A multi-substrate single-file model for ion-coupled transporters
Constitutive mRNA expression and protein activity levels of nine ABC efflux transporters in seven...
Transcript of Constitutive mRNA expression and protein activity levels of nine ABC efflux transporters in seven...
Ctr
SKa
b
c
d
e
f
a
ARR1A
KAMMPCEO
1
t1cA
H
0d
Aquatic Toxicology 101 (2011) 438–446
Contents lists available at ScienceDirect
Aquatic Toxicology
journa l homepage: www.e lsev ier .com/ locate /aquatox
onstitutive mRNA expression and protein activity levels of nine ABC effluxransporters in seven permanent cell lines derived from different tissues ofainbow trout (Oncorhynchus mykiss)
tephan Fischera,b,c, Jovica Loncard, Roko Zajad, Sabine Schnell e,ristin Schirmerb,c,f, Tvrtko Smitald, Till Luckenbacha,∗
Department of Bioanalytical Ecotoxicology, UFZ – Helmholtz Centre for Environmental Research, 04318 Leipzig, GermanyEawag, Swiss Federal Institute of Aquatic Science and Technology, 8600 Dübendorf, SwitzerlandETH Zürich, Institute of Biogeochemistry and Pollutant Dynamics, 8092 Zürich, SwitzerlandRuder Boskovic Institute, Laboratory for Molecular Ecotoxicology, Division for Marine and Environmental Research, Bijenicka 54, 10001 Zagreb, CroatiaEnvironmental Chemistry Department, IDAEA-CSIC, Barcelona, SpainEPF Lausanne, School of Architecture, Civil and Environmental Engineering, 1015 Lausanne, Switzerland
r t i c l e i n f o
rticle history:eceived 18 September 2010eceived in revised form1 November 2010ccepted 20 November 2010
eywords:BC efflux transportersultixenobiotic resistanceXR
ermanent rainbow trout cell linesonstitutive mRNA expressionfflux transporter activityncorhynchus mykiss
a b s t r a c t
Permanent fish cell lines have become common model systems for determining ecotoxicological effectsof pollutants. For these cell lines little is known on the cellular active transport mechanisms that con-trol the amount of a compound entering the cell, such as the MXR (multixenobiotic resistance) systemmediated by ATP binding cassette (ABC) transport proteins. Therefore, for toxic evaluation of chemi-cals with those cells information on MXR is important. We here present data on constitutive mRNAexpression and protein activity levels of a series of ABC efflux transporters in seven permanent celllines derived from liver (RTL-W1; R1) and liver hepatoma (RTH-149), gill (RTgill-W1), gonad (RTG-2),gut (RTgutGC) and brain (RTbrain) of rainbow trout (Oncorhynchus mykiss). In addition to known trans-porters abcb1 (designated here abcb1a), abcb11, abcc1-3, abcc5 and abcg2, we quantified expression levelsof a newly identified abcb1 isoform (abcb1b) and abcc4, previously unknown in trout. Quantitative realtime PCR (qPCR) indicated that mRNA of the examined ABC transporters was constitutively expressedin all cell lines. Transporter mRNA expression patterns were similar in all cell lines, with expressionlevels of abcc transporters being 80 to over 1000 fold higher than for abcg2, abcb1a/b and abcb11 (abcc1-5 > abcg2 > abcb1a/b, 11). Transporter activity in the cell lines was determined by measuring uptake oftransporter type specific fluorescent substrates in the presence of activity inhibitors. The combinationof the ABCB1 and ABCC transporter substrate calcein-AM with inhibitors cyclosporine A, PSC833 and
MK571 resulted in a concentration-dependent fluorescence increase of up to 3-fold, whereas reversin205 caused a slight, but not concentration-dependent fluorescence increase. Accumulation of the dyesHoechst 33342 and 2′,7′-dichlorodihydrofluorescein diacetate was basically unchanged in the presenceof Ko134 and taurocholate, respectively, indicating low Abcg2 and Abcb11 activities, in accordance withnscrit cell
low abcg2 and abcb11 traterns in the different trouof cell cultivation.
. Introduction
ATP binding cassette (ABC) transporters represent one impor-
ant line of cellular defence (Gottesman and Pastan, 1988; Kurelec,992; Leslie et al., 2005). These transporters pump a broad array ofhemical compounds against a concentration gradient in an active,TP dependent process out of the cell, mediating the so-called∗ Corresponding author at: Department of Bioanalytical Ecotoxicology, UFZ –elmholtz Centre for Environmental Research, 04318 Leipzig, Germany.
E-mail address: [email protected] (T. Luckenbach).
166-445X/$ – see front matter © 2010 Elsevier B.V. All rights reserved.oi:10.1016/j.aquatox.2010.11.010
pt levels. Our data indicate that transporter expression and activity pat-lines are irrespective of the tissue of origin, but are determined by factors
© 2010 Elsevier B.V. All rights reserved.
multixenobiotic resistance (MXR) phenotype, equivalent to themultidrug resistance (MDR) of cancer cells and pathogens in aclinical context (Kurelec, 1992). The efflux pumps act as a “firstline of defence” by preventing chemicals from entering the cell(phase 0 of cellular detoxification) and pump out phase 1 andphase 2 metabolites, such as oxidized molecules and glutathione,glucuronic or sulfate water soluble conjugates (phase 3). Conse-
quently, this efflux activity controls the amount of toxicants in thecells (Sarkadi et al., 2006).In humans, 49 ABC transporters have been identified, which,based on sequence homology and protein organisation, are dividedinto seven sub-families designated ABCA–ABCG (Dean et al., 2001).
oxicolo
O(Aae0lsaAfo2nb1abskaxm2dp
pias(actAlacRa
stAo(fiwkvswo
2
2
r
cS
S. Fischer et al. / Aquatic T
nly members of the ABCB (ABCB1, P-glycoprotein, P-gp), ABCCABCC1-2, multidrug resistance-associated proteins, MRPs) andBCG families (ABCG2, breast cancer resistance protein, BCRP)ppear to be associated with MXR/MDR (Epel et al., 2008; Lesliet al., 2005). Substrates of ABCB1 are unmodified xenobiotics (phase), whereas the ABCCs and ABCG2 recognize phase 1 and 2 metabo-
ites as substrates. ABCCs also transport physiological substratesuch as hormones and bile salts (Leslie et al., 2005), whereas thereppear to be no endogenous substrates of ABCB1 (Higgins, 2007).general characteristic of all MXR transporters is their specificity
or multiple substrates and there is overlap of substrate spectraf different transporter types to a certain degree (Litman et al.,001). In mammals, MXR transporters are expressed in inter-al barrier tissues, such as intestine, kidney, liver, blood–brainarrier, blood–testes barrier and placenta (Cordon-Cardo et al.,990; Leslie et al., 2005). In aquatic organisms, ABC transportersre also part of active, selectively permeable “environment-tissuearriers” (Luckenbach and Epel, 2008). Similar to mammals, expres-ion of ABC transporters has been detected in liver, intestine,idney, exocrine pancreas, brain and gill of several fish speciesnd the transporters have been found to function in extrusion ofenobiotics and physiological substrates in isolated tissue or pri-ary cultures of fish hepatocytes (reviewed in Sturm and Segner,
005). However, there is still comparatively little known about thisefence system in fish with regard to specific substrate spectra andhysiological function.
A pre-requisite to overcome the limited knowledge of ABC trans-orter function in fish is availability of an array of well characterised
n vitro systems. Tissue preparations and primary cultures havelready proven useful, but these preparations are cumbersome,hort-lived and undergo rapid changes caused by isolation stressBols et al., 2005). Therefore, permanent fish cell lines may serves alternative experimental systems, but ABC transporters in thoseells have so far only been little studied. Zaja et al. (2007) quan-ified transcript levels and characterized functional properties ofbcb1 (Pgp-1) and Abcc3 (MRP3) in the topminnow (Poeciliopsis
ucida) hepatoma cell line, PLHC-1. They subsequently establishedn Abcb1 over-expressing PLHC-1 sub-clone by exposing wild typeells to doxorubicine, a toxic substrate of ABCB1 (Zaja et al., 2008a).ecently, Fischer et al. (2010) confirmed the presence of abcc1 andbcc3 mRNA in the rainbow trout gill cell line, RTgill-W1.
Considering the limited knowledge of ABC transporter expres-ion and function in permanent fish cell lines, it was the aim ofhis work to investigate a set of ABC transporters belonging to thebcb, c and g subfamilies (Table 1) in seven rainbow trout cell linesriginating from liver (RTL-W1, R1, RTH-149), gonad (RTG-2), gillRTgill-W1), intestine (RTgutGC) and brain (RTbrain). We quanti-ed transporter transcript levels and carried out functional testsith different fluorescent transporter substrates and inhibitors
nown from the mammalian literature (Table 1) in order to pro-ide a measure of ABC transporter protein activity. We presentequences of abcc4 and an abcb1 isoform (named here abcb1b) thatere previously unknown from trout, and extended the sequence
f trout abcc5.
. Materials and methods
.1. Chemicals
Cyclosporine A (CsA), reversin 205 (REV205), sodium tau-ocholate hydrate (TAU), Hoechst 33342 (H33342), Triton
1 (our designation of gene and protein names is based on the Zebrafish Nomen-lature Guidelines (http://zfin.org/zf info/nomen.html); fish: shh/Shh, human:HH/SHH, mouse: Shh/SHH (gene/protein)).
gy 101 (2011) 438–446 439
X-100, dimethyl sulfoxide (DMSO), fetal bovine serum (FBS)and penicillin–streptomycin solution (10 mg/mL streptomycin,10000 IU/mL penicillin) were acquired from Sigma–Aldrich(Schnelldorf, Germany). PSC833 was a gift from Novartis (Basel,Switzerland). Ko134, a tetracyclic fumitremorgin C analog, wasordered from Solvo Biotechnology (Budapest, Hungary). Calcein-acetoxymethylester (Ca-AM) and MK571 were purchased fromBiozol (Eiching, Germany). 2′,7′-Dichlorodihydrofluorescein diac-etate (DCDHFDA) and Leibovitz’s L-15 medium were purchasedfrom Invitrogen (Karlsruhe, Germany).
2.2. Rainbow trout and MDCKII cell lines
Seven cell lines derived from five different rainbow trout(Oncorhynchus mykiss) tissues were chosen for this study. The celllines RTL-W1 (Lee et al., 1993) and R1 (Ahne, 1985) were derivedfrom liver; RTH-149 is derived from a liver-hepatoma (Lannanet al., 1984) (ATCC no. CRL-1710); RTG-2 from gonad (Wolf andQuimby, 1962) (ATCC no. CCL-55); RTgill-W1 from gill (Bols et al.,1994) (ATCC no. CRL-2523), RTgutGC from intestine (establishedby Schirmer and Bols) (Kawano et al., in press) and RTbrain frombrain (kindly provided by N. C. Bols). All cell lines were rou-tinely cultured in 75 cm2 culture flasks at 19–21 ◦C in normalatmosphere, in Leibovitz’s L-15 medium supplemented with 1%penicillin–streptomycin solution and 5% (RTL-W1 cell line) and 10%(all other cell lines) FBS.
Madin-Darby canine kidney (MDCKII) cells overexpressinghuman Pgp (MDCKII-Pgp [=ABCB1]) and BCRP (MDCKII-BCRP[=ABCG2]), respectively, were used as positive controls for func-tional transporter assays (see below) along with non-transfectedMDCKII cells. The MDCKII cell lines were a kind gift from Dr.Piet Borst from the Netherlands Cancer Institute – Antoni vanLeeuwenhoek Hospital. Culture conditions were according toEvers et al. (2000).
2.3. RNA isolation and cDNA synthesis
Total RNA was extracted from ∼4 × 106 cells using the QiagenRNeasy Protect MiniKit. Genomic DNA contaminations wereremoved using DNase from the RNase-Free DNase kit (Qiagen,Hilden, Germany). RNA quantities and qualities were determinedusing a NanoDrop spectrophotometer (PEQLAB BiotechnologieGMBH, Erlangen, Germany) and by agarose gel electrophoresis.The cDNA was synthesized from total RNA using the High CapacitycDNA ReverseTranscription Kit (Applied Biosystems, Darmstadt,Germany).
2.4. Identification of abcc4, 5 and abcb1b sequences
For obtaining partial sequences of abcc4, 5 and abcb1b, totalRNA from RTL-W1 cells (abcc5) and mRNA from a RTL-W1 clone(abcc4 and abcb1b) were used and reverse transcriptase (RT)-PCRwas performed with Advantage 2 polymerase mix (Clontech, Palo
Alto, USA) for 35 cycles at 94 ◦C (20 s), 58 ◦C (20 s), and 72 ◦C(2 min). For the nested RT-PCR for abcc4 (see Table S1), PCR prod-uct from the first reaction was 100× diluted and 3 �L were usedin a second PCR with inner primers. Primers were designed basedon expressed sequence tags (ESTs) that were identified with NCBIBLAST searches with sequences of human ABCC4, ABCC5 and ABCB1as listed in supplemental information (Table S1). PCR products werecloned and sequenced; sequences were edited and assembled usingSequencher (version 4.8).440 S. Fischer et al. / Aquatic Toxicology 101 (2011) 438–446
Table 1Overview of tissue distributions, substrates and pharmacologic inhibitors of ABC transporters investigated in this study from literature on mammalian ABC transporters.
Protein name andsynonyms
Tissue distribution Examples of endogenoussubstrates
Examples of exogenoussubstrates
Used fluorescentsubstrates
Used pharmaceuticalinhibitors
ABCB1/Pgp/MDR1 Ubiquitous Glucosylceramide (Thomas andBrown, 2010)
Amphiphilic drugs,hydrophobic cations,anthracyclines, anthracenes,vinca alkaloids, antibiotics,antivirals, insecticides,herbicides, fungicides (Litmanet al., 2001; Szakacs et al.,2008; Sarkadi et al., 2006;Leslie et al., 2005)
Calcein-AM Cyclosporine A,PSC-833, reversine 205
ABCB11/BSEP Liver Bile salts (Gerloff et al., 1998) DCDHFDA TaurocholateABCC1/MRP1 Ubiquitous Organic anions, folates,
hormones, glutathione,glutathione conjugates,glutathione disulfide (gssg),glucuronide and sulfateconjugates, prostaglandin a,bilirubin, lipid analogs (Haimeuret al., 2004; Deeley et al., 2006)
Anthracyclines, vinca alkaloids,metalloids, antivirals,antibiotics, drug/xenobioticconjugates with glutathioneand glucuronide insecticides,herbicides, fungicides (Litmanet al., 2001; Szakacs et al.,2008)
Calcein-AM MK571
ABCC2/MRP2 Liver, gut, kidney,placenta
Organic anions, hormones,glutathione, glutathioneconjugates, glucuronide andsulfate conjugates, bilirubin, bileacids (Haimeur et al., 2004;Deeley et al., 2006)
Anthracyclines, vinca alkaloids,epipodophyllotoxins,drug/xenobiotic conjugateswith glutathione and sulfate(Litman et al., 2001; Borst et al.,2000; Deeley et al., 2006)
Calcein-AM MK571
ABCC3/MRP3 Gut, pancreas,placenta, liver,kidney, prostate
Organic anions, glutathione andglucuronate conjugates,hormones, monoanionic bileacids, bilirubin (Haimeur et al.,2004; Deeley et al., 2006)
Anthracyclines, vinca alkaloids,antifolate drugs,drug/xenobiotic conjugateswith glucuronide and sulfate(Litman et al., 2001; Borst et al.,2000; Deeley et al., 2006)
Calcein-AM MK571
ABCC4/MRP4 Ovary, testis,kidney, lung,prostate
Lipophilic anions, cyclicnucleotides (cgmp, camp),glutathione, folates, glucuronideand glutathione conjugates(Haimeur et al., 2004; Deeleyet al., 2006)
Antifolate drugs, nucleotideanalogs, paramethoxyethylam-phetamine (PMEA) (Litmanet al., 2001; Borst et al., 2000;Deeley et al., 2006)
Calcein-AM MK571
ABCC5/MRP5 Ubiquitous Organic anions, glutathioneconjugates glutamate andphosphate conjugates, cyclicnucleotides, hyaluronan(Haimeur et al., 2004; Deeleyet al., 2006)
Anthracyclines, antifolatedrugs, nucleotide analogs(Litman et al., 2001; Borst et al.,2000; Deeley et al., 2006)
Calcein-AM MK571
ABCG2/BCRP Blood–brainbarrier, placenta,
Folic acids, bile salts, porphyrins,heme sulfated conjugates of
Anthracyclines, antifolatedrugs, anthracenedione(Lit200
Hoechst 33342 Ko134
2
daB2pp
Rtmr9A1siwm
where MNE stands for mean normalized expression; Eref is theamplification efficiency for the reference gene; Etarget is target gene
liver, gut, breast steroids (Doyle and Ross, 2003;Latunde-Dada et al., 2006;Haimeur et al., 2004)
.5. Quantitative real-time PCR (qPCR)
qPCR primers for reference and ABC transporter genes wereesigned either against the self obtained sequences (abcc4 and 5,bcb1b) or against mRNA sequences available from GenBank usingeacon Designer (Palo Alto, USA) and PrimerExpress software, Ver.,0 (Applied Biosystems) (see Table S1 for accession numbers,rimers and fragment lengths). RT-PCR products obtained with therimers were verified with gel electrophoresis and sequencing.
qPCR was performed on an Applied Biosystems StepOnePlusTM
eal-Time PCR System with SYBR Green PCR Master Mix (Quan-ace, Berlin, Germany). Samples contained 1× SYBR green master
ix, 0.5 �L of each primer and 2 �L of cDNA template for a finaleaction volume of 12.5 �L. Cycling parameters were as follows:5 ◦C (10 min), 40 cycles of 95 ◦C (15 s), 55 ◦C (20 s) and 72 ◦C (20 s).melting curve analysis was performed (95 ◦C for 15 s, 60 ◦C for
min, 0.3 ◦C increases for 15 s up to 95 ◦C) after each run. Tran-cript levels were measured in cDNAs of three independent RNAsolations for each cell line. From five candidate genes 18S rRNA
as selected as reference gene since it showed a low variability ofRNA expression among different cell lines (Supplemental Fig. S2).
man et al., 2001; Borst et al.,0; Sarkadi et al., 2006)
Data generated by qPCR were compiled and collected usingStepOne software v2.1 (Applied Biosystems). Samples with thresh-old cycles above 35 were considered below the level of detectionand not included into data analysis. Results of the qPCR for ABCtransporters were calculated relative to the reference gene, 18S,according to the normalization procedure of the Q-Gene Core Mod-ule (http://www.qgene.org/) (Muller et al., 2002; Simon, 2003). Theadvantage of this calculation is that it takes varying PCR amplifica-tion efficiencies into account (Table S2), according to:
MNE = (Eref)Ctref, mean
(Etarget)Cttarget, mean
amplification efficiency; Ctref, mean is mean Ct value for the refer-ence gene; and Cttarget, mean is the mean Ct value for the target gene.Expression data for target genes were presented relative to the ref-erence gene and expression was multiplied by the factor 105 forclearer presentation.
oxicolo
2a
tttiaitt(P(ods(e(ds
iotwLvceaf0Gtf
McMThp
eecomIm
3
3
tGi1C2
S. Fischer et al. / Aquatic T
.6. Dye uptake assays for determining cellular ABC protein effluxctivities
For detection of Abcb1, Abcc and Abcg2 transport activities inhe cells, fluorescent transporter substrates were used in combina-ion with pharmacologic transporter inhibitors (Table 1) accordingo Zaja et al. (2007). Efflux activity of a specific transporter isndicated if accumulation of a fluorescent substrate in the cells –nd thus the fluorescent signal – increases in the presence of annhibitor compound. The following substrate/inhibitor combina-ions were used, based on reported specificities for mammalian ABCransporters: Ca-AM as substrate of ABCB1 and ABCC transportersEssodaigui et al., 1998; Hollo et al., 1994) combined with CsA,SC833 (cyclosporine derivate) and REV205 as inhibitors of ABCB1Mayer et al., 1997; Sharom et al., 1999), and MK571 as inhibitorf ABCC type transporters (Gekeler et al., 1995); dichlorodihy-rofluorescein diacetate (DCDHFDA) as ABCB11 specific fluorescentubstrate (Wang et al., 2003) combined with ABCB11 inhibitor TAUNoe et al., 2002); H33342 as fluorescent substrate of ABCG2 (Jonkert al., 2002) in combination with Ko134 as ABCG2 specific inhibitorAllen et al., 2002). All fluorescent substrates and inhibitors wereissolved in DMSO, except for TAU and H33342 which were dis-olved in water.
For the functional assay, 200 �L per well of cell suspensionn L-15 medium supplemented with 5% (RTL-W1) or 10% (allther cell lines) FBS were seeded in 96 multi-well plates so aso achieve confluent monolayers. After 24 h, cells were washedith 200 �L of PBS (with Mg2+ and Ca2+) before 100 �L of fresh
-15 medium without FBS were added. Inhibitors were added in aolume of 50 �L and incubated for 5 min before 50 �L of fluores-ent substrate was added. Final concentrations of solvents neverxceeded 0.5%, which was found not to affect cellular viabilitynd efflux activity. Cells were incubated at 19–21 ◦C in the darkor 60 min, washed with 200 �L of PBS and lysed in 200 �L of.2% Triton X-100/PBS. Fluorescence was measured with a Tecanenios microplate reader (Tecan, Männedorf, Switzerland) with
he following excitation/emission wavelengths: 485 nm/530 nmor Ca-AM and DCDHFDA; 360 nm/465 nm for H33342.
As positive controls for the efflux protein activity dye assays,DCKII-Pgp (over-expressing human ABCB1) and MDCKII-BCRP
ells (over-expressing human ABCG2) were used. Non-transfectedDCKII cells (low efflux activity) were used as negative controls.
he dye assay was performed in serum-free DMEM at 37 ◦C in aumidified atmosphere with 5% (v/v) CO2. Otherwise, the assayrocedure was as described above.
Dye accumulation in the presence of various inhibitors wasxpressed as % fluorescence compared to the control, i.e., cellsxposed to the fluorescent substrate but no inhibitor. The con-entration of transporter inhibitor yielding 50% of the maximalbserved accumulation of fluorescent dye was determined as aeasure for potency of ABC transporter activity inhibition (IC50).
C50 values were determined using a non-linear regression, sig-oidal dose–response curve fitting module (GraphPad).
. Results
.1. Identification of partial abcc4, abcc5 and abcb1b sequences
For abcc4 (accession no. GU480583) we obtained a par-ial sequence of 1615 bp (537 aa) and for abcc5 (accession no.
U480582) a partial sequence of 1009 bp [913 bp (303 aa) cod-ng sequence and 96 bp 3′UTR]. For abcb1b we assembled a816 bp contig from ESTs (accession nos. CU069755.1, BX861573.3,A363431.1, CA366489.1, CX145430.1, CX145431.1) and cloned a29 bp sequence (accession no. HQ400613) with primers based on
gy 101 (2011) 438–446 441
the contig. Identities as abcc4, abcc5 and abcb1b orthologs wereindicated by NCBI BLAST analyses of the nucleotide and translatedamino acid sequences that showed close matches with respectiveABC transporter sequences from various other organisms. Degreesof identity of amino acid sequences with respective sequencesof human orthologs are 75% for abcc4 and 83% for abcc5. Thetrout abcc5 sequence contains the already identified sequencestretch of the gene (accession no. GU079635.1). Having 51 and48% identities with the accordant nucleotide sequence stretches,the abcb1b sequence is clearly distinct from the already identi-fied trout abcb1a (accession no. AY863423) and abcb11 (accessionno. NM 001124656.1) sequences, respectively. For alignments seeFig. S1.
3.2. Relative quantification of ABC transporter mRNA abundances
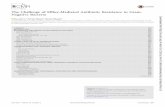
The qPCR data indicated that transcripts of all studied trans-porters were present in all seven investigated rainbow trout celllines (Table 2 and Supplemental Fig. S3). Generally, transcript abun-dance of abcc1, abcc2 and abcc3 transporters was comparativelyhigh. Moderate mRNA expression was determined for abcc4, abcc5and abcg2, and low for abcb1a, abcb1b, abcb11. Few distinct dif-ferences appeared in transcript abundance for some of the celllines. Namely, all liver-based cell lines (RTL-W1, R1 and RTH-149)showed low abundance in abcc4; the gill-derived cell line, RTgill-W1, was characterised by an overall high level of ABC transportertranscript abundance; and RTbrain cells showed comparatively lowlevels of abcc1-2 but among the highest observed transcript levelsfor abcb1a and abcc5.
3.3. ABC protein activity determined by dye uptake andaccumulation assay
Among the different fluorescent substrates and inhibitors used,general changes in dye accumulation (i.e., increase in fluorescence)were only observed for combinations of Ca-AM with inhibitors CsA,PSC833, REV205 and MK571 (Fig. 1). In all trout cell lines, CsA,PSC833 and MK571 caused concentration-dependent increases ofdye accumulation, with significant changes of fluorescence gener-ally occurring at 3–10 �M of inhibitor and fluorescence maxima of2.5–3.0-fold from control levels at concentrations ≥ 10 �M (Fig. 1,Emax/Econ ratios in Table 3). Significant fluorescence changesat inhibitor concentrations < 3 �M were only seen in RTgutGCand RTG-2 cells, indicating that Ca-AM efflux was most sensi-tive in these two cell lines. This was also reflected by IC50 values(inhibitory concentration causing 50% of maximal inhibition) thatwere generally lower in the two cell lines (Table 3). Thus, IC50 val-ues for CsA were 1.21 �M and 2.65 �M for RTG-2 and RTgutGC,respectively, whereas they were 3.12 �M for RTgill-W1 and above4 �M for RTL-W1, R1, RTH-149 and RTbrain (Table 3). IC50 valuesfor RTG-2 were lowest for PSC833 and MK571 compared to theother cell lines, whereas – unlike with CsA – they were among thehighest for RTgill-W1. The reason for substantial decreases of fluo-rescence at 50 �M MK571 that were observed in most cell lines isnot known (Fig. 1). Precipitation of MK571 or cytotoxicity was notobserved. Changes in calcein fluorescence were less pronouncedwith REV205 and there was no concentration-dependent effect inany of the trout cell lines (Fig. 1). In contrast, MDCKII-Pgp cellsshowed concentration-dependent increases of fluorescence withCsA, PSC833 and REV205 and to a considerably lower extent withMK571. Maximal fluorescence increases were almost 20-fold with
CsA and PSC833, 10-fold with REV205 and 2.6-fold with MK571(Table 3). IC50 values were similar for MDCKII-Pgp and trout cellsfor CsA and PSC833, indicating similar sensitivities of efflux in thecell lines. In contrast, the IC50 values for MK571 were consider-ably higher for the MDCKII-Pgp cells, indicating the need for higher442 S. Fischer et al. / Aquatic Toxicology 101 (2011) 438–446
Table 2Basal relative mRNA abundance of ABC transporters in 7 rainbow trout cell lines. Expression data were obtained from three independentRNA isolations of each cell line, normalized to 18S rRNA (18S). Means ± SD of the independent isolations are shown. Different grey intensitiesillustrate the magnitude of mRNA abundance levels. Expression values were multiplied by 105 for clarity of presentation.
±15.92
±0.12
±0.54
±0.11
±0.26
±0.15
±1.45
abcg2abcc5abcc4abcc3abcc2abcc1abcb11abcb1b
11.61
±2.71
144.69
±21.01
29.42
±6.63
292.68
±133.77
76.01
±20.11
42.95
±7.86
1.04
±0.68
0.73
±0.28
12.75
±3.88
32.71
±9.86
12.38
±4.53
375.00
±367.14
1080.07
±385.04
337.70
±59.32
0.60
±0.37
0.28
±0.14
±2.92
121.66
±48.01
24.25
±9.63
591.86
±230.05
1805.91
±397.72
580.62
±125.95
0.17
±0.06
4.18
±2.67
4.05
±1.30
15.27
±9.48
52.21
±13.02
353.35
±127.73
346.47
±162.12
249.42
±108.33
0.47
±0.11
1.44
±0.51
5.28
±2.39
57.30
±15.01
7.35
±3.47
167.39
±41.09
590.32
±309.55
321.06
±159.82
1.76
±0.88
0.31
±0.11
6.83
±3.28
16.37
±4.27
3.18
±1.63
361.78
±242.33
1528.70
±627.17
71.00
±29.44
3.51
±0.36
0.53
±0.09
18.23
±3.85
16.35
±2.48
1.52
±0.10
141.83
±15.45
642.99
±289.69
141.93
±79.60
1.25
±0.38
1.92
±0.46
abcb1a
72.98
1.34
5.70
0.26
0.89
0.46
2.64
RTbrain
RTgutGC
33.39RTgill-W1
RTG-2
RTH-149
R1
RTL-W1
ABC Transporters RT cell lines
vels
medi
iA
mRtlctccr
l2Acfl(lt
TItr
ABC transporter expression le
low
nhibitor concentrations to induce significant accumulation of Ca-M in this cell line compared to the fish cells (Table 3).
We tested the activity of Abcg2 transporter with H33342 asodel fluorescent substrate and Ko134 as model inhibitor. In the
TL-W1 and the RTgill-W1 cell lines we found no (RTL-W1) or lit-le (RTgill-W1) changes in Hoechst fluorescence, indicating veryow Abcg2 type transporter activity (Fig. 2). H33342 fluorescencehanges upon Ko134 exposures were similarly low in MDCKII wildype cells used as negative control, whereas the MDCK2-BCRPells, which over-express human ABCG2 and were used as positiveontrol, showed significant Ko134 concentration-dependent fluo-escence increases as a consequence of reduced dye efflux activity.
Abcb11 transporter activity was explored, in the three liver cellines RTL-W1, R1 and RTH-149 and one non-liver cell line, RTG-, using DCDHFDA as fluorescent substrate and TAU as inhibitor.ccumulation of DCDHFDA was altered slightly in the fish liverell lines upon TAU exposure but variability of data was high and
uorescence changes showed no TAU concentration-dependencySupplement, Fig. S4). An ABCB11 over-expressing MDCKII celline was not available as positive control for this transporterype.able 3nhibitory concentrations (IC50 values, �M) and the ratios of maximal inhibitor effect to coransporter activity in trout cell lines. MDCKII-Pgp cells were used as control for Ca-AM effluatio of maximal emission and emission in controls; n: number of independent experime
Fluorescent substrate Calcein-AM
Inhibitor CsA PSC833
IC50 Emax/Econ2 n IC50 Emax
RTL-W1 4.49 ± 1.18 2.48 6 2.35 ± 1.33 2.30R1 4.69 ± 1.16 3.00 5 3.72 ± 1.17 2.45RTH-149 4.01 ± 1.20 2.35 5 3.08 ± 1.40 2.17RTG-2 1.21 ± 1.44 2.89 4 0.57 ± 1.58 2.66RTgill-W1 3.12 ± 1.24 2.19 5 4.14 ± 1.24 2.04RTgutGC 2.65 ± 1.16 2.71 3 2.98 ± 1.45 2.78RTbrain 4.63 ± 1.13 3.08 4 2.76 ± 1.46 2.09MDCKII-Pgp 5.03 ± 1.28 19.71 2 1.35 ± 1.39 17.55
(relative to 18S expression) n=3
um strong
4. Discussion
A range of permanent cell lines derived from various rain-bow trout tissues are available and useful for studying bioactiveand toxic potentials of environmental contaminants. We hereexamined nine ABC efflux transporters in order to evaluate theirpresence, gene expression and transport activities as putativeintegral elements of the MXR defence system in permanentrainbow trout cell lines. Based on homology to mammaliantransporters, the examined fish transporters are thought to beinvolved in phase 0 (Abcb1, Abcg2) and phase 3 (Abcc1 and 2,Abcg2) of cellular detoxification. These four transporters areconsidered as toxicologically relevant “multixenobiotic” trans-porters (Leslie et al., 2005). It is less clear if and how the otherfish efflux transporters examined (Abcb11, Abcc3-5) are involvedin cellular detoxification. However, it is conceivable that theiractivity is also of toxicologic relevance. Taken together, we found
that (1) transcripts of all examined transporters were present inall cell lines, (2) transcript levels of transporters differed, withAbcc transporters generally more highly expressed, (3) mRNAexpression patterns of transporters were rather similarntrol effect of model inhibitors with Ca-AM as substrate as a measure of Abcb1/Abccx and ABCB1 activity. IC50 values are given as means ± standard errors; Emax/Econ:
nts; n.d.: not determined.
REV 205 MK 571
/Econ n IC50 Emax/Econ n IC50 Emax/Econ n
5 n.d. 1.59 5 1.37 ± 1.29 2.35 65 n.d. 1.74 3 2.93 ± 1.15 3.31 54 n.d. 1.70 4 1.20 ± 1.42 2.04 54 n.d. 2.31 3 0.64 ± 1.51 2.87 34 n.d. 1.65 3 2.19 ± 1.25 2.14 52 n.d. 1.73 2 1.37 ± 1.18 3.05 23 n.d. 1.84 3 1.41 ± 1.29 3.58 42 n.d. 10.27 3 8.98 ± 1.56 2.63 2
S. Fischer et al. / Aquatic Toxicology 101 (2011) 438–446 443
A) RTL-W1
450
500
ol
B) R1
450
500
0
50
100
150
200
250
300
350
400
% f
luo
resc
ence
vs.
co
ntr
o*
* ***
** **
***
0
50
100
150
200
250
300
350
400
+ SDControl
- SD
**
* **
** *
**
*
*
**
*
D) RTG-2
350
400
450
500
*** *
*
**
*
C) RTH-149
350
400
450
500
s. c
on
tro
l
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
[CsA] (µM) [PSC833] (µ ( ]175KM[)M µM)[REV205] (µM)
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
[CsA] (µM) [PSC833] (µ ( ]175KM[)M µM)[REV205] (µM)
0
50
100
150
200
250
300
+ SDControl- SD
***
**
**
*
0
50
100
150
200
250
300
% f
luo
resc
enc
e v
s
*
* ** *
****
**
** *
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
[CsA] (µM) [PSC833] (µ ( ]175KM[)M µM)[REV205] (µM)
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
[CsA] (µM) [PSC833] (µ ( ]175KM[)M µM)[REV205] (µM)
F) RTgutGC
250
300
350
400
450
500
*
** *
*
*
*
*
*
**
*
*
E) RTgill-W1
250
300
350
400
450
500
en
ce v
s. c
on
tro
l
***
***
** * * * *
H) MDCKII-Pgp
0
50
100
150
200
+ SDControl- SD
**
* *
*
***
* * *
G) RTbrain
0
50
100
150
200
% f
luo
resc
e * ***
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
[CsA] (µM) [PSC833] (µ ( ]175KM[)M µM)[REV205] (µM)
0. 3 1 3
10
20
30
50
0. 3 1 3
10
20
30
50
0. 3 1 3
10
20
30
50
0. 3 1 3
10
20
30
50
[CsA] (µM) [PSC833] (µM) [MK571] (µM)[REV205] (µM)
H) MDCKII Pgp
1000
1500
2000
2500
*
*
*
* *
*
**
**
*
* * *
)
150
200
250
300
350
400
450
500
ore
scen
ce
vs. c
on
tro
l
** * *
***
*
*
*
*
*
**
+ SD
0
500
+ SDControl- SD
**
* * *
0
50
100
% f
lu + SDControl- SD
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0.3 1 3
10
20
30
50
0. 1
0.3 1
2.5 5 10
30
F lationt means ce (A
icttCt
maqat
[CsA] (µM) [PSC833] (µ ( ]175KM[)M µM)[REV205] (µM)
ig. 1. Effects of transporter inhibitors CsA, PSC833, REV205 and MK571 on accumuhe fluorescence values in control cultures (no inhibitor) set to 100%. The values areignificant differences to controls were determined with one-way analysis of varian
n the different cell lines with few exceptions and (4) aoncentration-dependent intracellular increase of accumula-ion of fluorescent substrate in the presence of pharmacologicransporter inhibitors was consistently observed for all cells whena-AM was used as proxy dye, indicating Abcb1 and/or Abcc effluxransporter activities.
Transcript abundances were comparatively high for abcc1-3,
oderate for abcc4-5 and abcg2, and low for abcb1a, abcb1b andbcb11. ABC transporter transcript levels have so far only beenuantified in one other permanent fish cell line, the P. lucida hep-toma cell line (PLHC-1) (Zaja et al., 2007). In agreement with therout liver and liver hepatoma cell lines (RTL-W1, R1, RTH-149),
[CsA] (µM) [PSC833] (µ ( ]175KM[)M µM)[REV205] (µM)
of Ca-AM in the permanent trout cell lines. The values are expressed as percent ofs ± standard deviations from independent experiments (n, see Table 3). StatisticallyNOVA) and Dunnett’s test and are indicated by asterisks (p < 0.05).
abcb1 and abcc3 mRNA levels were also found to be low in the PLHC-1 cells. Considering that abcc1 and abcc2 mRNAs were relativelyhighly expressed in the trout cells, but have not been quantified infish cell lines before, it will be interesting to determine whethermRNAs of those transporters are also more highly expressed inPLHC-1 cells.
Both the relative transcript abundance of the ABC transporters
and the expression patterns observed in the different rainbow troutcell lines are distinct from those recently identified in freshly iso-lated tissues from rainbow trout (Loncar et al., 2010). Transcriptlevels were particularly high for abcb11 in liver tissue, in analogyto mammalian liver. In contrast, abcb11 mRNA was among the low-444 S. Fischer et al. / Aquatic Toxicology 101 (2011) 438–446
300
350MDCK2-WTRtgill-W1RTL-W1 MDCK2-BCRP
** *
200
250
*
*
*
*
*
50
100
150
% f
luo
res
ce
nc
e v
s. c
on
tro
l
+ SDControl- SD
* * * ** *
0
[Ko134] (µM)
F st3334A rcentm gend o
eaotsom2rasaltad
dltSialmrpdptas
stccbo1sfa
ig. 2. Effects of the Abcg2 transporter inhibitor Ko134 on accumulation of HoechBCG2 transfected MDCKII-BCRP (positive control). The values are expressed as peeans ± standard deviations from three independent experiments. Statistics see le
st expressed in all rainbow trout cell lines in our study. Moreover,bcg2 transcripts were highly abundant in gonad tissue but werebserved at low levels in the RTG-2 cell line. Accordingly, no respec-ive transporter protein activities assayed with dye substrates andpecific inhibitors were detected, suggesting absence or low levelsf Abcb11 and Abcg2 proteins in the cell lines. Interestingly, abcc1RNA is present at very low levels in all trout tissues (Loncar et al.,
010), which was not observed for the cell lines. On the other hand,elative transcript expression levels were corresponding in tissuend the respective cell line for abcb1, which is equal to abcb1a in ourtudy. It was found at high levels in the brain (Loncar et al., 2010)nd was also found at comparatively high levels in the RTbrain celline. Further, for abcc4, low mRNA levels were found in rainbowrout liver samples and all investigated liver cell lines. This is inccordance to humans; ABCC4 has been reported to have barelyetectable mRNA levels in human liver (Lee et al., 1998).
Several scenarios should be considered when discussing theifferences in transcript levels in the tissues compared to cell
ines. First, monolayer cell cultures obviously do not possess thehree-dimensional structure of the tissue they were derived from.econdly, immortalization and dedifferentiation processes thatnevitably take place in stabilization of permanent cell lines maylso affect the mRNA expression of ABC transporters. Along theseines, Zaja et al. (2008b) found three- to four-fold lower levels of
RNA abundance for abcb1, abcb11 and abcc2 in 24 h old primaryainbow trout hepatocytes compared to freshly isolated liver sam-les. A time-dependent down-regulation of mRNA levels was alsoescribed for an influx transporter, the organic anion transporterolypeptide (OATP) in primary rainbow trout hepatocytes; in fact,his transporter was not detectable at mRNA levels in the RTgutGCnd RTL-W1 cell lines (Boaru et al., 2006). Thus, the differentiationtatus appears to have a strong influence on transporter expression.
A third aspect to consider is the absence or presence ofubstrates of the respective transporters in the in vitro cell cul-ure environment. Cells are cultured in media rich in definedomponents, such as amino acids and antibiotics, or undefinedomponents from the serum supplement, such as hormones andilirubin (see Table 1). These are known physiological substrates
f mammalian ABCC1-3 (Deeley and Cole, 2006; Keppler et al.,998) (see also Table 1) and the comparatively high mRNA expres-ion of these transporters indicates maintenance of physiologicalunctions in the rainbow trout cells. In analogy, low expression ofbcb1a/b and abcg2 mRNAs may be explained by a lack of xenobiotic2 in the permanent lines RTL-W1, RTgill-W1, MDCKII (wild type) and the humanof fluorescence values in control cultures (no inhibitor) set to 100%. The values aref Fig. 1.
substrates in routine cell culture. Finally, the low mRNA expres-sion levels of abcb11 in the cell lines, in opposite to its typicallyhigh expression in liver tissue (Gerloff et al., 1998; Loncar et al.,2010), may be due to both lack of three-dimensional structure andsubstrate availability. One of the primary functions of ABCB11 is theexcretion of bile acids into the canaliculus after bile acids have beenimported into hepatocytes basolaterally by transporters such asOATP (Alrefai and Gill, 2007). Thus, both the lack of OATP, prevent-ing uptake of bile acids, as well as the lack of the three-dimensionalstructure of the canaliculus likely explain the low level of mRNAabundance of abcb11.
Our study is the first to identify transcripts of abcb1b, the prod-uct of which, based on sequence similarity, can be classified asmember of the Abcb subfamily, but is distinct from the previouslypublished abcb1 (abcb1a) and the abcb11 sequences. The finding ofan additional putative abcb1 isoform is in line with the finding oftwo copies of the abcb1 gene in the zebrafish genome (Annilo et al.,2006). It is not yet clear how the two abcb1 isoforms functionallydiffer. It is conceivable that one of the two isoforms is comparableto mammalian ABCB4, which has a high degree of sequence simi-larity with ABCB1, but in contrast to ABCB1 has a narrow spectrumof substrates (van Helvoort et al., 1996).
We performed transporter activity assays based on theassumption that specific fluorescent substrates and pharmacologicinhibitors of mammalian transporters are also indicative of activ-ity of respective fish ABC transporter homologs. This approachhas been used previously to identify specific constitutive ABCtransporter activities in fish cells, e.g. in trout primary hepato-cytes (Sturm et al., 2001; Zaja et al., 2008b) and PLHC cells (Zajaet al., 2007). Indeed, a doxorubicin-selected, Abcb1 over-expressingPLHC cell clone revealed similarity of fish vs. mammalian ABCB1substrates and inhibitors (Zaja et al., 2008a). Specifically, Ca-AMaccumulation was significantly greater in this cell line comparedto its wild type counterpart when CsA and PSC833 were added asinhibitors, demonstrating inhibition of the fish Abcb1 activity asexpected for mammalian ABCB1. MK571, inhibitor of mammalianABCC type efflux pumps, did on the other hand not enhance Ca-AM accumulation in the Abcb1-overexpressing fish cell line, which
suggests a lack of interaction of MK571 also with fish Abcb1.The inhibitors CsA, PSC833 and MK571 led to an increased accu-mulation of Ca-AM in all seven wild type rainbow trout cell linesinvestigated here. According to Zaja et al. (2008a), CsA and PSC833are indicative of fish Abcb1 activity. Yet, considering the virtual
oxicolo
aaasiTAemcCfdefact
ieratOo2ttAlc
etApbRl
dwawawtwtee2fipiere
A
S
S. Fischer et al. / Aquatic T
bsence of abcb1 transcripts in the trout cell lines, the effects of CsAnd PSC833 were surprising. One possible explanation is discrep-ncy of transcript and protein expression levels, with protein beingufficiently abundant for mediating efflux. Another interpretations interference of the inhibitors with additional ABC transporters.hus, CsA has been found to also block mammalian ABCC andBCG2 transporters (Germann et al., 1997; Leier et al., 1994; Qadirt al., 2005) and, to a lesser extent, PSC833 suppresses activity ofammalian ABCC transporters (Leier et al., 1994). Additional indi-
ation for unspecific blocking of other transporters than Abcb1 bysA and PSC833 in our experiments can be taken from IC50 values
or Ca-AM efflux inhibition. Considering far lower protein abun-ance in the trout than in the MDCKII-Pgp cell lines one wouldxpect lower IC50 values in the trout cells. However, IC50 valuesor CsA and PSC833 were in comparable ranges, indicating specificction of the inhibitors on ABCB1 in the transfected MDCKII-Pgpells, but less specific targeting of Ca-AM mediating transporters inhe trout cell lines.
MK571 was comparatively potent as inhibitor of Ca-AM effluxn all trout cell lines, in accordance with high mRNA transcript lev-ls for the abcc transporter types (Fig. 1). Analogous results wereeported for primary rainbow trout hepatocytes where MK571 wasn inhibitor of Ca-AM efflux and transcript levels of the studied abccransporter (abcc2) were higher than for abcb1 (Zaja et al., 2008a).n the other hand, MK571 effects on the Abcb1 activity in Abcb1ver-expressing PLHC cells were far less pronounced (Zaja et al.,008a), similar to the MK571 effects on the MDCKII-Pgp cells inhis study (Fig. 1H). Thus, MK571 appears to be specific for Abccransporters in fish cells, implying that the MK571 effects on Ca-M efflux in our experiments indicate Abcc activity in the trout cell
ines. However, as mentioned above, the Abcc transporters are alsoandidates to be additionally targeted by CsA and PSC833.
Both CsA and PSC833 were more potent inhibitors of Ca-AMfflux than REV205 (Fig. 1). Similar results have been reported withhe PLHC1 fish cell line where REV205 showed little effect on Ca-M efflux (Zaja et al., 2007). This may be due to the lower inhibitoryotency of REV205 with regard to Abcb1, which was also indicatedy our experiments with the MDCKII-Pgp cells (Fig. 1H). In addition,EV205 may be more specific to Abcb1 efflux activity with no or
ittle interference with other transporter types.In conclusion, our transporter activity studies indicated pre-
ominance of Abcc type efflux in the trout cell lines, in accordanceith mRNA levels. Abcc efflux activities are probably mainly medi-
ted by Abcc1-3, as Abcc4 and Abcc5 mRNA expression levelsere substantially lower. Physiological substrates of ABCC1-3 are
bundantly present in routine cell culture media (see Table 1)hich may lead to the maintenance of these physiological func-
ions in the cells. Also for human cell lines ABCC transportersere found to be more highly expressed compared to respec-
ive tissues (Vellonen et al., 2010) and general “background” ABCCfflux activity was found in non-MDR cell lines that were suppos-dly sensitive to toxic MDR substrates (Yeheskely-Hayon et al.,009). Thus, a pronounced ABCC/Abcc activity may be a generaleature of cell lines. This cellular “background” efflux activity maynfluence the dynamics of uptake, retention and extrusion of com-ounds and their metabolites, which may be important to consider
n toxicological experiments. Substrate/inhibitor specificities andnvironmental relevance of single fish ABC efflux transportersemain to be addressed in future work utilizing appropriate het-rologous expression systems and recombinant protein assays.
cknowledgments
This study was supported by the German Academic Exchangeervice (DAAD) and the Croatian Ministry of Science, Education
gy 101 (2011) 438–446 445
and Sport (Project No. 098-0982934-2745). We wish to thank MirkoPietsch for his contribution to obtain the abcc4 and abcc5 sequences,Ute Lohse for technical support with DNA sequencing, Peggy Well-ner for her great technical support in culturing of all the cell linesand Marta Popovic for helpful discussions.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, inthe online version, at doi:10.1016/j.aquatox.2010.11.010.
References
Ahne, W., 1985. Untersuchungen über die Verwendung von Fischzellkulturen fürToxizitätsbestimmungen zur Einschränkung und Ersatz des Fischtests. Zen-tralbl. Bakteriol. Mikrobiol. Hyg. B 180, 480–504.
Allen, J.D., van Loevezijn, A., Lakhai, J.M., van der Valk, M., van Tellingen, O., Reid, G.,Schellens, J.H., Koomen, G.J., Schinkel, A.H., 2002. Potent and specific inhibitionof the breast cancer resistance protein multidrug transporter in vitro and inmouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 1,417–425.
Alrefai, W.A., Gill, R.K., 2007. Bile acid transporters: structure, function, regulationand pathophysiological implications. Pharm. Res. 24, 1803–1823.
Annilo, T., Chen, Z.Q., Shulenin, S., Costantino, J., Thomas, L., Lou, H., Stefanov, S.,Dean, M., 2006. Evolution of the vertebrate ABC gene family: analysis of genebirth and death. Genomics 88, 1–11.
Boaru, D.A., Dragos, N., Schirmer, K., 2006. Microcystin-LR induced cellular effects inmammalian and fish primary hepatocyte cultures and cell lines: a comparativestudy. Toxicology 218, 134–148.
Bols, N.C., Barlian, A., Chirinotrejo, M., Caldwell, S.J., Goegan, P., Lee, L.E.J., 1994.Development of a cell line from primary cultures of rainbow trout, Oncorhynchusmykiss (Walbaum), gills. J. Fish Dis. 17, 601–611.
Bols, N.C., Dayeh, V.R., Lee, L.E.J., Schirmer, K., 2005. Use of fish cell lines in thetoxicology and ecotoxicology of fish. In: Mommsen, T.P., Moon, T.W. (Eds.), Bio-chemistry and Molecular Biology of Fishes. Elsevier Science, Amsterdam, pp.43–84.
Borst, P., Evers, R., Kool, M., Wijnholds, J., 2000. A family of drug transporters: themultidrug resistance-associated proteins. J Natl. Cancer Inst. 92, 1295–1302.
Cordon-Cardo, C., O’Brien, J.P., Boccia, J., Casals, D., Bertino, J.R., Melamed, M.R., 1990.Expression of the multidrug resistance gene product (P-glycoprotein) in humannormal and tumor tissues. J. Histochem. Cytochem. 38, 1277–1287.
Dean, M., Rzhetsky, A., Allikmets, R., 2001. The human ATP-binding cassette (ABC)transporter superfamily. Genome Res. 11, 1156–1166.
Deeley, R.G., Westlake, C., Cole, S.P., 2006. Transmembrane transport of endo- andxenobiotics by mammalian ATP-binding cassette multidrug resistance proteins.Physiol. Rev. 86, 849–899.
Deeley, R.G., Cole, S.P., 2006. Substrate recognition and transport by multidrug resis-tance protein 1 (ABCC1). FEBS Lett. 580, 1103–1111.
Doyle, L.A., Ross, D.D., 2003. Multidrug resistance mediated by the breast cancerresistance protein BCRP (ABCG2). Oncogene. 22, 7340–7358.
Epel, D., Luckenbach, T., Stevenson, C.N., Macmanus-Spencer, L.A., Hamdoun, A., Smi-tal, T., 2008. Efflux transporters: newly appreciated roles in protection againstpollutants. Environ. Sci. Technol. 42, 3914–3920.
Essodaigui, M., Broxterman, H.J., Garnier-Suillerot, A., 1998. Kinetic analysis ofcalcein and calcein-acetoxymethylester efflux mediated by the multidrug resis-tance protein and P-glycoprotein. Biochemistry 37, 2243–2250.
Evers, R., Kool, M., Smith, A.J., van Deemter, L., de Haas, M., Borst, P., 2000. Inhibitoryeffect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-,MRP1- and MRP2-mediated transport. Br. J. Cancer 83, 366–374.
Fischer, S., Pietsch, M., Schirmer, K., Luckenbach, T., 2010. Identification ofmulti-drug resistance associated proteins MRP1 (ABCC1) and MRP3 (ABCC3)from rainbow trout (Oncorhynchus mykiss). Mar. Environ. Res. 69 (Suppl.),S7–S10.
Gekeler, V., Ise, W., Sanders, K.H., Ulrich, W.R., Beck, J., 1995. The leukotriene LTD4receptor antagonist MK571 specifically modulates MRP associated multidrugresistance. Biochem. Biophys. Res. Commun. 208, 345–352.
Gerloff, T., Stieger, B., Hagenbuch, B., Madon, J., Landmann, L., Roth, J., Hofmann, A.F.,Meier, P.J., 1998. The sister of P-glycoprotein represents the canalicular bile saltexport pump of mammalian liver. J. Biol. Chem. 273, 10046–10050.
Germann, U.A., Ford, P.J., Shlyakhter, D., Mason, V.S., Harding, M.W., 1997.Chemosensitization and drug accumulation effects of VX-710, verapamil,cyclosporin A, MS-209 and GF120918 in multidrug resistant HL60/ADR cellsexpressing the multidrug resistance-associated protein MRP. Anticancer Drugs8, 141–155.
Gottesman, M.M., Pastan, I., 1988. The multidrug transporter, a double-edged sword.
J. Biol. Chem. 263, 12163–12166.Haimeur, A., Conseil, G., Deeley, R.G., Cole, S.P., 2004. The MRP-related andBCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity andregulation. Curr Drug Metab. 5, 21–53.
Higgins, C.F., 2007. Multiple molecular mechanisms for multidrug resistance trans-porters. Nature 446, 749–757.
4 oxicolo
H
J
K
K
K
L
L
L
L
L
L
L
L
L
M
M
N
46 S. Fischer et al. / Aquatic T
ollo, Z., Homolya, L., Davis, C.W., Sarkadi, B., 1994. Calcein accumulation as a fluo-rometric functional assay of the multidrug transporter. Biochim. Biophys. Acta1191, 384–388.
onker, J.W., Buitelaar, M., Wagenaar, E., Van Der Valk, M.A., Scheffer, G.L., Scheper,R.J., Plosch, T., Kuipers, F., Elferink, R.P., Rosing, H., Beijnen, J.H., Schinkel, A.H.,2002. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. U.S.A. 99,15649–15654.
awano, A., Haiduk, C., Schirmer, K., Hanner, R., Lee, L., Dixon, B., Bols,N. Development of a rainbow trout intestinal epithelial cell line and itsresponse to lipopolysaccharide. Aquacult. Nutr, in press, doi:10.1111/j.1365-2095.2010.00757.x.
eppler, D., Jedlitschky, G., Leier, I., 1998. Transport function and substrate specificityof multidrug resistance protein. Methods Enzymol. 292, 607–616.
urelec, B., 1992. The multixenobiotic resistance mechanism in aquatic organisms.Crit. Rev. Toxicol. 22, 23–43.
annan, C.N., Winton, J.R., Fryer, J.L., 1984. Fish cell lines: establishment and charac-terization of nine cell lines from salmonids. In Vitro 20, 671–676.
atunde-Dada, G.O., Simpson, R.J., McKie, A.T., 2006. Recent advances in mammalianhaem transport. Trends Biochem Sci. 31, 182–188.
ee, K., Belinsky, M.G., Bell, D.W., Testa, J.R., Kruh, G.D., 1998. Isolation of MOAT-B, a widely expressed multidrug resistance-associated protein/canalicularmultispecific organic anion transporter-related transporter. Cancer Res. 58,2741–2747.
ee, L.E., Clemons, J.H., Bechtel, D.G., Caldwell, S.J., Han, K.B., Pasitschniak-Arts, M.,Mosser, D.D., Bols, N.C., 1993. Development and characterization of a rainbowtrout liver cell line expressing cytochrome P450-dependent monooxygenaseactivity. Cell Biol. Toxicol. 9, 279–294.
eier, I., Jedlitschky, G., Buchholz, U., Cole, S.P., Deeley, R.G., Keppler, D., 1994. TheMRP gene encodes an ATP-dependent export pump for leukotriene C4 and struc-turally related conjugates. J. Biol. Chem. 269, 27807–27810.
eslie, E.M., Deeley, R.G., Cole, S.P., 2005. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl.Pharmacol. 204, 216–237.
itman, T., Druley, T.E., Stein, W.D., Bates, S.E., 2001. From MDR to MXR: newunderstanding of multidrug resistance systems, their properties and clinicalsignificance. Cell. Mol. Life Sci. 58, 931–959.
oncar, J., Popovic, M., Zaja, R., Smital, T., 2010. Gene expression analysis of theABC efflux transporters in rainbow trout (Oncorhynchus mykiss). Comp. Biochem.Physiol. C: Toxicol. Pharmacol. 151, 209–215.
uckenbach, T., Epel, D., 2008. ABCB- and ABCC-type transporters confer multixeno-biotic resistance and form an environment-tissue barrier in bivalve gills. Am. J.Physiol. Regul. Integr. Comp. Physiol. 294, R1919–1929.
ayer, U., Wagenaar, E., Dorobek, B., Beijnen, J.H., Borst, P., Schinkel, A.H., 1997. Fullblockade of intestinal P-glycoprotein and extensive inhibition of blood–brainbarrier P-glycoprotein by oral treatment of mice with PSC833. J. Clin. Invest.
100, 2430–2436.uller, P.Y., Janovjak, H., Miserez, A.R., Dobbie, Z., 2002. Processing of geneexpression data generated by quantitative real-time RT-PCR. Biotechniques 32,1372–1374, 1376, 1378–1379.
oe, J., Stieger, B., Meier, P.J., 2002. Functional expression of the canalicular bile saltexport pump of human liver. Gastroenterology 123, 1659–1666.
gy 101 (2011) 438–446
Qadir, M., O’Loughlin, K.L., Fricke, S.M., Williamson, N.A., Greco, W.R., Minderman,H., Baer, M.R., 2005. Cyclosporin A is a broad-spectrum multidrug resistancemodulator. Clin. Cancer Res. 11, 2320–2326.
Sarkadi, B., Homolya, L., Szakacs, G., Varadi, A., 2006. Human multidrug resistanceABCB and ABCG transporters: participation in a chemoimmunity defense sys-tem. Physiol. Rev. 86, 1179–1236.
Sharom, F.J., Yu, X., Lu, P., Liu, R., Chu, J.W., Szabo, K., Muller, M., Hose, C.D., Monks,A., Varadi, A., Seprodi, J., Sarkadi, B., 1999. Interaction of the P-glycoproteinmultidrug transporter (MDR1) with high affinity peptide chemosensitizers inisolated membranes, reconstituted systems, and intact cells. Biochem. Pharma-col. 58, 571–586.
Simon, P., 2003. Q-Gene: processing quantitative real-time RT-PCR data. Bioinfor-matics 19, 1439–1440.
Sturm, A., Segner, H., 2005. P-glycoproteins and xenobiotic efflux transport in fish.In: Mommsen, T.P., Moon, T.W. (Eds.), Biochemistry and Molecular Biology ofFishes. Elsevier, Amsterdam, pp. 495–533.
Sturm, A., Ziemann, C., Hirsch-Ernst, K.I., Segner, H., 2001. Expression and functionalactivity of P-glycoprotein in cultured hepatocytes from Oncorhynchus mykiss.Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1119–1126.
Szakacs, G., Varadi, A., Ozvegy-Laczka, C., Sarkadi, B., 2008. The role of ABC trans-porters in drug absorption, distribution, metabolism, excretion and toxicity(ADME-Tox). Drug Discov. Today 13, 379–393.
Thomas, N.K., Brown, T.J., 2010. ABC transporters do not contribute to extracellulartranslocation of hyaluronan in human breast cancer in vitro. Exp. Cell Res. 316,1241–1253.
van Helvoort, A., Smith, A.J., Sprong, H., Fritzsche, I., Schinkel, A.H., Borst, P., vanMeer, G., 1996. MDR1 P-glycoprotein is a lipid translocase of broad specificity,while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell87, 507–517.
Vellonen, K.S., Mannermaa, E., Turner, H., Hakli, M., Wolosin, J.M., Tervo, T.,Honkakoski, P., Urtti, A., 2010. Effluxing ABC transporters in human cornealepithelium. J. Pharmacol. Sci. 99, 1087–1098.
Wang, E.J., Casciano, C.N., Clement, R.P., Johnson, W.W., 2003. Fluorescent substratesof sister-P-glycoprotein (BSEP) evaluated as markers of active transport andinhibition: evidence for contingent unequal binding sites. Pharmacol. Res. 20,537–544.
Wolf, K., Quimby, M.C., 1962. Established eurythermic line of fish cells in vitro.Science 135, 1065–1066.
Yeheskely-Hayon, D., Regev, R., Katzir, H., Eytan, G.D., 2009. Competition betweeninnate multidrug resistance and intracellular binding of rhodamine dyes. FEBSJ. 276, 637–648.
Zaja, R., Caminada, D., Loncar, J., Fent, K., Smital, T., 2008a. Development andcharacterization of P-glycoprotein 1 (Pgp1, ABCB1)-mediated doxorubicin-resistant PLHC-1 hepatoma fish cell line. Toxicol. Appl. Pharmacol. 227,207–218.
Zaja, R., Klobucar, R.S., Smital, T., 2007. Detection and functional characterization
of Pgp1 (ABCB1) and MRP3 (ABCC3) efflux transporters in the PLHC-1 fish hep-atoma cell line. Aquat. Toxicol. 81, 365–376.Zaja, R., Munic, V., Klobucar, R.S., Ambriovic-Ristov, A., Smital, T., 2008b. Cloning andmolecular characterization of apical efflux transporters (ABCB1, ABCB11 andABCC2) in rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 90,322–332.