrainingGrant T32-MH18399, and the Price Foundation. U.F.B. was fundedby an Erwin-Schrödinger-Fellowship of the Austrian Science Fund(nos. J 2188 and J 2359-B02).
References
American Psychiatric Association (1994) Diagnostic and statisticalmanual of mental disorders, 4 edn. American PsychiatricAssociation
Bailer UF, Price JC, Meltzer CC, Mathis CA, Frank GK, Weissfeld L,McConaha CW, Henry SE, Brooks-Achenbach S, Barbarich NC,Kaye WH (2004) Altered 5-HT2A receptor binding after recoveryfrom bulimia-type anorexia nervosa: relationships to harmavoidance and drive for thinness. Neuropsychopharmacology29:1143–1155
Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Weissfeld L,Mathis CA, Drevets WC, Wagner A, Hoge J, Ziolko SK,McConana CW, Kaye WH (2005) Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosameasured by positron emission tomography and [11C]WAY100635. Arch Gen Psychiatry 62:1032
Bailer UF, Frank G, Henry S, Price J, Meltzer C, Mathis C, Wagner A,Thornton L, Hoge J, Ziolko SK, Becker C, McConaha C, KayeWH (2007) Exaggerated 5-HT1A but normal 5-HT2A receptor
Psychopharmacology
activity in individuals ill with anorexia nervosa. Biol. Psychiatry61:1090–1099
Barratt ES, Patton JH (1983) Impulsivity: cognitive, behavioral, andpsychophysiological correlates. In: Zuckerman M (ed) Biologicalbases of sensation seeking, impulsivity, and anxiety. LawrenceEarlbaum Associates, Hillsdale, NJ, p 85
Beck AT, Ward M, Mendelson M, Mock J, Erbaugh J (1961) Aninventory for measuring depression. Arch Gen Psychiatry 4:53–63
Blundell JE (1984) Serotonin and appetite. Neuropharmacology23:1537–1551
Brewerton TD, Jimerson DC (1996) Studies of serotonin function inanorexia nervosa. Psychiatry Res 62:31–42
Cho R, Kapur S, Du L, Hrdina PD (1999) Relationship betweencentral and peripheral serotonin 5-HT2A receptors: a positronemission tomography study in healthy individuals. Neurosci Lett261:139–142
Cloninger CR (1987) A systematic method for clinical description andclassification of personality variants. A proposal. Arch GenPsychiatry 44:573–588
Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD (1994) TheTemperament and Character Inventory (TCI): a guide to itsdevelopment and use. Center for Psychobiology of Personality,Washington University, St. Louis, MO
Company MLI (1959) New weight standards for men and women.Stat Bull Metrop Insur Co, pp 1–11
Cooper SJ (1996) Cholecystokinin modulation of serotonergic controlof feeding behavior. Ann N Y Acad Sci 780:213–222
Di Bella DD, Catalano M, Cavallini MC, Riboldi C, Bellodi L (2000)Serotonin transporter linked polymorphic region in anorexianervosa and bulimia nervosa. Mol Psychiatry 5:233–234
Drevets W, Gautier C, Price J, Kupfer D, Kinahan P, Grace A, Price J,Mathis C (2001) Amphetamine-induced dopamine release inhuman ventral striatum correlates with euphoria. Biol Psychiatry49:81–96
Edenberg H, Reynolds J (1998) Improved method for detecting thelong and short promoter alleles of the serotonin transporter geneHTT (SLC6A4). Psychiatric Genetics 8:193–195
Fichter MM, Pirke KM, Pollinger J, Wolfram GM, Brunner E (1990)Disturbances in the hypothalamo-pituitary-adrenal and otherneuroendocrine axes in bulimia. Biol Psychiatry 27:1021–1037
First MB, Gibbon M, Spitzer RL, Williams JBW (1996) Users guide forthe structured clinical interview for DSM-IV Axis I disorders—research version (SCID-I, version 2.0, February 1996 FinalVersion). Biometrics Research Department, New York StatePsychiatric Institute, New York
Fluoxetine Bulimia Nervosa Collaborative Study Group (1992)Fluoxetine in the treatment of bulimia nervosa. A multicenter,placebo-controlled, double-blind trial. Arch Gen Psychiatry49:139–147
Frank GK, Kaye WH, Weltzin TE, Perel J, Moss H, McConaha C,Pollice C (2001) Altered response to meta-chlorophenylpiper-azine in anorexia nervosa: support for a persistent alteration ofserotonin activity after short-term weight restoration. Int J EatDisord 30:57–68
Frank GK, Kaye WH, Meltzer CC, Price JC, Greer P, McConaha C,Skovira K (2002) Reduced 5-HT2A receptor binding afterrecovery from anorexia nervosa. Biol Psychiatry 52:896–906
Frank G, Bailer UF, Henry S, Drevets W, Meltzer CC, Price JC,Mathis C, Wagner A, Hoge J, Ziolko SK, Barbarich N, WeissfeldL, Kaye W (2005) Increased dopamine D2/D3 receptor bindingafter recovery from anorexia nervosa measured by positronemission tomography and [11C]raclopride. Biol Psychiatry58:908–912
Frankle W, Huang Y, Hwang D, Talbot P, Slifstein M, Van Heertum R,Abi-Dargham A, Laruelle M (2004) Comparative evaluation of
serotonin transporter radioligands 11C-DASB and 11C-McN5652 in healthy humans. J Nucl Med 45:682–694
Frankle W, Lombardo I, New AS, Goodman M, Talbot P, Huang Y,Hwang D, Slifstein M, Curry S, Abi-Dargham A, Lauruelle M,Siever L (2005) Brain serotonin transporter distribution insubjects with impulsive aggressivity: a positron emission studywith [11C]McN 5652. Am J Psychiatry 162:915–923
Frost RO, Marten P, Lahart C, Rosenblate R (1990) The dimensions ofperfectionism. Cognitive Therapy & Research 14:449–468
Fumeron F, Betoulle D, Aubert R, Herbeth B, Siest G, Rigaud D(2001) Association of a functional 5-HT transporter genepolymorphism with anorexia nervosa and food intake. MolPsychiatry 6:9–10
Garner DM (1990) Eating disorder inventory-2 professional manual.Psychological Assessment Resources, Odessa, FL
Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P,Heninger GR, Charney DS (1989a) The Yale-Brown obsessivecompulsive scale. II. Validity. Arch Gen Psychiatry 46:1012–1016
Goodman WK, Price LH, Rasmussen SA, Mazure C, FleischmannRL, Hill CL, Heninger GR, Charney DS (1989b) The Yale-brownobsessive compulsive scale. I. Development, use, and reliability.Arch Gen Psychiatry 46:1006–1011
Gorwood P (2004) Eating disorders, serotonin transporter polymor-phisms and potential treatment response. Am J Pharmacogenomics4:9–17
Hajos M, Gartside SE, Varga V, Sharp T (2003) In vivo inhibition ofneuronal activity in the rat ventromedial prefrontal cortex bymidbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharma-cology 45:72–81
Heinz A, Jones D, Mazzanti C, Goldman D, Ragan P, Hommer D,Linnoila M, Weinberger D (2000) A relationship betweenserotonin transporter genotype and in vitro protein expressionand alcohol neurotoxicity. Biol Psychiatry 48:334–335
Herzog DB, Field AE, Keller MB, West JC, Robbins WM, Staley J,Colditz GA (1996) Subtyping eating disorders: is it justified. JAm Acad Child Adolesc Psychiatry 35:928–936
Hesse S, Barthel H, Schwarz J, Sabri O, Muller U (2004) Advances inin vivo imaging of serotonergic neurons in neuropsychiatricdisorders. Neurosci Biobehav Rev 29:1119
Hesse S, Muller U, Lincke T, Barthel H, Villman T, Angermeyer M,Sabri O, Stengler-Wenzke K (2005) Serotonin and dopaminetransporter imaging in patients with obsessive–compulsivedisorder. Psych Res 140:63–72
Higley JD, Linnoila M (1997) Low central nervous system seroto-nergic activity is traitlike and correlates with impulsive behavior.A nonhuman primate model investigating genetic and environ-mental influences on neurotransmission. Ann NY Acad Sci836:39–56
Hinney A, Barth N, Ziegler A, von Prittwitz S, Hamann A,Hennighausen K, Pirke KM, Heils A, Rosenkranz K, Roth H,Coners H, Mayer H, Herzog W, Siegfried A, Lehmkuhl G,Poustka F, Schmidt MH, Schafer H, Grzeschik KH, Lesch KP,Lentes KU, Remschmidt H, Hebebrand J (1997) Serotonintransporter gene-linked polymorphic region: allele distributionsin relationship to body weight and in anorexia nervosa. Life Sci61:PL295–PL303
Kaye WH, Gwirtsman HE, George DT, Ebert MH (1991a) Alteredserotonin activity in anorexia nervosa after long-term weightrestoration. Does elevated cerebrospinal fluid 5-hydroxyindole-acetic acid level correlate with rigid and obsessive behavior?Arch Gen Psychiatry 48:556–562
Kaye WH, Weltzin TE, Hsu LK, Bulik CM (1991b) An open trial offluoxetine in patients with anorexia nervosa. J Clin Psychiatry52:464–471
Psychopharmacology
KayeWH, Frank GK,Meltzer CC, Price JC, McConaha CW, Crossan PJ,Klump KL, Rhodes L (2001a) Altered serotonin 2A receptoractivity in women who have recovered from bulimia nervosa. AmJ Psychiatry 158:1152–1155
Kaye WH, Nagata T, Weltzin TE, Hsu LK, Sokol MS, McConaha C,Plotnicov KH, Weise J, Deep D (2001b) Double-blind placebo-controlled administration of fluoxetine in restricting- and restrict-ing-purging-type anorexia nervosa. Biol Psychiatry 49:644–652
Kaye WH, Barbarich NC, Putnam K, Gendall KA, Fernstrom J,Fernstrom M, McConaha CW, Kishore A (2003) Anxiolyticeffects of acute tryptophan depletion in anorexia nervosa. Int JEat Disord 33:257–267
Kaye W, Strober M, Jimerson D (2004) The neurobiology of eatingdisorders. In: Charney DS, Nestler EJ (eds) The neurobiology ofmental illness. Oxford Press, New York, pp 1112–1128
Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ(1995) The structure of the genetic and environmental risk factorsfor six major psychiatric disorders in women. Phobia, generalizedanxiety disorder, panic disorder, bulimia, major depression, andalcoholism. Arch Gen Psychiatry 52:374–383
Kuikka JT, Tammela L, Karhunen L, Rissanen A, Bergstrom KA,Naukkarinen H, Vanninen E, Karhu J, Lappalainen R,Repo-Tiihonen E, Tiihonen J, Uusitupa M (2001) Reducedserotonin transporter binding in binge eating women. Psycho-pharmacology (Berl) 155:310–314
Lauzurica N, Hurtado A, Escarti A, Delgado M, Barrios V, Morande G,Soriano J, Jauregui I, Gonzalez-Valdemoro M, Garcia-Camba E,Fuentes J (2003) Polymorphisms within the promoter and the intron2 of the serotonin transporter gene in a population of bulimicpatients. Neurosci Lett 352:226–230
Leibowitz SF, Shor-Posner G (1986) Brain serotonin and eatingbehavior. Appetite 7:1–14
Lesch K, Bengel D, Heils A, Sabol S, Greenberg B, Petri S, Benjamin J,Muller C, Hamer D, Murphy D (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter generegulatory region. Science 274:1527–1531
Lilenfeld LR, Kaye WH, Greeno CG, Merikangas KR, Plotnicov K,Pollice C, Rao R, Strober M, Bulik CM, Nagy L (1998) Acontrolled family study of anorexia nervosa and bulimia nervosa:psychiatric disorders in first-degree relatives and effects ofproband comorbidity. Arch Gen Psychiatry 55:603–610
Little K, McLaughlin D, Zhang L, Livermore C, Dalack G, McFinton P,DelProposto A, Hill E, Cassin B, Watson S, Cook E (1998)Cocaine, ethanol, and genotype effects on human midbrainserotonin transporter binding sites and mRNA levels. Am J Psych155:207–213
Lopresti B,Mathis C, Price J, Villemagne V,Meltzer C, Holt D, Smith G,Moore R (2001) Serotonin transporter binding in vivo: furtherexamination of [11C]McN5652 molecular and pharmacologicalbrain imaging with positron emission tomography. Academic, SanDiego, pp 265–271
Lucki I (1998) The spectrum of behaviors influenced by serotonin.Biol Psychiatry 44:151–162
Mann JJ (1999) Role of the serotonergic system in the pathogenesis ofmajor depression and suicidal behavior. Neuropsychopharmacol-ogy 21:99S–105S
Matsushita S, Suzuki K, Murayama M, Nishiguchi N, Hishimoto A,Takeda A, Shirakawa O, Higuchi S (2004) Serotonin transporterregulatory region polymorphism is associated with anorexianervosa. Am J Med Genet B Neuropsychiatr Genet 128B:114–117
Mazure CM, Halmi KA, Sunday SR, Romano SJ, Einhorn AM (1994)The Yale-Brown-Cornell eating disorder scale: development, use,reliability and validity. J Psych Res 28:425–445
Meltzer CC, Zubieta JK, Links JM, Brakeman P, Stumpf MJ, Frost JJ(1996) MR-based correction of brain PET measurements for
heterogeneous gray matter radioactivity distribution. J CerebBlood Flow Metab 16:650–657
Meltzer CC, Kinaham P, Nichols TE, Greer PJ, Comtat C, Cantwell MN,Price J (1999) Comparative evaluation ofMR-based partial-volumecorrection schemes for PET. J Nucl Med 40:2053–2065
Monteleone P, Santonastaso P, Tortorella A, Favaro A, Fabrazzo M,Castaldo E, Caregaro L, Fuschino A, Maj M (2005) Serotonintransporter polymorphism and potential response to SSRIs inbulimia nervosa. Mol Psychiatry 10:716–718
Murphy D, Lerner A, Rudnick G, Lesch K (2004) Serotonintransporter: gene, genetic disorders, and pharmacogenetics. MolInterv 4:109–123
Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A,Mawlawi O, Slifstein M, Van Heertum RL, Mann JJ, Laruelle M(2000) In vivo quantification of brain serotonin transporters inhumans using [11C]McN 5652. J Nucl Med 41:1465–1477
Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang Y,Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum R,Arango V, Mann JJ (2006) Effect of a triallelic functionalpolymorphism of the serotonin-transporter-linked promoter re-gion on expression of serotonin transporter in the human brain.Am J Psych 163:48–51
Pogarell O, Hamann C, Popperl G, Juckel G, Chouker M, Zaudig M,Riedel M, Moller H, Hegerl U, Tatsch K (2003) Elevated brainserotonin transporter availability in patients with obsessive-compulsive disorder. Biol Psychiatry 54:1406–1413
Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, HiranoM, Shinobara M, Kagami M, Okubo Y, Nankai M, Kanba S(2003) No association between genotype of the promoter regionof serotonin transporter gene and serotonin transporter binding inhuman brain measured by PET. Synapse 15:184–188
Simpson H, Lombardo I, Slifstein M, Huang H, Hwang D,Abi-Dargham A, Liebowitz MR, Laruelle M (2003) Serotonintransporters in obsessive-compulsive disorder: a positron emis-sion tomography study with [11C]McN 5652. Biol Psychiatry54:1414–1421
Smith KA, Morris JS, Friston KJ, Cowen PJ, Dolan RJ (1999) Brainmechanisms associated with depressive relapse and associatedcognitive impairment following acute tryptophan depletion. Brit JPsychiatry 174:525–529
Soubrie P (1986) Reconciling the role of central serotonin neurons inhuman and animal behavior. Beh Brain Sci 9:319
Spielberger CD, Gorsuch RL, Lushene RE (1970) STAI manual forthe state trait anxiety inventory. Consulting Psychologists Press,Palo Alto, CA
Steiger H, Joober R, Israel M, Young S, Ng Ying Kin NM, Gauvin L,Bruce K, Joncas J, Torkaman-Zehi A (2005a) The 5HTTLPRpolymorphism, psychopathological symptoms, and platelet [3H-]paroxetine binding in bulimic syndromes. Int J Eat Disord 37:57–60
Steiger H, Richardson J, Israel M, Ng Ying Kin NM, Bruce K,Mansour S, Marie Parent A (2005b) Reduced density of platelet-binding sites for [3H]paroxetine in remitted bulimic women.Neuropsychopharmacology 30:1028–1032
Stengler-Wenzke K, Muller U, Angermeyer M, Sabri O, Hesse S(2004) Reduced serotonin transporter-availability in obsessive–compulsive disorder (OCD). Eur Arch Psychiatry Clin Neurosci254:252–255
Strober M, Freeman R, Lampert C, Diamond J, Kaye W (2000)Controlled family study of anorexia nervosa and bulimia nervosa:evidence of shared liability and transmission of partial syn-dromes. Am J Psychiatry 157:393–401
Sundaramurthy D, Pieri LF, Gape H, Markham AF, Campbell DA(2000) Analysis of the serotonin transporter gene linkedpolymorphism (5-HTTLPR) in anorexia nervosa. Am J MedGenet Neuropsychiatr Genet 96:53–55
Psychopharmacology
Sunday SR, Halmi KA, Einhorn A (1995) The Yale-Brown-Cornelleating disorder scale: a new scale to assess eating disordersymptomatology. Int J Eat Disord 18:237–245
Tauscher J, Pirker W, Willeit M, de Zwaan M, Bailer U, NeumeisterA, Asenbaum S, Lennkh C, Praschak-Rieder N, Brücke T,Kasper S (2001) [123I]beta-CIT and single photon emissioncomputed tomography reveal reduced brain serotonin trans-porter availability in bulimia nervosa. Biol Psychiatry 49:326–332
Uebelhack R, Franke L, Herold N, Plotkin M, Amthauer H, Felix R(2006) Brain and platelet serotonin transporter in humans–correlation between [123I]-ADAM SPECT and serotonergicmeasurements in platelets. Neurosci Lett 406:153–158
Wagner A, Barbarich N, Frank G, Bailer UF, Weissfeld L, Henry S,Achenbach S, Vogel V, Plotnicov K, McConaha C, Kaye W,Wonderlich S (2006) Personality traits after recovery fromeating disorders: Do subtypes differ. Int J Eat Disord 39:276–284
Walsh BT (1991) Psychopharmacologic treatment of bulimia nervosa.J Clin Psychiatry 52Suppl:34–38
Walsh BT, Devlin MJ (1998) Eating disorders: progress and problems.Science 280:1387–1390
Walsh B, Kaplan A, Attia E, Olmsted M, Parides M, Carter J, Pike K,Devlin M, Woodside B, Roberto C, Rocket W (2006) Fluoxetine
after weight restoration in anorexia nervosa: a randomizedcontrolled trial. JAMA 295:2605–2612
Ward A, Brown N, Lightman S, Campbell IC, Treasure J (1998)Neuroendocrine, appetitive and behavioural responses to d-fenfluramine in women recovered from anorexia nervosa. Br JPsychiatry 172:351–358
Willeit M, Stastny J, Pirker W, Praschak-Rieder N, Neumeister A,Asenbaum S, Tauscher J, Fuchs K, Sieghart W, Hornik K, AschauerH, Brucke T, Kasper S (2001) No evidence for in vivo regulation ofmidbrain serotonin transporter availability by serotonin transporterpromoter gene polymorphism. Biol Psychiatry 50:8–12
Wolfe BE, Metzger ED, Jimerson DC (1997) Research update onserotonin function in bulimia nervosa and anorexia nervosa.Psychopharmacol Bull 33:345–354
Yatham L, Steiner M, Liddle P, Shiah I, Lam R, Zis A, Coote M(2000) A PET study of brain 5-HT2A receptors and theircorrelation with platelet 5-HT2 recptors in healthy humans.Psychopharmacology (Berl) 15:424–427
Zitterl W, Aigner M, Stompe T, Zitterl-Eglseer K, Gutierrez-Lobos K,Schmidl-Mohl B, Wenzel T, Demal U, Zettinig G, Hornik K,Thau K (2007) [(123)I]-beta-CIT SPECT imaging shows reducedthalamus–hypothalamus serotonin transporter availability in 24drug-free obsessive–compulsive checkers. Neuropsychopharma-cology 32(8):1661–1668
Psychopharmacology