Final Exam solution - Prexams.com
-
Upload
khangminh22 -
Category
Documents
-
view
1 -
download
0
Transcript of Final Exam solution - Prexams.com
Introduction To Materials Science, Chapter 1, Introduction
University of Virginia, Dept. of Materials Science and Engineering 18
Polymer composite materials:
reinforcing glass fibers in a
polymer matrix.
Composites
Introduction to Materials Science, Chapter 15, Polymer Structures
University of Virginia, Dept. of Materials Science and Engineering 24
Polymer Crystals
Thin crystalline platelets grown from solution - chains fold
back and forth: chain-folded model
Polyethylene
The average chain length is much greater than the
thickness of the crystallite
Introduction To Materials Science, Chapter 3, The structure of crystalline solids
University of Virginia, Dept. of Materials Science and Engineering 9
Body-Centered Cubic (BCC) Crystal Structure (I)
Atom at each corner and at center of cubic unit cell
Cr, !-Fe, Mo have this crystal structure
Spring 2006
Final ExamMonday, August 18, 2006
Duration: 2 hrs
Open Book Exam (Book and Class Slides only)
Write clearly your derivations and answers on the question sheet
Instructions:
1. Do not open this exam until instructed to do so.
2. Complete the name and identification portion on your sheet.
3. Put all answers on the this question sheet.
4. No questions are allowed during the duration of the exam. If you feel that assumptions are needed to solve a certain problem, state these assumption clearly in your answer.
Name:
ID#:
Engineering Material
Summer 2006
Problem 1 [20 Points]
1. Regarding steels, a larger carbon content generally means that: (1 point)
a. The steel will not be as expensive.
b. Ductility will probably be unaffected.
c. The strength will be larger.
d. a and c.
e. all of the above.
2. When quenching a piece of steel in water, for example, the surface portions of the steel
piece will generally: (1 point)
a. cool faster than interior portions
b. have more ductility than interior portions
c. have a smaller hardness than interior portions
d. be unusable.
3. Suppose that Li2O is added as an impurity to MgO. If Li+ substitutes for Mg2+, what defects would you expect to form if several Li2O molecules are added? (1 point)
a. Li vacancies
b. Mg vacancies
c. O vacancies.
4. You want to increase the tensile strength of a semi-crystalline polymer such as polyethylene. Which of the following will increase tensile strength? (1 point)
a. a decrease in molecular weight
b. an increase in % crystallinity
c. fatigue loading of the polymer
d. a decrease in cross linking.
5. Below is a diagram of an edge dislocation. State which region in the diagram is expected to have a tensile stress. (1 point)
a. region A
b. region B
c. both A and B
d. none of the above.
Mech340: Engineering Material 2/9
!"#$%&'())*((+(,(-(
+.( /%012("3(*(#"*4&*'(15(*6(%#4%(#"3017*$"16.((8$*$%(29"79(&%4"16("6($9%(#"*4&*'("3(%:;%7$%#($1(
9*<%(*($%63"0%(3$&%33.(
*.( &%4"16(=(
>.( &%4"16(/(
7.( >1$9(=(*6#(/(
#.( 616%(15($9%(*>1<%.(
((
?.( @9"79(15($9%(510012"64(2"00(ABC(&%3D0$("6(*6("67&%*3%("6(E"%0#(3$&%64$9F(
*.( &%#D7"64($9%(4&*"6(3"G%(
>.( *##"64("';D&"$E(*$1'3($1(51&'(*(310"#(310D$"16(
7.( 710#(21&H"64(
#.( &%7&E3$*00"G*$"16.(
(
I.( =(;10E7&E3$*00"6%(3;%7"'%6(2"$9(&*6#1'0E(1&"%6$%#(7&E3$*03("3(01*#%#("6(3"';0%($%63"16(*6#(
E"%0#("3(1>3%&<%#.((C&D%(1&(5*03%J((C9%("6#"<"#D*0(7&E3$*03("6($9%(3;%7"'%6(*00(E"%0#(*$($9%(3*'%(
$"'%(#D&"64($9%($%3$.(
*.( $&D%(
>.( 5*03%((
(
K.( @9*$("3(*(0"H%0E(1D$71'%(15(D3"64(*('%$*0(*001E(*$(*(9"49%&($%';%&*$D&%F(
*.( C9%(#D7$"0"$E("3(0"H%0E($1(#%7&%*3%.(
>.( L"3017*$"163('*E(61$(>%(*>0%($1('1<%.(
7.( C9%(E"%0#(3$&%64$9(*6#($%63"0%(3$&%64$9(*&%(0"H%0E($1(#%7&%*3%.(
(
M.( @9"79(15($9%(510012"64($%63"0%(>*&3("00D3$&*$%3($9%(5*"0D&%(15(*(>&"$$0%('*$%&"*0F(
*.( >*&(=(
>.( >*&(/.(
(( ( /*&(=( /*&(/(
(
N%4"16(=(
N%4"16(/(
Summer 2006
6. Which of the following will NOT result in an increase in yield strength? (1 point)
a. reducing the grain size
b. adding impurity atoms to form a solid solution
c. cold working
d. re-crystallization.
7. A polycrystalline specimen with randomly oriented crystals is loaded in simple tension and yield is observed. True or false: The individual crystals in the specimen all yield at the same time during the test. (1 point)
a. true
b. false
8. What is a likely outcome of using a metal alloy at a higher temperature? (1 point)
a. The ductility is likely to decrease.
b. Dislocations may not be able to move.
c. The yield strength and tensile strength are likely to decrease.
9. Referring to Figure 7.17, what range of cold working will result in a copper alloy with a minimum yield strength of 290 MPa and a minimum ductility of 5%EL? (1 point)
a. 10-20%CW
b. 20-30%CW
c. 30-40%CW
d. unable to determine from the given information.
10. Real engineering materials rarely reach the strength of perfect materials since (1 point)
a. there is rarely enough ionic bonding
b. engineering materials are not cold worked enough
c. the coordination number is too small
d. flaws cause premature failure.
11. You are given a lead-tin (Pb-Sn) alloy of composition 40 wt% Sn – 60 wt% Pb. The phase diagram is given in figure 9.7. As we cool a sample of the material from an initial temperature of 400˚C, at what temperature will the LAST bit of the liquid solidify? (1 point)
a. 327˚C
b. 250˚C
c. 232˚C
d. 183˚C
e. 20˚C.
Mech340: Engineering Material 3/9
12.For the Pb-Sn alloy in question 11, what phases will be present at 150˚C? (1 point)
a. α only
b. β only
c. L only
d. α + β.
13. For the Pb-Sn alloy in question 11, what is the equilibrium mass fraction of α phase at
100˚C? (2 points)
a. 0.27
b. 0.38
c. 0.62
d. 0.73.
14. Austenite at 750˚C is rapidly quenched to 625˚C and held at that temperature for 10 s.
Then, it is rapidly cooled to 250˚C and held for an additional 100 s before it is finally
quenched to room temperature. What will the final microstructure be? Refer to the
isothermal transformation diagram in figure 10.13. (2 points)
a. 50% pearlite + 50% martensite
b. 50% pearlite + 50% austenite
c. martensite with a trace of austenite
d. 100% bainite
15.Which Fe-C product below is expected to have the largest hardness? (2 points)
a. spheroidite
b. pearlite
c. bainite
d. tempered martensite
e. martensite
16. Shown is a plot of an engineering property (Y) versus the composition of a Cu-Ni alloy. Unfortunately, the label for Y is missing. What possible engineering property(s) are likely for Y? (2 points)
a. ductility
b. tensile strength
c. yield strength
d. b and c.
Mech340: Engineering Material 4/9
!"#"$"
%&'"()*+,"-."/"0*+1"+2"/3")34-3))5-34"05+0)516"789":)5.;."1<)"=+>0+.-1-+3"+2"/"?;@A-"/**+6'""
B32+51;3/1)*6C"1<)"*/D)*"2+5"8"-.">-..-34'""E</1"0+..-D*)")34-3))5-34"05+0)5167.9"/5)"*-F)*6"
2+5"8G"
/'" H;=1-*-16"
D'" 1)3.-*)".15)341<"
='" 6-)*H".15)341<"
H'" D"/3H"='"
""
IJ'"K;.1)3-1)"/1"!LJ"M?"-."5/0-H*6"N;)3=<)H"1+"OIL"M?"/3H"<)*H"/1"1</1"1)>0)5/1;5)"2+5"%J".'""
P<)3C"-1"-."5/0-H*6"=++*)H"1+"ILJ"M?"/3H"<)*H"2+5"/3"/HH-1-+3/*"%JJ"."D)2+5)"-1"-."2-3/**6"
N;)3=<)H"1+"5++>"1)>0)5/1;5)'""E</1",-**"1<)"2-3/*">-=5+.15;=1;5)"D)G""Q)2)5"1+"1<)"
-.+1<)5>/*"15/3.2+5>/1-+3"H-/45/>"D)*+,'"
/'" LJR"0)/5*-1)"S"LJR">/51)3.-1)"
D'" LJR"0)/5*-1)"S"LJR"/;.1)3-1)"
='" >/51)3.-1)",-1<"/"15/=)"+2"/;.1)3-1)"
H'" %JJR"D/-3-1)"
""
8"
Summer 2005
Problem 2 [15 Points]
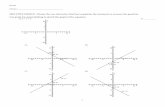
You are designing a turbine engine part made of an FCC single crystal. By using the Schmid law, determine the τc necessary for the part to have a uniaxial yield strength of 200 MPa in the [331] crystallographic direction?
—~ —CI,,>-z
-~ c//u2
+Iie S4L~-I Q+#L~L~ +A~ +~“‘
~CA+t~A- ~?1eY~3y
tS OY~7OyI—iOVlc~.¶ —(-0 ~bI,’. )
2£71-t L -f~
~~2~ 3
2
~fe~e5-~e~ foxor~~J~ LL
You are designing a turbine engine part made of an FCC single crystal. By using the Schmid
law1 determine the r~ necessary for the part to have a uniaxial yield strength of 200 MPa in
the {33 13 crystallographic direction?
Let us determine the Schinid factor of a FCC crystal pulled in the [331] direction.
SbpSystems
(111)
[110] [OIl] [101]
(Ill)
[110] [Oil] [101]
(111)
[110] [011] [101]
(111)
[110] [011] [101]Tensile cosp —~ 7
Axis cosA ~
~
0 — —
[331] M T T~7~ Th~ ~9V6 Vh”6 i9v’~ iRV6 T~7~ i~Vfi ig,R i~V6
The Schrrnd factor is thus ~ (tensile test in [331] direction).
~2
.F0~- -f~tLL c4,~y~ rec~cAo~ +0 #4~ pIace,0-Jr
-(-4
2~
ci
0So T~=~ ~ x200~z86 MPaj
Mech340: Engineering Material 5/9
Summer 2005
Problem 3 [30 Points]
Consider the gas carburizing of a gear of 1020 steel at 927˚C (1700˚F).
a. Calculate the diffusivity (diffusion coefficient) at 927˚C
b. Calculate the time in minutes necessary to increase the carbon content to 0.40% at 0.50 mm below the surface.
c. Calculate the carbon content at 0.50 mm beneath the surface of the gear after 5 hrs carburizing time.
Assume that the carbon content of the surface of the gear is 0.9% and that the steel has a nominal carbon content of 0.20%.
1-4tAI 6
Cs — Co
C5 = 0.90%
Co 0.20%
0.40%
x 0.5 mm 5.0 x 10~ m
D927~~ = 1.28 x 10—” m2/s
t =
Substituting the above values in Eq. (~) gives
0.90 — 0.40
0.90 — 0.20
0.50
0.70
( 69 88’\— 0rf = 0.7143
.1
69.88
Cc4~t 72 ~.
then erfZ = 0.7143
2
viA7¶S0L~Q~TQN S
Let
C)
Mech340: Engineering Material 6/9
c)
ortZ Z
o 7112o 7143o 7421
0.7143 — 0.71 12 x — 0.75
0.7421 — 0.7112 0.80 — 0.75
x — 0.75 (0.1003)(0.05)0,75
x0.80
x = 0.75 + 0.005 = 0.755
Thus
C5 = 0.90%
C0 = 0.20%
0.90 — C5
0.90 — 0.20
0.90 — C5
0.70
6%8 0755vi6988
92.60.755
= 8567 s = 143 mm 4
1.28 x 10—” m2/s
Cs—Cs _ / x N—erf
x = 0.50 mm = 5.0 x 10-a m
D92rc = 1.28 x 10-” m2/s
5 h 5 h x 3600 s/h 1.8 x 10~ s
=erf ~2”1.28 5.Ox 104m 1
L x 10-” rnls)(1.8 x jQ4 s)J
= erf 0.521
LetZ = 0.521. We need to know what the corresponding en-or function for the Z valueof 0.521 is. To determine this number from Table 4.1, we must interpolate the data ~shown in the accompanying table.
Z erfZ
0.500 0.52050.521 x0.550 0.5633
0.521 — 0.500 x — 0.5205
0.550 — 0.500 0.5633 — 0.5205
— x — 0.52050.42 0.0428
x — 0.5205 = (0.42)(0.0428)
x = 0.0180 + 0.5205
= 0.538
Therefore
00.90
—
0.70 = erf 0.521 = 0.538
C5 0.90 — (0.70)(0.538)
= 0.52% 4
Mech340: Engineering Material 7/9
Summer 2005
Problem 4 [20 Points]
a. On the basis of microstructure, explain why grey cast irons are recommended for compressive loading conditions and not for tensile loading conditions.
Grey cast irons contain graphite flakes in a ferrite or pearlite matrix. Graphite offers no strength and graphite flakes act as cracks in the matrix of grey cast irons. This makes the alloy very brittle and weak in tension. However, under compression cracks do not open and thus are not effective in reducing the mechanical strength of the material.
b. Can low carbon steels be quench hardened? No. Quenching hardens steels by forming martensite. The formation of martensite requires a minimum carbon concentration (usually ~ 0.4%C) to cause a reasonable level of lattice distortion. The carbon concentration in low carbon steels is too low to form martensite. c. How are low carbon steels strengthened? Low carbon steels are usually strengthened by cold working.
d. "Steel is made hard by quenching." List at least three requirements that must be met to justify this statement.
"Steel is made hard by quenching” because of the formation of martensite. The essential conditions for martensite to form in plain carbon steels are: (1) high enough carbon concentration (usually >0.4%) to cause lattice distortion, (2) high enough temperature to form austenite, and (3) fast enough cooling (quenching) to suppress C diffusion.
Mech340: Engineering Material 8/9
Summer 2005
Problem 5 [15 Points]
Suppose that CaO is added as an impurity to Li2O. If Ca2+ substitutes for Li+, what kind of vacancies would you expect to form? How many of these vacancies are formed for every Ca2+ added?
Suppose that CaO is added as an impurity to CaCl2. If the O2- substitutes for Cl- what kind of vacancies would you expect to form? How many of these vacancies are formed for every O2- added?
Mech340: Engineering Material 9/9