Ten New Genera of Oryzomyine Rodents (Cricetidae: Sigmodontinae)
-
Upload
independent -
Category
Documents
-
view
5 -
download
0
Transcript of Ten New Genera of Oryzomyine Rodents (Cricetidae: Sigmodontinae)
PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY
CENTRAL PARK WEST A T 79TH STREET, NEW YOR K, NY 10024
Number 3537, 29 pp., 1 figure, 3 tables October 19, 2006
Ten New Genera of Oryzomyine Rodents(Cricetidae: Sigmodontinae)
MARCELO WEKSLER,1 ALEXANDRE REIS PERCEQUILLO,2
AND ROBERT S. VOSS3
ABSTRACT
In order to achieve a monophyletic classification of oryzomyine rodents, 10 new genera aredescribed for species or species groups previously referred to the polyphyletic genus Oryzomys. Thefollowing names are proposed: Aegialomys, n.gen. (for the ‘‘xanthaeolus group’’ of authors);Cerradomys, n.gen. (for the ‘‘subflavus group’’); Eremoryzomys, n.gen. (for polius); Euryoryzomys,n.gen. (for the ‘‘nitidus group’’); Hylaeamys, n.gen. (for the ‘‘megacephalus group’’); Mindomys,n.gen. (for hammondi); Nephelomys, n.gen. (for the ‘‘albigularis group’’); Oreoryzomys, n.gen. (forbalneator); Sooretamys, n.gen. (for angouya); and Transandinomys, n.gen. (for bolivaris andtalamancae). All of the new genera thus constituted are morphologically diagnosable and havedistinct ecogeographic distributions. Pending revisionary work that is currently in progress byother researchers, six species belonging to the ‘‘alfaroi group’’ (herein construed as includingalfaroi, chapmani, melanotis, rhabdops, rostratus, and saturatior) are provisionally referred toHandleyomys. As a result of these changes, the genus Oryzomys is restricted to the ‘‘palustrisgroup’’ of authors, and the tribe Oryzomyini now comprises 28 genera.
INTRODUCTION
A striking characteristic of muroid rodentclassification at the middle of the last centurywas the large number of species assigned to
a few geographically widespread and morpho-logically undiagnosable genera. AlthoughRattus (sensu lato) is perhaps the mostnotorious example (Musser, 1981; Musserand Newcomb, 1983; Musser and Holden,
Copyright E American Museum of Natural History 2006 ISSN 0003-0082
1 Division of Vertebrate Zoology (Mammalogy), American Museum of Natural History. Present address: Institute ofArctic Biology and University of Alaska Museum, University of Alaska Fairbanks, Fairbanks, AK 99775 ([email protected]).
2 Departamento de Sistematica e Ecologia, Centro de Ciencias Exatas e da Natureza, Universidade Federal da Paraıba,Caixa Postal 5133, 58051-970 Joao Pessoa, Paraiba, Brazil ([email protected]).
3 Division of Vertebrate Zoology (Mammalogy), American Museum of Natural History ([email protected]).
1991), several New World genera have alsoserved as convenient receptacles for superfi-cially similar but phylogenetically heteroge-neous species. In Neotropical mammalogy,this was the traditional role of such elastictaxa as Akodon, Oryzomys, and Thomasomys.Thanks to revisionary research in the lastseveral decades (reviewed by Musser andCarleton, 1993, 2005), each of these genera isnow recognized in a much more restrictedsense than formerly, but many nomenclaturalproblems remain. The purpose of this report isto complete the transition from traditionalusage to a phylogenetic classification of thespecies hitherto referred to Oryzomys.
As treated in influential mid-20th-centurychecklists (e.g., Tate, 1932; Gyldenstolpe,1932; Ellerman, 1941; Hall and Kelson, 1959;Cabrera, 1961), the genus Oryzomys containedanywhere from 60 to 120 nominal taxa in fiveto seven subgenera that collectively rangedfrom Patagonia to New Jersey. The artifici-ality of this usage was subsequently empha-sized by karyotypic and morphological re-searchers (e.g., Gardner and Patton, 1976;Carleton and Musser, 1989) who raised all ofthe subgenera recognized by midcenturyauthors to generic rank. The species that arestill referred to Oryzomys, however, do notcomprise a monophyletic group (fig. 1), and itis intolerable that this situation should persist.
In order to achieve a monophyletic classi-fication, we now name 10 new genera forspecies currently classified as Oryzomys.Pending the description of other new genera(by M.D. Carleton and G.G. Musser, personalcommun.), we provisionally transfer membersof the ‘‘alfaroi group’’ (herein understood toinclude alfaroi, chapmani, melanotis, rhabdops,rostratus, and saturatior) to Handleyomys,a suboptimal but phylogenetically defensiblenomenclatural option previously discussed byWeksler (2006). Table 1 lists all of theoryzomyine genera that we recognize as valid,including those described as new herein.
NEW GENERA
Most of the clades for which we providenew generic names have been recognized inone form or another for many years, usuallyas informally designated species ‘‘groups’’ or
‘‘complexes’’ (e.g., by Goldman, 1918; Tate,1932; Ellerman, 1941; Gardner and Patton,1976; Weksler, 1996; Musser et al., 1998;Percequillo, 1998, 2003; Sanchez et al., 2001).In the accounts that follow, we designatea type species for each new genus, list the validspecies (and synonyms) referred to it, describeits geographic distribution, provide morpho-logical diagnoses and comparisons, andbriefly comment on the criteria we used todetermine the assignment of species notrepresented in published analyses of characterdata. Throughout these accounts, morpho-logical characters are described using termi-nology defined and illustrated by Voss (1988,1993), Carleton and Musser (1989), Voss andCarleton (1993), Musser et al. (1998), Voss etal. (2002), and Weksler (2006).
COMMON ATTRIBUTES
The taxa named below share many attri-butes that it would be pointless to repeat ineach diagnosis. For example, insofar as known(postcranial skeletons have not been examinedfrom all taxa), they resemble other members ofthe cricetid subfamily Sigmodontinae byhaving a double articulation of the first rib,lacking an entepicondylar foramen of thehumerus, and lacking an entoglossal processof the basihyal. Similarly, the material weexamined indicates that they consistently re-semble other members of the tribe Oryzomyiniin lacking a posterior suspensory process ofthe squamosal, having 12 ribs, having uniloc-ular-hemiglandular stomachs, and lackinga gall bladder.
Some characters that vary within Oryzo-myini are likewise uninformative in the con-text of these comparisons and need not berepeated below. Like most oryzomyines (withexceptions as noted), all of the taxa describedherein have soft fur (Neacomys and Scolomyshave spiny fur); the manual claws are smalland unkeeled (Lundomys has long, ventrallykeeled manual claws); the hind feet lack well-developed natatory fringes and interdigitalwebs (well-developed natatory fringes and/orwebbing are present in Amphinectomys,Holochilus, Lundomys, Nectomys, Oryzomyspalustris, and Pseudoryzomys); the mammarycomplement consists of eight teats in inguinal,
2 AMERICAN MUSEUM NOVITATES NO. 3537
abdominal, postaxial, and pectoral pairs(Handleyomys and Scolomys have six mam-mae because they lack pectoral teats); thesparsely haired tail is covered with more or
less conspicuous epidermal scales and lacksa terminal tuft of long hairs (the well-hairedtail of Nesoryzomys does not appear scaly, andsome species of Oecomys have prominently
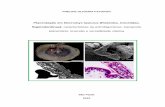
Fig. 1. Phylogenetic relationships of oryzomyines based on a heuristic parsimony analysis of sequencedata from the Interphotoreceptor Retinoid Binding Protein (IRBP, 1266 bp from exon 1) and 99morphological characters (after Weksler, 2006: fig. 37). Numbers above and below branches representjackknife support (.50%) and decay indices (.1), respectively. Vertical bars on the right-hand side of thefigure indicate taxon membership in clades A–D. See Weksler (2006: 14–17) for methodological details.
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 3
tufted tails); the zygomatic plate lacks ananterodorsal spinous process (a spinous pro-cess is present in Pseudoryzomys, Lundomys,and Holochilus); the nasal bones have roundedor squared posterior margins (Nectomys,Scolomys, and Sigmodontomys have acutelyangled posterior nasal margins); the posteriorwall of the orbit is smooth (Holochilus has
a well-developed postorbital ridge); the bonypalate between the molar rows is smooth orweakly sculpted (the palates of Holochilus andLundomys have a well-developed median keelflanked by deep lateral gutters); the alisphe-noid canal has a large anterior opening (theanterior opening of the alisphenoid canal isabsent or very small in Scolomys); the upper
TABLE 1Contents of the Tribe Oryzomyini
4 AMERICAN MUSEUM NOVITATES NO. 3537
incisors have smoothly rounded enamel bands(the upper incisor enamel is distinctly faceted inHolochilus); the molars are low-crowned andbunodont or terraced (Holochilus has high-crowned, planar molars); the labial flexi areenclosed by a cingulum (the labial flexi areunenclosed in Holochilus and Lundomys); themaxillary toothrows are parallel (Holochilusand Lundomys have anteriorly convergenttoothrows); mesolophs are present on all uppermolars (Holochilus, Lundomys, Pseudoryzomys,Scolomys, and Zygodontomys lack mesolophson one or more upper teeth); the median mureis connected to the protocone on M1 (themedian mure is connected to the paracone inHolochilus); the paracone of M2 lacks anaccessory loph (an accessory loph is presentin Oecomys); and a posteroflexid is present onm3 (posteroflexids are absent on m3 inHolochilus, Lundomys, Pseudoryzomys, andZygodontomys). Likewise, all dissected oryzo-myines (except Nesoryzomys) have male acces-sory reproductive gland complements thatinclude one pair each of bulbourethral, dorsalprostate, anterior prostate, vesicular, andampullary glands, and two pairs of ventralprostate glands.
In effect, the species that still remain inOryzomys are those that lack the conspicu-ously divergent morphological traits of ory-zomyines hitherto referred to other genera.However, the taxa named below differ in othercharacters that provide an unambiguous basisfor the following diagnoses and comparisons.
Aegialomys, new genus
TYPE SPECIES: Oryzomys xanthaeolus Tho-mas, 1894.
CONTENTS: galapagoensis Waterhouse,1839 (including bauri J.A. Allen, 1892) andxanthaeolus Thomas, 1894 (including baroniJ.A. Allen, 1897, and ica Osgood, 1944).
DISTRIBUTION: In the lowland dry forestsof western Ecuador (including the GalapagosIslands) and western Peru, but also at higherelevations (to about 2500 m) in the upperMaranon valley of northern Peru.
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagecoarsely grizzled yellowish- or grayish-brown;ventral pelage abruptly paler (superficiallywhitish or pale yellow), but ventral hairsalways gray-based. Pinnae small, not reaching
eye when laid forward. Mystacial and super-ciliary vibrissae not extending posteriorlybeyond pinnae when laid back. Hind footwith conspicuous tufts of ungual hairs at basesof claws on dI–dV; plantar surface denselycovered with distinct squamae distal to thenarpad; hypothenar pad present and large; clawof dI extending beyond middle of phalange 1(almost to first interphalangeal joint) of dII;claw of dV extending just beyond first in-terphalangeal joint of dIV. Tail about as longas head and body in A. galapagoensis butdistinctly longer than head and body in A.xanthaeolus; weakly to distinctly bicolored(dark above, pale below).
Skull with stout rostrum flanked by deepzygomatic notches; interorbital region anteri-orly convergent with strongly beaded supra-orbital margins; braincase oblong, usuallywith well-developed temporal crests; lambdoi-dal and nuchal crests often well developed inolder adults. Posterior margin of zygomaticplate dorsal to M1 alveolus in some examinedspecimens, anterior to M1 alveolus in others.Jugal present but small (the maxillary andsquamosal zygomatic processes broadly over-lapping in lateral view but not in contact).Nasals extending posteriorly behind lacrimalsin A. galapagoensis but shorter (extending tobut usually not behind lacrimals) in A.xanthaeolus; lacrimals usually with longermaxillary than frontal sutures. Fronto-squamosal suture usually colinear with fron-toparietal suture. Parietals with broad lateralexpansions. Incisive foramina long, typicallyextending posteriorly to or between M1alveoli; almost parallel-sided (in A. galapa-goensis) or widest at midlength and taperingsymmetrically anteriorly and posteriorly (in A.xanthaeolus). Posterolateral palatal pits large,complex, and recessed in deep fossae; mesop-terygoid fossa penetrating anteriorly betweenmaxillae in A. galapagoensis but often not inA. xanthaeolus; bony roof of mesopterygoidfossa perforated by very large sphenopalatinevacuities. Alisphenoid strut absent (buccina-tor-masticatory foramen and accessory fora-men ovale confluent). Stapedial foramen andposterior opening of alisphenoid canal small;squamosal-alisphenoid groove and spheno-frontal foramen absent; secondary anastomo-sis of internal carotid crosses dorsal surface of
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 5
pterygoid plate (5 carotid circulatory pattern3 of Voss, 1988). Postglenoid foramen largeand rounded; subsquamosal fenestra smallbut distinct in most forms, but vestigial orabsent in an unnamed species from coastalEcuador. Periotic exposed posteromediallybetween ectotympanic and basioccipital, butusually not extending anteriorly to carotidcanal; mastoid unfenestrated or with a smallbut distinct posterodorsal fenestra (in speci-mens from coastal Ecuador). Capsular processof lower incisor alveolus well developed inmost fully adult specimens; superior andinferior masseteric ridges conjoined anteriorlyas single crest below m1.
Labial and lingual flexi of M1 and M2 notinterpenetrating. First upper molar (M1)anterocone divided into anterolabial andanterolingual conules by distinct anterome-dian flexus in some forms (e.g., A. galapa-goensis and an undescribed species fromcoastal Ecuador), undivided in others (e.g.,A. xanthaeolus, which, however, has a smallinternal fossette that seems to representa vestigial anteromedian flexus); anterolophwell developed and fused with anterostyle onlabial cingulum, separated from anterocone bypersistent anteroflexus in some species (e.g., A.xanthaeolus) but fused with anterocone (ante-roflexus reduced or absent) in others; proto-style absent; paracone usually connected byenamel bridge to posterior moiety of proto-cone. Second upper molar (M2) protoflexuspresent; mesoflexus present as single internalfossette. Third upper molar (M3) with poster-oloph and diminutive hypoflexus (the lattertending to disappear with moderate to heavywear). Accessory labial root of M1 oftenpresent.
First lower molar (m1) anteroconid usuallywithout an anteromedian flexid; anterolabialcingulum present on all lower molars; ante-rolophid present on m1 but absent on m2 andm3; ectolophid absent on m1 and m2;mesolophid distinct on unworn m1 but re-duced on m2; m2 hypoflexid short. Accessorylingual and labial roots of m1 present; m2 andm3 each with two small anterior roots and onelarge posterior root.
Fifth lumbar (17th thoracicolumbar) verte-bra with well-developed anapophysis. Hemalarch between second and third caudal verte-
brae with posterior spinous process. Sup-ratrochlear foramen of humerus present.
Stomach without extension of glandularepithelium into corpus. One pair of preputialglands present. Distal bacular cartilage ofglans penis small and trifid (with a short andslender central digit); nonspinous tissue on rimof terminal crater does not conceal bacularmounds; dorsal papilla spineless; urethralprocesses without subapical lobules.
COMPARISONS: Aegialomys was representedby ‘‘Oryzomys’’ xanthaeolus4 in the phyloge-netic analyses of Weksler (2003, 2006), whoconsistently recovered it as a member of cladeD. Within clade D, ‘‘O.’’ xanthaeolus usuallyappeared as the sister taxon of a groupcomposed of Amphinectomys, Melanomys,Nectomys, and Sigmodontomys (as in fig. 1).Phenetically, however, Aegialomys more close-ly resembles Oryzomys sensu stricto (the‘‘palustris group’’ of authors), Cerradomys(the ‘‘subflavus group’’), and Eremoryzomys(the ‘‘polius group’’). Comparisons withOryzomys sensu stricto and Cerradomys areprovided here, and comparisons withEremoryzomys are included in the accountfor that genus (below). Table 2 summarizeskey morphological comparisons among all ofthe new taxa belonging to clade D.
Aegialomys differs from Oryzomys in nu-merous traits, among which the most note-worthy are its large, distinct hypothenar padon the hind foot (the hypothenar pad is absentor vestigial in Oryzomys); conspicuous tufts oflong ungual hairs at the bases of the claws onpedal digits II–V (the ungual hairs are sparseand short in Oryzomys); M1 anteromedianflexus present or vestigial (the anteromedianflexus is unambiguously absent in Oryzomys);M1 paracone usually attached by an enamelbridge to the posterior moiety of the proto-cone (the attachment is usually to the anteriormoiety in Oryzomys); M2 mesoflexus form-ing a single internal fossette (the M2 meso-flexus usually forms two internal fossettes inOryzomys); lack of distinct anterolophids on
4 Subsequent study indicates that this terminal taxon wasa composite based on material of the unnamedEcuadorean species mentioned in the preceding diagnosistogether with Aegialomys xanthaeolus sensu stricto.Taxonomic differences therefore account for some of thecharacter variation scored as polymorphisms of A.xanthaeolus in Weksler’s (2006) analyses.
6 AMERICAN MUSEUM NOVITATES NO. 3537
m2 and m3 (anterolophids are usually distincton unworn m2 and m3 in Oryzomys); andmesolophids that tend to disappear as distinctstructures with only moderate wear (mesolo-phids are persistent as distinct structures inOryzomys). In addition, A. xanthaeolus hasa well-developed anapophysis on the fifthlumbar vertebra that is absent in O. couesiand O. palustris; a tridigitate bacular cartilagewith a short and slender central digit (thecentral digit is robust in O. couesi and O.palustris); a spineless dorsal papilla (the dorsalpapilla is provided with spines in O. couesi andO. palustris); and urethral processes that lacksubapical lobules (subapical lobules are pres-ent on the urethral processes of O. couesi andO. palustris).
Although Aegialomys xanthaeolus and Cer-radomys subflavus differ in numerous mor-phological characters and were never recov-ered as sister taxa in Weksler’s (2003, 2006)phylogenetic analyses, only a few traits distin-guish their respective genera as recognizedherein. This difficulty arises from substan-tial character variation among species within
each genus: for example, as documented inLangguth and Bonvicino’s recent (2002) de-scriptions of new species of Cerradomys, andby our remarks about character variation inAegialomys (above). In fact, Aegialomys andCerradomys do not appear to differ consis-tently in any integumental or cranial featurethat we have been able to identify. Severaldental and genitalic characters, however,suggest that these are distinct taxa that meritformal recognition. Because they are so few,each character merits particular attention.
In Aegialomys galapagoensis, the unwornanterocone of M1 is divided into subequalanterolabial and anterolingual conules by ananteromedian flexus, but in A. xanthaeolus(from coastal Peru) the anterocone is un-divided and the anteromedian flexus is presentonly as an internal fossette whose faintconnection to a shallow median sulcus in theanterior face of the anterocone is transient andcan only be seen on minimally worn teeth(e.g., AMNH 10111, 42398). By contrast, theanterocone of M1 in Cerradomys is neverdivided into labial and lingual conules by an
TABLE 2Selected Morphological Comparisons Among New Genera from Clade D
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 7
anteromedian flexus, and the internal fossetteof the procingulum that is visible in someunworn dentitions (e.g., AMNH 134566,illustrated by Musser et al., 1998: fig. 144) isclearly derived from the anteroflexus, a labialenamel infolding.
The mesoflexus of M2 is present as a singleinternal fossette in Aegialomys. Althoughoccasional rare variants are to be expected insuch traits, this morphology appears to beexhibited consistently by examined specimensof A. galapagoensis, A. xanthaeolus (includingbaroni), and the unnamed form from coastalEcuador. The mesoflexus of Cerradomys,however, is usually represented by two in-ternal fossettes, of which one is labial andother is nearer the midline of the tooth (asillustrated for ‘‘Oryzomys’’ subflavus byMusser et al., 1998: fig. 144).
The male genitalia of Aegialomys galapa-goensis (‘‘Oryzomys bauri’’) and A. xanthaeo-lus (‘‘O. xantheolus’’) were described andillustrated by Patton and Hafner (1983). Inboth species, the distal bacular cartilage isunambiguously trifid, with a slender butdistinct central digit. By contrast, the glanspenis of Cerradomys scotti, C. subflavus, andan undescribed congener from northeasternBrazil have a bifid distal bacular cartilagebecause the middle digit is vestigial or absent.
REMARKS: Although ‘‘Oryzomys’’ galapa-goensis and ‘‘O.’’ xanthaeolus have long beenrecognized as closely related species (e.g., byThomas, 1894; Gardner and Patton, 1976;Patton and Hafner, 1983), no publishedphylogenetic analysis of biochemical or mor-phological data is currently available tosupport the monophyly of Aegialomys asconstituted herein. The presence of at leastone undescribed species among the materialwe examined, together with questions thathave been raised elsewhere concerning thetaxonomic status of ica (by Musser andCarleton, 2005: 1156) and our own reserva-tions about baroni, suggest that a revision ofthis group is needed to identify the terminaltaxa that should be represented in futurephylogenetic analyses.
ETYMOLOGY: From aegialos (Greek for theseashore), in reference to the predominantlycoastal distribution of these species in westernSouth America.
Cerradomys, new genus
TYPE SPECIES: Hesperomys subflavus Wag-ner, 1842.
CONTENTS: maracajuensis Langguth andBonvincino, 2002; marinhus Bonvincino,2003; scotti Langguth and Bonvincino, 2002(including andersoni Brooks et al., 2004); andsubflavus Wagner, 1842 (including vulpinusLund, 1840; vulpinoides Schinz, 1845; andlaticeps Winge, 1888 [not Lund, 1840]).
DISTRIBUTION: In dry tropical and sub-tropical forests of the Caatinga, Cerrado,and Chaco from northeastern Brazil to easternBolivia.
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagecoarsely grizzled, usually some shade ofreddish- or yellowish-brown; ventral pelageabruptly paler in some species (superficiallywhitish or yellowish) or not (the ventralcoloration merging gradually with that of thedorsum), but ventral hairs always gray-based.Pinnae small, not extending to eye when laidforward. Mystacial and superciliary vibrissaenot extending posteriorly beyond pinnae whenlaid back. Hind foot with conspicuous tufts oflong ungual hairs at bases of claws on dI–dV;plantar surface densely covered with distinctsquamae distal to thenar pad; hypothenar padsmall but distinct; claw of dI extending beyondmiddle of phalange 1 but not quite to firstinterphalangeal joint of dII; claw of dVextending to but not beyond first interphalan-geal joint of dIV. Tail longer than combinedlength of head and body, weakly bicolored inmost species but distinctly bicolored in others(e.g., C. scotti).
Skull with long, tapering rostrum flankedby deep zygomatic notches; interorbital regionanteriorly convergent, with strongly beadedsupraorbital margins; braincase oblong, withwell-developed temporal crests; lambdoidaland nuchal crests well developed in olderadults. Posterior margin of zygomatic plateusually anterior to M1 alveolus. Jugal presentbut small (maxillary and squamosal zygomaticprocesses overlapping in lateral view but notin contact). Nasals not extending posteriorlybeyond lacrimals; lacrimals usually suturedequally to maxillary and frontal bones (exceptin C. maracajuensis, which has longer maxil-lary than frontal sutures). Frontosquamosalsuture usually colinear with frontoparietal
8 AMERICAN MUSEUM NOVITATES NO. 3537
suture. Parietals with broad lateral expan-sions. Incisive foramina long, usually extend-ing posteriorly to or between M1 alveoli;usually widest at midlength and taperingsymmetrically anteriorly and posteriorly.Posterolateral pits large, complex, and re-cessed in deep fossae; mesopterygoid fossapenetrating anteriorly between maxillae butusually not between molar rows; bony roof ofmesopterygoid completely ossified in somespecies (e.g., C. marinhus and C. maracajuen-sis) but perforated by large sphenopalatinevacuities in others (e.g., C. scotti). Alisphenoidstrut absent (buccinator-masticatory foramenand accessory foramen ovale confluent) inmost species, but present (foramina separate)in C. scotti. Stapedial foramen and posterioropening of alisphenoid canal vestigial orabsent; squamosal-alisphenoid groove andsphenofrontal foramen absent; secondaryanastomosis of internal carotid crosses dorsalsurface of pterygoid plate (5 carotid circula-tory pattern 3 of Voss, 1988). Postglenoidforamen large and rounded; small subsqua-mosal fenestra distinct and patent in mostspecies but absent or vestigial (not patent) inC. scotti. Periotic exposed posteromediallybetween ectotympanic and basioccipital butusually not extending anteriorly to carotidcanal; mastoid completely ossified or fenes-trated (variation observed within and amongspecies). Capsular process of lower incisoralveolus strongly developed below base ofcoronoid process; superior and inferior mas-seteric ridges converge anteriorly as openchevron below m1.
Labial and lingual flexi of M1 and M2 notinterpenetrating. First upper molar (M1) ante-rocone not divided into labial and lingualconules (anteromedian flexus absent); antero-loph well developed and fused with anterostyleon labial cingulum, usually separated fromanterocone by persistent anteroflexus; proto-style usually absent; paracone connected byenamel bridge to middle or to posterior moietyof protocone. Second upper molar (M2) pro-toflexus present; mesoflexus usually present astwo internal fossettes. Third upper molar (M3)with posteroloph; hypoflexus present or absent.Accessory labial root of M1 present.
First lower molar (m1) anteroconid withoutan anteromedian flexid; anterolabial cingulum
present on all lower molars; anterolophidpresent on m1 but absent on m2 and m3;ectolophid usually absent on m1 and m2;mesolophid well developed on m1 and m2 inmost species, but mesolophid (and mesostylid)reduced or absent in C. scotti; m2 hypoflexidshort. Accessory lingual and labial rootspresent on m1; m2 and m3 each with onelarge anterior root and one large posteriorroot.
Fifth lumbar (17th thoracicolumbar) verte-bra with well-developed anapophysis. Hemalarch between second and third caudal verte-brae with posterior spinous process. Supra-trochlear foramen of humerus present.
Stomach without extension of glandularepithelium into corpus. Distal bacular carti-lage of glans penis bifid (the central digit isvestigial or absent); nonspinous tissue oncrater rim does not conceal bacular mounds;dorsal papilla spineless; urethral processeswithout subapical lobules.
COMPARISONS: Cerradomys was represent-ed by ‘‘Oryzomys’’ subflavus in the phyloge-netic analyses of Weksler (2003, 2006), whoconsistently recovered it as a member of cladeD (as in fig. 1). Although its phylogeneticposition within clade D was never stronglysupported in any analytic permutation basedon morphological and/or IRBP sequencecharacters, Cerradomys never appeared asthe sister taxon of any other species ofOryzomys sensu lato. However, it is pheneti-cally most similar to Aegialomys (the‘‘xanthaeolus group’’ of authors), Oryzomyssensu stricto (the ‘‘palustris group’’), andSooretamys (‘‘Oryzomys’’ angouya). Compar-isons with Oryzomys are provided here,whereas comparisons with Aegiolomys andSooretamys are provided in the accounts forthose taxa (above and below, respectively).
Cerradomys differs from Oryzomys sensustricto by its distinct hypothenar pad on thehind foot (the hypothenar is absent or vestigialin Oryzomys); conspicuous tufts of long un-gual hairs at the bases of the claws on pedaldigits II–V (the short ungual hairs ofOryzomys do not form distinct tufts); lacri-mals that are usually sutured equally to themaxillary and frontal bones (the lacrimals areprimarily sutured with the maxillaries inOryzomys); shorter palate (the mesopterygoid
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 9
fossa does not extend anteriorly between themaxillary bones in Oryzomys); absence ofan anterolophid on m2 and m3 (the ante-rolophid is distinct on unworn m2 and m3 inOryzomys); presence of an anapophysis on thefifth lumbar vertebra (absent in Oryzomys);bifid distal bacular cartilage (the distal bacularcartilage is trifid in Oryzomys); dorsal papillaof glans penis spineless (spinous in Oryzomys);and urethral processes without subapicallobules (present in Oryzomys).
REMARKS: Compelling evidence for themonophyly of Cerradomys is provided byparsimony and maximum likelihood anal-yses of cytochrome b mtDNA sequences(Bonvicino and Moreira, 2001; Bonvicino,2003). The highly distinctive anatomy of theglans penis, which lacks a central baculardigit, likewise supports this conclusion.Hopefully, the flurry of recently publisheddescriptions of new species of Cerradomys(e.g., by Langguth and Bonvicino, 2002;Bonvicino, 2003; Brooks et al., 2004) willsoon be followed by more synthetic andcomprehensive studies to convincingly docu-ment species identifications and geographicdistributions across the entire range of thisobviously diverse clade. At least some of themany unstudied specimens representing thisgenus from Bolivia and Paraguay are likely tobelong to taxa recently described fromBrazilian material, but others may representnew forms. Unfortunately, no taxonomicstudy published to date has effectively trans-cended national boundaries.
ETYMOLOGY: For the Cerrado, a vast mo-saic of savannas and dry forests, where manyspecies of this clade are found.
Eremoryzomys, new genus
TYPE SPECIES: Oryzomys polius Osgood,1913.
CONTENTS: polius Osgood, 1913.DISTRIBUTION: Known only from a few
localities in the upper Rıo Maranon valley ofnorthern Peru.
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagecoarsely grizzled-grayish (but brownish- oryellowish-gray in some old and possibly soiledspecimens); ventral pelage paler (superficiallywhitish), but ventral hairs always gray-based.Pinnae small, not reaching eye when laid
forward. Mystacial and superciliary vibrissaenot extending posteriorly beyond pinnae whenlaid back. Hind foot with conspicuous tufts oflong ungual hairs at bases of claws on dI–dV;plantar surface densely covered with distinctsquamae distal to thenar pad; hypothenar padlarge and distinct; claw of dI extending almostto first interphalangeal joint of dII; claw of dVextending just beyond first interphalangealjoint of dIV. Tail longer than combined lengthof head and body; distinctly bicolored (darkabove, pale below).
Skull with long, stout rostrum flanked bymoderately deep zygomatic notches; interor-bital region anteriorly convergent, withbeaded supraorbital margins; braincaserounded, with more or less distinct temporalcrests; lambdoidal and nuchal crests devel-oped in older adults. Posterior margin ofzygomatic plate dorsal to M1 alveolus; jugalpresent and large (the maxillary and squamo-sal zygomatic processes widely separated, notoverlapping in lateral view). Nasals short, notextending posteriorly beyond lacrimals; lacri-mals equally sutured to maxillary and frontalbones. Frontosquamosal suture usually co-linear with frontoparietal suture. Parietalswith broad lateral expansions. Incisive foram-ina very long, usually extending posteriorlybetween M1 anterocones or protocones; withsubparallel lateral margins. Posterolateralpalatal pits large, complex, and recessed indeep fossae; mesopterygoid fossa penetratinganteriorly to or slightly between molar rows;bony roof of mesopterygoid fossa perforatedby large sphenopalatine vacuities. Alisphenoidstrut usually present (buccinator-masticatoryforamen and accessory foramen ovale sepa-rate), but strut unilaterally absent on someskulls. Stapedial foramen and posterior open-ing of alisphenoid canal small; squamosal–alisphenoid groove and sphenofrontal fora-men absent; secondary anastomosis of internalcarotid crosses dorsal surface of pterygoidplate (5 carotid circulatory pattern 3 of Voss,1988). Postglenoid foramen large and round-ed; subsquamosal fenestra large and patent.Periotic exposed posteromedially betweenectotympanic and basioccipital but not ex-tending anteriorly to carotid canal; mastoidperforated by small or large posterodorsalfenestra. Capsular process of lower incisor
10 AMERICAN MUSEUM NOVITATES NO. 3537
alveolus indistinct or absent. Superior andinferior masseteric ridges usually conjoinedanteriorly as single crest below m1.
Labial and lingual flexi of M1 and M2 notinterpenetrating. First upper molar (M1)anterocone not divided into labial and lingualconules (but a small internal fossette obviouslyderived from the anteromedian flexus ispresent); anteroloph usually well developedand fused with anterostyle on labial cingulum,separated from anterocone by persistent ante-roflexus; protostyle absent; paracone con-nected by enamel bridge to middle or toposterior moiety of protocone. Second uppermolar (M2) protoflexus present but shallow;mesoflexus present as one or more internalfossettes (both conditions occurring on oppo-site sides of some specimens: e.g., AMNH64054). Third upper molar (M3) with postero-loph and diminutive hypoflexus (the lattertending to disappear with moderate to heavywear). Labial accessory root of M1 absent.
First lower molar (m1) anteroconid withoutan anteromedian flexid; anterolabial cingulumand anterolophid present on all lower molars;ectolophid absent on m1 and m2; mesolophidvariably developed on m1 and m2, large anddistinct in some specimens but much reducedor absent in others; m2 hypoflexid short.Accessory roots absent on m1; m2 and m3each with one large anterior root and onelarge posterior root.
COMPARISONS: ‘‘Oryzomys’’ polius was con-sistently recovered as the most basal lineageof clade D and not as the sister group of anyother terminal taxon in the phylogeneticanalyses of Weksler (2003, 2006). In hisoriginal description of ‘‘O.’’ polius, Osgood(1913) contrasted it with ‘‘O.’’ xanthaeolus,a geographically adjacent species, but heemphasized the lack of any close resemblancebetween them. Indeed, the genera to whichwe now refer these species are strikinglydivergent in several characters.
Among other contrasts, Eremoryzomys dif-fers from Aegialomys by its much grayerdorsal pelage (the dorsal fur is distinctlyyellowish or brownish in Aegialomys); largerjugal (the jugal of Aegialomys is muchsmaller); longer incisive foramina (these open-ings never extend posteriorly between the M1protocones in Aegialomys); shorter palate (the
mesopterygoid fossa never extends anteriorlyto the molar rows in Aegialomys); alisphenoidstrut separating the buccinator–masticatoryand accessory oval foramina (the alisphenoidstrut is invariably absent and the foramina areconfluent in Aegialomys); absence of a distinctcapsular process of the lower incisor alveolus(the capsular process is well developed inAegialomys); absence of accessory roots onM1/m1 (accessory roots are normally presenton these teeth in Aegialomys); and presence ofthe anterolophid on m2 and m3 (the anterolo-phid is absent on these teeth in Aegialomys).
Comparisons with other new genera be-longing to clade D, none of which appear tobe closely related to Eremoryzomys, aresummarized in table 2.
ETYMOLOGY: From eremia (Greek fora lonely place), in reference to the isolateddistribution of this monotypic genus.
Euryoryzomys, new genus
TYPE SPECIES: Oryzomys macconnelli Tho-mas, 1910.
CONTENTS: emmonsae Musser et al., 1998;lamia Thomas, 1901; legatus Thomas, 1925;macconnelli Thomas, 1910 (including incertusJ.A. Allen, 1913, and mureliae J.A. Allen,1915); nitidus Thomas, 1884 (including boliviaeThomas, 1901); and russatus Wagner, 1848(including physodes Brants, 1827, intermediaLeche, 1886, coronatus Winge, 1887, kelloggiAvila-Pires, 1959, and moojeni Avila-Pires,1959).
DISTRIBUTION: In moist (evergreen andsemi-evergreen) forests throughout the cis-Andean tropical and subtropical lowlands ofSouth America, including Amazonia, theGuianas, southeastern Brazil, eastern Bolivia,northern Argentina, and eastern Paraguay (seeMusser et al., 1998: figs. 78, 79).
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagefinely grizzled yellowish- to reddish-brown;ventral pelage abruptly paler (superficiallywhitish), but ventral hairs always gray-based.Pinnae large, reaching eye when laid forward.Mystacial and superciliary vibrissae not ex-tending posteriorly beyond pinnae when laidback. Hind foot with conspicuous tufts of longungual hairs at bases of claws on dII–dV;plantar surface smooth or sparsely coveredwith indistinct squamae distal to thenar pad;
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 11
hypothenar pad distinct; claw of dI extendingjust beyond base of phalange 1 of dII; claw ofdV extending to middle of phalange 1 of dIV.Tail about as long as combined length of headand body in some species (e.g., E. nitidus) butusually longer than head and body in others(e.g., E. macconnelli); distinctly bicolored(dark above, pale below).
Skull with long, tapering rostrum flankedby deep zygomatic notches; interorbital regionanteriorly convergent, with beaded supraor-bital margins; braincase oblong, with weaklydeveloped temporal crests; lambdoidal andnuchal crests developed in older adults.Posterior margin of zygomatic plate usuallydorsal to M1 alveolus; jugal present but smallin most species, but absent in E. lamia. Nasalsnot extending posteriorly beyond lacrimalbones in some species (e.g., E. macconnelli)but often extending beyond lacrimals in others(e.g., E. lamia); lacrimals equally sutured tomaxillaries and frontals. Frontosquamosalsuture usually colinear with frontoparietalsuture. Parietals without lateral expansionsin some species (e.g., E. russatus) or lateralexpansions present but usually not very broad(e.g., in E. macconnelli). Incisive foraminaranging from moderately short and posteriorlybroad (e.g., in E. macconnelli) to moderatelylong and widest near their midlength (e.g., inE. russatus), but never extending posteriorlybetween M1 anterocones. Posterolateral pala-tal pits small to moderately large, but usuallynot recessed in distinct fossae; mesopterygoidfossa extending anteriorly between maxillae insome species (e.g., E. russatus) but not inothers (e.g., E. macconnelli); bony roof ofmesopterygoid fossa completely ossified orperforated by small (slit-like) sphenopalatinevacuities. Alisphenoid strut usually absent(buccinator-masticatory foramen and accesso-ry oval foramen confluent) in most species butoften present in others (e.g., E. nitidus).Stapedial foramen, squamosal–alisphenoidgroove and sphenofrontal foramen present(5 carotid circulatory pattern 1 of Voss,1988). Postglenoid foramen large and round-ed, subsquamosal fenestra large and patent.Periotic exposed posteromedially betweenectotympanic and basioccipital, but usuallynot extending anteriorly to carotid canal;mastoid completely ossified in most fully adult
specimens. Capsular process of lower incisoralveolus well developed in adult specimens ofmost species but absent in E. macconnelli andE. emmonsae; superior and inferior massetericridges converge anteriorly as open chevronbelow m1.
Labial and lingual flexi of M1 and M2 not(or shallowly) interpenetrating. First uppermolar (M1) anterocone not divided into labialand lingual conules (anteromedian flexusabsent); anteroloph well developed and fusedwith anterostyle on labial cingulum, separatedfrom anterocone by persistent anteroflexus;protostyle absent; paracone connected byenamel bridge to posterior moiety of proto-cone except in E. macconnelli (where theattachment is usually to the middle of theprotocone). Second upper molar (M2) proto-flexus present; mesoflexus usually present astwo internal fossettes. Upper third molar (M3)without posteroloph; hypoflexus well devel-oped (persisting with moderate to heavy wear:e.g., in E. lamia) or absent (e.g., in E. nitidus).Labial accessory root of M1 usually absent.
First lower molar (m1) anteroconid withoutan anteromedian flexid; anterolabial cingulumpresent on all lower molars; anterolophidpresent on m1 but usually absent on m2 andm3; ectolophid variably present or absent onm1 and m2; mesolophid well developed on m1and m2; m2 hypoflexid short. Accessory rootsusually absent on m1; m2 and m3 each withone large anterior root and one large posteriorroot.
Fifth lumbar (17th thoracicolumbar) verte-bra with well-developed anapophysis. Hemalarch between second and third caudal verte-brae with or without posterior spinous pro-cess. Supratrochlear foramen of humeruspresent.
Stomach without extension of glandularepithelium into corpus. Male accessory re-productive glands not dissected, unknown.Distal bacular cartilage of glans penis largeand trifid (with robust central digit); shelf ofnonspinous tissue on crater rim does notconceal bacular mounds; dorsal papilla spine-less; urethral processes without subapicallobules.
COMPARISONS: Weksler’s (2003, 2006) phy-logenetic analyses of morphological andmolecular characters consistently recovered
12 AMERICAN MUSEUM NOVITATES NO. 3537
Euryoryzomys (represented by ‘‘Oryzomys’’lamia, ‘‘O.’’ macconnelli, and ‘‘O.’’ russatus)as a member of clade B together withHylaeamys, Transandinomys, Nephelomys,Oecomys, Handleyomys, and the ‘‘alfaroigroup’’. Within clade B, however, the relation-ships of Euryoryzomys were not consistentlyindicated by different analytic permutations.Whereas some analyses suggested that thisgenus may be the sister group of Mindomys +Oecomys, others recovered it as a basal lineageor as the sister group of Transandinomys, butnone of these alternatives was strongly sup-ported. Phenetically, Euryoryzomys resemblesother lowland moist-forest taxa that also havelarge pinnae, short outer digits on the hindfoot, and that normally lack accessory rootson M1/m1. Comparisons of Euryoryzomyswith Hylaeamys and Transandinomys are ofspecial interest because the species that weassign to these genera have traditionally beentreated as members of the so-called ‘‘capito’’complex of Oryzomys sensu lato (Musser etal., 1998).
Although fully adult specimens of Eur-yoryzomys are usually larger than those ofHylaeamys (see measurement data summa-rized by Musser et al., 1998) and tend to havebrighter (tawny or ochraceous versus brown-ish) dorsal fur, these genera are externallysimilar and are often confused in the field.Among the integumental contrasts summa-rized by Musser et al. (1998: table 52), tailcoloration most consistently distinguishesEuryoryzomys (their ‘‘nitidus group’’) fromHylaeamys (their ‘‘megacephalus’’ and ‘‘yun-ganus’’ groups): In Euryoryzomys, the tail isalmost always distinctly bicolored, whereas itis indistinctly bicolored or unicolored (all-dark) in Hylaeamys.
Euryoryzomys also differs strikingly fromHylaeamys by its primitive pattern of carotidcirculation (pattern 1 of Voss, 1988), whichincludes both the supraorbital and infraorbitalbranches of the stapedial artery. The supraor-bital branch leaves a prominent translucentgroove across the squamosal and alisphenoidon the inside of the braincase and exits theskull via the sphenofrontal foramen; both ofthese osteological features are lacking inHylaeamys, amost all specimens of whichclearly lack the supraorbital branch of the
stapedial artery (pattern 2 of Voss, 1988).Illustrations of these alternative conditionsas expressed by representative species ofEuryoryzomys and Hylaeamys are providedin Musser et al. (1998: fig. 27).
The only other cranial trait that usefullydistinguishes these genera is mastoid fenestra-tion. The occipital surface of the mastoidcapsule that houses the paraflocculus iscompletely ossified in almost all examinedspecimens of Euryoryzomys, but it is promi-nently fenestrated in most specimens ofHylaeamys. Illustrations of these contrastingcharacter states, exemplified by other taxabut resembling the conditions seen inEuryoryzomys and Hylaeamys, are providedin Weksler (2006: fig. 22).
Fully adult specimens of Euryoryzomysaverage larger than those of Transandinomys,but they are otherwise similar in most exter-nal features. Indeed, the integumental pig-mentation of T. talamancae strikingly resem-bles that of Euryoryzomys. Apparently, theonly nonmetric external feature by whichEuryoryzomys and Transandinomys can bedistinguished is the length of the superciliaryvibrissae. These tactile hairs, rooted just abovethe eye, extend well behind the pinnae in bothspecies of Transandinomys, although they aremuch longer in T. bolivaris than in T.talamancae (see Musser et al., 1998: fig. 53).By contrast, the superciliary vibrissae donot extend beyond the posterior marginsof the pinnae in examined specimens ofEuryoryzomys.
Euryoryzomys and Transandinomys alsodiffer in several dental traits. Of these, themost striking concerns the upper second molar(M2) mesoflexus, which is consistently repre-sented by two internal fossettes (labial andmedial) in Euryoryzomys, whereas only a singleinternal fossette represents the M2 mesoflexusin Transandinomys (see Musser et al., 1998:figs. 29, 151). On the upper third molar (M3),the hypoflexus tends to be deeper and morepersistent in Euryoryzomys than it is inTransandinomys, but this trait is not suffi-ciently constant to permit unambiguous iden-tifications by itself. On the second lower molar(m2), however, the hypoflexid is distinctivelyshorter in Euryoryzomys (see Musser et al.,1998: fig. 32A,B) than it is in Transandinomys
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 13
(see Musser et al., 1998: fig. 64, left andmiddle).
REMARKS: This clade has usually beencalled the ‘‘nitidus group’’ by authors(e.g., Weksler, 1996; Musser et al., 1998;Percequillo, 1998; Patton et al., 2000), butwe designate macconnelli as the type species ofEuryoryzomys because it is represented bymore complete character data than any of theother congeneric forms whose relationshipshave been analyzed to date. Although onlythree of the species that we refer toEuryoryzomys were analyzed by Weksler(2003, 2006), more taxonomically inclusivephylogenetic studies based on morphologicaldata also support generic monophyly(Weksler, 1996). This clade is likewise re-covered by parsimony analyses of cytochromeb sequence data that exclude third-positiontransitions and by neighbor-joining and max-imum-likelihood analyses when transversionsare weighted more heavily than transitions(Bonvicino and Moreira, 2001).
ETYMOLOGY: From eurus (Greek for far-reaching or far-spread), in reference to theextensive distribution of this genus.
Hylaeamys, new genus
TYPE SPECIES: Mus megacephalus Fischer,1814.
CONTENTS: acritus Emmons and Patton,2005; laticeps Lund, 1840 (including saltatorWinge, 1888, and seuanezi Weksler et al.,1999); megacephalus Fischer, 1814 (includingcapito Olfers, 1818, cephalotes Desmarest,1819, velutinus J.A. Allen and Chapman,1893, goeldii Thomas, 1897, and modestus,J.A. Allen, 1899); oniscus Thomas, 1904;perenensis J.A. Allen, 1901; tatei Musser etal., 1998; and yunganus Thomas, 1902.
DISTRIBUTION: In moist (evergreen andsemi-evergreen) forests of cis-Andean tro-pical and subtropical lowlands and foot-hills (to about 1500 m above sea level)from Venezuela and the Guianas southwardthroughout Amazonia and the Atlantic rain-forest to Paraguay and northern Argentina.Numerous records from drier landscapes (e.g.,in the Chaco and Cerrado) are probably allfrom gallery forests or relictual moist forestfragments formerly continuous with eitherAmazonian or coastal Brazilian rain forests.
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagefinely grizzled-brownish, typically drab gray-ish-brown in young adults but often tawny orbuffy in mature specimens; ventral pelageusually abruptly paler (superficially whitish),but ventral hairs always gray-based. Pinnaelarge, extending to eye when laid forward.Mystacial and superciliary vibrissae not ex-tending posteriorly beyond pinnae when laidback. Pes with tufts of long ungual hairs atbases of claws on dII–dV (also on dI of H.acritus); plantar surface smooth or sparselycovered with indistinct squamae distal tothenar pad; hypothenar pad distinct in mostspecies but often very small in H. megacepha-lus and sometimes absent in H. yunganus; clawof dI extending just beyond base of phalange 1of dII; claw of dV extending almost to firstinterphalangeal joint of dIV. Tail usuallyabout as long as or slightly shorter thancombined length of head and body; unicolored(all dark) or weakly bicolored (dark above,pale below) near base.
Skull with moderately long, tapering ros-trum flanked by deep zygomatic notches;interorbital region usually convergent anteri-orly with weakly beaded supraorbital margins(some specimens of H. laticeps and H. mega-cephalus have almost hourglass-shaped inter-orbits with squared dorsolateral margins);braincase oblong, usually with distinct tempo-ral crests; lambdoidal and nuchal crests de-veloped in older adults. Posterior margin ofzygomatic plate usually dorsal to M1 alveolus;jugal present but small (maxillary and squa-mosal zygomatic processes overlapping inlateral view but not in contact). Nasals short,not extending posteriorly beyond lacrimal;lacrimals equally sutured to maxillary andfrontal bones in some species (e.g., O.yunganus) or sutured primarily to maxilla inothers (e.g., O. megacephalus). Parietals withsmall lateral expansions. Incisive foraminashort, not extending posteriorly between M1alveoli, and broader posteriorly than anteri-orly. Posterolateral palatal pits variable insize, number, and morphology, but usuallylarge and recessed in shallow fossae; mesop-terygoid fossa usually not extending anteriorlybetween maxillae; roof of mesopterygoid fossacompletely ossified or perforated by small(slit-like) sphenopalatine vacuities. Alisphe-
14 AMERICAN MUSEUM NOVITATES NO. 3537
noid strut absent (buccinator–masticatoryforamen and accessory oval foramen conflu-ent). Stapedial foramen and posterior openingof alisphenoid canal large, squamosal–ali-sphenoid groove and sphenofrontal foramenabsent (5 carotid circulatory pattern 2 ofVoss, 1988). Postglenoid foramen large androunded; subsquamosal fenestra large andpatent. Periotic exposed posteromedially be-tween ectotympanic and basioccipital butusually not extending anteriorly to carotidcanal; mastoid perforated by conspicuousposterodorsal fenestra. Distinct capsular pro-cess of lower incisor alveolus absent; superiorand inferior masseteric ridges converge ante-riorly as open chevron below m1.
Labial and lingual flexi of M1 and M2 not(or shallowly) interpenetrating except in H.tatei (which has more deeply interpenetratingflexi). First upper molar (M1) anterocone notdivided into labial and lingual conules (ante-romedian flexus absent); anteroloph well de-veloped and fused with anterostyle on labialcingulum, separated from anterocone byanteroflexus in minimally worn dentitions;protostyle absent; paracone connected byenamel bridge to posterior moiety of proto-cone. Second upper molar (M2) protoflexuspresent or absent; mesoflexus present as singleinternal fossette in some species (e.g., H.megacephalus) or as two fossettes (e.g., in H.yunganus). Third upper molar (M3) withoutposteroloph; hypoflexus deep and persistent insome species, shallow and transitory in other.Labial accessory root of M1 absent.
First lower molar (m1) anteroconid withoutan anteromedian flexid; anterolabial cingulumpresent on all lower molars; anterolophidusually distinct on m1 but absent on m2 andm3; ectolophid often present on m1 and m2;mesolophid present and distinct on m1 andm2; m2 hypoflexid usually short in somespecies (e.g., H. yunganus) but usually longin others (e.g., H. megacephalus). Accessoryroots absent on m1; m2 and m3 each with onelarge anterior root and one large posteriorroot.
Fifth lumbar (17th thoracicolumbar) verte-bra usually without anapophysis. Hemalarch between second and third caudal verte-brae without posterior spinous process.Supratrochlear foramen of humerus present.
Stomach without extension of glandularepithelium into corpus. One pair of preputialglands present. Distal bacular cartilage ofglans penis large and trifid (with robustcentral digit); shelf of nonspinous tissue oncrater rim does not conceal bacular mounds;dorsal papilla spineless; urethral processeswithout subapical lobules.
COMPARISONS: Weksler’s (2003, 2006) phy-logenetic analyses of morphological andmolecular characters consistently recoveredHylaeamys (represented by ‘‘Oryzomys’’ mega-cephalus, and ‘‘O.’’ yunganus) as part of clade Balong with Euryoryzomys, Transandinomys,Nephelomys, Oecomys, and Handleyomys.Phenetically, Hylaeamys is most similar totwo other genera that contain species formerlyreferred to the so-called ‘‘capito complex’’ ofOryzomys sensu lato (Musser et al., 1998),namely Euryoryzomys and Transandinomys.Because comparisons with Euryoryzomyshave already been provided in the accountfor that genus (above), only comparisons withTransandinomys are discussed here.
Hylaeamys is similar to Transandinomys insize and in most qualitative external features,but its superciliary vibrissae are shorter, notextending posteriorly behind the pinnae.Although T. talamancae has much shortersuperciliary vibrissae than T. boliviae (seeMusser et al., 1998: fig. 53), these tactile hairsextend posteriorly behind the pinnae in bothspecies of Transandinomys and are diagnosti-cally longer than they are in Hylaeamys.Otherwise, the two genera are difficult (if notimpossible) to distinguish per se based onintegumental comparisons.
In cranial features, the two genera aremost readily distinguished by their alterna-tive patterns of carotid circulation. WhereasHylaeamys possesses only the infraorbitalbranch of the stapedial artery, Transandi-nomys also has an intact supraorbital branch.The presence of the latter vessel is indicated bya translucent groove across the internalsurfaces of the squamosal and alisphenoidbones and by the presence of a sphenofrontalforamen. Both of these osteological markersare constant features of examined skulls ofTransandinomys, but they are just as consis-tently absent in Hylaeamys (see Musser et al.,1998: fig. 151).
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 15
Hylaeamys and Transandinomys do notappear to differ consistently in any othercharacter that we have been able to score in allmember species. However, the potential di-agnostic value of an anapophysis on the fifthlumbar (17th thoracicolumbar) vertebra,a process that is usually absent in H. mega-cephalus but present in T. talamancae, meritsevaluation as postcranial skeletal materialbecomes available for other congeneric taxa.
REMARKS: The monophyly of Hylaeamysis not supported by analyses of morphologicalcharacter data (Weksler, 2006: figs. 34, 35) ormtDNA sequences (Bonvincino and Moreira,2001). Instead, compelling evidence for gener-ic monophyly comes primarily from nuclearsequences (Weksler, 2003). Because the latterare only available from two species (H.megacephalus and H. yunganus), our conceptof Hylaeamys is primarily based on theabsence of the supraorbital branch of thestapedial artery. This trait was optimized as anunambiguous synapomorphy of H. megace-phalus + H. yunganus in Weksler’s combinedanalyses of morphological and IRBP charac-ters; within clade B, it is uniquely shared bythe species that we refer to Hylaeamys.
ETYMOLOGY: For the hylaea, Humboldt’sname for the the rainforested lowlands of cis-Andean South America, the principal habitatof species belonging to this clade.
Mindomys, new genus
TYPE SPECIES: Nectomys hammondi Tho-mas, 1913.
CONTENTS: hammondi Thomas, 1913.DISTRIBUTION: Currently known from just
nine specimens, eight of which were collectedin the vicinity of Mindo in the western Andeanfoothills of Pichincha province, Ecuador.Another specimen, labeled as having beencollected in the eastern (Amazonian) lowlandsof Ecuador, represents a remarkable andsomewhat problematic range disjunction. Inconsequence, the geographic distribution ofthis taxon is difficult to interpret.5
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagecoarsely grizzled yellowish-brown; ventralpelage abruptly paler (whitish or yellowishsuperficially) in most specimens, but ventralhairs always gray-based. Pinnae small, notreaching eye when laid forward. Mystacial and
superciliary vibrissae very long, extendingposteriorly well beyond caudal margins ofpinnae when laid back. Pes with sparse tufts ofrather short ungual hairs at bases of claws ondII–dV; plantar surface sparsely covered withindistinct squamae distal to thenar pad;hypothenar pad present and distinct; claw ofdI extending to or just beyond first interpha-langeal joint of dII; claw of dV extending tomiddle of phalange 2. Tail unicolored (alldark), and much longer than combined lengthof head and body.
Skull with long, stout rostrum flanked byvery shallow zygomatic notches; interorbitalregion anteriorly convergent, with stronglybeaded supraorbital margins; braincase elon-gate, with well-developed temporal, lambdoi-dal, and nuchal crests developed in olderadults. Posterior margin of zygomatic platedorsal to M1 alveolus; jugal present and large(the maxillary and squamosal zygomatic pro-cesses widely separated, usually non-overlap-ping in lateral view). Nasals not extendingposteriorly beyond lacrimal bones; lacrimalsequally sutured to maxillary and frontalbones. Frontosquamosal suture anterior to
5 The eight specimens of ‘‘Oryzomys’’ hammondi fromMindo (0u029S, 78u489W, 1264 m above sea level; Paynter,1993) were taken by three different collectors—G.Hammond, L. Soderstrom, and R.S. Voss—from 1913to 1980; seven of these specimens are at the BMNH andthe eighth is at the UMMZ. The single Amazonian recordis based on MCZ 52543, an adult female whose skin labelstates that it was collected by the Olallas (a family ofprofessional collectors) on 27 July 1929 at ‘‘Concepcion,Oriente, Ecuador’’. According to Paynter (1993),Concepcion is at 0u489S, 77u259W, about 50 km NE ofTena between 300 and 500 m above sea level in Napoprovince. Several aspects of these distributional data areproblematic. First, we are not aware of any other speciesof small nonvolant native mammal that occurs belowabout 1500 m on both sides of the Ecuadorean Andes.Second, the lowlands and foothills around Tena have beenintensively worked over for many years by numerouscollectors, none of whom have taken additional materialof this species. Thus, there is some reason to questionwhether Mindomys really occurs in Amazonia.Additionally, it is not known if Mindo represents theupper or the lower limit of the elevational distribution ofthis taxon in western Ecuador, or if Mindo is somewherein the middle of its elevational range. If Mindomys ispredominantly a lowland taxon, then it might moreappropriately be classified as a trans-Andean rather thanas an Andean clade (sensu Weksler, 2006). Only futurefieldwork can resolve such uncertainties, which areobviously relevant to reconstructing oryzomyine historicalbiogeography.
16 AMERICAN MUSEUM NOVITATES NO. 3537
frontoparietal suture (dorsal facet of frontalin broad contact with squamosal). Parietalswith broad lateral expansions. Incisive fora-mina short, not extending posteriorly to levelof M1 alveoli, usually widest posteriorlyand converging anteriorly (teardrop-shaped).Posterolateral palatal pits small and unre-cessed in some specimens but larger andrecessed in moderately deep fossae in others;mesopterygoid fossa extending anteriorly be-tween maxillae but not between molar rows;bony roof of mesopterygoid fossa usuallycompletely ossified (some specimens have verynarrow sphenopalatine vacuities flanking thepresphenoid or the presphenoid/basisphenoidsuture). Alisphenoid strut absent (buccinator–masticatory foramen and accessory oval fora-men confluent). Stapedial foramen, squamo-sal–alisphenoid groove, and sphenofrontalforamen present (5 carotid circulatory pattern1 of Voss, 1988). Postglenoid foramen smalland dorsoventrally compressed; subsquamosalfenestra vestigial (not patent) or absent.Periotic broadly exposed posteromedially be-tween ectotympanic and basioccipital, extend-ing anteriorly to carotid canal; mastoidcompletely ossified, not fenestrated. Distinctcapsular process of lower incisor alveolusabsent; superior and inferior masseteric ridgesconverge anteriorly as open chevron belowm1.
Labial and lingual flexi of M1 and M2interpenetrating. First upper molar (M1)anterocone not divided into labial and lingualconules (anteromedian flexus absent); ante-roloph well developed, fused with anterostyleon labial cingulum, and separated fromanterocone by persistent anteroflexus; proto-style absent; paracone connected by enamelbridge to posterior moiety of protocone.Second upper molar (M2) protoflexus absent;mesoflexus present as two internal fossettes.Third upper molar (M3) with posteroloph andpersistent hypoflexus. Labial accessory root ofM1 absent.
Lower first molar (m1) anteroconid withoutan anteromedian flexid; anterolabial cingulumpresent on m1 and m2, occasionally absent onm3; anterolophid present on m1 but absent onm2 and m3; ectolophid present on m1 but noton m2 and m3; mesolophid well developed onall lower molars; m2 hypoflexid short.
Accessory roots absent on m1; m2 and m3each with one large anterior root and onelarge posterior root.
Postcranial skeletal characters unknown.Stomach without extension of glandular
epithelium into corpus. Male reproductivetracts not examined.
COMPARISONS: Weksler (2006) recovered‘‘Oryzomys’’ hammondi either as the mostbasal oryzomyine lineage (as in fig. 1) or asa member of clade B. Within clade B, ‘‘O.’’hammondi was sometimes recovered as thesister taxon to Oecomys, but its relationshipswere unresolved in other analytic permuta-tions. Because the phylogenetic position of‘‘O.’’ hammondi was not strongly supported inany analysis, no compelling evidence exists forthe membership of this taxon in any mono-phyletic group less inclusive than the tribeOryzomyini. The following comparisons aretherefore motivated in part by historicalconcepts of taxonomic affinity.
Among other characters, Mindomys differsfrom both Nectomys (the genus to whichhammondi was originally referred by Thomas[1913]) and Oryzomys (the genus to whichhammondi was transferred by Hershkovitz[1948]) by its much longer vibrissae (thevibrissae do not extend posteriorly behindthe pinnae in Nectomys or Oryzomys); posses-sion of a distinct hypothenar pad on the hindfoot (the hypothenar is absent or vestigial inNectomys and Oryzomys); unwebbed pedaldigits (Nectomys and Oryzomys have par-tially webbed hindfeet); very long fifthdigit (the claw of dV does not extendbeyond the first interphalangeal joint inNectomys or Oryzomys); much shallowerzygomatic notches; much larger jugals; small,simple, unrecessed posterolateral palatal pits(Nectomys and Oryzomys have large, complexposterolateral palatal pits that are deeplyrecessed in conspicuous fossae); completestapedial circulation (the stapedial circulationis absent in Nectomys and Oryzomys); absenceof accessory roots on M1 and m1 (upper andlower first molars have accessory roots inNectomys and Oryzomys); and possession ofan ectolophid on m1 (Nectomys and Oryzomyslack ectolophids). Given the large number ofadditional characters by which Mindomysdiffers individually from Nectomys (represent-
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 17
ed by N. squamipes in Weksler [2006: table 5])and Oryzomys (represented by O. couesi andO. palustris), the morphological distinctness ofthese taxa is not arguable.
Several authors (Ray, 1962; Hershkovitz,1970; Steadman and Ray, 1982) have sug-gested a close relationship between ‘‘Oryzo-mys’’ hammondi and extinct Antillean giantrats of the genus Megalomys. We have notexamined material of Megalomys, but Musserand Carleton’s (2005) statements that itsmetatarsal pads are vestigial, that accessoryroots are present on M1 and m1, and that ithas a derived carotid circulation suggests thatthe genus is a member of clade D and not, infact, a close relative of Mindomys.
Mindomys appears to differ consistentlyfrom Oecomys (a speciose genus that exhibitstaxonomic variation in many characters) by itsmuch smaller interdigital pads on the hindfoot (the interdigital pads are very large inOecomys); indistinct plantar squamae (the soleof the hind foot is entirely smooth inOecomys); sparse tufts of short ungual hairson pedal digits II–V (ungual tufts are denserand longer in Oecomys); much longer rostrum(all species of Oecomys have short rostrums);frontosquamosal suture anterior to the fron-toparietal suture (the two sutures are morenearly colinear in Oecomys); shorter palate(the mesopterygoid fossa does not extendanteriorly between the maxillae in Oecomys);small, simple, unrecessed posterolateral pala-tal pits (Oecomys has large, often complexposterolateral palatal pits that are usuallydeeply recessed in conspicuous fossae); morelophodont upper molars (the labial andlingual flexi do not interpenetrate deeply onthe upper molars of Oecomys); suppression ofthe protoflexus on M2 (the protoflexus isdistinct on unworn M2s in Oecomys); absenceof a paralophule on M2 (a distinct para-lophule is present on the M2 of all examinedspecies of Oecomys); and absence of ananterolabial cingulum from m3 (the anterola-bial cingulum is distinct on the unworn m3 ofOecomys).
REMARKS: Hershkovitz (1948) designatedhammondi as the type species of Macrurory-zomys, but the latter was not diagnosed andthe name is therefore unavailable (Pine andWetzel, 1975). Although Hershkovitz (1970)
acknowledged this situation, he effectivelydid nothing to correct it, so Macruroryzomysremains a nomen nudum.
The possibly basal position of this extraor-dinary rat within the oryzomyine radiation,together with its enigmatic distribution andthe absence of preserved tissues suitable forDNA extraction and sequencing, will hope-fully impel future collectors to make specialefforts to obtain more material.
ETYMOLOGY: For Mindo, a tiny agricul-tural community in the western Andean foot-hills of Pichincha province, Ecuador.
Nephelomys, new genus
TYPE SPECIES: Hesperomys albigularis To-mes, 1860.
CONTENTS: albigularis Tomes, 1860; auri-venter Thomas, 1899; caracolus Thomas, 1914;childi Thomas, 1895 (including oconnelli J.A.Allen, 1913); devius Bangs, 1902; keaysi J.A.Allen, 1900 (including obtusirostris J.A. Allen,1900); levipes Thomas, 1902; maculiventer J.A.Allen, 1899; meridensis Thomas, 1894; moerexThomas, 1914; nimbosus Anthony, 1926;pectoralis J.A. Allen, 1912; and pirrensisGoldman, 1913.
DISTRIBUTION: In humid montane (‘‘cloud’’)forests between about 900 and 3500 m abovesea level from Bolivia northward along theAndes and Central American cordilleras toCosta Rica, and eastward along the Caribbeancoastal mountains to eastern Venezuela(Percequillo, 2003).
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagefinely grizzled yellowish- to reddish-brown;ventral pelage abruptly paler (gray-basedwhitish) in some species, but not in others(which have gray-based ochraceous under-parts); with irregular patches of self-whitishfur variably present on throat, chest, abdo-men, and/or groin in some species. Ears small,not quite extending to eye when laid forward.Mystacial vibrissae usually extending posteri-orly for several millimeters beyond caudalmargins of pinnae when laid back, butsuperciliary vibrissae shorter (not extendingbeyond pinnae). Pes with conspicuous tufts oflong ungual hairs at bases of claws on dII–dV;plantar surface smooth, without plantar squa-mae; hypothenar pad large and distinct; clawof dI extending to or just beyond middle of
18 AMERICAN MUSEUM NOVITATES NO. 3537
phalange 1 of dII; claw of dV extending to orjust beyond first interphalangeal joint of dIV.Tail longer than combined length of head andbody, weakly to distinctly bicolored (darkabove, pale below).
Skull with long, stout rostrum flanked byrelatively shallow to moderately deep zygo-matic notches; interorbital region variable,hourglass-shaped with rounded supraorbitalmargins in some species (e.g., N. albigularis)but anteriorly convergent with beaded in-terorbital margins in others (e.g., N. auriven-ter); braincase rounded, usually with indistincttemporal crests in species having roundedsupraorbital margins but with better-devel-oped temporal crests in species having beadedsupraorbital margins; lambdoidal and nuchalcrests moderately developed in older adults.Posterior margin of zygomatic plate dorsal toM1 alveolus; jugal bone present but small inmost species (the maxillary and squamosalzygomatic processes overlapping in lateralview but not in contact; N. auriventer,however, has a large jugal). Nasals usuallynot extending posteriorly beyond lacrimals;lacrimals sutured equally to maxillae andfrontals in some species (e.g., N. albigularis)or primarily to maxillae in others (e.g., N.moerex). Frontosquamosal suture usually co-linear with frontoparietal suture. Parietalswith or without large lateral expansions (vari-able within and among species). Incisiveforamina long (extending posteriorly to orbetween the alveoli of M1) and usually widestat midlength (e.g., in N. levipes) or muchshorter (never approaching the molar rows)and wider posteriorly than anteriorly (e.g., inN. moerex). Posterolateral palatal pits usuallylarge, complex, and recessed in deep fossae(but much reduced and inconspicuous in N.caracolus and N. nimbosus); mesopterygoidfossa extending anteriorly between maxillae inmost species, but often extending betweentoothrows in N. levipes; bony roof of mesop-terygoid fossa completely ossified or per-forated by small sphenopalatine vacuities.Alisphenoid strut usually absent (buccinator–masticatory foramen and accessory oval fora-men confluent) in most species, but strutusually present in N. moerex and variablypresent in others (N. auriventer, N. levipes, andN. keaysi). Stapedial foramen, squamosal–
alisphenoid groove and sphenofrontal fora-men present (5 carotid circulatory pattern 1of Voss, 1988). Postglenoid foramen large androunded; subsquamosal fenestra large andpatent in most species but much narrower inN. auriventer. Periotic exposed posterome-dially between ectotympanic and basioccipitalbut usually not extending anteriorly to carotidcanal; mastoid completely ossified (e.g., in N.auriventer) or usually fenestrated (e.g., in N.levipes). Capsular process of lower incisoralveolus indistinct or absent; superior andinferior masseteric ridges converge anteriorlyas open chevron below m1.
Labial and lingual flexi of M1 and M2interpenetrating. First upper molar (M1)anterocone divided into labial and lingualconules by distinct anteromedian flexus; ante-roloph well developed and fused with ante-rostyle on labial cingulum, separated fromanterocone by persistent anteroflexus; proto-style absent; paracone connected by enamelbridge to posterior moiety of protocone.Second upper molar (M2) protoflexus present;mesoflexus present as single internal fossette.Third upper molar (M3) posteroloph present;hypoflexus shallow and transitory (disappear-ing with moderate wear). Labial accessoryroot of M1 absent.
First lower molar (m1) anteroconid usuallywithout an anteromedian flexid (a shallowmedian crease visible on some newly eruptedteeth is quickly obliterated by wear); ante-rolabial cingulum present on all lower molars;anterolophid usually distinct on unworn m1but absent on m2 and m3; ectolophid presentor absent on m1 and m2 (variable within andamong species); mesolophid present and dis-tinct on m1 and m2; m2 hypoflexid short.Accessory labial root of m1 usually present,accessory lingual root absent; m2 and m3 eachwith one large anterior root and one largeposterior root.
Fifth lumbar (17th thoracicolumbar) verte-bra usually with anapophysis. Hemal archbetween second and third caudal vertebraewithout posterior spinous process. Supra-trochlear foramen of humerus present.
Stomach without extension of glandularepithelium into corpus. Macroscopic preputialglands absent. Distal bacular cartilage of glanspenis large and trifid (with robust central
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 19
digit); shelf of nonspinous tissue on crater rimdoes not conceal bacular mounds; dorsalpapilla spineless; urethral processes withoutsubapical lobules.
COMPARISONS: Phylogenetic analyses ofnuclear gene sequences and morphology con-sistently recovered Nephelomys, representedby ‘‘Oryzomys’’ albigularis and ‘‘O.’’ levipes inWeksler (2003, 2006), as a member of clade Balong with Euryoryzomys, Handleyomys,Hylaeamys, Oecomys, and Transandinomys(as in fig. 1). Within clade B, the relationshipsof Nephelomys were often unresolved, butsome analytic permutations weakly supporteda sister-group relationship with Transandi-nomys. Comparisons between Nephelomysand Transandinomys are summarized below,whereas comparisons among Nephelomys andother new taxa belonging to clade B aresummarized in table 3.
Species of Nephelomys are larger-bodiedthan species of Transandinomys, a contrastthat is apparent in most external dimen-sions. The adult hind foot (including claws),for example, averages about 32 mm or more
in species of Nephelomys, whereas this di-mension averages about 30 mm or less inTransandinomys (see measurements in Musseret al., 1998). In addition, species of Nephe-lomys have relatively shorter superciliaryvibrissae, smaller pinnae, longer fifth pedaldigits, and longer tails than Transandinomys(see the diagnoses of both taxa for ratios orlandmark comparisons that document thesedifferences).
The most conspicuous qualitative cranio-dental difference between Nephelomys andTransandinomys concerns the anterocone ofM1, which is deeply divided into labial andlingual conules by a persistent anteromedianflexus in the former genus. By contrast,the anterocone of M1 is undivided and notrace of an anteromedian flexus is present inTransandinomys. The size difference betweenthese genera indicated by external dimensionsis also apparent in craniodental comparisons:length of the maxillary toothrow, for example,consistently averages .5.5 mm in species ofNephelomys, but this measurement averages,4.7 mm in species samples of Transandi-
TABLE 3Selected Morphological Comparisons Among New Genera from Clade B
20 AMERICAN MUSEUM NOVITATES NO. 3537
nomys. The accessory root(s) that are usuallypresent on m1 in Nephelomys are apparentlynever developed in Transandinomys.
Due to morphological variation among con-generic taxa (as noted above in this accountand below in the account that follows), con-sistent differences between Nephelomys andTransandinomys are not apparent in any othercharacters that we have been able to scorein all member species. However, the poten-tially diagnostic value of preputial morpholo-gy deserves future study. Among the materialdissected by Voss and Linzey (1981), a singlepair of large preputial glands was found inTransandinomys talamancae (represented bytheir Panamanian specimens of ‘‘Oryzomyscapito’’), but no macroscopic preputial glandswere detected in Nephelomys devius (repre-sented by their specimens of ‘‘Oryzomysalbigularis’’); unfortunately, no additionalspecies from either genus were included inthat study and none have been dissected byother investigators.
REMARKS: All of the taxa that we refer toNephelomys were treated as synonyms orsubspecies of ‘‘Oryzomys’’ albigularis byHershkovitz (1944: 72) and Cabrera (1961:380–383), but subsequent karyotypic andmorphological research has shown that mostof these are valid species (Gardner and Patton,1976; Patton et al., 1990; Aguilera et al., 1995;Marquez et al., 2000; Percequillo, 2003). Ofthe characters that Weksler (2006) identifiedas unambiguous synapomorphies of ‘‘O.’’albigularis + ‘‘O.’’ levipes, only the dividedanterocone of M1 seems to be exhibitedconsistently by other species of Nephelomys.Although no additional analytic results arecurrently available to support generic mono-phyly, we are not aware of any evidence thatcontradicts this hypothesis.
ETYMOLOGY: From nephele (Greek forclouds or mist), in reference to the cloud-forest habitat of these montane species.
Oreoryzomys, new genus
TYPE SPECIES: Oryzomys balneator Thomas,1900.
CONTENTS: balneator Thomas, 1900 (in-cluding hesperus Anthony, 1924).
DISTRIBUTION: In humid montane(‘‘cloud’’) forest on the Andean slopes ofsouthern Ecuador and northern Peru.
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagedark olive-brown; ventral pelage abruptly paler(superficially whitish or yellowish), but ventralhairs mostly gray-based (a few specimens haveirregular gular and/or pectoral patches of self-whitish fur). Pinnae small, not reaching eyewhen laid forward. Mystacial vibrissae extend-ing posteriorly beyond caudal margins ofpinnae when laid back against cheeks; super-ciliary vibrissae shorter, not extending tocaudal margins of pinnae when laid back. Peswith conspicuous tufts of long ungual hairs atbases of claws on dII–dV; plantar surfacecovered with distinct squamae distal to thenarpad; hypothenar pad present and distinct; clawof dI extends to middle of phalange 1 of dII;claw of dV extends to first interphalangeal jointof dIV. Tail unicolored (all-dark) or indistinctlybicolored basally, much longer than combinedlength of head and body.
Skull with short, narrow rostrum flanked byshallow zygomatic notches; interorbital regionhourglass shaped, with rounded supraorbitalmargins; braincase rounded and globose,without temporal, lambdoidal, or nuchalcrests. Posterior margin of zygomatic plateusually anterior to M1 alveolus. Jugal absent(maxillary and squamosal zygomatic processesin contact). Nasals extending posteriorlybeyond lacrimals; lacrimals small, equallysutured to maxillary and frontal bones.Frontosquamosal suture usually colinear withfrontoparietal suture. Parietals with or with-out small lateral expansions. Incisive foraminashort, sometimes extending posteriorly to butnot between M1 alveoli, and widest posteri-orly (with anteriorly convergent lateral mar-gins). Posterolateral palatal pits large andrecessed in shallow fossae; mesopterygoidfossa extending anteriorly between maxillaebut not between molar rows; bony roof ofmesopterygoid fossa usually perforated bynarrow sphenopalatine vacuities (the type ofbalneator has a completely ossified mesopter-ygoid roof). Alisphenoid strut absent (bucci-nator–masticatory foramen and accessoryforamen ovale confluent). Stapedial foramen,squamosal–alisphenoid groove and spheno-frontal foramen present (5 carotid circulatory
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 21
pattern 1 of Voss, 1988). Postglenoid foramenlarge and rounded, subsquamosal fenestralarge and patent. Periotic broadly exposedposteromedially between ectotympanic andbasioccipital, extending anteriorly to carotidcanal; mastoid perforated by conspicuousposterodorsal fenestra. Capsular process oflower incisor alveolus strongly developedbelow base of coronoid process; superior andinferior masseteric ridges converge anteriorlyas open chevron below m1.
Labial and lingual flexi of M1 and M2 notinterpenetrating. First upper molar (M1) withanterocone divided into labial and lingualconules by anteromedian flexus; anterolophwell developed and fused with anterostyle onlabial cingulum, separated from anterocone bypersistent anteroflexus; protostyle absent;paracone connected by enamel bridge toanterior moiety of protocone. Second uppermolar (M2) protoflexus present; mesoflexususually present as single internal fossete. Thirdupper molar (M3) without a posteroloph, butwith persistent hypoflexus. Labial accessoryroot of M1 absent.
Lower first molar (m1) anteroconid withouta distinct anteromdian flexid (an indistinctflexid visible in some newly erupted dentitionsis presumably obliterated with light wear);anterolabial cingulum present on all lowermolars; anterolophid distinct on unworn m1but absent on m2 and m3; ectolophid absenton all lower molars; mesolophid present anddistinct on m1, reduced or absent on m2; m2hypoflexid short. Accessory roots absent onm1; m2 and m3 each with one large anteriorroot and one large posterior root.
Postcranial skeletal characters unknown.Stomach with extension of glandular epi-
thelium into corpus. Distal bacular cartilageof glans penis large and trifid (with a robustcentral digit); nonspinous tissue on crater rimconcealing bacular mounds; dorsal papillanonspinous; urethral processes without sub-apical lobules.
COMPARISONS: ‘‘Oryzomys’’ balneator wasconsistently recovered as a member of clade Cin the phylogenetic analyses of Weksler (2003,2006). Although a sister-group relationshipbetween Oreoryzomys and Microryzomys wasmoderately well supported in some analyses,Oreoryzomys was recovered as the sister taxon
of Neacomys (albeit with only trivial support)in other analytic permutations. In effect, itsrelationships within clade C remain to beresolved convincingly, and comparisons withall three member genera (including Oligory-zomys) seem appropriate.
As noted by Musser and Carleton (2005),Oreoryzomys and Microryzomys exhibit note-worthy similarities, but they also differ inmany characters. Among the most usefulmorphological features for distinguishingthese taxa, the pelage of Oreoryzomys isdistinctly countershaded (the pelage is notcountershaded in Microryzomys); the claw ofpedal digit V extends only to the first in-terphalangeal joint of dIV (the claw of dVextends well beyond the first interphalangealjoint of dIV in Microryzomys); the tail isunicolored or indistinctly bicolored basally(the tail is more or less distinctly bicolored inMicroryzomys); the premaxillae do not extendas far posteriorly as the nasals do (thepremaxillae and nasals extend posteriorly toabout the same extent in Microryzomys); theincisive foramina do not extend posteriorlybetween the M1 alveoli (as they usually do inMicroryzomys); the foramen magnum is morecaudally oriented (versus more ventrally ori-ented in Microryzomys); and the anteroconidof m1 is undivided (the anteroconid of m1 isdeeply divided into anterolabial and antero-lingual conulids by a persistent anteromedianflexid in Microryzomys).
Oreoryzomys differs from Neacomys by itssoft fur (Neacomys has spiny fur); muchlonger tail (the tail of Neacomys seldomexceeds the combined length of head andbody); shallower zygomatic notch (the zygo-matic notch is moderately deep in Neacomys);hourglass-shaped interorbital region withrounded supraorbital margins (the interorbitalregion is anteriorly convergent with beadedsupraorbital margins in Neacomys); absence ofthe jugal (the jugal is small but present inNeacomys); and shorter palate (the mesopter-ygoid fossa extends anteriorly between themaxillary bones in Oreoryzomys but not inNeacomys). We observed additional charac-ter differences between Oreoryzomys andsome species of Neacomys, but none thatappear to represent generically consistentdistinctions.
22 AMERICAN MUSEUM NOVITATES NO. 3537
Oreoryzomys differs from Oligoryzomys byits unicolored or indistinctly bicolored tail (thetail is distinctly bicolored in Oligoryzomys);premaxillae that do not extend as far posteri-orly as the nasals do (the premaxillae andnasals extend posteriorly to about the samelevel in Oligoryzomys); shallower zygomaticnotch (the zygomatic notch is moderately deepin Oligoryzomys); shorter palate (the meso-pterygoid fossa does not extend anteriorlybetween the maxillae in Oligoryzomys); moredeeply excavated parapterygoid fossae (theflat parapterygoid fossae of Oligoryzomys arealmost level with the palate); smaller spheno-palatine vacuities (these openings are verylarge in Oligoryzomys); complete stapedialcirculation (the dorsal ramus of the stapedialartery is absent in Oligoryzomys); moreposterior position of the masseteric crest(which extends anterior to m1 in Oli-goryzomys); absence of spines on the dorsalpapilla of the gland penis (the dorsal papilla isspinous in examined species of Oligoryzomys);and extension of glandular epithelium into thegastric corpus (the gastric corpus is entirelylined by cornified epithelium in examinedspecies of Oligoryzomys).
REMARKS: The available morphologicalcharacter data for Oreoryzomys is incompletebecause postcranial skeletons and male acces-sory reproductive gland dissections are cur-rently unavailable. Although new morpholog-ical information from these (and other)systems might help resolve the relationshipsof this genus within clade C, the trenchantdifferences it exhibits with other member taxasuggests that such resolution will not affect itsstatus as a distinct clade. Oreoryzomys iscurrently unrepresented by sequence datafrom any gene other than IRBP, hence thelack of prior information concerning phyloge-netic relationships in published molecularstudies of oryzomyines (e.g., Myers et al.,1995; Bonvincino and Moreira, 2001).
ETYMOLOGY: From oros (Greek for moun-tain), in reference to the montane distributionof this distinctive taxon.
Sooretamys, new genus
TYPE SPECIES: Mus angouya Fischer, 1814.CONTENTS: angouya Fischer, 1814 (in-
cluding buccinatus Olfers, 1818; angouya
Desmarest, 1819; leucogaster Wagner, 1845;ratticeps Hensel, 1872; rex Winge, 1887;paraganus Thomas, 1924; and topicius Tho-mas, 1924).
DISTRIBUTION: In tropical and subtropicalmoist forests of the Atlantic littoral insoutheastern Brazil and in interior subtropicalmoist forests of eastern Paraguay and north-ern Argentina.
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagecoarsely grizzled-brownish (the overall effectvarying from drab grayish-brown to warmreddish- or yellowish-brown); ventral pelageabruptly paler (superficially whitish or yellow-ish), but ventral hairs always gray-based.Pinnae small, not reaching eye when laidforward. Mystacial vibrissae extending poster-iorly well beyond pinnae when laid backagainst cheeks; superciliary vibrissae muchshorter, not extending posteriorly to caudalmargins of pinnae. Pes with conspicuoustufts of long ungual hairs at bases of clawson dI–dV; plantar surface densely coveredwith distinct squamae distal to thenar pad;hypothenar pad present and distinct; claw ofdI extending to middle of phalange 1 ofdII; claw of dV extending to or just beyondfirst interphalangeal joint of dIV. Tail uni-colored (all dark) in most specimens andmuch longer than combined length of headand body.
Skull with long, broad rostrum flanked bydeep zygomatic notches; interorbital regionhourglass-shaped, with squared supraorbitalmargins; braincase oblong and usually with-out distinct temporal crests, but lambdoidaland nuchal crests often well developed in olderadults. Posterior margin of zygomatic platedorsal to M1 alveolus. Jugal present but notlarge (the maxillary and squamosal zygomaticprocesses overlapping in lateral view but notin contact). Nasals short, not extendingposteriorly beyond lacrimals; lacrimals equallysutured to maxillary and frontal bones.Frontosquamosal suture colinear with fronto-parietal suture in most specimens. Parietalswith broad lateral expansions. Incisive foram-ina long, usually extending posteriorly to orbetween M1 alveoli; usually widest at mid-length and tapering symmetrically anteriorlyand posteriorly. Posterolateral palatal pitslarge, usually complex, and recessed in deep
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 23
fossae; mesopterygoid fossa penetrating ante-riorly between maxillae but not between molarrows; bony roof of mesopterygoid fossaperforated by large sphenopalatine vacuities.Alisphenoid strut absent (buccinator–mastica-tory foramen and accessory foramen ovaleconfluent). Stapedial foramen and pos-terior opening of alisphenoid canal small;squamosal–alisphenoid groove and spheno-frontal foramen absent; secondary anastomo-sis crosses dorsal surface of pterygoid plate (5carotid circulatory pattern 3 of Voss, 1988).Postglenoid foramen large and rounded; sub-squamosal fenestra large and patent. Perioticexposed posteromedially between ectotympa-nic and basioccipital but usually not extend-ing anteriorly to carotid canal; mastoid per-forated by conspicuous posterodorsal fenes-tra. Capsular process of lower incisor alveolusstrongly developed posteroventral to base ofcoronoid process; superior and inferior mas-seteric ridges converge anteriorly as openchevron below m1.
Labial and lingual flexi of upper molars not(or shallowly) interpenetrating. First uppermolar (M1) anterocone not divided into labialand lingual conules (anteromedian flexusabsent); anteroloph well developed and fusedwith anterostyle on labial cingulum, separatedfrom anterocone by persistent anteroflexus;protostyle present but often inconspicuous;paracone connected by enamel bridge tomiddle or to anterior moiety of protocone.Second upper molar (M2) protoflexus pre-sent; mesoflexus usually present as two in-ternal fossettes. Third upper molar (M3) withposteroloph and persistent hypoflexus. Labialaccessory root of M1 absent.
First lower molar (m1) anteroconid withoutan anteromedian flexid; anterolabial cingulumpresent on all lower molars; anterolophidpresent on m1 but indistinct or absent on m2and m3; ectolophid variably developed on m1and m2; mesolophid present and distinct onm1 and m2; m2 hypoflexid short. Accessorylabial root of m1 usually present, accessorylingual root usually absent; m2 and m3 eachwith one large anterior root and one largeposterior root.
Fifth lumbar (17th thoracicolumbar) verte-bra with well-developed anapophysis. Hemalarch between second and third caudal verte-
brae with posterior spinous process. Supra-trochlear foramen of humerus absent.
Stomach without extension of glandularepithelium into corpus. One pair of preputialglands present. Distal bacular cartilage ofglans penis large and trifid (with robustcentral digit); nonspinous tissue of crater rimdoes not conceal bacular mounds; dorsalpapilla spineless; urethral processes withoutsubapical lobules.
COMPARISONS: ‘‘Oryzomys’’ angouya wasconsistently recovered as an isolated lineagewithin clade D by Weksler (2003, 2006), butsome analyses of mtDNA sequences weaklysupport a sister-group relationship withCerradomys (see Bonvicino and Moreira,2001; Bonvicino, 2003). The hypothesis thatSooretamys and Cerradomys might be sistertaxa was not explicitly stated by Musser et al.(1998), but it is implicit in their concept of the‘‘subflavus group’’ of Oryzomys sensu lato.Despite such indications, we are not aware ofany compelling morphological evidence thatSooretamys and Cerradomys are more closelyrelated to one another than they are to othermembers of clade D; indeed, they are strik-ingly divergent in many respects.
Among other anatomical contrasts, Soore-tamys differs from Cerradomys by its muchlonger vibrissae (the mystacial vibrissae ofCerradomys do not extend posteriorly beyondthe pinnae); unicolored tail (the tail is moreor less bicolored in Cerradomys); hourglass-shaped interorbital region with squared su-praorbital margins (the interorbital region isanteriorly convergent and the supraorbitalmargins are strongly beaded in Cerradomys);lack of distinct temporal crests (temporalcrests are well developed in Cerradomys);lack of a labial accessory root on M1 (alabial accessory root is present on M1 inCerradomys); lack of a lingual accessory rooton m1 (a lingual accessory root is presenton m1 in Cerradomys); lack of a supratro-chlear foramina of the humerus (this fora-men is present in Cerradomys); and a trifidbacular cartilage bearing a robust centraldigit (the bacular cartilage is bifid becausethe central digit is vestigial or absent inCerradomys).
REMARKS: The assumption that all of thenominal taxa currently synonymized with S.
24 AMERICAN MUSEUM NOVITATES NO. 3537
angouya are conspecific has not been tested byany revisionary study.
ETYMOLOGY: From sooretama, the oldTupian name for the Atlantic rainforest regionof eastern Brazil (Por, 1992).
Transandinomys, new genus
TYPE SPECIES: Oryzomys talamancae J.A.Allen, 1891.
CONTENTS: bolivaris J.A. Allen, 1901 (in-cluding castaneus J.A. Allen, 1901; rivularisJ.A. Allen, 1901; bombycinus Goldman, 1912;alleni Goldman, 1915; and orinus Pearson,1939); and talamancae J.A. Allen, 1891 (in-cluding mollipilosus J.A. Allen, 1899; magda-lenae J.A. Allen, 1899; villosus J.A. Allen,1899; sylvaticus Thomas, 1900; panamensisThomas, 1901; medius Robinson and Lyon,1901; and carrikeri J.A. Allen, 1908).
DISTRIBUTION: In tropical lowland andpremontane trans-Andean rain forests (toabout 1500 m above sea level) from north-eastern Nicaragua throughout much of CostaRica and Panama to Colombia, westernEcuador, and northern Venezuela (seeMusser et al., 1998: figs. 50, 66).
MORPHOLOGICAL DIAGNOSIS: Dorsal pelagefinely grizzled brownish (in T. bolivaris) ortawny (in T. talamancae); ventral pelageabruptly paler (superficially whitish), butventral hairs uniformly gray-based overthroat, chest, abdomen, and groin. Pinnaelarge, reaching eye when laid forward.Mystacial vibrissae long (extending posteri-orly to or slightly beyond caudal margins ofpinnae when laid back against cheeks in T.talamancae) or very long (extending wellbehind pinnae in T. bolivaris); superciliaryvibrissae extending behind pinnae in bothspecies (but much longer in T. bolivaris thanin T. talamancae). Pes with conspicuous tuftsof long ungual hairs at bases of claws on dII–dV; plantar surface entirely smooth (in T.bolivaris) or sparsely covered with indistinctsquamae distal to thenar pad (in T. talaman-cae); hypothenar pad present and distinct;claw of dI extending almost to middle ofphalange 1 of dII; claw of dV extendingalmost to first interphalangeal joint of dIV.Tail about as long as or slightly longer thancombined length of head and body; unicolored
in T. bolivaris but often distinctly bicolored(dark above, pale below) in T. talamancae.
Skull with long, tapering rostrum flankedby moderately deep zygomatic notches; in-terorbital region anteriorly convergent, withbeaded supraorbital margins; braincaserounded, with moderately well-developedtemporal crests; lambdoidal and nuchal crestsdeveloped in older adults. Posterior margin ofzygomatic plate usually dorsal to M1 alveolus.Jugal present but small (maxillary and squa-mosal zygomatic processes overlapping inlateral view but not in contact). Nasals notextending posteriorly beyond lacrimals; lacri-mals equally sutured to maxillary and frontalbones. Frontosquamosal suture usually co-linear with frontoparietal suture. Parietalswith broad lateral expansions (in T. talaman-cae) or lateral expansions reduced or absent(in T. bolivaris). Incisive foramina short (notextending posteriorly between the alveoli ofM1) and usually wider posteriorly thananteriorly (teardrop-shaped). Posterolateralpalatal pits usually simple and recessed (if atall) in shallow fossae; mesopterygoid fossausually not extending anteriorly betweenmaxillae; bony roof of mesopterygoid fossacompletely ossified or perforated by smallsphenopalatine vacuities. Alisphenoid strutabsent (buccinator–masticatory foramen andaccessory oval foramen confluent). Stapedialforamen, squamosal–alisphenoid groove, andsphenofrontal foramen present (5 carotidcirculatory pattern 1 of Voss, 1988).Postglenoid foramen large and rounded, sub-squamosal fenestra usually large and patent.Periotic exposed posteromedially betweenectotympanic and basioccipital but not ex-tending anteriorly to carotid canal; mastoidusually unfenestrated in T. bolivaris butfenestrated in T. talamancae. Capsular processof lower incisor alveolus indistinct or absent;superior and inferior masseteric ridges con-verge anteriorly as open chevron below m1.
First upper molar (M1) anterocone notdivided into labial and lingual conules (ante-romedian flexus absent); anteroloph well de-veloped, fused with anterostyle on labialcingulum, separated from anterocone bypersistent anteroflexus; protostyle absent;paracone usually connected by enamel bridgeto posterior end of protocone. Second upper
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 25
molar (M2) protoflexus present; mesoflexuspresent as single internal fossette. Third uppermolar (M3) posteroloph absent; hypoflexusshallow and transitory (usually disappearingwith moderate wear). Labial accessory root ofM1 absent.
First lower molar (m1) anteroconid withoutan anteromedian flexid; anterolabial cingulumpresent on all lower molars; anterolophiddistinct on m1 but absent on m2 and m3;ectolophid often present on m1 and m2;mesolophid present on m1 and m2; m2hypoflexid long and deep, almost bisectingtooth. Accessory labial and lingual rootsabsent on m1; m2 and m3 each with one largeanterior root and one large posterior root.
Fifth lumbar (17th thoracicolumbar) verte-bra with well-developed anapophysis. Hemalarch between second and third caudal verte-brae without posterior spinous process.Supratrochlear foramen of humerus present.
Stomach without extension of glandularepithelium into corpus. Distal bacular carti-lage of glans penis large and trifid (with robustcentral digit); shelf of nonspinous tissue oncrater rim does not conceal bacular mounds;dorsal papilla spineless; urethral processeswithout subapical lobules.
COMPARISONS: Weksler (2003, 2006) con-sistently recovered Transandinomys (represent-ed by ‘‘O.’’ talamancae) as a member of cladeB together with Euryoryzomys, Handleyomys,Hylaeamys, Nephelomys, and Oecomys (as infig. 1). Within clade B, Transandinomys wasvariously recovered by different analytic per-mutations as the sister taxon of Euryoryzomys,of Nephelomys, or of Handleyomys intectus +Nephelomys. Comparisons of Transandinomyswith Euryoryzomys, Hylaeamys, and Nephe-lomys have already been provided in theaccounts for those genera, salient aspects ofwhich are summarized in table 3. The mor-phology of Handleyomys intectus (redescribedby Voss et al., 2002) is sufficiently distinctivethat direct comparisons seem unnecessary inthis context.
REMARKS: Although Transandinomys boli-varis and T. talamancae are pheneticallysimilar (as remarked by Musser et al., 1998:323), no analytic results are currently availableto support the monophyly of this genus. Theonly unambiguously derived trait that appears
to be uniquely shared by these species is theirpossession of unusually long supraorbitalvibrissae, a character that was not scored forphylogenetic analysis by Weksler (2006).
ETYMOLOGY: For the trans-Andean distri-bution of these species.
DISCUSSION
Given the analytic results in hand (Weksler,2003, 2006), several phylogenetically defensi-ble nomenclatural options could be adopted.One would be to keep all of the speciescurrently referred to Oryzomys sensu lato ina single genus that would necessarily alsoinclude all the descendants of their mostrecent common ancestor (the root node infig. 1). However, the oldest available namefor this clade is Holochilus (see table 1), soOryzomys would effectively disappear frombinomial usage, as would Neacomys, Nec-tomys, Oecomys, and many other well-estab-lished names.
Other options would involve naming fewernew genera than the 10 proposed herein bycombining clades for which there is anyevidence of a sister-group relationship. Forexample, one might combine the ‘‘albigularisgroup’’ (our Nephelomys) with ‘‘Oryzomys’’bolivaris and ‘‘O.’’ talamancae (together com-prising our Transandinomys) to form one newgenus instead of two new genera. However,doing so would effectively formalize a weaklysupported hypothesis and obscure the usefulfact that each of these groups is a morpholog-ically diagnosable and ecogeographically dis-tinct collection of species. Because weaklysupported groups are more likely to berejected by future taxonomic studies thanwell-supported groups, and because biologistsneed names for clades with distinct morphol-ogies and distributions, two new names ratherthan one better serve the larger community ofzoological researchers in this case and inothers like it discussed by Weksler (2006: 75–77).
Thanks to ongoing revisionary research andthe advent of character-based phylogeneticanalyses, complex patterns of muroid rodentrelationships are now emerging from the fogof prephylogenetic nomenclature. The aban-donment of traditional usage for Oryzomys is
26 AMERICAN MUSEUM NOVITATES NO. 3537
a necessary consequence of advancing knowl-edge about how species are actually related toone another, a process previously exemplifiedby the restriction of such long-abused namesas Rattus and Peromyscus to smaller groups ofclosely related species (e.g., by Musser, 1981;Carleton, 1989). In both of those cases, theresult was a richer lexicon of generic namesthat enabled muroid researchers in Asia andNorth America to communicate more effec-tively about a wide range of biological topicsthan they had previously been able to do. Weexpect that a similar benefit will now accrue tomuroid researchers in the Neotropics.
ACKNOWLEDGMENTS
We thank Paula Jenkins (at the NaturalHistory Museum, London), Bruce Patterson(Field Museum of Natural History, Chicago),and Jim Patton (Museum of VertebrateZoology, Berkeley) for timely specimen loansthat helped us evaluate character variation inseveral taxa that are not well represented atthe AMNH. Al Gardner, Guy Musser, andJim Patton read a preliminary draft of ourmanuscript and provided thoughtful reviewsthat helped us improve the text, but they arenot responsible for any factual errors orflawed arguments that might remain uncor-rected herein. Mary Knight kindly checkedour Greek etymologies, for which we thankher, too.
REFERENCES
Aguilera, M., A. Perez-Zapata, and A. Martino.1995. Cytogenetics and karyosystematics ofOryzomys albigularis (Rodentia, Cricetidae)from Venezuela. Cytogenetics and CellGenetics 69: 44–49.
Bonvicino, C.R. 2003. A new species of Oryzomys(Rodentia, Sigmodontinae) of the subflavusgroup from the Cerrado of central Brazil.Mammalian Biology 68: 78–90.
Bonvicino, C.R., and M.A.M. Moreira. 2001.Molecular phylogeny of the genus Oryzomys(Rodentia: Sigmodontinae) based on cyto-chrome b DNA sequences. Molecular Phylo-genetics and Evolution 18: 282–292.
Brooks, D.M., R.J. Baker, R.J. Vargas, M. T.Tarifa, H. Aranibar, and J.M. Rojas. 2004. Anew species of Oryzomys (Rodentia: Muridae)from an isolated pocket of Cerrado in eastern
Bolivia. Occasional Papers Museum of TexasTech University 241: 1–11.
Cabrera, A. 1961. Catalogo de los mamıferos deAmerica del Sur. Revista del Museo Argentinode Ciencias Naturales ‘‘Bernardino Rivadavia’’(Ciencias Zoologicas) 4(2): 309–732.
Carleton, M.D. 1989. Systematics and evolution. InG.L. Kirkland, Jr., and J.N. Layne (editors),Advances in the study of Peromyscus: 7–141.Lubbock: Texas Tech University Press.
Carleton, M.D., and G.G. Musser. 1989.Systematic studies of oryzomyine rodents(Muridae, Sigmodontinae): a synopsis ofMicroryzomys. Bulletin of the AmericanMuseum of Natural History 191: 1–83.
Ellerman, J.R. 1941. The families and genera ofliving rodents, vol. 2. Family Muridae.London: British Museum (Natural History).
Emmons, L.H., and J.L. Patton. 2005. A newspecies of Oryzomys from eastern Bolivia.American Museum Novitates 3478: 1–26.
Emmons, L.H., V. Chavez, N. Rocha, B. Phillips, I.Phillips, L.F. del Aguila, and M. Swarner. Inpress. The non-flying mammals of ParqueNacional Noel Kempff Mercado, Bolivia.Revista Boliviana de Ecologıa y ConservacionAmbiental.
Gardner, A.L., and J.L. Patton. 1976. Karyotypicvariation in oryzomyine rodents (Cricetinae)with comments on chromosomal evolution inthe Neotropical cricetine complex. OccasionalPapers of the Museum of Zoology LouisianaState University 49: 1–48.
Goldman, E.A. 1918. The rice rats of NorthAmerica (genus Oryzomys). North AmericanFauna 43: 1–100.
Gyldenstolpe, N. 1932. A manual of Neotropicalsigmodont rodents. Kunglinga SvenskaVetenskapsakademiens Handlingar 11: 1–164.
Hall, E.R., and K.R. Kelson. 1959. The mammalsof North America, 2 vols. New York: RonaldPress.
Hershkovitz, P. 1944. A systematic review of theNeotropical water rats of the genus Nectomys(Cricetinae). Miscellaneous PublicationsMuseum of Zoology, University of Michigan58: 1–88, pls. I–IV, gazetteer, + folding map.
Hershkovitz, P. 1948. Mammals of northernColombia preliminary report no. 3: water rats(genus Nectomys), with supplemental notes onrelated forms. Proceedings of the United StatesNational Museum 98: 49–56.
Hershkovitz, P. 1970. Supplementary notes onNeotropical Oryzomys dimidiatus andOryzomys hammondi (Cricetinae). Journal ofMammalogy 51: 789–794.
Langguth, A., and C.R. Bonvincino. 2002. TheOryzomys subflavus species group, with descrip-
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 27
tions of two new species (Rodentia, Muridae,Sigmodontinae). Arquivos do Museu Nacional,Rio de Janeiro 60: 285–294.
Marquez, E.J., M. Aguilera M, and M. Corti. 2000.Morphometric and chromosomal variation inpopulations of Oryzomys albigularis (Muridae:Sigmodontinae) from Venezuela: multivariateaspects. Zeitschrift fur Saugetierkunde 65:84–99.
McFarlane, D.A., and A.O. Debrot. 2001. A newspecies of extinct oryzomyine rodent from theQuaternary of Curacao, Netherlands Antilles.Caribbean Journal of Science 37: 182–184.
Morgan, G.S. 1993. Quaternary land vertebrates ofJamaica. In R.M. Wright and E. Robinson(editors), Biostratigraphy of Jamaica. Geolo-gical Society of America Memoir 182: 417–442. Boulder, CO: Geological Society ofAmerica.
Musser, G.G. 1981. Results of the Archboldexpeditions. No. 105. Notes on systematics ofIndo-Malayan murid rodents, and descriptionsof new genera and species from Ceylon,Sulawesi, and the Philippines. Bulletin of theAmerican Museum of Natural History 168:225–334.
Musser, G.G., and M.D. Carleton. 1993. FamilyMuridae. In D.E. Wilson and D.M. Reeder(editors), Mammal species on the world, 2nded.: 501–755. Washington, DC: SmithsonianInstitution Press.
Musser, G.G., and M.D. Carleton. 2005.Superfamily Muroidea. In: D.E. Wilson andD.M. Reeder (editors), Mammal species of theworld, 3rd ed., vol. 2: 894–1531. Baltimore:Johns Hopkins University Press.
Musser, G.G., M.D. Carleton, E.M. Brothers, andA.L. Gardner. 1998. Systematic studies oforyzomyine rodents (Muridae, Sigmodon-tinae): diagnoses and distributions of speciesformerly assigned to Oryzomys ‘‘capito’’.Bulletin of the American Museum of NaturalHistory 236: 1–376.
Musser, G.G., and M.E. Holden. 1991. Sulawesirodents (Muridae: Murinae): morphologicaland geographical boundaries of species in theRattus hoffmanni group and a new species fromPulau Peleng. Bulletin of the AmericanMuseum of Natural History 206: 322–413.
Musser, G.G., and C. Newcomb. 1983. Malaysianmurids and the giant rat of Sumatra. Bulletin ofthe American Museum of Natural History 174:327–598.
Myers, P., B. Lundrigan, and P.K. Tucker. 1995.Molecular phylogenetics of oryzomyine ro-dents: the genus Oligoryzomys. MolecularPhylogenetics and Evolution 4: 372–382.
Osgood, W.H. 1913. New Peruvian mammals. FieldMuseum of Natural History Zoological Series10: 93–100.
Patton, J.L., and M.S. Haffner. 1983. Bio-systematics of the native rodents of theGalapagos Archipelago, Ecuador. In R.I.Bowman, M. Benson and A.E. Leviton (edi-tors), Patterns of evolution in Galapagosorganisms: 539–568. San Francisco: PacificDivision American Association for theAdvancement of Science.
Patton, J.L., M.N.F. da Silva, and J.R. Malcolm.2000. Mammals of the Rio Jurua and theevolutionary and ecological diversification ofAmazonia. Bulletin of the American Museumof Natural History 244: 1–306.
Patton, J.L., P. Myers, and M.F. Smith. 1990.Vicariant versus gradient models of diversifica-tion: the small mammal fauna of easternAndean slopes of Peru. In G. Peters and R.Hutterer (editors), Vertebrates in the tropics:355–371. Bonn: Museum Alexander Koenig.
Paynter, R.A., Jr. 1993. Ornithological gazetteer ofEcuador, 2nd ed. Cambridge, MA: Museum ofComparative Zoology (Harvard University).
Percequillo, A.R. 1998. Sistematica de OryzomysBaird, 1858 do Leste do Brasil (Muroidea,Sigmodontinae). Unpublished Master’s thesis,Universidade de Sao Paulo.
Percequillo, A.R. 2003. Sistematica de OryzomysBaird, 1858: definicao dos grupos de especies erevisao taxonomica do grupo albigularis(Rodentia, Sigmodontinae). UnpublishedDoctoral thesis, Universidade de Sao Paulo.
Pine, R.H., and R.M. Wetzel. 1975. A new sub-species of Pseudoryzomys wavrini (Mammalia:Rodentia: Muridae: Cricetinae) from Bolivia.Mammalia 39: 649–655.
Por, F.D. 1992. Sooretama, the Atlantic rainforestof Brazil. The Hague: SPB AcademicPublishers.
Ray, C.E. 1962. Oryzomyine rodents of theAntillean Subregion. Unpublished Doctoralthesis, Harvard University.
Sanchez, H.J., J. Ochoa, G., and R.S. Voss. 2001.Rediscovery of Oryzomys gorgasi (Rodentia,Muridae), with notes on taxonomy and naturalhistory. Mammalia 65: 205–214.
Steadman, D.W., and C.E. Ray. 1982. The relation-ships of Megaoryzomys curioi, an extinctcricetine rodent (Muroidea: Muridae) fromthe Galapagos Islands, Ecuador. SmithsonianContributions to Paleobiology 51: 1–23.
Tate, G.H.H. 1932. The taxonomic history of theSouth and Central American cricetid rodents ofthe genus Oryzomys. Part 1: subgenusOryzomys. American Museum Novitates 579:1–18.
28 AMERICAN MUSEUM NOVITATES NO. 3537
Thomas, O. 1894. Descriptions of some newNeotropical Muridae. Annals and Museum ofNatural History ser. 6, vol. 14: 346–366.
Thomas, O. 1913. New mammals from SouthAmerica. Annals and Magazine of NaturalHistory ser. 8, vol. 12: 567–574.
Voss, R.S. 1988. Systematics and ecology ofichthyomyine rodents (Muroidea): patterns ofmorphological evolution in a small adaptiveradiation. Bulletin of the American Museum ofNatural History 188: 259–493.
Voss, R.S. 1993. A revision of the Brazilian muroidrodent genus Delomys with remarks on ‘‘tho-masomyine’’ characters. American MuseumNovitates 3073: 1–44.
Voss, R.S., and M.D. Carleton. 1993. A new genusfor Hesperomys molitor Winge and Holochilusmagnus Hershkovitz (Mammalia, Muridae)with an analysis of its phylogenetic relation-ships. American Museum Novitates 3085: 1–39.
Voss, R.S., and A.V. Linzey. 1981. Comparativegross morphology of male accessory glandsamong Neotropical Muridae (Mammalia:Rodentia) with comments on systematic im-
plications. Miscellaneous Publications Museumof Zoology, University of Michigan 159: i–iv,1–41.
Voss, R.S., M. Gomez-Laverde, and V. Pacheco.2002. A new genus for Aepeomys fuscatusAllen, 1912, and Oryzomys intectus Thomas,1921: enigmatic murid rodents from Andeancloud forests. American Museum Novitates3373: 1–42.
Weksler, M. 1996. Revisao sistematica do grupo deespecies nitidus do genero Oryzomys (Rodentia:Sigmodontinae). Unpublished Master’s thesis,Universidade Federal do Rio de Janeiro.
Weksler, M. 2003. Phylogeny of Neotropicaloryzomyine rodents (Muridae: Sigmodon-tinae) based on the nuclear IRBP exon.Molecular Phylogenetics and Evolution 29:331–349.
Weksler, M. 2006. Phylogenetic relationships oforyzomyine rodents (Muroidea: Sigmo-dontinae): separate and combined analyses ofmorphological and molecular data. Bulletin ofthe American Museum of Natural History 296:1–149.
2006 WEKSLER ET AL.: TEN NEW ORYZOMYINE GENERA 29