Sonoporation: Mechanistic insights and ongoing challenges for gene transfer
Transcript of Sonoporation: Mechanistic insights and ongoing challenges for gene transfer
Gene xxx (2013) xxx–xxx
GENE-38471; No. of pages: 9; 4C:
Contents lists available at SciVerse ScienceDirect
Gene
j ourna l homepage: www.e lsev ie r .com/ locate /gene
Review
Sonoporation: Mechanistic insights and ongoing challenges for gene transfer
Anthony Delalande a, Spiros Kotopoulis b, Michiel Postema b, Patrick Midoux a, Chantal Pichon a,⁎a Centre de Biophysique Moléculaire, CNRS, Orléans, Franceb Department of Physics and Technology, University of Bergen, Bergen, Norway
Abbreviations: DMPC, 1,2-dimyristoyl-sn-glycero-3distearoyl-sn-glycero-3-phosphocholine; eGFP, enhancHIFU, High Intensity Focused Ultrasound; VEGF, Vascula⁎ Corresponding author. Tel.: +33 238255595; fax: +
E-mail address: [email protected] (C. P
0378-1119/$ – see front matter © 2013 Elsevier B.V. Allhttp://dx.doi.org/10.1016/j.gene.2013.03.095
Please cite this article as: Delalande, A., et al.dx.doi.org/10.1016/j.gene.2013.03.095
a b s t r a c t
a r t i c l e i n f oArticle history:Accepted 7 March 2013Available online xxxx
Keywords:UltrasoundMicrobubblesPhysical gene delivery methodGene therapy
Microbubbles first developed as ultrasound contrast agents have been used to assist ultrasound for cellulardrug and gene delivery. Their oscillation behavior during ultrasound exposure leads to transient membranepermeability of surrounding cells, facilitating targeted local delivery. The increased cell uptake of extracellularcompounds by ultrasound in the presence ofmicrobubbles is attributed to a phenomenon called sonoporation.In this review, we summarize current state of the art concerning microbubble–cell interactions and cellulareffects leading to sonoporation and its application for gene delivery. Optimization of sonoporation protocoland composition of microbubbles for gene delivery are discussed.
© 2013 Elsevier B.V. All rights reserved.
Contents
1. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 02. Ultrasound as a physical method for delivery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 03. Microbubbles and sonoporation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0
3.1. Microbubble–cell interactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 03.2. Cellular effects of ultrasound application . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 03.3. Cargo size and cellular localization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0
4. Optimization of gene delivery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 05. Microbubble features for efficient gene delivery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 06. Conclusion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0Acknowledgments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0
1. Introduction
The principle of gene therapy is to introduce gene or nucleic acidsinto cells to cure genetic deficiencies. The success of gene therapyobtained with the use of viral vectors demonstrates unambiguouslythe feasibility of this innovative therapy (Bennett et al., 2012;Fischer et al., 2000; Nathwani et al., 2011). To date, viral vectorsremain the best vehicles to introduce genes into cells. Nevertheless,there are still drawbacks inherent to the use of a viral molecule ob-served in gene therapy clinical trials raising serious safety concerns(Hacein-Bey-Abina et al., 2003; Raper et al., 2003). In addition, sizelimitation capacity, cell targeting and manufacturing issues are still
-phosphocholine; DSPC, 1,2-ed Green Fluorescent Protein;r Endothelium Growth Factor.33 238631517.ichon).
rights reserved.
, Sonoporation: Mechanistic i
difficult to handle despite tremendous progress made on viral vectorbioengineering. Therefore, there is still some room for the develop-ment of alternative approaches of high safety, low immunogenicityand easy manufacture. This last decade, many efforts have beendone to search for non-viral options. The goal is to design syntheticgene delivery systems that incorporate viral-like features to transfectefficiently cells (Mahato, 1999; Midoux et al., 2009; Wagner et al.,1998). Among non-viral systems, chemical vectors are themost widelyused. These vectors have to face several extracellular and cellular bar-riers to efficiently reach the target cells. One of the main challenges isthe lack of selectivity towards target tissues explaining their narrowtherapeutic index. Lack of specificity causes high toxicity which ham-pers the efficacy at the target site. For that, designing an efficienttargeted delivery strategy is of importance to further improve the deliv-ery systems while reducing side effects. To target chemical vectors, it ispossible to couple themwith ligands specific to receptors present at thecellular surface of target cells. A second option is to use an externallyapplied trigger to control the gene delivery in the targeted area. These
nsights and ongoing challenges for gene transfer, Gene (2013), http://
2 A. Delalande et al. / Gene xxx (2013) xxx–xxx
two strategies are not mutually exclusive and could be combined. Aphysical trigger can be used either alone or combined with chemicalor viral vectors to improve the targeting and/or gene expression effi-ciency. There are several physical methods starting fromhydrodynamicinjection to more sophisticated systems such as electroporation basedon electric fields or ultrasound-mediated delivery.
2. Ultrasound as a physical method for delivery
Ultrasound can be used for imaging (ultrasonography) and for phys-ical therapy (pulsed ultrasound mode) (Lindner, 2004; Mitragotri,2005). These last years, therapeutic applications of ultrasound havegained new interests as a result of its exploitation for drug or genedelivery. Depending on the energy delivered by ultrasound, two typesof effects can be produced either thermal or non-thermal each ofthemhaving their own application. High ultrasound intensities produceheating due to the absorption of acoustic energy by tissues; this proper-ty is employed in high-intensity focused ultrasound (HIFU) surgery orultrasound-based physiotherapy. The “World Federation for Ultrasoundin Medicine and Biology Temperature” has stated that an elevation of1.5 °C is considered safe while an elevation of 4–5 °C during 5 mincould be dangerous (Barnett et al., 2000). At low ultrasound intensities,cavitation, mechanical streaming and radiation forces are the mainnon-thermal effects obtained. These effects can induce some benefits
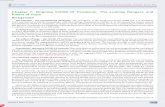
Fig. 1. Five mechanisms of pore formation provoked by microbubbles oscillating under ultrbrane surface, possibly pushing it apart. (B) Pull: During the contraction phase of an oscillaticell membrane towards the microbubble, possibly disrupting the plasma membrane. (C) Jettthrough the bubble which is directed towards a boundary. (D) Streaming: If a microbubble isenough shears that could induce cell membrane rupture. (E) Translation: Owing to radiatiomicrobubble may lose part of its shell whilst passing through the cell membrane. Figure ad
Please cite this article as: Delalande, A., et al., Sonoporation: Mechanistic idx.doi.org/10.1016/j.gene.2013.03.095
such as tissue healing or ultrasound-mediated delivery. Mechanicalstreaming and radiation forces could be used to enhance the diffusionof drugs or nanometer sized bubbles across the vessel wall (Stieger etal., 2007; Wang et al., 2010). Inertial cavitation is the process of forma-tion, oscillation and collapse of gaseous bubbles driven by an acousticfield. The presence of preformed microbubbles in the environmentallows reducing the threshold of energy needed for cavitation. Used asan external trigger, ultrasound permits to spatiotemporally control therelease of a drug encapsulated in microbubbles or in their surroundingin a non-invasive manner (Frenkel, 2008; Kinoshita et al., 2006; Kostet al., 1989; O'Neill and Li, 2008; Rapoport et al., 2007; Schroederet al., 2007, 2009).
3. Microbubbles and sonoporation
Microbubbles are gas-filled particles consisting of a gas core encap-sulated by a stabilizing shell. They have been first developed as ultra-sound contrast agents to differentiate blood and their surroundingsunder ultrasound due to their low acoustic impedance difference.When microbubbles are driven by ultrasound at a frequency close totheir resonance frequency, they oscillate and produce sound (Daytonet al., 2002; Morgan et al., 2000). These oscillations lead to an increasedpermeability of surrounding cells allowing a targeted local drug deliv-ery. The increased cellular uptake has been attributed to the formation
asound. (A) Push: During its expansion phase, a microbubble might touch a cell mem-ng microbubble, the plasma filling the void left by the contracting bubble might pull theing: Jetting is the asymmetric collapse of a bubble, creating a funnel-shaped protrusionattached to a cell membrane, the fluid streaming around the oscillating bubbles createsn forces, lipid-encapsulated microbubbles could translate through cell membrane. Theapted from (Delalande et al., 2011a).
nsights and ongoing challenges for gene transfer, Gene (2013), http://
3A. Delalande et al. / Gene xxx (2013) xxx–xxx
of transient pores in the cell membrane facilitating trans-membranetransport of drugs into the cell (Deng et al., 2004; Kaddur et al., 2010;Mehier-Humbert et al., 2005a; Zhou et al., 2009). This transient perme-abilization of a cell membrane is called sonoporation.
3.1. Microbubble–cell interactions
Microbubble interactions with cells under ultrasound are the keystep for the sonoporation process. To date, five types of interactionshave been described (Fig. 1). Microbubble oscillations near a plasmamembrane could lead to a cell “massage”, the “push and pull”phenomena (Marmottant and Hilgenfeldt, 2003; van Wamel et al.,2004) and microbubble jetting through the plasma membrane(Prentice et al., 2005). The latter is less likely to be the dominantmechanism as shown by in silico studies, in vitro by high-speed opti-cal observations, and in cellulo experiments (Postema and Gilja,2010). A microbubble attached to a cell membrane, could also createenough shear to rupture the membrane due to the fluid streamingaround the oscillating bubbles. Recently, we have observed a newevent: a penetration of a microbubble into a cell during sonoporationprocess under a specific ultrasound setting (Delalande et al., 2011a).
t=621
+375m
+750m
A
C
B
Fig. 2. Microbubble entry in cells under sonoporation. (A) Z-stack of fluorescent-labelemicrobubbles (circle) close to a cell under 6.6 MHz pulsed ultrasound. The microbubbles wtheir disappearance (arrows). (C) Fluorescence images of HeLa cells after sonoporation, theIntact microbubbles were still in the media after sonoporation.
Please cite this article as: Delalande, A., et al., Sonoporation: Mechanistic idx.doi.org/10.1016/j.gene.2013.03.095
The tracking of fluorescent-labeled microbubbles inside cells aftersonoporation has proved this phenomenon. These phenomena arenon-exclusive and can occur even at very low acoustic amplitudes.Only jetting occurs at relatively high pressures, which makes thisphenomenon a less suitable candidate for therapeutic delivery. Thetranslation phenomenon is a promising candidate for ultrasound-guided delivery in cancer therapy, as the microbubble delivers (partof) its load inside the target cell. Fig. 2 shows two microbubbles(black circle) entering into a cell during ultrasound stimulation, onemicrobubble was pushed toward the cell deforming the plasmamem-brane before its cellular penetration. While entering, the microbubbleunderwent shrinking. This phenomenon took place 6.2 s after ultra-sound stimulation and 850 ms later, the microbubble penetratedinside the cell. This event is slow, compared to the other phenomenadescribed above. Indeed, jetting occurs in themillisecond time scale. Fluo-rescent microbubbles were observed inside the cells after sonoporationproving their penetration (Fig. 2C, black arrows). However, it is hardto state if the microbubble has only fused with the plasma membraneor if the structure of the microbubble was preserved after entry. Never-theless, these results reinforce the strategy on using microbubbles asdrug or gene carriers.
3ms +125ms +250ms
s +500ms +625ms
s +875ms +2830ms
d microbubbles observed by confocal microscopy. (B) High-speed imaging of twoere seen to push the plasma membrane and entered (frame +875 ms) in the cell untilfluorescence observed was due to DiD-labeled microbubbles entered in a cell (arrows).
nsights and ongoing challenges for gene transfer, Gene (2013), http://
4 A. Delalande et al. / Gene xxx (2013) xxx–xxx
We have also tracked the early interactions between cells andmicrobubbles at the beginning of the ultrasound stimulation (Delalandeet al., 2011a). In this study, we have used several phospholipid-basedmicrobubbles (SonoVue®, Micromarker™, Definity®). Before ultra-sound stimulation, microbubbles were randomly distributed allaround cells. When ultrasound was turned on, microbubbles imme-diately interacted with each other forming small clusters (Fig. 3) asdescribed by Kotopoulis and Postema (2010). These clusters couldbe observed approximately 15 ms after ultrasound application. In-terestingly, every cluster present in the field seemed to be attractedto cells and was found at the vicinity of their plasma membrane sev-eral seconds after ultrasound stimulation independently of the ultra-sound field direction. It is worth noticing that we did not observesuch behavior for hard-shelled microbubbles like Quantison™ withthe same ultrasound settings. Quantison™ microbubbles interacted
Fig. 3.Microbubble–cell interaction during sonoporation. Observation ofmicrobubble behaviorbefore ultrasound (0 s). After 15 ms of ultrasound stimulation, clusters of microbubbles couldmembrane after 40 ms suggesting a specific attraction between microbubbles and cells. Movrepresented by green circles, the line next to them corresponds to the analysis of their displac
Please cite this article as: Delalande, A., et al., Sonoporation: Mechanistic idx.doi.org/10.1016/j.gene.2013.03.095
also to each other by clustering but they moved only in the directionof the ultrasound field. Since sonoporation is based on microbubbleand cell interactions, these observations indicate the importanceof microbubble features (composition and acoustic activity). It alsoraises the question if microbubbles are attracted to specific areas ofthe plasma membrane which could be worth determining.
3.2. Cellular effects of ultrasound application
Pore formation has been reported to be essential for sonoporation-mediated delivery (Mehier-Humbert et al., 2005a; Mukherjee et al.,2000; Taniyama et al., 2002). The presence of 100 nm-sized cell mem-brane pores was observed by electron microscopy and confirmed byflow cytometry with the use of 75 nm-sized fluorescent nanospheres.Using an electro-diffusion model, Zhou and colleagues have evaluated
during sonoporation by high-speed imaging (A).Microbubbleswere randomly distributedbe observed around the cell membrane and all them were found in contact with plasmaements of microbubbles were analyzed using a tracking software (B). Microbubbles areement during 30 ms of ultrasound.
nsights and ongoing challenges for gene transfer, Gene (2013), http://
5A. Delalande et al. / Gene xxx (2013) xxx–xxx
the pore size at 110 ± 40 nm during a sonoporation process in thepresence of Definity® microbubbles (Zhou et al., 2009). Concomitantto pore formation, a transient intracellular calcium entrance has beenobserved during ultrasound application in the absence or presence ofmicrobubbles (Deng et al., 2004; Fan et al., 2010; Juffermans et al.,2009; Kumon et al., 2007, 2009; Meijering et al., 2009; Park et al.,2011; Paula et al., 2011). This intracellular calcium entrance hasbeen supposed to promote the pore closure (Deng et al., 2004) and toinduce the endocytosis and exocytosis processes (Eliasson et al., 1996;MacDonald et al., 2005). The pore closure time has been estimatedat 5 s after the ultrasound stimulation by flow cytometry and patchclamp experiments (Mehier-Humbert et al., 2005a; Zhou et al., 2009).Several studies have reported the pore formation after sonoporation,however, it is still not known if these pores are directly responsiblefor drug entry. In addition, a production of hydrogen peroxide (H2O2)was detected after sonoporation and it seems to be important for calci-um entrance hence for pore formation (Juffermans et al., 2006). Somereports have suggested that this H2O2 production could be related tothe cavitation phenomenon (Bao et al., 1997; Miller et al., 1991; Paulaet al., 2011).
Another cellular effect observed during sonoporation was an hyper-polarization state of cells that can occur in the absence (Paula et al.,2011) or presence of microbubbles (Deng et al., 2004; Juffermans etal., 2008; Tran et al., 2008). This hyperpolarization is directly attributedto the opening of the BKCa channels (Juffermans et al., 2008; Tran et al.,2008). It is related to a mechanical stress of the plasmamembrane sim-ilar to that obtained when applying mechanical pressure on the mem-brane using a glass probe (Tran et al., 2007). Both hyperpolarizationand calcium signaling could increase macromolecule uptake. Besidesthese phenomena, we have observed an outward transport of small in-tracellular molecules likely due to membrane destabilization (Kadduret al., 2010). A transient release of small molecules such as enhancedgreen fluorescent protein (eGFP) was observed from the cytosol ofHeLa cells stably expressing eGFP gene while preserving cell viability.These results reinforce the hypothesis of transient pore formation in-duced by sonoporation.
3.3. Cargo size and cellular localization
Sonoporation has been used to deliver drugs in vitro (Escoffreet al., 2011; Yoshida et al., 2007) and in vivo (Iwanaga et al., 2007).The presence of microbubbles is a requirement for an efficient delivery.The delivery mechanism by sonoporation appeared to be dependenton the size of the molecules to transfer. The cell membrane perme-abilization and viability are highly dependent on the ultrasound param-eters used during sonoporation (Karshafian et al., 2009). Followingsonoporation, fluorescent labeled molecules have a different localiza-tion profile depending on their molecular size (Meijering et al., 2009).
Fig. 4. Influence of the cargo size on its cellular localization after sonoporation. Observation of t
Please cite this article as: Delalande, A., et al., Sonoporation: Mechanistic idx.doi.org/10.1016/j.gene.2013.03.095
A dextran with a small size of 3 kDa had a diffuse localization in thecytosol and in the nucleus whilst 70 kDa dextran was only found inthe cytosol. For dextrans with sizes over 150 kDa, they were observedas patchy structures localized in the cytoplasm suggesting either aggre-gates in the cytosol or localization inside endosomes (Fig. 4). Aftersonoporation, a similar localization was observed for plasmid DNA of7.5 kb which has an estimated size of approximately 4.95 MDa. Fig. 5shows the localization of fluorescent-labeled plasmid DNA 10, 30 and45 min post sonoporation. Ten minutes after sonoporation, plasmidDNA was still found at the plasma membrane. It went closer to the nu-cleus from 30 to 45 min. These observations suggest slow plasmid DNAtrafficking inside the cells. Taking into account that naked plasmid DNAinjected inside the cytosol does not diffuse at all (Lukacs et al., 2000) it islikely that thesefluorescent spots corresponded to plasmidDNA locatedinside endosomes. Overall, these data indicate that pore formation isnot a uniquemechanism occurring during sonoporation and that endo-cytosis might also be involved in the uptake (Meijering et al., 2009).However, it is still not clear if the type of mechanism(s) involvedcould be both dependent on the microbubble chemical compositionand on the type of tissue insonified.
4. Optimization of gene delivery
The number of publications relative to the use of sonoporationfor gene delivery gives evidence of the potentiality of this method.Ultrasound-enhanced gene delivery has been successfully demon-strated both in vitro (Bao et al., 1997; Mehier-Humbert et al., 2005b;Miller et al., 1999) and in vivo (Pichon et al., 2008; Lu et al., 2003;Shen et al., 2008; Delalande et al., 2011b). The efficacy is dependenton acoustic parameters, the presence of microbubbles and the localconcentration of plasmid DNA. Commercial microbubbles as wellas lipid- or polymer-shelled customized bubbles have been used inthose studies. In most cases, plasmid DNA was not complexed withmicrobubbles rendering it prone to degradation. As a consequence,the level of gene expression is still not as much as expected eventhough it is sometimes 1 or 2 orders of magnitude higher than thelevel obtained with plasmid DNA alone (Newman and Bettinger,2007; Pichon et al., 2008). Note that it is rather difficult to make com-parisons between different reported studies because of the ultra-sound set-up and acoustic condition diversity used by each group.Biological effects and high spatial targeting of transgene expressionwere achieved but again not as high as that obtained with electropo-ration for instance. Still, the specific targeting of the gene expressionobserved upon local or systemic administration in addition to lowtoxicity demonstrates the potentiality of this delivery system (Luet al., 2003; Shen et al., 2008). Our recent data showed remarkablythat it is possible to get an efficient and sustained gene transfer upto 100 days in Achilles tendons by BR14 lipid shelled MB as shown
he dextran of different molecular size (3, 70 and 150 kDa) in HeLa cells after sonoporation.
nsights and ongoing challenges for gene transfer, Gene (2013), http://
Fig. 5. Plasmid DNA intracellular trafficking after sonoporation. Fluorescent-labeled pDNA (Cy3) was followed in HeLa cells after sonoporation during 45 min under confocalmicroscopy (dot lines correspond to cell boundaries).
6 A. Delalande et al. / Gene xxx (2013) xxx–xxx
in Fig. 6 (Delalande et al., 2010, 2011b). Optimized gene transfer wasobtained with 1 MHz US frequency, 200 kPa and 40% duty cycle in thepresence of 10 μg plasmid DNA and 5 × 105 BR 14 microbubbles. Thelevel of gene transfer was 130-fold more than that obtained withnaked plasmid DNA. The level of gene expression obtained here wasas good as with adenoviral vectors in tendons highlighting the poten-tial of this system (Lou, 2000; Rickert, 2008).
In addition to acoustic parameters andmicrobubbles, sonoporationprotocols could also be optimized to achieve a good gene transfer.After optimizing acoustic parameters and set-up to transfect HeLacells, we have assessed if the number of ultrasound applicationscould improve gene transfer efficiency since microbubbles interactedwith cells and vanished in a few seconds. With our setup, optimal pa-rameters for an efficient gene transfer were 1 MHz frequency, 40%duty cycle, 10 kHz pulse repetition frequency, 150 kPa during 60 s inthe presence of 2.5 μg of plasmid DNA encoding the GFP and 5 μl ofMicromarker microbubbles. Gene transfer efficiency was evaluatedby flow cytometry 48 h after sonoporation on HeLa cells. As shownin Fig. 7, when the ultrasound stimulation was made sequentially inmore than one step (the total amount of microbubbles and plasmidDNA being equally dispatched in two or three sequential injections),
Bio
lum
ines
cenc
e [p
/s]
104
105
106
107
108
Time [days]
0
pLuc+US+MBpLuc
10 20 30 40 50 60 70 80 90 100 110
Fig. 6. Kinetic of luciferase gene expression after sonoporation in mouse Achilles tendons. Mnot with 5 × 105 of microbubbles (MB) followed by ultrasound (US) exposure at 1 MHz, 200per second) that correspond to the luciferase activity were measured after luciferin injectiononly (circle), pLuc mixed withMB and exposed to US (square). Values aremeans ± SEM. DatRight panel: representative CCD image showing luciferase expression in tendons injectedAdapted from (Delalande et al., 2011b).
Please cite this article as: Delalande, A., et al., Sonoporation: Mechanistic idx.doi.org/10.1016/j.gene.2013.03.095
the gene transfer efficiency was enhanced. Here, the ultrasound stim-ulation total time was preserved (1 × 60 s, 2 × 30 s or 3 × 20 s).A two-time insonation enhanced by 2.2-fold the gene transferefficiency (16.2% vs 7.3% for single exposure sonoporation) and athree-time sequential insonation improved the efficiency to reach19.9% of GFP expressing cells. This effect could be explained by an im-provement of the number of stimulated cells due to a new supply ofactivable microbubbles. It is worth noticing that the mean fluores-cence intensity of transfected cells was not significantly differentbetween all sonoporation conditions validating this hypothesis.
5. Microbubble features for efficient gene delivery
While the range of sizes (mean diameter > 1 μm) of microbubblesclinically used is appropriate for imaging, they are nevertheless too bigand present a polydispersity unsuitable for therapeutic applications(Zhao et al., 2005; for extensive reviews see Ferrara, 2008; Huang,2008; Schroeder et al., 2009; Unger et al., 2004). Under ultrasoundexposure, lipids are compliant to dilatation and compression andthis renders gas-filled liposomes more echogenic. The developmentof acoustically active liposomes has been optimized (for a review,
2.5
0.5
1.0
1.5
2.0
x107 photons/sec
Day 64
120
L : pLuc+US+MBR : pLuc
ice Achilles tendons were injected either with 10 μg pLuc only or 10 μg pLuc mixed orkPa, 40% duty cycle during 10 min. Bioluminescence signals (expressed in p/s: photonsin the tendon area. Luciferase gene expression of mice groups injected with either pLuca are representative of two experiments with fivemice per group. (***: p-value b 0.001).with pLuc mixed with MB and US exposed (left tendon) or pLuc only (right tendon).
nsights and ongoing challenges for gene transfer, Gene (2013), http://
Micromarker + US
A
BMicromarker + 2x US Micromarker + 3x US
Fig. 7. Gene transfer efficiency by sonoporation. Sonoporation was performed using 2.5 μg of pGFP and Micromarker microbubbles using the following acoustic parameters: 1 MHz,150 kPa, 40% duty cycle, 10 kHz pulse repetition frequency and 60 s total exposure time. (A) Percentage of transfected HeLa cells 48 h after sonoporation. US: ultrasound, 2×:two-times insonation, 3×: three-times insonation. Data represents means ± SD. (B) Representative fluorescence images of HeLa sonoporated cells in several conditions.
7A. Delalande et al. / Gene xxx (2013) xxx–xxx
see Huang, 2008) but efforts have been mainly focused to get lipo-some bubbles with a high acoustic response. These microbubblesare made of classical phospholipids without any specific feature.Recently, more complex molecular architectures of liposome basedmicrobubbles have been described: bubble liposomes that consistof PEG-modified liposomes that encapsulate perfluorocarbon gasenclosed in PEG-lipid micelles (Suzuki et al., 2007a,b; Un et al., 2010)and a hybrid particle made with a microbubble loaded with liposomesthat are made of thousands of small unilamellar biotinylated lipo-somes attached via an avidin molecule to a biotinylated microbubble(Kheirolomoom et al., 2010). These bubble liposomes (BLs) have asmaller size (b1 μm) than conventional microbubbles, increasingtheir possibility to be extravasated from blood wall (Suzuki et al.,2007a).
To achieve improvements in the sonoporation method, the devel-opment of new microbubbles with reduced size and able to reachspecifically the target and locally deliver the nucleic acid is also need-ed (Fig. 8). Targeting microbubbles with antibodies that can recog-nize a specific antigen present on a cell membrane has allowedmicrobubble binding even under flow (Klibanov, 2007). For example,microbubbles targeted to the P-selectin and to the single chain VEGFwere used to analyze the endothelium inflammation (Lindner et al.,2001) and to investigate tumor angiogenesis (Anderson et al., 2010),respectively. These microbubbles mainly used in molecular imaging
Please cite this article as: Delalande, A., et al., Sonoporation: Mechanistic idx.doi.org/10.1016/j.gene.2013.03.095
were only based on the acoustic properties and are not able to bind orcarry nucleic acids.
Some studies have reported the use of cationic microbubbles to di-rectly complex plasmid DNA (Anwer et al., 2000; Christiansen et al.,2003; Hayashi et al., 2009; Tlaxca et al., 2010; Vannan et al., 2002).They consist of gas-made cationic liposomes made with neutral clas-sical phospholipids (1,2-dimyristoyl-sn-glycero-3-phosphocholine;DMPC or1,2-distearoyl-sn-glycero-3-phosphocholine; DSPC) andcationic lipids (1,2-distearoyl-3-trimethylammoniumpropane). Twomain strategies have been proposed to produce microbubbles ableto bind nucleic acids: (i) a cationic lipid-based microbubble allowingelectrostatic interactions between the nucleic acid and microbubbles,and (ii) compaction of the nucleic acid using polymers or liposomeslinked to the microbubble by biotin–streptavidin interaction (Delalandeet al., 2012). Despite a good acoustic response of these microbubbles,the level of gene transfer obtained in those studies was rather lowlikely due to different limitations such as a large bubble size andthe intracellular fate of delivered plasmid DNA. If in addition topore formation, endocytosis could be also involved in sonoporation,the compartmentalization of therapeutic molecules, and most im-portantly genes, may affect their efficiency and should be takeninto consideration. Therefore, it is necessary to rethink microbubblecomposition and to pay more attention on the intracellular fate ofboth bubbles and their payload.
nsights and ongoing challenges for gene transfer, Gene (2013), http://
Vessel ultrasound stimulation
ultrasound probe
insonified region
2. Fixation of drug/gene loaded microbubbles on the specific cellular target
1. IV injection of targeted microbubbles
3. Ultrasound stimulation of theregion to treat
4. Local delivery of the drug/gene
Fig. 8. General overview of the sonoporation strategy for in vivo drug/gene delivery. The drug/gene loaded targeted microbubbles are injected in the systemic circulation. Targetedmicrobubbles can bind specifically to cells and ultrasound stimulation of the region of interest can activate these fixed microbubbles leading to the delivery of their payload throughthe plasma membrane or inside the cells depending on microbubbles composition.
8 A. Delalande et al. / Gene xxx (2013) xxx–xxx
6. Conclusion
The non-invasiveness of sonoporation renders it superior to otherphysical methods as electroporation. The combination of ultrasoundwith targeted gas microbubbles as gene carriers holds great promiseby offering double targeting controlling both gene release and genetransfer location (Frenkel, 2008; Kinoshita et al., 2006; Kost et al.,1989; O'Neill and Li, 2008; Rapoport et al., 2007; Schroeder et al.,2007, 2009). Nevertheless, there are some challenges that have tobe tackled to improve its efficiency. It is important to combine a judi-cious choice ofmicrobubble composition and plasmidDNA compositionto deliver gene in a specific cell type. So far, cationic microbubbles didnot reach the expectations in terms of gene delivery efficiency. Thisis not surprising because if plasmid DNA is internalized duringsonoporation, it has to overcome the same cellular barriers as thoseencountered by chemical vectors (Pichon et al., 2010). Therefore,plasmid DNA must escape from endosomes and be routed insidethe nucleus. This could be tackled by fine tuning microbubble com-position and reducing their size.
Most importantly, we need to improve our knowledge concerningmicrobubble–cell interactions and how they impact on gene deliveryto fully exploit this method in a safe and efficient way. This wouldallow us to establish a rational design of sonoporation protocols andto propose sonoporation for possible clinical applications.
Acknowledgments
We are grateful to the Region Centre (Nanodeliv project) and theFrench Agence Nationale de la Recherche (ANR-MEDDU project) fortheir financial support.
References
Anderson, C.R., et al., 2010. scVEGF microbubble ultrasound contrast agents: a novelprobe for ultrasound molecular imaging of tumor angiogenesis. Investig. Radiol.45 (10), 579–585.
Please cite this article as: Delalande, A., et al., Sonoporation: Mechanistic idx.doi.org/10.1016/j.gene.2013.03.095
Anwer, K., et al., 2000. Ultrasound enhancement of cationic lipid-mediated genetransfer to primary tumors following systemic administration. Gene Ther. 7 (21),1833–1839.
Bao, S., Thrall, B.D., Miller, D.L., 1997. Transfection of a reporter plasmid into culturedcells by sonoporation in vitro. Ultrasound Med. Biol. 23 (6), 953–959.
Barnett, S.B., et al., 2000. International recommendations and guidelines for the safeuse of diagnostic ultrasound in medicine. Ultrasound Med. Biol. 26 (3), 355–366.
Bennett, J., et al., 2012. AAV2 gene therapy readministration in three adults with con-genital blindness. Sci. Transl. Med. 4 (120), 120ra15.
Christiansen, J.P., et al., 2003. Targeted tissue transfection with ultrasound destruction ofplasmid-bearing cationic microbubbles. Ultrasound Med. Biol. 29 (12), 1759–1767.
Dayton, P.A., Allen, J.S., Ferrara, K.W., 2002. The magnitude of radiation force on ultra-sound contrast agents. J. Acoust. Soc. Am. 112 (5 Pt 1), 2183–2192.
Delalande, A., et al., 2010. Ultrasound-assisted microbubbles gene transfer in tendonsfor gene therapy. Ultrasonics 50 (2), 269–272.
Delalande, A., et al., 2011a. Sonoporation at a low mechanical index. Bubble Sci. Eng.Technol. 3 (1), 3–11.
Delalande, A., et al., 2011b. Ultrasound and microbubble-assisted gene delivery inAchilles tendons: long lasting gene expression and restoration of fibromodulinKO phenotype. J. Control. Release 156 (2), 223–230.
Delalande, A., et al., 2012. Ultrasound and microbubble-assisted gene delivery: recentadvances and ongoing challenges. Ther. Deliv. 3 (10), 1199–1215.
Deng, C.X., et al., 2004. Ultrasound-induced cell membrane porosity. Ultrasound Med.Biol. 30 (4), 519–526.
Eliasson, L., et al., 1996. Endocytosis of secretory granules in mouse pancreatic beta-cellsevoked by transient elevation of cytosolic calcium. J. Physiol. 493 (Pt 3), 755–767.
Escoffre, J.M., et al., 2011. Doxorubicin delivery into tumor cells with ultrasound andmicrobubbles. Mol. Pharm. 8 (3), 799–806.
Fan, Z., et al., 2010. Intracellular delivery and calcium transients generated in sonoporationfacilitated by microbubbles. J. Control. Release 142 (1), 31–39.
Ferrara, K.W., 2008. Driving delivery vehicles with ultrasound. Adv. Drug Deliv. Rev. 60(10), 1097–1102.
Fischer, A., et al., 2000. Gene therapy of severe combined immunodeficiencies. Immunol.Rev. 178, 13–20.
Frenkel, V., 2008. Ultrasound mediated delivery of drugs and genes to solid tumors.Adv. Drug Deliv. Rev. 60 (10), 1193–1208.
Hacein-Bey-Abina, S., et al., 2003. A serious adverse event after successful gene ther-apy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 348 (3),255–256.
Hayashi, S., et al., 2009. Effect of sonoporation on cationic liposome-mediated IFNbetagene therapy for metastatic hepatic tumors of murine colon cancer. Cancer GeneTher. 16 (8), 638–643.
Huang, S.L., 2008. Liposomes in ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev.60 (10), 1167–1176.
Iwanaga, K., et al., 2007. Local delivery system of cytotoxic agents to tumors by focusedsonoporation. Cancer Gene Ther. 14 (4), 354–363.
nsights and ongoing challenges for gene transfer, Gene (2013), http://
9A. Delalande et al. / Gene xxx (2013) xxx–xxx
Juffermans, L.J., et al., 2006. Transient permeabilization of cell membranes by ultrasound-exposed microbubbles is related to formation of hydrogen peroxide. Am. J. Physiol.Heart Circ. Physiol. 291 (4), H1595–H1601.
Juffermans, L.J., et al., 2008. Low-intensity ultrasound-exposed microbubbles provokelocal hyperpolarization of the cell membrane via activation of BK(Ca) channels.Ultrasound Med. Biol. 34 (3), 502–508.
Juffermans, L.J., et al., 2009. Ultrasound and microbubble-targeted delivery of therapeuticcompounds: ICIN Report Project 49: drug and gene delivery through ultrasound andmicrobubbles. Neth. Heart J. 17 (2), 82–86.
Kaddur, K., et al., 2010. Transient transmembrane release of green fluorescent proteinswith sonoporation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 57 (7), 1558–1567.
Karshafian, R., et al., 2009. Sonoporation by ultrasound-activated microbubble contrastagents: effect of acoustic exposure parameters on cell membrane permeability andcell viability. Ultrasound Med. Biol. 35 (5), 847–860.
Kheirolomoom, A., et al., 2010. Enhanced in vivo bioluminescence imaging using lipo-somal luciferin delivery system. J. Control. Release 141 (2), 128–136.
Kinoshita,M., et al., 2006. Targeted delivery of antibodies through the blood–brain barrier byMRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 340 (4), 1085–1090.
Klibanov, A.L., 2007. Ultrasoundmolecular imaging with targeted microbubble contrastagents. J. Nucl. Cardiol. 14 (6), 876–884.
Kost, J., Leong, K., Langer, R., 1989. Ultrasound-enhanced polymer degradation and releaseof incorporated substances. Proc. Natl. Acad. Sci. U. S. A. 86 (20), 7663–7666.
Kotopoulis, S., Postema, M., 2010. Microfoam formation in a capillary. Ultrasonics 50(2), 260–268.
Kumon, R.E., et al., 2007. Ultrasound-induced calcium oscillations and waves in Chinesehamster ovary cells in the presence of microbubbles. Biophys. J. 93 (6), L29–L31.
Kumon, R.E., et al., 2009. Spatiotemporal effects of sonoporation measured by real-timecalcium imaging. Ultrasound Med. Biol. 35 (3), 494–506.
Lindner, J.R., 2004. Molecular imagingwith contrast ultrasound and targetedmicrobubbles.J. Nucl. Cardiol. 11 (2), 215–221.
Lindner, J.R., et al., 2001. Ultrasound assessment of inflammation and renal tissue injurywith microbubbles targeted to P-selectin. Circulation 104 (17), 2107–2112.
Lou, J., 2000. In vivo gene transfer into tendon by recombinant adenovirus. Clin. Orthop.Relat. Res. (379 Suppl.), S252–S255.
Lu, Q.L., et al., 2003. Microbubble ultrasound improves the efficiency of gene transductionin skeletal muscle in vivowith reduced tissue damage. Gene Ther. 10 (5), 396–405.
Lukacs, G.L., et al., 2000. Size-dependent DNAmobility in cytoplasm and nucleus. J. Biol.Chem. 275 (3), 1625–1629.
MacDonald, P.E., Eliasson, L., Rorsman, P., 2005. Calcium increases endocytotic vesiclesize and accelerates membrane fission in insulin-secreting INS-1 cells. J. Cell Sci.118 (Pt 24), 5911–5920.
Mahato, R.I., 1999. Non-viral peptide-based approaches to gene delivery. J. Drug Target.7 (4), 249–268.
Marmottant, P., Hilgenfeldt, S., 2003. Controlled vesicle deformation and lysis by singleoscillating bubbles. Nature 423 (6936), 153–156.
Mehier-Humbert, S., et al., 2005a. Plasma membrane poration induced by ultrasoundexposure: implication for drug delivery. J. Control. Release 104 (1), 213–222.
Mehier-Humbert, S., et al., 2005b. Ultrasound-mediated gene delivery: kinetics of plasmidinternalization and gene expression. J. Control. Release 104 (1), 203–211.
Meijering, B.D., et al., 2009. Ultrasound and microbubble-targeted delivery of macro-molecules is regulated by induction of endocytosis and pore formation. Circ. Res.104 (5), 679–687.
Midoux, P., et al., 2009. Chemical vectors for gene delivery: a current review on polymers,peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br. J.Pharmacol. 157 (2), 166–178.
Miller, D.L., Thomas, R.M., Frazier, M.E., 1991. Ultrasonic cavitation indirectly inducessingle strand breaks in DNA of viable cells in vitro by the action of residual hydro-gen peroxide. Ultrasound Med. Biol. 17 (7), 729–735.
Miller, D.L., Bao, S., Morris, J.E., 1999. Sonoporation of cultured cells in the rotating tubeexposure system. Ultrasound Med. Biol. 25 (1), 143–149.
Mitragotri, S., 2005. Healing sound: the use of ultrasound in drug delivery and othertherapeutic applications. Nat. Rev. Drug Discov. 4 (3), 255–260.
Morgan, K.E., et al., 2000. Experimental and theoretical evaluation ofmicrobubble behavior:effect of transmitted phase and bubble size. IEEE Trans. Ultrason. Ferroelectr. Freq.Control 47 (6), 1494–1509.
Mukherjee, D., et al., 2000. Ten-fold augmentation of endothelial uptake of vascularendothelial growth factor with ultrasound after systemic administration. J. Am.Coll. Cardiol. 35 (6), 1678–1686.
Nathwani, A.C., et al., 2011. Long-term safety and efficacy following systemic adminis-tration of a self-complementary AAV vector encoding human FIX pseudotypedwith serotype 5 and 8 capsid proteins. Mol. Ther. 19 (5), 876–885.
Please cite this article as: Delalande, A., et al., Sonoporation: Mechanistic idx.doi.org/10.1016/j.gene.2013.03.095
Newman, C.M., Bettinger, T., 2007. Gene therapy progress and prospects: ultrasoundfor gene transfer. Gene Ther. 14 (6), 465–475.
O'Neill, B.E., Li, K.C., 2008. Augmentation of targeted delivery with pulsed high intensityfocused ultrasound. Int. J. Hyperthermia 24 (6), 506–520.
Park, J., Fan, Z., Deng, C.X., 2011. Effects of shear stress cultivation on cell membranedisruption and intracellular calcium concentration in sonoporation of endothelialcells. J. Biomech. 44 (1), 164–169.
Paula, D.M., et al., 2011. Therapeutic ultrasound promotes plasmid DNA uptake byclathrin-mediated endocytosis. J. Gene Med. 13 (7–8), 392–401.
Pichon, C., et al., 2008. Recent advances in gene deliverywith ultrasound andmicrobubbles.J. Exp. Nanosci. 3 (1), 17–40.
Pichon, C., Billiet, L., Midoux, P., 2010. Chemical vectors for gene delivery: uptake andintracellular trafficking. Curr. Opin. Biotechnol. 21 (5), 640–645.
Postema, M., Gilja, O.H., 2010. Jetting does not cause sonoporation. Biomed. Eng. 55 (S1),19–20.
Prentice, P., et al., 2005.Membranedisruptionby optically controlledmicrobubble cavitation.Nat. Phys. 1 (2), 107–110.
Raper, S.E., et al., 2003. Fatal systemic inflammatory response syndrome in a ornithinetranscarbamylase deficient patient following adenoviral gene transfer. Mol. Genet.Metab. 80 (1–2), 148–158.
Rapoport, N., Gao, Z., Kennedy, A., 2007. Multifunctional nanoparticles for combiningultrasonic tumor imaging and targeted chemotherapy. J. Natl. Cancer Inst. 99 (14),1095–1106.
Rickert, M., 2008. BMP-14 gene therapy increases tendon tensile strength in a ratmodel of Achilles tendon injury. J. Bone Joint Surg. Am. 90 (2), 445 (authorreply 445-6).
Schroeder, A., et al., 2007. Controlling liposomal drug releasewith low frequency ultrasound:mechanism and feasibility. Langmuir 23 (7), 4019–4025.
Schroeder, A., Kost, J., Barenholz, Y., 2009. Ultrasound, liposomes, and drug delivery:principles for using ultrasound to control the release of drugs from liposomes. Chem.Phys. Lipids 162 (1–2), 1–16.
Shen, Z.P., et al., 2008. Ultrasound with microbubbles enhances gene expression ofplasmid DNA in the liver via intraportal delivery. Gene Ther. 15 (16), 1147–1155.
Stieger, S.M., et al., 2007. Enhancement of vascular permeability with low-frequencycontrast-enhanced ultrasound in the chorioallantoic membrane model. Radiology243 (1), 112–121.
Suzuki, R., et al., 2007a. Gene delivery by combination of novel liposomal bubbles withperfluoropropane and ultrasound. J. Control. Release 117 (1), 130–136.
Suzuki, R., et al., 2007b. Effective gene delivery with liposomal bubbles and ultrasoundas novel non-viral system. J. Drug Target. 15 (7–8), 531–537.
Taniyama, Y., et al., 2002. Local delivery of plasmid DNA into rat carotid artery usingultrasound. Circulation 105 (10), 1233–1239.
Tlaxca, J.L., et al., 2010. Analysis of in vitro transfection by sonoporation using cationicand neutral microbubbles. Ultrasound Med. Biol. 36 (11), 1907–1918.
Tran, T.A., et al., 2007. Effect of ultrasound-activated microbubbles on the cell electro-physiological properties. Ultrasound Med. Biol. 33 (1), 158–163.
Tran, T.A., et al., 2008. Characterization of cell membrane response to ultrasoundactivated microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 55 (1),43–49.
Un, K., et al., 2010. Development of an ultrasound-responsive and mannose-modifiedgene carrier for DNA vaccine therapy. Biomaterials 31 (30), 7813–7826.
Unger, E.C., et al., 2004. Therapeutic applications of lipid-coated microbubbles. Adv.Drug Deliv. Rev. 56 (9), 1291–1314.
van Wamel, A., et al., 2004. Micromanipulation of endothelial cells: ultrasound–microbubble–cell interaction. Ultrasound Med. Biol. 30 (9), 1255–1258.
Vannan, M., et al., 2002. Ultrasound-mediated transfection of canine myocardium byintravenous administration of cationic microbubble-linked plasmid DNA. J. Am.Soc. Echocardiogr. 15 (3), 214–218.
Wagner, E., Ogris, M., Zauner, W., 1998. Polylysine-based transfection systems utilizingreceptor-mediated delivery. Adv. Drug Deliv. Rev. 30 (1–3), 97–113.
Wang, Y., et al., 2010. Preparation of nanobubbles for ultrasound imaging and intracel-lular drug delivery. Int. J. Pharm. 384 (1–2), 148–153.
Yoshida, T., et al., 2007. Combination of doxorubicin and low-intensity ultrasoundcauses a synergistic enhancement in cell killing and an additive enhancementin apoptosis induction in human lymphoma U937 cells. Cancer Chemother.Pharmacol. 61 (4), 559–567.
Zhao, Y.Z., et al., 2005. Preparation, characterization and in vivo observation ofphospholipid-based gas-filled microbubbles containing hirudin. Ultrasound Med.Biol. 31 (9), 1237–1243.
Zhou, Y., et al., 2009. The Size of Sonoporation Pores on the Cell Membrane. UltrasoundMed. Biol. 35 (10), 1756–1760.
nsights and ongoing challenges for gene transfer, Gene (2013), http://