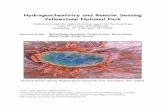
A geothermal-linked biological oasis in Yellowstone Lake, Yellowstone National Park, Wyoming
-
Upload
independent -
Category
Documents
-
view
2 -
download
0
Transcript of A geothermal-linked biological oasis in Yellowstone Lake, Yellowstone National Park, Wyoming
A geothermal-linked biological oasis in Yellowstone Lake,Yellowstone National Park, WyomingD. LOVALVO,1 ,* S. R. CLINGENPEEL,2 ,* S. MCGINNIS,3 R. E. MACUR,2 J . D. VARLEY,3
W. P. INSKEEP,2 J . GLIME,4 K. NEALSON5 AND T. R. MCDERMOTT2
1Eastern Oceanics, West Redding, CT, USA2Thermal Biology Institute, Montana State University, Bozeman, MT, USA3Big Sky Institute, Montana State University, Bozeman, MT, USA4Department of Biological Sciences, Michigan Technological University, Houghton, MI, USA5Department of Earth Sciences, University of Southern California, Los Angeles, CA; and JC. Venter Institute, La Jolla, CA, USA
ABSTRACT
Hundreds of active and dormant geothermal vents have been located on the floor of Yellowstone Lake, although
characterization of the associated biology (macro or micro) has been extremely limited. Herein, we describe an
aquatic moss (Fontinalis) colony closely associated with vent emissions that considerably exceeded known
temperature maxima for this plant. Vent waters were supersaturated with CO2, likely accommodating a CO2
compensation point that would be expected to be quite elevated under these conditions. The moss was colo-
nized by metazoa, including the crustaceans Hyalella and Gammarus, a segmented worm in the Lumbriculidae
family, and a flatworm specimen tentatively identified as Polycelis. The presence of these invertebrates suggest
a highly localized food chain that derives from the presence of geothermal inputs and thus is analogous to the
deep marine vents that support significant biodiversity.
Received 17 December 2009; accepted 5 April 2010
Corresponding authors: Timothy R. McDermott. Tel.: 406-994-2190; fax: 406-994-3933; e-mail: timmcder@
montana.edu; John D. Varley. Tel.: 406-994-2320; fax: 406-994-5122; e-mail: [email protected]
INTRODUCTION
Yellowstone Lake contains hundreds of hydrothermal vents
(Morgan et al., 2007) that contribute roughly 10% to the
total geothermal venting activity in the Yellowstone geother-
mal complex (Balistrieri et al., 2007). The lake bottom has
been mapped three different times over the last 136 years,
with vent exploration being the focus of work during the last
quarter century. The majority of the Yellowstone Lake hydro-
thermal vents have been documented using bathymetric and
seismic approaches, and a submersible remote operating
vehicle (ROV) (see Morgan et al., 2007, for comprehensive
summary). The vents are primarily clustered in the northern
half of the lake, spanning from the West Thumb to the Mary
Bay regions, and all appear to be within the boundary of the
current Yellowstone caldera (Morgan et al., 2007).
Some vent activity has been located visually as gas bubbles
or turbulence on the lake surface. Evidence of high output
vents in the West Thumb area (Fig. S1) originally derived
from observations of open ice during the winter, which sug-
gested subsurface geothermal vent activity was maintaining
water column temperatures above freezing. Subsequent ROV
dives discovered a large vent cone and a rock outcrop shelf
structure that emits large volumes of warm water and gas.
Interestingly, this particular vent seemed unique relative to all
other active vents thus far observed in the lake in that it is
robustly colonized by plants. In all cases, the plants appeared
very closely associated with vent emissions (http://www.
tbi.montana.edu/media/Fontinalis_vent.html); i.e. only
where water and gas venting activity was visually obvious.
The occurrence of higher plants so closely associated with
venting activity was of interest not only because of plant–
temperature relationships, but also because of the relative
depth and very low light conditions at this site. Also, while
microbiological diversity and novelty associated with Yellow-
stone’s diverse terrestrial geothermal features has been well
documented (e.g., Brouns et al., 2005; Inskeep & McDer-
mott, 2005; Reysenbach et al., 2005; Sheehan et al., 2005;
Spear et al., 2005; Ward & Cohan, 2005; Young et al., 2005;
Madigan et al., 2005), except for cursory visual descriptions*Both authors contributed equally.
� 2010 Blackwell Publishing Ltd 327
Geobiology (2010), 8, 327–336 DOI: 10.1111/j.1472-4669.2010.00244.x
of microbial mats and a report of unidentified macrophytes in
the vicinity of vents at SCUBA depths (Remsen et al., 2002),
relatively little is known about the biology, micro or macro,
associated with the hydrothermal features on the Yellowstone
Lake floor. The study described herein provides the first
in-depth identification of the biology associated with any vent
in this lake, focusing in this case on the above-mentioned
plant colony and the mesofauna found to inhabit it.
MATERIALS AND METHODS
Sampling
The sampling site was in the West Thumb region of Yellow-
stone Lake, approximately 0.5 km offshore from the West
Thumb geyser basin (Fig. S1), with the coordinates
44�24.720¢ N, 110�33.616¢ W. The ROV used in this work
(Fig. 1) was first mentioned by Cuhel et al. (2002), but
additional description is provided here (see below) to more
thoroughly document the ROV’s instrumentation and
sampling capability important for the work conducted in this
study. The National Park Service boat ‘Cutthroat’ was used
for all sampling work.
Geochemical and light analyses
Geochemical analyses were either performed immediately or
samples were appropriately preserved and stored until further
analyses could be conducted at the field-based laboratory
located at Lake Village or at Montana State University in
Bozeman. Several redox sensitive species were immediately
analyzed onboard the ship, including FeII and FeIII using the
Ferrozine method with filtered samples (0.2 lm; To et al.,
1999) and total dissolved sulfide (DS) using the amine sulfuric
acid method (APHA, 1998) with unfiltered samples [to avoid
rapid degassing of H2S(aq) upon filtration]. Aqueous pH val-
ues were obtained using a Fisher Accumet AP-71 meter and
AP-55 probe equipped with temperature compensation.
Additional aqueous samples were filtered (0.2 lm) directly
into sterile 50 mL Falcon tubes and refrigerated at 4 �C. Two
tubes were preserved with trace metal grade HNO3 (1%) and
HCl (0.5%) for analysis using inductively coupled plasma
instrumentation [ICP-OES and ICP-MS (Aligent Model
7500)] for total dissolved elements including Ag, Al, As, Ba,
Be, Bi, B, Ca, Cd, Ce, Co, Cr, Cs, Cu, Dy, Er, Eu, Fe, Ga,
Gd, Ge, Hf, Ho, In, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd,
Ni, P, Pb, Pr, Re, Rb, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Te, Tb,
Th, Ti, Tl, Tm, U, V, W, Y, Yb, Zn, and Zr. One unacidified
tube was analyzed for predominant inorganic anions (F),
Cl), SO42), NO3
), CO32), S2O3
2), AsO43)) using anion
exchange chromatography (Dionex DX 500; AS16-4 mm
column), and aqueous NH4+ using the phenolate colorimetric
(A630nm) procedure on a flow injection analyzer (APHA,
1998). Dissolved inorganic C (DIC) and dissolved organic C
(DOC) were determined on separate samples taken in closed
headspace serum bottles (previously baked at 500 �C) using a
Shimatzu Model TOC-VCSH total C analyzer.
Concentrations of dissolved O2 in vent fluids were deter-
mined immediately after collection using the Winkler method
(APHA, 1998). Dissolved H2, CH4, and CO2 were deter-
mined using headspace gas chromatography as described
previously by Inskeep et al. (2005). Closed headspace
aqueous samples were collected by using either the ROV
syringe or a peristaltic pump to push vent fluids through
an inline 140 mm diameter filter (0.2 lm) and into sterile
(autoclaved) 160-mL serum bottles. After a three head space-
volume purge, the serum bottles were capped with zero
headspace using sterile (autoclaved) butyl stoppers. A known
Sampling arm
Sampling cup w/temp probe
Tether
Water/sample Water
A B
CBuoyancy
Sampling arm(deployed)
Tether
Water/sampleintake exit
Video
35 mm camera &
strobe Samplingsyringe
Tether
SonarSonarRunning light
Samplecamera basket
Fig. 1 Photographs of the ROV used for sampling
of the geothermal vent and moss. (A) Functional
description of ROV equipment. (B) Vacuum sampler
collection can. (C) Deployment of the ROV immedi-
ately after release from the boat tether.
328 D. LOVALVO et al .
� 2010 Blackwell Publishing Ltd
volume of liquid (�30 mL) was withdrawn and replaced with
an equivalent volume of filter-sterilized (0.2 lm) air and the
bottles were incubated with intermittent shaking for 60 min.
Samples of the gas headspace were injected into a portable
dual-channel Varian gas chromatograph (Model CP2900)
equipped with thermal conductivity detectors. One of the
channels utilized a 10-m 5-A molecular sieve column (60 �C)
and UHP Ar carrier gas (21 psi) to separate H2 and the other
channel utilized a 10 m PPQ (PoraPlotQ) column (70 �C)
and UHP He carrier gas (18 psi) to separate CH4 and CO2.
Gas concentrations measured in the headspace were then used
to calculate the gas concentrations in the original solution
using temperature-corrected Henry’s Law constants and a
mass balance equation:
Henry’s Law:
KH ¼ c(aq)=c(g)
Mass Balance:
½c(aq)sampleVL� ¼ ½c(g)equilVg;eq� þ ½c(aq)equilVL;eq�;
where the KH is Henry’s Law constant at the incubation tem-
perature (KH calculated using thermodynamic constants
reported in Amend & Shock, 2001), c is concentration in
either aqueous or gas phases (mol L)1), and V is volume of
liquid or gas.
Light intensity was measured using an International Light
Technologies Model ILT 1700 radiometer by lowering a light
sensor to various depths in the water column overlying the
vent. The light sensor was calibrated to read visible light at
400–700 nm, with primary sensitivity at 555 nm.
Scanning electron microscope energy-dispersive X-ray
analysis
Portions of the solid phase material collected were air-dried,
mounted and sputter-coated with carbon, and imaged with
the SEM (JEOL 6100). Elemental spectra were acquired from
representative spots using Energy Dispersive X-rays (NORAN
detector with ROENTEC software, Bruker AXS Microanalysis,
Ewing, NJ, USA). Quantitative analysis from each spectrum
was run to obtain an elemental profile.
Moss identification
Moss samples were stored frozen until identified. Initial iden-
tification was based on leaf morphology features followed by
phylogenetic assessments. For the latter, the chloroplast rbcL
gene, tRNA-Leu ⁄ Phe spacer, and nuclear ITS region DNA
were PCR cloned and sequenced. DNA was extracted from
washed leaves using methods previously described by Botero
et al. (2005), and then used as templates for 50 lL PCRs
containing 1.5 mM MgCl2, 20 lg BSA, 0.2 mM each dNTP,
1 lM each primer, and 1.25 u Taq polymerase. The PCR
programs were as follows:
1. The rbcL gene was amplified as two overlapping
fragments. The first fragment used the primers rbcL-73F (5¢-ATACCAAAGATGTTTTTTTATAAG-3¢) and rbcL804hR
(5¢-TGCAGTAAAACCACCTG-3¢) (Tsubota et al., 1999).
The PCR program was: 94 �C for 5 min, 30 cycles of 94 �Cfor 1 min, 40 �C for 1 min, and 72 �C for 1:30 min, 72 �Cfor 7 min, and 4 �C hold. The second fragment used primers
FontF (5¢-TYATGCGTTGGMGWGAYCGT-3¢) and FontR
(5¢-TCTAAGGCAACTCTATTAGCAAC-3¢). PCR condi-
tions were: 94 �C for 5 min, 30 cycles of 94 �C for 1 min,
50 �C for 1 min, and 72 �C for 1 min, 72 �C for 7 min, and
4 �C hold.
2. The tRNA-Leu ⁄ Phe region was amplified using primers
trnC (5¢-CGAAATCGGTAGACGCTACG-3¢) and trnF (5¢-ATTTGAACTGGTGACACGAG-3¢), using the program:
94 �C for 5 min, 30 cycles of 94 �C for 45 s, 52 �C for 45 s,
and 72 �C for 45 s, 72 �C for 7 min, and 4 �C hold.
3. The ITS region was amplified using primers BMBC-R
(5¢-GTACACACCGCCCGTCG-3¢) and LS4-R (5¢-TCAA
GCACTCTTTGACTCTC-3¢) (Shaw & Allen, 2000), using
the program: 94 �C for 5 min, 30 cycles of 94 �C for 1 min,
50 �C for 1:45 min, and 72 �C for 1 min, 72 �C for 7 min,
and 4 �C hold. In addition to these primers, sequencing
was accomplished using the internal primers ITS2
(5¢-GCTGCGTTCTTCATCGATGC-3¢), ITS3 (5¢-GCATC-
GATGAAGAACGCAGC-3¢), ITS4 (5¢-TCCTCCGCTTAT-
TGATATGC-3¢), and ITS5 (5¢-GGAAGTAAAAGTCGTAA-
CAAGG-3¢) (Baldwin, 1992).
Mesofauna identification
All samples were immediately preserved in 80% ethanol on
the boat. As with the plant, identification followed two
approaches: (i) traditional Linnean taxonomy and (ii)
molecular phylogeny. For the Linnean taxonomic approach,
the Pennak (1989) key was used for the crustacean and
flatworm specimens, whereas the segmented worm was
keyed from Kathman & Brinkhurst (1998). For phylo-
genetic analysis, DNA was obtained by using the FastDNA
Spin Kit for Soil (MP Biomedicals, Solon, OH, USA) on
tissue dissected from the organisms, and then PCRs ampli-
fied the 5¢ end of the 18S rRNA gene and a portion of
the cytochrome c oxidase subunit I (COI) gene using the
following protocols:
18S rRNA gene: Amplification used primers SSU F04
(5¢-GCTTGTCTCAAAGATTAAGCCC-3¢) and SSU R22
(5¢-GCCTGCTGCCTTCCTTGGA-3¢) (Meldal et al., 2007)
with a PCR program of 94 �C for 5 min, 30 cycles of 94 �Cfor 45 s, 50 �C for 45 s, and 72 �C for 45 s, 72 �C for 7 min,
and 4 �C hold.
Geothermal-linked biological oasis 329
� 2010 Blackwell Publishing Ltd
COI gene: A portion of the COI gene was amplified using
primers pr-a2 (5¢-AGCTGCAGTTTTGGTTTTTTGGA-3¢)and pr-b2 (5¢-ATGAGCAACAACATAATAAGTATCATG-
3¢) for the planarian (Bessho et al., 1992) and primers
LCO1490 (5¢-GGTCAACAAATCATAAAGATATTGG-3¢)and HCO2198 (5¢-TAAACTTCAGGGTGACCAAAAAA-
TCA-3¢) for the other organisms (Hou et al., 2007). Both
used the PCR program given above for the 18S rRNA gene.
All amplicon sequences were compared against public data-
bases using BLAST 2.2.22 (Altschul et al., 1997). Phyloge-
netic analysis was performed on the amplicons. Sequences
were aligned using CLUSTALW (Thompson et al., 1997) and
then a Maximum Likelihood phylogenetic trees were con-
structed using PAUP 4.0b10 (Sinauer Associates Inc., Sunder-
land, MA, USA).
Accession numbers
GenBank accession numbers for all gene sequences generated
and described in this study are as follows: Gammarus 18S
rRNA gene, GU066807; Hyalella 18S rRNA gene,
GU066808; Lamprodrilus 18S rRNA gene, GU066809;
Polycelis 18S rRNA gene, GU066810; Gammarus COI (cyto-
chrome c), GU066811; Hyalella COI, GU066812; Lampro-
drilus COI, GU066813; Polycelis COI, GU066814;
Fontinalis rbcL, GU066815; Fontinalis ITS, GU066816;
Fontinalis tRNA, GU066817.
RESULTS
ROV Description
The ROV (Fig. 1) is fully operator controlled via thrusters that
allow for lake floor reconnaissance and precise ROV position-
ing for sampling, and is equipped with a mechanical arm that
allows for probing and sampling of vent openings. A sampling
cup at the end of the mechanical arm encloses suction tube
openings as well as two calibrated temperature sensors (deli-
berate redundancy) that allows for real-time temperature
determination. Live feed video allows for real-time viewing,
vent identification, and guides sampling efforts. Operator-
controlled syringes (1.0 L capacity each) are located on the
port and starboard sides of the ROV (Fig. 1A), and allow water
samples to be taken for gas and solute chemistry determina-
tions. In addition, a separate suction tube located in the
sampling cup is connected to a vacuum device (Fig 1B) that
passes water and sample into a 1.0-L container outfitted with a
screen covering (1 mm mesh) the exit port to allow water to
pass through while collecting solid phase material. This instru-
ment was used to obtain samples of plant branches and leaves,
and to glean vent emission sediment and metazoa attached to
the moss leaves and branches. Also, a continuous Norprene�
tubing connection from the sampling cup to the boat allows
for large volume (e.g. 300 L) water samples brought to the
surface via a peristaltic pump located on the boat deck. The
ROV is tethered to the boat via an electrical ⁄ optical umbilical,
which facilitates operator communication with the ROV and
also includes the peristaltic pump tubing. The ROV also
carries a solid sample collection basket, a color sector scan
sonar system, and a 35-mm camera and strobe.
Plant identification
This report focuses on the intriguing and robust colony of a
macrophyte that visually appeared to be of a single type and
that was closely associated with vent emissions (Fig. 2)
(http://www.tbi.montana.edu/media/Fontinalis_vent.html).
Initial inspection of leaf architecture identified the plant as the
moss Fontinalis. Moss leaf morphology included an absence
of keel, twist at upper part of leaf, and 30–40� spread of leaves.
These characteristics are consistent with Fontinalis novae-
angliae (Welch, 1960). Genetic characterization based first
on the PCR cloned rbcL (1251-bp amplicon) suggested it was
99.9% identical to GenBank accession AB050949, annotated
A
B
Fig. 2 Video excerpt and photograph of the Fontinalis moss. (A) Part of Fonti-
nalis colony adjacent to the high output vent and illuminated by the running
lights on the ROV. The ROV sampling arm is shown inserted into the vent hole
while sampling water from the vent. (B) Close up of moss leaf structure.
330 D. LOVALVO et al .
� 2010 Blackwell Publishing Ltd
as Fontinalis antipyretica rbcL. Having placed the moss in the
genus Fontinalis, subsequent genetic analysis focused on the
ITS region spanning from the 3¢ portion of the 18S rRNA
gene and including ITS1, the 5.8S rRNA gene ITS2, and a 5¢portion of the 26S rRNA gene. In addition, the tRNA-
Leu ⁄ Phe spacer DNA was also PCR cloned and sequenced.
Both nuclear genomic DNA regions are well represented
among the Fontinalis entries. A maximum-likelihood analysis
of ITS DNA concatenated with tRNA spacer sequence (1807
nucleotides total) suggests that the Yellowstone Lake speci-
men is most closely related to a clade comprised of F. anti-
pyretica, Fontinalis gigantea, and Fontinalis chrysophylla
(Fig. 3), all of which differ from this moss by having keeled
leaves.
Mesofauna identification
The mesofauna found associated with the moss are shown in
Fig. 4. As with the moss, Linnean taxonomic approaches were
used initially to identify the organism, which then directed
DNA target selection and guided PCR primer design.
Sequence availability for the 18S rRNA and COI genes
differed for various species. A summary maximum-likelihood
tree for the 18S rRNA gene is shown in Fig. 5. Yellowstone
Lake specimen I4 (Fig. 4A) keyed out as Hyalella azteca,
which was in agreement with 18S rRNA gene phylogenetic
analysis that clearly placed it within the genus Hyalella
(Fig. 5), and an COI-based analysis which confirmed this
specimen as the species azteca (Fig. 6). Morphological fea-
tures of crustacean specimen I3 identified it as Gammarus
lacustris (Fig. 4B), which was also supported by phylogenetic
analyses of the 18S rRNA (Fig. 5) and COI (Fig. 7) genes.
The segmented worm specimen I6 (Fig. 4C) was sexually
immature so visual identification could only assign it to the
family Lumbriculidae; a genus designation was not possible.
Phylogenetic analysis (18S rRNA) confirmed it as a member
of the Lumbriculidae and potentially a member of the genus
Lamprodrilus (Fig. 5). The flatworm specimen I7 was appar-
ently damaged during suctioning from the moss leaves and
thus only a tentative identification was made as Polycelis coro-
nata. Phylogenetic analysis (18S rRNA) confirms that it is a
species of Polycelis (Fig. 5), but there are few sequences from
that genus available in GenBank, leaving us uncertain as to a
species level identification. Additional COI work with the
putative Lamprodrilus (Fig. S2) and Polycelis (Fig. S3) speci-
mens are in agreement with these genus-level designations.
Vent environment characterization
Vent water temperature was 34.6 �C, but slightly cooler at
32.3 �C within the moss cluster adjacent to the vent. Light
intensity was assessed throughout the water column above the
vent site. Incremental lowering of a light meter showed a
nearly two order of magnitude decrease in light irradiance in
the first 10 m (Fig. 8). Near vent depth (28 m), light irradi-
ance decreased further (total of >8000-fold decrease) and was
likely due in part to the constant emission of solid phase mate-
rial from the vent (http://www.tbi.montana.edu/media/
Fontinalis_vent.html) that resulted in the surrounding water
being somewhat clouded. SEM electron dispersive X-ray anal-
ysis of this beige-colored material found it to be comprised
primarily of an alumino-silicate material (% atom composition:
O, 62.3; Si, 17.7; Al, 5.1). The close association of the moss
with the vent resulted in the moss being covered with this
material.
Additional analysis of the vent water was directed towards
ascertaining whether it could be the source of important
nutrients for the moss. Total inorganic CO2(aq) concentra-
tion was 10.22 mM (Table 1) and 1.11 mM upon mixing with
96
65
81
59
94
83
67
67
51
55
95
51
0.005 substitutions per site
Fontinalis antipyretica (AF192108, AF191517)
Fontinalis neomexicana (AF192105, AF191514)
Fontinalis hypnoides (AF192101, AF191510)
Fontinalis antipyretica (AF192117, AF191526)
Fontinalis duriaei (AF192126, AF191535)
Fontinalis squamosa (AF192111, AF191520)
Fontinalis antipyretica (AF192113, AF191522)
Fontinalis chrysophylla (AF192103, AF191512)
Fontinalis gigantea (AF192127, AF191536)
Yellowstone Lake sample (GU066816, GU066817)
Fontinalis redfearnii (AF192098, AF191507)
Fontinalis dalecarlica (AF192125, AF191534)
Fontinalis flaccida (AF192104, AF191513)
Fontinalis novae-angliae (AF192131, AF191540)
Fontinalis sullivantii (AF192102, AF191511)
Fontinalis missourica (AF192106, AF191515)
Fontinalis welchiana (AF192133, AF191542)
Fig. 3 Maximum-likelihood tree illustrating the phylogenetic relationship of
the Yellowstone Fontinalis ITS-tRNA DNA concatenate. Bootstrap values
>50% are shown. Tree was rooted with Brachelyma subulatum (AF192094,
AF191503), Dichelyma falcatum (AF192096, AF191505), and Dichelyma
uncinatum (AF192095, AF191504).
Geothermal-linked biological oasis 331
� 2010 Blackwell Publishing Ltd
lake water approximately 1 m above the vent. Presumably,
similar mixing might occur within the moss stand immediately
adjacent to the vent opening. Vent water was acidic (pH 5.5)
and significant levels of some important macronutrients were
present. Specifically, a constant flux of �24 lM NH4+ and
0.75 lM NO3) (Table 1) combine to provide significant levels
of available nitrogen. The same can be said for sulfur and
potassium (60 and 140 lM, respectively), although total phos-
phorus was below detection, which in this analysis was
<10 lM. Among the trace elements, B and Li were present at
significant concentrations, and the metalloids Sb and As were
also present, with the latter at significant levels (see below).
DISCUSSION
The literature contains numerous inventories and phy-
logenetic characterizations of the microbial communities
intimately linked with geothermal features, whereas such
relationships with eukaryotic organisms have been much less
common. The proliferation of complex higher organisms in
close association with a Yellowstone Lake geothermal vent
parallels that documented for deep marine vents (Rona et al.,
1986; Fustec et al., 1987; Galkin & Moskalev, 1990;
Cann et al., 1994; Desbruyeres et al. 1994; Chevaldonne
et al., 1997), although to our knowledge this is the first such
documentation for a freshwater habitat, and thus this report
extends and expands awareness of such ecological relation-
ships. Unidentified macrophytes have been reported in the
general vicinity of vents at SCUBA depths (Remsen et al.,
2002); however, the frequency of the moss-vent association
documented here is unknown at present. Given the lake’s vio-
lent volcanic past and relative youth (�11 000 years; Morgan
et al., 2007), fossil evidence of this or any other association
would likely be rare.
CBA
Fig. 4 Photos of mesofauna collected from the branches of the Fontinalis moss. (A) Hyalella azteca specimen, (B) Gammarus lacustris, and (C) the Lamprodrilus sp.
All samples are preserved in 70% ethanol (bar = 1 mm in each panel).
Lake Organism I7 Accession
Polycelis felina DQ665996
Polycelis tenuis Z99949
Phagocata vitta DQ6659998
Crenobia alpina M58345
Lake Organism I6 Accession
Lamprodrilus achaetus DQ31323
Lamprodrilus stigmatias DQ313
Lumbriculus variegatus AF2094
Styloscolex baicalensis AJ308
Helobdella paranensis AF11598
Tubifex tubifex EU126846
Lake Organism I4 Accessions
Hyalella sp GreenHy AJ966716
Hyalella sp LGB1 AJ966715
Hyale nilssoni AY826958
Parhyale hawaiensis AY826957
Lake Organism I3 Accession
Gammarus lacustris AY926786
Gammarus troglophilus AF20298
Dikerogammarus villosus EF582
Chaetogammarus stoerensis AY9
81 88
67
100
51
90
100
100
71
54
99
53
93
51 79
63
0.05 substitutions per site Chaetogammarus stoerensis (AY926762)
Dikerogammarus villosus (EF582898)
Gammarus troglophilus (AF202983) Gammarus lacustris (AY926786) Yellowstone Lake sample I3 (GU066807)
Parhyale hawaiensis (AY826957) Hyale nilssoni (AY826958)
Yellowstone Lake sample I7 (GU066810) Polycelis felina (DQ665996)
Polycelis tenuis (Z99949) Phagocata vitta (DQ665998)
Crenobia alpina (M58345) Yellowstone Lake sample I6 (GU066809)
Lamprodrilus achaetus (DQ313235) Lamprodrilus stigmatias (DQ313236) Lumbriculus variegatus (AF209457)
Styloscolex baicalensis (AJ308513) Helobdella paranensis (AF115987)
Tubifex tubifex (EU126846) Yellowstone Lake sample I4 (GU066808)Hyalella sp. GreenHy (AJ966716) Hyalella sp. LGB1 (AJ966715)
Fig. 5 Abbreviated maximum-likelihood tree illustrating the phylogenetic rela-
tionship of the 18S rRNA genes PCR cloned from the Yellowstone Lake mesofauna
associated with the moss. Phylogeny is based on alignments of nucleotides. Boot-
strap values >50% are shown. Tree was rooted with Dalatias licha (AY049827),
Pristiophorus cirratus (AY049849), and Squalus acanthias (M91179).
Yellowstone Lake sample I4 (GU066812)
Hyalella azteca (DQ464710)
Hyalella sp. isolate GreenHy (AJ968918)
Hyalella sp. isolate BeulCf (AJ968916)
Hyalella sp. isolate LGB1 (AJ968917)
Hyalella sp. isolate BeulHy (AJ968915)
Hyale nilssoni (AF520435)
Parhyale hawaiensis (EF989709)
Orchestia cavimana (EF989708)
98
100
100
100
96
53
0.05 substitutions per site
Fig. 6 A maximum-likelihood tree illustrating the phylogenetic relationship of
the COI gene PCR cloned from the Yellowstone Lake specimen I4. Phylogeny is
based on alignments of 626 nucleotides, representing the 65–690 bp region of
the gene. Bootstrap values >50% are shown. Tree was rooted with Gammarus
lacustris (AY926671).
332 D. LOVALVO et al .
� 2010 Blackwell Publishing Ltd
Water temperature in the vent examined in this study
was significantly lower than that documented for marine vents
and is also much lower than most vents described for this lake
(Morgan et al., 2007). Nevertheless, the temperature
observed at this vent is considerably greater than the sustained
temperature range of the coldwater moss Fontinalis (Glime,
1987). Even tropical bryophytes do poorly above 25 �C(Frahm, 1990) and truly aquatic bryophytes are rare there.
However, we note the report of geothermal bryophytes:
Bryum japanense can grow at 40 �C, and Philonotis laxiretis
and Bryum cyclophyllum at 38 �C (Watanabe, 1957). Previous
physiology work with F. antipyretica demonstrated this moss
experiences a net carbon loss at 20 �C (Carballeira et al.,
1998). And while Fontinalis appears to have some adaptive
capacity to environmental temperature (Fornwall & Glime,
1982), the extremes noted for the Fontinalis colony described
herein exceeds the environmental plasticity documented for
this bryophyte. Consequently, at the temperatures observed
in this study CO2 lost due to respiration would be expected to
greatly exceed photosynthetically captured carbon (Sommer
& Winkler, 1982) under conditions where this moss is
normally documented to occur; i.e. not associated with a geo-
thermal vent.
In addition to high temperature stress, water turbidity lim-
ited light penetration at this location. As a group, bryophytes
are tolerant of shade (Martin, 1980; Martin & Churchill,
1982), and indeed Fontinalis has been documented to occur
at depths of up to 120 m in Crater Lake, where water clarity
Yellowstone Lake sample I3 (GU066811)
G. pseudolimnaeus (EF570333)
G. troglophilus (EF570350)
G. lacustris (AY926671)
G. bousfieldi (EF570299)
G. emeiensis (EF570306)
G. pulex (EF570334)
G. tigrinus (EF570348)
G. abstrusus (EF570304)
G. martensi (EF570325)
G. nipponensis (EF570312)
G. sinuolatus (EF570339)
G. duebeni (AY926669)
G. locusta (EF570324)
G. roeseli (EF570337)
G. glabratus (EF570307)
G. koreanus (EF570314)
G. lichuanensis (EF570356)
G. curvativus (EF570302)
G. gregoryi (EF570311)
G. comosus (EF570300)
G. nekkensis (EF570331)
56
81
100
100
0.05 substitutions/site
Fig. 7 A maximum-likelihood tree illustrating the phylogenetic relationship of
the COI gene PCR cloned from the Yellowstone Lake specimen I3 and to
Gammuras accessions available in GenBank. Phylogeny is based on alignments
of 593 nucleotides, representing the 95–687 bp region of the gene. Bootstrap
values >50% are shown. Tree was rooted with Sinogammarus chuanhui
(EF570355).
Fig. 8 Light penetration in the Yellowstone Lake water column above the
vent. Light irradiance is shown as percent relative to that recorded at the surface
(�800 W m)2) and as a function of depth.
Table 1 Geochemical analysis of the vent water
Major cations and neutrals
Na+ K+ Ca+2 Si NH4+ Mg+2
mM mM mM mM lM lM
1.23 0.06 0.12 0.27 27.4 70.5
Anions
Cl) SO4)2 F) NO3
) S2O3)2 PO4
)3
mM mM lM lM mM mM
0.55 0.14 95 0.75 bd bd
Dissolved gases
CO2 (aq) DIC S)2 (aq) O2 (aq) CH4 (aq) H2 (aq)
mM mM lM lM lM nM
10.22 10.2 bd 81.8 7.6 14
Trace elements
As Se Rb Sr Mo Sb
lM lM lM lM lM lM
1.85 bd 0.15 0.58 0.11 0.03
B Al Mn Fe Zn Ga
lM lM lM lM lM lM
28.62 3.23 bd bd 0.22 bd
Cs Ba W Pb V Li
lM lM lM lM lM lM
0.21 0.11 0.08 bd 0.25 32.20
bd, below detection.
Geothermal-linked biological oasis 333
� 2010 Blackwell Publishing Ltd
allows light to penetrate to considerable depths (Hasler,
1938). This is in contrast to Yellowstone Lake, which is quite
turbid owing to lake sediments being carried by subsurface
currents generated by significant daily winds (Benson, 1961).
In addition to lake turbidity, mineral vent emissions no doubt
contributed to reduce light levels at vent depth by nearly four
orders of magnitude relative to the lake surface waters
(Fig. 8). Vent emissions also generated a constant showering
of mineral material that covered leaf surfaces of the moss
(http://www.tbi.montana.edu/media/Fontinalis_vent.html),
which presumably would further reduce access to light and
thus present additional physiological challenges to incorporat-
ing CO2 via photosynthesis.
All of the above apparent vent-associated growth con-
straints notwithstanding, occurrence of this moss appeared
restricted to the immediate areas surrounding vent emissions,
suggesting the latter provides a positive influence which
overcomes the presumably negative vent-associated effects of
high temperature, low light, and epiphytic mineral covering.
The high concentration of CO2(aq) in the vent water
(Table 1) and in the area immediately surrounding the vent
probably offers an explanation. Under these conditions,
the moss’ Rubisco enzyme is likely constantly saturated,
permitting it to efficiently capture and fix carbon at rates
that exceed respiratory costs, and thus allowing for biomass
accumulation and moss growth. Availability of substantial
fixed nitrogen (Table 1) would presumably also be a favorable
influence.
Seemingly, the vent environment would also be atypical for
the mesofauna found associated with the moss. The organisms
identified here are normally found in the littoral zone as
opposed to being found in the profundal zone. In this vent-
associated environment, these organisms presumably experi-
ence significantly elevated temperature and CO2 concentra-
tions, acidic pH, and depleted O2; conditions that depart
from optimum conditions known for these species. Further,
concentrations of aluminum and arsenic (Table 1) in the vent
waters are similar in magnitude to the LC50 reported for
members of Hyalella (Borgmann et al., 2005) and Gamma-
rus (Spehar et al., 1980). Although these animals are motile,
they are nevertheless subjected to lake currents that could
potentially carry them in and out of the vent water plume by
the swaying of the moss. Thus, at a minimum, they would be
exposed to these extreme conditions for brief periods. It is
currently unknown how these organisms are coping with this
environment.
Phylogenetic analysis of the mesofauna specimens resulted
in maximum-likelihood estimates that were in agreement
with Linnean-based approaches. Assessments of specimen I4
based on the 18S rRNA and the COI genes were consistent
with each other and with the classification based on
morphological features: all classified this organism as Hyalella
azteca (Figs 5 and 6). The same can be said for crustacean
specimen I3, which was identified as Gammarus lacustris
(Figs 4B, 5 and 7). The sexual immaturity of the segmented
worm specimen I6 (Fig. 4C) constrained visual identifica-
tion to a family-level (Lumbriculidae) designation. Phylo-
genetic analysis (18S rRNA) confirmed it as a member of
the Lumbriculidae and potentially within the genus Lampro-
drilus (Fig. 5), and was consistent with the results of the
COI-based analysis (Fig. S2). However, we note with
interest that the latter suggested significant phylogenetic
relatedness of segmented worms previously identified as
Agriodrilus and Teleuscolex (Fig. S2). This has been noted
previously with specimens collected from Lake Baikal
(Kaygorodova & Sherbakov, 2006), and is consistent with
the suggestion that Agriodrilus and Teleuscolex may actually
be subgenera of, or cogeneric with, Lamprodrilus based on
some key morphological features used in Linnean taxonomy
(Brinkhurst, 1989). To the best of our knowledge, the
aforementioned segmented worm genera have never been
reported in North America.
Identification of the moss using Linnean and molecular
genetic techniques yielded somewhat different results. Both
approaches clearly placed the moss in the genus Fontinalis,
however more precise taxonomic resolution was less certain.
Initial phylogenetic analysis of the plant based on rbcL sug-
gested high relatedness to F. antipyretica, although because
Fontinalis rbcL is not well represented in GenBank, subse-
quent analysis examined ITS and tRNA-Leu ⁄ Phe spacer DNA
sequences for which Fontinalis is much more robustly repre-
sented. A maximum likelihood-based assessment (Fig. 3)
illustrated F. antipyretica to be paraphyletic and thus similar
to that reported by Shaw & Allen (2000). The Yellowstone
specimen occupied the same clade with at least one F. antipy-
retica accession, but was separate from F. novae-angliae
(Fig. 2). Lack of congruency between the leaf morphology
and genetic characterization has been noted previously (Shaw
& Allen, 2000).
In summary, the vent water properties represent a paradox
with respect to bryophyte photosynthesis, but nevertheless
appears foundational to a food web comprised of Fontinalis
upon whose surfaces reside metazoa that in turn are known to
be consumed by fish. This linkage between a food web and
raw geochemical inputs from the Yellowstone caldera parallels
that documented for deep marine vents, where geochemical
energy is converted to biochemical energy that enables and
sustains complex communities in interesting, if not extraordi-
nary, environments. Ecologically, these deeply submerged
hydrothermal vents are oases that are essentially islands (sensu
MacArthur and Wilson) from which biological diversity
emerges.
ACKNOWLEDGMENTS
This research was supported primarily by a grant from the
Gordon and Betty Moore Foundation (Grant #1555), and
additional funding from the National Park Service Centennial
334 D. LOVALVO et al .
� 2010 Blackwell Publishing Ltd
Challenge Match Program (PMIS #137808). Work was con-
ducted under NPS research permit No. 5700.
REFERENCES
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W,
Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new genera-tion of protein database search programs. Nucleic Acids Research25, 3389–3402.
Amend JP, Shock EL (2001) Energetics of overall metabolic reactions
of thermophilic and hyperthermophilic Archaea and Bacteria. FEMSMicrobiol Revs 25, 175–243.
APHA (1998) In Standard Methods for the Examination of Water andWastewater (eds Clesceri LS, Greenberg AE, Eaton AD). American
Public Health Association, Washington, DC.Baldwin BG (1992) Phylogenetic utility of the internal transcribed
spacers of nuclear ribosomal DNA in plants: an example from the
compositae. Molecular Phylogenetics and Evolution 1, 3–16.Balistrieri LS, Shanks WC III, Cuhel RL, Aguilar C, Klump JV (2007)
The influence of sub-lacustrine hydrothermal vents on the geo-
chemistry of Yellowstone Lake. In Integrated Geoscience Studies inthe Greater Yellowstone Area – Volcanic, Tectonic, and Hydrother-mal Processes in the Yellowstone Geoecosystem (ed. Morgan LA). US
Geological Survey Professional Paper 1717, pp. 169–199.
Benson NG (1961) Limnology of Yellowstone Lake in relation to the
cutthroat trout. United States Department of the Interior Fish andWildlife Service, Bureau of Sport Fisheries and Wildlife. Research
Report 56.
Bessho Y, Ohama T, Osawa S (1992) Planarian mitochondria I. Het-erogeneity of cytochrome c oxidase subunit I gene sequences in the
freshwater planarian, Dugesia japonica. Journal of Molecular Evolu-tion 34, 324–330.
Borgmann U, Couillard Y, Doyle P, Dixon DG (2005) Toxicity ofsixty-three metals and metalloids to Hyalella azteca at two levels of
water hardness. Environmental Toxicology and Chemistry 24,
641–652.
Botero LM, D’Imperio S, Burr M, McDermott TR, Young M,Hassett DJ (2005) Poly(A) polymerase modification and reverse
transcriptase PCR amplification of environmental RNA. Appliedand Environmental Microbiology 71, 1267–1275.
Brinkhurst RO (1989) A phylogenetic analysis of the Lumbriculidae
(Annelida, Oligochaeta). Canadian Journal of Zoology 67,
2731–2739.
Brouns SJJ, Ettema TJG, Stedman KM, Walther J, Smidt H, SnijdersAPL, Young M, Bernander R, Wright PC, Siebers B, van der Oost J
(2005) The hyperthermophilic archeon Sulfolobus: from explora-
tion to exploitation. In Geothermal Biology and Geochemistry inYellowstone National Park (eds Inskeep WP, McDermott TR).Proceedings Thermal Biology Workshop, Yellowstone National
Park, WY, October, 2003, pp. 261–276.
Cann J, Van Dover C, Walker C, Dando P, Murton B (1994) Diver-
sity of vent ecosystems (DOVE). Bridge Newsletter 4, 1–39.Carballeira A, Dı¢az S, Va¢zquez MD, Lopez J (1998) Inertia and
resilience in the responses of the aquatic bryophyte Fontinalisantipyretica Hedw. to thermal stress. Archives EnvironmentalContamination and Toxicology 34, 343–349.
Chevaldonne P, Jollivet D, Vangriesheim A, Desbruyeres D (1997)
Hydrothermal-vent alvinellid polychaete dispersal in the eastern
Pacific. 1. Influence of vent site distribution, bottom currents, andbiological patterns. Limnology and Oceanography 42, 67–80.
Cuhel RL, Aguilar C, Anderson PD, Maki JS, Paddock RW, Remsen
CC, Val Klump J, Lovalvo D (2002) Underwater domains in
Yellowstone Lake hydrothermal vent geochemistry and bacterial
chemosynthesis. In Yellowstone Lake: Hotbed of Chaos or Reservoir ofResilience? (eds Anderson RJ, Harmon D). Proceedings of the 6th
Biennial Scientific Conference on the Greater Yellowstone Ecosys-tem. October 8–10, 2001 Mammoth Hot Springs Hotel, Yellow-
stone National Park and Hancock, MI, Yellowstone Center for
Resources and George Wright Society, pp. 27–53.Desbruyeres D, Alayse-Danet A-M, Ohta S, STARMER and BIO-
LAU cruises participants (1994) Deep-sea hydrothermal communi-
ties in Southern Pacific back-arc basins (the North Fiji and Lau
Basins): composition, microdistribution and food-web. MarineGeology 116, 227–242.
Fornwall MD, Glime JM (1982) Cold and warm-adapted phases in
Fontinalis duriaei Schimp. As evidenced by net assimilatory and
respiratory responses to temperature. Aquatic Botany 13,165–177.
Frahm J-P (1990) Bryophyte phytomass in tropical ecosystems.
Journal of the Linnean Society Botany 104, 23–33.
Fustec A, Desbruyeres D, Juniper SK (1987) Deep-sea hydrothermalvent communities at 13�N on the East Pacific Rise: microdistribu-
tion and temporal variations. Biological Oceanography 4,
121–164.Galkin SV, Moskalev LI (1990) Hydrothermal fauna of the Mid-
Atlantic Ridge. Oceanology 30, 624–627.
Glime JM (1987) Phytogeographic implications of a Fontinalis (Bry-
opsida) growth model based on temperature and flow conditionsfor six species. Memoirs of the New York Botanical Garden 45,
154–170.
Hasler AD (1938) Fish biology and limnology of Crater Lake, Ore-
gon. The Journal of Wildlife Management 2, 94–103.Hou Z, Fu J, Li S (2007) A molecular phylogeny of the genus Gamm-
arus (Crustacea: Amphipoda) based on mitochondrial and nuclear
gene sequences. Molecular Phylogenetics and Evolution 45,596–611.
Inskeep WP, McDermott TR, eds (2005) Geomicrobiology of acid-
sulfate-chloride springs in Yellowstone National Park. In Geother-mal Biology and Geochemistry in Yellowstone National Park.Proceedings of Thermal Biology Workshop, Yellowstone National
Park, WY, October, 2003, pp. 143–162.
Inskeep WP, Ackerman GG, Taylor WP, Kozubal M, Korf S, Macur
RE (2005) On the energetics of chemolithotrophy in nonequilibri-um systems: case studies of geothermal springs in Yellowstone
National Park. Geobiology 3, 297–317.
Kathman RD, Brinkhurst RO (1998) Guide to Freshwater Oligochaetesof North America. Aquatic Resources Center, College Grove, TN.
Kaygorodova IA, Sherbakov DYu (2006) Molecular phylogenetic
study of the systematic position of Baikalian oligochaetes in Clitella-
ta. Russian Journal of Genetics 42, 1390–1397.Madigan MT, Jung DO, Karr EA, Sattley WM, Achenbach LA, van
der Meer MTJ (2005) Diversity of anoxygenic phototrophs in con-
trasting extreme environments. In Geothermal Biology and Geo-chemistry in Yellowstone National Park (eds Inskeep WP,McDermott TR). Proceedings of Thermal Biology Workshop,
Yellowstone National Park, WY, October, 2003, pp. 203–220.
Martin CE (1980) Chlorophyll a ⁄ b ratios of eleven North Carolina
mosses. Bryologists 83, 84–87.Martin CE, Churchill SP (1982) Chlorophyll concentrations and a ⁄ b
ratios in mosses collected from exposed and shaded habitats in Kan-
sas. Journal of Bryology 12, 297–304.Meldal BHM, Debenhamb NJ, De Ley P, De Ley IT, VanXeteren JR,
Vierstraete AR, Bert W, Borgonie G, Moens T, Tyler PA, Austen
MC, Blaxter ML, Rogers AD, Lambshead PJD (2007) An
improved molecular phylogeny of the Nematoda with specialemphasis on marine taxa. Molecular Phylogenetics and Evolution42, 622–636.
Geothermal-linked biological oasis 335
� 2010 Blackwell Publishing Ltd
Morgan LA, Shanks WCP III (2005) Influences of rhyolytic lava flows
on hydrothermal processes in Yellowstone Lake and on the Yellow-
stone plateau. In Geothermal Biology and Geochemistry in Yellow-stone National Park (eds Inskeep WP, McDermott TR).
Proceedings of Thermal Biology Workshop, Yellowstone National
Park, WY, October, 2003, pp. 31–52.Morgan LA, Shanks WC III, Pierce KL, Lovalvo DA, Lee GK,
Webring MW, Stephenson WJ, Johnson SY, Harlan SS, Schulze B,
Finn CA (2007) The floor of Yellowstone Lake is anything but
quiet – new discoveries from high-resolution sonar imaging,seismic-reflection profiling, and submersible studies. In IntegratedGeoscience Studies in the Greater Yellowstone Area – Volcanic,Tectonic, and Hydrothermal Processes in the Yellowstone Geoecosystem(ed. Morgan LA). US Geological Survey Professional Paper 1717,pp. 95–126.
Pennak RW (1989) Fresh-Water Invertebrates of the United States, 3rd
edn. Wiley-Interscience, Hamilton Printing Co., New York, NY,
USA.Remsen CC, Maki JS, Val Klump J, Aguilar C, Anderson PD,
Buchholz L, Cuhel RL, Lovalvo D, Paddock RW, Waples J,
Bruckner JC, Schroeder CM (2002) Sublacrustrine geothermalactivity in Yellowstone Lake: studies past and present. In YellowstoneLake: Hotbed of Chaos or Reservoir of Resilience? (eds Anderson RJ,
Harmon D). Proceedings of the 6th Biennial Scientific Conference
on the Greater Yellowstone Ecosystem. October 8–10, 2001 Mam-moth Hot Springs Hotel, Yellowstone National Park and Hancock,
MI, Yellowstone Center for Resources and George Wright Society,
pp. 192–212.
Reysenbach A-L, Banta A, Civello S, Daly J, Mitchel K, Lalonde S,Konhauser K, Rodman A, Rusterholz K, Takacs-Vesbach C (2005)
Aquificales in Yellowstone National park. In Geothermal Biologyand Geochemistry in Yellowstone National Park (eds InskeepWP, McDermott TR). Proceedings of Thermal Biology
Workshop, Yellowstone National Park, WY, October, 2003,
pp. 129–142.
Rona PA, Klinkhammer G, Nelsen TA, Trefry JH, Elderfield H(1986) Black smokers, massive sulphides and vent biota at the
Mid-Atlantic Ridge. Nature 321, 33–37.
Shaw AJ, Allen B (2000) Phylogenetic relationships, morphological
incongruence, and geographic speciation in the Fontinalaceae(Bryophyta). Molecular Phylogenetics and Evolution 16, 225–237.
Sheehan KB, Fagg JA, Ferris MJ, Henson JM (2005) Thermophilic
amoebae and Legionella in hot springs in Yellowstone and GrandTeton National Parks. In Geothermal Biology and Geochemistry inYellowstone National Park (eds Inskeep WP, McDermott TR).
Proceedings of Thermal Biology Workshop, Yellowstone
National Park, WY, October, 2003, pp. 317–326.Sommer C, Winkler S (1982) Reaktionen im Gaswechsel von
Fontinalis antipyretica Hedw. nach experimentellen Belastungen
mit Schwermetallverbindungen. Archiv fur Hydrobiologie 93,
503–514.Spear JR, Walker JJ, Pace NR (2005) Hydrogen and primary produc-
tivity: inference of biogeochemistry from phylogeny in a geother-
mal ecosystem. In Geothermal Biology and Geochemistry inYellowstone National Park (eds. Inskeep WP, McDermott TR).
Proceedings of Thermal Biology Workshop, Yellowstone National
Park, WY, October, 2003, pp. 113–128.
Spehar RL, Fiandt JT, Anderson RL, DeFoe DL (1980) Comparativetoxicity of arsenic compounds and their accumulation in inverte-
brates and fish. Archives of Environmental Contaminant and Toxi-cology 9, 53–63.
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG
(1997) The CLUSTAL_X windows interface: flexible strategies for
multiple sequence alignment aided by quality analysis tools. NucleicAcids Research 25, 4876–4882.
To TB, Nordstrom DK, Cunningham KM, Ball JW, McCleskey RB
(1999) New method for the direct determination of dissolved
Fe(III) concentration in acid mine waters. Environmental Scienceand Technology 33, 807–813.
Tsubota H, Nakao N, Arikawa T, Yamaguchi T, Higuchi M, Deguchi
H, Seki T (1999) A preliminary phylogeny of Hypnales (Musci) as
inferred from chloroplast rbcL sequence data. Bryoogical Research7, 233–248.
Ward DM, Cohan FM (2005) Microbial diversity in hot spring cyano-
bacterial mats: pattern and prediction. In Geothermal Biology andGeochemistry in Yellowstone National Park (eds Inskeep WP,McDermott TR). Proceedings of Thermal Biology Workshop,
Yellowstone National Park, WY, October, 2003, pp. 185–202.
Watanabe R (1957) On some mosses growing in hotspring water.
Miscellanea Bryologica et Lichenologica 13, 2.Welch WH (1960) A Monograph of the Fontinalaceae,’’ Nijhoff,
Dordrecht.
Young M, Wiedenheft B, Snyder J, Spuhler J, Roberto F, Douglas T
(2005) Archael viruses from Yellowstone’s high temperature envi-ronments. In Geothermal Biology and Geochemistry in YellowstoneNational Park (eds Inskeep WP, McDermott TR). Proceedings of
Thermal Biology Workshop, Yellowstone National Park, WY,October, 2003, pp. 289–304.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the
online version of this article:
Fig. S1 Maps relating location of study.
Fig. S2 A maximum-likelihood tree illustrating the phylogenetic relationship of
the COI gene PCR cloned from the Yellowstone Lake specimen I6.
Fig. S3 A maximum-likelihood tree illustrating the phylogenetic relationship of
the COI gene PCR cloned from the Yellowstone Lake specimen I7.
Please note: Wiley-Blackwell are not responsible for the
content or functionality of any supporting materials supplied
by the authors. Any queries (other than missing material)
should be directed to the corresponding author for the article.
336 D. LOVALVO et al .
� 2010 Blackwell Publishing Ltd