Morphine and substance P release in the spinal cord
Transcript of Morphine and substance P release in the spinal cord
Exp Brain Res (1990) 82:89-96
nResearch �9 Springer-Verlag 1990
Morphine and substance P release in the spinal cord
C.R. Morton, W.D. Hutchison*, A.W. Dnggan **, and I.A. Hendry
Division of Neuroscience, John Curtin School of Medical Research, G.P.O. Box 334, Australian National University, Canberra, A.C.T. 2601, Australia
Received December 18, 1989/Accepted March 23, 1990
Summary. In anaesthetised cats, antibody microprobes were used to measure the release of immunoreactive substance P (irSP) in the lumbar dorsal horn during noxious cutaneous stimulation or high-intensity electrical stimulation of a hind limb nerve. The major region of irSP release detected was centred on the substantia gelatinosa, with lesser release at the dorsal cord surface. Release at these sites was unchanged by systemic administration of morphine, or of morphine followed by naloxone. During superfusion of the dorsal cord surface with high concentra- tions of morphine, irSP release in the substantia gelatinosa region was slightly reduced and surface release was not observed, effects not reversed by systemic naloxone ad- ministration. The results suggest that the analgesic action of morphine does not involve reduced release of SP in the spinal cord.
Key words: Morphine - Substance P release - Substantia gelatinosa Antibody microprobe- Cats
Introduction
There is considerable evidence that a significant pro- portion of the antinociceptive effects of #-opioid receptor agonists such as morphine are produced by a direct action in the spinal cord. The topical administration of morphine to the spinal cord surface produces analgesia in animals and humans (reviewed in Yaksh 1981; Yaksh and Noueihed 1985). There is evidence that the important intraspinal site of action for this effect is the region of the substantia gelatinosa. In spinalised cats, morphine admin- istered either microelectrophoretically into the substantia gelatinosa or systemically in analgesic doses, selectively
Present addresses: *Physiologisches Institut der Universitfit W/irzburg, D-8700 W/irzburg, Federal Republic of Germany ** Department of Preclinical Veterinary Sciences, University of Edinburgh, Summerhall, Edinburgh, UK Offprint requests to: C.R. Morton (address see above)
reduces the nociceptive excitation of deeper (laminae IV/N) dorsal horn neurones, effects which are reversed by microelectrophoretic administration of naloxone into this spinal region (Duggan et al. 1977, 1981; Johnson and Duggan 1981). In addition, systemically administered opiates bind intensely in the substantia gelatinosa (Pert et al. 1975).
There are several ways in which these analgesic and selective neuronal inhibitory effects of morphine could occur. One favoured hypothesis is presynaptic inhibition, whereby opioids have been proposed to reduce the amount of transmitter released from the central terminals of nociceptive primary afferent fibres (Jessell and Iversen 1977). Although the identity of the compound(s) mediating transmission from nociceptive primary afferents is not certain, immunoreactive substance P (irSP) is released in the superficial dorsal horn during noxious cutaneous stimulation or electrical stimulation of unmyelinated pri- mary afferent fibres (Duggan and Hendry 1986; Duggan et al. 1987, 1988b). Therefore, presynaptic inhibition of trans- mission from nociceptors by opioids might be expected to reduce SP release in the spinal cord, an expectation supported by both in vitro and in vivo studies. The potassium-evoked release of irSP from rat trigeminal nucleus slices (Jessell and Iversen 1977) and the potassium- or electrically-evoked irSP release from cultures of chick dorsal root ganglion neurones (Mudge et al. 1979; Chang et al. 1989) was inhibited by morphine and other opioids. In rats and cats, the in vivo release ofirSP evoked by high intensity peripheral nerve stimulation and detected in superfusates of the lumbar spinal cord, was reduced by superfusion of the cord with morphine (Yaksh et al. 1980; Go and Yaksh 1987). In rabbits, the release of irSP in the spinal and medullary dorsal horns detected with the push- pull cannula technique during nociceptive afferent stimu- lation was reduced by local administration of opioids (Hirota et al. 1985; Yonehara et al. 1986).
A significant limitation of all these investigations has been the inability to localise the site of irSP release. Experiments utilising the antibody microprobe technique in the feline lumbar cord have revealed that the intraspinal release of irSP in response to nociceptive primary afferent
90
inpu t is cent red on the region of the subs tan t i a ge la t inosa (Duggan and H e n d r y 1986; D u g g a n et al. 1987, 1988b). Therefore, the present exper iments have used this tech- nique to examine the influence of m o r p h i n e on i rSP release in the spinal cord in vivo.
Methods
Antibody microprobes were prepared to detect irSP as previously described (Duggan and Hendry 1986; Duggan et al. 1987, 1988a). The outer surface of glass micropipettes was coated with a siloxane polymer by several immersions in a 10%o solution of 3-aminopropyl- triethoxysilane (Aldrich) in toluene. The micropipettes were then immersed in glutaraldehyde (2.5%) to link Protein A (Sigma) to the polymer. Antibodies to SP were subsequently bound to the Protein A by incubation of the tips in dilutions of either rabbit antiserum (1:400, a gift from Professor R. Helme, Monash University, Melbourne) or monoclonal antibody concentrate (1:100, Com- monwealth Serum Laboratories, Melbourne). Comparable results were obtained with both antibodies, which were C-terminal-directed but did not cross-react with neurokinin A at 10-5M.
The experiments were performed on 16 cats (2.0-3.8 kg) anaes- thetised with pentobarbitone sodium (35 mg/kg i.p. initially). During surgery, the level of anaesthesia was frequently assessed and pento- barbitone supplements (2 3 mg/kg i.v. via a cephalic vein) were administered as required. A carotid artery was cannulated to enable continuous recording of blood pressure. Following a lumbar lamin- ectomy, the spinal cord was transected at the thoracolumbar junc- tion. The dura mater was slit dorsally and the exposed cord surface covered with a thin layer of Ringer-agar. In some experiments, the left and right tibial nerves were exposed and mounted on bipolar platinum electrodes under warm mineral oil. Anaesthesia and neuro- muscular paralysis were maintained throughout the experiments by the continuous i.v. infusion of pentobarbitone sodium (3 mg/kg/h) and gallamine triethiodide (4 mg/kg/h), respectively. The animals were artificially ventilated with air via a tracheal cannula, with end- tidal CO2 levels maintained at 4%. Adequacy of anaesthesia was regularly confirmed by the absence of a hypertensive response to noxious stimulation cephalic to the level of cord transection.
The Ringer-agar overlying the lower lumbar spinal cord was removed for several centimetres to form a pool and the dorsal cord surface thus exposed was continuously irrigated with sterile artificial cerebrospinal fluid (CSF) at 37~ pH 7.4. Microprobes were inserted into the cord in regions somatotopically appropriate for the site of peripheral stimulation, the ipsilateral hind limb. To allow micro- probe entry, small perforations were made in the pia mater about midway between the lateral and median dorsal sulci at sites of maximum cord dorsum potential evoked by innocuous mechanical stimulation of the hind paw or low intensity tibial nerve stimulation. Prior to microprobe insertion, the suitability of these sites was assessed by recording, with a 4M NaCl-filled microelectrode, excit- atory responses of neurones in lamina IV or V to the aforementioned peripheral stimuli. Microprobes were inserted in pairs to a depth of either 2.0 mm or 3.0 mm.
For noxious thermal stimulation, the hind paw was immersed in a water bath maintained at 52~ For noxious mechanical stimula- tion, strong alligator clips were applied to the digital pads. For peripheral nerve stimulation, the tibial nerve was electrically stimu- lated en passage, either with trains of 3 pulses at 333 Hz, 0.5 ms duration, repeated at 10 Hz, or with 30 Hz continuous stimulation. The stimulus intensity was sufficient to excite both myelinated (A) fibres and unmyelinated (C) fibres (200-300 • threshold for the lowest threshold fibres). All these stimulation procedures have previously been shown to evoke irSP release in the substantia gelatinosa region of the lumbar cord (Duggan and Hendry 1986; Duggan et al. 1987, 1988b). Microprobes were inserted, and stimuli applied, for selected periods within the range 15-30 min, times over which consistent intraspinal release of irSP has been detected with
this technique (Duggan and Hen, dry 1986; Duggan et al. 1988b). Only one type of stimulation (thermal, mechanical or electrical) and one period of stimulation (min) were used throughout any one experi- ment. Morphine (sulphate or hydrochloride) was administered sys- temically by slow intravenous injection at doses within the range 1-20 mg/kg (calculated as free morphine base), or applied topically to the cord by irrigating the dorsal cord surface with artificial CSF containing morphine at concentrations of base within the range 1-5 mM. Naloxone hydrochloride was administered intravenously at doses within the range 0.3-5.0 mg/kg (calculated as naloxone base).
After removal from the spinal cord, the microprobe tips (about 15 mm) were incubated in 125I-SP (2000 mCi//~mol) (Amersham), diluted to 2/~Ci/ml, for 36 h, and subsequently mounted on paper next to single emulsion X-ray film (Kodak NMC). Computerised microdensitometric analysis of each autoradiographic image pro- duced an image density scan for each microprobe, consisting of digitised optical density values plotted with respect to distance from the tip (Hendry et al. 1988). Regions of in vivo peptide release along the length of each microprobe were identifiable as relative deficits of tracer binding, producing zones of reduced optical density (peaks) on the scan. Mean image density scans were calculated for selected groups of microprobes subjected to the same experimental proce- dure, and differences in release between such groups were assessed by computerised analyses of the differences between their mean scans (Duggan et al. 1988b; Hendry et al. 1988). Peptide release was also quantified by ~easurement of the area of defined peaks of release on individual scans. For particular groups of microprobes, mean areas were statistically compared (Morton et al. 1988, 1989; Morton and Hutchison 1989).
Microprobes were also used in in vitro assays of sensitivity in conjunction with each experiment. In each assay, microprobe tips were incubated at 37~ in known concentrations of SP within the range 10-5M to 10-SM for 15 30 min, prior to incubation in 12sI- SP for 36 h. The radioactivity bound to the microprobe tips was then measured in a gamma counter. An in vitro concentration of un- labelled SP of 10-7M or greater consistently suppressed the sub- sequent binding of 12sI-SP.
Results
A to ta l of 394 a n t i b o d y mic rop robes were ana lysed in this study. Of these, 282 were used to detect i rSP release in vivo, and 112 were used for para l le l in vi t ro assays.
Systemic morphine, systemic naloxone
In the first series of exper iments (143 microprobes) , micro- p robes were used ini t ial ly to measure i rSP release in the spinal cord dur ing a pa r t i cu la r noxious pe r iphera l s t imu- la t ion p rocedure ( thermal , mechanica l or electrical) ap- pl ied for a pa r t i cu la r t ime (15-30 min). M i c r o p r o b e s were then used to measure i rSP release in response to the same per iphera l s t imulus, app l ied for the same time, a t var ious t imes fol lowing systemic m o r p h i n e admin i s t ra t ion . There was a poss ibi l i ty tha t only high systemic doses of mor - phine might reduce in t rasp ina l i rSP release (Kura ish i et al. 1983), an effect which could be masked by ca lcula t ing a mean image dens i ty scan of all m ic rop robes inser ted fol lowing m o r p h i n e admin i s t ra t ion . Consequent ly , the effects of m o r p h i n e on i rSP release were measu red with mic rop robes inser ted 4 - 9 0 min fol lowing low doses of m o r p h i n e (1 -6 mg /kg to ta l dose) and 4 - 1 8 0 min fol lowing high doses (10-20 mg/kg to ta l dose), t imes at which there
91
is pronounced suppression of feline spinal nociceptive transmission by intravenous morphine administration (Le Bars et al. 1975; Jurna and Grossmann 1976; Johnson and Duggan 1981, 1984). Subsequently, irSP release was meas- ured following naloxone administration (0.3-4.0 mg/kg total dose). Mean scans for low and high morphine dose ranges were calculated and compared with mean scans of the appropriate pre-morphine microprobes, and of post- naloxone microprobes.
A o-
B
C
D
E
13_ I1)
Co~ (0
if)
Q - � 9
(j (0
C ~ ro o~
I1)
C03 ro
m ~
Oj r'4 (j fO
c ~ ~o o~ 43
n
col fO 4~
m
t500"
3000
O"
PRE LOW DOSE MORPHINE
POST LOW DOSE MORPHINE
1500" " ~
300q 3 ~ i 6
0- PRE HIGH DOSE MORPHINE
t500"
3001 3 2 i 6
0]. POST HIGH DOSE MORPHINE ts00j~r ~
3 2 t 0
o-
POST NALOXONE
t500"
3ooo 3 ;~ i 6
Depth in Spinal Cord [mm)
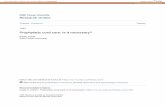
Figures 1A and 1C illustrate mean scans of micro- probes inserted 3 mm into the spinal cord during noxious peripheral stimulation (thermal, mechanical or electrical) prior to morphine administration. Both mean scans have a peak centred 1.1 mm from the dorsal cord surface, re- presenting irSP release in the region of the substantia gelatinosa in response to the noxious stimulation, as reported previously (Duggan and Hendry 1986; Duggan et al. 1988b). This noxiously-evoked release of irSP in the substantia gelatinosa was not reduced following intraven- ous administration of morphine in either the low dose range (1-6mg/kg) (Fig. 1B) or the high dose range (10-20 mg/kg) (Fig. 1D). Figure 1E illustrates the mean scan of microprobes inserted after the administration of naloxone (0.3-4.0 mg/kg) subsequent to all doses of mor- phine (1-20 mg/kg), where comparable irSP release in the substantia gelatinosa region was also detected. In addi- tion, all mean scans feature a smaller zone of release just beneath the dorsal cord surface, as described previously (Duggan et al. 1988b). This zone was also of similar magnitude in all scans. Thus, there were no significant differences among any of the mean scans, indicating comparable intraspinal irSP release in all microprobe groups.
The effect of systemic morphine and naloxone on irSP release in the substantia gelatinosa was analysed further by calculation of the area of the peak of release in this spinal region on individual scans. Such areas are a meas- ure of the total amount of peptide released at a particular site (Morton et al. 1988, 1989). The mean areas (and S.E.M.) under the peaks of irSP release in the substantia gelatinosa for each group of microprobes are plotted as histograms in Fig. 2. There were no significant differences in these mean areas.
Morphine superfusion, systemic naloxone
In the second series of experiments (139 microprobes), irSP release evoked by peripheral noxious stimuli was meas- ured firstly during irrigation of the dorsal cord surface
Fig. IA-E. Systemic administration of morphine, and morphine followed by naloxone, does not affect the intraspinal release of irSP produced by activation of nociceptive primary afferent fibres. The solid lines are mean image density scans of autoradiographs of antibody microprobes; the broken lines are the S,E.M. for each mean scan. Ordinates, digitised optical density values expressed as an arbitrary grey scale (see Methods); abscissae, depth of insertion into spinal cord (mm), with the cord surface at 0. All microprobes were inserted 3.0 mm into the lower lumbar spinal cord during nociceptive afferent stimulation (noxious cutaneous stimulation or high-inten- sity tibial nerve stimulation). A The mean scan (and S,E.M.) of 22 microprobes detecting irSP release prior to systemic administration of low doses of morphine. B The mean scan (and S.E.M.) of 19 microprobes detecting irSP release following morphine administra- tion (1-6 mg/kg i.v.). C The mean scan (and S.E.M.) of 22 micro- probes detecting irSP release prior to systemic administration of high doses of morphine. D The mean scan (and S.E.M.) of 17 microprobes detecting irSP release following morphine administra- tion (10-20 mg/kg i.v.). E The mean scan (and S.E.M.) of 24 microprobes detecting irSP release following administration of naloxone 0.3-4.0 mg/kg i.v.) subsequent to morphine (1 20 mg/kg i.v.)
92
400
E E X
r m" m
C
300
200
100
(59)
Control
(28)
(56)
)( O~))()l)()())))ji )0 ))))())Oli)(l)i)())i)( )F)O))():I
))))))))))f i!(F))))))))O))[)))) )0(0)))))))))))))))))) f)] ))))~))))))))))i))))) )?)J))) )))i))))))))))J!~))J))l
iiiil i!i !i!i!i:i'i!i) Morphine IV Naloxone IV
Fig. 2. Noxiously-evoked release of irSP in the substantia gelati- nosa region before and after systemic administration of morphine, and after naloxone administration subsequent to morphine. The histo- grams plot the mean area (and S.E.M.) under the peaks of irSP release in the substantia gelatinosa on individual image density scans of microprobes. The units of area are grey scale (GS) values x millimetres (mm). Microprobes were inserted 2.0 or 3.0 mm into
the lumbar cord prior to morphine administration (CONTROL histogram), following morphine administration 1-20 mg/kg (MOR- PHINE IV histogram), and following morphine (1-20 mg/kg) and naloxone (0.3 4.0 mg/kg) administration (NALOXONE IV histo- gram). Numbers of microprobes are shown in parentheses. Statistical comparisons by the Student's t-test for unpaired data showed no significant differences in the 3 mean areas
with n o r m a l CSF , and then dur ing superfus ion of the cord with C S F con ta in ing morph ine , commenc ing at least 30 min fol lowing the changeover f rom C S F to morph ine - CSF . Since s imi lar results were ob ta ined with all concen- t ra t ions of m o r p h i n e used (range 1-5 mM), m i c r o p r o b e scans f rom all exper iments have been pooled. F u r t h e r
Fig. 3 ~ D . The effect of morphine (1 5 mM), superfused over the lumbar cord dorsal surface, on intraspinal irSP release produced by activation of nociceptive primary afferents. All microprobes were inserted 2.0 mm into the lower lumbar spinal cord. A The mean image density scan (and S.E.M.) of 30 microprobes detecting irSP release in response to nociceptive afferent stimulation (cutaneous or electrical) during cord superfusion with normal CSF. Ordinate and abscissa as for Fig. 1. B The mean scan (and S.E.M.) of 41 microprobes detecting irSP release in response to nociceptive stimu- lation during cord superfusion with morphine-CSF. C The differ- ences between the mean scans plotted in A and B. The digitised optical density values of mean scan B were subtracted from the corresponding values of mean scan A, in 16 #m intervals along the scans, so that decrements in irSP release during morphine superfu- sion are represented as upward deflections. D The t-values calculated for the differences between the mean scans A and B. The broken line is the t-value for significance at P=0.05
measurement s of i rSP release were m a d e fol lowing in t ra- venous admin i s t r a t i on of na loxone (1-5 mg /kg to ta l dose) dur ing con t inued superfusion of the cord with morphine .
As before, the nox ious pe r iphera l s t imula t ion evoked i rSP release at 2 in t rasp ina l sites; a m a j o r zone in the subs tan t i a ge la t inosa region and a smal ler zone jus t benea th the dorsa l cord surface (Fig. 3A). Dur ing superfu- sion with m o r p h i n e - C S F , the smal ler zone of i rSP release at the cord do r sum was not observed, and the peak of release in the subs tan t i a ge la t inosa region, a l though of
A
B
C
D
o] PRE MORPHINE SUPERFUSION
1 o �9 i!!iiiiiiiiiii - / ............ "-... = o !ii!ii!iiii!i ;i;i, C0 ~ 1500
=8 m
30001 , , 2 t 0
n 111
i1~ ,--.4
COO
ffl
Q- QJ Q)~-4
C03 ~0
CO
EL (J
QJ.~J L) 03 C . r - I
4J f0
03
o 1
t500-
3000
MORPHINE SUPERFUSION
i 6
750 -
0-
-750
DIFFERENCE V V "
i 6
3.0- t-VALUES
0.0,~- t 0
Depth in Spinal Cord (ram)
93
similar height, was not as broad (Fig. 3B). These differ- ences are readily seen in the plot of the differences between the mean scans of Figs. 3A,B, where the reduction in the shoulders of the substantia gelatinosa peak of release have been highlighted with arrowheads (Fig. 3C). This probably reflects a reduction in the spread of released irSP dorsal and ventral from the actual release site. The differences between the means were statistically significant at the cord dorsum, but not in the substantia gelatinosa (Fig. 3D). Administration of naloxone during continued morphine superfusion did not reverse these effects of morphine on irSP release in the substantia gelatinosa region or at the cord surface. Thus, the mean scan of microprobes detect- ing irSP release following naloxone administration (not illustrated) was not significantly different from that of microprobes inserted prior to naloxone administration (Fig. 3B).
The effect of cord superfusion with morphine on irSP release in the substantia gelatinosa was also examined by calculation of the mean area under the peak of release in this spinal region on single scans of microprobes. Figure 4 illustrates these mean area data (and S.E.M.) as histograms for each group of microprobes. The mean areas under the
400
E E X (/) (9
r
300
200
100
(66)
iii~i~ii!~ii~i~i~L~ii~i~i~i~iiiiii!iiiii!iiiiiiiLiiii!iiii!iiiiiii I ii!i!iiii!iiiiiiiiiiiiiiiiiiii!iiiiiiii
iiiiiiiiiiii ii!iiiiiiiiiiii iii iiiiiii :i!iii iiiiiiiiiiiiiiiiii!iii!iii!@iiiii~iiiiiiiiiiiiiiiiiii:ii~i~i~ilil
iiiiiiiiiiiiiiiiiiiiiili!i!iiiiiiiiiiiiii@ii}iiiiiiiiii;iiii !iiii!iiiiiiii!iiiiiii!i!iiiii!iiiii!iiiiiiiiiiiiiiiiiiiiiiiili~:~:~,l i!iiiii!iiiiiii!!i!!!iiiii!iiiililili!iii!iiiiiiiiiiiiiii!iiii:i:t
ii?iiii!iiiii!?iii!ii!iiiiiiii?iiii';ii!iiiiiiiiiiiiiiiiiil
Control Morphine SF
(22)
7// / / / /~ s / - ' ~ / / J l i ~ . , , ' / / / . J / / / j , ' 7 / / / / / / /
~///////A , " / / / / / / / / / / / / / / / / / / / / / / / / / /
Naloxone IV
Fig. 4. Noxiously-evoked release of irSP in the substantia gelati- nosa region during lumbar cord superfusion with morphine, and following systemic naloxone administration during continued mor- phine superfusion. The histograms plot the mean area (and S.E.M.) under the peaks of irSP release in the substantia gelatinosa on single image density scans of microprobes inserted 2.0 or 3.0 mrn into the lower lumbar cord. The units of area are as for Fig. 2. Mean areas were calculated for evoked irSP release prior to morphine superfu- sion (CONTROL histogram), during morphine superfusion (pooled data from all concentrations, 1-5 mM) (MORPHINE SF histo- gram), and following naloxone administration (1 5 mg/kg i.v.) dur- ing morphine superfusion (NALOXONE IV histogram). Numbers of microprobes are shown in parentheses. Statistical comparisons by the Student's t-test for unpaired data revealed no significant differ- ences in the 3 mean areas
substantia gelatinosa peak during morphine superfusion and following naloxone administration during morphine superfusion were 91% and 89% of the pre-morphine mean area, respectively. These small decreases were not statis- tically significant.
Discussion
These experiments indicate that the release of irSP in the substantia gelatinosa of the lumbar cord of cats produced by impulses in nociceptive primary afferents is not altered by systemically administered morphine in doses more than adequate to produce analgesia (McKenzie and Beechey 1962; Watts et al. 1973), and to suppress the nociceptive excitation of lumbar dorsal horn neurones (Le Bars et al. 1975; Johnson and Duggan 1981), in this species. More- over, superfusion of the dorsal cord surface with millimo- lar concentrations of morphine only slightly reduced the evoked release ofirSP in this spinal region. The irSP in the superficial laminae of the dorsal horn is largely of primary afferent origin; a proportion, however, is derived from intrinsic spinal neurones (Takahashi and Otsuka 1975; Ljungdahl et al. 1978; Barber et al. 1979). Although the present experiments cannot determine relative contribu- tions of primary afferents and spinal neurones to the irSP release observed, there is considerable other evidence that irSP release in the substantia gelatinosa during nocicep- tion originates from the central terminals of primary afferent fibres (see Discussion and references in Duggan et al. 1988b). The present findings have important implica- tions for current concepts of the mechanisms of the antinociceptive action of opioids in the spinal cord, as discussed below.
Due to technical limitations, much of the evidence for an opioid modulation of SP release from the central terminals of nociceptors has been indirect and the findings are not conclusive. In some in vitro studies, opioids including morphine have reduced the potassium- or elec- trically-evoked release of irSP from rat trigeminal slices (Jessell and Iversen 1977) and isolated rat dorsal spinal cord (Lembeck and Donnerer 1985). However, more recent studies with rat lumbar cord slices have shown that irSP release evoked by superfusion with potassium, vera- tridine, or capsaicin was increased by #-opioid agonists including morphine and decreased by 6-opioid agonists (Mauborgne et al. 1987; Pohl et al. 1989). The interpreta- tion of these differing findings is complicated by un- certainties regarding the source of the released irSP and by the type of stimulus employed to produce release. In rats virtually devoid of SP-containing afferent fibres due to neonatal capsaicin treatment, although irSP release evoked from lumbar cord slices by capsaicin was markedly decreased, that evoked by potassium was much less affec- ted, suggesting a neuronal origin of irSP other than from nociceptor terminals (Pohl et al. 1989). Thus, it may be unwise to infer that opioid modulation of SP release evoked from in vitro preparations by high potassium concentrations or electrical field stimulation necessarily occurs by altered SP release from the central terminals of nociceptors. Opioids have also produced some inhibition
94
of irSP release from cultured dorsal root ganglion neu- rones, associated with a decreased duration of somatic calcium-dependent action potentials (Mudge et al. 1979; Werz and Macdonald 1982; Chang et al. 1989; Shen and Crain 1989), but it is difficult to evaluate the extent to which these observations on a cultured heterogeneous cell population reflect the action of opioids in the spinal cord under in vivo conditions.
The present results are in partial agreement with previous findings of in vivo release experiments using the push-pull cannula technique. In spinalised rabbits, release of irSP in the dorsal horn evoked by noxious cutaneous stimulation was not affected by morphine administered intravenously in analgesic (1 mg/kg) or supra-analgesic (10 mg/kg) doses (Kuraishi et al. 1983), but was reduced, in a naloxone-reversible manner, by the administration of morphine or met-enkephalin (10 #M) through the perfu- sion system (Hirota et al. 1985). The size of the push-pull cannula relative to the dorsal horn, however, not only precluded identification of the intraspinal region of release (Kuraishi et al. 1983; Hirota et al. 1985), but its use is associated with substantial trauma to the cord (Sorkin et al. 1988). In rats and cats, the release of irSP into super- fusates of the lumbar cord surface following electrical stimulation of unmyelinated primary afferent fibres was blocked by the addition of morphine (0.1-1.0 mM) to the superfusing artificial CSF solution, an effect reversed by naloxone administered systemically (Yaksh et al. 1980) or added to the perfusing solution (Go and Yaksh 1987). As with the push-pull cannula experiments, the location of the irSP release site could not be determined. It is noteworthy that in the present and previous (Duggan et al. 1988b) experiments, microprobes have detected a zone of appar- ent irSP release at or just beneath the cord dorsum. The significance of this release is not clear, but it has been suggested to originate from pial afferents active in a localised inflammatory response in the area of pial perfora- tion (see discussion in Duggan et al. 1988b). The abolition of this region of irSP release by morphine superfusion of the cord raises the possibility that at least part of mor- phine-susceptible irSP release detected in cord super- fusates (Yaksh et al. 1980; Go and Yaksh 1987) may have derived from pial nerves rather than the terminals of nociceptors in the substantia gelatinosa, although a con- tribution from the latter cannot be excluded. Placement of a spinal catheter is likely to produce some trauma to the pia arachnoid. The naloxone reversal of the effect of morphine on irSP release in previous superfusion studies (Yaksh et al. 1980; Go and Yaksh 1987) was, however, not observed in the present experiments with either zone of irSP release. It is therefore unlikely that in this study, the effects of cord superfusion with morphine, which were not observed with intravenous morphine over a wide dose range, resulted from an action on opioid receptors. A more plausible explanation is that the high local concentrations of morphine may have abolished surface release and slightly decreased substantia gelatinosa release by a par- tial block of conduction in. primary afferent fibres. From studies of peripheral nerve function during the topical application of morphine, it appears that while low concen- trations (1.3-20 #M) have no effect on conduction (Yuge et
al. 1985; Senami et al. 1986), increased concentrations (I00 ~tM) can produce alterations in compound action potentials and refractory periods in cutaneous nerve (Jurna and Grossmann 1977). High morphine concentra- tions (2.8-8.6 mM) can produce complete block of C-fibre conduction, reversible by washout but not by naloxone (Gilly et al. 1985), and these concentrations were similar to those used for the present cord superfusion experiments.
The question of presynaptic modulation of transmis- sion from nociceptive primary afferents by opioids has also been investigated in studies of the excitability of the terminal regions of these fibres. Release of GABA at axo- axonic synapses onto the terminals of myelinated primary afferents produces depolarisation and increased terminal excitability, decreasing the amount of transmitter released by afferent impulses (presynaptic inhibition) (Schmidt 1971). In the cat, opioids administered intravenously (morphine, meperidine) or microelectrophoretically in the (presumed) region of the central terminals of cutaneous A6 and C fibres (morphine, met-enkephalin) decreased the terminal excitability of these fibres and increased primary afferent depolarisation induced by peripheral nerve stimu- lation, effects which were naloxone-reversible in some (Sastry 1978, 1979a,b) but not all (Carstens et al. 1979) studies. Although it was suggested that opioids might thus potentiate presynaptic inhibition of nociceptive pathways (Sastry 1978, 1979a,b), there are considerable problems with the interpretation of such studies, such as the circula- tory effects of systemic administration of opioids and opioid antagonists and the uncertain relevance of concen- trations following microelectrophoretic administration to those attained by systemic administration of analgesic doses (see Duggan and North 1984). These difficulties indicate that the use of more direct methods of release measurement (such as the antibody microprobe) has con- siderable advantages for investigations of pharmacolog- ical modulation of release from nociceptive primary affer- ent fibres.
The presence of opioid receptors on primary afferent fibres appears to be indicated since dorsal rhizotomy or neonatal capsaicin treatment produces a 30-50% loss of opioid binding sites in the superficial dorsal horn and on dorsal roots (Lamotte et al. 1976; Fields et al. 1980; Nagy et al. 1980; Ninkovic et al. 1981). These sites are unlikely to comprise the post-synaptic elements of axo-axonic syn- apses since these structures are rare in the superficial dorsal horn (Barber et al. 1978; Duncan and Morales 1978; Zhu et al. 1981), and although it is conceivable that circulating opioids could act at these sites, the functional significance of activation of receptors remote from ter- minals is uncertain. Moreover, it is not known what proportion of nociceptive primary afferent fibres bear opioid receptors, and what relationships exist between irSP content and the presence or absence of these re- ceptors. Assuming that the irSP release observed in the present experiments was of primary afferent origin, our findings suggest either that sensory fibres with opioid receptors do not contain irSP, or that activation of these receptors does not suppress irSP release from their central terminals. A fundamental problem is that the extent to which SP participates in nociceptive transmission is still
95
not clear, and it is conceivable that opioid analgesia might involve a reduction in release of other compounds from the central terminals of nociceptive primary afferents. These could include other neuropeptides known to be released within the dorsal horn during nociception such as somatostatin (Morton et al. 1989), calcitonin gene-related peptide (Morton and Hutchison 1989), and neurokinin A (Duggan et al. 1990), or amino acids such as glutamate which occurs in vesicles within terminals of small diameter afferent fibres (De Biasi and Rustioni 1988). Whether this is the function of primary afferent opioid receptors re- mains to be determined.
In view of the present results and the existing literature, we believe that the antinociceptive effect of morphine in the spinal cord is most likely to be mediated by an action on structures located beyond the first central synapse. A substantial proport ion (50-70%) of opioid binding sites in the superficial dorsal horn remain after deafferentation (Lamotte et al. 1976; Ninkovic et al. 1981), and intrinsic spinal neurones, with their cell bodies in the substantia gelatinosa, have been found to project to neurones of laminae III, IV and V (Light and Kavookjian 1988). Moreover, many neurones of the substantia gelatinosa are hyperpolarised by opioids including normorphine (Yoshimura and Nor th 1983). A post-synaptic action of opioids on these superficial cells would interrupt the transmission of nociceptive information to deeper dorsal horn neurones, thereby selectively inhibiting their nocicep- tive excitation (Duggan et al. 1977, 1981). Alternatively, opioids could act directly on nociceptive projection neu- rones, since a significant proport ion of spinothalamic tract cells in laminae I and V are contacted by enkephalin- containing terminals (Ruda et al. 1984). Moreover, the caudally-directed scratching/biting behaviour evoked in unanaesthetised rodents by intrathecal SP administration, a behavioural response suggestive of nociception but independent of SP release, is inhibited by intrathecal administration of opioids (Hylden and Wilcox 1983). Finally, a proposed post-synaptic action of morphine in the spinal cord is further supported by the observation that although morphine reduced the excitatory responses of dorsal horn neurones to noxious cutaneous stimulation, inhibitory responses to such stimulation were unaffected, a result inconsistent with an action presynaptically on noci- ceptive primary afferents (Harris and Ryall 1988).
Apart from opioids, there is evidence that the nox- iously-evoked release of irSP in the substantia gelatinosa is not under the control of descending fibres (Duggan et al. 1988c) nor of segmental afferent inputs (Hutchison and Mor ton 1989). The results of the present study suggest that such release of irSP is also unaffected by morphine, and therefore offer no support for the notion that the analgesic actions of this opiate in the spinal cord occur through reduced release of SP in the superficial dorsal horn.
Acknowledgements. This work was supported by grants from the National Health and Medical Research Council of Australia and the Wenkart Foundation. The authors wish to thank A. Turudic, J. McGovern and S. Ford for expert assistance with this work, and R. M. Crane for the manuscript preparation.
References
Barber RP, Vaughn JE, Saito K, McLaughlin BJ, Roberts E (1978) GABA-ergic terminals are presynaptic to primary afferent ter- minals in the substantia gelatinosa of the rat spinal cord. Brain Res 141:35-55
Barber RP, Vaughn JE, Slemmon JR, Salvaterra PM, Roberts E, Leeman SE (1979) The origin, distribution and synaptic relation- ships of substance P axons in rat spinal cord. J Comp Neurol 184: 331-352
Carstens E, Tulloch I, Zieglg/insberger W, Zimmermann M (1979) Presynaptic excitability changes induced by morphine in single cutaneous afferent C- and A-fibers. Pfl/igers Arch 379:143-147
Chang HM, Berde CB, Holz GG, Steward GF, Kream RM (1989) Sufentanil, morphine met-enkephalin, and kappa-agonist (U-50, 488H) inhibit substance P release from primary sensory neurons: a model for presynaptic spinal opioid actions. Anesthesiology 70: 672-677
De Biasi S, Rustioni A (1988) Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci USA 85:7820 7824
Duggan AW, Hall JG, Headley PM (1977) Suppression of transmis- sion of nociceptive impulses by morphine: selective effects of morphine administered in the region of the substantia gelatinosa. Br J Pharmacol 61:65-76
Duggan AW, Hendry IA (1986) Laminar localization of the sites of release of immunoreactive substance P in the dorsal horn with antibody-coated microelectrodes. Neurosci Lett 68:134-140
Duggan AW, Hendry IA, Green JL, Morton CR, Hutchison WD (1988a) The preparation and use of antibody microprobes. J Neurosci Meth 23:241-247
Duggan AW, Hendry IA, Morton CR, Hutchison WD, Zhao ZQ (1988b) Cutaneous stimuli releasing immunoreactive substance P in the dorsal horn of the cat. Brain Res 451:261-273
Duggan AW, Hope P J, Jarrott B, Schaible HG, Fleetwood-Walker SM (1990) Release, spread and persistence of immunoreactive neurokinin A in the dorsal horn of the cat following noxious cutaneous stimulation: studies with antibody microprobes. Neuroscience (in press)
Duggan AW, Johnson SM, Morton CR (1981) Differing distribu- tions of receptors for morphine and met5-enkephalinamide in the dorsal horn of the cat. Brain Res 229:379-387
Duggan AW, Morton CR, Hutchison WD, Hendry IA (1988c) Absence of tonic supraspinal control of substance P release in the substantia gelatinosa of the anaesthetized cat. Exp Brain Res 71: 597-602
Duggan AW, Morton CR, Zhao ZQ, Hendry IA (1987) Noxious heating of the skin releases immunoreactive substance P in the substantia gelatinosa of the cat: a study with antibody micro- probes. Brain Res 403:345-349
Duggan AW, North RA (1984) Electrophysiology of opioids. Phar- macol Rev 35:219-281
Duncan D, Morales R (1978) Relative numbers of several types of synaptic connections in the substantia gelatinosa of the cat spinal cord. J Comp Neurol 182:601 610
Fields HL, Emson PC, Leigh BK, Gilbert RFT, Iversen LL (1980) Multiple opiate receptor sites on primary afferent fibres. Nature 284:351-353
Gilly H, Kramer R, Zahorovsky I (1985) "Lokalanaesthetische" Effekte von Morphin und Naloxon. Anaesthesist 34: 619-626
Go VLW, Yaksh TL (1987) Release of substance P from the cat spinal cord. J Physiol (Lond) 391:141-167
Harris NC, Ryall RW (1988) Opiates distinguish spinal excitation from inhibition evoked by noxious heat stimuli in the rat: relevance to theories of analgesia. Br J Pharmacol 94:185-191
Hendry IA, Morton CR, Duggan AW (1988) Analysis of antibody microprobe autoradiographs by computerized image processing. J Neurosci Meth 23:249-256
Hirota N, Kuraishi Y, Hino Y, Sato Y, Satoh M, Takagi H (1985) Met-enkephalin and morphine but not dynorphin inhibit nox- ious stimuli-induced release of substance P from rabbit dorsal
96
horn in situ. Neuropharmacology 24:567-570 Hutchison WD, Morton CR (1989) Electrical stimulation of primary
afferent A fibres does not reduce substance P release in the dorsal horn of the cat. Pain 37:357 363
Hylden JLK, Wilcox GL (1983) Pharmacological characterisation of substance P-induced nociception in mice: modulation by opioid and noradrenergic agonists at the spinal level. J Pharmacol Exp Ther 226:398-404
Jessell TM, Iversen LL (1977) Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature 268:549-551
Johnson SM, Duggan AW (1981) Evidence that the opiate receptors of the substantia gelatinosa contribute to the depression, by intravenous morphine, of the spinal transmission of impulses in unmyelinated primary afferents. Brain Res 207:223-228
Johnson SM, Duggan AW (1984) Dependence in the absence of tolerance to morphine. Eur J Pharmacol 97:305-308
Jurna I, Grossmann W (1976) The effect of morphine on the activity evoked in ventrolateral tract axons of the cat spinal cord. Exp Brain Res 24:473 484
Jurna I, Grossmann W (1977) The effect of morphine on mammalian nerve fibres. Eur J Pharmacol 44:339-348
Kuraishi Y, Hirota N, Sugimoto M, Satoh M, Takagi H (1983) Effects of morphine on noxious stimuli-induced release of sub- stance P from rabbit dorsal horn in vivo. Life Sci 33 Suppl 1: 693-696
Lamotte C, Pert CB, Snyder SH (1976) Opiate receptor binding in primate spinal cord: distribution and changes after dorsal root section. Brain Res 112:407-412
Le Bars D, Menetrey D, Conseiller C, Besson JM (1975) Depressive effects of morphine upon lamina V cells activities in the dorsal horn of the spinal cat. Brain Res 98:261-277
Lembeck F, Donnerer J (1985) Opioid control of the function of primary afferent substance P fibres. Eur J Pharmacol 114: 241-246
Light AR, Kavookjian AM (1988) Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neu- rons with axons that terminate in deeper dorsal horn laminae (Ill-V). J Comp Neurol 267:172-189
Ljungdahl A, H6kfelt T, Nilsson G (1978) Distribution of substance P-like immunoreactivity in the central nervous system of the rat. I. Cell bodies and nerve terminals. Neuroscience 3:861-943
Mauborgne A, Lutz O, Legrand JC, Hamon M, Cesselin F (1987) Opposite effects of delta and mu opioid receptor agonists on the in vitro release of substance P-like material from the rat spinal cord. J Neurochem 48:529-537
McKenzie JS, Beechey NR (1962) The effects of morphine and pethidine on somatic evoked responses in the midbrain of the cat, and their relevance to analgesia. Electroenceph Clin Neuro- physiol 14:501-519
Morton CR, Hutchison WD (1989) Release of sensory neuropeptides in the spinal cord: studies with calcitonin gene-related peptide and galanin. Neuroscience 31:807-815
Morton CR, Hutchison WD, Hendry, IA (1988) Release of immuno- reactive somatostatin in the spinal dorsal horn of the cat. Neuropeptides 12:189-197
Morton CR, Hutchison WD, Hendry IA, Duggan AW (1989) Somatostatin: evidence for a role in thermal nociception. Brain Res 488:89-96
Mudge AW, Leeman SE, Fischbach GD (1979) Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci USA 76: 526-530
Nagy JI, Vincent SR, Staines WmA, Fibiger HC, Reisine TD,
Yamamura HI (1980) Neurotoxic action of capsaicin on spinal substance P neurons. Brain Res 186:435-444
Ninkovic M, Hunt SP, Kelly JS (1981) Effect of dorsal rhizotomy on the autoradiographic distribution of opiate and neurotensin receptors and neurotensin-like immunoreactivity within the rat spinal cord. Brain Res 230:111-119
Pert CB, Kuhar M J, Snyder SH (1975) Autoradiographic localisa- tion of the opiate receptor in rat brain. Life Sci 16:1849-1853
Pohl M, Mauborgne A, Bourgoin S, Benoliel J J, Hamon M, Cesselin F (1989) Neonatal capsaicin treatment abolishes the modulations by opioids of substance P release from rat spinal cord slices. Neurosci Lett 96:102-107
Ruda MA, Coffield J, Dubner R (1984) Demonstration of postsyn- aptic opioid modulation of thalamic projection neurons by the combined techniques of retrograde horseradish peroxidase and enkephalin immunocytochemistry. J Neurosci 4:2117-2132
Sastry BR (1978) Morphine and met-enkephalin effects on sural A6 afferent terminal excitability. Eur J Pharmacol 50:269-273
Sastry BR (1979a) Presynaptic effects of morphine and methionine- enkephalin in feline spinal cord. Neuropharmacology 18: 367-375
Sastry BR (1979b) Potentiation of presynaptic inhibition of nocicep- tive pathways as a mechanism for analgesia. Can J Physiol Pharmacol 58:97 101
Schmidt RF (1971) Presynaptic inhibition in the vertebrate central nervous system. Rev Physiol Biochem Pharmacol 63:20-101
Senami M, Aoki M, Kitahata LM, Collins JG, Kumeta Y, Murata K (1986) Lack of opiate effects on cat C polymodal nociceptive fibers. Pain 27:81-90
Shen K-F, Crain SM (1989) Dual opioid modulation of the action potential duration of mouse dorsal root ganglion neurons in culture. Brain Res 491:227-242
Sorkin LS, Steinman JL, Hughes MG, Willis WD, McAdoo DJ (1988) Microdialysis recovery of serotonin released in spinal cord dorsal horn. J Neurosci Meth 23:131 138
Takahashi T, Otsuka M (1975) Regional distribution of substance P in the spinal cord and nerve roots of the cat and the effect of dorsal root section. Brain Res 87:1-11
Watts S J, Slocombe RF, Harbison WD, Stewart GA (1973) Assess- ment of analgesia and other effects of morphine and thiambutene in the mouse and cat. Aust Vet J 49:525-529
Werz MA, Macdonald RL (1982) Heterogeneous sensitivity of cultured dorsal root ganglion neurones to opioid peptides selec- tive for/t- and 6-opiate receptors. Nature 299:730-733
Yaksh TL (1981) Spinal opiate analgesia: characteristics and prin- ciples of action. Pain 11 : 293-346
Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman SE (1980) Intrathecal morphine inhibits substance P release from mamma- lian spinal cord in vivo. Nature 286:155-157
Yaksh TL, Noueihed R (1985) The physiology and pharmacology of spinal opiates. Ann Rev Pharmacol Toxicol 25:433-462
Yonehara N, Shibutani T, Tsai HY, Inoki R (1986) Effects of opioids and opioid peptide on the release of substance P-like material induced by tooth pulp stimulation in the trigeminal nucleus caudalis of the rabbit. Eur J Pharmacol 129:209-216
Yoshimura M, North RA (1983) Substantia gelatinosa neurones hyperpolarised in vitro by enkephalin. Nature 305:529-530
Yuge O, Matsumoto M, Kitahata LM, Collins JG, Senami M (1985) Direct opioid application to peripheral nerves does not alter compound action potentials. Anesth Analg 64:667-671
Zhu CG, Sandri C, Akert K (1981) Morphological identification of axo-axonic and dendro-dendritic synapses in the rat substantia gelatinosa. Brain Res 230:25-40