physical understanding of ferrite grain growth during welding ...
Influence of Ni 2+ substitution on the structural, dielectric and magnetic properties of Cu–Cd...
Transcript of Influence of Ni 2+ substitution on the structural, dielectric and magnetic properties of Cu–Cd...
This article appeared in a journal published by Elsevier. The attachedcopy is furnished to the author for internal non-commercial researchand education use, including for instruction at the authors institution
and sharing with colleagues.
Other uses, including reproduction and distribution, or selling orlicensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of thearticle (e.g. in Word or Tex form) to their personal website orinstitutional repository. Authors requiring further information
regarding Elsevier’s archiving and manuscript policies areencouraged to visit:
http://www.elsevier.com/authorsrights
Author's personal copy
Influence of Ni2+ substitution on the structural, dielectric and magneticproperties of Cu–Cd ferrite nanoparticles
Mohd. Hashim a,⇑, Alimuddin a, Sagar E. Shirsath b, R.K. Kotnala c, Sher Singh Meena d, Shalendra Kumar e,Aashis Roy f, R.B. Jotania g, Pramod Bhatt d, Ravi Kumar h
a Department of Applied Physics, Aligarh Muslim University, Aligarh 202 002, Indiab Spin Device Technology Centre, Department of Information Engineering, Shinshu University, Nagano 380-8553, Japanc National Physical Laboratory (CSIR), Dr. K.S. Krishnan Road, New Delhi 110 012, Indiad Solid State Physics Division, Bhabha Atomic Research Centre, Mumbai 400 085, Indiae Department of Physics, Pohang University of Science and Technology, Pohang 790 784, Republic of Koreaf Department of Materials Science, Gulbarga University, Gulbarga 585106, Karnataka, Indiag Department of Physics, University School of Sciences, Gujarat University, Ahmedabad 380 009, Indiah Centre for Material Science Engineering, National Institute of Technology, Hamirpur, HP 171 005, India
a r t i c l e i n f o
Article history:Received 12 January 2013Received in revised form 4 March 2013Accepted 9 March 2013Available online 19 March 2013
Keywords:NanoparticlesSpinel ferriteDielectric responseM–T
a b s t r a c t
Nanoparticles with compositions of Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 (0.0 6 x 6 0.5) were successfully syn-thesized by a citrate–nitrate sol–gel auto combustion route. The combusted powder was calcinated atfour hours in a furnace and then slowly cooled to room temperature. The analysis methods of FTIR,XRD, FESEM, VSM and dielectric measurements were used to characterize prepared magnetic particles.The effect of Ni2+ substitution on structural, magnetic and dielectric properties of Cu–Cd ferrite nanopar-ticles was studied. The comprehensive studies on compositional and frequency dependent dielectricproperties were carried out by means of AC conductivity (rac), imaginary dielectric constant (e0 0), loss tan-gent (tand), impedance and dielectric modulus (real and imaginary) measurements in frequency range of50 Hz–5 MHz at room temperature. The structural properties investigated by using X-ray diffraction,scanning electron microscopy, and Fourier transform infrared spectroscopy. X-ray diffraction patternand FTIR results revealed that synthesized samples are in single phase. It is observed that the dielectricconstant (e0 0) and dielectric loss (tand) value decreases with increase in Ni2+ concentration (x). At low fre-quency the Maxwell type interfacial polarization was observed. Magnetization measurement shows thatthe Curie temperature of the samples increases with Ni2+ concentration, which is explained by a changein the A–O–B super exchange interaction.
� 2013 Published by Elsevier B.V.
1. Introduction
Nanomaterials are materials possessing one or more dimen-sional features having a length on the order of a nanometer. Theymanifest extremely fascinating and useful properties due to nanodimensions and which can be exploited for a variety of engineeringtechnologies, nano photonics, solar energy conversion, opto-elec-tronics, sensors, labeling, catalysis, and in medical applications[1,2]. The volume to surface area ratio is high in nanomaterials effi-ciently enhance the physical, chemical, and magnetic propertiesand often differ from those of bulk materials [3]. Ferrites are tech-nologically important materials because of their high magneticpermeability and low core losses. Nanocrystalline ferrites studiedintensively due to their potential applications in radio-frequency,
high quality noise filters, resonant devices, transformers chokeand digital read-write heads. Nickel ferrite is one of the mostimportant spinel ferrites which have attractive properties for theapplications [4]. Nickel ferrite crystallizes in an inverse spinelstructure and exhibits tunable conducting behavior [5]. The copperferrite CuFe2O4 is also early known to be mixed inverse spinel(CuxFe1�x)[Cu1�xFe1+x]O4, depending on the method of preparationand cooling process. The possibility of the reduction of Cu2+ andCu1+ in the process of preparation of copper-containing ferritescould probably have influenced the behavior of these compounds.The presence of Cu1+ enhances the appearance of p-type conductiv-ity of these ferrites which pointed out, that the variation of theslopes in the r vs. 1/T curve leads to the phase transition fromtetragonal to the cubic phase. This fact determines the abnormalbehavior of the dielectric parameters as a function of frequencyand temperature [6]. It has been observed that the electricalresistivity of many ferrites changes remarkably by controlling the
0925-8388/$ - see front matter � 2013 Published by Elsevier B.V.http://dx.doi.org/10.1016/j.jallcom.2013.03.029
⇑ Corresponding author. Tel.: +91 9359380185.E-mail address: [email protected] (Hashim).
Journal of Alloys and Compounds 573 (2013) 198–204
Contents lists available at SciVerse ScienceDirect
Journal of Alloys and Compounds
journal homepage: www.elsevier .com/locate / ja lcom
Author's personal copy
sintering temperature and substitution with magnetic and nonmagnetic ions. Investigation revealed that the Curie temperature,dielectric and magnetic properties of spinel ferrite nanoparticlesare sensitive to composition fraction and particle size. In this arti-cle we report the effect of Ni2+ substitution on the structural, Curietemperature, dielectric properties and site distribution of Cu–Cdspinel ferrite prepared by using a citrate–nitrate auto combustionsol–gel route. Systematic investigation on frequency dependentdielectric properties, AC conductivity and temperature dependentmagnetic properties would be very useful to understand types ofcharge carrier involved in polarization process and magneticordering.
2. Experimental procedure
2.1. Sample preparation
Nanoparticles of mixed ferrite Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 (0.0 6 x 6 0.5)were synthesized by an auto-combustion method by using Ni(NO3)2�6H2O,Cu(NO3)2�6H2O, Cd(NO3)2�4H2O, C6H8O7H2O and Fe(NO3)3�9H2O as precursors. Themetal nitrates were dissolved together in a minimum amount of water in orderto obtain a clear solution. The details of the synthesis process are given in our pre-vious publications [7,8]. Fig. 1 shows the actual photograph when the combustiontakes place in the sample.
2.2. Sample characterization
The structural analysis was performed on X-ray diffraction (XRD) by using theCu Ka radiation (k = 1.5405 Å) on a Rigaku X-ray diffractometer (Rigaku MiniflexII) operated at 40 kV and 35 mA). Scanning electron microscope (TESCAN; MIRA IILMH) was used to observe the microstructure of the fracture surface of the sinteredsamples. Fourier Transform Infra red spectra (FTIR) of the calcinated particles wererecorded from 400 to 800 cm�1 by the KBr pellet method using a Shimadzu FTIR-8400S spectrophotometer in order to study the functional groups. The pellets areprepared by mixing 0.8 mg of sample and 120 mg of KBr powder in order to elim-inate the scattering effects. The mixed powder is pressed into a pellet size appositefor the instrument. The capacitance, dielectric and impedance spectroscopy mea-surements were carried out between the frequency ranges of 50 Hz–5 MHz usingLCR HI-Tester (HIOKI 3532-50).
3. Results and discussion
3.1. Structural properties
Fig. 2 shows the X-ray diffraction pattern of calcinated Ni2+
substituted Cu–Cd ferrite samples synthesized by using a citrate–nitrate auto combustion sol–gel route. The existence of the (220),(311), (400), (422), (511), (440) and (533) major lattice planesin the XRD pattern confirms the formation of spinel cubic structure
with the Fd3m space group. The XRD pattern for lower concentra-tion of Ni2+ ions indicate that the powder is mainly composed oftetragonal structure of CuFe2O4 (JCPDS card no. 34-425). As the con-centration of Ni2+ substitution is increased, waning of diffraction
Fig. 1. Photographs of Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 ferrite nanoparticles when combustion takes place.
20 30 40 50 60 70 80
Inte
nsity
Fe2O
3
CuFe2O
4
(533
)
(422
)
(440
)
(511
)
(400
)
(311
)
(220
)
(f)
(e)
(d)
(c)
(b)
(a)
2 θ (degree)
Fig. 2. XRD patterns of Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 ferrite nanoparticles for (a)x = 0.0, (b) x = 0.1, (c) x = 0.2, (d) x = 0.3, (e) x = 0.4 and (f) x = 0.5.
0.0 0.1 0.2 0.3 0.4 0.5
22
23
24
25
26 t
Composition (x)
Cry
stal
lite
size
't' (
nm)
8.365
8.372
8.379
8.386
8.393
8.400
8.407
a
Lattice constant 'a' (Å
)
Fig. 3. Variation in lattice constant (a) and crystallite size (t) with the composition(x) of Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 (x = 0.0, 0.1, 0.2, 0.3, 0.4 and 0.5) ferritenanoparticles.
Mohd. Hashim et al. / Journal of Alloys and Compounds 573 (2013) 198–204 199
Author's personal copy
peaks attributed to the presence of CuFe2O4 in tetragonal crystalstructure. This tetragonal distortion is due to the Jahn–Teller effectof the Cu2+ ions located in the octahedral sites of spinel in a largeconcentration of Ni2+ ions [9,10]. Variation of lattice constant (a)and crystallite size (t) with composition (x) is shown in Fig. 3.
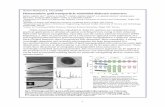
Fig. 4 shows the scanning electron micrographs (SEMs) of thesynthesized samples. The SEM images give the appearance of anon-uniform and a sponge structure with large number of pores.This might be due to the fact that the copper, nickel and cadmiumare immiscible with each other.
The FTIR bands of solids are usually assigned to the vibration ofions in the crystal lattice. Two main broad metal oxygen bands areobserved in the FTIR spectra for all spinels ferrites (Fig. 5). In spec-tra the important two characteristics absorption peaks were ap-peared (m1 and m2) at approximately 560 and 475 cm�1, whichare corresponds to the octahedral and tetrahedral sites [11] of spi-nel ferrite. Waldron [11] reported that the higher absorption band�600 cm�1 corresponds to the intrinsic vibrations of tetrahedralcomplexes and the lower absorption band at �400 cm�1 is attrib-uted to the vibrations of octahedral complexes. It is found thatthe change in band position is because of the difference in theFe3+–O2� distances for the tetrahedral and octahedral complexes.The FTIR shows small shifts in band position m1 and m2 withincreasing Ni2+ content. The absorption band m2 shifts to lower fre-quency side except for x = 0.4. This shift to lower frequency sidewith increase in Ni2+substitution is attributed to increase in bondlength on B-site. This suggests that, the Ni2+ ion occupies both tet-rahedral and octahedral site.
3.2. Dielectric properties
3.2.1. Frequency dependent dielectric measurementsIn case of ferrites, the revolution of Fe2+�Fe3+ dipoles can be
visualized as the exchange of electrons between the Fe ions so that
the diploes line up themselves with an applied AC field. The bond-ing of atoms that form a molecule may lead to a situation in whichthe molecule has a dipole moment even in the absence of an elec-tric field. A molecule with a permanent dipole moment suffers atorque in an electric field that tries to align the dipole with itself.Hence it is to be expected that the time constant in the processare longer than in those of other polarization mechanisms. The ori-entational polarization is indeed a low-frequency phenomenon,and optical waves do not contribute in polarizing a mediumthrough orientation of the molecules. An external field tends to ori-ent the permanent dipoles, but on the other hand their thermalmotion is a force that tries to preserve the randomness. The result-ing polarizability is linear for small field amplitudes and inverselyproportional to the temperature as was shown by Langevein andDebye [12,13]. A relaxation to the polarization is caused due tothe existence of inertia to charge moment. The frequency depen-dence of dielectric constant have been studied at room tempera-ture Figs. 6 and 7, shows the variation of imaginary dielectricconstant and dielectric loss tangent with the applied frequencies.It is observed that the dielectric dispersion occurs at lower fre-quency region for all the ferrite compositions and where imaginarydielectric constant (e0 0) decreases as the frequency increases from50 Hz to 1 MHz at that it remains constant. The dielectric constantdecreases rapidly in low frequency region and the velocity of de-crease is sluggish in high frequency region, the response ap-proaches almost to frequency independent behavior at higherfrequencies. This phenomenon of dielectric dispersion in ferritesis explained on the basis of Maxwell–Wagner two layer models[14,15] and Koops phenomenological theory of dielectric disper-sion [16]. In this model, a dielectric medium is supposed to bemade-up of well conducting path, which is separated by poorlyconducting grain boundaries. It has been noticed that in ferritesamples the permittivity is directly proportional to the square rootof its conductivity [17]. Consequently, the grains are exceedingly
0.3
0.4
0.2
0.5
Fig. 4. SEM images of Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 ferrite nanoparticles for x = 0.2, 0.3, 0.4 and 0.5.
200 Mohd. Hashim et al. / Journal of Alloys and Compounds 573 (2013) 198–204
Author's personal copy
conductive and contain elevated values of permittivity, while grainboundaries are less conductive and have slighter values of permit-tivity. The dielectric loss tangent (tand) also decreases rapidly inthe low frequency region, while at the middle frequency region itdecrease gradually and then at high frequency it becomes almostfrequency independent. Such a behavior can be explained on thebasis of that in low frequency region, which corresponds to low
conductivity of grain boundary, more energy is required for elec-tron exchange between Fe2+ and Fe3+ ions; as a result the loss ishigh. In high frequency region, which corresponds to high conduc-tivity of grain, a small energy is required for electron transfer be-tween the Fe2+ and Fe3+ ions at the octahedral site [18,19].
3.2.2. Composition dependence of dielectric propertiesThe variation of e0, e0 0, tand and rac as a function of Ni2+ compo-
sition with selected frequencies is shown in Fig. 8. In the presentstudy, it is proposed that the conduction process occurs as a conse-quence of electron exchange between Fe2+ and Fe3+ ions and holeexchange between Ni2+ and Ni3+ at the octahedral sites [20]. Thepresence of Ni and Cu at the octahedral sites may also cause con-duction according to the following exchange reaction:
Ni2þ þ Fe3þ $ Ni3þ þ Fe2þ ð1Þ
Cu2þ þ Fe3þ $ Cu3þ þ Fe2þ ð2Þ
3.2.3. The variation of AC conductivity with frequencyFig. 9 shows the variation of AC conductivity with frequency.
The conductivity is an increasing function of frequency in the caseof conduction by hopping and a decreasing function of frequency inthe case of band conduction. The complex conductivity of semicon-ductor materials can be expressed as:
r�ðxÞ ¼ r0AC þ ir00AC ð3Þ
The real part of this function consists of two components;
r0AC ¼ r1ðTÞ þ r2ðT;xÞ ð4Þ
The first term r1(T) is the DC electrical conductivity rdc(T); thatis associated with the drift mobility of electric charge carriers andobeys an Arrhenius relation [21]. The second term r2(T, x) is fre-quency and temperature refugee; it is attributed to the dielectricrelaxation caused by the localized electric charge carriers andobeys the power law form;
r2ðT;xÞ ¼ Axn ð5Þ
where A and ‘n’ are constants; depend upon both temperature andcomposition. The ‘n’ is a dimensionless quantity, whereas A having
800 700 600 500 400
ν2- (Fe3+-O2-)
x = 0.0 x = 0.1 x = 0.2 x = 0.3 x = 0.4 x = 0.5
ν1- (Fe3+-O2-)
Tra
nsm
ittan
ce
Wavenumber (cm)-1
Fig. 5. IR transmittance spectra of Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 (x = 0.0, 0.1, 0.2,0.3, 0.4 and 0.5) ferrite nanoparticles.
1 2 3 4 5 6 7 80
500
1000
1500
2000
2500
3000
3500
4000
Imag
inar
y di
elec
tric
con
stan
t ( ε
'')
log f (Hz)
x = 0.0 = 0.1 = 0.2 = 0.4 = 0.5
Fig. 6. Plot of dielectric constant (e0) vs. frequency (log f) for Cu0.4�0.5xCd0.2Ni0.4+-
xFe2�0.5xO4 (x = 0.0, 0.1, 0.2, 0.4 and 0.5) ferrite nanoparticles.
0.0 0.1 0.2 0.3 0.4 0.5
200
300
4000.0
0.2
0.4
0.60.0
2.0x10-6
8.0x10-6 0
100
200
ε'
Composition x
ε "T
an δ
σac
(Ω
- m
-1)
5KHz 10KHz100KHz 1MHz
Fig. 8. Compositional variation of dielectric constant (e0), dielectric loss (e0 0), losstangent (tand) and AC conductivity (rac) for Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 (x = 0.0,0.1, 0.2, 0.3, 0.4 and 0.5) ferrite nanoparticles.
1 2 3 4 5 6 7
0.0
0.5
1.0
1.5
2.0
2.5
3.0
Die
lect
ric
loss
tan
gent
(ta
nδ)
log f (Hz)
x = 0.0
x = 0.1
x = 0.2
x = 0.4
x = 0.5
Fig. 7. Plot of loss tangent (tand) vs. frequency (log f) for Cu0.4�0.5xCd0.2Ni0.4+-
xFe2�0.5xO4 (x = 0.0, 0.1, 0.2, 0.4 and 0.5) ferrite nanoparticles.
Mohd. Hashim et al. / Journal of Alloys and Compounds 573 (2013) 198–204 201
Author's personal copy
unit of conductivity. The exponent ‘n’ has been calculated as a func-tion of frequency for each composition, by plotting ln r versus lnx,which represents a straight line with slope equal to the exponent nand the intercept part equal to ln B on vertical axis at ln x = 0.
The variation of ac electrical conductivity with frequency ofnanocrystalline Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 ferrites has beenshown in Fig. 9. It can be seen that the conductivity is increasingslowly at low frequency region, while it increases sharply at highfrequency region. The increase in conductivity not indicate thatthe number of charge carriers increases, but only the rate of hop-ping of charge between the charge carriers, Fe2+ and Fe3+ increases.The rate of hopping of charge is enhanced once the frequency of anapplied field increases; resulting in an increase of conductivity. In ahopping model, it is probable to differentiate diverse characteristicregions of frequency; at low frequencies where the conductivity isstable, the mechanism of transport of charge takes place via manypaths. The hopping mechanism becomes the solitary involvementto conductivity progression in the frequency regions where con-ductivity is strongly dependent on frequencies. At tolerably highfrequencies, the resistivity remains constant because of the factthat rate of hopping of charge can no longer follow the frequencyof the applied electrical field leading to the saturation point. Inthe present study, it is believed that the conduction process occursas a result of electron exchange between Fe2+ and Fe3+ ions andhole exchange between Ni2+ and Ni3+ and also between Cu2+ andCu3+ at the octahedral sites.
The variation of exponent ‘n’ with composition is shown inFig. 10. Finally, small values of conductivity at room temperaturepoint out that the studied compositions may be excellent candi-dates for microwave applications that require negligible eddycurrents.
3.2.4. AC resistivityThe AC resistivity qac was calculated from dielectric data and
loss tangent using the following relation:
qac ¼ 1=e0e0x tan d ð6Þ
where e0 is the dielectric constant, e0 is permittivity of free space(8.854 � 10�12 F/m), x is the angular frequency and tan d is the lossfactor. The experimental results (Fig. 11) pointed out that the ACresistivity (qac) decreases with increasing frequency. It has beenalso found that at lower frequency region the resistivity decreasesfaster comparing to the higher frequency region and at much higherfrequency the resistivity become almost independent of frequency.This means that all the samples show typical ferromagnetic behav-ior with frequency. The conduction mechanism in ferrites is re-ported to be due to the hopping of electrons on application ofelectric field which is already discussed in the dielectric propertiesof ferrites. The increase of Fe2+ concentration in the sample in-creases the hopping probability resulting in decease of resistivity[22]. At high frequency region, the hopping frequency can no longerfollow the frequency of the applied AC field and hence the resistiv-ity becomes almost invariant. During the moment when the jump-ing frequency of hopping charge carriers are equal to that of theapplied field; resistivity becomes minimum and resonance takesplace.
Fig. 12 represents the variation of real part of impedance as afunction of frequency at room temperature. It can be seen that Z0
decreases as the frequency gradually increases. The maximum va-lue of Z0 is observed for the composition (x) = 0.5. The higher valueof the resistance means conductivity is lowered.
3.2.5. The variation of dielectric modulus with frequencyFigs. 13 and 14 show the real and imaginary part, respectively of
dielectric modulus with frequency at different composition of Ni2+.The real part of modulus spectra increases with increasing fre-quency and composition. The imaginary part of dielectric modulusspectra increases up to a certain frequency and followed bydecreasing trend with increasing frequency. In this case, electrodepolarization/interfacial polarization effect is seen to be completelyvanishing in contrast to the dielectric formalism. The appearanceof a peak in imaginary part of the dielectric modulus can be
Fig. 9. Plots of AC conductivity (rac) vs. frequency (log f) for Cu0.4�0.5xCd0.2Ni0.4+-
xFe2�0.5xO4 (x = 0.0, 0.1, 0.2, 0.4 and 0.5) ferrite nanoparticles.
0.0 0.1 0.2 0.3 0.4 0.5
0.50
0.55
0.60
0.65
n
Composition (x)
Fig. 10. The variation of exponent ‘n’ with composition for Cu0.4�0.5xCd0.2Ni0.4+x-
Fe2�0.5xO4 (x = 0.0, 0.1, 0.2, 0.3, 0.4 and 0.5) ferrite nanoparticles.
31 2 4 5 6 7
0
1x107
2x107
3x107
4x107
5x107
6x107
7x107
Log f (Hz)
AC
res
isti
vity
x = 0.5 x = 0.4 x = 0.2 x = 0.1 x = 0.0
Fig. 11. Variation of ‘AC resistivity’ with frequency (logf) for Cu0.4�0.5xCd0.2Ni0.4+x
Fe2�0.5xO4 (x = 0.0, 0.1, 0.2, 0.4 and 0.5) ferrite nanoparticles.
202 Mohd. Hashim et al. / Journal of Alloys and Compounds 573 (2013) 198–204
Author's personal copy
assumed to be related with the translation ionic dynamics and theconductivity relaxation of the mobile ions.
3.3. Temperature dependent magnetization
Temperature dependence of magnetization of Cu–Cd–Ni ferritenanoparticles with different Ni2+ concentration is measured in a
magnetic field of 10 kOe and is shown in Fig. 15. The magnetiza-tions of Cu–Cd–Ni ferrite particles monotonically decrease withincreasing temperature. The magnetization falls slowly when themagnetic state of the ferrite changes from the ferromagnetic ferriteto the paramagnetic state. The Curie temperatures, which aredetermined from the graphs are; 407, 478, and 509 �C for x = 0.1,0.3 and 0.5, respectively. The Curie temperature and magnetizationof Cu–Cd–Ni ferrite particles tends to increase with the increase inNi2+ substitution. The linear increase in the Curie temperature andmagnetization with the Ni2+ substitution may be explained by achange in the A–O–B super-exchange interaction due to the changeof the Fe3+ distribution between A and B sites. Ni2+ ions maystrengthen the super-exchange interaction between the magneticions in A and B sites. The stronger super-exchange interactionhas a stronger ability to endure thermal motion, resulting in a lin-ear increase in the Curie temperature with the Ni2+ substitution.
4. Conclusions
We have successfully synthesized Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5x-
O4 (0.0 6 x 6 0.5) ferrite nanoparticles using a citrate nitrate gelauto combustion route. XRD results confirm the formation ofsingle phase cubic spinel structure. SEM micrographs show thatall the particles have spherical shape and crystalline behavior.FTIR absorption spectra shows two fundamental bands m1 andm2 in the frequency range 600–400 cm�1, respectively, which cor-respond to tetrahedral and octahedral metal complexes, respec-tively. At low frequencies due to the heterogeneous structureof nano ferrites, Maxwell–Wagner type space charge polarizationhas been observed. The dielectric constant decreases and resis-tivity increases with enhancing the Ni concentration becauseNi2+ ion substitutes for Cu2+ and Fe3+ ions leading to decreaseof the hopping rate of electrons between Fe3+ and Fe2+ whichis caused by the change in dielectric and resistivity properties.Temperature dependent magnetization study shows that themagnetization and Curie temperature increases with increasein Ni2+ substitution, suggesting the strengthening in the A–Binteractions.
Acknowledgements
One of the authors Mohd. Hashim are highly thankful to Univer-sity grant commission (U.G.C.) for providing financial assistancethrough grant MANF-MUS-UTT-1092. This work is also supportedby Centre of Excellence in Material Science (Nanomaterials),Department of Applied Physics, Aligarh Muslim University, Aligarh.
300 400 500 600 700 8000
4
8
12
16
20
24 0.1
0.3
0.5
Mag
netiz
atio
n (e
mu/
g)
Temperature (K)
Fig. 15. Temperature dependent magnetization measurement of Cu0.4�0.5xCd0.2-
Ni0.4+xFe2�0.5xO4 (x = 0.1, 0.3, and 0.5) ferrite nanoparticles.
1 2 3 4 5 6 7
00
1x106
2x106
3x106
4x106
x = 0.5 = 0.4 = 0.2 = 0.1 = 0.0Z'
log f (Hz)
Fig. 12. Variation of real part of impedance (Z0) with frequency (log f) at roomtemperature for Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 (x = 0.0, 0.1, 0.2, 0.4 and 0.5) ferritenanoparticles.
41 2 3 5 6 70.0000
0.0003
0.0006
0.0009
0.0012
0.0015
0.0018
0.0021
0.0024
0.0027
0.0030 x = 0.0 = 0.1 = 0.2 = 0.4 = 0.5
Imag
inar
y M
odul
us
Log f (Hz)
Fig. 14. Variation of imaginary part of dielectric modulus with frequency (log f) atroom temperature for Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 (x = 0.0, 0.1, 0.2, 0.4 and 0.5)ferrite nanoparticles.
41 2 3 5 6 7
0.000
0.001
0.002
0.003
0.004
0.005
0.006
0.007
Rea
l Mod
ulus
Log f (Hz)
x = 0.5 = 0.4 = 0.2 = 0.1 = 0.0
Fig. 13. Variation of real part of dielectric modulus with frequency (log f) at roomtemperature for Cu0.4�0.5xCd0.2Ni0.4+xFe2�0.5xO4 (x = 0.0, 0.1, 0.2, 0.4 and 0.5) ferritenanoparticles.
Mohd. Hashim et al. / Journal of Alloys and Compounds 573 (2013) 198–204 203
Author's personal copy
References
[1] Alex Goldman, Modern Ferrite Technology, second ed., Springer, New York,2006.
[2] Aashis S. Roy, Satyajit Gupta, S. Sindhu, Ameena Parveen, Praveen C.Ramamurthy, Composites: Part B 47 (2013) 314–319.
[3] Sagar E. Shirsath, R.H. Kadam, Anil S. Gaikwad, Ali Ghasemi, AkimitsuMorisako, J. Magn. Magn. Mater. 323 (2011) 3104.
[4] Sagar E. Shirsath, B.G. Toksha, Maheshkumar L. Mane, V.N. Dhage, D.R.Shengule, K.M. Jadhav, Pow. Technol. 212 (2011) 218.
[5] Sagar E. Shirsath, B.G. Toksha, K.M. Jadhav, Mater. Chem. Phys. 117 (2009) 163.[6] M.A. Ahmed, N. Okasha, J. Magn. Magn. Mater. 321 (2009) 3436.[7] Mohd. Hashim, Alimuddin, Shalendra Kumar, B.H. Koo, Sagar E. Shirsath, E.M.
Mohammed, Jyoti Shah, R.K. Kotnala, H.K. Choi, H. Chung, Ravi Kumar, J. AlloyComp. 518 (2012) 11–18.
[8] Mohd. Hashim, Alimuddin, Shalendra Kumar, Sagar E. Shirsath, R.K. Kotnala,Hanshik Chung, Ravi Kumar, Pow. Technol. 229 (2012) 37.
[9] M.A. Gabal, Y.M. Al Angari, M.W. Kadi, Polyhedron 30 (2011) 1185–1190.
[10] D.R. Mane, D.D. Birajdar, Swati Patil, Sagar E. Shirsath, R.H. Kadam, J. Sol–GelSci. Technol. 58 (2011) 70.
[11] R.D. Waldron, Phys. Rev. 99 (1955) 1727.[12] D. Emin, Adv. Phys. 24 (1975) 305.[13] P. Debye, Polar Molecules, Dover, New York, 1929.[14] J.C. Maxwell, Electricity and Magnetism, vol. 1, Oxford University Press,
Oxford, 1929 (Section 328).[15] K.W. Wagner, Ann. Phys. 40 (1913) 817.[16] C.G. Koops, Phys. Rev. 83 (1951) 121–124.[17] Sagar E. Shirsath, Santosh S. Jadhav, B.G. Toksha, S.M. Patange, K.M. Jadhav,
Scripta Mater. 64 (2011) 773.[18] I.T. Rabinkin, Z.I. Novikova, Ferrites, Izv Acad, Nauk USSR Minsk, 1960.[19] J.C. Maxwell, Electricity and Magnetism, vol. 2, Oxford University Press, New
York, 1973.[20] Aashis S. Roy, Koppalkar R. Anil kumar, M.V.N. Ambika Prasad, J. Appl. Poly. Sci.
123 (2012) 1928–1934.[21] E.J. Verway, Heilman, J. Chem. Phys. 15 (1947) 174.[22] J. Smit, H.P.J. Wijn, Ferrites, Philips Technical Library, Netherlands, 1959.
204 Mohd. Hashim et al. / Journal of Alloys and Compounds 573 (2013) 198–204