Statistical consideration when adding new arms to ongoing ...
Chronic administration of antipsychotics attenuates ongoing and ketamine-induced increases in...
Transcript of Chronic administration of antipsychotics attenuates ongoing and ketamine-induced increases in...
Chronic administration of antipsychoticsattenuates ongoing and ketamine-inducedincreases in cortical γ oscillations
Paul M. Anderson1,2, Didier Pinault2, Terence J. O’Brien1 and Nigel C. Jones11Department of Medicine, Faculty of Medicine Dentistry and Health Sciences, University of Melbourne, Parkville 3010, Victoria, Australia2 INSERM U1114, Neuropsychologie cognitive et physiopathologie de la schizophrénie, Université de Strasbourg, Fédération de MédecineTranslationnelle de Strasbourg, 11 rue Humann, F-67085 Strasbourg, France
Abstract
Noncompetitive N-methyl-D-aspartate receptor (NMDAr) antagonists can elicit many of the symptoms observedin schizophrenia in healthy humans, and induce a behavioural phenotype in animals relevant to psychosis. Thesecompounds also elevate the power and synchrony of gamma (γ) frequency (30–80Hz) neural oscillations. Acutedoses of antipsychotic medications have been shown to reduce ongoing γ power and to inhibit NMDArantagonist-mediated psychosis-like behaviour in rodents. This study aimed to investigate how a chronicantipsychotic dosing regimen affects ongoing cortical γ oscillations, and the electrophysiological and behaviouralresponses induced by the NMDAr antagonist ketamine. Male Wistar rats were chronically treated withhaloperidol (0.25mg/kg/d), clozapine (5mg/kg/d), LY379268 (0.3mg/kg/d) or vehicle for 28 d, delivered by sub-cutaneous (s.c.) osmotic pumps. Weekly electrocorticogram (ECoG) recordings were acquired. On day 26,ketamine (5mg/kg, s.c.) was administered, and ECoG and locomotor activity were simultaneously measured.These results were compared with data generated previously following acute treatment with these antipsycho-tics. Sustained and significant decreases in ongoing γ power were observed during chronic administration ofhaloperidol (64%) or clozapine (43%), but not of LY379268 (2% increase), compared with vehicle. Acute ketamineinjection concurrently increased γ power and locomotor activity in vehicle-treated rats, and these effects wereattenuated in rats chronically treated with all three antipsychotics. The ability of haloperidol or clozapine to in-hibit ketamine-induced elevation in γ power was not observed following acute administration of these drugs.These results indicate that modulation of γ power may be a useful biomarker of chronic antipsychotic efficacy.
Received 13 February 2014; Reviewed 25 March 2014; Revised 12 May 2014; Accepted 16 May 2014;First published online 25 June 2014
Key words: Antipsychotics, ECoG, gamma oscillations, ketamine, locomotor activity, NMDA receptorantagonist, schizophrenia.
Introduction
Schizophrenia is a debilitating psychiatric disorder that ischaracterized by a range of symptoms, including halluci-nations, delusions, emotional disturbances and diversecognitive deficits (Harrison, 1999). In recent years therehas been a convergence of various lines of evidencesuggesting that the underlying pathophysiology ofschizophrenia involves disruptions of neural synchrony(Uhlhaas et al., 2008). In particular, it has been demon-strated that gamma (γ) frequency (30–80Hz) oscillationsare disrupted in patients with schizophrenia. These dis-ruptions appear to be complex, with studies report-ing both decreases in stimulus-evoked γ oscillations
(Kwon et al., 1999; Spencer et al., 2003; Ford and Roth,2004), as well as increases in ongoing γ oscillations (typi-cally associated with psychotic events or positive symp-toms) (Baldeweg et al., 1998; Gordon et al., 2001; Beckeret al., 2009; Spencer et al., 2009; Behrendt, 2010). The func-tional role of γ oscillations has been linked to a range ofhigher-order brain functions, including cognition (Engelet al., 2001), working-memory (Tallon-Baudry et al.,1998; Howard et al., 2003; Uhlhaas et al., 2008) and sen-sory perception (Lee et al., 2003; Spencer et al., 2004;Herrmann and Demiralp, 2005; Light et al., 2006;Uhlhaas et al., 2006; Gross et al., 2007; Krishnan et al.,2009; Maharajh et al., 2010). These same cognitive pro-cesses are disrupted in schizophrenia, suggesting thataberrant γ oscillations may be directly related to thepathophysiology of the disorder, although provingcausation of this is challenging.
Ketamine is a dissociative anaesthetic drug and a non-competitive antagonist of N-methyl-D-aspartate (NMDA)receptors. Although it has been shown to have a broad
Address for correspondence: N. C. Jones, Department of Medicine (RMH),University of Melbourne, Melbourne Brain Centre, 3052, Parkville,Australia.Tel.: +61 3 9035 6402 Fax: +61 3 9347 1863Email: [email protected]
International Journal of Neuropsychopharmacology (2014), 17, 1895–1904. © CINP 2014doi:10.1017/S1461145714000959
ARTICLE
pharmacological profile, including interactions at dopa-mine and serotonin receptors (Kapur and Seeman,2002), it is principally regarded as an NMDA receptorligand. Ketamine and other NMDA receptor (NMDAr)antagonists have been shown to induce hallucinationsin healthy humans, and exacerbate psychotic symp-toms in schizophrenic patients (Krystal et al., 1994).Subsequent studies described the ability of ketamineto promote affective symptoms and cognitive deficits(Hetem et al., 2000; Stone et al., 2008; Driesen et al.,2013), and some suggest that ketamine more closely mod-els negative and cognitive symptoms of schizophrenia(Newcomer et al., 1999). Acute ketamine administrationis hence widely considered to be a valid model of thepositive, negative and cognitive symptoms of schizo-phrenia (Frohlich and Van Horn, 2014). This and otherevidence has led to the development of the NMDArhypofunction hypothesis of schizophrenia, which positsthat reduced activity at NMDA receptors leads to theexpression of schizophrenia symptoms. We (Pinault,2008; Hakami et al., 2009) and others (Ehrlichman et al.,2009; Lazarewicz et al., 2010) previously demonstratedthat NMDAr antagonists dose-dependently increasethe power of ongoing γ cortical oscillations in rodentsand also recapitulate complex electrophysiologicalabnormalities seen in schizophrenia (Kulikova et al.,2012; Saunders et al., 2012). We further developed thismodel by examining the effects of antipsychotic com-pounds on ongoing γ oscillations, and in response to aketamine challenge (Jones et al., 2012). We tested a typical(haloperidol) and atypical (clozapine) antipsychoticand a preclinical metabotropic glutamate 2/3 receptor(mGluR2/3) agonist (LY379268) with antipsychotic proper-ties (Imre, 2007). Our results demonstrated that, on theirown, antipsychotic medications reduce the power ofongoing cortical γ oscillations in freely moving rodents.Antipsychotics also reduce oscillations of higher frequen-cies (100–180Hz) in the nucleus accumbens (Olszewskiet al., 2013). However, acute treatment with conventionalantipsychotics did not impact the ability of ketamine toincrease γ oscillations, a finding in contrast to the effectsof these drugs on ketamine-induced hyperlocomotoractivity (Jones et al., 2012).
The effect of antipsychotics to modulate γ oscillationsmay confound much of the literature examining theseneural rhythms which has been generated in clinicalpopulations. Although some studies have compareddrug-free and medicated patients (Gallinat et al., 2004),or examined first episode patients to eliminate the effectsof medication (Symond et al., 2005), the vast majority donot control for drug effects. This therefore remains anunderstudied area with important clinical implications.
Given the widespread reports of γ frequency altera-tions in schizophrenia, the ability of antipsychotic medi-cations to modulate neuronal oscillations may be centralto their efficacy, with the potency of modulation of γ ac-tivity representing a potential new biomarker of
antipsychotic efficacy. Previous studies have only exam-ined acute antipsychotic drug treatment, whereas in clini-cal situations, the efficacy of these drugs typically takeseveral weeks of administration to manifest (Gelderet al., 2000). This study aims to examine the effect ofchronic antipsychotic administration on ongoing and onthe acute challenge of low-dose ketamine-inducedincreases in γ oscillations.
Materials and methods
Animals
Male Wistar rats aged 10–12wk old (weighing 250–350 g)were used (total n=44). Animals were bred and housed(3–4 per cage) in the Biological Research Facility of theDepartment of Medicine, Royal Melbourne Hospital,University of Melbourne. Animals had access to foodand water ad libitum and were kept on a 12 h light–dark cycle (lights on at 06:00 hours). All experimentswere approved by the University of Melbourne AnimalEthics committee (Ethics #1011868) and adhered to theAustralian Code of Practice for the Care and Use ofAnimals for Scientific Purposes.
Surgery
Animals were anaesthetised by inhalation of isoflurane(5% induction, 1.5–2.5% maintenance) in equal parts ofmedical air and oxygen and implanted with an osmoticmini-pump (Model 2ML4, Alzet, USA) in the dorsalthoracic region by way of a single incision midway be-tween the scapulae. Animals were then positioned in astereotaxic frame as described previously (Hakamiet al., 2009) for implantation of electrocorticograph(ECoG) electrodes. Briefly, a single midline incisionwas made over the scalp and six holes were drilledthrough the skull with stereotaxic guidance (Paxinosand Watson, 1998) [2 mm anterior and 2mm lateral tobregma bilaterally (active electrodes); 2 mm posteriorand 2mm lateral to bregma bilaterally (ground electro-des); and 2mm posterior and 2mm lateral to lambdabilaterally (reference electrodes)]. Electrodes were thenscrewed into the skull without breaching the dura, anddental cement applied to the skull to fix the electrodesin place. After recovery from anaesthesia, animals werehoused in separate cages for the duration of theexperiment.
Drugs and vehicles
Clozapine, haloperidol and LY379268 were obtained fromTocris Bioscience (UK). Ketamine was obtained fromParnell Laboratories (Australia). Isoflurane was pur-chased from Abbott Pharmaceuticals (USA). Haloperidoland ketamine were diluted in 0.9% sterile saline, cloza-pine was dissolved in 10% acetic acid in sterile waterwith pH adjusted to 6.0 using 10 M NaOH. Control
1896 P. M. Anderson et al.
pumps were loaded with 10% acetic acid (n=5) or sterilesaline (n=3): there were no significant differences in theoutcome of these treatments so the two vehicle controlconditions were combined for analysis. Pumps wereweighed before and after filling, and residual volumewas checked at the end of the experiment to ensure ad-equate delivery (>95% expected volume) of drug. Cloza-pine was administered to give an approximate dose of5mg/kg/d, haloperidol at 0.25mg/kg/d and LY 79268 at0.3 mg/kg/d, based on the predicted weight of animalsat day 28. Dosages were selected on two criteria: to main-tain a clinically relevant plasma concentration (for halo-peridol and clozapine) (Kapur et al., 2003) and to havean equivalent effect on γ power (based on acute dosing(Jones et al., 2012)).
Assessment of ECoG power and locomotor activity
Animals underwent ‘baseline’ ECoG recordings at 7,14 and 21 d post-surgery. Animals had a recordingcable attached while in their home cages and after a30min acclimatisation period, 30min of ECoG activitywas recorded. On day 26 or 27 of the experiment animalsunderwent a ketamine ‘challenge’. Animals were broughtinto the Behavioural Testing Facility in the Department ofMedicine at least 30min before the start of the study toallow habituation to the environment. Rats were then in-dividually placed into an open arena (1m diameter),while attached to an ECoG recording cable suspendedfrom the ceiling. Each rat was allowed to acclimatise tothe arena for 30min at which point ECoG recordingbegan. Following a 30min baseline recording animalsreceived an injection of ketamine (5mg/kg s.c.) andrecorded for another 60min. Throughout the ECoG acqui-sition animals’ locomotor activity was video-tracked andobjectively assessed with Ethovision software (Noldus,The Netherlands), total distance travelled was calculatedfor each 2min interval.
ECoG acquisition and analysis
ECoG was acquired and analysed with a Synampamplifier and SCAN v4.5 software (Compumedics,Australia), ECoG was sampled at 2000 Hz with a band-pass of 0.5–1000 Hz. Drug effects on the power of differ-ent frequency bands was assessed as previouslydescribed (Hakami et al., 2009). Briefly, raw ECoG wassectioned into 2.048 s epochs, fast Fourier transformationswere performed to determine the average power in differ-ent frequency bands (δ – 1–4 Hz θ – 4–8 Hz; α – 8–12Hz;β – 13–30; γ – 30–80Hz) and average power was then cal-culated for each 2min interval and averaged over 30min.For the ketamine challenge experiments, to quantify bothγ power and locomotor activity, the first 15 data points(representing the 30min prior to ketamine) are averagedto give a baseline activity for each recording, all recordedvalues are then expressed as percentage of this activity.
Acute dosage experiment
Data generated from our previous acute dosage study(Jones et al., 2012) were reanalysed here to directly com-pare the effects of different dosage paradigms (i.e. acutevs. chronic). ECoG data from the acute experiment wereacquired with a MacLab amplifier and Chart v. 3.5 soft-ware (AD Instruments, Australia). 50 Hz line noise wasdetected and digitally subtracted from the recording inreal time using selective eliminators (Humbug noise elim-inators; Digitimer, UK) which do not affect the biologicalsignal. Single doses of clozapine (5mg/kg), haloperidol(0.25mg/kg) and LY379268 (0.3 mg/kg) and ketamine(5mg/kg) were used. ECoG and locomotor data wereacquired in the same behavioural facility using a protocolsimilar to the ketamine ‘challenge’ recording: first a30min acclimatisation period in the arena, followed by30min baseline recordings. This was followed by s.c. in-jection of antipsychotic and 30min post antipsychoticrecording, followed by injection of ketamine and 60minrecording. Electrophysiology data from the acute exper-iment were expressed as a percentage of the post-antipsychotic period average.
Statistical analyses
Differences between treatment groups in spectral powerwere assessed by averaging the 15 data points from the30min baseline recording and then comparing thesevalues using the Kruskal–Wallis test, with Dunn’smultiple comparisons post-hoc test. Both the electrophy-siological and locomotor response to ketamine challengewas assessed with two-way analysis of variance(ANOVA) with repeated measures (time) comparingdrug treatment to vehicle controls. The effects of ketamineon γ power were quantified by analysing the area underthe curve for each animal, normalised to the meanγ power in the 20min preceding ketamine injection.Comparisons between acute and chronic treatmentswere made with two-way ANOVA, with Bonferroni’spost-hoc test comparing treatments within a treatmentregime.
Results
Haloperidol and clozapine stably reduce the power ofongoing γ oscillations, while chronic LY379268increases low frequency power
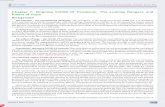
Chronic treatment with the conventional antipsychoticshaloperidol and clozapine caused pronounced and per-sistent decreases across the power spectrum (Fig. 1a).Conversely LY379268-treated animals displayed powercomparable to vehicle-treated animals. These changeswere stable over the 4-wk experimental period with thedecrease in θ, α and γ power caused by clozapine andhaloperidol persisting throughout. Robust differences
Chronic antipsychotics modulate γ oscillations 1897
were observed in the γ frequency band, two-way repeatedmeasures ANOVA showed a significant effect of drugtreatment (F(3,105) =11.92, p<0.0001). Post-hoc tests showedsignificant differences between haloperidol- and vehicle-treated animals at all time points (p<0.01), while cloza-pine was different to vehicle at all but week 2 (p<0.05).LY379268-treated animals did not have significantly
different γ power at any time-point. Theta and alphapower bands were also shown to be altered by antipsy-chotic treatment (two-way repeated measures ANOVA,theta: F(3,105) =3.161, p=0.036, alpha: F(3,105) =8.696,p=0.0002), while beta was not F(3,105) =0.706, p=0.555.No effects of the drugs were observed in the delta fre-quency band (all p>0.05).
1000(a)
(b)
Spe
ctra
l pow
er (µ
V2 )
Frequency (Hz)
Pow
er (µ
V2 )
100
10
20
Vehicle
150
100
50
0Week 1 Week 2 Week 3 Week 4 Week 1 Week 2 Week 3 Week 4
Week 1 Week 2 Week 3 Week 4
Week 1 Week 2 Week 3 Week 4
Week 1 Week 2 Week 3 Week 4
Pow
er (µ
V2 )
100
50
0
Pow
er (µ
V2 )
20
15
10
5
0
Pow
er (µ
V2 )
6
4
2
0
Pow
er (µ
V2 )
200
150
100
50
0
Haloperidol
Delta Theta
Alpha Beta
Gamma
Clozapine LY379268
40 60 80 100
1
Fig. 1. Average spectral power from electrocorticogram (ECoG) of rats undergoing chronic antipsychotic treatment. (a) Averagespectral power (from 0–100Hz) from all animals recorded on week 3 of treatment. The solid lines represent the mean, and thedashed lines represent the S.E.M for each treatment. (b) Spectral power in discrete frequency bands taken during each week oftreatment. LY379268-treated animals have elevated α power, but no change in γ power compared with vehicle. Clozapine- andhaloperidol-treated animals display reduced θ, α and γ power compared with vehicle treatment. Data represent mean±S.E.M;n=9–11/group.
1898 P. M. Anderson et al.
Chronic treatment with haloperidol or clozapinesignificantly attenuates the ketamine-induced rise inγ power, but acute treatment does not
The electrophysiological response to the ketamine chal-lenge injection (5mg/kg s.c.) manifested as a rapid andlarge increase in the power of ongoing γ oscillations,with vehicle-treated animals having an average maxi-mum response of 237±14% relative to baseline occurringat 10min post-injection. This effect of ketamine wasinhibited in the chronically treated animals, with all
treatments reducing ketamine’s effect. In the clozapine-and LY379268-treated animals, the power of ongoingγ oscillations returned to baseline levels sooner thanvehicle-treated animals (Fig. 2a). Peak responses for thethree treatment groups were haloperidol: 187±13% occur-ring at 10min post injection; clozapine: 213±9% at 10minpost injection; and LY379268: 177±7% at 12min post in-jection. ANOVA showed that treatment had a statisticallysignificant effect on γ power for haloperidol (F(1,20) =6.57,p=0.0185), clozapine (F(1,24) =25.30, p<0.0001) and LY379268 (F(1,22) =37.83, p<0.0001). Ketamine did not affect
300
200
Gam
ma
pow
er (%
of b
asel
ine)
100
300
8000
6000
4000
2000
0Acute Chronic
200
Gam
ma
pow
er (%
of b
asel
ine)
100
–15 0 15 30Time (min)
Chronic(a)
(b)
(c)
Acute
KET
KET
Vehicle
Haloperidol
Clozapine
LY379268
Vehicle
Haloperidol
Clozapine
LY379268
VehicleHaloperidolClozapineLY379268
45 60
–15 0 15 30Time (min)
Gam
ma
pow
er A
UC
(0–6
0 m
in p
ost-
keta
min
e)
45 60
Fig. 2. Effects of antipsychotic treatment on ketamine-induced increases in γ power. Chronic treatment (a) with all three drugsattenuated the ketamine-induced rise in γ power, compared withto vehicle treatment. In contrast, in the acute treatment paradigm(b), only LY379268 significantly attenuated ketamine’s effect. Comparison of the effects of chronic vs. acute antipsychotic treatmenton ketamine-induced γ power (c) found that chronic treatment with haloperidol and clozapine reduced the magnitude ofketamine-induced increases in γ power, but acute treatment with these drugs did not. However, both acute and chronic treatmentwith LY379268 induced similar magnitude of effects. Data represents mean±S.E.M; n=9–14/group. * indicates p<0.05 compared withvehicle within the same treatment regime (i.e. acute or chronic). AUC: area under curve.
Chronic antipsychotics modulate γ oscillations 1899
oscillations in the other frequency bands, and so nofurther analyses were conducted on these.
In order to compare the effects of chronic treatmentwith antipsychotic drugs to those induced by a singleacute dose, we reanalysed our previously publisheddata (Jones et al., 2012), examining single acute dosesof the same drugs. When comparing the time courseof effects of acute antipsychotics, only LY379268significantly reduced the electrophysiological effect ofketamine (F(1,24) =52.68, p<0.0001) compared withvehicle-treated animals (Fig. 2b). The electrophysiologicalresponse to ketamine was quantified by calculating thearea under the curve 0–60min post ketamine injection(Fig. 2c), and responses compared between the acuteand chronic treatment paradigms. ANOVA showed thatdrug treatment (p<0.0001) and treatment regime(p<0.0001) were significantly different, and that therewas a significant interaction between these variables(p=0.0032). Bonferroni post-hoc tests showed that forchronic treatment, all three antipsychotics significantlyreduced the effect of ketamine on γ power (p<0.05),whereas for acute treatment, LY379268 was the onlydrug to significantly affect this outcome (p<0.001). Assuch, there was a differential effect of haloperidol andclozapine, such that they were only effective whenchronically administered.
All three antipsychotics attenuate ketamine-inducedhyper-locomotion
The effects of chronic antipsychotic treatment on thepower of ongoing γ oscillations were mirrored in theoutcome of the locomotor response to ketamine injection.Ketamine caused a hyperlocomotor response in allinjected animals; in vehicle-treated animals, we observeda maximum response of 932±229% occurring at2min post injection (Fig. 3a). This response was signifi-cantly, but not completely, reduced in all three chronictreatment groups with maximum locomotor activitiesfor haloperidol: 441±92% at 8min post-injection, cloza-pine: 494±151% at 2min and LY379268: 313±105% at2min post injection. ANOVA showed that chronictreatment had a significant effect on the locomotor re-sponse for haloperidol (F(1,13) =4.69, p=0.0496), clozapine(F(1,14) =8.28, p=0.0122) and LY379268 (F(1,12) =9.96,p=0.0083). Acute treatment (Fig. 3b) evoked similarresults, with haloperidol (F(1,14) =22.29, p=0.0003), cloza-pine (F(1,13) =8.12, p=0.0137) and LY379268 (F(1,11) =5.68,p=0.0362) all significantly inhibiting the locomotor re-sponse of ketamine as compared with vehicle-treatedanimals.
In contrast to their effects on the electrophysiologicalresponse to ketamine, when assessing the area underthe curve (Fig. 3c), there were no differences betweenthe acute and chronic treatment regime (p>0.05), suchthat all treatments were equally effective at reducing thelocomotor response to ketamine.
Discussion
The primary finding of this study is that chronic treat-ment with the antipsychotic medications haloperidoland clozapine, and the pre-clinical drug LY379268,attenuate the effects of an acute ketamine challenge,as measured by electrophysiological and behaviouralresponses. Chronic treatment with haloperidol andclozapine attenuated the ketamine-induced increase inγ power, an effect not seen with single acute dosages.The mGluR2/3 agonist LY379268 reduced ketamine-induced locomotor and γ hyper-activities under bothacute and chronic treatment paradigms. FurthermoreLY379268 did not alter the power of ongoing physiologi-cal γ oscillations during the 4-wk treatment, whereas theconventional antipsychotics, clozapine and haloperidol,caused a pronounced depression of the ongoing back-ground γ activity that was sustained throughout thetreatment period.
The observation that chronic treatment for 4wk withthe conventional antipsychotics was effective in attenuat-ing the effects of ketamine to increase cerebral γ activity,while acute single dosing was not (Jones et al., 2012),is potentially important finding. In clinical practice,the efficacy of antipsychotic treatment is typically notseen until 2–3 weeks after treatment has been instituted(Gelder et al., 2000). Although some recent studieshave questioned this (Agid et al., 2003), it is clear thatin clinical settings antipsychotic treatment displays in-creasing efficacy over time. The findings of this study sug-gest that the cerebral γ activity response to ketamine mayrepresent an endophenotype of the efficacy of chronicantipsychotic treatment which is practical for use indrug screening in animal models.
There is a large body of evidence reporting alteredγ frequency oscillations in patients with schizophrenia,with studies generally, but not exclusively, reportingboth decreases in evoked γ oscillations (potentially relatedto the negative symptoms of schizophrenia) and increasesin ongoing γ oscillations (correlating with positive signs),as mentioned in the introduction. It has been proposedthat these findings represent different aspects of an over-all disruption in γ signal-to-noise in cortical informationprocessing (Gallinat et al., 2004; Flynn et al., 2008;Krishnan et al., 2009; Williams et al., 2009); and see(Gandal et al., 2012) for review). This conceptualises thatthe increase in ongoing γ power triggered by NMDArantagonists represent a diffuse network noise, disruptingcortical processing and leading to perceptual and cogni-tive deficits reminiscent of those seen in schizophrenia.The findings of this study support the proposition thatmodulation of γ power represents a key property of anti-psychotic medications, with both clozapine and haloperi-dol’s capacity to inhibit aberrant γ oscillations potentiallyreflecting their efficacy against positive symptoms.
The differential effects of LY379268 on spontaneous(ongoing) γ activity compared with the established
1900 P. M. Anderson et al.
clinical antipsychotic drugs, haloperidol and clozapine, isnoteworthy. LY379268 is a mGluR2/3 agonist, while mostclinical antipsychotic drugs are thought to primarily actvia dopamine D2 receptors (although clozapine has lesseffects at this receptor that haloperidol) (Seeman, 2002).The mGluR2/3 agonists have been shown to decrease therelease of glutamate and inhibit excitatory synaptic ac-tivity (Lovinger and McCool, 1995; Yoshino et al., 1996;Battaglia et al., 1997). Ketamine (and other NMDArantagonists) trigger release of glutamate in the pre-frontalcortex (Adams and Moghaddam, 1998) and LY379268pre-treatment can block this glutamate release following
PCP administration (Lorrain et al., 2003), providinga plausible mechanism of action of how this drugmay combat the behavioural and electrophysiologicaleffects of ketamine observed here. Furthermore, althoughLY379268 causes a transient decrease in ongoing γ powerfollowing acute administration (Jones et al., 2012), this ef-fect was not seen with chronic treatment. In contrast thetwo clinical antipsychotic drugs resulted in sustaineddecreases in ongoing γ activity. mGluR2/3receptor ago-nists have been shown in clinical trials to have a lowercognitive side effect profile (Adams et al., 2013). It ispossible that the lack of effect of LY379268 on the
Vehicle
2000
1500
1000
Dis
tanc
e tr
avel
led
(cm
)
500
0
2000
1500
1000
Dis
tanc
e tr
avel
led
(cm
)
500
0
Haloperidol
Clozapine
LY379268
Vehicle
Haloperidol
Clozapine
LY379268
–15 0 15 30Time (min)
45 60
–15 0 15 30
Acute
ChronicKET
KET
45 60
(a)
(b)
20000
15000
10000
Dis
tanc
e tr
avel
led
(0–6
0 m
in p
ost-
keta
min
e )
5000
0
VehicleHaloperidolClozapineLY379268
Acute Chronic
(c)
Fig. 3. Effects of antipsychotic treatment on ketamine-induced locomotion. Chronic treatment (a) with all three different drugsattenuated the ketamine-induced locomotor activity as compared with vehicle treatment. Similar effects on locomotion where seenwith acutely treated animals (b) where all three medications attenuated the hyperlocomotion effects of ketamine. For all drugs,these two treatment regimens were equally effective at reducing the ketamine-induced locomotor effect (c). Data representmean±S.E.M, n=6–8/group. * indicates p<0.05 compared with vehicle within the same treatment regime (i.e. acute or chronic). AUC:area under curve.
Chronic antipsychotics modulate γ oscillations 1901
physiological ongoing γmay be related to its reduced cog-nitive side effects, given the role they are thought to playon cognitive processing. Haloperidol has been shownto induce negative symptoms (Veselinovic et al., 2013),and a comprehensive assessment in healthy subjectsshowed a general effect of impairing attention andspeed of processing caused by antipsychotic adminis-tration (Ramaekers et al., 1999).
It is important to point out that our study was conduc-ted in healthy animals, displaying physiologically normalγ oscillations, not the chronic pathological abnormalitiesseen in schizophrenia. This limits any interpretationsmade, with the acute NMDA receptor antagonist treat-ment considered an acute psychotic precipitant. Furtherwork performed in genetic or chronic pharmacologicalmodels for the schizophrenic state should be conductedto validate the relevance of the findings of this study.Nonetheless, the findings of this study – that chronictreatment with antipsychotic medications attenuates theketamine-induced increase in γ power – supports the hy-pothesis that γ frequency abnormalities are a significantfeature of the psychotic-like state that is induced in theNMDAr model. In addition, our studies do not containa placebo injection arm as a control to the ketamine injec-tions. Our previous work has demonstrated that injectionof vehicle treatments can impact ongoing γ power, butthis is minimal and short-lived (<2min), and dwarfedby the effect of ketamine (Hakami et al., 2009).Although we should consider the potential of a placeboeffect here, our primary comparisons are the inhibitionof the effect of ketamine to increase γ frequency powerby antipsychotic drugs, so the lack of placebo for keta-mine should not compromise the interpretation of thedata in this regard.
In conclusion this study has demonstrated chronicantipsychotic administration causes a strong attenuationof the behavioural and electrophysiological effects ofketamine, as assessed through hyperlocomotion andECoG γ hyperactivity. The modulation of γ frequencyoscillations by NMDA receptor antagonists and antipsy-chotic medications provide insights into the role that γ fre-quency abnormalities may play in schizophrenia, andhave potential utility as a measure of antipsychoticefficacy and cognitive side effects.
Acknowledgment
Paul Anderson is supported by an Ian Scott PhDScholarship from the Australian Rotary HealthFoundation.
Funding and Disclosure Statement
The authors declare no competing interests, financial orotherwise, associated with this work.
References
Adams B, Moghaddam B (1998) Corticolimbic dopamineneurotransmission is temporally dissociated from the cognitiveand locomotor effects of phencyclidine. J Neurosci18:5545–5554.
Adams DH, Kinon BJ, Baygani S, Millen BA, Velona I,Kollack-Walker S, Walling DP (2013) A long-term, phase 2,multicenter, randomized, open-label, comparative safety studyof pomaglumetad methionil (LY2140023 monohydrate) vs.atypical antipsychotic standard of care in patients withschizophrenia. BMC Psychiatry 13:143.
Agid O, Kapur S, Arenovich T, Zipursky RB (2003)Delayed-onset hypothesis of antipsychotic action: ahypothesis tested and rejected. Arch Gen Psychiatry60:1228–1235.
Baldeweg T, Spence S, Hirsch SR, Gruzelier J (1998)Gamma-band electroencephalographic oscillations in a patientwith somatic hallucinations. Lancet 352:620–621.
Battaglia G, Monn JA, Schoepp DD (1997) In vivo inhibition ofveratridine-evoked release of striatal excitatory amino acids bythe group II metabotropic glutamate receptor agonistLY354740 in rats. Neurosci Lett 229:161–164.
Becker C, Gramann K, Muller HJ, Elliott MA (2009)Electrophysiological correlates of flicker-induced colorhallucinations. Conscious Cogn 18:266–276.
Behrendt RP (2010) Contribution of hippocampal region CA3 toconsciousness and schizophrenic hallucinations. NeurosciBiobehav Rev 34:1121–1136.
Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V,D’Souza DC, Gueorguieva R, He G, Ramachandran R,Suckow RF, Anticevic A, Morgan PT, Krystal JH (2013)Relationship of resting brain hyperconnectivity andschizophrenia-like symptoms produced by the NMDAreceptor antagonist ketamine in humans. Mol Psychiatry18:1199–1204.
Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT,Finkel LH, Contreras D, Turetsky BI, Siegel SJ (2009)N-methyl-D-aspartic acid receptor antagonist-inducedfrequency oscillations in mice recreate pattern ofelectrophysiological deficits in schizophrenia. Neuroscience158:705–712.
Engel AK, Fries P, Singer W (2001) Dynamic predictions:oscillations and synchrony in top-down processing. Nat RevNeurosci 2:704–716.
Flynn G, Alexander D, Harris A, Whitford T, Wong W,Galletly C, Silverstein S, Gordon E, Williams LM (2008)Increased absolute magnitude of gamma synchrony infirst-episode psychosis. Schizophr Res 105:262–271.
Ford JM, Roth WT (2004) Deficient response modulation ofevent-related brain potentials in schizophrenia. Curr OpinPsychiatry 17:91–96.
Frohlich J, Van Horn JD (2014) Reviewing the ketamine modelfor schizophrenia. J Psychopharmacol 28:287–302.
Gallinat J, Winterer G, Herrmann CS, Senkowski D (2004)Reduced oscillatory gamma-band responses in unmedicatedschizophrenic patients indicate impaired frontal networkprocessing. Clin Neurophysiol 115:1863–1874.
Gandal MJ, Edgar JC, Klook K, Siegel SJ (2012) Gammasynchrony: towards a translational biomarker for thetreatment-resistant symptoms of schizophrenia.Neuropharmacology 62:1504–1518.
1902 P. M. Anderson et al.
Gelder MG, Lopez-Ibor J, Andreasen NC (2000) New oxfordtextbook of psychiatry. New York, NY: Oxford UniversityPress.
Gordon E, Williams LM, Haig AR, Bahramali H, Wright J,Meares R (2001) Symptom profile and ‘gamma’ processing inschizophrenia. Cogn Neuropsychiatry 6:2001.
Gross J, Schnitzler A, Timmermann L, Ploner M (2007) Gammaoscillations in human primary somatosensory cortex reflectpain perception. PLoS Biol 5:e133.
Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J,Salzberg M, O’Brien TJ, Pinault D (2009) NMDA receptorhypofunction leads to generalized and persistent aberrantgamma oscillations independent of hyperlocomotion and thestate of consciousness. PLoS ONE 4:e6755.
Harrison PJ (1999) The neuropathology of schizophrenia.A critical review of the data and their interpretation. Brain 122(Pt 4):593–624.
Herrmann CS, Demiralp T (2005) Human EEG gammaoscillations in neuropsychiatric disorders. ClinNeurophysiology 116:2719–2733.
Hetem LAB, Danion JM, Diemunsch P, Brandt C (2000) Effectof a subanesthetic dose of ketamine on memory andconscious awareness healthy volunteers. Psychopharmacology152:283–288.
Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J,Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ(2003) Gamma oscillations correlate with working memoryload in humans. Cereb Cortex 13:1369–1374.
Imre G (2007) The preclinical properties of a novel group IImetabotropic glutamate receptor agonist LY379268. CNS DrugRev 13:444–464.
Jones NC, Reddy M, Anderson P, Salzberg MR, O’Brien TJ,Pinault D (2012) Acute administration of typical and atypicalantipsychotics reduces EEG gamma power, but only thepreclinical compound LY379268 reduces the ketamine-inducedrise in gamma power. Int J Neuropsychopharmacol15:657–668.
Kapur S, Seeman P (2002) NMDA receptor antagonists ketamineand PCP have direct effects on the dopamine D(2) andserotonin 5-HT(2)receptors-implications for models ofschizophrenia. Mol Psychiatry 7:837–844.
Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN (2003)Antipsychotic dosing in preclinical models is oftenunrepresentative of the clinical condition: a suggestedsolution based on in vivo occupancy. J Pharmacol Exp Ther305:625–631.
Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN,O’Donnell BF (2009) Steady state and induced auditorygamma deficits in schizophrenia. Neuroimage 47:1711–1719.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R,Bremner JD, Heninger GR, Bowers MB Jr., Charney DS (1994)Subanesthetic effects of the noncompetitive NMDA antagonist,ketamine, in humans. Psychotomimetic, perceptual, cognitive,and neuroendocrine responses. Arch Gen Psychiatry51:199–214.
Kulikova SP, Tolmacheva EA, Anderson P, Gaudias J, Adams BE,Zheng T, Pinault D (2012) Opposite effects of ketamine anddeep brain stimulation on rat thalamocortical informationprocessing. Eur J Neurosci 36:3407–3419.
Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW,Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME,McCarley RW (1999) Gamma frequency-range abnormalities
to auditory stimulation in schizophrenia. Arch Gen Psychiatry56:1001–1005.
Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ,Finkel LH, Siegel SJ (2010) Ketamine modulates theta andgamma oscillations. J Cogn Neurosci 22:1452–1464.
Lee KH, Williams LM, Haig A, Gordon E (2003) “Gamma (40Hz)phase synchronicity” and symptom dimensions inschizophrenia. Cogn Neuropsychiatry 8:57–71.
Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J,Swerdlow NR, Braff DL (2006) Gamma band oscillationsreveal neural network cortical coherence dysfunction inschizophrenia patients. Biol Psychiatry 60:1231–1240.
Lorrain DS, Schaffhauser H, Campbell UC, Baccei CS, Correa LD,Rowe B, Rodriguez DE, Anderson JJ, Varney MA,Pinkerton AB, Vernier JM, Bristow LJ (2003) Group II mGlureceptor activation suppresses norepinephrine release in theventral hippocampus and locomotor responses to acuteketamine challenge. Neuropsychopharmacology 28:1622–1632.
Lovinger DM, McCool BA (1995) Metabotropic glutamatereceptor-mediated presynaptic depression at corticostriatalsynapses involves mGLuR2 or 3. J Neurophysiol 73:1076–1083.
Maharajh K, Teale P, Rojas DC, Reite ML (2010) Fluctuation ofgamma-band phase synchronization within the auditorycortex in schizophrenia. Clin Neurophysiology 121:542–548.
Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G,Melson AK, Hershey T, Craft S, Olney JW (1999)Ketamine-induced NMDA receptor hypofunction as a modelof memory impairment and psychosis.Neuropsychopharmacology 20:106–118.
Olszewski M, Piasecka J, Goda SA, Kasicki S, Hunt MJ (2013)Antipsychotic compounds differentially modulatehigh-frequency oscillations in the rat nucleus accumbens: acomparison of first- and second-generation drugs. Int JNeuropsychopharmacol 16:1009–1020.
Paxinos G, Watson C (1998) The rat brain in stereotaxiscoordinates, 4th edn. USA: Academic Press, Inc.
Pinault D (2008) N-methyl D-aspartate receptor antagonistsketamine and MK-801 induce wake-related aberrant gammaoscillations in the rat neocortex. Biol Psychiatry 63:730–735.
Ramaekers JG, Louwerens JW, Muntjewerff ND, Milius H,de Bie A, Rosenzweig P, Patat A, O’Hanlon JF (1999)Psychomotor, cognitive, extrapyramidal, and affectivefunctions of healthy volunteers during treatment with anatypical (amisulpride) and a classic (haloperidol)antipsychotic. J Clin Psychopharmacol 19:209–221.
Saunders JA, Gandal MJ, Siegel SJ (2012) NMDA antagonistsrecreate signal-to-noise ratio and timing perturbations presentin schizophrenia. Neurobiol Dis 46:93–100.
Seeman P (2002) Atypical antipsychotics: mechanism of action.Can J Psychiatry 47:27–38.
Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF,Shenton ME, McCarley RW (2003) Abnormal neural synchronyin schizophrenia. J Neurosci 23:7407–7411.
Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA,Klump MC, Frumin M, Shenton ME, McCarley RW (2004)Neural synchrony indexes disordered perception andcognition in schizophrenia. Proc Natl Acad Sci U S A101:17288–17293.
Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME,McCarley RW (2009) Left auditory cortex gammasynchronization and auditory hallucination symptoms inschizophrenia. BMC Neurosci 10:85.
Chronic antipsychotics modulate γ oscillations 1903
Stone JM, Erlandsson K, Arstad E, Squassante L, Teneggi V,Bressan RA, Krystal JH, Ell PJ, Pilowsky LS (2008) Relationshipbetween ketamine-induced psychotic symptoms and NMDAreceptor occupancy: a [(123)I]CNS-1261 SPET study.Psychopharmacology (Berl) 197:401–408.
Symond MB, Harris AWF, Gordon E, Williams LM (2005)“Gamma synchrony” in first-episode schizophrenia: adisorder of temporal connectivity? Am J Psychiatry162:459–465.
Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J (1998)Induced gamma-band activity during the delay of a visualshort-term memory task in humans. J Neurosci 18:4244–4254.
Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M,Maurer K, Rodriguez E (2006) Dysfunctional long-rangecoordination of neural activity during Gestalt perception inschizophrenia. J Neurosci 26:8168–8175.
Uhlhaas PJ, Haenschel C, Nikolic D, Singer W (2008) The role ofoscillations and synchrony in cortical networks and theirputative relevance for the pathophysiology of schizophrenia.Schizophr Bull 34:927–943.
Veselinovic T, Schorn H, Vernaleken IB, Hiemke C, Zernig G,Gur R, Grunder G (2013) Effects of antipsychotic treatment oncognition in healthy subjects. J Psychopharmacol 27:374–385.
Williams LM, Whitford TJ, Nagy M, Flynn G, Harris AW,Silverstein SM, Gordon E (2009) Emotion-elicited gammasynchrony in patients with first-episode schizophrenia: aneural correlate of social cognition outcomes. J PsychiatryNeurosci 34:303–313.
Yoshino M, Sawada S, Yamamoto C, Kamiya H (1996) Ametabotropic glutamate receptor agonist DCG-IV suppressessynaptic transmission at mossy fiber pathway of the guinea pighippocampus. Neurosci Lett 207:70–72.
1904 P. M. Anderson et al.