Patologi Endometriosis
-
Upload
laurensia-erlina-natalia -
Category
Documents
-
view
163 -
download
1
description
Transcript of Patologi Endometriosis

A. Patologi
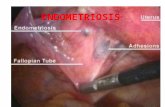
Organ yang biasa terkena endometriosis adalah ovarium, organ tuba dan salah
satu atau kedua ligamentum sakrouterinum, Cavum Douglasi, dan permukaan uterus
bagian belakang dapat ditemukan satu atau beberapa bintik sampai benjolan kecil yang
berwarna kebiru-biruan (Prawirohardjo, 2008).
Gambar 6. Kista cokelat yang pecah pada ovarium sebelah kiri
(http://en.wikipedia.org/wiki/file:Perforierte_EndometrioseZyte.jpg)
B. Gambaran kista endometriosis
Penampakan kasar endometriosis dapat berupa suatu penebalan atau kista yang
berisi darah baru, merah atau biru hitam. Semakin lama lesi-lesi tersebut berubah
menjadi rata dan berwarna coklat tua. Struktur kista besar bisa tetap berisi darah tua dan
disebut kista cokelat. Lesi-lesi yang sudah lama bisa tampak pucat, tersebar, dan
mengerutkan jaringan setempat. Ukuran lesi bervariasi dari kecil kurang dari 1 mm
sampai dengan kista besar berukuran lebih dari 10 cm (Rayburn, 2001). (Gambar 7 dan
Gambar 8.)

Gambar 7. Kista cokelat pada ovarium
(http://img.webmd.com/medscape/netbeacon.html)

Gambar 8. Lesi merah pada berbagai organ
(http://img.webmd.com/medscape/netbeacon.html)
C. Klasifikasi endometriosis
Berdasarkan visualisasi rongga pelvis dan volume tiga dimensi dari endometriosis
dilakukan penilaian terhadap ukuran, lokasi dan kedalaman invasi, keterlibatan ovarium
dan densitas dari perlekatan. Dengan perhitungan ini didapatkan nilai-nilai dari skoring
yang kemudian jumlahnya berkaitan dengan derajat klasifikasi endometriosis. Nilai 1-4
adalah minimal (stadium I), 5-15 adalah ringan (stadium II), 16-40 adalah sedang
(stadium III) dan lebih dari 40 adalah berat (stadium IV) (Rusdi, 2009).
Tabel 2. Derajat endometriosis berdasarkan skoring dari Revisi AFS

Sumber: American Fertility Society, 2007a.
Skema klasifikasi berdasarkan beratnya penyakit endometriosis menurut
American Fertility Society (2007a) dapat dilihat pada gambar dibawah.
Endometriosis <1cm 1-3 cm >1cm
Peritoneum Permukaan 1 2 4
Dalam 2 4 6
Ovarium Kanan Permukaan 1 2 4
Dalam 4 16 20
Kiri Permukaan 1 2 4
Dalam 4 16 20
Perlekatan kavum douglas Sebagian Komplit
4 40
Ovarium Perlekatan <1/3 1/3-2/3 >2/3
Kanan Tipis 1 2 4
Tebal 4 8 16
Kiri Tipis 1 2 4
Tebal 4 8 16
Tuba Kanan Tipis 1 2 4
Tebal 4 8 16
Kiri Tipis 1 2 4
Tebal 4 8 16

Gambar 9. Skema klasifikasi stage 1 sampai stage 3. (American Fertility
Society, 2007a)
Gambar 10. Skema klasifikasi stage 3 sampai stage 4. (American Fertility
Society, 2007a)
Diferensial Diagnosis

Ultrasound Features to Distinguish Endometrioma from Other Pelvic Masses
ANIS UR REHMAN, IFFAT MANSOOR*, MUHAMMAD AKRAM DOGAR**
Department of Radiology, NGHA, Dammam, & *Department of Gynaecology, Astoon
Hospital, Alkhobar, Kingdom of Saudi Arabia
**Department of Surgery, Mayo Hospital, Lahore
Correspondence to Dr. Anis ur Rehman, Radiologist Email: [email protected]
ABSTRACT
Purpose: To evaluate diagnostic accuracy of ultrasound findings in the diagnosis
and discrimination of endometriomas from other adnexal masses.
Materials and methods: Ultrasound examination of 120 adnexal masses in 98
women was done and findings were recorded on a check list. Philips HDI 5000 and
I21 ultrasound machines were used. Diagnostic features which can discriminate
endometriomas from other pelvic masses were reviewed.

Results: There were 30 endometriomas. Diffuse low-level internal echoes were
present in 29 (97%) of endometriomas and in 5 (17%) nonendometriomas.. The
presence of multilocularity or hyperechoic wall foci further increased the positive
likelihood ratio to 35, allowing the identification of 15 endometriomas (50%).
Conclusion: ultrasound features of diffuse low level echoes, echogenic wall and
multilocularity has a high positive predict value in the diagnosis of endometrioma
and helping it in discrimination from other pelvic masses. Endometriomas exhibits
wide range of ultrasound features, ranging from anechoic to echogenic cysts to
masses containing multiple septations and solid tissue.
Key words: Endometrioma, ultrasound, pelvic masses
INTRODUCTION
Endometriosis is defined as the presence of endometrial glandular tissue outside of the uterus.
Two main theories exist for the pathogenesis of endometriosis. One theory is that endometrial
tissue is spread by retrograde menstruation or by vascular and/or lymphatic spread. The
second theory holds that the serosal epithelium of the peritoneum undergoes metaplastic
differentiation into endometrium-like tissue.
The theory that retrograde menstruation causes endometriosis is supported by the
analysis of peritoneal fluid in women. As many as 90% of women have blood in the
peritoneal fluid during the perimenstrual period. In addition, endometrial cells have been
found in the peritoneal fluid. The pattern of endometriosis is consistent with retrograde
menstruation and is most common in the ovary, followed by the other dependent areas of the
pelvis. Vascular and/or lymphatic spread is supported by noting the occasional distal
(extraperitoneal) sites of endometriosis, including the lungs and central nervous system
(CNS). In addition, teenage girls with obstructive uterine or vaginal anomalies show
retrograde menstrual bleeding, and endometriosis is common in these patients.
This study is designed to evaluate the accuracy of conventional grey scale ultrasound
examination as a first line diagnostic modality and to evaluate its predictability for the need
of any further diagnostic modality.
MATERIALS AND METHODS

The study consisted of a retrospective review of the sonograms from 98 women with 120
adnexal masses. Two groups of patients were selected. First group of patients was reviewed
in National Guard hospital, Dammam (52 patients) and second group of patient (46 patients)
examined in Astoon Hospital Dammam. Study period was one year and seven months
(February 2007 to September 2008). Philips HDI 5000 and I21 ultrasound machines were
used. Trans-abdominal and endovaginal probes were used to asses these patients.
Inclusion criteria: All female patient of reproductive age with clinically suspicion of pelvic
mass.
Exclusion criteria: Women who were suspected of having an ectopic pregnancy or pelvic
inflammatory disease.
Two ultrasound examiners blinded to the clinical history of the patient, the information about
follow-up examinations, and the final diagnosis independently reviewed each sonogram. For
each mass, both sonologists recorded US features by using a standardized checklist:
Ultrasound Findings Check
List
Focal Acoustic Impedance
Regional Bright Echoes
Hyperechoic Lines And Dots
Fluid-Fluid Levels(gravity-
dependent linear interface
between two materials of
differing echogenicity)
Fibrinous strands
Retracting Clot (marginal
clumped echoes with concave
margins)
Diffuse Low-Level Internal
Echoes
Hyperechoic Wall Foci (punctate
peripheral echogenic foci)
Internal Septal Thickness

Masses were categorized as cystic or solid. The sensitivity, specificity, positive predictive
value, negative predictive value, negative likelihood ratio, and positive likelihood ratio were
analyzed for the diagnosis of endometrioma according to each US finding and selected
combinations of findings.
Our study design does not allow for determination of the true sensitivity of disease
detection because patients without ultrasonographically identified adnexal masses were not
surgically evaluated. The negative likelihood ratio is defined as (1−sensitivity)/specificity,
and the positive likelihood ratio is defined as sensitivity (1−specificity). Likelihood ratios are
useful because they do not change with the pretest probability of disease, unlike sensitivity
and specificity.
RESULTS
Among the 120 masses, there were 30 endometriomas (25% prevalence). Of the 90
nonendometriomas, there were 11 cystic teratomas, 17 benign neoplasms, 5 malignant
neoplasms, 29 hemorrhagic cysts, 26 other nonneoplastic cysts (16 histopathologically
proved nonneoplastic ovarian cysts, 7 self-resolving anechoic ovarian cysts, 2 cases of
hydrosalpinx, and 1paratubal cysts), 1 case of ovarian torsion, and 1 broad ligament myoma.
Two adnexal cystic masses showing diffuse internal low level echoes.
Hemorrhagic cyst of the right ovary in a 33-year-old woman, showing diffuse low level
internal echoes, difficult to differentiate on ultrasound features but usually resolves on follow
up ultrasounds.

Endometrioma in a 25-year-old woman, exhibits diffuse low-level internal echoes and
punctate peripheral echogenic foci
An endometrioma in a 30-year-old woman. Hyperechoic wall foci (arrow) and low-level
echoes
Multiloculated endometrioma showing low level internal echoes and thin septations in a 32
years old woman.
DISCUSSION
Ectopic endometrial glandular tissue is influenced by ovarian hormones and undergoes cyclic
bleeding. Over time, the repeated hemorrhaging can produce extensive fibrosis surrounding
the endometrial tissue, which can result in adhesions to adnexal structures or to bowel and
can obliterate the pelvic cul-de-sac.
When the ovaries are involved, they can become enlarged with cystic, blood-filled
spaces that, on gross examination, are termed chocolate cysts, or endometriomas.
Endometriomas can become large and multilocular. The endometrial-cell lining can become
obliterated over time, making the pathologic distinction between an endometrioma and a
hemorrhagic cyst difficult in some cases.

There is a wide spectrum of ultrasound features that can mimic endometrioma which
lead to this false impression that with ultrasound impossible to achieve a high positive
predictive value in the diagnosis of endometrioma. Several investigators have sought to
identify the diagnostic performance of US in distinguishing endometriomas from other
adnexal masses, with each group using features different than those used by the others.
Clearly, there is a range in what experts have defined as a “classic” endometrioma. These
studies have not addressed which of the criteria used are most important in discriminating
between an endometrioma and other adnexal lesions. While the presence of low-level echoes
is a uniform diagnostic criterion for all studies, diagnostic requirements for the thickness and
contour of the wall and shape and location of the lesion differ among the investigations.
Understanding the degree to which any particular feature or set of features increases or
decreases the likelihood ratio for the diagnosis of endometrioma is important, because one
can then use this knowledge to direct the evaluation toward identifying those features that
have the greatest relevance and avoid effort and confusion in characterizing those features
that have no relevance.
Our study systematically addressed these issues, and our findings confirm that the
presence of diffuse low-level internal echoes is the important feature that helps to
discriminate an endometrioma from other lesions; 97% of endometriomas exhibit diffuse
low-level internal echoes. While the absence of this finding does not exclude endometrioma,
it significantly decreases the likelihood of that diagnosis (negative likelihood ratio, 0.1).
Certain US features (focal acoustic impedance, regional bright echoes, and hyperechoic lines
and dots) are predictive of cystic teratomas . Because most endometriomas do not
demonstrate these features, excluding masses with these features improves diagnostic
performance.
Our data suggest that there is no diagnostic value to the assessment of wall thickness. On
the other hand, we demonstrate considerable benefit from the assessment of wall nodularity, a
feature that is associated with neoplasia. Excluding masses with wall nodularity from the
diagnosis of endometrioma helped to exclude neoplasm, especially malignancy, as a false-
positive diagnosis
Hemorrhagic ovarian cysts can also demonstrate diffuse low-level internal echoes and
typically do not demonstrate those features discussed above that are associated with dermoids
or other neoplasms. Using only these criteria to predict that a mass is an endometrioma yields
majority of false-positive diagnoses representing hemorrhagic cysts. Hemorrhagic cysts are
almost exclusively nonneoplastic. Thus, they resolve spontaneously and are surgically

removed only when patients have compelling acute symptoms. This has justifiably led to the
practice of follow-up US as a diagnostic strategy to use when a mass is thought most likely to
represent an endometrioma but that alternatively may represent an acutely hemorrhagic cyst,
a strategy that virtually eliminates the possibility that a patient will undergo an unnecessary
operation.
Our data document the usefulness of this strategy. Hemorrhagic cysts are
Demonstration of hyperechoic wall foci in a mass with low-level echoes and absence of
neoplastic features is strongly predictive of an endometrioma. We separately evaluated “wall
nodularity” from “hyperechoic wall foci.” Thirty-five percent of endometriomas in our series
demonstrated these foci, while only 6% of nonendometriomas did so. Thus, our data indicate
that a mass with low-level internal echoes, hyperechoic wall foci, and no neoplastic features
is 30 times more likely to be an endometrioma than another adnexal mass and that US follow-
up in such lesions is a low-yield course of action
Another feature of diagnostic usefulness is the presence of septations without nodularity,
also known as multilocularity. However, when used as a feature in addition to low-level
echoes and absence of neoplastic features, multilocularity increased the likelihood that a mass
was an endometrioma. As a result, a mass with low-level echoes, without neoplastic features,
and with multilocularity is 58 times more likely to represent an endometrioma than another
adnexal mass.
This study analyzed adnexal masses that were not related to pelvic inflammatory disease
or ectopic pregnancy. While these entities may manifest US features similar to those
discussed here, the clinical presentations in patients with pelvic inflammatory disease and
ectopic pregnancy differ.
CONCLUSION
Gray-scale US can achieve a high degree of accuracy in the diagnosis of endometrioma. An
adnexal mass with diffuse low-level internal echoes and absence of particular neoplastic
features is highly likely to be an endometrioma if there are no features of acute hemorrhage
and especially if multilocularity or hyperechoic wall foci are present.
In addition, a diagnosis of endometrioma is highly unlikely when no component of an
adnexal mass contains low-level internal echoes.. For those masses with low-level internal
echoes, follow-up US appears most useful when the mass has no wall nodularity, is
unilocular, and does not possess hyperechoic wall foci.

MR imaging may be useful in discriminating between endometriomas and neoplasms
when wall nodularity is present.
REFERENCES
1. Endometriosis: Radiologic-Pathologic Correlation Radiographics January 2001 21:193-
216
2. Fried AM, Rhodes RA, Morehouse IR. Endometrioma: analysis and sonographic
classification of 51 documented cases. South Med J 1993; 86: 297-301.
3. Pelvic Pain: Overlooked and Underdiagnosed Gynecologic Conditions Radiographics
January 2005 25:3-20; doi:10.1148/rg.251045511
4. Moran RE, Older RA, De Angelis GA, Baghdady BH, Chrisman HB, Ciambotti JM.
Genitourinary case of the day: endometrioma. AJR 1996; 167: 246, 248-249.
5. Mais V, Guerriero S, Ajossa S, Angiolucci M, Paoletti AM, Melis GB. The efficiency
of transvaginal ultrasonography in the diagnosis of endometrioma. Fertil Steril 1993; 60:
776-780.
6. Guerriero S, Mais V, Ajossa S, Paoletti AM, Angiolucci M, Melis GB. Transvaginal
ultrasonography combined with CA-125 plasma levels in the diagnosis of endometrioma.
Fertil Steril 1996; 65: 293-298.
7. Guerriero S, Ajossa S, Paoletti AM, Mais V, Angiolucci M, Melis GB. Tumor markers
and transvaginal ultrasonography in the diagnosis of endometrioma. Obstet Gynecol
1996; 88: 403-407.
8. Jain KA. Prospective evaluation of adnexal masses with endovaginal gray-scale and
duplex and color Doppler US: correlation with pathologic findings. Radiology 1994; 191:
63-67.
9. Volpi E, De Grandis T, Zuccaro G, La Vista A, Sismondi P. Role of transvaginal
sonography in the detection of endometriomata. J Clin Ultrasound 1995; 23: 163-167.
10. Alcazar JL, Laparte C, Jurado M, Lopez-Garcia G. The role of transvaginal
ultrasonography combined with color velocity imaging and pulsed Doppler in the
diagnosis of endometrioma. Fertil Steril 1997; 67: 487-491.
11. Outwater E, Schiebler ML, Owen RS, Schnall MD. Characterization of hemorrhagic
adnexal lesions with MR imaging: blinded reader study. Radiology 1993; 186: 489-494.

12. Takahashi K, Okada S, Okada M, Kitao M, Kaji Y, Sugimura K. Magnetic resonance
imaging and serum CA-125 in evaluating patients with endometriomas prior to medical
therapy. Fertil Steril 1996; 65: 288-292.
13. Radack KL, Rouan G, Hedges J. The likelihood ratio: an improved measure for
reporting and evaluating diagnostic test results. Arch Pathol Lab Med 1986; 110: 689-
693.
14. Atri M, Nazarnia S, Bret PM, Aldis AE, Kintzen G, Reinhold C. Endovaginal
sonographic appearance of benign ovarian masses. RadioGraphics 1994; 14: 747-760.
15. Fried AM, Kenney CM, III, Stigers KB, Kacki MH, Buckley SL. Benign pelvic masses:
sonographic spectrum. RadioGraphics 1996; 16: 321-334.
16. Patel MD, Feldstein VA, Lipson SD, Chen DC, Filly RA. Cystic teratomas of the ovary:
diagnostic value of sonography. AJR 1998; 171: 1061-1065.
17. Sassone AM, Timor-Tritsch IE, Artner A, Westhoff C, Warren WB. Transvaginal
sonographic characterization of ovarian disease: evaluation of a new scoring system to
predict ovarian malignancy. Obstet Gynecol 1991; 78: 70-76.
Endometriomas
Transvaginal sonography (TVS) is universally most frequently used imaging tool for
evaluation of endometriomas. USG features of chocolate cysts are diverse. The classical
appearance is that of a cystic structure with diffuse low level internal echoes and echogenic
wall foci. The cyst may be unilocular or multilocular. It may contain thin or thick septa.
Sometimes there may be wall nodularity. Wall nodularity if present requires further
investigation to rule out malignancy. Imaging alone cannot exclude malignant neoplasm.
It is interesting to note that out of 20% of the endometriomas exhibiting wall nodularity, 35%
had hyperechogenic wall foci (Patel et al). Effort should be made to distinguish between wall
nodularity and the hyperechogenic foci within the wall. The latter when present in lesion with
low level echoes and no features of malignancy is indicative of endometrioma.
Differential diagnoses of chocolate cyst include haemorrhagic cyst, dermoid cyst and cystic
neoplasms. Dermoid cyst usually exhibit either echogenic shadow due to its fat content or
acoustic shadowing due to calcium which aids in the diagnosis. To differentiate between
haemorrhagic cyst and chocolate cyst can be a difficult task. The haemorrhagic cyst usually

displays high level internal echoes within a thin walled cyst which may advance with time
and emerge as a more complex cyst. Formation of fibrin may imitate thin septa but these
lesions usually resolve on follow up.
The accuracy of USG can be further improved by color Doppler flow studies. Blood flow in
the endometrioma is through the regularly spaced vessels running in the hilar region and the
pericystic space.
MRimaging is another tool for identifying endometriomas. Due to the cyclical bleeding
endometriomas contain blood products at different age. They are seen as bright or
hyperintense lesions on T1 weighted image. On T2 they appear more hypointense or dark
with foci of hyperintensity, imparting it the classical appearance of ‘shading’. Shading is
effect of degenerated blood products present at different stage within the same cyst. It can
range from subtle layering to a complete signal void (black).
Since both the haemorrhagic cysts and the chocolate cyst contain blood products, it can be
difficult to distinguish between them except for the fact that hemorrhagic cysts do not display
shading, are mostly unilocular and resolve on interval imaging. In contrast dermoid cysts are
easily diagnosed on MRI since they lose the signals and become dark on fat suppressed
sequences.
After contrast administration, the periovarian peritoneal surface of the cyst can be enhanced
which can help in identification of torsion ovary. Endometrioma in an enlarged but poorly
enhancing ovary with peripherally located follicles is suggestive of torsion ovary on
MRimaging.