Investigating trophic-level variability in Celtic Sea fish predators
Habitat selection by two predators in an urban area: The stone marten and red fox in Wrocław (SW...
-
Upload
independent -
Category
Documents
-
view
3 -
download
0
Transcript of Habitat selection by two predators in an urban area: The stone marten and red fox in Wrocław (SW...
O
Ha
La
b
c
6
a
ARAbA
KMVHPI
I
ue(necuotsFC‘ta
a
z
1h
Mammalian Biology 79 (2014) 71– 76
Contents lists available at ScienceDirect
Mammalian Biology
j ourna l ho me page: www.elsev ier .com/ locate /mambio
riginal Investigation
abitat selection by two predators in an urban area: The stone martennd red fox in Wrocław (SW Poland)
eszek Dudusa,∗, Andrzej Zalewskib, Olga Kozioła, Zbigniew Jakubieca, Nina Królc
Lower-Silesian Field Station, Institute of Nature Conservation, Polish Academy of Sciences, Podwale 75, 50-449 Wrocław, PolandMammal Research Institute, Polish Academy of Sciences, 17-230 Białowieza, PolandDepartment of Microbial Ecology and Environmental Protection, Institute of Genetics and Microbiology, University of Wroclaw, ul. Przybyszewskiego3/77, 51-148 Wrocław, Poland
r t i c l e i n f o
rticle history:eceived 21 February 2013ccepted 2 August 2013y Luca Corlattivailable online 12 September 2013
eywords:
a b s t r a c t
We analyzed the habitat use of stone martens and red foxes based on incidental observations withinthe urbanized zone of Wrocław, SW Poland. We compared proportional habitat use at observation siteswith randomly selected points and evaluated differences in distance to the water sources and to urbanboundaries. Habitat use by both species was different from what we had expected from random points.Stone martens used high-density housing more frequently than red foxes and that expected from randompoints and avoided open and industrial areas, whereas red foxes used housing estates significantly more
artes foinaulpes vulpesabitat useredation avoidancenterspecific competition
often than expected and avoided high-density housing. Both species used the other habitats accordingto their availability. Stone martens often selected habitats located closer to the city centre, whereas redfoxes often selected habitats closer to urban borders than expected. The distribution of red foxes andstone martens is influenced by several factors including the availability of shelter and food, as well asthe opportunity to move around undetected. Interspecific competition may also play an important rolein habitat selection. Stone martens seem to be better adapted to urbanized areas than red foxes.
sellsc
© 2013 Deutsche Gentroduction
The process of “urban sprawl”, in which urban centres encroachpon surrounding ecosystems, leads to the degradation of thesecosystems but in turn, creates new and free ecological nichesMcKinney, 2002). Some species may respond positively to theew urban conditions. These are usually generalist species that canxploit both urban and non-urban resources, but also some spe-ialist species that persists in remnant habitat fragments withinrban systems (Francis and Chadwick, 2012). Greater availabilityf food sources and limited competition and/or predation meanshat some species benefit from urbanization, evidenced by higherurvival rates (Horak and Lebreton, 1998; Ditchkoff et al., 2006;rancis and Chadwick, 2012) and population densities (Gloor, 2002;ontesse et al., 2004; Tomiałojc, 2011). These species are referred as
synurbic’, which is a subcategory of ‘synanthropic’ and only referso populations associated with urban areas (Luniak, 2004; Francis
nd Chadwick, 2012).On the other hand, the new conditions created in urbanreas (e.g. human disturbance, street lighting, habitat loss and
∗ Corresponding author. Tel.: +48 781746978; fax: +48 12 632 24 32.E-mail addresses: [email protected] (L. Dudus),
[email protected] (A. Zalewski), [email protected] (N. Król).
616-5047/$ – see front matter © 2013 Deutsche Gesellschaft fü r Sä ugetierkunde. Publisttp://dx.doi.org/10.1016/j.mambio.2013.08.001
haft fü r Sä ugetierkunde. Published by Elsevier GmbH. All rights reserved.
modification, and increased exposure to domestic predators)have forced urban populations of some species to modify theirbehaviour, ecology and physiology in order to adapt to urbanstresses (Luniak, 2004; Ditchkoff et al., 2006). The most char-acteristic adjustments of synurbic populations include changedforaging behaviour, smaller home ranges leading to higher pop-ulation densities, longer breeding seasons, increased tolerance tohuman presence and the ability to exploit anthropogenic structuresas resting and breeding sites (Jakubiec-Benroth and Jakubiec, 2001;Gloor, 2002; Baker and Harris, 2004; Bozek et al., 2007; Herr et al.,2008; Bateman and Fleming, 2012; Francis and Chadwick, 2012).Species respond to environmental changes caused by urbanizationdifferently: “urban exploiters” are almost completely dependenton human subsidies and occur more frequently inside than out-side cities; “urban adapters” can utilize human subsidies withinsuburban areas but also predominantly rely on natural resourcesoutside developed areas; “urban avoiders” are very sensitive to thechanges in habitat caused by urbanization and are the first to disap-pear from urbanizing areas (Blair, 2001; McKinney, 2002). A betterunderstanding of how certain species use urbanized areas is likelyto aid our management and conservation of animals in both urban
and natural habitats.One group of animals that adapt well to urban environmentsare mammalian predators, many species of which have colonizedurban areas. These include small- to medium-sized mammals
hed by Elsevier GmbH. All rights reserved.
7 lian Biology 79 (2014) 71– 76
(l2viaou1ppmbt(
cacT(bpaf4cf2itprbe2
aWfsafrteaf1
M
(sotobocfpil
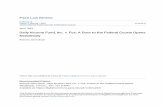
Fig. 1. Distribution of stone marten (Martes foina) (circles) and red fox (Vulpes
2 L. Dudus et al. / Mamma
Gloor, 2002; Randa and Yunger, 2006; Herr, 2008), but alsoarge-sized mammals (Rauer et al., 2003; Beckmann and Lackey,008). The colonization of urban environments by several carni-ore species may lead to strong interaction between them such asntraguild predation and competition (Hunter, 2009). These inter-ctions may affect the abundance, habitat selection, socio-spatialrganization and behaviour of the species that are being preyedpon or excluded (Voigt and Earle, 1983; Palomares and Caro,999; Randa and Yunger, 2006; Harrison et al. 2011). For exam-le, intraguild predation from red foxes (Vulpes vulpes) decreasesopulation densities of pine marten (Martes martes) and stoneartens (Martes foina) leading to the avoidance of certain habitats
y martens (Martes spp.) where there is a high risk of preda-ion, even if these habitats have abundant fundamental resourcesLindström et al., 1995; Papakosta et al., 2010).
Stone martens and red foxes are common in some Europeanities (Gloor, 2002; Herr, 2008). Their ecological flexibility hasllowed them to integrate into the urban environment and asso-iated food webs (Bissonette and Broekhuizen, 1995; Gloor, 2002;óth et al., 2009). The stone marten is medium size predatormean body mass: 1.7 kg, range: 1–2.5 kg; Buchalczyk, 1984), capa-le of climbing trees and walls. It is an omnivore and can uselant food besides preying on rodents, birds and insects whenvailable (Genovesi et al., 1996; Posłuszny et al., 2007). The redox is larger than stone marten (mean body mass: 6 kg, range:.7–8.7 kg; Buchalczyk, 1984), and is an omnivore mesopredatorapable of utilizing various types of food mainly rodents, birds,ruits or human refuse (Contesse et al., 2004; Papakosta et al.,010). At present both species inhabit urbanized habitats of var-
ous kinds (e.g. parks, housing estates, industrial areas) withinheir geographic range. However, little is known about the com-etition and predation interaction between stone marten anded fox in a single city and how this affects habitat selection byoth species (Cignini and Riga, 1997; Contesse et al., 2004; Salekt al., 2005; Marks and Bloomfield, 2006; Herr, 2008; Tóth et al.,009).
The objective of this study was to compare the distribution ofnd habitat use by stone martens and red foxes in the urban area ofrocław. Given the morphological, ecological and behavioural dif-
erences between these two species, we predict that stone martenshould select areas with high densities of buildings due to theirbility to use these as shelter (Herr et al., 2008). In contrast, redoxes should select low-density housing and green areas in whiched fox-human interactions are more limited and opportunitieso remain undetected are higher (Gloor, 2002). Furthermore, wexpect that the smaller-bodied mesopredator (stone marten) willvoid the habitat occupied by the larger-bodied mesopredator (redox) because of the risk of intraguild predation (Palomares and Caro,999).
aterial and methods
We gathered incidental data on the presence of stone martensN = 171 observations) and red foxes (N = 133 observations) in thetudy area between 2000 and 2010 (Fig. 1). Data on incidentalbservations were obtained from several sources: the interven-ion log of the local dog kennel (56 stone marten and 96 red foxbservations), questionnaires disseminated to local naturalists andiologists (42 stone marten and 32 red fox observations) and ourwn observations (16 stone marten and 5 red fox observations). Weollected additional information on the presence of stone martens
rom road kills (20), trapping (13) and latrine locations (25). Torevent repetition of observations, we used only one observationn cases when species were observed several times in the sameocality in the same year.
vulpes) (squares) observations in the urbanized area of Wrocław. The solid line marksthe border of the study area and grey colour denotes water.
All the locations were incorporated on a geographic informa-tion system in ArcMap (ver. 9.2; ESRI, Redlands, CA, USA). Weprepared a land cover map based on aerial photographs of thestudy area in which eight land covers were delimited (Table 1).To compare the habitat selection of both species we compared theproportion of habitat types used by predators with the proportionof habitat types available. To estimate habitat availability withinthe overall city area we generated 600 random points within thestudy environments, excluding water, using the ArcMap RandomPoint Generator. Furthermore, we created buffers with a radius of25 m around all the stone marten and red fox observation pointsto determine the proportion of habitat types used by predatorsand around random points to determine the proportion of avail-able habitat types. We used a 25 m radius plot (0.002 km2) tominimize overlap with other locations and cover only the near-est neighbourhood of the animal locations. Using large plots wouldinclude areas that were not used by animals (this is especiallythe case with observations made close to the edge of the habi-tat type where animals were observed), whereas using only theposition of each animal observation would relate to where theanimal was sighted rather than to the habitat used by the preda-tor. The total area of the buffers around the stone marten, red foxand random points was 0.33 km2, 0.26 km2 and 1.17 km2, respec-tively. We calculated the proportion of each habitat type per bufferand the overall proportion across all buffers of the used and avail-able habitat types. We then compared the proportion of the givenhabitat from buffers created around predator locations (observedproportion) in relation to the proportion of the habitat from bufferscreated around random points (expected proportion) using theChi-square test for goodness-of-fit (Zar, 1999). Next, we estimatedhabitat selection (preference or avoidance) by comparing habi-tat use and availability using Bonferroni 95% confidence intervals(Neu et al., 1974). To calculate differences in habitat use betweenred foxes and stone martens we compared the 95% confidenceintervals for the two species. In these calculations we excluded“water”, as no animals were observed in this environment and
because the amount of water in the buffer habitats was in anycase very small. We obtained only three stone marten observa-tions from open areas and used William’s correction (Zar, 1999),L. Dudus et al. / Mammalian Biology 79 (2014) 71– 76 73
Table 1Description of the habitat types in the study area in Wrocław (SE Poland).
Habitat type Description of habitat type Area (km2) Percentage Percentage of area covered by Humanpopulationper km2
Buildings Roads Trees Othera
Industrial areas Factories, warehouses, power stations,production facilities, industrial plants,transhipment stations and riverharbour
15.3 17 36.7 8.1 6.8 48.4 0
High-densityhousing
Multi-storey (max. 5 floors) oldtenement houses and some infillbuildings along streets with an innercourtyard, shopping centres, officebuildings
15.3 16 36.5 15.4 10.5 37.6 6300
Housing estates Detached, semidetached and terracedhouses, with numerous fruit plants andtrees in backyards
14.4 16 19.9 8.1 25.6 46.4 4300
High-riseresidential areas
Detached and terraced high buildings(from 4 to 16 floors) made of concretepanels, without attics
13.3 14 16.5 11.4 20.1 52.0 12,100
Allotment gardens Private gardens with many fruit trees,bushes and vegetables patches, closedduring night, without residentialbuildings
10.0 11 5.4 3.4 25.4 65.8 0
Green areas Tree-covered areas: parks, cemeteries,the Zoo and the Botanical Garden
10.8 12 0.5 1.8 75.5 22.2 0
Open areas Grasslands, fields, meadows, car parks,waste grounds and floodplains
10.5 11 0.2 0.2 6.7 92.9 0
Water Rivers, canals, an old moat, river docksand numerous small ponds
0.3 3
a ing ve
b(atattym
S
Wmmp2bm(cubfdailwaaA(
‘Other’ represents the percentage of area covered by open areas with low-grow
ecause pooling categories were incompatible with our objectivescomparing habitat selection by stone marten and red fox). Welso evaluated differences in distribution between species loca-ions and random points by distances to the closest water sourcend to the study site borders using nonparametric Mann–Whitneyest. Data sets were tested for normality using the Shapiro–Wilkest. The STATISTICA 8.0 package was used for all statistical anal-ses. Whenever averages were referred to they were presented asean ± SD.
tudy area
Located in south-western Poland (17◦02′16′′ E, 51◦07′56′′ N),rocław is the fourth most populous city in the country. Theean annual temperature during 2000–2010 was 9.8 ◦C (meanonthly temperature range: −3.6 to 25 ◦C) and the mean annual
recipitation was 578 mm (mean monthly precipitation range:30–1580 mm) (Komarowska, 2010). Wrocław has 623,240 inha-itants and has an area of 293 km2. This study covered the oldest,ost developed and most densely populated part of the city
92 km2). The basic structure and composition of this part of theity did not change during the study period as it was already highlyrbanized (Hawrylak and Hawrylak 2010). We defined the urbanorder of Wrocław (the outline of the study area, Fig. 1) as the lineollowing the edge of contiguous built-up area and other habitatsirectly associated with human activities (e.g. cemeteries, parks,llotment gardens). Beyond the study area, the urban habitat wasnterspersed with rural and suburban habitats like villages, farm-and, grassland, natural forests and adjoining housing estates that
ere under construction during the study period. In the study
rea we distinguished eight urban habitats with different cover-ge of buildings and trees and different human densities (Table 1).mong these habitats there were three types of residential areahigh-density housing, housing estates, high-rise residential areas;
getation or without vegetation.
see Table 1 for details), offering different ecological conditions(availability of food and shelter and disturbance from humans) toanimals.
Results
We found a significant difference between the habitat cate-gories around random points and the habitats surrounding thelocations of stone martens (�2 = 62.07, df = 6, P < 0.001) and redfoxes (�2 = 21.03, df = 6, P = 0.001). Stone martens used high-densityhousing more frequently than expected and avoided open areasthe most, followed by industrial areas. Housing estates, greenareas, allotment gardens and high-rise residential areas were usedaccording to their availability (listed in order of usage). Red foxesused housing estates significantly more often than expected andavoided high-density housing. Industrial areas, high-rise residen-tial areas, parks, allotment gardens and open areas were usedby red foxes according to availability (listed in order of usage)(Table 2).
Habitat use was also found to differ significantly between redfoxes and stone martens (�2 = 31.33, df = 6, P = 0.001). Stone martenswere observed significantly more often in high-density housingthan red fox, which avoided this type of habitat. In the other habitatsboth species were observed with similar frequency.
The average distance to water was 368.5 ± 255.5 m (N = 171)for stone martens, 350.4 ± 271.8 m (N = 133) for red foxes and392.3 ± 185.6 m for random points. There were no significant differ-ences in the distances to the closest water source between the twospecies (U = 10,636.5, Z = 0.9667, P = 0.336). However, both stonemartens and red foxes were located significantly closer to water
than the random points (for stone marten U = 14,786, Z = −2.2,P = 0.02; for red fox U = 10,343, Z = −3.4, P < 0.001).The average distance to urban borders was 1736.1 ± 952.5 m forstone martens, 1052.1 ± 671 m for red foxes and 1251.2 ± 663.5 m
74 L. Dudus et al. / Mammalian Biology 79 (2014) 71– 76
Table 2Habitat selection by stone martens and red foxes in the urbanized area of Wrocław between 2000 and 2010. For a description of the habitats, see Table 1.
Habitat type Area(km2)
Proportion ofavailablehabitatestimated fromrandom points
Stone marten Red fox
Na Confidenceintervals(lower-upper)b
Selectionc Rank ofhabitats
Na Confidenceintervals(lower-upper)b
Selectionc Rank ofhabitats
High-riseresidentialareas
13.3 0.14 22 0.06–0.20 0 5 21 0.07–0.23 0 3
Allotmentgardens
10.0 0.10 12 0.01–0.12 0 4 12 0.02–0.15 0 5
Open areas 10.5 0.13 3 0.06–0.10 − 7 12 0.02–0.14 0 6Green areas 10.8 0.14 20 0.06–0.19 0 3 18 0.05–0.21 0 4Industrial areas 15.3 0.17 13 0.02–0.13 − 6 21 0.08–0.25 0 2Housing estates 14.4 0.14 38 0.14–0.31 0 2 33 0.16–0.37 + 1High-density
housing15.3 0.18 63 0.26–0.46 + 1 16 0.04–0.176 − 7
a Number of observations in each habitat type. pred
ft(PuZ
D
WchwbwawtbfOpuiyiih
saikm1cpilop
b 95% Bonferroni confidence intervals represent the proportion of habitat used byc “0”, no selection; “+”, positive selection; “−”, negative selection (avoidance).
or random points. Stone martens were located significantly fur-her from urban borders (closer to the city centre) than red foxesU = 6603, Z = 6.3, P < 0.001) and random points (U = 12,024, Z = 4.9,
< 0.001). In contrast, red foxes were located significantly closer torban borders than stone martens and random points (U = 11,033,
= 2.6, P = 0.008).
iscussion
Stone martens and red foxes are found throughout the city ofrocław but their distribution is uneven. Stone martens prefer the
ity centre, far from the urban border, whereas red foxes preferousing estates and underdeveloped areas close to the city borderith a low human population density. Our results may be biased
y the uneven numbers of observers in different habitats whichere higher in densely populated areas. Assuming that the prob-
bility of seeing red foxes and stone martens in different habitatsas relatively similar (the same number of residents, similar noc-
urnal activity of both species), the bias will have been similar foroth species. We were therefore able to compare the habitat pre-erences of the two species in every major type of urban habitat.ur results displayed opposing trends in both species, for exam-le, in the preference for high-density housing and distance to therban border. Another factor which may have affected our results
s the fluctuation in stone marten and red fox densities over 10ears; however no data are available on predator density dynamicsn Wrocław or other cities. Nevertheless, the number of sightingsn each year was low, so the inter-year variation in density shouldave only a limited influence on habitat selection.
We consider that the main factors influencing distribution oftone marten and red fox are predation or persecution risk andccess to resources (food, rest and breeding sites). The possibil-ty of remaining concealed during resting and activity periods isnown to be an important factor limiting the distribution of stoneartens and red foxes (Sacchi and Meriggi, 1995; Saunders et al.,
997; Gloor, 2002; Rondinini and Boitani, 2002). However, theonstant presence of people in built-up areas may not limit theresence of stone martens if these areas provide suitable cover. For
nstance, high-density housing was the second most densely popu-ated habitat in our study area, yet it was still the preferred habitatf stone martens. This preference has been observed in other Euro-ean cities (Salek et al., 2005; Tóth et al., 2009). In this habitat
ator.
stone martens move from house to house through attics and onroofs (Dudus, personal observation). The stone marten’s nocturnalbehaviour, small size and ability to climb may therefore explaintheir apparent tolerance of densely populated urban areas. Pre-sumably it is because they go unnoticed by humans, so draw lessattention.
In turn red foxes avoid the most densely populated areasbecause they have limited possibilities of remaining undetected inthese environments (Gloor, 2002). In addition red foxes are consid-ered vectors of zoonotic diseases and predators of domestic animals(e.g. pets, poultry), therefore there is a lot of public persecutionagainst them (Bontadina et al., 2001; König, 2008). The presenceof humans is therefore the main factor preventing red foxes frombecoming established in cities. To reduce the probability of beingnoticed, red foxes prefer areas on the outskirts of densely popu-lated, urbanized zones.
The establishment of both species in urbanized areas is alsorestricted by the availability of suitable places for diurnal and nataldens. Stone marten resting sites and dens are located almost exclu-sively in buildings, especially in attics or roof spaces (Herr et al.,2008). Therefore, parts of the city such as high-density housingand housing estates potentially provide the most suitable sites ofall the urban habitat types, where there are a large number ofunfrequented attics. These structures offer good thermal insulationduring winter, minimal disturbance and protection from predators(Herr et al., 2008). Red foxes have their diurnal shelters and nataldens almost exclusively in burrows or dense vegetation (Saunderset al., 1997; Marks and Bloomfield, 2006). Back gardens in residen-tial districts, despite being the most frequently selected habitat byred foxes, are not suitable places for natal dens because they areexposed and may be destroyed by the landowner for fear that theanimals may attack children or pets. In this study we found onlyone natal red fox den in a back garden on an abandoned property,but it was soon destroyed by the new owner. Red foxes are ableto travel long distances each night (Goszczynski, 1989) and maymove between different habitats in a short time, depending on thedistribution of diurnal and natal dens, human presence and foodaccessibility.
Stone martens and red foxes are omnivores, capable of consum-ing diverse types of food (Genovesi et al., 1996; Contesse et al.,2004; Posłuszny et al., 2007). In urban areas, anthropogenic food isthe main component of the red fox’s diet (Doncaster et al., 1990;
lian B
Cbttaf(aipppe
mrslrs2ieoLffc(epom
afcmfsos1hbaatmls
A
paflco
R
B
L. Dudus et al. / Mamma
ontesse et al., 2004) whereas fruit are the main items consumedy stone martens (Holisová and Obrtel, 1982). Stone martens, withheir small size and climbing ability, are likely to have greater accesso diverse food supplies than red foxes. Stone martens can hunt inttics, on roofs and trees, eat fruit directly in tree crowns or searchor food in dustbins. Red foxes can only forage at ground levelwindfalls, composters, food provided for pets) and have limitedccess to dustbins. The red foxes’ preference for housing estatess due to the foraging opportunities presented by gardens, whichrovide an abundant supply of fruit, waste in composters and foodut out for pets, birds and other animals. Our observations sup-ort those of Gloor (2002), who found that red foxes in Zurich onlyntered residential areas for foraging.
Interestingly, housing estates were not preferred by stoneartens. A similar type of habitat in villages provides very good
esting sites and large amounts of various food types, and thetone marten density is very high there (A. Zalewski, unpub-ished data). This may be explained by the avoidance of largered fox. Larger predators may kill smaller ones if they use theame resources (Palomares and Caro, 1999; Donadio and Buskrik,006; Hunter, 2009; Hall, 2011). There is a considerable overlap
n the diets of red foxes and stone martens (Brangi, 1995; Padialt al., 2002; Papakosta et al., 2010). Interspecific killings have beenbserved between pine martens and red foxes (Storch et al., 1990;indström et al., 1995), American mink (Neovison vison) and redoxes (Carlsson et al., 2010), or stoats (Mustela erminea) and redoxes (Mulder, 1990). In all of these cases there was a negativeorrelation between the abundance of small and large predatorsMulder, 1990; Storch et al., 1990; Lindström et al. 1995; Carlssont al., 2010). In response to the higher predation risk, the meso-redator may change its use of space or activity patterns. The riskf interspecific killings by red foxes in housing estates in Wrocławight also affect habitat selection by stone martens there.In summary, the distribution of red foxes and stone martens
ppears to be influenced by several factors, including shelter andood availability, the possibility of moving around unseen, interspe-ific competition, or human attitudes. Our results show that stoneartens appear to be better adapted to urbanized areas than red
oxes. Due to their small size, climbing ability and omnivorous diet,tone martens have been able to colonize the most urbanized partf the city, which is devoid of larger carnivores but rich in food. Thispecies colonized Wrocław long before red foxes did so (Göppert,857; Jakubiec-Benroth and Jakubiec, 2001); hence, they will havead more time to adapt to constant human presence. Red foxeseing larger than stone martens, and with their terrestrial lifestylend bad reputation, tend to remain on the outskirts of urbanizedreas, which provide cover against people. Red foxes may overcomeheir fear of humans in time and the persecution for stone marten
ay increase (e.g. because of damage to car engines or roof insu-ation) which could lead to changes in distribution and populationize of both species in urban ecosystem.
cknowledgements
We thank all the persons who provided information aboutredator locations, especially the staff at the local dog kennels. Were grateful to A.V. Stronen, P. Senn and two anonymous reviewersor the English corrections and useful critical comments on an ear-ier draft. Special thanks go to M. Slimakowska for her considerableontribution to this work. This study was financed by the Ministryf Science and Higher Education (Grant No. NN 304049734).
eferences
aker, P.J., Harris, S., 2004. Red foxes. The behavioral ecology of red foxes in urbanBristol. In: MacDonald, D.W., Sillero-Zubiri, C. (Eds.), Biology and Conservationof Wild Canids. Bloomsbury Publishing Inc, London, pp. 207–216.
iology 79 (2014) 71– 76 75
Bateman, P.W., Fleming, P.A., 2012. Big city life: carnivores in urban environments.J. Zool. 287, 1–23.
Beckmann, J.P., Lackey, C.W., 2008. Carnivores, urban landscapes, and longitudinalstudies: a case history of black bears. Hum. Wildl. Confl. 2, 168–174.
Bissonette, J.A., Broekhuizen, S., 1995. Martens populations as indicators of habi-tat spatial patterns: the need of multiscale approach. In: Lidicker, W.Z. (Ed.),Landscape Approaches in Mammalian Ecology and Conservation. University ofMinnesota Press, Minnesota, pp. 95–121.
Blair, R., 2001. Birds and butterflies along urban gradients in two ecoregions of theU.S. In: Lockwood, J.L., McKinney, M.L. (Eds.), Biotic Homogenization. Kluwer,New York, pp. 33–56.
Bontadina, F., Contesse, P., Gloor, S., 2001. How does personal involvement influenceattitudes towards urban foxes? For. Snow Landsc. Res. 76, 255–266.
Bozek, C.K., Prange, S., Gehrt, S.D., 2007. The influence of anthropogenic resourceson multi-scale habitat selection by raccoons. Urban Ecosyst. 10, 413–425.
Brangi, A., 1995. Seasonal changes of trophic niche overlap in the stone marten(Martes foina) and the red fox (Vulpes vulpes) in a mountainous area of theNorthern Apennines (N-Italy). Hystrix 7, 113–118.
Buchalczyk, T., 1984. Rzad: Drapiezne–Carnivora (Order: Carnivora). In: Pucek, Z.(Ed.), Klucz do oznaczania ssaków Polski (A Key to Identify Mammals of Poland).PWN, Warszawa, pp. 256–310.
Carlsson, N.O.L., Jeschke, J.M., Holmqvist, N., Kindberg, J., 2010. Long-term dataon invaders: when the fox is away, the mink will play. Biol. Invasions 12,633–641.
Cignini, B., Riga, F., 1997. Red fox sightings in Rome. Hystrix 9, 1–74.Contesse, P., Hegglin, D., Gloor, S., Bontadina, F., Deplazes, P., 2004. The diet of urban
foxes (Vulpes vulpes) and the availability of anthropogenic food in the city ofZurich, Switzerland. Mamm. Biol. 69, 81–95.
Ditchkoff, S., Saalfeld, S., Gibson, C., 2006. Animal behavior in urban ecosystems:modifications due to human-induced stress. Urban Ecosyst. 9, 1–12.
Donadio, E., Buskrik, S.W., 2006. Diet, morphology, and interspecific killing in Car-nivora. Am. Nat. 167, 534–536.
Doncaster, C.P., Dickman, C.R., Macdonald, D.W., 1990. Feeding ecology of red foxes(Vulpes vulpes) in the city of Oxford, England. J. Mamm. 71, 188–194.
Francis, R.A., Chadwick, M.A., 2012. What makes a species synurbic? Appl. Geogr.32, 514–521.
Genovesi, P., Secchi, M., Boitani, L., 1996. Diet of stone martens: an example ofecological flexibility. J. Zool. 238, 545–555.
Gloor, S., 2002. The Rise of Urban Foxes (Vulpes vulpes) in Switzerland and Ecologicaland Parasitological Aspects of a Fox Population in the Recently Colonised City ofZurich. Universität Zürich, Zürich (PhD thesis).
Goszczynski, J., 1989. Population dynamics of the red fox in central Poland. ActaTheriol. 34, 141–154.
Göppert, H.R., 1857. Der Königliche botanische Garten der Universität Breslau (TheRoyal Botanical Garden of Wroclaw University). Heyn’sche Buchhandlung, Göer-litz.
Hall, R.J., 2011. Intraguild predation in the presence of a shared natural enemy.Ecology 92, 352–361.
Harrison, W.S.R., Cypher, B.L., Bremner-Harrison, S., Job, C.L.V.H., 2011. Resource useoverlap between urban carnivores: implications for endangered San Joaquin kitfoxes (Vulpes macrotis mutica). Urban Ecosyst. 14, 303–311.
Hawrylak, M., Hawrylak, P., 2010. Gospodarka przestrzenna. In: Lewicki, Z.(Ed.), Srodowisko Wrocławia (Environment of Wrocław). ATUT, Wrocław, pp.62–76.
Herr, J., 2008. Ecology and Behavior of Urban Stone Martens (Martes foina) in Lux-embourg. University of Sussex, Brighton (PhD thesis).
Herr, J., Schley, L., Engel, E., Roper, T.J., 2008. Den preferences and denning behaviorin urban stone martens (Martes foina). Mamm. Biol. 75, 138–145.
Holisová, V., Obrtel, R., 1982. Scat analytical data on the diet of urban stone martens,Martes foina (Mustelidae, Mammalia). Folia Zool. 31, 21–30.
Horak, P., Lebreton, J.D., 1998. Survival of adult Great Tits Parus major in relationto sex and habitat; a comparison of urban and rural populations. Ibis 140,205–209.
Hunter, M.D., 2009. Trophic promiscuity, intraguild predation and the problem ofomnivores. Agric. Forest Entomol. 11, 125–131.
Jakubiec-Benroth, D., Jakubiec, Z., 2001. Synanthropisation of the red fox Vulpesvulpes in Wrocław. Przegl. Zool. 55, 21–126.
Komarowska, D., 2010. Rocznik Miasta Wrocławia 2010 (Statistical yearbook ofWrocław city 2010). Statistical Office in Wrocław – Printing Department, Leg-nica.
König, A., 2008. Fears, attitudes and opinions of suburban residents with regards totheir urban foxes. Eur. J. Wildlife. Res. 54, 101–109.
Luniak, M., 2004. Synurbization–adaptation of animal wildlife to urban develop-ment. In: Proceedings of the 4th International Urban Wildlife Symposium,Tucson, Arizona, pp. 50–55.
Lindström, E.R., Brainerd, S.M., Helldin, J.O., Overskaug, K., 1995. Pine marten–redfox interactions: a case of intraguild predation? Ann. Zool. Fenn. 32,123–130.
Marks, C.A., Bloomfield, T.E., 2006. Home-range size and selection of natal den anddiurnal shelter sites by urban red foxes (Vulpes vulpes) in Melbourne. Wild. Res.33, 339–347.
McKinney, M.L., 2002. Urbanization, biodiversity, and conservation. Bioscience 52,883–890.
Mulder, J.L., 1990. The stoat Mustela erminea in the Duch dune region, its localextinction, and possible cause: the arrival of the red fox Vulpes vulpes. Lutra33, 1–21.
7 lian B
N
P
P
P
P
R
R
6 L. Dudus et al. / Mamma
eu, C.W., Byer, C.R., Peek, J.M., 1974. A technique for analysis of utilization-availability data. J. Wild. Manage. 38, 541–545.
adial, J., Ávila, E., Gil-Sánchez, J.M., 2002. Feeding habits and overlap among red fox(Vulpes vulpes) and stone marten (Martes foina) in two Mediterranean mountainhabitats. Mamm. Biol. 67, 137–146.
alomares, F., Caro, T.M., 1999. Interspecific killing among mammalian Carnivores.Am. Nat. 153, 492–508.
apakosta, M., Vlachos, C., Bakaloudis, D., Kitikidou, K., Chatzinikos, E., 2010. Dietaryoverlap among seasons and habitats of red fox and stone marten in CentralGreece. Eur. J. Sci. Res. 45, 122–127.
osłuszny, M., Pilot, M., Goszczynski, J., Gralak, B., 2007. Diet of sympatric pinemarten (Martes martes) and stone marten (Martes foina) identified by genotypingof DNA from faeces. Ann. Zool. Fenn. 44, 269–284.
anda, L., Yunger, J., 2006. Carnivore occurrence along an urban–rural gradient: alandscape-level analysis. J. Mamm. 87, 1154–1164.
auer, G., Kaczensky, P., Knauer, F., 2003. Experiences with aversive conditioningof habituated brown bears in Austria and other European Countries. Ursus 14,215–224.
iology 79 (2014) 71– 76
Rondinini, C., Boitani, L., 2002. Habitat use by beech martens in a fragmented land-scape. Ecography 25, 257–264.
Sacchi, O., Meriggi, A., 1995. Habitat requirements of the stone marten (Martes foina)on the Tyrrhenian slopes of the northern Apennines. Hystrix 7, 99–104.
Salek, M., Sicova, P., Sedlacek, F., 2005. Stone martens Martes foina in urban environ-ment: abundance and distribution. Lynx 36, 111–116.
Saunders, G., White, P.C.L., Harris, S., 1997. Habitat utilisation by urban foxes (Vulpesvulpes) and the implications for rabies control. Mammalia 61, 497–510.
Storch, I., Lindstrom, E., De Jounge, J., 1990. Diet and habitat selection of the pinemarten in relation to competition with the red fox. Acta Theriol. 35, 311–320.
Tomiałojc, L., 2011. Changes in breeding bird communities of two urban parks inWrocław across 40 years (1970–2010): before and after colonization by impor-tant predators. Ornis Polonica 52, 1–25.
Tóth, M., Bárány, A., Kis, R., 2009. An evaluation of stone marten records in the cityof Budapest, Hungary. Acta Zool. Hung. 55, 199–209.
Voigt, D.R., Earle, B.D., 1983. Avoidance of coyotes by red fox families. J. Wild. Man-age. 47, 852–857.
Zar, J.H., 1999. Biostatistical Analysis, 4th edn. Prentice Hall Inc, London.