Anatomical and physiological evidence of white blush on baby carrot surfaces
-
Upload
independent -
Category
Documents
-
view
1 -
download
0
Transcript of Anatomical and physiological evidence of white blush on baby carrot surfaces
Postharvest Biology and Technology 55 (2010) 45–52
Contents lists available at ScienceDirect
Postharvest Biology and Technology
journa l homepage: www.e lsev ier .com/ locate /postharvbio
Anatomical and physiological evidence of white blush on baby carrot surfaces
Adriano do N. Simõesa, Marília C. Ventrellaa, Celso L. Morettib, Marcelo A.G. Carnelossi c, Rolf Puschmanna,∗
a Plant Biology Department, Federal University of Vicosa, 36571-000 Minas Gerais, Brazilb Postharvest Laboratory, Embrapa Vegetables, 70.359-970 Federal District, Brazilc Chemical Engineering Department, Federal University of Sergipe, 49.100-000 Sergipe, Brazil
a r t i c l e i n f o
Article history:Received 9 December 2008Accepted 19 July 2009
Keywords:Daucus carotaMinimally processedCell structureWhite blushDehydrationLignificationSuberization
a b s t r a c t
This study characterizes dehydration and white blush processes, structural alterations and synthesisof phenolic compounds (lignin and suberin), in relation to development of white blush on baby carrotsurfaces. Carrots were minimally processed as baby carrots and kept on polypropylene trays with orwithout polyvinyl chloride (PVC) film at 5 ± 2 ◦C, 90 ± 5% RH. During storage, baby carrots that were notwrapped with PVC film were rehydrated 1, 1.5, 15 and 17 h after minimal processing. Fresh-cut baby carrotswere evaluated for white blush index, sensory analysis (visual scores), fresh matter loss, phenylalanineammonia-lyase (PAL) activity and structural and histochemical changes. Increases in white blush indexand subjective visual scores on the carrot surface occurred in the first hours, when the material waskept on trays without PVC film and after 3 and 6 d, when stored on trays covered with PVC film. Visualassessment of white blush resulted in a more accurate assessment than instrumental evaluation becauseit allowed the perception of minor differences between distinct white blush levels, especially at the tips.Hydrating baby carrot surfaces for 1 and 1.5 h after processing allowed partial absorption of water bytissues and the orange color was reestablished on the surface. Fifteen hours after processing, even afterrehydration, the color did not return to the original orange standard. The rapid increase in PAL activity
in the secondary phloem of baby carrots, compared to that of whole carrots, suggested a physiologicalresponse as a result of abrasion. Dehydration and structural alterations of the more superficial cell layerswere the main causes of white blush in baby carrots that was not related to lignin accumulation, but-stru
1
ci1sa
bwcbbrto
0d
rather to synthesis of non
. Introduction
Baby carrots are processed raw carrots that are selected, rinsed,ut into cylindrical pieces 5–6 cm long, put through a lathe, washedn cold chlorinated water, centrifuged and packed (Bolin and Huxoll,991; Avena-Bustillos et al., 1994). Technological problems duringtorage, such as white blush on the surface, can reduce acceptancend consequently commercialization.
Early studies by Tatsumi et al. (1991, 1993) suggested that whitelush occurred as result of superficial dehydration and thereforeas a physical phenomenon. Later, Cisneros-Zevallos et al. (1995)
onfirmed the close relationship between dehydration and whitelush. The same authors concluded that, in the short term, white
lush was reversible, that is, when baby carrots were hydrated theyeturned to their original orange color. However, in the long term,his process would not happen, suggesting that there was a physi-logical event taking place.∗ Corresponding author. Tel.: +55 31 3899 2591.E-mail address: [email protected] (R. Puschmann).
925-5214/$ – see front matter © 2009 Elsevier B.V. All rights reserved.oi:10.1016/j.postharvbio.2009.07.007
ctural phenolic compounds.© 2009 Elsevier B.V. All rights reserved.
Further research by Bolin and Huxoll (1991), Bolin (1992),Howard and Griffin (1993) and Howard et al. (1994), suggested thatthe superficial whitening of peeled carrots was related to alterationin phenolic metabolism and resulted in lignin deposition on the sur-face, involving key enzymes such as phenylalanine ammonia-lyase(PAL), peroxidase (POD) and others (Howard and Griffin, 1993). Onthe other hand, Avena-Bustillos et al. (1994) suggested that whiteblush in peeled carrots resulted essentially from dehydration andthey reported that other authors could have misunderstood thephenomenon, since dehydration and lignification can occur inde-pendently and would not result in white blush.
Although white blush in fresh-cut baby carrots has been exten-sively studied, on most occasions physico-chemical, biochemicaland physiological tools have been used. Few studies have con-sidered anatomical issues. In some studies reddish purple spotshave been reported on the surface of mini carrots, characterized
by the reaction between lignin and phloroglucinol without show-ing the anatomical cut (Bolin and Huxoll, 1991; Howard and Griffin,1993). Furthermore, recent results obtained by scanning electronicmicroscopy have shown lignin in minimally processed carrot tis-sues (Rico et al., 2007). Histochemical studies allow identification of4 iology
chSfboloplb
2
2
tCk
2
limt
fr6wcs
2
iis1
2
afdpv
2
(fimcbbha
6 A.d.N. Simões et al. / Postharvest B
hemical substances and their location in plant cells and tissues andave been used in potatoes and carrots to identify lignin (Walter andchadel, 1983). Fifteen years after the first studies on white blush,ew studies currently have focussed on this subject, and the contri-ution of lignin to white blush is still doubtful, because this eventccurs rapidly, with almost no time for the induction of a physio-ogical response. In considering the aspects mentioned above, thebjective of the present study was to characterize the dehydrationrocess, white blush and the histological location of suberin, pheno-
ic compounds and lignin and to assess their contribution to whitelush on baby carrot surfaces.
. Materials and methods
.1. Raw material
Carrot plants (Daucus carota L. cv. Esplanada) were cultivated athe Federal University of Vicosa and collected 90 d after planting.arrots were washed in running water, with one batch of carrotsept whole and another minimally processed.
.2. Minimal processing
Carrots up to 2 cm in diameter were cut into pieces (5–6 cmong), selected and rounded using a PCE SKYMSEN® ETERNA® lathe,n two phases. The first phase worked with abrasive sandpapers (60
esh) and the second with small mesh files (100 mesh) both for 60 so obtain baby carrots (Lana et al., 2001).
Baby carrots were rinsed (rapid immersion in water at 5 ◦C)ollowed by immersion in water containing 200 mg L−1 active chlo-ine (dehydrated sodium dichloride-S-triazinatrion), at 5 ± 2 ◦C, for00 s. The final rinse was carried out by immersing baby carrots inater containing 3 mg L−1 active chlorine at 5 ± 2 ◦C for 600 s. Baby
arrots were then centrifuged for 15 s in a 251.33 rad s−1 angularpeed centrifuge without load.
.3. Refrigerated storage without PVC film
Whole carrots and baby carrots were stored without PVC filmn display cases at 5 ± 2 ◦C and 90 ± 5% RH for 36 h. White blushndex (WI), visual white blush, fresh matter loss, PAL activity andtructural and histochemical alterations were assessed at 0, 2, 4, 6,2, 24, and 36 h.
.4. Rehydration of baby carrots storage without PVC film
Baby carrots 5 cm long, 1.5 ± 0.1 cm in diameter, were selectednd stored at 5 ± 2 ◦C and 90 ± 5% RH, without PVC film. The sur-ace was hydrated 1.0, 1.5, 15 and 17 h after processing, using 3 mListilled water on each baby carrot (the volume was defined in areliminary test, using a 5 mL syringe). White blush index (WI),isual white blush and fresh matter were determined.
.5. Cold storage with PVC film
Baby carrots were stored on 90 mm × 170 mm × 25 mmwidth × length × height) polypropylene trays wrapped in PVC
lm (12 �m thick) with 300 g m−2 d−1 atm−1 water vapor per-eability, oxygen permeability of 15.68 mL m−2 d−1 atm−1, andarbon dioxide permeability of 80.52 mL m−2 d−1 atm−1. Wrappedaby carrots were kept at 5 ± 2 ◦C and 90 ± 5% RH for 21 d. Whitelush index (WI), fresh matter loss, PAL activity and structural andistochemical variations in whole carrots and baby carrots weressessed every 3 d.
and Technology 55 (2010) 45–52
2.6. White blush index (WI)
WI was determined according to Bolin and Huxoll (1991), usingthe parameters “L”, “a” and “b” and calculated indirectly by theformula WI = 100 − [(100 − L)2 + a2 + b2]1/2 given by a digital col-orimeter (Color reader CR-10 Minolta).
2.7. Visual white blush
Visual sensory assessments used scores ranging from 1 to 4.Score 1 represented the surface with the original orange coloring,without white blush; 2, the start of white blush, 1–15% of the surfacearea and the extremities, without quality damage; 2.5, moderatewhite blush (16–50%); 3, advanced white blush (51–70%), accep-tance limit; 4, extremely whitened surface (71–100%).
2.8. Fresh matter
Fresh matter was determined by gravimetry on a semi-analyticalscale.
2.9. PAL (EC 4.3.1.5) analysis
Samples (2.5 mm × 2.5 mm × 2.5 mm; width × length × height)of the carrot surface, without the periderm, were obtained. Sec-ondary phloem extraction and analyses were carried out accordingto Ke and Saltveit (1986), with adaptations. The enzyme extract wasobtained by homogenizing 2.0 g of plant material with 5 mL sodiumborate buffer (0.1 M) pH 8.8, containing �-mercaptanol (5 mM),EDTA (2 mM) and 1% insoluble polyvinyl pyrrolidone (PVPP) (p/v).The extract was then filtered through two layers of gauze and cen-trifuged at 25,000 × g, for 1200 s at 4 ◦C.
In the experiment 1.5 mL l-phenylalanine (60 mM) in boratebuffer (0.1 M) pH 8.8 was kept before the reaction at 40 ◦C, for 900 s.Then 0.5 mL of the enzyme extract was added and after 1 h incu-bation at 40 ◦C, absorbance was measured at 290 nm. Previouslyboiled extract was used as the control.
2.10. Structural and histochemical assessment
Surface tissues were collected from whole carrots and recentlyprocessed baby carrots, stored without wrapping for 1 and 36 h afterprocessing under conditions of 5 ± 2 ◦C and 90 ± 5% RH, withoutPVC film. Surface tissues were also collected from recently pro-cessed whole carrots and baby carrots stored for 10, 25 and 30 dafter minimal processing at 5 ± 2 ◦C and 90 ± 5% RH, with PVC film.
All plant material was fixed in FAA50 for 48 h and kept in 70%ethanol (Johansen, 1940) until samples were processed. For struc-tural analysis, 0.125 cm3 sections of the surface region of wholecarrots and baby carrots were blocked in methacrylate (Historesin-Leica) according to the manufacturer’s recommendations, and crosscut 8 �m thick in a rotating microtome. The material was stainedwith toluidine blue (O’Brein et al., 1964) for metachromasia or withtoluidine blue and lugol (Johansen, 1940), to show the phenoliccompounds and starch, and mounted in synthetic resin (Permount).
For histochemical analysis, 1 cm3 tissue pieces from the surfaceregion of whole and fresh-cut baby carrots after 30 d of storage were
sectioned with a table microtome and submitted to histochemi-cal tests: acid phloroglucin (Johansen, 1940) to detect lignin andsudan scarlet (Brundrett et al., 1991) to detect lipid compounds, andmounted in glycerinated gelatin (Sass, 1958). Sudan scarlet was alsoused in the material blocked in methacrylate and mounted in glyc-erinated gelatin (O’Brien and McCully, 1981). Images were obtainedby photomicroscopy (Olympus AX 70) using the U-Photo system.iology and Technology 55 (2010) 45–52 47
2
w(
3
3
aaatafi3t
bPtwP(
cgoaWsr
aowbs2
auormTt(lmc
tpTtmahTcib
Fig. 1. White blush index (A), visual white blush (B) and fresh matter loss (C) in
A.d.N. Simões et al. / Postharvest B
.11. Statistical analyses
A complete randomized experimental design was used. Dataere submitted to analysis of variance and Tukey’s test was applied
5% probability) to evaluate differences among treatments.
. Results and discussion
.1. White blush and dehydration
The white blush index of whole carrots did not alter during stor-ge (Fig. 1A), but was perceptible in baby carrots after 1 h, mainlyt the tips when they were kept on trays without PVC film (Fig. 1And B, arrows; see Fig. 4B). In the first 2 h there were increases inhe WI and in the visual assessment of surface white blush (Fig. 1And B). When baby carrots were stored on trays covered with PVClm, it was observed that WI mean values did not alter in the firstd (Fig. 2). After this period, white blush was observed also at the
ips.The variation in time to white blush perception, after 1 h for
aby carrots stored without PVC, and after 3 d when stored withVC film, showed the importance of a physical barrier to reducehe incidence of white blush. White blush appeared in parallelith the intense increase in WI, after 2 h, when stored without
VC (Fig. 1A) and up to 9 d, when stored wrapped with PVC filmFigs. 1A and 2).
On the other hand, for visual assessment, white blush scoreshanged from 3 to 4 (maximum score, Fig. 1B) after 2 h. It is sug-ested that visual sensory assessment provided a better perceptionf levels of white blush on baby carrot surfaces, resulting in a moreccurate assessment. Instrumental assessment showed increases inI with the perception of white blush on the surface under both
torage conditions (Figs. 1A and 2) with a Pearson correlation of= 0.61 at a level of 1% significance.
In general, orange color loss on the surface of baby carrots startedt the tips probably because it is a region where exposed sec-ndary xylem predominates. This tissue has lignin in the secondaryalls of some cells (Esaú, 1940), which in turn presents hydropho-ic properties (Laschimke, 1989). Furthermore, recent results havehown evidence of lignification in fresh-cut carrots (Rico et al.,007).
Fresh matter loss was similar in the two types of carrots 2 hfter minimal processing (Fig. 1C). However, when fresh matter val-es were examined, losses of about 1.25 ± 0.1% fresh matter werebserved for baby carrots and around 1.23 ± 0.1% for whole car-ots. Furthermore, at the end of 36 h, the difference between freshatter losses for the two types of carrots was approximately 3%.
hese results indicate the importance of the peridermis in main-aining high resistance to water vapor diffusion in whole carrotsSoliday et al., 1979). There were marked differences in fresh matteross between the two types of carrots, intact and fresh-cut, which
ay have influenced the level of white blush observed in babyarrots.
Dehydration also increased when baby carrots were stored onrays wrapped in PVC film (Fig. 2, internal graphic). The PVC filmrovided a 3 d delay for the beginning of white blush symptoms.his occurred because of the greater dehydration speed charac-erized by high water loss values (Fig. 2, internal graphic). Fresh
atter loss values of carrots stored without PVC were between 10
nd 12% at the end of 36 h (Fig. 1C), whereas those stored wrappedad lost about 3% fresh matter after 20 d (Fig. 2, internal graphic).hese results show the importance of wrapping to form a physi-al barrier to water vapor that minimized dehydration, althought did not contribute totally to the non-manifestation of whitelush.whole carrots (�) and baby carrots (�) stored for 36 h without PVC film at 5 ◦C and90 ± 5% RH. The arrows indicate the start of visual white blush. The bars representthe standard deviation from the mean from the three replications.
3.2. Baby carrot rehydration
When baby carrot surfaces were rehydrated at 1.0 and 1.5 h afterprocessing, an immediate reduction was observed in WI values andsubjective scores, followed by an absence of white blush. Fifteenhours after processing, fresh-cut baby carrots presented extensivewhite blush, with high values for WI and visual scores (Figs. 3A
and B). When rehydration took place at 15 and 17 h, return to theoriginal orange color was not observed; some seconds after rehy-dration, white blush was perceptible again (Fig. 3B, arrows), evenwith reductions in the WI values (Fig. 3A, arrows).48 A.d.N. Simões et al. / Postharvest Biology and Technology 55 (2010) 45–52
FsR
Imtt(
Fbai2
Fig. 4. PAL activity in the whole carrot ((�) superficial 0.25 cm secondary phloem,without peridermis); and baby carrots ((�) 0.25 cm secondary phloem) stored forup to 36 h, without PVC film at 5 ◦C and 90 ± 5% RH. The internal graphic represents
ig. 2. White blush index and fresh matter loss (internal graphic) in baby carrotstored on polypropylene trays wrapped in PVC film and stored at 5 ◦C and 90 ± 5%H for 21 d.
Fresh-cut baby carrot fresh matter reduced gradually (Fig. 3C).
n all the rehydrations there was an immediate increase in freshatter. However, the mass of water absorbed/adsorbed in the firstwo rehydrations was higher, between 0.28 and 0.25 g, comparedo the last two, which was 0.20 and 0.22 g on average, respectivelyFig. 3C). In general, the first two rehydrations enabled a clear return
ig. 3. White blush index (WI, A) visual white blush (B) and fresh weight (FW, C) inaby carrots stored up to 17.5 h after minimal processing, without PVC film, at 5 ◦Cnd 90 ± 5% RH. Dotted arrows indicate the course of white blush and regular arrowsndicate fresh weight changes immediately after rehydration 1, 1.5, 15 and 17 h (1st,nd, 3rd and 4th rehydration, respectively).
the PAL activity in a portion of the baby carrot secondary phloem (surface 0.25 cm)stored on PVC trays for 21 d at 5 ◦C and 90 ± 5% RH. The vertical bars represent thestandard deviation from the mean and the minimal significant difference (MSD) at5%. Data for three replications.
to the original orange color on the surface, whereas in the lasttwo rehydrations this event did not occur (Fig. 3B). The absenceof orange color recovery after the last two rehydrations suggestedthat, in part, a physiological event took place. This conclusion is inline with many authors who believe white blush to be the result oflignin deposits on the cell walls adjacent to the cut surface (Bolinand Huxoll, 1991; Howard and Griffin, 1993).
Immediate increases in fresh matter were generally observedafter rehydration (Fig. 3C) followed by reductions in WI and visualscores (Fig. 3A and B). This suggested an inverse relationshipbetween fresh matter and white blush index, although in differentproportions, once a small water gain resulted in an intense changein color in the first two rehydrations. This performance, however,was not observed in the last two rehydrations.
These results indicated that white blush occurred extensively inbaby carrots after minimal processing, in the first 2 h after process-ing and between 3 and 9 d in baby carrots stored with or withoutPVC, respectively. From a practical point of view, handling babycarrots as quickly as possible, during processing and packagingoperations, can minimize white blush on the surface. Furthermore,rehydration may be an alternative for baby carrots during minimalprocessing, once it allows the recovery of the original orange color,if the baby carrots have shown any of these symptoms for a shortperiod of time.
3.3. PAL (EC 4.3.1.5)
An intense increase in PAL activity, corresponding to the first0.25 cm on the surface, without the periderm, was observed in thefirst 36 h for baby carrots when compared to whole roots (Fig. 4).When baby carrots were stored for long periods, a parabola-shapedcurve was observed, with maximum activity at 9 d (Fig. 4, inter-nal graphic). These results indicated alteration in phenylpropanoidmetabolism. The processing operation of abrasion, may haveinduced the production of an elicitor of an unknown nature, which
accounted for the intercellular communication that increased PALactivity in the tissues adjacent to the cut surface, as suggested bySaltveit (2000), Saltveit et al. (2005) and Choi et al. (2005). PALactivation is well known in minimally processed carrot tissue andA.d.N. Simões et al. / Postharvest Biology and Technology 55 (2010) 45–52 49
F nd babfi as stab enoli
iH
rsatabsip
3
scmw
ig. 5. Cross-section photomicrographs of whole carrot surface regions (A and B) alm (D), after 10 and 30 d with PVC film (E and F, respectively). (A and C–E) Tissue wlue and lugol. S, suber; SC, phenolic compound secretory canal. Arrow indicates ph
ts response begins in the first days of storage (Babic et al., 1993;oward and Griffin, 1993).
These results did not indicate that the increase in PAL activityesulted in lignin accumulation, although this enzyme is the firsttep in the phenylpropanoid pathway (Dixon and Paiva, 1995) and islso essential for lignin synthesis (Monteiro et al., 2004). However,his biosynthetic pathway produces a variety of soluble phenoliccids in carrots (Klaiber et al., 2005) that are precursors for ligniniosynthesis (Brett and Waldron, 1996). Furthermore, although thetart of white blush coincides with the period of increased PAL activ-ty, there appears to be no correlation because they have differenthysiological and physical origins.
.4. Light microscopy
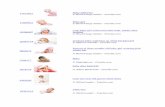
The peridermis was discrete in whole carrots (Fig. 5A) and con-isted of unistratified phelogen, phelloderm, and suber with fewell layers. There was a parenchyma region internally to the perider-is where there were many secretory canals. The greenish stainingith toluidine blue confirmed the presence of phenolic compounds
y carrots (C–F). Baby carrots recently processed (C), after 36 h at 5 ◦C without PVCined with toluidine blue. (B) Stained with sudan scarlet. (F) Stained with toluidine
c compounds.
in the canal secretion that is very common in the Apiaceae family(Esaú, 1940; Metcalfe and Chalk, 1957). The general structure of car-rot root peridermis was in line with that described by Esaú (1940)and O’Rear and Flore (1983) for the species.
In the suber, cell walls presented phenolic compounds, shownby the greenish staining (Fig. 5A) characteristic of toluidine blue(O’Brien and McCully, 1981), whereas lignin was identified by red-dish staining with acid phloroglucin. Suberin was also present insuber cell walls, confirmed by the orange-reddish staining char-acteristic of Sudan scarlet (Fig. 5B). This confirmed the presenceof phenolic compounds linked to the lipid material in the peri-dermis walls, as shown by Riley and Kolattukudy (1975). Ligninand suberin deposits in the cell walls of the carrot root arealso found in other species such as sweet potato (Walter andSchadel, 1983). Histochemistry can help identify the structural and
chemical changes, as shown in alfalfa lignification (Vallet et al.,1996).Immediately after carrots were abraded (Fig. 5C) the more super-ficial cell layers were eliminated to a depth of approximately 2 mm,including all the peridermis, the parenchyma region containing the
50 A.d.N. Simões et al. / Postharvest Biology and Technology 55 (2010) 45–52
Table 1Histochemical tests on intact and baby carrots in different storage conditions at 5 ◦C and 90 ± 5% RH.
Test Color Intact carrot (suber) Baby carrot (superficial cell layers)
Time of storage
Without PVCa film With PVCa film
Recently processed 36 h 10 d 25 d 30 d
Sudan scarlet for lipids Orangy-red + − − − − −Phloroglucin for lignin Red + − − − − −LT
spbiactrt
echtscn
ipbosttac
FcCa(
ugol for starch Brown −oluidine blue for phenolics Greenish +
a Polyvinylchloride.
ecretory canals and the more external layers of the secondaryhloem. The more external secondary phloem cells now coveredaby carrot surfaces and also became turgid shortly after process-
ng, although the most superficial cells were broken. The irregularspect of the new surface, formed by voluminous cells with pecto-ellulosic walls that were usually broken, probably contributed tohe superficial roughness of baby carrots. This roughness was notelated to white blush, because at this stage the product still main-ained its intense orange coloring.
After 36 h (Fig. 5D), baby carrot surfaces presented many lay-rs of collapsed and tangentally flattened cells. Cell collapse andell density was higher towards the surface, probably because ofigher dehydration, confirmed by the fresh matter loss (Fig. 1C) inhis period. Therefore, it can be considered that there were onlytructural modifications in the cells most exposed by abrasion andollapsed by dehydration and there was no accumulation of phe-olic compounds or lignin and/or suberin impregnation.
White blush in baby carrots, already observed 36 h after process-ng, may be associated with the formation of a physical barrier toigments visualization in the still hydrated inner cells. This physicalarrier consists basically of wall material and protoplasm residuesf collapsed and tangentially compressed cells. The considerablepreading of light on the irregular surface may also be related to
he perception of white blush. When the surface cells rehydrated,he spread of reflected light is reduced, resulting in a translucentppearance that enables the visualization of the original orangeolor (Cisneros-Zevallos et al., 1995).ig. 6. Proposed mechanism regarding the participation of some physical and physiologiarried out, in large part with minimally processed carrots (underlined letters) and on thisneros-Zevallos (2003); (b) Lafuente et al. (1996); (c) Picchioni et al. (1996); (d) Seljasend Alarcón (1995); (h) Campos-Vargas and Saltveit (2002); (i) Klaiber et al. (2005); (j) Ta1998); (m) Jiang and Zhang (2002); (n) Morris and Mann (1955); (o) Avena-Bustillos et a
− − − + ++− − + ++ ++
Superficial cell layer compaction was less evident in baby car-rots kept for 10 d in trays wrapped with PVC film. While compactingand cell collapse in the superficial layers presented a gradient fromthe peripheral to the center up to 36 h of storage, for the materialstored for 10 d this gradient was no longer observed. The band ofcollapsed cells was straighter and more compact, directly in con-tact with turgid parenchyma cells and more voluminous than on therecently processed surface (Fig. 5E). Although there were no ligninor suberin deposits, there was a discrete accumulation of phenoliccompounds in the collapse cell band and the physical barrier estab-lished started to restrict water loss from the more internal tissues,maintaining hydration of adjacent cells.
After 10 d of storage, the damage from dehydration in the col-lapsed cell band was more extensive and probably affected themembranes, because there was no reestablishment of the originalorange color when the baby carrots were rehydrated. This eventsuggested that color did not recover in an undefined manner, as ina purely physical system, but rather as a function of the recoverycapacity of the biological system. Extreme drying can cause lesionsin biological membranes (Berjak and Pammenter, 2000) and there-fore lead to the sudden and irreversible collapse of the cell osmoticsystem and water absorbing capacity.
After 30 d of storage (Fig. 5F) the accumulation of phenolic
compounds in the collapsed and condensed cells was more evi-dent, indicated by the greenish coloring of the toluidine blue andcounter-staining with lugol. This staining technique also permittedthe identification of small starch grains, stained brown, associatedcal responses that characterize white blush. The diagram was based on the studiese results obtained in this study. The references underlined are: (a) Surjadinata andn et al. (2001); (e) Czepa and Hofmann (2003); (f) Reyes et al. (2007); (g) Malonetsumi et al. (1991); (k) Cisneros-Zevallos et al. (1995); (l) Barry-Ryan and O’Beirne
l. (1994); (p) Artschwager and Starret (1931); (q) Babic et al. (1993).
iology
ttta(
fdp(tptd
tfigdwswvcistt
cpfrbdccl
A
fi
R
A
A
B
B
B
B
B
B
B
C
A.d.N. Simões et al. / Postharvest B
o the phenolic compound deposits (Fig. 5F and Table 1). The loca-ion and the negative reaction with acid phloroglycin showed thathe accumulated phenolic compounds were not structural, suchs lignin. Suberin deposits did not occur in this storage periodTable 1).
In general, the alterations that took place on baby carrot sur-aces during storage can be defined as the collapse and progressiveehydration of the most superficial cell layers, with phenolic com-ounds and the presence of small starch grains, also shown by Esaú1940) in carrot, without lignin and suberin deposits, as occurred inhe peridermis of whole carrots. Therefore, the alterations that tooklace did not characterize a scar-forming peridermis, as in potatoubers (Walter and Schadel, 1982; Thomson et al., 1995) with theevelopment of phelogen, and suber formation.
Based on the information in the literature and on the results ofhe present study, Fig. 6 shows a diagram proposing a hypothesisor the mechanism of physical and physiological responses that arenvolved in white blush in baby carrots. The results obtained sug-ested that white blush on baby carrot surface was the result ofehydration. It was also verified that white blush was reversiblehen dehydration was not extreme but irreversible when the tis-
ue suffered intense dehydration. The irreversibility in color occurshen water stress is more long-lasting and greater compacting is
isualized in the collapsed cell layers on the surface. This responsean result in cells that are physiologically incapable of absorb-ng/adsorbing water because of the damage to the cell membraneystem, that are associated with physical impairment generated byhe dehydrated walls, which can explain the incapacity to recoverhe natural orange color.
It was also concluded that recovering the orange color in babyarrots was not a process that occurs in an undefined manner. Thisrocess depends directly on the intensity of water loss and physical
actors, such as structural changes in the cell structure and indi-ectly, on a set of physiological factors related to the cell wateralance, because the biological membrane functionality has beenamaged by intense dehydration. Therefore white blush on babyarrots consists of a physical process, caused by dehydration asso-iated to the collapse of surface cells and is not associated withignin or suberin deposition.
cknowledgements
The authors are grateful to CNPq, PRODETAB and FAPEMIG fornancial support.
eferences
rtschwager, E., Starret, R.G., 1931. Suberization and wound periderm formation insweet potatoes and gladiolus as affected by temperature and relative humidity.J. Agric. Res. 43, 353–354.
vena-Bustillos, R.J., Cisneros-Zevallos, L.A., Krochta, J.M., Saltveit, M.E., 1994. Appli-cation of casein-lipid edible film emulsions to reduce white blush on minimallyprocessed carrots. Postharvest Biol. Technol. 4, 319–329.
abic, I., Amiot, M.J., Nguyen-the, C., Aubert, S., 1993. Changes in phenolics contentin fresh ready-to-use shredded carrots during storage. J. Food Sci. 58, 351–356.
arry-Ryan, C., O‘Beirne, D., 1998. Quality and shelf-life of fresh cut carrot slices asaffected by slicing method. Eng. Process. 63, 851–856.
erjak, P., Pammenter, N., 2000. What ultrastructure has told us about recalcitrantseeds. Rev. Bras. Fisiol. Veg., Brasília 12, 22–55.
olin, H.R., Huxoll, C.C., 1991. Control of minimally processed carrot (Daucus carota)surface discoloration caused by abrasion peeling. J. Food Sci. 56, 416–418.
olin, H.R., 1992. Retardation of surface lignification on fresh peeled carrots. J. FoodProcess. 16, 99–103.
rett, C., Waldron, K., 1996. Physiology and Biochemistry of Plant Cell Walls, seconded. Printed in Great Britain at the University Press, Cambridge, 255 pp.
rundrett, M.C., Kendrick, B., Peterson, C.A., 1991. Efficient lipid staining inplant material with Sudan Scarlet 7B ou Fluoral Yellow 088 in polyethyleneglycol–glycerol. Biotech. Histochem. 66, 111–116.
ampos-Vargas, R., Saltveit, M.E., 2002. Involvement of putative chemical woundsignals in the induction of phenolic metabolism in wounded lettuce. Physiol.Plant. 114, 73–84.
and Technology 55 (2010) 45–52 51
Czepa, A., Hofmann, T., 2003. Structural and sensory characterization of compoundscontributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree.J. Agric. Food Chem. 51, 3865–3873.
Choi, Y.J., Tomás-Barberán, F.A., Saltveit, M.E., 2005. Wound-induced phenolic accu-mulation and browning in lettuce (Lactuca sativa L.) leaf tissue is reduced byexposure to n-alcohols. Postharvest Biol. Technol. 37, 47–55.
Cisneros-Zevallos, L., Saltveit, M.E., Krochta, J.M., 1995. Mechanism of surface whitediscoloration of peeled (minimally processed) carrots during storage. J. Food Sci.60, 320–323.
Dixon, R.A., Paiva, N., 1995. Stress-induced phenylpropanoid metabolism. Plant Cell7, 1085–1097.
Esaú, K., 1940. Developmental anatomy of the fleshy storage organ of Daucus carota.Hilgardia 13, 175–226.
Howard, L.R., Griffin, L.E., 1993. Lignin formation and surface discoloration of mini-mally processed carrot sticks. J. Food Sci. 58, 1065–1068.
Howard, L.R., Griffin, L.E., Lee, Y., 1994. Steam treatment of minimally processedcarrots sticks to control surface discoloration. J. Food Sci. 59, 356–358.
Jiang, M., Zhang, J., 2002. Water stress-induced abscisic acid accumulation trig-gers the increased generation of reactive oxygen species and up-regulatesthe activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 53, 2401–2410.
Johansen, D.A., 1940. Plant Microtechnique. McGraw-Hill, New York, 423 pp.Ke, D., Saltveit, M.E., 1986. Effects of calcium and auxin on russet spotting and
phenylalanine ammonia-lyase activity in iceberg lettuce. HortScience 21, 1169–1171.
Klaiber, R.G., Baur, S., Koblo, A., Carle, R., 2005. Influence of washing treatmentand storage atmosphere on phenylalanine ammonialyase activity and pheno-lic acid content of minimally processed carrots sticks. J. Agric. Food Chem. 53,1065–1072.
Lafuente, M.T., López-Galvéz, G., Cantwell, M., Yang, S.F., 1996. Factors influencingethylene-induced isocoumarin formation and increasing respiration in carrot. J.Am. Soc. Hort. Sci. 121, 537–542.
Lana, M.M., Veira, J.V.V., Silva, J.B.C., Lima, D.B., 2001. Cenourete e catetinho: minicenouras brasileiras. Hort. Bras. 19, 376–379.
Laschimke, R., 1989. Investigation of the wetting behaviour of natural lignin-a con-tribution to the cohesion theory of water transport in plants. Thermochim. Acta151, 35–56.
Malone, M., Alarcón, J.J., 1995. Only xylem-borne factors can account for systemicwound signaling in the tomato plant. Planta 196, 740–746.
Metcalfe, C.R., Chalk, L., 1957. Anatomy of the Dicotyledons: Leaves, stem, and Woodin Relation to Taxonomy with Notes on Economic Uses. Clarendon Press, Oxford,2v.
Monteiro, M.B.O., Pereira, R.P.W., Abreu, H.S., 2004. Bioquímica da lignificacão decélulas xilemáticas. Floresta Ambiente 11, 48–57.
Morris, L.L., Mann, L.L., 1955. Wound healing, keeping quality and compositionalchanges during curing and storage of sweet potatoes. Hilgardia 24, 143–183.
O’Brein, T.P., Feder, N., McCully, M.E., 1964. Polychromatic staining of plant cell wallsby toluidine blue O. Protoplasma 59, 368–373.
O’Brien, T.P., McCully, M.E., 1981. The Study of Plant Structure: Principles and SelectedMethods. Termarcarphy Pty, Melburne.
O’Rear, L., Flore, J., 1983. Quantitative and qualitative characterization of carrot rootperiderm during development. J. Am. Soc. Hort. Sci. 108, 923–928.
Picchioni, G.A., Watada, A.E., Whitaker, B.D., Reyes, A., 1996. Calcium delayssenescence-related membrane lipid changes and increases net synthesis ofmembrane lipid components in shredded carrots. Postharvest Biol. Technol. 9,235–245.
Reyes, L.F., Villareal, E., Cisneros-Zevallos, L., 2007. The increase in antioxidant capac-ity after wounding depends on the type of fruit or vegetables tissue. Food Chem.101, 1254–1262.
Rico, D., Martín-Diana, A.B., Frias, J.M., Barat, J.M., Henehan, G.T.M., Barry-Ryan, C.,2007. Improvement in texture using calcium lactate and heat-shock treatmentsfor stored ready-to-eat carrots. J. Food Eng. 79, 1196–1206.
Riley, R.G., Kolattukudy, P.E., 1975. Evidence for covalently attached p-coumaric acidand ferrulic acid in cutins and suberins. Plant Physiol. 56, 650–654.
Sass, J.E., 1958. Botanical Microtechnique. The Iowa State University Press, Ames, IA.Saltveit, M.E., 2000. Wound induced changes in phenolic metabolism and tissue
browning are altered by heat shock. Postharvest Biol. Technol. 21, 61–69.Saltveit, M.E., Choi, Y.J., Tomás-Barberán, F.A., 2005. Involvement of components
of the phospholipid-signaling pathway in wound-induced phenylpropanoidmetabolism in lettuce (Lactuca sativa) leaf tissue. Physiol. Plant. 125, 345–355.
Seljasen, R., Bengtsson, G.B., Hoftun, H., Vogt, G., 2001. Sensory and chemical changesin five varieties of carrot (Daucus carota L.) in response to mechanical stress atharvest and post-harvest. J. Sci. Food Agric. 81, 436–447.
Soliday, C.L., Kolattukudy, P.E., Davis, R.W., 1979. Chemical and ultrastructural evi-dence that waxes associated with the suberin polymer constitute the mayordiffusion barrier to water vapor potato tuber (Solanum tuberosum L.). Planta 166,207–214.
Surjadinata, B.B., Cisneros-Zevallos, L., 2003. Modeling wound-induced respirationof fresh-cut carrots (Daucus carota L.). J. Food Sci. 68, 2735–2740.
Tatsumi, Y., Watada, A.E., Wergin, W.P., 1991. Scanning electron microscopy of carrotstick surface to determined cause of white translucent appearance. J. Food Sci.56, 1357–1359.
Tatsumi, Y., Watada, A.E., Wergin, W.P., 1993. Sodium chloride treatment or water-jet slicing effects on white tissue development of carrot sticks. J. Food Sci. 58,1390–1392.
5 iology
T
V
2 A.d.N. Simões et al. / Postharvest B
homson, N., Evert, R.F., Kelman, A., 1995. Wound healing in whole potato tubers:a cytochemical, fluorescence, and ultrastructural analysis of cut and bruisewounds. Can. J. Bot. 73, 1436–1450.
allet, C., Chabbert, B., Czaninski, Y., Monties, B., 1996. Histochemistry of lignindeposition during sclerenchyma differentiation in alfalfa stems. Ann. Bot. 78,625–632.
and Technology 55 (2010) 45–52
Walter Jr., W.M., Schadel, W.E., 1982. A rapid method for evaluating curing progressin sweet potatoes. J. Am. Soc. Hort. Sci. 107, 1129–1133.
Walter Jr., W.M., Schadel, W.E., 1983. Structure and composition of normal skin (peri-derm) and wound tissue from cure sweet potatoes. J. Amer. Soc. Hort. Sci. 108(6), 909–914.